Abstract
Background
Medication reviews for people transitioning from one healthcare setting to another potentially improve health outcomes, although evidence for outcome benefits varies. It is unclear when and why medication reviews performed by pharmacists in primary care for people who return from hospital to the community lead to beneficial outcomes.
Objective
A realist synthesis was undertaken to develop a theory of what works, for whom, why and under which circumstances when pharmacists conduct medication reviews in primary care for people leaving hospital.
Methods
The realist synthesis was performed in accordance with Realist And MEta-narrative Evidence Syntheses: Evolving Standards reporting standards. An initial programme theory informed a systematic literature search of databases (PubMed, Embase, Cumulative Index of Nursing and Allied Health Literature, International Pharmaceutical Abstracts, OpenGrey, Trove), augmented by agency and government sources of information. Documents were synthesised by exploring interactions between contexts, intervention, outcomes and causal mechanisms.
Results
The synthesis identified 9 contexts in which 10 mechanisms can be activated to influence outcomes of pharmacist medication reviews conducted in primary care postdischarge. For a medication review to take place these include trust patients have in healthcare professionals, their healthcare priorities postdischarge, capacity to participate, perceptions of benefit and effort, and awareness required by all involved. For the medication review process, mechanisms which issue an invitation to collaborate between healthcare professionals, enable pharmacists employing clinical skills and taking responsibility for medication review outcomes were linked to more positive outcomes for patients.
Conclusions
Medication reviews after hospital discharge seem to work successfully when conducted according to patient preferences, programmes promote coordination and collaboration between healthcare professionals and establish trust, and pharmacists take responsibility for outcomes. Findings of this realist synthesis can inform postdischarge medication review service models.
Keywords: health services research, medication reconciliation, healthcare quality improvement, pharmacists, transitions in care
Introduction
During the immediate period after leaving a hospital people experience heightened vulnerability and a risk of clinical deterioration.1 They may have been prescribed new medicines or changes applied to their regimen, which are intended to be temporary or permanent. A lack of, or inadequate, communication between those involved in care can contribute to adverse outcomes from medicines when transferring from one care setting to another.2 3 These factors account for only some of the problems in the delivery of healthcare but contribute to the complexity which influences the benefits or harms people can experience from the prescription and use of medicines.
In order to reduce the risk of adverse effects from medicines when people move between various healthcare settings, many governing bodies or quality agencies recommend strategies which ensure timely and accurate transfer of information and continuity of care.3 4 Pharmacists’ role in the care of people returning to primary care settings from hospital contributes to bridging information gaps, optimising outcomes and minimising harms.5 Their engagement at transitions of care ranges from performing medication reconciliation to medication reviews (MR).3 6 7 Both are supported by relatively robust evidence of avoidance of adverse events and cost reduction, mainly after patient admission into hospital, and are endorsed through healthcare standards.8–10
It is less clear what benefits can be derived for patients and health systems through medication reconciliation or MR when people transition from hospitals back to primary care. Although systematic reviews seem to show some benefit ‘on average’, for example, a reduction in medication-related problems (MRP),11 12 they have not identified a preferred, effective pharmacist-led intervention13 or established a consensus for best practice.14 This may be related to variations of how MR are executed as interventions by individual practitioners or implemented as programmes within health services. Although most jurisdictions supporting medication reconciliation or MR for people moving from hospitals into the community provide procedural guidance on how to conduct a reconciliation or review, the reality of practice introduces variations.15 16 Results from individual studies vary significantly, some indicating that MR after hospital discharge may have benefit in reducing hospital readmissions or emergency hospital visits,17–20 others casting doubt that tangible outcome benefits can be achieved21 or producing evidence of a negative effect.22 23
The majority of studies and subsequent systematic reviews focus on outcomes as a result of an MR, they provide limited detail as to how these outcomes have been achieved or explore the differing contexts that may have led to contradictory results. Adding to systematic reviews in the area,11–14 24 this realist synthesis was undertaken to clarify what works, for whom, why and under which circumstances in relation to MR performed face-to-face by pharmacists in primary care settings for people who return from hospital into the community. For the purpose of this review, the definition of MR by National Institute for Health and Care Excellence, UK, was adopted.3
Realist synthesis is a theory-driven approach to evaluating complex programmes, through an investigation of contexts, details of the intervention and identification of causal principles which explain why, for example, an MR programme works.25 26 It draws on academic and grey literature as well as policy documents, in this case relating to pharmacists providing some form of MR as an intervention in primary care for people who return from hospital into the community. The aim of the synthesis was to establish a programme theory of why, for whom, how and under which circumstances pharmacist-conducted MR succeed or fail to make differences to peoples’ health and healthcare utilisation after leaving hospital.
Methods
Realist synthesis
Realist research explores the causal links between the context in which healthcare interventions or programmes take place, in this case MR, the mechanisms which are triggered by the intervention in specific contexts and certain outcomes, which may be intermediate or final. A realist approach to data synthesis endeavours to unpack the relationships between context, mechanisms and outcomes (CMO), providing an explanatory account and programme theory of why an intervention or programme achieves what it does.27 As outlined previously, a realist approach to analysis will provide greater insight compared with existing systematic reviews examining when a pharmacist conducted MR after leaving hospital is likely to be successful.
Research questions
To support the generation of programme theory the following research questions were formulated using a Context-Intervention-Mechanism-Outcome (CIMO) framework.28
Context: what are the contexts in which a pharmacist-conducted MR may create an outcome benefit for patients after moving from hospital to primary care?
Intervention: what are the exact details of an MR performed by pharmacists in primary care for people who have been discharged from hospital as described in the literature?
Mechanism: what are the context-specific mechanisms which are likely to contribute to outcome benefits?
Outcome: what are the outcomes (related to C&M) achieved by pharmacist-conducted MR in primary care postdischarge?
The synthesis was registered on PROSPERO (2019 CRD42019123825) and conducted by implementing practical guidance to realist research25 29 and applying Realist And MEta-narrative Evidence Syntheses: Evolving Standards (RAMESES) quality standards for publication of realist reviews.30
Development of initial programme theory
Scoping of the literature (based on prior knowledge by the research team and a PubMed search) facilitated the development of an initial programme theory around MR for people who move from hospitals to primary care. Documents which used the terms medication review, hospital discharge and community pharmacy were surveyed initially. At this point, literature was not analysed in depth nor data extracted, but was read for an overview and description of the medication review process to inform the generation of an initial programme theory. Documents were examined to identify any contexts and potential mechanisms which may explain when, why and how the MR process works for people who have left hospital. Regular discussions and comparisons of notes made on documents revealed the general assumptions underlying an MR, which are that pharmacists are skilled at identifying problems with medicines, including MRPs or prescriber, health system and patient-related issues and then identify and recommend appropriate solutions to these problems.4 As a result of identifying contexts and outcomes of interest, the process of medication review was separated into three steps to break down some of its inherent complexity. First, the MR had to be initiated or ‘made’ to happen, then the actual MR itself performed by a pharmacist and the last step considers what occurs after the MR has been performed. The initial programme theory with contextual and interventional factors which were hypothesised to influence these three steps are described in online supplemental appendix 1.
bmjqs-2020-011418supp001.pdf (183.8KB, pdf)
Data collection for realist synthesis
After designing and trialling the most appropriate search strategies in collaboration with a specialist librarian, a systematic search of PubMed, EMBASE, International Pharmaceutical Abstracts and Cumulative Index of Nursing and Allied Health Literature was performed in December 2018. Alerts for new articles which fit the search terms were set in PubMed, to facilitate timely additions of new publications during the synthesis, and the original search was repeated in February 2020. A start date was not set, as most relevant studies were conducted after the year 2000 but relevant discussion and policy documents possibly published earlier. Search strategies for each database are detailed in the supplementary materials (online supplemental appendix 2). OpenGrey and Trove databases were searched for grey literature as were the websites of pharmacy organisations and relevant government agencies in the UK, the USA, Canada and Australia. The focus of this realist synthesis was on MR performed by pharmacists working in community practice or primary care settings (eg, community pharmacy, general practice, community clinic), with no limitations to review locations. MR conducted in a hospital setting were excluded. Narrowing the scope of the review, searches did not include terms of ‘transition of care’ to minimise capturing MR performed by pharmacists working in hospital settings. Documents describing medication reconciliation were included if this was not the sole intervention. Programme evaluation reports were identified to add conceptual and contextual richness.
bmjqs-2020-011418supp002.pdf (9.7KB, pdf)
At times, the terms medication reconciliation and MR were used interchangeably or loosely in studies and reports, for thoroughness medication reconciliation as well as MR review articles were screened for further relevant studies.5 8 11–14 24 31 32 Backward and forward reference tracking of included articles was performed and sibling papers identified. Multiple papers or reports could be related to the same original study and reported in conference abstracts, full research reports, evaluation reports, and trial protocols which often outline the underlying assumptions of how the programme under investigation is supposed to work.
Two members of the research team (KL, MJT) screened titles and abstracts for inclusion for full-text review. All three authors then read a proportion of full-texts, with a random overlap between each, which ensured 15% of full-texts were screened by all researchers. Any discrepancies in screening were resolved through regular discussion. In line with realist approaches to data collection any publication or document with potential to inform the generation of programme theories was considered, this included policies and procedures of established MR programmes. A conventional quality appraisal of studies was not performed but all documents were assessed for their relevance, trustworthiness and rigour. An assessment of rigour was made by examining whether the methods used to generate data were appropriate to answer the research questions, were employed with fidelity and consistency and could credibly produce presented results. Trustworthiness of data was established by assessing whether they had been empirically obtained and cross-examining outcomes of similar studies. Documents were regarded as relevant if they were deemed to contribute to the development of programme theory or disputed it, with relevance at times shifting depending on the developmental phase of theory. For documents which seemed highly relevant but lacked adequate detail, authors were contacted to obtain clarification and additional detail to support judgements of trustworthiness and rigour.29
Data analysis
Based on the research questions framed via CIMO, literature and factors considered in the initial programme theory data were extracted from the finally included articles, as described in table 1, and considered in conjunction with extractions of texts from included documents as well as interpretive and reflective notes researchers kept on all included documents.27 28 33 All data were managed using Microsoft Excel.
Table 1.
Template for data extraction
| Date extracted from articles included in review | |
| Study type | Study type (eg, cohort, RCT) |
| Participants | Patient characteristics, patient-specific inclusion criteria, healthcare professionals and their characteristics |
| Intervention | Timing, location, funding, medication review model and activities, communication, follow-up, reporting, referrals |
| Comparator | Characteristics of comparator groups if present |
| Outcomes | All outcome measures (process, patient-focused) |
| Context | Individuals, interpersonal relations, institutional settings, infrastructure33 |
| Mechanism | Qualitative data, theory-based discussions |
RCT, randomised controlled trial.
Outcomes were categorised into proximal and distal, contexts were themed into relevant subcategories and mechanisms initially inferred, before refinement of both during the synthesis.
Data synthesis and refining programme theory
Causal links, contexts, mechanisms and outcomes were then configured iteratively into CMO configurations (CMOCs) to refine the programme theory through regular discussions by the research team. Working backwards from outcomes started the generation of CMOCs, followed by interpretation of extracted data and author notes in relation to contexts and/or mechanisms. Contexts were examined for significance to each of the three stages and synthesised after ascertaining which outcomes they linked to. Some mechanisms were inferred to start with, as they often were not articulated clearly or remained hidden until relevant data from all included documents had been examined. In addition, the relationships between contexts, mechanisms and outcomes were established within a specific and across all documents. Once a COMC was established, all documents were iteratively scrutinised for confirmation or disconfirmation. This process identified the presence and an absence of mechanisms as triggers for outcomes and CMOCs were formulated in positive and negative terms.
CMOCs were then examined for demi-regularities and abstracted to establish mid-range theories. An additional literature search for more general studies and documents on MR as well as substantive theory was performed to adjudicate and confirm mechanisms and the final programme theory.
Results
Sixty-six documents were included, as described in the flow chart for their identification and selection in figure 1 and listed in online supplemental appendix 3.
Figure 1.
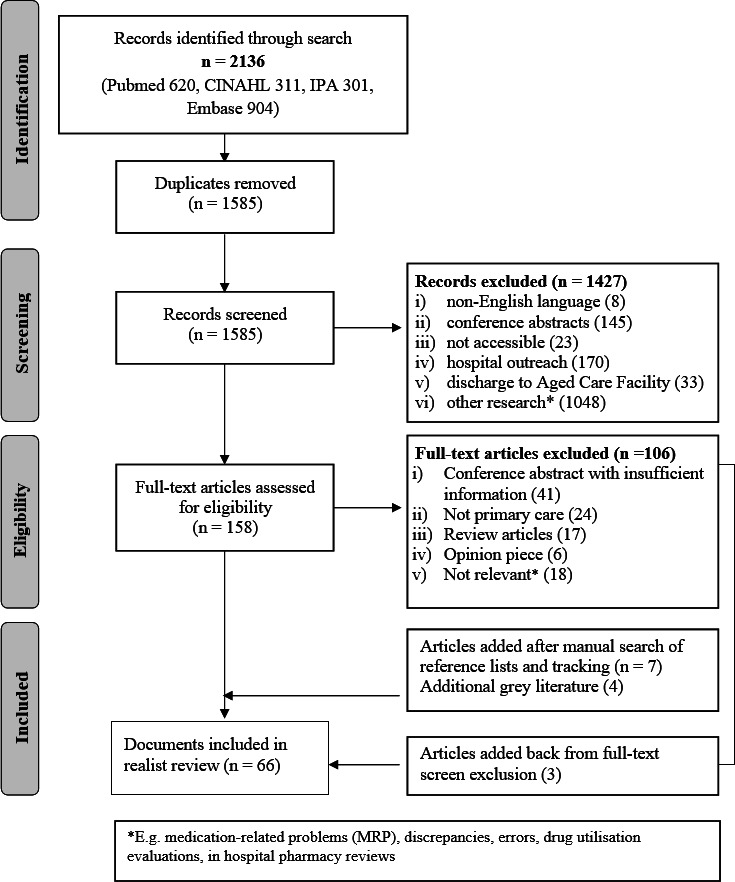
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart. CINAHL, Cumulative Index of Nursing and Allied Health Literature; IPA, International Pharmaceutical Abstracts.
bmjqs-2020-011418supp003.pdf (61.5KB, pdf)
Of the 33 primary studies which were reported in conference abstracts, trial protocols, journal articles and evaluation reports, 13 were conducted in the UK,20 23 34–44 10 in the USA,18 19 45–52 6 in Australia,17 22 53–56 3 in the Netherlands57–59 and 1 in Canada.21 Multiple papers or reports could be related to the same original study. Findings were reported for 16 distinct studies of MR performed by pharmacists in community pharmacies18–21 34–44 50 (described in 22 articles), 15 involved home visits17 22 23 46 47 49 51–59 (described in 32 articles) and 2 in-clinic review.45 48 Outcome measures are described in table 2.
Table 2.
Outcome measures of studies
| Outcomes | ||
| Proximal | Process outcomes | Interest by patients, patient recruitment/participation (rates), attrition rate after consent, referral rates (by in-hospital healthcare professional, doctors), uptake of medication reviews by pharmacists. |
| Identification of issues | Identification of medication-related problems, adherence issues, supply, guideline recommended treatment, patient understanding, healthcare needs. | |
| Resolving issues | Reported differences of medication-related problems before and after medication reviews. | |
| Distal | Hospital readmissions | Rate of readmissions. |
| Healthcare utilisation | Emergency department visits, visits to general practitioners. | |
Fourteen studies reported patient-focused outcomes, for example, readmission rates, visits to emergency departments rather than the identification or rate of resolving MRPs. Seven of those showed positive outcomes or trends in reducing hospital readmissions or other healthcare utilisation,17–20 45 46 60 four showed no differences between intervention and control groups21 35 47 48 and three reported increases in hospital admissions or other healthcare utilisation for the intervention group.22 23 57 61
Contexts which were identified as potentially influencing outcomes are described in table 3.
Table 3.
Contexts influencing the MR process and outcomes
| Context identified from the literature as having potential to influence participation | ||
| Context | Description | |
| Broadly applicable to medication review | Awareness and knowledge of MR programmes and referral pathways by HCPs | Policies, care models and practices promoting the existence of pharmacist-conducted MR programmes determine HCPs’ awareness of the programme and knowledge of how and who to refer for a medication review (MR).42 49 71 79 85
Referral pathways were established when existing MR programmes are leveraged or extended for MR after discharge18 19 50 51 54 65 76 and/or a triaging system is in place.53 57 |
| Patients’ experience and attitudes to pharmacists’ clinical role | Patients have varying experiences of pharmacists exercising their clinical role.36 37 74 77 | |
| System and organisational structures support MR and facilitate role integration | When postdischarge MR programmes were introduced de novo pharmacists only slowly integrated them into their practice or business.42 86
Postdischarge MR were implemented as variations of existing programmes, for example, Comprehensive Medication Review, Medication Therapy Management in the USA or Home Medicines Review in Australia.18 19 21 53 60 78 85 87 |
|
| Location of MR appointment | Most MR were performed in a community pharmacy (CP).18–21 35–41 50 71 86
Several studies where MR was performed in a CP screened out patients who could not access the CP.38–41 50 72 74 Patients were visited at home.17 22 23 46 47 49 51–53 57–59 66 MR was performed in a physician’s practice.45 48 |
|
| Specific to postdischarge MR | Location and timing of patient recruitment for participation | Patients are approached and recruited to an MR programme while in hospital and preparing for discharge.17–20 22 35 41 43 49 59 66 78 79 86 88
Patients receive information about MR while in hospital and are recruited before or when they are visiting their CP or before they visit their doctor.40 48 89 Planning and recruitment for MR occurs at a distance from the professional providing MR, who is not aware that a patient left hospital.34 38 39 42–44 72 |
| Postdischarge environment, being back home | Contact and engagement with patients was difficult to establish once they returned home.18–20 41 50 75
Patients were asked to organise their appointment once they returned home.20 37 40 41 43 Patients declined to attend an MR appointment.18–20 35 40 41 50 72 74 75 |
|
| Contexts identified from the literature as having potential to influence MR process and outcomes | ||
| Broadly applicable to MR | Information available to pharmacist before and during the MR | The pharmacist has access to comprehensive clinical information provided by the hospital17 19 and/or a referring primary care provider (eg, doctor)53 60 65 and/or access to an electronic health record.18 45 47 48 51 52
Pharmacist has only access to discharge medication list and/or summary.20 23 35 37 38 43 44 49 57 58 72 |
| Regulations, standards and funding models guiding MR | These determine the extent and expectations of pharmacists’ involvement and scope of practice. MR is expected to be mainly an assessment of MRP, which the pharmacist documents and/or communicates to other HCPs (eg, the patient’s doctor, community pharmacy).23 57 59 Pharmacist addresses and resolves issues with patient.17–19 Pharmacist identifies and resolves MRPs (eg, dose adjustments) or follows up with other HCPs who can resolve them.17–19 66 |
|
| Specific to postdischarge MR | Professional communication collaboration, coordination and networks | A network of HCPs coordinates the MR, for example, scheduling of an appointment is not left to the patient, appointments are made, doctors are prompted to refer, records are shared. This could be through a hospital pharmacist, care-navigator (primary care), discharge coordinator (in hospital), GP, community pharmacist.17–19 45 46 48 53 55 56 59 60 65 67 87 90
Pharmacists who are tasked to perform MR are informed that patients have been discharged.18–20 23 35 46 47 49 50 57–59 86 Pharmacists who are tasked to perform MR are not informed that patients have been discharged.39 42 44 71 |
GP, general practitioner; HCP, healthcare professional; MR, medication reviews; MRP, problem.
Many studies provided limited detail on the intervention. When it was based on an existing MR programme, relevant policies and procedures were consulted for further guidance and detail, for example, for Medication Utilisation Reviews (MUR) in the UK, MedsCheck in Ontario and the Home Medicine Review (HMR) programme in Australia.62–64
Only two aspects of the intervention seemed to consistently trigger mechanisms which facilitated favourable outcomes, these were the degrees of active problem solving by the pharmacist and mode and degree of communication with other healthcare providers. Other aspects, for example, timing of the MR, credentialing or specific training received by pharmacists, seemed to be of little relevance.
Numerous mechanisms were identified as causal processes generating outcomes when activated within the various contexts, as listed in table 4.
Table 4.
Mechanisms influencing outcomes
| 1. Mandate | HCPs have a mandate to recruit patients to MR, for example, in hospitals or community pharmacy. Pharmacists are given a mandate through regulation and funding and perceive they have a mandate to perform MR by patients and other HCPs. |
| 2. Effort required | Effort any participant has to make to obtain information to organise or participate in the MR process, for example, patients to organise appointments, pharmacists to recruit and organise, doctors to refer. |
| 3. Trust in HCPs | Patients trust a referral by a doctor or hospital staff and trust the pharmacist performing MR. |
| 4. Recognition of pharmacists’ clinical and professional role | Pharmacists’ competence and skill to perform MR is recognised by pharmacists themselves, other HCPs and patients. |
| 5. Perception of benefit from MR by HCPs and patients | Patients and HCPs perceptions of benefit from an MR influences their willingness to participate in, refer to or conduct an MR. |
| 6. Patient preference | Accessibility (6a), acceptability (6b) and convenience (6c) of location and time for MR and who performs it (un/familiar pharmacist) (6d). |
| 7. Prioritisation of health and social care needs | Patients balance the benefit of MR against other priorities and commitments. MR is not always a priority for patients after leaving hospital. |
| 8. Invitation to collaborate | Pharmacists personally communicate with or contact doctors about MR, doctors refer patients to pharmacists, inviting each other to collaborate. |
| 9. Potential to employ clinical skill | Pharmacists are enabled to employ their clinical skills and judgement. |
| 10. Taking responsibility | Pharmacists take responsibility for MR outcomes, resolving the issues they can or take responsibility to get the ones who can to resolve them. HCPs take responsibility for recruiting patients to MR. |
HCP, healthcare professional; MR, medication reviews.
Finally, CMOCs, which were constructed in positive and negative terms, describing outcomes when mechanisms were present in a particular context versus their absence, are detailed in table 5.
Table 5.
CMOCs for final programme theory
| CMOCs—participation | |||
| Context | Mechanism | Outcomes when M present | Outcomes when M not present |
| 1. Recruitment: time point and location (community pharmacy (CP), hospital, clinic) of patient recruitment for participation. Organisational support for recruitment. |
M1: mandate, perception of benefit by recruiters. M3: patients trust HCPs who recommends MR. M10: pharmacists or designated recruiters take responsibility for identifying and consenting patients. |
O1: patients provide informed consent to participate in MR.17–19 53 65 66 | O2: patient recruitment/participation rates are low.37 42 |
| 2. Patients’ experience and attitudes to pharmacists’ clinical role. | M3: trust in pharmacist skill. M5: perception of benefit from pharmacist-led MR by patients. |
O1: acceptance of referral to and participation in pharmacist-led MR.37 | O2: low rates of referral to and participation in pharmacist-led MR.36 46 53
O3: patients seek out specialists, GP or community pharmacist to address concerns or concerns are not met.37 |
| 3. Awareness of MR programmes and referral pathways by healthcare professionals. | M4: recognition of pharmacists’ clinical and professional role by HCPs. M5: pharmacists, doctors, other HCPs perceive benefit of an MR. |
O4: increased referral to and/or uptake of MR.49 51 57 65 66 | O5: low referral and/or uptake of MR.37–39 53 71–73 |
| 4. Systems and organisational structures support MR and facilitate its role integration. | M1: mandate to perform MR. M4: recognition and acceptance of MR by pharmacists as professional role. M5: pharmacists’ perceive benefit from MR for patients and their professional standing. |
O6: pharmacists perform MR as part of their routine practice.18–21 45 48 53 57 | O7: pharmacists do not perform MR as part of routine practice.40 42 |
| 5. Location of MR appointment. | M2: less effort required. M6: patient preferences. |
O1: patients participate in MR.17 23 76 | O2: patients decline to participate in MR.37 38 40 41 52 74 75 |
| 6. Life after hospital has to be reorganised, regained. | M7: patients’ weigh priorities against perceptions of benefit. | O1: patients participate (schedule and attend) in MR.37 | O2: patients do not participate (schedule or attend) in MR they agreed to in hospital.18 19 37 50 51 53 78 |
| CMOC related to participation and process | |||
| 7. Communication, collaboration, coordination and networks | M2: less effort required. M8: invitation to collaborate by pharmacists to doctors, or doctors to pharmacists. |
O1: patients are more likely to participate.17 48 53 65
O4: doctors refer to MR.53 56 65–67 O8: all or most issues, for example, medication-related problems or patient problems, are identified and strategies to resolve them suggested or actioned.17–19 45 48 60 O10: pharmacists performing MR are aware when people are discharged.18–20 46 48 57–59 79 80 89 |
O9: issues may be missed and strategies to resolve issues cannot be easily actioned.22 23 75 80
O11: pharmacists or patients do not schedule appointments after discharge.40 41 72 O12: pharmacists are not aware when people have left hospital.39 42 44 |
| CMOCs process | |||
| 8. Information available to pharmacist before and during the MR | M8: invitation to collaborate by other HCPs. M9: pharmacists enabled to employ clinical skills and judgement. |
O8: all or most issues, for example, medication-related problems or patient problems, are identified and strategies to resolve them suggested or actioned.17–19 45 48 60 65 | O9: issues may be missed and strategies to resolve issues cannot be easily actioned.22 23 57 75 77 80 |
| 9. Regulations and standards guiding MR | M10: pharmacist takes responsibility for MR outcomes. | O13: MR becomes more than an assessment-focused service. 17–19 46 48 |
O14: MR constitutes an administrative rather than clinical assessment of medicines, limiting positive process and patient outcomes.22 23 57–59 75 80 |
CMOC, CMO configuration; GP, general practitioner; HCP, healthcare professional; MR, medication reviews.
The contribution of the individual CMOCs to the overall programme theory is detailed below, separated into the steps of an MR as outlined previously.
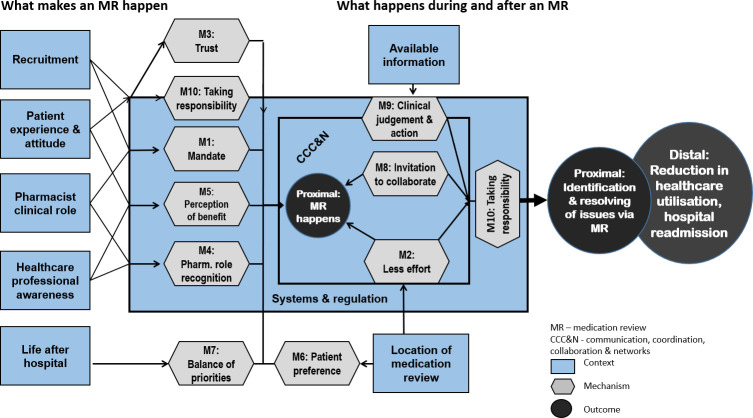
Step 1: what makes an MR happen?
The first step to the success of any intervention or programme is the engagement of the intended participants, in this case patients (and their carers), pharmacists, doctors and other healthcare professionals (HCPs). Information on demi-regularities explaining which contexts and mechanisms are likely to be in place for an MR to happen in the first place was easily obtainable. The following CMOCs relate to ‘what makes an MR happen’:
For patients:
Patient recruitment
Patients were generally amenable to considering an MR after leaving hospital, in most studies 60%–70% of those approached in hospital agreed to participate, when approached by designated personnel.17–19 53 65 66A specific role for recruitment was more commonly assigned in hospitals and contingent on the study design gave recruiters a mandate, encouraging them to take responsibility. Recruitment in community pharmacies was less successful when added on to other roles.37 42 Patients consented to MR when recruited by HCPs they knew, trusting their recommendation.17 53 65 However, reliance on referrals by doctors significantly reduced the reach of potential patients compared with hospital or community pharmacy initiated MR processes, unless the referral process was highly coordinated and supported, linking to CMOC 7.53 65–67 A combination of starting recruitment in hospital and follow-up by those involved in the actual MR process seemed to maximise patient engagement.18 53 65 These findings are abstracted into the following CMOCs:
CMOCs 1: when patients are offered an MR in a healthcare environment they are likely to consent because they trust the HCP recommending the MR.
When recruitment of patients to an MR is supported by an organisation, for example in hospital, recruitment rates increase because ‘recruiters’ have a mandate and take responsibility for identifying and consenting patients.
Patient experience
Particularly during the early implementation of MR programmes, patients were unfamiliar with pharmacists’ extended and clinical roles and lacked trust in pharmacists’ skill.36 68 In addition, patients’ perception of little benefit and low positive outcome expectancy of an MR are mechanisms reducing patients’ willingness to participate.36 46 53 69 70
CMOC 2: depending on patients’ experience and attitudes to pharmacists’ clinical roles, acceptance of an MR referral and participation increases if patients perceive a benefit from a pharmacist-led MR and/or trust the pharmacist’s skill.
For pharmacists and other HCPs:
Healthcare professional awareness
HCPs, in particular pharmacists and doctors, had to gain familiarity with MR programmes. Referrals to and/or uptake of MR increased with awareness and familiarity because of increasing recognition of pharmacists’ clinical and professional role and changing perceptions of patients’ benefit from an MR.49 51 57 65 66 These mechanisms were confirmed by observation or low referral and/or uptake of MR in their absence.37–39 53 71–73
CMOC 3: when MR programmes and systems have raised HCPs’ awareness and experience, referral and uptake increase because they recognise pharmacists’ professional role and may perceive a benefit of pharmacist-conducted MR for patients.
Role integration
Interlinking with the previous CMOCs additional factors influencing pharmacists’ uptake of MR were observed. Organisational, policy and procedural support had to be in place before pharmacists integrated MR into routine practice. The legitimisation of their professional, clinical roles created a mandate to perform MR and a positive perception of their professional standing.18–21 45 48 53 57 Policies and formalised MR programmes also signal to pharmacists (and other HCPs) that MR is of benefit to patients. When pharmacists did not accept MR as a professional responsibility and doubted benefits to patients, MR were not incorporated into their practice.40 42
CMOC 4: when systems and organisational structures support MR and facilitate role integration pharmacists undertake them as routine practice because they accept it as part of their professional role and perceive a benefit for patients and their own professional standing.
Location of MR
Despite prescreening, many patients were unable to access the location of the MR or relied on carers for access to appointments due to poor mobility and morbidity, leading to attrition rates of 50% or higher after recruitment.18–20 40 41 50 72 74 75 Most programmes are inflexible about the location for postdischarge MRs, for example, community pharmacies for discharge MUR and MedsChecks, homes when leveraging HMR programmes. Although not all patients feel comfortable attending an MR conducted by a pharmacist they do not know or welcome a pharmacist into their home,52 participation increased when accessing the location required little effort.17 23 76 Patients declined participation when the location of the MR did not meet their preferences, which was often the case for community pharmacy-based programmes.37 38 40 41 52 74 75
CMOC 5: when the location of the MR is flexible patients are more likely to participate because their preferences in terms of accessibility, acceptability and convenience are met and less effort is required.
Life after hospital
Patients preferences and priorities changed once they were home, when they had to reorganise their lives and juggle multiple appointments.53 77 Patients participated (scheduled and attended) an MR when perceived benefits outweighed their health and other priorities and effort required to attend,37 but frequently that balance did not tilt in favour of the MR.18 19 37 50 51 53 78
CMOC 6: when life after hospital has to be reorganised, patients may or may not schedule and attend MR appointments because they weigh their priorities and effort against perceptions of benefit differently.
Steps 2 and 3: what happens and what happens next?
Details on contexts, interventions and mechanisms describing what happens during the MR process and after the MR was performed were more limited, therefore CMOCs outlining these two steps were combined. Mechanisms identified for the three CMOCs generated outcomes along a continuum of activation, the stronger mechanisms were activated and the more of them were present, the more likely positive outcomes were achieved. The following CMOCs relate to the MR process: ‘what happens during and after the MR’. They frequently acted interdependently, with CMOC 7 creating favourable conditions for CMOCs 1, 3, 8 and 9 to develop successfully, and CMOC 8 particularly linked to CMOC 9.
Communication and networks
Community pharmacists who were supposed to undertake the MR often were not aware patients had left the hospital.39 42 44 Independently of the location where the MR was undertaken and how patients had been engaged, communication within networks and inviting pharmacist collaboration increased awareness and performance of MRs.18–20 46 48 57–59 79 80 An invitation to collaborate, either through a referral from a prescriber to the pharmacist or the pharmacist contacting prescribers to inform them of their involvement or a discussion of MR findings, initiated degrees of shared care for a patient and resulted in higher referral53 65–67 and participation rates17 48 53 65 and achieved positive patient outcomes.17 45 48
CMOC 7: because it reduces the effort for those involved in the MR process and invites their collaboration, communication and coordination increase the likelihood of doctors referring, patients and pharmacists scheduling/performing MR and facilitates reductions in MRPs or readmissions to hospital.
Available information
Information available to pharmacists before and during the MR varied between studies, partially dependent on regulation and standards, from a hospital discharge medication letter to comprehensive referrals including relevant clinical information. A referral from a doctor constituted an invitation to collaborate, facilitating sharing of or requests for information.45 60 65 When pharmacists had access to adequate relevant information they were enabled to identify most MRPs or patient-related problems as well as strategies to resolve these, either by action or recommendation.17–19 45 48 60 65 When only medication information was available, pharmacists were hindered in identifying clinically relevant issues and identifying or enacting appropriate strategies to resolve them.22 23 57 75 77 80
CMOC 8: when pharmacists either receive, or are able to easily access, comprehensive and relevant clinical information about patients, MR achieves positive outcomes because they are enabled to recognise more relevant issues and make recommendations to resolve them.
Regulations and standards
While governance of MR programmes influence how pharmacists’ can address issues they identify, they allow for flexibility and discretion in taking action. Pharmacists conducting MR as a holistic clinical assessment, at times including social considerations, and taking responsibility for outcomes achieved significantly higher rates of MRP resolution and patient-focused outcomes, for example, reductions in readmissions,17–19 46 48 compared with identifying MRPs, making recommendations only and leaving the solving of problems to someone else without follow-up.22 23 57–59 75 80
CMOC 9: better outcomes are achieved when standards for MR facilitate pharmacists taking responsibility for solving issues identified during the MR, either themselves, in collaboration with the patient, or ensuring prescribers resolve any issues they could not address.
The predominant absence of the enabling mechanisms of the three CMOCs described above was particularly observed in the three studies with detrimental outcomes, that is, an increase in hospital admissions, where a pharmacist, without access to comprehensive information about patients was ‘parachuted in’ to undertake an MR and/or sent a medication list and additional reporting notes to the doctor without two-way communication or formalised follow-up.22 23 57
The final programme theory and connections between CMOCs is depicted in figure 2.
Figure 2.
Final programme theory.
Discussion
This realist synthesis of MR performed by pharmacists in primary care for people who have left hospital provides insight into which contexts have potential to influence outcomes and which mechanisms have to be activated to achieve positive outcomes for all participants. Policies, standards, systems and organisational structures link to contexts which raise HCPs’ awareness of MR, provide a mandate to recruit and perform MR and contribute to role integration for pharmacists. They also influence communication, collaboration and networks positively.
These more or less generic conditions seem necessary but are insufficient in achieving patient-focused outcomes. The following discussion will focus on the specific findings with most potential to inform further shaping of MR programmes and their implementation.
Patient recruitment and participation
Patients are likely to perceive benefit from an MR differently than HCPs, framing it according to their needs and preferences will increase their participation. Deducing from what does not work, increased flexibility for the reviewing pharmacist to conduct the MR at a location and time which suits patients’ preferences and capacity will ensure that those who perceive benefit and may benefit are receiving an MR. Compelling evidence for a particular time frame in which the MR achieves outcome benefit is so far lacking, flexibility and meeting preferences in combination with factors discussed below seem of greater importance.
Establishment of trust
Doctors, who in many MR programmes are asked to implement pharmacists’ MR recommendations, have to perceive a benefit for their patients and trust the pharmacist’s professional expertise. As ‘trust pertains to relationships with specific others over specific matters’81 (italics in original), pharmacists cannot assume they are inherently regarded as trustworthy and will have to build trust through communication and invitations to collaborate.
When implementing MR programmes patients and HCPs have to gain trust into the pharmacists’ clinical role and skills, and perceive a benefit for an MR. Trust in pharmacists can be conferred by a HCP patients already trust, with doctors in particular exerting significant positive social influence over the willingness to use a medication management service.82
Communication, coordination, collaboration and networks
Communication and coordination between participating HCPs and patients increases the likelihood of participation and positive outcomes because it reduces required effort to engage in the MR process. Invitations to collaborate between HCPs, for example, doctors and pharmacists, enhance collaboration and information sharing. Availability of clinical information has previously been hypothesised as a factor influencing outcomes for an MR in a positive manner.5 The more pertinent detail pharmacists have access to the more enabled they seemingly are to employ their clinical skill and identify MRPs, social issues and other patient factors which may contribute to negative outcomes. MR programmes have to ensure the quality and relevance of information pharmacists receive before or can collect during an MR.
Pharmacists taking responsibility
The manner in which issues are consequently addressed influence whether positive outcomes are achieved. Lower reductions in MRPs and no changes in healthcare utilisation were observed when pharmacists sent reports unsolicited or unannounced to prescribers or did not follow-up on their MR findings with patients or reports to doctors, confirming that an underlying mechanism of initiating or established collaboration comes into play. A patient-focused approach and follow-up care has previously been suggested to enhance the effectiveness of MR.83 Pharmacists resolving issues they can resolve themselves with patients, for example, access to medicines, adherence, understanding, social support, increases the chances of success, and ideally is encouraged by policies and systems surrounding MR programmes.
The term ‘review’ unfortunately implies ‘the act of considering something in order to make changes to it, give an opinion or appraise it’. We hypothesise that when pharmacists moved from providing only an opinion, assessment or consult to collaborative problem solving because they took responsibility for the outcome of the postdischarge MR they performed, positive outcomes were more likely for people leaving hospital.
Strength and limitations
A strength of this synthesis is the inclusion of recent research in the area of post-discharge MR.
Many included studies reported outcomes which were not necessarily patient-focused and provided limited detail on contexts and potential mechanisms.
Issues with patients’ capacity to access the MR point to limitations of studies where patients who agreed to participate in an MR when approached in hospital but could not be contacted after their return home were allocated into the usual care or control group. Any differences in outcomes might have partly been a function of patients’ capacity to attend the MR compared with those who did not attend, for example, related to lesser morbidity and higher mobility, being more engaged or self-efficacious in their healthcare, rather than attributable solely to the MR.19 20 84 It is also plausible that some of these usual care group patients were not contactable due to readmission into hospital shortly after their previous discharge.75
The intervention, in this case the MR either as a process or programme, was often not described in a way to assist a realist approach, for example, detail was limited20 21 47 or too complex or multifaceted41 57–59 to understand with certainty which components were essential to achieving a positive outcome.
Available data allow confidence in theory of what makes an MR happen, particularly in the early phases of MR programme implementation, for example, why and how stakeholders engage in the MR process or not. Due to the above limitations, the programme theory relating to tangible outcomes of MR developed via this realist synthesis still has to be tested.
Conclusions
This realist synthesis provides guidance for the future development of service models in which pharmacists provide MR in primary care for people who have left hospital. In order to make it a successful health service multiple, interacting elements need to be in place. Programmes which offer flexibility in MR location and timing, are attuned to patients’ priorities and preferences and facilitate coordination and information exchange between all participants increase uptake and participation. Coordination and collaboration between HCPs and pharmacists taking responsibility for MR outcomes seem to create conditions in which the MR leads to patient-relevant outcomes, for example, reductions in hospital readmissions. This synthesis provides a basis for future (realist) evaluations examining MR in general and for people who have left hospital to strengthen and confirm the proposed causal explanations.
Acknowledgments
The authors would like to thank stakeholders and authors of original articles for discussions and clarifications.
Footnotes
Contributors: KL and MJT conceived the study. KL, MJT and DR undertook abstract and full-text screening. KL undertook data extraction. All authors performed the synthesis. KL drafted the manuscript, all authors revised the manuscript critically for intellectual content and agreed and approved the final version to be published. Disagreements were resolved by regular discussion until all authors reached consensus.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information, further data related to searches and screening will be available from the submitting author on request.
References
- 1. Dharmarajan K, Krumholz HM. Risk after hospitalization: we have a lot to learn. J Hosp Med 2015;10:135–6. 10.1002/jhm.2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Howard R, Avery A, Bissell P. Causes of preventable drug-related hospital admissions: a qualitative study. Qual Saf Health Care 2008;17:109–16. 10.1136/qshc.2007.022681 [DOI] [PubMed] [Google Scholar]
- 3. National Institute for Health and Care Excellence . Medicines optimisation: the safe and effective use of medicines to enable the best possible outcomes (NG5), 2015. Available: https://www.nice.org.uk/guidance/ng5 [Accessed 20 Apr 2020]. [PubMed]
- 4. Australian Commission for Safety and Quality in Health Care . Medication safety standard. Available: https://www.safetyandquality.gov.au/standards/nsqhs-standards/medication-safety-standard [Accessed 20 Apr 2020].
- 5. Ensing HT, Stuijt CCM, van den Bemt BJF, et al. Identifying the optimal role for pharmacists in care transitions: a systematic review. J Manag Care Spec Pharm 2015;21:614–36. 10.18553/jmcp.2015.21.8.614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organisation . Standard operating protocol assuring medication accuracy at transitions in care, 2014. Available: https://www.who.int/patientsafety/solutions/patientsafety/PS-Solution6.pdf [Accessed 20 Apr 2020].
- 7. Australian Commission for safety and quality in health care healthcare. Available: https://www.safetyandquality.gov.au/our-work/medication-safety/medication-reconciliation [Accessed 20 Apr 2020].
- 8. Mekonnen AB, McLachlan AJ, Brien J-AE. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis. BMJ Open 2016;6:e010003. 10.1136/bmjopen-2015-010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graabaek T, Kjeldsen LJ. Medication reviews by clinical pharmacists at hospitals lead to improved patient outcomes: a systematic review. Basic Clin Pharmacol Toxicol 2013;112:359–73. 10.1111/bcpt.12062 [DOI] [PubMed] [Google Scholar]
- 10. Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database Syst Rev 2016;2:CD008986. 10.1002/14651858.CD008986.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nazar H, Nazar Z, Portlock J, et al. A systematic review of the role of community pharmacies in improving the transition from secondary to primary care. Br J Clin Pharmacol 2015;80:936–48. 10.1111/bcp.12718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McNab D, Bowie P, Ross A, et al. Systematic review and meta-analysis of the effectiveness of pharmacist-led medication reconciliation in the community after hospital discharge. BMJ Qual Saf 2018;27:308–20. 10.1136/bmjqs-2017-007087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bethishou L, Herzik K, Fang N, et al. The impact of the pharmacist on continuity of care during transitions of care: a systematic review. J Am Pharm Assoc 2020;60:163–77. 10.1016/j.japh.2019.06.020 [DOI] [PubMed] [Google Scholar]
- 14. Kooyman CDA, Witry MJ. The developing role of community pharmacists in facilitating care transitions: a systematic review. J Am Pharm Assoc 2019;59:265–74. 10.1016/j.japh.2018.11.009 [DOI] [PubMed] [Google Scholar]
- 15. Patton SJ, Miller FA, Abrahamyan L, et al. Expanding the clinical role of community pharmacy: a qualitative ethnographic study of medication reviews in Ontario, Canada. Health Policy 2018;122:256–62. 10.1016/j.healthpol.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 16. Silva RdeOS, Macêdo LA, Santos GAdos, et al. Pharmacist-participated medication review in different practice settings: service or intervention? an overview of systematic reviews. PLoS One 2019;14:e0210312–24. 10.1371/journal.pone.0210312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naunton M, Peterson GM. Evaluation of home-based follow-up of high-risk elderly patients discharged from hospital. J Pharm Pract Res 2003;33:176–82. 10.1002/jppr2003333176 [DOI] [Google Scholar]
- 18. Heaton PC, Frede S, Kordahi A, et al. Improving care transitions through medication therapy management: a community partnership to reduce readmissions in multiple health-systems. J Am Pharm Assoc 2019;59:319–28. 10.1016/j.japh.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 19. Luder HR, Frede SM, Kirby JA, et al. TransitionRx: impact of community pharmacy postdischarge medication therapy management on hospital readmission rate. J Am Pharm Assoc 2015;55:246–54. 10.1331/JAPhA.2015.14060 [DOI] [PubMed] [Google Scholar]
- 20. Mantzourani E, Nazar H, Phibben C, et al. Exploring the association of the discharge medicines review with patient Hospital readmissions through national routine data linkage in Wales: a retrospective cohort study. BMJ Open 2020;10:e033551. 10.1136/bmjopen-2019-033551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lapointe-Shaw L, Bell CM, Austin PC, et al. Community pharmacy medication review, death and re-admission after hospital discharge: a propensity score-matched cohort study. BMJ Qual Saf 2020;29:41–51. 10.1136/bmjqs-2019-009545 [DOI] [PubMed] [Google Scholar]
- 22. Barker A, Barlis P, Berlowitz D, et al. Pharmacist directed home medication reviews in patients with chronic heart failure: a randomised clinical trial. Int J Cardiol 2012;159:139–43. 10.1016/j.ijcard.2011.02.034 [DOI] [PubMed] [Google Scholar]
- 23. Holland R, Lenaghan E, Harvey I, et al. Does home based medication review keep older people out of hospital? the Homer randomised controlled trial. BMJ 2005;330:293–5. 10.1136/bmj.38338.674583.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Melody KT, McCartney E, Sen S, et al. Optimizing care transitions: the role of the community pharmacist. Integr Pharm Res Pract 2016;5:43–51. 10.2147/IPRP.S87947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pawson R. Evidence-Based policy: a realist perspective. London, UK: Sage, 2006. [Google Scholar]
- 26. Wong G, Westhorp G, Pawson R, et al. Realist synthesis RAMESES training materials. National Institute for health research health services and delivery research program, 2013. Available: https://www.ramesesproject.org/media/Realist_reviews_training_materials.pdf [Accessed 5 Aug 2020].
- 27. Pawson R, Tilley N, Tilley N. Realistic evaluation. London, UK: Sage, 1997. [Google Scholar]
- 28. Booth A, Wright J, Briscoe S. Scoping and searching to support realist approaches. : Emmel NGJ, Manzano A, Monaghan M, et al., . Doing realist research. London, UK: Sage, 2018. [Google Scholar]
- 29. Wong G. Data gathering in realist reviews: Looking for needles in haystacks. : Emmel N, Greenhalgh J, Manzano A, et al., . Doing realist research. London, UK: Sage, 2018. [Google Scholar]
- 30. Wong G, Greenhalgh T, Westhorp G, et al. RAMESES publication standards: realist syntheses. BMC Med 2013;11:21. 10.1186/1741-7015-11-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ellitt GR, Brien J-anneE, Aslani P, et al. Quality patient care and pharmacists' role in its continuity--a systematic review. Ann Pharmacother 2009;43:677–91. 10.1345/aph.1L505 [DOI] [PubMed] [Google Scholar]
- 32. Bayoumi I, Howard M, Holbrook AM, et al. Interventions to improve medication reconciliation in primary care. Ann Pharmacother 2009;43:1667–75. 10.1345/aph.1M059 [DOI] [PubMed] [Google Scholar]
- 33. Pawson R. The science of evaluation a realist manifesto. London, UK: SAGE, 2013. [Google Scholar]
- 34. Mantzourani E, Way CM, Hodson KL. Does an integrated information technology system provide support for community pharmacists undertaking discharge medicines reviews? an exploratory study. Integr Pharm Res Pract 2017;6:145–56. 10.2147/IPRP.S133273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramsbottom H, Fitzpatrick R, Rutter P. Hospital referral of older patients to community pharmacy: outcome measures in a feasibility study. Int J Clin Pharm 2020;42:18–22. 10.1007/s11096-019-00961-w [DOI] [PubMed] [Google Scholar]
- 36. Gwynn BF, Blenkinsopp A, Armitage G, et al. Missed opportunities: the role of community pharmacy after discharge from cardiology wards. Int J Pharm Pract 2014;22:7–8. [Google Scholar]
- 37. Lam MYY, Dodds LJ, Corlett SA. Engaging patients to access the community pharmacy medicine review service after discharge from Hospital: a cross-sectional study in England. Int J Clin Pharm 2019;41:1110–7. 10.1007/s11096-019-00838-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baqir W, Desai N, Harker N, et al. Can targeted medicines use reviews support safe transfer of care from hospital to community? Int J Pharm Pract 2012;20:82–3. [Google Scholar]
- 39. Bhatti N, Devlin L, Farooq H, et al. Community pharmacists' experiences of managing patients' medicines after discharge from Hospital: a preliminary study of discharge medicines use reviews (DMURs). Int J of Pharm Pract 2013;21:86–7. [Google Scholar]
- 40. Evans A, Wheeler L, Hood K, et al. Is medicines support at discharge with follow up in community pharmacy for patients admitted to hospital with an acute exacerbation of COPD feasible? the PICMeUP study. Int J of Pharm Pract 2013;21:37–8. [Google Scholar]
- 41. Elson R, Cook H, Blenkinsopp A. Patients' knowledge of new medicines after discharge from Hospital: what are the effects of hospital-based discharge counseling and community-based medicines use reviews (MURs)? Res Social Adm Pharm 2017;13:628–33. 10.1016/j.sapharm.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 42. Corlett S, Goel P, Kothari S, et al. Are sufficient efforts being made by hospital pharmacy teams to encourage patients to access a medicines use review after discharge? Int J Pharm Pract 2014;22:8–9. [Google Scholar]
- 43. Hodson K. Evaluation of the discharge medicines review service. UK: Cardiff University; Cardiff, 2014. [Google Scholar]
- 44. Veeren JC, Rogers PJ, Taylor ADJ, et al. Improving communication across the primary-secondary care interface: a survey of the attitudes and experiences of community pharmacists in England. Int J Pharm Pract 2019;27:9–10. [Google Scholar]
- 45. Bellone JM, Barner JC, Lopez DA. Postdischarge interventions by pharmacists and impact on hospital readmission rates. J Am Pharm Assoc 2012;52:358–62. 10.1331/JAPhA.2012.10172 [DOI] [PubMed] [Google Scholar]
- 46. Polinski JM, Moore JM, Kyrychenko P, et al. An insurer’s care transition program emphasizes medication reconciliation, reduces readmissions and costs. Health Aff 2016;35:1222–9. 10.1377/hlthaff.2015.0648 [DOI] [PubMed] [Google Scholar]
- 47. Shcherbakova N, Tereso G. Clinical pharmacist home visits and 30-day readmissions in Medicare advantage beneficiaries. J Eval Clin Pract 2016;22:363–8. 10.1111/jep.12495 [DOI] [PubMed] [Google Scholar]
- 48. Tedesco GW, McConaha JL, Skomo ML, et al. A pharmacist's impact on 30-day readmission rates when compared to the current standard of care within a patient-centered medical home: a pilot study. J Pharm Pract 2016;29:368–73. 10.1177/0897190014568671 [DOI] [PubMed] [Google Scholar]
- 49. Tuttle KR, Alicic RZ, Short RA, et al. Medication therapy management after hospitalization in CKD: a randomized clinical trial. Clin J Am Soc Nephrol 2018;13:231–41. 10.2215/CJN.06790617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kelling S, Bright D, Sullivan D, et al. Preliminary results of community pharmacy transitions of care program for patients with managed Medicaid. J Am Pharm Assoc 2013;53:e67. [Google Scholar]
- 51. Pherson EC, Shermock KM, Efird LE, et al. Development and implementation of a postdischarge home-based medication management service. Am J Health Syst Pharm 2014;71:1576–83. 10.2146/ajhp130764 [DOI] [PubMed] [Google Scholar]
- 52. Kogut SJ, Goldstein E, Charbonneau C, et al. Improving medication management after a hospitalization with pharmacist home visits and electronic personal health records: an observational study. Drug Healthc Patient Saf 2014;6:1–6. 10.2147/DHPS.S56574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Angley M. Implementing and evaluating a parallel post-discharge home medicines review (HMR) model, 2015. Available: http://6cpa.com.au/resources/fourth-agreement/implementing-and-evaluating-a-parallel-post-discharge-home-medicines-review-hmr-model/
- 54. Bernal DDL, Stafford L, Bereznicki LRE, et al. Home medicines reviews following acute coronary syndrome: study protocol for a randomized controlled trial. Trials 2012;13:30. 10.1186/1745-6215-13-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stafford L, Peterson GM, Bereznicki LRE, et al. A role for pharmacists in community-based post-discharge warfarin management: protocol for the 'the role of community pharmacy in post hospital management of patients initiated on warfarin' study. BMC Health Serv Res 2011;11:16. 10.1186/1472-6963-11-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ellitt GR, Chen TF, Brien JA, et al. From hospital to community: a multidisciplinary ‘continuity of care’ model for cardiovascular patients, involving community pharmacists, 2005. Available: http://6cpa.com.au/resources/third-agreement/from-hospital-to-community-a-multidisplinary-continuity-of-care-model-for-cardiovascular-patients-involving-community-pharmacists/ [Accessed 20 Apr 2020].
- 57. van der Heijden AAWA, de Bruijne MC, Nijpels G, et al. Cost-Effectiveness of a clinical medication review in vulnerable older patients at hospital discharge, a randomized controlled trial. Int J Clin Pharm 2019;41:963–71. 10.1007/s11096-019-00825-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Daliri S, Hugtenburg JG, Ter Riet G, et al. The effect of a pharmacy-led transitional care program on medication-related problems post-discharge: a before-after prospective study. PLoS One 2019;14:e0213593. 10.1371/journal.pone.0213593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ensing HT, Koster ES, Dubero DJ, et al. Collaboration between hospital and community pharmacists to address drug-related problems: the HomeCoMe-program. Res Social Adm Pharm 2019;15:267–78. 10.1016/j.sapharm.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 60. Stafford L, Peterson GM, Bereznicki LRE, et al. Clinical outcomes of a pharmacist-led post-discharge warfarin management service. J Thromb Thrombolysis 2011;31:370. [Google Scholar]
- 61. Pacini M, Smith RD, Wilson ECF, et al. Home-Based medication review in older people: is it cost effective? Pharmacoeconomics 2007;25:171–80. 10.2165/00019053-200725020-00008 [DOI] [PubMed] [Google Scholar]
- 62. Ministry of Health and Long-Term Care . Professional pharmacy services Guidebook 3.0, 2016. Available: http://www.health.gov.on.ca/en/pro/programs/drugs/medscheck/docs/guidebook.pdf [Accessed 20 Apr 2020].
- 63. Pharmaceutical Services Negotiating Committee . Medicines use review and prescription intervention service, 2013. Available: https://psnc.org.uk/wp-content/uploads/2013/06/MUR-service-spec-Aug-2013-changes_FINAL.pdf [Accessed 20 Apr 2020].
- 64. Pharmaceutical Society of Australia . Guidelines for pharmacists providing home medicines review (HMR) services., 2011. Available: https://my.psa.org.au/s/article/Guidelines-for-Home-Medicines-Reviews [Accessed 20 Apr 2020].
- 65. Bernal DDL. A randomised controlled trial of home medicines reviews following acute coronary syndromes. Hobart, Australia: University of Tasmania, 2017. [Google Scholar]
- 66. Angley M, Ponniah AP, Spurling LK, et al. Feasibility and timeliness of alternatives to Post‐Discharge home medicines reviews for High‐Risk patients. J Pharm Pract Res 2011;41:27–32. 10.1002/j.2055-2335.2011.tb00062.x [DOI] [Google Scholar]
- 67. Bollella G, Angley MT, Pink JA, et al. Optimal level of liaison pharmacist intervention to facilitate a post-discharge home medicines review. J Pharm Pract Res 2008;38:107–10. 10.1002/j.2055-2335.2008.tb00813.x [DOI] [Google Scholar]
- 68. McMillan SS, Kelly F, Sav A, et al. Australian community pharmacy services: a survey of what people with chronic conditions and their carers use versus what they consider important. BMJ Open 2014;4:e006587. 10.1136/bmjopen-2014-006587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Carter SR, Moles R, White L, et al. Patients’ willingness to use a pharmacist-provided medication management service: The influence of outcome expectancies and communication efficacy. Res Soc Admin Pharm 2012;8:487–98. 10.1016/j.sapharm.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 70. Gidman W, Ward P, McGregor L. Understanding public trust in services provided by community pharmacists relative to those provided by general practitioners: a qualitative study. BMJ Open 2012;2:e000939. 10.1136/bmjopen-2012-000939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hodson K, James D, Smith M, et al. Evaluation of the discharge medicines review service in Wales: community and hospital pharmacists' views. Int J Pharm Pract 2014;22:6. [Google Scholar]
- 72. Stafford L, Peterson GM, Bereznicki LRE, et al. Clinical outcomes of a collaborative, home-based postdischarge warfarin management service. Ann Pharmacother 2011;45:325–34. 10.1345/aph.1P617 [DOI] [PubMed] [Google Scholar]
- 73. Ponniah A, Shakib S, Doecke CJ, et al. Post-Discharge medication reviews for patients with heart failure: a pilot study. Pharm World Sci 2008;30:810–5. 10.1007/s11096-008-9230-7 [DOI] [PubMed] [Google Scholar]
- 74. Ramsbottom HF, Fitzpatrick R, Rutter P, et al. Post discharge medicines use review service for older patients: recruitment issues in a feasibility study. Int J Clin Pharm 2016;38:208–12. 10.1007/s11096-015-0243-8 [DOI] [PubMed] [Google Scholar]
- 75. Ramsbottom H, Rutter P, Fitzpatrick R. Post discharge medicines use review (dMUR) service for older patients: cost-savings from community pharmacist interventions. Res Social Adm Pharm 2018;14:203–6. 10.1016/j.sapharm.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 76. Salter C, Holland R, Harvey I, et al. "I haven't even phoned my doctor yet." The advice giving role of the pharmacist during consultations for medication review with patients aged 80 or more: qualitative discourse analysis. BMJ 2007;334:1101. 10.1136/bmj.39171.577106.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. MYY L, Corlett SA, Dodds LJ. Poor uptake of dMURS is not the fault of hospital pharmacy services. Pharmacoepidemiol Drug Saf 2016;25:15–16. [Google Scholar]
- 78. Rutter P, Ramsbottom H, Fitzpatrick R. Community pharmacist perceptions of delivering post-hospital discharge medicines use reviews for elderly patients. Int J Clin Pharm 2017;39:33–6. 10.1007/s11096-016-0400-8 [DOI] [PubMed] [Google Scholar]
- 79. Ahmad A, Hugtenburg J, Welschen LMC, et al. Effect of medication review and cognitive behaviour treatment by community pharmacists of patients discharged from the hospital on drug related problems and compliance: design of a randomized controlled trial. BMC Public Health 2010;10:133. 10.1186/1471-2458-10-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Holland R, Lenaghan E, Smith R, et al. Delivering a home-based medication review, process measures from the Homer randomised controlled trial. Int J Pharm Pract 2006;14:71–9. 10.1211/ijpp.14.1.0009 [DOI] [Google Scholar]
- 81. Farrell H. The political economy of trust: institutions, interests, and inter-firm cooperation in Italy and Germany. Cambridge University Press, 2009. [Google Scholar]
- 82. Carter SR, Moles RJ, White L, et al. Consumers' willingness to use a medication management service: the effect of medication-related worry and the social influence of the general practitioner. Res Social Adm Pharm 2013;9:431–45. 10.1016/j.sapharm.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 83. Rodrigues CR, Harrington AR, Murdock N, et al. Effect of Pharmacy-Supported Transition-of-Care interventions on 30-day readmissions: a systematic review and meta-analysis. Ann Pharmacother 2017;51:866–89. 10.1177/1060028017712725 [DOI] [PubMed] [Google Scholar]
- 84. Nazar H, Brice S, Akhter N, et al. New transfer of care initiative of electronic referral from hospital to community pharmacy in England: a formative service evaluation. BMJ Open 2016;6:e012532. 10.1136/bmjopen-2016-012532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Blennerhassett JD, Cusack BM, Smith CD, et al. A novel medicines management pathway. J Pharm Pract Res 2006;36:175–80. 10.1002/j.2055-2335.2006.tb00601.x [DOI] [Google Scholar]
- 86. Yu K, Nguyen A, Shakib S, et al. Enhancing continuity of care in therapeutics: development of a post-discharge home medicines review model. J Pharm Pract Res 2007;37:22–6. 10.1002/j.2055-2335.2007.tb00652.x [DOI] [Google Scholar]
- 87. Padhye V, Ponniah AP, Spurling LK, et al. Alternatives to Post-Discharge Home Medication Reviews for High-Risk Patients: Doctors’ and Pharmacists’ Views. J Pharm Pract Res 2012;42:273–7. 10.1002/j.2055-2335.2012.tb00187.x [DOI] [Google Scholar]
- 88. Angley M, Ponniah AP, Spurling LK, et al. Alternatives to post-discharge HMRS for high-risk patients. Aust J Pharm 2011;92:76–9. [Google Scholar]
- 89. Foot H, Freeman C, Hemming K, et al. Reducing medical admissions into Hospital through optimising medicines (remain home) study: protocol for a stepped-wedge, cluster-randomised trial. BMJ Open 2017;7:e01530. 10.1136/bmjopen-2016-015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ponniah A, Anderson B, Shakib S, et al. Pharmacists' role in the post-discharge management of patients with heart failure: a literature review. J Clin Pharm Ther 2007;32:343–52. 10.1111/j.1365-2710.2007.00827.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjqs-2020-011418supp001.pdf (183.8KB, pdf)
bmjqs-2020-011418supp002.pdf (9.7KB, pdf)
bmjqs-2020-011418supp003.pdf (61.5KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information, further data related to searches and screening will be available from the submitting author on request.