Abstract
A 57-year-old man with lumbar pain and fever was diagnosed with spondylodiscitis. Afterward, he acquired full paraplegia. Image studies showed a mass extending from D9 to the vertebral canal, plus numerous adjacent osteolytic lesions. Serum immunoelectrophoresis was normal, bone marrow had 0.5% of monoclonal plasmocytes, but D9’s biopsy found a plasmacytoma. Despite bone marrow aspiration results, skeleton osteolytic lesions made multiple myeloma (MM) a more plausible diagnosis, later confirmed by the biopsy. The absence of classical MM findings, alongside a medullary compression syndrome, suggested an oligosecretory MM, which was proved by an altered FLC essay. This delayed diagnosis, with multiple diagnostic misguiding leads, also presents rare IgA and lambda chains production and normal levels of uninvolved immunoglobulins. Oligosecretory MM can lead to an inaccurate and delayed diagnosis, with devastating consequences to patient’s morbidity and mortality. Therefore, FLC essay is essential in early assessment of potential MM patients.
Keywords: malignant and benign haematology, oncology
Background
Multiple myeloma (MM) is responsible for approximately 10% of all haematological cancers and 1% of all malignancies.1 Being a neoplastic disorder characterised by clonal proliferation of plasma cells, MM disrupts bone marrow function, leading to anaemia in up to 73% of patients. Other common symptoms, like bone pain (in 58%) or hypercalcaemia (in 28%), result from invasion and destruction of adjacent bone or, like renal impairment with elevation of creatine (in up to 58%), from excessive production of monoclonal protein (M-protein) by clonal plasma cells.2 This M-protein is usually detectable by serum electrophoresis in up to 82% of patients or immunofixation in 93%.2
The first described non-secretory version of MM (NSMM) lacked a measurable monoclonal component in the serum or urine.3 However, using a more recent assay capable of measuring free light chains (FLC), three-fourths are now described as oligosecretory. Their monoclonal components are under the electrophoretic threshold of 500–1000 mg/L,3 4 but they present altered FLC k/λ ratio (normal values ranging from 0.26 to 1.65) and/or elevated FLC kappa (k) or lambda (λ),4 as showed by table 1.3 Therefore, without MM classical findings and with symptomatic bone lesions as the most frequent symptom, the diagnostic of the previously called NSMM can be delayed.3
Table 1.
A nomenclature proposed by Dimopoulos et al for myeloma patients without ‘measurable disease’ in immunoelectrophoresis3
| Serum M-protein | Urine M-protein | FLC ratio | FLC involved | ||
| Oligosecretory myeloma | Measurable | <1 g/L | <200 mg/dL | Abn | >10 mg/dL |
| Non-measurable | <1 g/L | <200 mg/dL | N or Abn | <10 mg/dL | |
| Serum FLC-only myeloma | Measurable | Neg IF | Neg IF | Abn | >10 mg/dL |
| Non-measurable | Neg IF | Neg IF | Abn | <10 mg/dL | |
| True non-secretory myeloma | Neg IF | Neg IF | N | N | |
All parameters are obligatory for the definition of each entity.
Abn, Abnormal; FLC, free light chains; IF, Immunofixation; N, Normal; Neg, negative.
We hereby present a case of oligosecretory MM producing IgA λ chains, initially diagnosed as an infectious spondylodiscitis and presenting itself as a spinal cord compression (SCC). We decided to report this case of an infrequent subtype of MM because it presented with atypical symptoms and clinical findings, posing a true diagnostic challenge.
Case presentation
A 57-year-old male patient, with history of spine surgery and under methotrexate treatment for psoriasis in the last 9 years, presented to the ER with 1-month persistent lumbar pain and fever of 38°C for the past 3 days. Also, in the clinical history, there were no other exposures or intakes besides frequent consumption of non-pasteurised dairy products. On physical examination, he described pain when pressure was applied to spinous process of lumbar vertebrae 2 and 3, but without any neurological deficits. An X-ray image disclosed the presence osteosynthesis material in lumbar spine without any other relevant findings. A biochemical routine and a complete haemogram with leucogram were also performed. They showed a slight elevation of leucocytes (10.4×109/L) and neutrophils (7.83×109/L), but with a normal platelet count (431×109/L) and no anaemia (haemoglobin of 14.5 g/L). Erythrocyte sedimentation rate was elevated (56 mm/hour), as well as C reactive protein (5.39 mg/dL). Serum creatinine (0.87 mg/dL), blood urea nitrogen (17 mg/dL), calcium (9.5 mmol/L) and remaining serum ions were all within normal range. Albumin was decreased (3.2 mg/dL) and lactate dehydrogenase (LDH) was normal (193 U/L).
Empiric antibiotic therapy was applied only for 6 days for suspected spondylodiscitis, because following haemocultures were negative, as well as Interferon Gama Released Assay and serological studies for Brucella melitensis, Ricketsia conorii, Coxiella burnetii and Borrelia burgdorferi. Also, the patient started to present neurological symptoms with loss of strength in both lower limbs, progressing during the period of 2 weeks to full paraplegia with vesical retention and faecal incontinence.
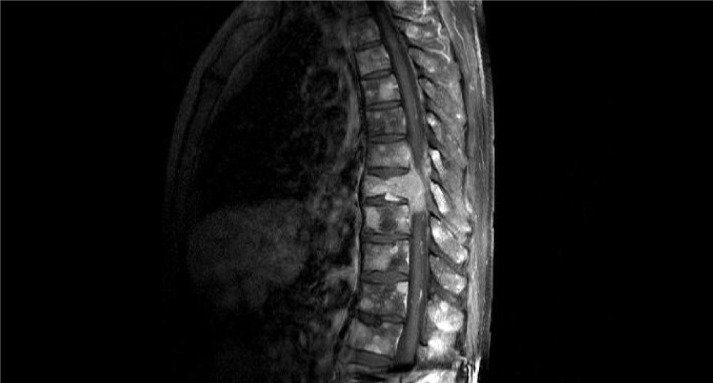
A conducted CT study of chest, abdomen and pelvis showed a mass in the body of thoracic vertebrae D9 with extension to the vertebral canal and a pathologic fracture, as well as multiple osteolytic lesions of the adjacent vertebra. In addition, subsequent Positron Emission Tomography (PET) scan only showed uptake in bone territory, specifically many osteolytic lesions throughout the vertebral column, cranium, sternum, ribs and pelvic bones. Following MRI of the lumbosacral area confirmed the CT findings, showing substantial medullary compression with myelopathy at D9 level (figure 1).
Figure 1.
MRI showing a D9 vertebral lesion with medullary compression and other osteolytic lesions in adjacent vertebra.
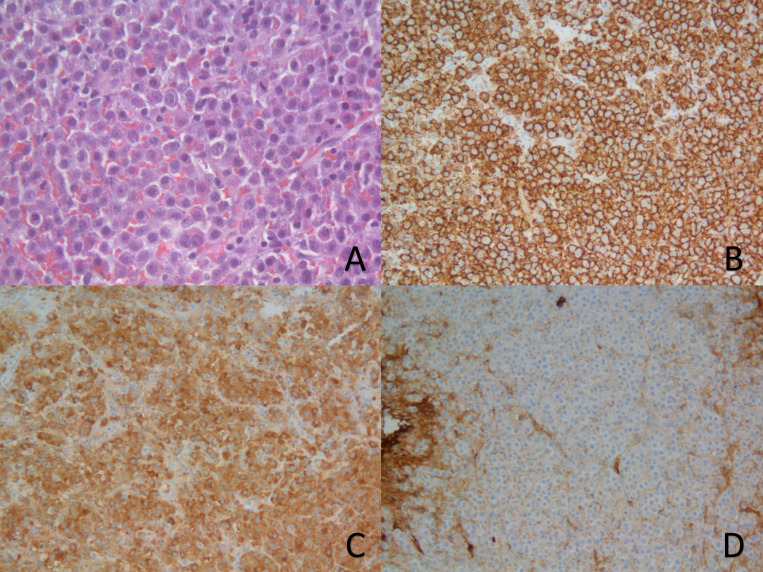
Serum electrophoresis showed no monoclonal spike and all immunoglobulins, as well as K and λ chains levels in the serum, were within normal range. Urine immunofixation was inconclusive. Nevertheless, serum immunofixation revealed heavy chains alfa (IgA) and light λ with monoclonal appearance. Also, in the serum, β2 microglobulin was normal (2.00 mg/L) and FLC assay showed increased λ chains (310 mg/L) and normal values of k (6.7 mg/dL). Considering these results, bone marrow aspiration was performed. It exhibited 0,5% of plasmocytes, from which 62% had abnormal phenotype, with positive λ, CD56, CD38 and CD138. Fluorescent in situ hybridisation (FISH) only showed 1q21 amplifications. A subsequent biopsy of the D9 lesion, with a histopathological study showing a plasmacytoma that marked positively for CD138 and λ chains, made the definitive diagnosis of a MM (figure 2).
Figure 2.
Slides from the plasmacytoma sample obtained from surgical biopsy. (A) A x400 magnification of a H&E stain; (B, C) x200 magnification with immunohistochemical (IHC) staining for CD138 and lambda chains. (D) x200 magnification showing no IHC staining for kappa chains.
Treatment
After the biopsy, the patient was subjected to five sessions of radiotherapy (RT) and started physical rehabilitation. Then, he was admitted to the haematology department to conduct chemotherapy with a scheme contaning Bortezomib, Dexamethasone and Lenalidomide.
Outcome and follow-up
At this time, the patient has already started chemotherapy and is regaining some function of the inferior limbs, while functional prognosis remains unclear.
Discussion
The MM subtypes covered by the previously nomenclature of NSMM may lack the typical MM symptomatic and laboratorial findings, requiring a strong clinical suspicion to lead the physician to an appropriate and detailed evaluation, as required.3 In MM, fever is a rare finding, so in this case, only back pain favoured this diagnosis.2 Hence, those symptoms together with the analytic results showing elevated C reactive protein and erythrocyte sedimentation rate, taking into account the patient’s risk factors, immunosuppression, presence of surgical implants of the spine and consumption of non-pasteurised dairy products, made spondylodiscitis, with probable infection by B. melitensis, a more probable aetiology.5 6
With negative blood cultures and no serological evidence of brucellosis and neither tuberculosis, the probability of this diagnosis declined. Empiric antibiotic therapy was suspended within few days, after the CT scan showed a vertebral mass with pathological fracture and multiple nearby osteolytic lesions, with ensuing PET scan revelling similar lesions throughout the appendicular and axial skeleton, extending to the skull base, without extrabone involvement. Despite M-protein not having been detected in immunoelectrophoretic studies, immunofixation showed an IgA λ monoclonal component. Therefore, all these findings grounded the diagnostic hypothesis of a MM, namely of the oligosecretory subtype.
Nevertheless, the updated diagnostic criteria for MM requires 10% or more clonal plasma cells in a bone marrow sample or presence of plasmacytoma in biopsy of an extramedullar or bony lesion, associated with evidence of end-organ damage and biomarkers of malignancy, according to table 2.7
Table 2.
Revised diagnostic criteria for multiple myeloma according to International Myeloma Working Group7
| Clonal bone marrow plasma cells ≥10% or biopsy-proven bony or extramedullary plasmacytoma and any one or more of the following myeloma defining events: | |
| Evidence of end organ damage that can be attributed to the underlying plasma cell proliferative disorder, specifically: |
Hypercalcaemia Serum calcium >2.25 mmol/L (>1 mg/dL) higher than the upper limit of normal or >2.75 mmol/L (>11 mg/dL). |
|
Renal insufficiency Creatinine clearance (measured or estimated) <40 mL/min or serum creatinine >177 μmol/L (>2 mg/dL) | |
|
Anaemia Haemoglobin value of >20 g/L below the lower limit of normal, or a haemoglobin value <100 g/L. | |
|
Bone lesions ≥1 osteolytic lesion on skeletal radiography, CT or PET-CT (more than one if bone marrow has less than 10% clonal plasma cells). | |
| Any one or more of the following biomarkers of malignancy: | Clonal bone marrow plasma cell percentage ≥60%. |
| Involved/uninvolved serum free light chain ratio ≥100. | |
| >1 focal lesion with size ≥5 mm on MRI studies. | |
Lacking a measurable M-protein, patients with non-mensurable MM mostly presents themselves with symptomatic bone lesions and less anaemia, hypercalcaemia or renal impairment.3 Osteolytic lesions can be present at diagnosis in up to 80% of patients with MM, increasing the probability of skeleton-related complications.8 All of this was proven true in our case, specially when the patient started to develop progressive neurologic symptoms, compatible with one complication of this nature, namely an SCC, later confirmed by MRI. Leading to severe disability and decreasing severely the prognosis, this medical emergency occurs in approximately 5% of all MM patients.9
Continuing the diagnostic investigation, a bone marrow aspiration didn’t show enough plasma clonal cells to allow the diagnosis of MM, which can be attributed to a non-representative sample of a patchy bone marrow, or to a real absence of generalised bone marrow involvement. We could correlate the absent symptoms of bone marrow malfunction to this last alternative, however, there is no proven relation between these symptoms and bone marrow plasmacytosis.7 Nevertheless, with the discovery of a plasmacytoma producing light λ chains in a surgical biopsy of the D9 lesion, it is possible to fulfil the previously mentioned diagnostic criteria for MM.
FLC assay validated the λ light chains as the involved ones, with a value of 310 mg/L and an abnormal FLC ratio of 0.02, fulfilling the criteria for a measurable oligosecretory MM, according to the nomenclature proposed in table 1.3 Usually, the monoclonal component of MM is made of heavy and light immunoglobulin’s chains, being IgG (52%) or IgA (21%) the most common types, alongside with reduced levels of one or more uninvolved immunoglobulins in up to 91% of patients.2 In this case, all immunoglobulin levels (involved and uninvolved) were normal, and an IgA λ monoclonal component is present, which, according to a retrospective study in Mayo Clinic in Rochester, represents 8% of all MM.2
Nowadays, a combination of chromosomal abnormalities of bone marrow plasmocytes studied by FISH, serum LDH and International Staging System for MM (ISS), known as revised-ISS, improved the assessment of prognosis for MM bearing patient.1 With an ISS stage II due to normal β2 microglobulin and decreased albumin, normal range LDH and no high-risk chromosomal abnormalities, this patient can be classified as revised-ISS stage II, which predicts an overall survival of 83 months and a median progression-free survival of 42 months.1 However, the presence of 1q21 amplification in FISH is considered as an independent poor prognostic factor in some studies.10
Being a relatively young patient, an induction therapy with bortezomib–dexamethasone with addition of a third agent (in this case, lenalidomide), is considered to be the front line treatment for MM, prior to stem cell transplantation, according to European Society for Medical Oncology guidelines.1 However, regarding this patient’s treatment, the priority was addressing the medical emergency of SCC. Considering the patient’s history of vertebral intervention and imaging of the existing fracture, a surgical approach was considered, however, pondering patient‘s status, tumour radio sensitivity and the lack of evidence about surgery in MM patients with SCC, it was discarded.8 9 Therefore, due to the extent of the disease and its rapid progression, a short course RT was selected for this patient, since there is no statistical difference in overall survival and functional outcome, when compared with long courses of RT. Additionally, a short course of high-dose corticosteroids was performed, due to its proven benefits in SCC, alongside opioid treatment for pain control and prophylactic anticoagulation.9
Learning points.
Without traditional signs of multiple myeloma (MM) and lacking a measurable M-protein by immunoelectrophoresis, oligosecretory MM can lead to an inaccurate and, therefore, delayed diagnosis.
Because this type of MM presents itself predominantly with bone lesions, which are related with a high incidence of bone related consequences, it is associated with possible devastating outcomes to patient’s morbidity and mortality.
With a various range and combinations of laboratory findings in MM patients, free light chains (FLC) essay may be essential to early assessment of patients with suspected myeloma.
Since up to three fourths of all MM without measurable disease have a M-protein detectable by FLC, the previous non-secretory version of MM can be redefined into multiple subtypes, being the oligosecretory MM an entity requiring further investigation efforts.
Acknowledgments
We thank MD Olinda Rebelo, of the Neuropathology Department of Coimbra Hospital University Center, for preparing and providing the pathology slides and their captions. We thank MD António Aragão, of the Internal Medicine Department of Coimbra Hospital University Center, for coordinating patient care and diagnostic approach.
Footnotes
Contributors: RR was mainly responsible for information selection and organisation, design conception and the draft preparation. Scientific research was conducted by FC and DF, and LM was in charge of assuring scientific correction. Patient’s follow up and clinical information acquisition was responsibility of all coauthors, was well as data interpretation, analysis and discussion. All authors revised and accepted the final manuscript. Substantial contributions to the conception or design of the work, or the acquisition, analysis or interpretation of data.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Moreau P, San Miguel J, Sonneveld P, et al. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv52–61. 10.1093/annonc/mdx096 [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003;78:21–33. 10.4065/78.1.21 [DOI] [PubMed] [Google Scholar]
- 3.Dimopoulos MA, Kastritis E, Terpos E. Non-Secretory myeloma: one, two, or more entities? Oncology 2013;27:930–2. [PubMed] [Google Scholar]
- 4.Shaw GR. Nonsecretory plasma cell myeloma--becoming even more rare with serum free light-chain assay: a brief review. Arch Pathol Lab Med 2006;130:1212–5. 10.5858/2006-130-1212-NPCMEM [DOI] [PubMed] [Google Scholar]
- 5.Sobottke R, Seifert H, Fätkenheuer G, et al. Current diagnosis and treatment of spondylodiscitis. Dtsch Arztebl Int 2008;105:181–7. 10.3238/arztebl.2008.0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother 2010;65:iii11–24. 10.1093/jac/dkq303 [DOI] [PubMed] [Google Scholar]
- 7.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International myeloma Working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014;15:e538–48. 10.1016/S1470-2045(14)70442-5 [DOI] [PubMed] [Google Scholar]
- 8.Terpos E, Morgan G, Dimopoulos MA, et al. International myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. J Clin Oncol 2013;31:2347–57. 10.1200/JCO.2012.47.7901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sen E, Yavas G. Annals of Hematology & Oncology The Management of Spinal Cord Compression in Multiple Myeloma. Ann Hematol Oncol 2016;3:id1090. [Google Scholar]
- 10.Chng WJ, Dispenzieri A, Chim C-S, et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia 2014;28:269–77. 10.1038/leu.2013.247 [DOI] [PubMed] [Google Scholar]