Abstract
This case demonstrates pneumatosis intestinalis and small bowel perforation in a paediatric patient with multisystem inflammatory syndrome in children (MIS-C). Our patient presented with fever, abdominal pain and shortness of breath. She progressed to haemodynamic failure and small bowel perforation approximately 1 week after admission. Patients with suspected or confirmed MIS-C should be monitored closely for abdominal catastrophe, especially when critically ill in the intensive care unit.
Keywords: COVID-19, paediatric surgery
Background
Multisystem inflammatory syndrome in children (MIS-C) is a hyperinflammatory process that is temporally associated with the SARS-CoV-2.1 Although the acute infection of SARS-CoV-2 in children is often relatively mild,2 MIS-C is a potentially life-threatening illness. Children who meet the Center for Disease Control (CDC) clinical guidelines demonstrate elevated inflammatory markers, alterations in cardiac function evidenced by elevated troponin-T and pro-Basic Naturetic Peptide (BNP) levels, and ventricular or coronary abnormalities on echocardiogram.3 Patients with MIS-C commonly exhibit involvement of gastrointestinal, cardiovascular, haematologic, mucocutaneous and respiratory systems.1 Gastrointestinal catastrophe; however, is not well described.
This case is a presentation of MIS-C with acute pneumatosis intestinalis and small bowel perforation.
Case presentation
The patient is a morbidly obese 17-year-old African-American girl who presented to an emergency department with a 4-day history of fever, difficulty breathing and abdominal pain with vomiting and diarrhoea. She was noted to have leukocytosis (white blood count 22.3) with a left shift, and a chest X-ray with peri-bronchial thickening. She received a single dose of ceftriaxone for presumed pneumonia before transferring care to our general paediatrics ward for further evaluation.
The patient tested negative for COVID-19 via RT-PCR on admission. However, 1 month earlier a family member tested positive for COVID-19. The patient had reportedly developed similar symptoms to her family member at that time but did not get tested. The patient also had family history of an aunt who died in her 30s from a pulmonary embolism, but the patient had not previously been worked up for hypercoagulability. Social history was positive for vaping.
Due to worsening dyspnoea and need for escalated respiratory support, the patient was transferred to the paediatric intensive care unit (PICU) on the day of admission. She arrived in the PICU requiring 15 L of oxygen via a simple face mask and was tachypneic to the 60 s and tachycardic to 110 s. Because of this, she was transitioned to non-invasive ventilation (NIV). On physical examination, she was awake and anxious, had coarse breath sounds and decreased aeration of the lungs, bounding peripheral pulses and a non-tender abdomen. The patient transiently improved with NIV, and a CT angiogram (CTA) was obtained due to her family history of pulmonary embolism and her clinical presentation of tachypnea, hypoxemia and tachycardia. She had further respiratory decompensation during the scan, and was urgently intubated on arrival back in the PICU. Her CTA demonstrated bilateral diffuse opacities in the lower lobes but no evidence of a pulmonary embolism. She was started on prophylactic enoxaparin at this point. Her differential diagnosis at that time included e-cigarette or vaping use-associated lung injury (EVALI), bacterial pneumonia, acute respiratory distress syndrome and MIS-C. Due to suspected vaping history, EVALI was pursued and she was started on methylprednisolone therapy. She had initial stabilisation, however, on hospital day (HD) 4, the patient developed atrial fibrillation, which required cardioversion. Her initial echocardiogram was normal for age.
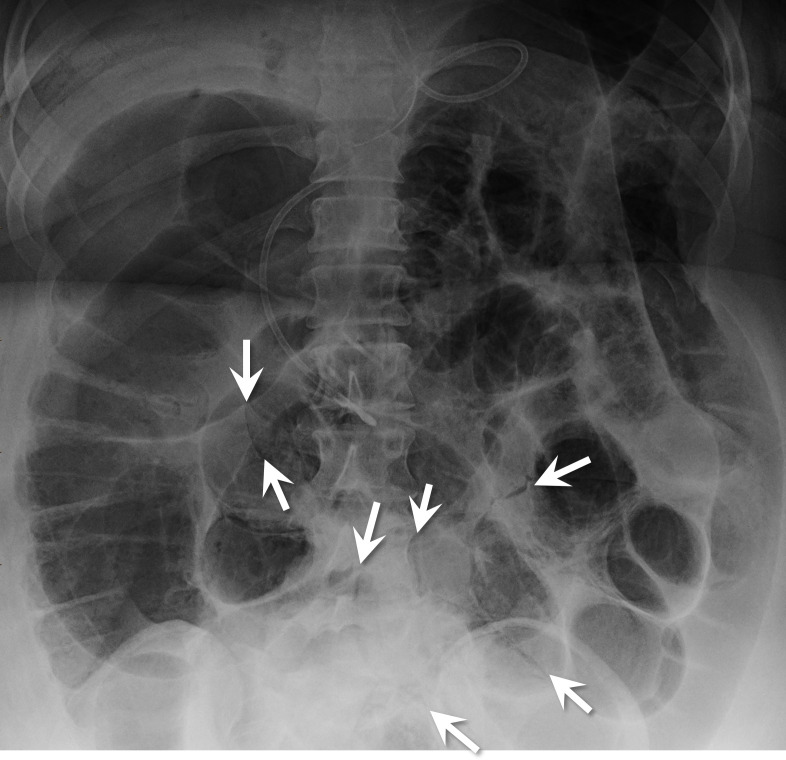
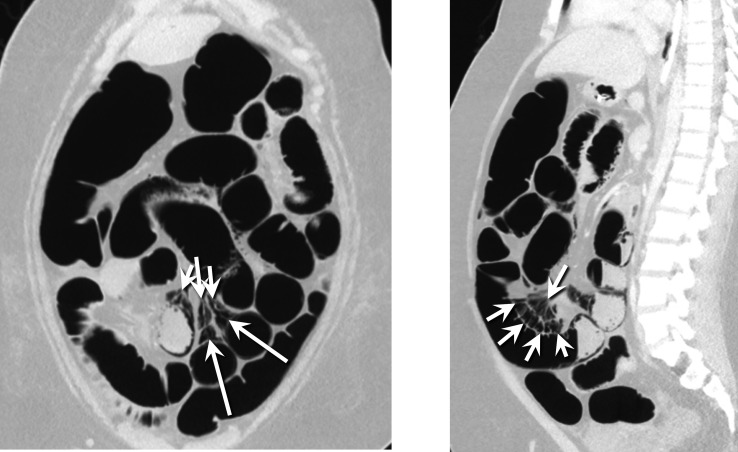
On the HD 7, the patient developed haemodynamic failure and signs of shock with decreased urine output, hypotension and acute kidney injury. She was also tachypneic and tachycardic. Her abdomen was firm on examination, and an abdominal X-ray demonstrated pneumatosis intestinalis and portal venous gas (figure 1). The follow-up abdominal CT confirmed pneumatosis with a pneumoperitoneum (figure 2). The patient subsequently underwent a resection of perforated, necrotic small bowel. A transesophageal echocardiogram at the time of surgery detected irregular masses, likely thrombi, in her right atrium that were attached to the atrial walls and to one another with smaller fibrinous connections. The bubble study was negative.
Figure 1.
Abdominal X-ray shows gas-dilated bowel loops and multifocal pneumatosis intestinalis. Arrows point out some of these areas.
Figure 2.
CT of the abdomen: coronal (left) and sagittal (right) images show air dissecting from the pneumatosis into the mesenteric venous system (arrows).
The patient remained haemodynamically unstable after surgery. She was started on continuous renal replacement therapy (CRRT) due to oliguric renal failure (Blood Urea Nitrogen 53, creatinine 3.00), hyperkalaemia (5.9) and metabolic acidosis (pH 7.21 and bicarbonate 17). She remained on CRRT for 3 days until renal recovery. She weaned off of vasoactive support the day after surgery.
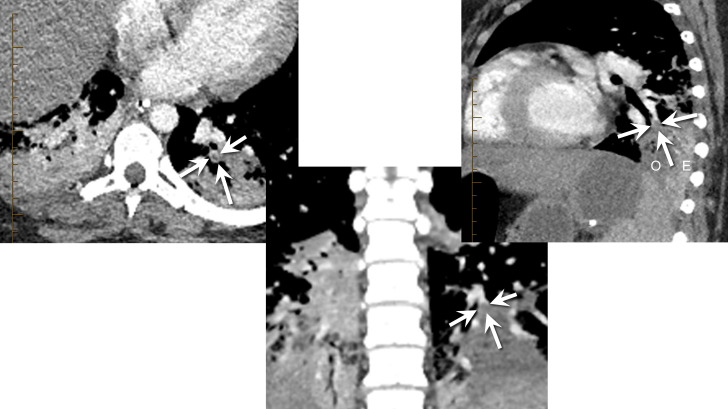
Following surgery, the patient underwent a CT head, chest, abdomen and pelvis that showed new bilateral lower lobe segmental and subsegmental pulmonary emboli (figure 3). Ultrasounds of the extremities were inconclusive due to body habitus. She was started on argatroban due to her renal failure for anticoagulation, which was ultimately changed to bivalarudin.
Figure 3.
Axial (left), coronal (middle) and sagittal (right) contrast-enhanced CTA images show a filling defect in a segmental basal branch of the left lower lobe. Arrows show the thin rim of enhancement surrounding the darker embolus. The adjacent consolidated lung is hypodense (O on sagittal) relative to the enhancing but consolidated lung more peripherally (E on sagittal), indicating hypoperfusion/infarction. Similar hypoperfused lung was seen on the right, suggesting additional pulmonary emboli, though they were not clearly seen. CTA, CT angiogram.
The patient returned to the operating room twice for removal of additional necrotic small bowel and eventual jejunal–ileal anastomosis with abdominal closure on HD 12. After all resections, she was left with approximately 100 cm of small bowel.
Investigations
Admission work-up on the general paediatric floor included a repeat chest X-ray that showed bilateral basal opacities and no cardiomegaly. Echocardiogram at that time was normal for age. Admission labs were significant for elevated erythrocyte sedimentation rate (ESR), C reactive protein (CRP), fibrinogen, lactate dehydrogenase and lactic acid. Urine drug screen was positive for cannabinoids and acetone, raising initial concern for EVALI. Following collection of urine, blood and respiratory cultures as well as a respiratory viral panel, the patient was empirically started on vancomycin and ceftriaxone.
Despite a negative COVID-19 PCR on admission, the patient’s recent COVID-19 exposure, chest X-ray findings, respiratory distress, gastrointestinal symptoms and fever prompted further work-up. The SARS-CoV2 IgG antibody test was reactive. The institution’s MIS-C algorithm guided which laboratory tests and studies to order. Critically ill patients in this algorithm are tested for both our screening and second tier labs. Initial labs were indicative of significant inflammation (table 1). Normal lab values are noted in parentheses. The ECG was read as normal sinus rhythm. The venous blood gas was unremarkable. Urine, respiratory and blood cultures were all negative.
Table 1.
Laboratory values
| Tier 1 labs | Admission | Day 7 (shock) | Day 8 |
| ALT (≤44 unit/L) | 16 | 163 | |
| AST (12–50 unit/L) | 37 | 127 | |
| CRP (0–1 mg/dL) | 26.3 | 4 | 24.3 |
| ESR (0–19 mm/hour) | 80 | 26 | |
| Blood culture | No growth | No growth | |
| WBC (4.5–11×109/L) | 20.36 | 28.88 | |
| Plt (150–450×103/mcL) | 322 | 590 | |
| Hgb (12–16 g/L) | 10.9 | 13.1 | |
| Hct (36%–46%) | 34.8 | 44.5 | |
| pH (7.36–7.44) | 7.34 | 7.34 | |
| pCO2 (32–45 mm Hg) | 45 | 49 | |
| pO2 (80–105 mm Hg) | 39 | 32 | |
| HCO3 (19–29 mmol/L) | 24 | 26 |
| Tier 2 labs | Admission | Day 7 (shock) | Day 8 | Day 9 |
| Troponin (0–0.03 ng/mL) | <0.01 | <0.01 | ||
| NT-Pro-BNP (0–125 pg/mL) | 37.1 | 92.8 | ||
| D-dimer (≤0.49 mcg/mL) | 1.7 | 6.01 | 5.74 | 2.66 |
| Fibrinogen (164–382 mg/dL) | 863 | 686 | 512 | 458 |
| Ferritin (13–146 ng/mL) | 68 | 204 | ||
| CPK (45–230 units/L) | 61 | 52 | 658 | |
| PT (11.3–15.6 s) | 16.6 | 15.7 | 21 | 81.5 |
| PTT (24.5–37.5 s) | 34 | 20.5 | 103.4 | |
| INR (>5) | 1.26 | 1.17 | 1.69 | 9.64 |
| LDH (370–645 units/L) | 1768 | 1534 | ||
| Lactate (0.7–2.1 mmol/L) | 5.4 | 3.2 | 2.7 | 1.9 |
| Triglycerides (30–200 mg/dL) | 84 | 144 |
*Laboratory Values
ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; CPK, Creatine Phosphokinase; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; hct, hematocrit; hgb, hemoglobin; INR, International normalized ratio; LDH, lactate dehydrogenase; plt, platelets; PT, protime; PTT, Partial thromboplastin time; WBC, White Blood Count.
Differential diagnosis
The initial differential diagnoses for this patient included EVALI, MIS-C, pulmonary embolism and pneumonia.
The CDC case definition for MIS-C requires age <21, fever, laboratory evidence of inflammation, clinically severe illness requiring hospitalisation, involvement of ≥2 organ systems, no alternative plausible diagnosis and positive for current or recent SARS-CoV-2 infection by RT-PCR, serology or antigen test or COVID-19 19 exposure within 4 weeks prior to onset of symptoms.4 On admission, the patient had significant inflammation evidenced by elevated CRP, ESR, fibrinogen and leukocytosis. The patient presented with involvement of both gastrointestinal and respiratory systems, fever of 39.6°C for at least 24 hours prior to admission, and SARS-CoV-2 IgG antibody test was reactive. However, on admission, the patient did not satisfy the criteria of having no alternative plausible diagnosis due to the possible diagnosis of EVALI.
The positive family history for PE and respiratory symptoms on presentation necessitated a CTA to rule out a pulmonary embolism. The results of this imaging study were initially negative for pulmonary embolism and more indicative of EVALI with diffuse ground glass opacities throughout the lungs but primarily in the lower lobes. The patient’s urine drug screen was positive for cannabinoids and acetone. The imaging findings, in conjunction with the urine drug screen results and gastrointestinal symptoms, initially supported the diagnosis of EVALI.
Although the imaging was not suggestive of a lobar pneumonia, the respiratory findings and inflammatory response made atypical pneumonia a possible diagnosis. Mycoplasma PCR and legionella antigen were negative. Mycoplasma antibody test was positive, indicative of previous exposure. HIV antibody screen was negative. Urine, respiratory and blood cultures on admission were negative.
On HD 7 when the patient suddenly decompensated due to shock and abdominal catastrophe, MIS-C was reconsidered. The atrial clots seen on tracheoesophageal echocardiogram, pulmonary emboli and tier 1 and 2 labs (table) were indicative of MIS-C and the infectious disease department concurred with this diagnosis.
Treatment
In the PICU, the patient was empirically started on vancomycin and ceftriaxone, but switched to levofloxacin on HD 2 for better atypical bacterial coverage. Pulmonology and rheumatology consultants recommended methylprednisolone to treat the working diagnosis of EVALI, realising this would also be an appropriate treatment for MIS-C. The coagulation team initiated prophylactic enoxaparin due to the patient’s obesity, family history and anticipated immobility.
The patient’s respiratory status improved on methylprednisolone until her acute decompensation on HD 7.
When the patient rapidly decompensated due to shock on HD 7, she underwent fluid resuscitation, and was started on vasoactive medications including epinephrine and norepinephrine. Her antibiotics were broadened to cefepime, metronidazole and clindamycin. She underwent a small bowel resection with removal of 120 cm of jejunum after the discovery of pneumatosis and free air on abdominal imaging.
Following surgery and detection of the atrial clots and pulmonary emboli, the patient was started on therapeutic systemic anticoagulation, first with argatroban, then with bivalirudin.
The patient transitioned from methylprednisolone to hydrocortisone after surgery for stress dosing. Rheumatology was consulted to discuss treatment for the probable MIS-C diagnosis. Although intravenous immunoglobulin is indicated for the myocardial dysfunction associated with MIS-C, it is a pro-thrombotic agent. Therefore, its use was contraindicated considering the patient’s significant clot burden. Anakinra is another option in MIS-C but was not advised due to the patient’s recent surgery. She continued on hydrocortisone and tapered off the systemic steroids approximately 8 days after her abdominal catastrophe.
Outcome and follow-up
On HD 20, the patient transferred out of the PICU to a general floor team for gastrointestinal rehabilitation. She had a gastrostomy tube placed on HD 57. She was then discharged from the hospital on HD 68. Discharge medications included amlodipine, clonidine, enoxaparin, fluticasone and norethindrone. She has not yet been seen for follow-up.
Discussion
Cardiac involvement is frequently reported in cases of MIS-C and includes myocardial dysfunction, coronary involvement and arrhythmias. Left ventricular systolic dysfunction necessitating inotropic support has been repeatedly described. The mechanism behind myocardial involvement is not well understood although hypothesised to be a result of the immune response to the virus and systemic hyperinflammation. Coronary artery involvement lies on a spectrum from mild dilation to large aneurysms. The most common ECG findings are non-specific but first and second-degree atrioventricular blocks and atrial fibrillation have been documented.5 6 The patient’s cardiac involvement first with arrhythmia then with haemodynamic failure, although likely secondary to her abdominal catastrophe are consistent with previous MIS-C reports.
Similarly, derangement in the clotting cascade leading to thrombosis is described in SARS-CoV-2 patients as well as MIS-C patients. Our patient’s atrial and pulmonary clots are also consistent with these reports.
However, ischaemic colitis and pneumatosis intestinalis are not well reported in patients with active SARS-CoV-2 infection7–11 nor MIS-C. One published case attributes the pneumatosis to direct mucosal damage8 while another hypothesises the cause to be occlusive ischaemic colitis secondary to the hypercoagulopathy associated with COVID-19.11 While we did not find mesenteric thrombosis on imaging, ischaemia leading to pneumatosis intestinalis and perforation was suspected. As none of the available case reports of pneumatosis intestinalis report intestinal perforation, our case is novel in its presentation.
The severity of her bowel catastrophe was profound and will leave her with short gut syndrome.
This case demonstrates the previously reported pneumatosis intestinalis complication but also the possibility of bowel perforation.
Learning points.
Multisystem inflammatory syndrome in children (MIS-C) should remain in the differential diagnosis even when clinical findings initially point to a possible alternative diagnosis.
Presenting symptoms of MIS-C including fever, abdominal pain and shortness of breath may improve but clinicians should remain cognizant of the possibility for abdominal catastrophe in the disease course.
In patients with presumed MIS-C, haemodynamic instability and shock could warrant a work-up for pneumatosis and small bowel perforation.
Acknowledgments
Special thanks to Grace Mitchell for assistance with radiological findings.
Footnotes
Twitter: @AlizaOlive
Contributors: AO contributed to conception and design of the case report, acquisition of information and revision of the article, as well as final approval of the version being submitted. LH contributed to conception and design of the case report and acquisition of information. LH performed the majority of the research on background, and drafted the initial article, then revised it. JM contributed to conception and design of the case report, as well as acquisition of information, and revision of the article. JM also contributed to final approval of the version being submitted. JM is also responsible for overall content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med Overseas Ed 2020;383:334–46. 10.1056/NEJMoa2021680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liguoro I, Pilotto C, Bonanni M, et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr 2020;179:1029–46. 10.1007/s00431-020-03684-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radia T, Williams N, Agrawal P, et al. Multi-system inflammatory syndrome in children & adolescents (MIS-C): A systematic review of clinical features and presentation. Paediatr Respir Rev 2020. 10.1016/j.prrv.2020.08.001. [Epub ahead of print: 11 Aug 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Center for Disease Control . Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). CDC website, 2020. [Google Scholar]
- 5.Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020;324:259. 10.1001/jama.2020.10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperotto F, Friedman KG, Son MBF, et al. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr 2021;180:307–22. 10.1007/s00431-020-03766-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aiello P, Johnson S, Ramos Mercado A, et al. Pneumatosis intestinalis in a patient with COVID-19. BMJ Case Rep 2020;13. 10.1136/bcr-2020-237564. [Epub ahead of print: 07 Sep 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kielty J, Duggan WP, O'Dwyer M, O’Dwyer M. Extensive Pneumatosis intestinalis and portal venous gas mimicking mesenteric ischaemia in a patient with SARS-CoV-2. Ann R Coll Surg Engl 2020;102:e145–7. 10.1308/rcsann.2020.0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meini S, Zini C, Passaleva MT, et al. Pneumatosis intestinalis in COVID-19. BMJ Open Gastroenterol 2020;7:e000434. 10.1136/bmjgast-2020-000434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lakshmanan S, Toubia N. Pneumatosis intestinalis in COVID-19. Clin Gastroenterol Hepatol 2020. 10.1016/j.cgh.2020.05.048. [Epub ahead of print: 30 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan KH, Lim SL, Damati A, et al. Coronavirus disease 2019 (COVID-19) and ischemic colitis: an under-recognized complication. Am J Emerg Med 2020;38:2758.e1–2758.e4. 10.1016/j.ajem.2020.05.072 [DOI] [PMC free article] [PubMed] [Google Scholar]