Abstract
Objective
In the Netherlands, the threshold for offering active treatment for spontaneous birth was lowered from 25+0 to 24+0 weeks’ gestation in 2010. This study aimed to evaluate the impact of guideline implementation on survival and causes and timing of death in the years following implementation.
Design
National cohort study, using data from the Netherlands Perinatal Registry.
Patients
The study population included all 3312 stillborn and live born infants with a gestational age (GA) between 240/7 and 266/7 weeks born between January 2011 and December 2017. Infants with the same GA born between January 2007 and December 2009 (N=1400) were used as the reference group.
Main outcome measures
Survival to discharge, as well as cause and timing of death.
Results
After guideline implementation, there was a significant increase in neonatal intensive care unit (NICU) admission rate for live born infants born at 24 weeks’ GA (27%–69%, p<0.001), resulting in increased survival to discharge in 24-week live born infants (13%–34%, p<0.001). Top three causes of in-hospital mortality were necrotising enterocolitis (28%), respiratory distress syndrome (19%) and intraventricular haemorrhage (17%). A significant decrease in cause of death either complicated or caused by respiratory insufficiency was seen over time (34% in 2011–2014 to 23% in 2015–2017, p=0.006).
Conclusions
Implementation of the 2010 guideline resulted as expected in increased NICU admissions rate and postnatal survival of infants born at 24 weeks’ GA. In the years after implementation, a shift in cause of death was seen from respiratory insufficiency towards necrotising enterocolitis and sepsis.
Keywords: neonatology, mortality
What is already known on this topic?
The Dutch approach towards preterm infants treatment is more conservative compared with other countries; therefore, survival outcomes of international studies may not be applicable.
In 2010, the Dutch guideline on treatment of extreme preterm birth was revised to offer active treatment of infants born from 24+0 weeks onwards.
Respiratory distress syndrome, necrotising enterocolitis, intraventricular haemorrhage and infection have been reported earlier as main causes of death in extremely preterm infants.
What this study adds?
Implementation of the 2010 guideline, supporting active treatment of infants born at 24+0 weeks’ gestation, resulted in increased neonatal intensive care unit admission rates and postnatal survival.
Cause-specific survival curves show different periods of vulnerability of death for different causes of death.
A decrease in death complicated or caused by respiratory insufficiency was seen in the last 3 years.
Introduction
In the past two decades, the Dutch guideline for active treatment of extremely preterm infants has been modified twice. Until 2006, the guideline had been very restrictive, and in the majority of the centres, infants born below 26+0 weeks of gestation were not actively treated unless they were considered viable. In 2006, this guideline was changed to include active treatment of infants born with a gestational age (GA) between 25+0 and 25+6.1 In 2010, the perinatal guideline was revised again, lowering the threshold to offer active treatment from 25+0 to 24+0 weeks of gestation.
Since the national guideline implementation in 2010, knowledge on the impact of change in perinatal treatment approach on survival is limited.2 International studies published during the last decade have shown variability in survival rates and outcomes among healthcare settings as well as within countries.3–13 Furthermore, survival data of international studies may not be generalisable due to differences in practices, healthcare system, outcome definitions and study period.9 13–15 The availability of up-to-date longitudinal and GA stratified data on survival is important as this influences antenatal counselling, resuscitation policies in the delivery room or future revision of the perinatal guideline.
In the presurfactant era, most extremely preterm infants died within a few days after birth due to immaturity and respiratory failure. A recent study has shown that changes in neonatal care may have led to death at a later time point after birth.16 Also changes and innovations in neonatal care may have led to a shift from death attributable to pulmonary causes towards death attributable to non-pulmonary causes.16 To further evaluate survival rates in preterm infants, more insight in causes and timing of death of extremely preterm infants is necessary.
This study aimed to evaluate survival in extremely preterm infants in the Netherlands in the past decade. As a first aim, the impact of the guideline implementation in 2010 on survival was assessed. As secondary aims, causes and timing of death in the years following guideline implementation were evaluated.
Methods
Patient population
This population-based study included all stillborn and live born extremely preterm infants born between 24+0 and 26+6 weeks of gestation between 1 January 2011 and 31 December 2017 in the Netherlands. As a reference group, all infants born between 24+0 and 26+6 weeks of gestation in the period 1 January 2007 until 31 December 2009 in the Netherlands were used. Infants born in 2010 were excluded, since this was the year the guideline was implemented.
Data collection
For this study, data from the Netherlands Perinatal Registry (Perined) were used.17 This registry contains linked population-based information regarding pregnancy, delivery, (re)admissions and pregnancy outcomes, as registered by midwives (LVR1 registration), obstetricians (LVR2 registration) and paediatricians/neonatologists (LNR registration). All 10 perinatal centres with a level III neonatal intensive care unit (NICU) facility export data to this national registry. The LVR1-2 covers approximately 99% of all births ≥160/7 weeks’ gestation in the Netherlands, including delivery room deaths and stillbirths. The LNR covers 100% of all live-born infants admitted to one of the ten Dutch intensive care units, as these units are obligated to register all admissions. Variables used from the registry for this study included information on birth weight, sex, method of birth and multiplicity of birth. Small for gestational age was defined as a birth weight below 10th percentile.18
Outcome measurements
Several outcome measurements were used, including live births (as a percentage of total births), NICU admission rate (defined as having at least 1 day of NICU admission registered in the LNR as a percentage of live births) and survival to discharge (as a percentage of live birth as well as NICU admissions). In all NICU admitted infants who died, cause of death was classified according to Patel et al.16 The primary cause of death was identified and defined as the single underlying, proximate disease that initiated the series of events leading to the final cause of death. The principal investigator of each centre classified cause of death for each subject based on information in the medical records. If the principal investigator could not decide on the primary cause of death, consensus was reached through a discussion in an expert panel group consisting of three neonatologists (JLvH, WO and PA).
Statistical analysis
Continuous variables were compared between the study period and reference period using the Student’s t-test. Categorical and dichotomous variables were compared using the χ2 test. To evaluate possible shifts in causes of death, two periods (2011–2014 and 2015–2017) were compared using a χ2 test. Kaplan-Meier curves were used to show 3-month survival, and a proportional hazard test was performed to analyse difference in survival between 2011–2014 and 2015–2017. A p value <0.05 was considered statistically significant. Calculations were performed using R V.3.5.1.
Results
Patient population
The total study population (stillborn and live born, gestation between 240/7 and 266/7 weeks, 2011–2017) consisted of 3312 infants, with 2569 live born infants (78%). Table 1 shows outcome measurements of all infants born with a GA between 24+0 and 26+6 weeks of gestation between 2011 and 2017, compared with infants born in the reference period 2007–2009. A total of 2121 infants (83% of live born infants) were admitted to a NICU in 2011–2017, compared with 694 infants (70% of live born infants) in 2007–2009 (p value <0.001). Table 2 shows the neonatal baseline characteristics for the admitted infants. Of the 2121 admitted infants in 2011–2017, 1518 (72%) infants survived until discharge home, compared with 491 (71%) of the admitted infants in 2007–2009 (p value 0.714).
Table 1.
Outcome measurements for all infants born with a gestational age between 24+0 and 26+6 weeks between 2011 and 2017, compared with infants born between 2007 and 2009.
| 24 weeks | 25 weeks | 26 weeks | Total cohort | |||||||||
| 2007–2009 | 2011–2017 | P value | 2007–2009 | 2011–2017 | P value | 2007–2009 | 2011– 2017 | P value | 2007–2009 | 2011–2017 | P value | |
| n=406 | n=1013 | n=442 | n=999 | n=522 | n=1300 | n=1370 | n=3312 | |||||
| Live born | 255 (62.8) | 697 (68.8) | 0.035* | 328 (74.2) | 757 (75.8) | 0.569 | 405 (77.6) | 1115 (85.8) | <0.001* | 988 (72.1) | 2569 (77.6) | <0.001* |
| Admitted to NICU | 69 (27.1) | 480 (68.9) | <0.001* | 264 (80.5) | 625 (82.6) | 0.465 | 361 (89.1) | 1016 (91.1) | 0.283 | 694 (70.2) | 2121 (82.6) | <0.001* |
| Survival (% admissions) | 32 (46.4) | 240 (50.0) | 0.664 | 181 (68.6) | 445 (71.2) | 0.479 | 278 (77.0) | 833 (82.0) | 0.053 | 491 (70.7) | 1518 (71.6) | 0.714 |
| Survival (% live born) | 32 (12.5) | 240 (34.4) | <0.001* | 181 (55.2) | 445 (58.8) | 0.300 | 278 (68.6) | 833 (74.7) | 0.024* | 491 (49.7) | 1518 (59.1) | <0.001* |
*Significant at a 0.05 level.
NICU, neonatal intensive care unit.
Table 2.
Baseline characteristics of infants admitted to a neonatal intensive care unit (NICU) in the Netherlands, born with a gestational age between 24+0 and 26+6 weeks between 2011 and 2017, compared with infants born between 2007 and 2009
| 24 weeks | 25 weeks | 26 weeks | Total cohort | |||||||||
| 2007–2009 | 2011–2017 | P value | 2007–2009 | 2011–2017 | P value | 2007–2009 | 2011–2017 | P value | 2007–2009 | 2011–2017 | P value | |
| n=69 | n=480 | n=264 | n=625 | n=361 | n=1016 | n=694 | n=2121 | |||||
| Birth weight (g) | 705 (96) | 687 (98) | 0.172 | 806 (115) | 789 (131) | 0.068 | 869 (177) | 880 (237) | 0.445 | 829 (158) | 810 (200) | 0.020* |
| Sex (male) | 38 (55.1) | 257 (53.5) | 0.913 | 152 (57.6) | 358 (57.3) | 0.994 | 195 (54.0) | 560 (55.1) | 0.764 | 385 (55.5) | 1175 (55.4) | 1.000 |
| SGA | 6 (8.7) | 53 (11.0) | 0.704 | 35 (13.3) | 115 (18.4) | 0.076 | 116 (32.1) | 262 (25.8) | 0.024* | 157 (22.6) | 430 (20.3) | 0.205 |
| Caesarean section | 2 (2.9) | 89 (18.5) | 0.001* | 33 (12.5) | 205 (32.8) | <0.001* | 166 (46.0) | 510 (50.2) | 0.140 | 201 (29.0) | 804 (37.9) | <0.001* |
| Multiple birth | 21 (30.4) | 166 (34.6) | 0.586 | 91 (34.5) | 192 (30.7) | 0.309 | 86 (23.8) | 293 (28.8) | 0.078 | 198 (28.5) | 651 (30.7) | 0.303 |
Birth weight is presented as mean (SD), and other variables are presented as N (%). SGA defined as birth weight below 10th percentile.18
*Significant at a 0.05 level.
SGA, small for gestational age.
Effect of the guideline implementation
The guideline implementation led to an increase in live births at 24 weeks’ gestation (from 63% to 69%, p value 0.035), as well as in NICU admissions (27%–69%, p value <0.001, table 1). Comparing the years before and after guideline implementation in 2010, no statistically significant difference was seen in survival in NICU admitted infants (50% vs 46%, p value 0.66), but more live born infants born at 24 weeks survived (34% vs 13%, p value <0.001). Simultaneously, there was an increase in survival in live born infants (63% to 68%, p value 0.007) and NICU-admitted infants (73% to 78%, p value 0.029) born at 25 and 26 weeks’ gestation. There was an increase in caesarean section rate in infants born at 24 week and 25 weeks, from 3% to 19% (24 weeks, p value 0.001) and from 13% to 33% (25 weeks, p value <0.001), with an increase from 29% to 38% for the total cohort (p value <0.001) (table 2). Figure 1 shows survival rates for subgroups of GA in the years following the implementation of the guideline, relative to live born infants (figure 1A) as well as relative to admitted infants (figure 1B).
Figure 1.
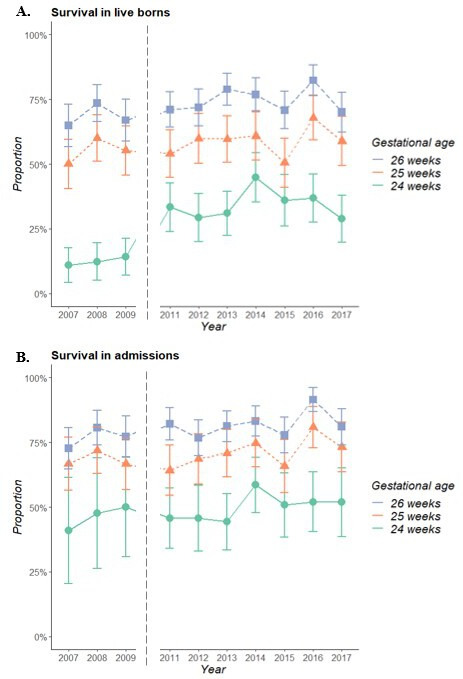
Survival in all live born infants (A) and NICU-admitted infants (B) born with a gestational age between 24+0 and 26+6 weeks between 2007 and 2009 (reference period) and 2011–2017 (study period). Bars reflect 95% CIs. The year 2010 is not presented, as this was the year of implementation of the guideline and was therefore considered as a transition period. NICU, neonatal intensive care unit.
Timing of death
Figure 2A shows 90-day survival in admitted infants for subgroups of GA, showing better survival for each additional completed week of gestation. Comparing the periods 2011–2014 and 2015–2017, no statistically significant difference in 90-day survival could be seen (HR 0.88 (0.75–1.04) for those born in 2015–2017 compared with 2011–2014, data not shown). Figure 2B shows cause-specific survival curves, showing different periods of vulnerability of death for each cause of death. Deaths due to respiratory distress syndrome (RDS) and severe intracranial haemorrhage (IVH) are likely to occur early in life, followed by deaths due to sepsis or necrotising enterocolitis (NEC) in the subsequent weeks, while bronchopulmonary dysplasia (BPD) is main cause of death in the second and third months.
Figure 2.
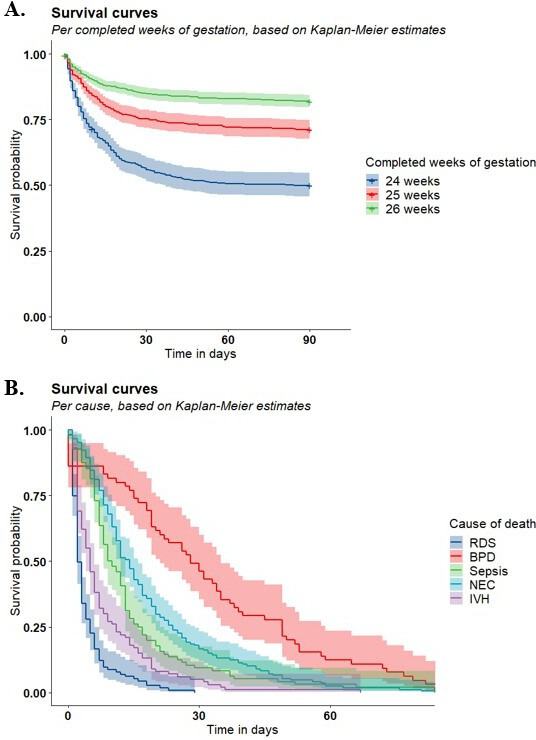
(A) Three-month survival with 95% CI for admitted infants born with a gestational age between 24+0 and 26+6 weeks between 2011 and 2017 for different completed weeks of gestational age. (B) Three-months survival with 95% CI for NICU admitted infants born with a gestational age between 24+0 and 26+6 weeks between 2011 and 2017 for different causes of death. BPD, bronchopulmonary dysplasia; IVH, intracranial haemorrhage; NEC, necrotising enterocolitis; RDS, respiratory distress syndrome.
Causes of death
Table 3 shows causes of death for all NICU admitted infants, for the subgroups of GA. No statistically significant difference in causes of death was detected between all GAs, with NEC (28%), RDS (19%), IVH (17%) and infection (16%) as main causes of death. Comparing the time periods 2011–2014 and 2015–2017, no differences were seen in main cause of death between the two periods (online supplemental 1). Combining RDS and BPD to respiratory problems showed that there was a decrease from 2011 to 2014 to 2015–2017 in deaths complicated or caused by respiratory problems (34%–23%, p value 0.006).
Table 3.
Causes of death using the classification by Patel et al 16 for admitted infants born with a gestational age between 24+0 and 26+6 weeks between 2011 and 2017, compared between different completed weeks of gestation
| Variable | Total | 24 weeks | 25 weeks | 26 weeks | P value |
| N admissions | 2121 | 480 | 625 | 1016 | |
| N died (%) | 603 (28.4) | 240 (50.0) | 180 (28.8) | 183 (18.0) | |
| Cause of death | 0.759 | ||||
| NEC | 168 (27.9) | 67 (27.9) | 51 (28.3) | 50 (27.3) | |
| RDS | 115 (19.1) | 49 (20.4) | 36 (20.0) | 30 (16.4) | |
| Severe intracranial haemorrhage | 100 (16.6) | 40 (16.7) | 31 (17.2) | 29 (15.8) | |
| Infection | 96 (15.9) | 39 (16.2) | 29 (16.1) | 28 (15.3) | |
| BPD | 65 (10.8) | 23 (9.6) | 19 (10.6) | 23 (12.6) | |
| Other | 41 (6.8) | 16 (6.7) | 10 (5.6) | 15 (8.2) | |
| Congenital malformation | 8 (1.3) | 1 (0.4) | 1 (0.6) | 6 (3.3) | |
| Immaturity | 5 (0.8) | 2 (0.8) | 2 (1.1) | 1 (0.5) | |
| Non-classifiable | 5 (0.8) | 3 (1.2) | 1 (0.6) | 1 (0.5) | |
| Death complicated or caused by: | |||||
| Infection | 210 (34.8) | 87 (36.2) | 66 (36.7) | 57 (31.0) | 0.455 |
| Respiratory problems | 180 (29.9) | 72 (30.0) | 55 (30.6) | 53 (29.0) | 0.944 |
| Central nervous system injury | 160 (26.5) | 72 (30.0) | 45 (25.0) | 43 (23.5) | 0.278 |
Results are presented as N (%).
Death complicated or caused by infection includes categories RDS with infection, BPD with infection, suspected sepsis/infection, proven sepsis/infection, NEC with sepsis and severe IVH with infection culture proven or suspected; death complicated or caused by respiratory problems includes all categories with RDS or BPD; death complicated or caused by central nervous system injury includes categories RDS with severe IVH, BPD with severe IVH, severe IVH, severe IVH with infection culture proven or suspected and from category ‘other’ cPVL, severe cerebral damage, damage due to asphyxia and congenital CMV.
BPD, bronchopulmonary dysplasia; CMV, cytomegalovirus; cPVL, cystic periventricular leukomalacia; IVH, intraventricular haemorrhage; NEC, necrotising enterocolitis; RDS, respiratory distress syndrome.
archdischild-2020-318978supp001.pdf (383.5KB, pdf)
Discussion
In this study, survival in extremely preterm infants in the past decade in the Netherlands was evaluated following a major policy change in 2010 concerning treatment of infants born at 24 weeks. After guideline implementation, more infants born at 24 weeks’ GA were live born and admitted to the NICU, leading to an increased survival of live born infants born at 24 weeks’ GA. Top three causes of death were NEC, RDS and IVH. Compared with 2011–2014, less infants died because of respiratory problems in the period 2015–2017.
Quick implementation of the guideline occurred nationwide, with an admission rate of approximately 29% in infants born at 24 weeks before, compared with approximately 69% after the implementation of the guideline. Relative to the number of live born infants, almost three times more infants survived to discharge after implementation of the guideline, although survival in admitted infants did not change. However, no further increase in survival was seen after the first year of implementation of the guideline. It is known that the current Dutch approach towards treatment decisions for extremely preterm infants is more restrictive than the approach in other countries.19 In addition to lowering the GA of active treatment, the new guideline also stated the need for parental consent when initiating early intensive care at 24 weeks’ GA and taking individual factors into account when counselling parents.20 Above factors might explain the flattening of survival rates of extremely preterm infants in the Netherlands.
The survival rates for extremely preterm infants presented in this study in the period 2011–2017 are within the range of survival rates in other developed countries.21 Other national population-based cohorts reporting on survival to discharge in live born infants showed survival rates varying from 31% to 67% in 24-week infants and varying from 59% to 81% in 25-week infants, compared with 34% (24 weeks) and 59% (25 weeks) in our cohort.6 8 9 22 Nevertheless, international comparison of survival in extremely preterm infants remain limited by differences in data collection, time span of survival and selection of denominator.23 To illustrate, several examples of international comparisons of preterm mortality can be found on national and regional levels.24 25 Such efforts on international collaboration should ensure more consistent reporting of outcomes in extremely preterm infants.23
As demonstrated in our study, an additional result of lowering the GA threshold is increased survival in higher GA’s too. Active management of extremely preterm infants seems to improve also survival for those born at higher GAs.6 This is also reflected by higher survival rates in countries that also offer active treatment to infants born below 24 weeks’ GA, which underlies that decision regarding treatment have major influence on outcome data.26 When the threshold of active treatment is lowered, probably also more mature infants might benefit.
In 2016, another Dutch paper was published, evaluating the previous guideline implementation in 2006.27 They reported overall results comparable with our study, with an increased proportion of live births and NICU admission and a decreased survival after guideline implementation. Comparing the current study with this previously published study, we demonstrated further increased proportions of live born and admitted infants for all GAs with similar survival rates in admitted infants.
The low survival rate at 24 weeks’ gestation might reflect the lack of consensus and heterogeneity of perinatal management for these infants.6 Moreover, this may be reflected in the low caesarean section rate, which remained low even after implementation of the guideline, compared with other studies where active care is routinely given at 24 weeks. Caesarean section rates in 24-week infants in countries offering active care to infants born at 22–23 weeks’ GA are comparable or even higher than the caesarean section rate in 26-week infants in this current study.14 28 29 Recently, in the Netherlands, an evidence-based, nationwide framework for prenatal counselling was developed, with the advantage to exclude interprofessional variance based on different values of doctors and to support personalisation allowing variation in parental preferences in decision making.30 Counselling recommendations, treatment recommendations and outcome data are inevitably linked. The 2010 guideline on perinatal care in extremely preterm infants is currently being revised, taking all the above factors into account. Outcome data will need evaluation again after implementation of this new guideline.
Most infants die within the first 2 weeks of life, according to our study, but there were clear differences in period of vulnerability for each cause of death. Deaths due to RDS and IVH were more likely to occur at an earlier stage, while NEC mainly occurred in the subsequent weeks. This was in line with two studies reporting that infants who die from acute respiratory illness and IVH usually die within the first two to 3 weeks and that the onset of NEC starts after 2–3 weeks of age.21 31 In contrast to Patel et al, the current study hardly reported any deaths attributed to immaturity, which is the result of different methodological choices. While Patel et al included all infants born alive, this study only included infants who survived to 24-hour postnatal age and were admitted to a NICU, which has resulted in eliminating most deaths attributable to immaturity.
No differences in cause of death were found between all GAs. Top three causes of death were NEC, RDS and IVH, with similar cause-dependent incidences of death compared with other studies.32–34 Combining RDS and BPD to respiratory problems, similar to the study of Patel et al, less infants died because of respiratory insufficiency in the period 2015–2017 compared with 2011–2014. This has been reported before and might be a result of more aggressive respiratory care in the NICU with increased use of high-frequency ventilation.16 However, new techniques including minimally invasive surfactant therapy may have led to increased avoidance of mechanical ventilation, which is known to be associated with lower incidences of BPD.35 Simultaneously with decreasing respiratory insufficiency, Patel et al showed an increase in deaths attributed to NEC. Although not significant, our study also reported a proportion of death attributable to NEC of 25% in 2011–2014, compared with 32% in 2015–2017, and a proportion of death complicated or caused by infection of 33% in 2011–2014 compared with 38% in 2015–2017. Efforts to increase NICU survival in extremely preterm infants should therefore focus on research on optimising therapies that may decrease NEC and infection.
Strengths and limitations
Strengths of this study include the use of a large national population-based cohort using detailed population information and inclusion of infants over an 8-year period. However, this study has several limitations.
Causes of death have been classified by different people, namely the principal investigator of the centre where infants were born. To maximally reduce interobserver bias, an expert panel group was held to discuss unclear cases. Furthermore, determining a single cause of death when multiple causes may play a role can be difficult and subjective, so misclassifications might have occurred. Lack of postmortem data might also have obscured the causes of death. To minimise bias, we used standard definitions as used by Patel et al 16, we combined pulmonary causes RDS and BPD and combined deaths that were coded either directly attributed to or complicated by infection or CNS injury. It needs to be taken into account that determining cause of death as done in this paper reflects current daily practice of registering cause of death in the Netherlands. Third, as active care also includes interventions prior to delivery such as administration of antenatal corticosteroids, it would have been of great value to include such variables. Unfortunately, this information was not available in the registry. Lastly, for this study, it was decided that infants had to be admitted to a NICU for at least 1 day to be included in this study. Therefore, this study provides no information on cause of death for infants born alive but not admitted to a NICU and does not provide any information on decisions in the delivery room. These decisions might have influenced resuscitation and therefore might have influenced distribution of cause of death.
Conclusions
In conclusion, this study showed that offering active treatment from 24 weeks’ GA onwards resulted in an increase in NICU admissions rate and postnatal survival of infants born at 24 weeks’ GA in the past decade. In the years after implementation, a shift in cause of death was seen from respiratory insufficiency towards NEC and sepsis.
Acknowledgments
The EPI-DAF study group (steering committee members: Monique Rijken, Guid Oei and Peter Andriessen) and EPI-DAF PhD student (Pauline van Beek) are indebted to all collaborators, including paediatricians, psychologists and physiotherapists of all perinatal centres in the Netherlands: Amsterdam UMC location AMC (PI: Aleid van Wassenaer-Leemhuis), Amsterdam UMC location VUmc (PI: Céleste Laarman), Erasmus MC (PI: Renate Swarte), Isala Clinics (PI: Susanne Mulder-de Tollenaer), LUMC (PI: Monique Rijken), Maastricht UMC+ (PI: Elke van Westering-Kroon), Máxima MC (PI: Ellen de Kort), Radboud UMC (PI: Katerina Steiner), UMCG (PI: Henk ter Horst) and UMC Utrecht (PI: Corine Koopman-Esseboom). The EPI-DAF study group is also grateful for the support by the Netherlands Perinatal Registry (Perined; Lisa Broeders, Ger de Winter), the Dutch working group on Neonatal Follow-up (LNF; Cornelieke Aarnoudse-Moens, Monique Rijken, Renate Swarte), the Dutch National Neonatal Registry Working Group (LNR; Floris Groenendaal, René Kornelisse) and the Neonatology Network Netherlands (N3; Debbie Nuytemans, Wes Onland).
Footnotes
Collaborators: EPI-DAF study group.
Contributors: PA, FG, LB and PvB had a substantial contribution to the methodological design of the study. FG, PD, KPD, FvdD, AFvH, RFK, WO, FABAS, EvW-K and RSGMW had a substantial contribution to data acquisition, being the principal investigators of the 10 Dutch perinatal centres. PA, FG, LB and PvB had a substantial contribution to the analysis and interpretation of the data. PA, WO and JLvH participated in the expert panel group. PvB wrote the first draft of the manuscript. All authors contributed to drafting the manuscript or revised it critically with respect to its intellectual content.
Funding: This project has been funded by an unrestricted grant from Stichting Tiny & Anny van Doorne Fonds.
Disclaimer: The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. the Netherlands Association of Pediatrics & the Netherlands Association of Obstetrics and Gynaecology. Guideline: Threatening preterm birth 2007.
- 2. de Kluiver E, Offringa M, Walther FJ, et al. [Perinatal policy in cases of extreme prematurity; an investigation into the implementation of the guidelines]. Ned Tijdschr Geneeskd 2013;157:A6362. [PubMed] [Google Scholar]
- 3. Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 2015;314:1039–51. 10.1001/jama.2015.10244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shah PS, Sankaran K, Aziz K, et al. Outcomes of preterm infants. J Perinatol 2012;32:132–8. [DOI] [PubMed] [Google Scholar]
- 5. Berger TM, Steurer MA, Woerner A, et al. Trends and centre-to-centre variability in survival rates of very preterm infants (. Arch Dis Child Fetal Neonatal Ed 2012;97:323. [DOI] [PubMed] [Google Scholar]
- 6. Ancel P-Y, Goffinet F, et al. , EPIPAGE-2 Writing Group . Survival and morbidity of preterm children born at 22 through 34 weeks' gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr 2015;169:230–8. 10.1001/jamapediatrics.2014.3351 [DOI] [PubMed] [Google Scholar]
- 7. Tommiska V, Heinonen K, Lehtonen L, et al. No improvement in outcome of nationwide extremely low birth weight infant populations between 1996-1997 and 1999-2000. Pediatrics 2007;119:29–36. 10.1542/peds.2006-1472 [DOI] [PubMed] [Google Scholar]
- 8. Serenius F, Sjörs G, Blennow M, et al. Express study shows significant regional differences in 1-year outcome of extremely preterm infants in Sweden. Acta Paediatr 2014;103:27–37. 10.1111/apa.12421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Costeloe KL, Hennessy EM, Haider S, et al. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ 2012;345:e7976. 10.1136/bmj.e7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Numerato D, Fattore G, Tediosi F, et al. Mortality and length of stay of very low birth weight and very preterm infants: a EuroHOPE study. PLoS One 2015;10:e0131685. 10.1371/journal.pone.0131685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia-Munoz Rodrigo F, Diez Recinos AL, Garcia-Alix Perez A, et al. Changes in perinatal care and outcomes in newborns at the limit of viability in Spain: the EPI-SEN study. Neonatology 2015;107:120–9. [DOI] [PubMed] [Google Scholar]
- 12. Grisaru-Granovsky S, Reichman B, Lerner-Geva L, et al. Population-Based trends in mortality and neonatal morbidities among singleton, very preterm, very low birth weight infants over 16 years. Early Hum Dev 2014;90:821–7. 10.1016/j.earlhumdev.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 13. Fellman V, Hellstrom-Westas L, et al. , EXPRESS Group, . One-Year survival of extremely preterm infants after active perinatal care in Sweden. JAMA 2009;301:2225–33. [DOI] [PubMed] [Google Scholar]
- 14. Mehler K, Oberthuer A, Keller T, et al. Survival among infants born at 22 or 23 weeks' gestation following active prenatal and postnatal care. JAMA Pediatr 2016;170:671–7. 10.1001/jamapediatrics.2016.0207 [DOI] [PubMed] [Google Scholar]
- 15. Johnson S, Marlow N. Early and long-term outcome of infants born extremely preterm. Arch Dis Child 2017;102:97–102. 10.1136/archdischild-2015-309581 [DOI] [PubMed] [Google Scholar]
- 16. Patel RM, Kandefer S, Walsh MC, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med 2015;372:331–40. 10.1056/NEJMoa1403489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dutch national perinatal registry. Available: www.perined.nl
- 18. Hoftiezer L, Hof MHP, Dijs-Elsinga J, et al. From population reference to national standard: new and improved birthweight charts. Am J Obstet Gynecol 2019;220:383.e1–383.e17. 10.1016/j.ajog.2018.12.023 [DOI] [PubMed] [Google Scholar]
- 19. Gallagher K, Martin J, Keller M, et al. European variation in decision-making and parental involvement during preterm birth. Arch Dis Child Fetal Neonatal Ed 2014;99:F245–9. 10.1136/archdischild-2013-305191 [DOI] [PubMed] [Google Scholar]
- 20. the Netherlands Association of Pediatrics & the Netherlands Association of Obstetrics and Gynaecology. Guideline: Reference to a perinatal centrecollaboration between second and third line 2010.
- 21. Patel RM, Rysavy MA, Bell EF, et al. Survival of infants born at Periviable gestational ages. Clin Perinatol 2017;44:287–303. 10.1016/j.clp.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boland RA, Davis PG, Dawson JA, et al. Outcomes of infants born at 22-27 weeks' gestation in Victoria according to outborn/inborn birth status. Arch Dis Child Fetal Neonatal Ed 2017;102:F153–61. [DOI] [PubMed] [Google Scholar]
- 23. Patel RM. Short- and long-term outcomes for extremely preterm infants. Am J Perinatol 2016;33:318–28. 10.1055/s-0035-1571202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shah PS, Lee SK, Lui K, et al. The International network for evaluating outcomes of very low birth weight, very preterm neonates (iNeo): a protocol for collaborative comparisons of international health services for quality improvement in neonatal care. BMC Pediatr 2014;14:110. 10.1186/1471-2431-14-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zeitlin J, Manktelow BN, Piedvache A, et al. Use of evidence based practices to improve survival without severe morbidity for very preterm infants: results from the EPICE population based cohort. BMJ 2016;354:i2976. 10.1136/bmj.i2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rysavy MA, Li L, Bell EF, et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med 2015;372:1801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zegers MJ, Hukkelhoven CWPM, Uiterwaal CSPM, et al. Changing Dutch approach and trends in short-term outcome of periviable preterms. Arch Dis Child Fetal Neonatal Ed 2016;101:F391–6. 10.1136/archdischild-2015-308803 [DOI] [PubMed] [Google Scholar]
- 28. Norman M, Hallberg B, Abrahamsson T, et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004-2007 and 2014-2016. JAMA 2019;321:1188–99. 10.1001/jama.2019.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ishii N, Kono Y, Yonemoto N, et al. Outcomes of infants born at 22 and 23 weeks' gestation. Pediatrics 2013;132:62–71. [DOI] [PubMed] [Google Scholar]
- 30. Geurtzen R, van Heijst AFJ, Draaisma JMT, et al. Development of nationwide recommendations to support prenatal counseling in extreme prematurity. Pediatrics 2019;143:e20183253. 10.1542/peds.2018-3253 [DOI] [PubMed] [Google Scholar]
- 31. Schindler T, Koller-Smith L, Lui K, et al. Causes of death in very preterm infants cared for in neonatal intensive care units: a population-based retrospective cohort study. BMC Pediatr 2017;17:59–3. 10.1186/s12887-017-0810-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jacob J, Kamitsuka M, Clark RH, et al. Etiologies of NICU deaths. Pediatrics 2015;135:e59–65. 10.1542/peds.2014-2967 [DOI] [PubMed] [Google Scholar]
- 33. Park JH, Chang YS, Sung S, et al. Trends in overall mortality, and timing and cause of death among extremely preterm infants near the limit of viability. PLoS One 2017;12:e0170220. 10.1371/journal.pone.0170220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Corchia C, Ferrante P, Da Frè M, et al. Cause-Specific mortality of very preterm infants and antenatal events. J Pediatr 2013;162:1125–32. 10.1016/j.jpeds.2012.11.093 [DOI] [PubMed] [Google Scholar]
- 35. Janssen LC, Van Der Spil J, van Kaam AH, et al. Minimally invasive surfactant therapy failure: risk factors and outcome. Arch Dis Child Fetal Neonatal Ed 2019;104:F636–42. 10.1136/archdischild-2018-316258 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
archdischild-2020-318978supp001.pdf (383.5KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.


