Abstract
Background:
Disturbances of the bodily self are fundamental to the phenomenological experience of individuals with schizophrenia, a population at risk for social isolation. Both proprioception and exteroception contribute to a sense of consistent body boundary that contains the self across time and space, and this process is influenced by self-other (social) interactions. However, the relationship between social isolation, exteroception, and in-the-moment changes in body representation has not been elucidated. We investigated susceptibility to anomalous bodily experiences with a phantom nose induction procedure that elicits a sensation that one’s nose is changing (Pinocchio Illusion: PI) in relation to exteroceptive awareness and social isolation.
Methods:
25 individuals with schizophrenia (SZ) and 15 matched controls (CO) participated in a PI induction procedure to quantify susceptibility to bodily aberrations and a tactile discrimination task to assess exteroception. Clinical symptoms in SZ and schizotypy in CO were assessed, in addition to a self-report measure of perceived social isolation.
Results:
Compared to CO, SZ showed increased PI and impaired tactile discriminability. SZ reported greater loneliness than CO. PI scores were correlated with increased loneliness and decreased tactile discriminability.
Conclusions:
Greater susceptibility to anomalous bodily experiences, together with reduced exteroceptive awareness and increased loneliness, is compatible with the framework of Hoffman’s Social Deafferentation Hypothesis, which posits that a functional “amputation” from one’s social environment could lead to a reorganization of the social brain network, resulting in hallucinations and delusions. These findings underscore the importance of the relationship between social isolation and self-disturbances in schizophrenia.
Keywords: Body aberration, Self-disturbances, Proprioception, Exteroception, Pinocchio Illusion, Social isolation
1. Introduction
A tacit understanding of one’s body as a continuously unified entity with fixed boundaries allows one to distinguish self from other, and this experienced unity of self and body is necessary for adaptive social functioning (Petkova et al., 2011; Postmes et al., 2014; Park and Nasrallah, 2014). Self-disturbances and anomalous beliefs concerning one’s own body were important to Bleuler’s (1911) conceptualization of schizophrenia. Clinical descriptions of schizophrenia are replete with sudden alterations in size and shape of the body, abnormal body ownership, anomalous agency, and even out-of-body experiences (e.g., Chapman et al., 1978; Priebe and Röhricht, 2001; Saks, 2008; Kean, 2011). These self-disturbances are present during the prodromal stage and remain salient throughout the course of the illness (Sass and Parnas, 2003; Lysaker and Lysaker, 2010; Nelson et al., 2012; Nasrallah, 2012; Koren et al., 2013; Brent et al., 2014). These phenomenological accounts of self-disturbances in schizophrenia are supported by empirical studies of two aspects of self-disturbances: anomalous agency (Frith et al., 2000; Fourneret et al., 2002; Graham et al., 2015; Garbarini et al., 2016; Hur et al., 2014) and abnormal body ownership (Peled et al., 2000; Peled et al., 2003; Thakkar et al., 2011; Ferri et al., 2014; Gallese and Ferri, 2014). However, more elusive yet crucial experiences of temporary changes in the shape, size, or location of one’s own body parts (see Chapman et al., 1978) have not been extensively investigated in the laboratory.
Since bodily aberrations tend to occur spontaneously, they are difficult to objectively measure, and interview-based assessments may not be optimal for quantifying subtle anomalies in real time. However, susceptibility to bodily self-aberration can be reliably demonstrated via a family of proprioceptive paradigms, such as the Rubber Hand Illusion (e.g., Botvinick and Cohen, 1998; Albrecht et al., 2011; Thakkar et al., 2011), the Full Body Illusion (Ehrsson, 2007; Blanke and Metzinger, 2009; Mizumoto and Ishikawa, 2005), and the Pinocchio Illusion (PI: Lackner, 1988; Burrack and Brugger, 2005).
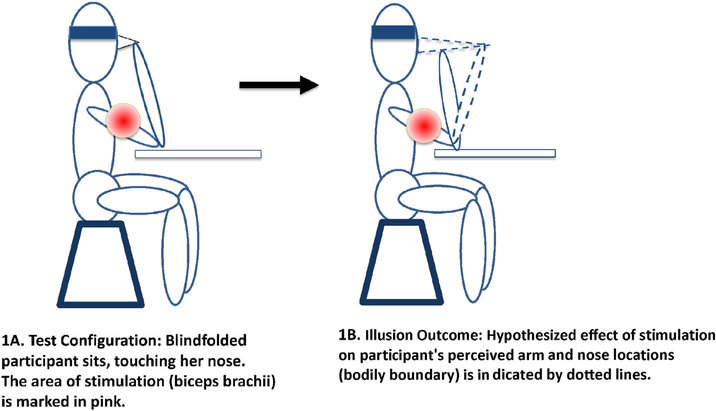
The PI is a proprioceptive illusion that engenders the feeling that one’s nose is growing. To induce it, a vibrator is applied to the biceps brachii of the participant while she touches her nose with the index finger of the stimulated arm (Fig. 1). The vibrator stimulates muscle spindles in the biceps that would normally be activated by the stretching muscle, but since the arm position is fixed by requiring the subject to touch her nose, this procedure creates a kinesthetic illusion that the arm is moving away from the face. Because the finger touching the nose is still giving tactile information of being in contact with the nose, while the arm is perceived to be moving forward, the participant feels that the nose is also moving away from the face, as the brain works to reconcile conflicting proprioceptive and tactile sensations. Thus, the perceived shape, size, and orientation of one’s own body parts can be manipulated to generate false information about limb position. In contrast to the RHI, the PI does not include an “other” component, and, therefore, is more suitable for investigating representation of bodily boundaries without influences of altered agency or body ownership.
Fig. 1.
Pinocchio Illusion induction.
Proprioception links one’s bodily movement with the body’s location, contributing to the spatial and peri-personal sense of self. Tactile perception (i.e., exteroception) contributes to one’s sense of body boundary (i.e., self versus non-self) through interpretation of sensory stimuli on the skin. Reduced exteroceptive awareness or misinterpreted tactile perception exacerbates self-disturbances and psychosis risk (Nelson et al., 2008; Postmes et al., 2014). Indeed, tactile sensitivity is reduced in the schizophrenia-spectrum (Chang and Lenzenweger, 2001, 2005).
The bodily self does not exist in a vacuum. Infants’ sense of the body schema and bodily self develop via dynamic multisensory (especially proprioceptive and tactile) interactions with the environment (Piaget and Inhelder, 1967; Morgan and Rochat, 1997). Thus, our sense of the bodily self and awareness of embodied psychological self are significantly shaped by our interactions with the world. Specifically, social interactions provide us with a framework to interpret and disambiguate somatic signals (Cioffi, 1991; Russell, 2003). When these constant interactions with the world are removed, ensuing social isolation and loneliness lead to adverse consequences (Cacioppo and Hawkley, 2009). Specifically, social isolation and withdrawal are thought to trigger or exacerbate delusions and hallucinations in vulnerable individuals across diagnostic categories (Brugger et al., 1999; Hoffman, 2007; Jiang et al., 2013; Selten et al., 2013; El Haj et al., 2016) and worsen positive symptoms (Grassian, 1983; Siegel, 1984; Hoffman, 2007; De Sousa et al., 2015). Hoffman’s (2007) Social Deafferentation Hypothesis posits that a loss of social connectedness leads to a tendency to create humanlike agents (i.e. hallucination) and increased belief in the supernatural (i.e. delusion) through neural re-organization. These tendencies may be magnified by the unmet need for social support and connectedness (Epley et al., 2008) and impaired self-perception (Bastian & Haslam, 2010). Moreover, there are neuroanatomical consequences of social isolation. Loneliness exerts a detrimental impact on the left posterior superior temporal sulcus (pSTS) that normally supports social cognition (Kanai et al., 2012). Abnormal function and reduced volume of the pSTS are associated with hallucinations, delusions, and thought disorder in schizophrenia (Shenton et al., 1992; Kim et al., 2011). Furthermore, the areas that contribute to the social brain network (e.g., temporo-parietal junction (TPJ) and the inferior temporal region) and the areas implicated in bodily processing overlap (Blanke et al., 2005; Wible, 2012). Taken together, these findings suggest an important role of impoverished social interactions in clinical symptoms and the sense of bodily self, including both proprioceptive and exteroceptive abnormalities.
In the present study, we aimed to fill in important gaps in the literature by quantifying subjective bodily experiences (i.e. aberrant proprioception and exteroception) and identifying potential underlying mechanisms (i.e. social isolation). We hypothesized increased susceptibility to anomalous bodily experiences and impaired exteroceptive awareness would be associated with the severity of clinical symptoms in schizophrenia. Furthermore, we hypothesized that social isolation and reduced exteroceptive awareness would be associated with increased susceptibility to the PI.
2. Methods
2.1. Participants
Twenty-five outpatients who met the DSM-IV criteria for schizophrenia (SZ) were recruited from outpatient facilities in Nashville. All, except for one, were medicated. Symptoms were assessed with the Brief Psychiatric Rating Scale (BPRS; Overall and Gorham, 1962), the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984), and the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1983). Fifteen healthy controls (CO) without history of DSM-IV Axis I disorder in themselves or in their families were recruited from Nashville by advertisement. Schizotypy in CO was assessed with the Schizotypal Personality Questionnaire (SPQ) (Raine, 1991).
All participants were screened for substance use within the past 6 months, neurological disorders, and past head injuries. Participants were not taking any pain medication. The two groups were matched in age, handedness, and gender but not on IQ or education. However, the mean IQ and education level of SZ were well within the normal range. All participants gave written informed consent and were paid. The protocol was approved by the Vanderbilt Institutional Review Board. Please see Table 1 for demographic and clinical information.
Table 1.
Demographic and clinical information.
| Schizophrenia Mean (SD) | Controls Mean (SD) | Statistical test (t-test/chi sq) | p value | |
|---|---|---|---|---|
| Age | 44.44 (9.06) | 44.93 (7.56) | t = 0.18 | 0.85 |
| Sex | 11F/14M | 5F/10M | χ2 = −0.11 | 0.74 |
| Years of education | 13.36 (2.18) | 15.67 (2.06) | t = 3.36 | 0.001 |
| IQ (NART) (Nelson, 1982) | 102.68 (10.12) | 108.56 (7.23) | t = 2.13 | 0.04 |
| Edinburgh Handedness (Oldfield, 1971) | +71.04 (41.86) | +60.67 (69.07) | t = −0.52 | 0.61 |
| BPRS | 21.44 (13.60) | N/A | ||
| SAPS | 18.9 (15.78) | N/A | ||
| SANS | 40.32 (16) | N/A | ||
| SPQ - total | N/A | 10.53 (8.31) | ||
| SPQ-positive | N/A | 3.47 (4.50) | ||
| SPQ-negative | N/A | 4.8 (3.78) | ||
| SPQ-disorganized | N/A | 3 (3.66) | ||
| Chlorpromazine equivalent dose (mg/kg/day) | 400.23 (341.63) | N/A | ||
2.2. Procedure
The Pinocchio Illusion task (PI; Burrack and Brugger, 2005), the two-point discrimination task (2-PT; Chang and Lenzenweger, 2005), and the self-report questionnaires were administered with the order of presentation counterbalanced across participants.
After informed consent and instructions, the participant wore a blindfold and rested her elbow on a table with the forearm slightly angled towards the body so that the index finger could touch the nose (see Fig. 1). The experimenter then stimulated the bicep brachii tendon of the upper arm at 120 Hz with a physiotherapy vibrator (Novafon SK 1/1). The participant was instructed to report the onset of any sensations she might feel, apart from the vibrations. If the participant reported the onset of an unusual sensation, the stimulation was continued for another minute. If the participant did not report any unusual sensations, the stimulation stopped after 2 min. Immediately after stimulation, an 11-item questionnaire (see Supplement 1) was given to quantify susceptibility to the PI. Each item was a statement about proprioceptive and tactile sensations (e.g., position changes of the arm, position or shape changes of the nose), which was rated on a scale from 0 (no experience) to 100 (full endorsement of experience). There were two amplitude settings on the vibrator: the lowest setting reaching a depth of 1.5 mm and the highest setting reaching a depth of 1.6 mm. Both the right and left arms were stimulated. The order of stimulation of the arm was counterbalanced across participants, and the low setting was used first on each arm.
The two-point discrimination task (2-PT) measures tactile sensitivity (Chang and Lenzenweger, 2005). An anesthesiometer was used to deliver precise tactile stimulation. The blindfolded participant laid her hand flat on the table, palm facing upwards, and was asked to determine whether she felt one point or two points on her palm. The participant responded by saying “one” or “two.” There were three tactile stimulus conditions: one point, 6 mm between the two points (hard), and 10 mm between the two points (easy). There were 50 trials per hand, consisting of 30 one-point trials, 10 hard two-point trials, and 10 easy two-point trials. The order of these trials was randomized for each hand, and the order of the stimulation of the hand was counterbalanced across participants.
The 20-item UCLA Loneliness Scale (Russell, 1996) was used to estimate loneliness and perceived social isolation. This questionnaire estimates both the number of social interactions and overall subjective satisfaction with the social environment.
The 74-item Schizotypal Personality Questionnaire (SPQ; Raine, 1991) was used to measure schizotypy in CO. The SPQ consists of the positive (Cognitive-Perceptual), negative (Interpersonal), and disorganized subscales.
A survey recorded the total number of Out of Body Experiences (OBEs) experienced over lifetime by each participant.
3. Results
The PI data from one patient were incomplete and, therefore, not included in the analysis. The Loneliness score was missing for one CO. All significance tests were 2-tailed, unless otherwise indicated.
Matched pairs t-tests found no effect of amplitude setting (low or high) (t(38) = 1.16, p = 0.25) or arm (right or left) (t(38) = 1.64, p = 0.11) on the PI scores, so these scores were collapsed into one PI score (the mean of the four scores from each trial). For the 2-PT task, matched pairs t-tests found no difference in performance between left and right palms for easy trials (t(39) = 1.77, p = 0.8), hard trials (t(39) = 0.88, p = 0.39), one point trials (t(39) = 0.81, p = 0.42), or overall accuracy (t(39) = 0.91, p = 0.37). Therefore, the accuracy scores for the both palms were averaged into a total 2-PT accuracy score.
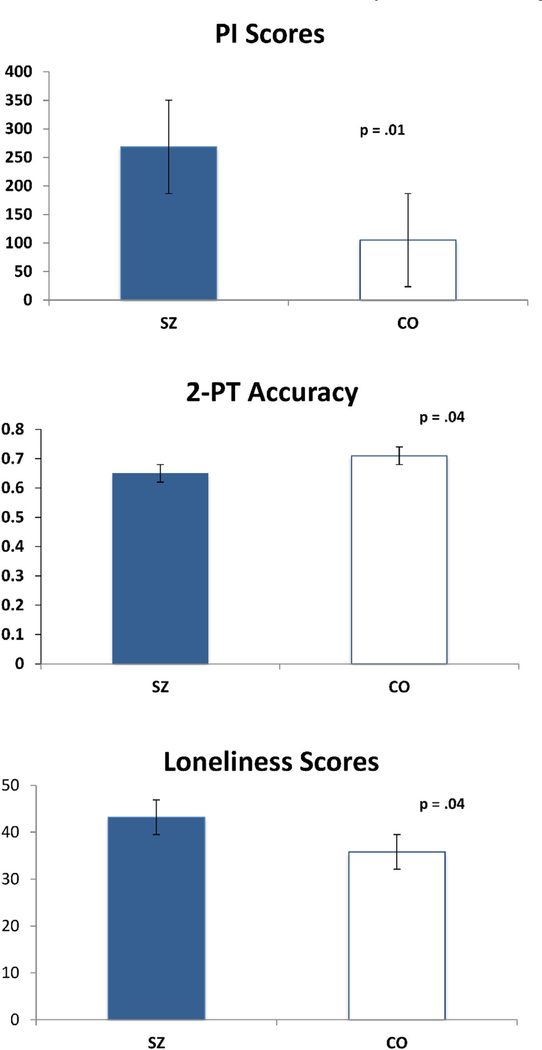
SZ and CO differed significantly on PI (F(1,38) = 6.39, p = 0.016, d = 0.826), with SZ scoring higher. The two groups also differed on the 2-PT task (F(1,39) = 4.60, p = 0.04, d = 0.701), with SZ showing reduced accuracy for both hands. SZ were lonelier than CO (F(1,38) = 4.3, p = 0.04, d = 0.678) (Fig. 2). Although the mean lifetime OBEs appeared to be elevated in SZ (SZ = 1.44 vs CO = 0.8), the two groups did not differ statistically (F(1,39) = 0.63, p = 0.43).
Fig. 2.
Group differences in Pinocchio Illusion, tactile accuracy, and loneliness.
Spearman’s correlations were used to test associations between task performances, self-report measures, and symptoms. PI scores positively correlated with Loneliness scores across both groups (rs(38) = 0.41, p < 0.01). PI scores were also correlated with the SAPS-Hallucinations subscale in SZ (r(24) = 0.41, p < 0.05), and the positive factor of the SPQ in CO (r(15) = 0.52, p < 0.04). PI was associated with lifetime OBEs across both groups (r(38) = 0.28, p < 0.04, 1-tailed). PI was negatively correlated with the 2-PT accuracy across both groups (r(39) = −0.31, p < 0.05) and SANS-Affective Flattening subscale in SZ (rs(24) = −0.71, p < 0.0002). Loneliness was positively correlated with the SAPS-Hallucinations (rs(24) = 0.58, p < 0.002) and SAPS-Delusions subscales (rs(24) = 0.55, p < 0.004) in SZ. Medication dose (CPZ) was not associated with PI, 2-PT, Loneliness scores, or symptoms.
4. Discussion
In this study, we demonstrated that SZ are more prone to anomalous bodily experiences, as investigated via a tactile-proprioceptive illusion induction. This finding supports earlier studies of flexible bodily representations (Peled et al., 2003; Thakkar et al., 2011; Germine et al., 2013) and impaired tactile discriminability (Chang and Lenzenweger, 2001, 2005) in schizophrenia-spectrum individuals. Moreover, we found an inverse relationship between the strength of the PI and the measure of tactile discriminability.
Unsurprisingly, the PI was associated with self-report measures of abnormal bodily experiences: positive symptoms in SZ, positive schizotypy in CO, and the increased lifetime prevalence of OBEs. Loneliness and the PI were strongly associated across both groups such that perceived social isolation and susceptibility to bodily aberrations were linked. Loneliness was also correlated with the severity of hallucinations and delusions in SZ. Although tactile discrimination and loneliness were not correlated, each measure was associated with the PI.
There are limitations to our study. First, although the effect sizes for group comparisons were robust, the sample size was modest. Thus, independent replications are warranted. Second, our measure of the PI relied on self-report ratings. However, these ratings were consistent and there were no practice effects; PI scores for the right and left arms were not different regardless of order of stimulation. Moreover, PI scores were associated with lifetime OBEs, which suggests a consistency of responses concerning abnormal bodily experiences across lifespan and in the lab. Third, there was no “control” condition in the PI task. We opted to maximize the sample size for PI induction, but including a sham stimulation condition is an important factor to consider in future replications of the PI. Fourth, we cannot infer directionality from the correlations between loneliness and PI. We do not know if chronic social isolation led to self-disturbance, or vice versa. However, a preliminary study in our laboratory suggests that even a temporary induction of social exclusion can impair social perception in healthy persons (Hieber and Park, 2015). It seems likely that social isolation and abnormal self-experiences interact to co-incubate adverse consequences over lifetime.
Lastly, given the association between negative symptoms such as anhedonia and social isolation, it may seem unintuitive from some theoretical viewpoints to conceptualize social isolation and bodily hallucinations as related issues. Social dysfunctions, including abnormal social interactions, isolation, and withdrawal, could arise from a lack of motivation and anhedonia. However, there are multiple routes to social impairments. The crucial role of hallucinations, delusions, and thought disorder (i.e., positive symptoms) in social impairments does not receive much attention. Hallucinations and delusions make normal social interactions difficult, and, therefore, these positive symptoms are likely to contribute to social impairments. Schizophrenia patients could be socially withdrawn because they have anhedonia or because they are pre-occupied by their internal experiences of hallucinations and delusions. Our findings demonstrate that these factors are inter-related. There is also solid evidence from previous work to suggest that positive symptoms such as hallucinations and delusions are linked to social impairments (e.g., McIntosh and Park, 2014; Thakkar et al., 2011; Kim et al., 2011). Moreover, patients who feel lonely are less likely to be socially anhedonic since they desire social interactions. If someone were socially anhedonic, being socially isolated would not be aversive, and she would not desire or seek other people. Feelings of loneliness suggest that there is longing and yearning for social companionship, but these desires are not being met. Therefore, feeling lonely contradicts social anhedonia, a central feature of negative symptoms. In the Social Deafferentation Hypothesis framework, perceived social isolation would predict hallucinations and delusions.
Thus, increased susceptibility to experiencing bodily aberrations may be catalyzed by loneliness in schizophrenia patients. Loneliness was clearly linked to the severity of hallucinations and delusions in our study, supporting Hoffman’s (2007) finding that increased social isolation preceded hallucination onset in 73% of interviewed patients. These findings are consistent with his Social Deafferentation Hypothesis, which proposes that social isolation leads to compensatory activity of the social brain network in response to an absence of social stimulation, producing social hallucinations and delusions in vulnerable individuals. In a study by El Haj et al. (2016), the authors use this neural compensation mechanism to explain why social isolation predicts hallucinations in a sample of Alzheimer’s patients. In our work, we extended Hoffman’s hypothesis to account for non-auditory, anomalous bodily hallucinatory experiences. The principle is the same. If one is cut off from the social world (i.e. amputated from the social environment and, therefore, socially isolated), the social brain network will prompt social cognitive programs to over-activate and increase the likelihood of experiencing spurious bodily self-experiences. The social brain network in this context includes the superior temporal sulcus and the TPJ (Thakkar et al., 2014; Wible et al., 2009). Since the TPJ is central to automatic and tacit bodily processing, such as maintaining an accurate map of body location, perception of self-body unity, and sense of body ownership (Wible, 2012; Blanke et al., 2005), spurious TPJ activation could elicit abnormal proprioceptive and tactile experiences. Furthermore, reduced opportunities for social interactions could limit the lifetime exposure to proprioceptive and tactile experiences with detrimental consequences for maintaining a continuous sense of self and body.
In conclusion, this study examined the relationship between three common issues in schizophrenia: anomalous self-experiences, decreased tactile sensitivity, and social isolation. The results support previous work regarding the prevalence of aberrant proprioceptive and exteroceptive perception in schizophrenia and also extend theoretical and empirical studies linking social isolation to abnormalities in these domains. The directionality of the associations among bodily disturbances, loneliness, and symptomatology cannot be determined from the current dataset, but future prospective and intervention studies could bridge these gaps. It will also be important to independently replicate our results using different methodologies to highlight the central role of bodily aberrations in schizophrenia etiology and further elucidate mediating and exacerbating factors. Given the prevalence of self-disturbances throughout the course of schizophrenia and associated bodily, proprioceptive abnormalities, it is of utmost importance to track disruptions of the phenomenological self in high-risk individuals to better characterize the prodromal signs and to develop interventional strategies that directly target bodily aberrations in this population.
Supplementary Material
Acknowledgement
We thank Lindsey McIntosh, Taylor Benson, and Joel Peterman for their help with recruitment, interviews, and testing and Peter Brugger for his expert guidance on the Pinocchio Illusion. We would like to dedicate this paper to the memory of Ralph Hoffman, whose visionary ideas and seminal work inspired us to delve deeper and look further: beyond the end of one’s own nose.
Role of the funding source
This work was supported in part by the Gertrude Conaway Vanderbilt Endowment.
Footnotes
Conflict of interest
Both authors declare that they have no conflicts of interest.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.schres.2016.06.013.
References
- Albrecht MA, Martin-Iverson MT, Price G, Lee J, Iyyalol R, Waters F, 2011. Dexamphetamine effects on separate constructs in the rubber hand illusion test. Psychopharmacology 217 (1), 39–50. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, 1983. Scale for the Assessment of Negative Symptoms. University of Iowa, Iowa City. [Google Scholar]
- Andreasen NC, 1984. Scale for the Assessment of Positive Symptoms. University of Iowa, Iowa City. [Google Scholar]
- Bastian B, Haslam N, 2010. Excluded from humanity: The dehumanizing effects of social ostracism. J. Exp. Soc. Psychol. 46 (1), 107–113. [Google Scholar]
- Blanke O, Metzinger T, 2009. Full-body illusions and minimal phenomenal selfhood. Trends Cogn. Sci. 13, 7–13. [DOI] [PubMed] [Google Scholar]
- Blanke O, Mohr C, Michel CM, Pascual-Leone A, Brugger P, Seeck M, Landis T, Thut G, 2005. Linking out-of-body experience and self processing to mental own-body imagery at the temporoparietal junction. J. Neurosci. 25 (3), 550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuler E, 1911. Dementia Praecox oder gruppe der Schizophrenien. In: Aschaffenburg G (Ed.), Handbuch der Psychiatrie. Spezieller Teil, 4. Abteilung, 1. Hälfte. Deuticke, Leipzig. B. [Google Scholar]
- Botvinick M, Cohen JD, 1998. Rubber hands ‘feel’ touch that eyes see. Nature 391, 756. [DOI] [PubMed] [Google Scholar]
- Brent BK, Seidman LJ, Thermenos HW, Holt DJ, Keshavan MS, 2014. Self disturbances as a possible premorbid indicator of schizophrenia risk: a neurodevelopmental perspective. Schizophr. Res. 152 (1) (73–80. C). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugger P, Regard M, Landis T, Oelz O, 1999. Hallucinatory experiences in extreme-altitude climbers. Cogn. Behav. Neurol. 12 (1), 67–71. [PubMed] [Google Scholar]
- Burrack A, Brugger P, 2005. Individual differences in susceptibility to experimentally induced phantom sensations. Body Image 2 (3), 307–313. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, 2009. Perceived social isolation and cognition. Trends Cogn. Sci. 13 (10), 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BP, Lenzenweger MF, 2001. Somatosensory processing in the biological relatives of schizophrenia patients: a signal detection analysis of two-point discrimination. J. Abnorm. Psychol. 110, 433–442. [DOI] [PubMed] [Google Scholar]
- Chang BP, Lenzenweger MF, 2005. Somatosensory processing and schizophrenia liability: proprioception, exteroceptive sensitivity, and graphesthesia performance in the biological relatives of schizophrenia patients. J. Abnorm. Psychol. 114, 85–95. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML, 1978. Body image aberration in schizophrenia. J. Abnorm. Psychol. 87 (399), 407. [DOI] [PubMed] [Google Scholar]
- Cioffi D, 1991. Beyond attentional strategies: a cognitive-perceptual model of somatic interpretation. Psychol. Bull. 109 (1), 25. [DOI] [PubMed] [Google Scholar]
- De Sousa P, Spray A, Sellwood W, Bentall RP, 2015. ‘No man is an island’. Testing the specific role of social isolation in formal thought disorder. Psychiatry Res. 230 (2), 304–313. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, 2007. The experimental induction of out-of-body experiences. Science 317 (5841), 1048. [DOI] [PubMed] [Google Scholar]
- El Haj M, Jardri R, Larøi F, Antoine P, 2016. Hallucinations, loneliness, and social isolation in Alzheimer’s disease. Cogn. Neuropsychiatry 7, 1–13. [DOI] [PubMed] [Google Scholar]
- Epley N, Akalis S, Waytz A, Cacioppo JT, 2008. Creating social connection through inferential reproduction loneliness and perceived agency in gadgets, gods, and greyhounds. Psychol. Sci. 19 (2), 114–120. [DOI] [PubMed] [Google Scholar]
- Ferri F, Costantini M, Salone A, Di Iorio G, Martinotti G, Chiarelli A, Merla A, Di Giannantonio M, Gallese V, 2014. Upcoming tactile events and body ownership in schizophrenia. Schizophr. Res. 152 (1), 51–57. [DOI] [PubMed] [Google Scholar]
- Fourneret P, Vignemont FD, Franck N, Slachevsky A, Dubois B, Jeannerod M, 2002. Perception of self-generated action in schizophrenia. Cogn. Neuropsychiatry 7 (2), 139–156. [DOI] [PubMed] [Google Scholar]
- Frith CD, Blakemore S-J, Wolpert DM, 2000. Explaining the symptoms of schizophrenia: abnormalities in the awareness of action. Brain Res. Rev. 31, 357–363. [DOI] [PubMed] [Google Scholar]
- Gallese V, Ferri F, 2014. Psychopathology of the bodily self and the brain: the case of schizophrenia. Psychopathology 47 (6), 357–364. [DOI] [PubMed] [Google Scholar]
- Garbarini F, Mastropasqua A, Sigaudo M, Rabuffetti M, Piedimonte A, Pia L, Rocca P, 2016. Abnormal sense of agency in patients with schizophrenia: evidence from bimanual coupling paradigm. Front. Behav. Neurosci. 10, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germine L, Benson TL, Cohen F, Hooker CIL, 2013. Psychosis-proneness and the rubber hand illusion of body ownership. Psychiatry Res. 207 (1), 45–52. [DOI] [PubMed] [Google Scholar]
- Graham KT, Martin-Iverson MT, Holmes NP, Jablensky A, Waters F, 2015. Deficits in agency in schizophrenia, and additional deficits in body image, body schema, and internal timing, in passivity symptoms. Cognition across the Psychiatric Disorder Spectrum: From Mental Health to Clinical Diagnosis, p. 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassian S, 1983. Psychopathological effects of solitary confinement. Am. J. Psychiatr. 140 (11), 1450–1454. [DOI] [PubMed] [Google Scholar]
- Hieber LL, Park S, 2015. Making meaning: social isolation, loneliness and social perception acuity. 29th Annual Meeting of the Society for Research in Psychopathology. New Orleans, LA. [Google Scholar]
- Hoffman RE, 2007. A social deafferentation hypothesis for induction of active schizophrenia. Schizophr. Bull. 33 (5), 1066–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J-W, Kwon JS, Lee TY, Park S, 2014. The crisis of minimal self-awareness in schizophrenia: a meta-analytic review. Schizophr. Res. 152 (1), 58–64. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Rompala GR, Zhang S, Cowell RM, Nakazawa K, 2013. Social isolation exacerbates schizophrenia-like phenotypes via oxidative stress in cortical interneurons. Biol. Psychiatry 73 (10), 1024–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Bahrami B, Duchaine B, Janik A, Banissy MJ, Rees G, 2012. Brain structure links loneliness to social perception. Curr. Biol. 22 (20), 1975–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean C, 2011. Battling with the life instinct: the paradox of the self and suicidal behavior in psychosis. Schizophr. Bull. 37 (1), 4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Park S, Blake RB, 2011. Perception of biological motion in schizophrenia and healthy individuals: a behavioral and fMRI study. PLoS One 6 (5), e19971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren D, Reznik N, Adres M, Scheyer R, Apter A, Steinberg T, Parnas J, 2013. Disturbances of basic self and prodromal symptoms among non-psychotic help-seeking adolescents. Psychol. Med. 43, 1365–1376. [DOI] [PubMed] [Google Scholar]
- Lackner JR, 1988. Some proprioceptive influences on the perceptual representation of body shape and orientation. Brain 111, 281–297. [DOI] [PubMed] [Google Scholar]
- Lysaker PH, Lysaker JT, 2010. Schizophrenia and alterations in self-experience: a comparison of 6 perspectives. Schizophr. Bull. 36 (2), 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh LG, Park S, 2014. Social trait judgment and affect recognition from static faces and video vignettes in schizophrenia. Schizophr. Res. 158 (1), 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto M, Ishikawa M, 2005. Immunity to error through misidentification and the bodily illusion experiment. J. Conscious. Stud. 12 (7), 3–19. [Google Scholar]
- Morgan R, Rochat P, 1997. Intermodal calibration of the body in early infancy. Ecol. Psychol. 9 (1), 1–23. [Google Scholar]
- Nasrallah HA, 2012. Impaired mental proprioception in schizophrenia. Curr. Psychiatry 11 (8), 4. [Google Scholar]
- Nelson HE, 1982. National Adult Reading Test (NART): for the assessment of premorbid intelligence in patients with dementia. Test Manual. NFER-Nelson. [Google Scholar]
- Nelson B, Yung AR, Bechdolf A, McGorry PD, 2008. The phenomenological critique and self-disturbance: implications for ultra-high risk (“prodrome”) research. Schizophr. Bull. 34 (2), 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B, Thompson A, Yung AR, 2012. Basic self-disturbance predicts psychosis onset in the ultra high risk for psychosis “prodromal” population. Schizophr. Bull. 38 (6), 1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC, 1971. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 9 (1), 97–113. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR, 1962. The brief psychiatric rating scale. Psychol. Rep. 10 (3), 799–812. [Google Scholar]
- Park S, Nasrallah HA, 2014. The varieties of anomalous self experiences in schizophrenia: splitting of the mind at a crossroad. Schizophr. Res. 152 (1), 1–4. [DOI] [PubMed] [Google Scholar]
- Peled A, Ritsner M, Hirschmann S, Geva AB, Modai I, 2000. Touch feel illusion in schizophrenic patients. Biol. Psychiatry 48 (11), 1105–1108. [DOI] [PubMed] [Google Scholar]
- Peled A, Pressman A, Geva AB, Modai I, 2003. Somatosensory evoked potentials during a rubber-hand illusion in schizophrenia. Schizophr. Res. 64 (2), 157–163. [DOI] [PubMed] [Google Scholar]
- Petkova VI, Björnsdotter M, Gentile G, Jonsson T, Li TQ, Ehrsson HH, 2011. From part- to whole-body ownership in the multisensory brain. Curr. Biol. 21 (13), 1118–1122. [DOI] [PubMed] [Google Scholar]
- Piaget J, Inhelder B, 1967. The Child’s Conception of Space (Langdon FJ & Lunzer JL, Trans) (New York: ). [Google Scholar]
- Postmes L, Sno HN, Goedhart S, van der Stel J, Heering HD, de Haan L, 2014. Schizophrenia as a self-disorder due to perceptual incoherence. Schizophr. Res. 152 (1), 41–50. [DOI] [PubMed] [Google Scholar]
- Priebe S, Röhricht F, 2001. Specific body image pathology in acute schizophrenia. Psychiatry Res. 101 (3), 289–301. [DOI] [PubMed] [Google Scholar]
- Raine A, 1991. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr. Bull. 17 (4), 555. [DOI] [PubMed] [Google Scholar]
- Russell DW, 1996. UCLA loneliness scale (version 3): reliability, validity, and factor structure. J. Pers. Assess. 66 (1), 20–40. [DOI] [PubMed] [Google Scholar]
- Russell JA, 2003. Core affect and the psychological construction of emotion. Psychol. Rev. 110 (1), 145–172. [DOI] [PubMed] [Google Scholar]
- Saks ER, 2008. The center cannot hold: my journey through madness. Hyperion. [DOI] [PubMed] [Google Scholar]
- Sass LA, Parnas J, 2003. Schizophrenia, consciousness, and the self. Schizophr. Bull. 29 (3), 427–444. [DOI] [PubMed] [Google Scholar]
- Selten JP, van der Ven E, Rutten BP, Cantor-Graae E, 2013. The social defeat hypothesis of schizophrenia: an update. Schizophr. Bull. 39 (6), 1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW, 1992. Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N. Engl. J. Med. 327 (9), 604–612. [DOI] [PubMed] [Google Scholar]
- Siegel RK, 1984. Hostage hallucinations: Visual imagery induced by isolation and lifethreatening stress. J. Nerv. Ment. Dis. 172 (5), 264–272. [PubMed] [Google Scholar]
- Thakkar KN, Nichols HS, McIntosh LG, Park S, 2011. Disturbances in body ownership in schizophrenia: evidence from the rubber hand illusion and case study of a spontaneous out-of-body experience. PLoS One 6 (10), e27089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar KN, Peterman JS, Park S, 2014. Altered brain activation during action imitation and observation in schizophrenia: a translational approach for studying social dysfunction. Am. J. Psychiatry 171, 539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wible CG, 2012. Hippocampal temporal-parietal junction interaction in the production of psychotic symptoms: a framework for understanding the schizophrenic syndrome. Front. Hum. Neurosci. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wible CG, Preus AP, Hashimoto R, 2009. A cognitive neuroscience view of schizophrenic symptoms: abnormal activation of a system for social perception and communication. Brain Imaging Behav. 3 (1), 85–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.