Abstract
Introduction:
Fragile X Tremor and Ataxia Syndrome is a progressive neurodegenerative disorder that develops in some FMR1 premutation carriers. The objective of this study is to characterize how cytokine levels are altered in the FXTAS brain.
Methods:
Fresh frozen cerebellar tissue from FXTAS cases and controls was homogenized and analyzed for 12 different cytokines using a commercially available ELISA panel.
Results:
Relative to controls, FXTAS cases showed large and significant increases in the cytokines IL-12 and TNFα. There were large but non-significant increases in the levels of IL-2, IL-8, and IL-10 in FXTAS cases. The cytokines IL-1A, IL-1B, IL-4 IL-6, IL-17A, IFNγ, and GM-CSF were not different between FXTAS and control subjects.
Conclusions:
For the first time, we demonstrate an increase in the pro-inflammatory cytokines TNFα and IL-12 in the FXTAS brain, both of which are implicated in the pathogenesis of Multiple Sclerosis, another neurodegenerative disorder that predominantly consists of white matter disease.
Keywords: FXTAS, FMR1 gene, premutation, neurodegeneration
Introduction
Individuals with a CGG expansion in the FMR1 gene in the premutation range (55–200 CGG repeats) are at risk of developing the progressive neurodegenerative disorder Fragile X-Associated Tremor and Ataxia Syndrome (FXTAS) [1]. The disorder typically develops in the 6th decade of life, and the predominant symptoms include intention tremor, cerebellar ataxia, peripheral neuropathy, autonomic dysfunction, and cognitive impairment [1]. CGG expansion in the premutation range confers a toxic gain of function on the encoded RNA [1] – in FXTAS, this leads to the formation of inclusion bodies, significant white matter disease, gliosis, and cerebral atrophy [1].
Neuroinflammation is a common neuropathological alteration across most neurodegenerative disorders. Among those are Multiple Sclerosis (MS), Alzheimer’s disease (AD), and Parkinson’s disease (PD) that typically present with astrogliosis, microglial activation, and an upregulation in cytokine synthesis and release [2]. Cytokines are key mediators of immune response and inflammation - they are soluble signaling peptides secreted primarily from immune cells that regulate a coordinated response to tissue trauma and/or infection [3]. As the primary resident immune cells of the brain, microglia are the main source of cytokine release and neuroinflammation in the CNS [4]. However, astrocytes and neurons [3, 4] can also release cytokines, and thus contribute directly to neuroinflammatory processes as well. In FXTAS there are high levels of astrogliosis, sometimes forming in numerous discrete patches within the parenchyma [5]. There is also profound microglial activation in most cases, and microglial senescence in half of patients [6]. However, while both astrocytes and microglia are in an activated state in FXTAS, indicative of neuroinflammation, the corresponding profile of cytokine synthesis and release in the FXTAS brain has never been investigated. Accordingly, here we characterized for the first time how the levels of 12 cytokines are altered in the FXTAS brain, relative to healthy controls, utilizing fresh-frozen postmortem human brain tissue. We found significant elevations (approximately 3-fold) in the cytokines Interleukin-12 and TNFα. We also found 2-fold elevations in the cytokines IL2, IL8, and IL10, although these alterations did not meet statistical significance. Thus, for the first time, we showed that FXTAS patients present alterations in central cytokine levels, consistent with a FXTAS neuroinflammatory profile that involves microglial activation and astrogliosis.
Methods
Subjects and Tissue Processing.
Fresh frozen postmortem cerebellar tissue from 11 FXTAS cases and 11 healthy controls were utilized in this experiment (See Table 1). All included FXTAS subjects were clinically diagnosed with FXTAS during life. Premutation status, clinical symptomology, and the presence of intranuclear ubiquitin+ inclusions (for the 8 cases with fixed tissue available) were further assessed in all FXTAS cases to confirm diagnosis. FXTAS tissue was procured from the Fragile X Brain Repository at the University of California Davis Medical Center in Sacramento, California. Control tissue was procured from the UC Davis Department of Pathology, also located at the UCD Medical Center. Brain tissue was collected from donors at autopsy, after obtaining informed consent from the next of kin, and with the approval of the UC Davis Institutional Review Board (IRB). All control cases were free of any brain disease or brain injury - as assessed by autopsy and a review of patient medical records at the time of brain donation/intake. Postmortem Interval (PMI) was recorded for all subjects, which refers to the latency between the time of death and the time of autopsy in hours (Table 1). All samples utilized here were frozen on dry-ice immediately upon brain removal at autopsy, and stored at −80C until processed for this experiment. FMR1 CGG repeat length was assessed by PCR for FXTAS subjects, as described previously, to verify premutation status.
Table 1. Characteristics of Included Subjects.
Postmortem brain tissue from 12 FXTAS cases and 12 Controls (CTL) was utilized in this study to assess alterations in cytokine profile. Age (years), sex, PMI (hours), and CGG repeat length are shown here for included subjects. For FXTAS cases, all available information relating to inclusion bodies, presence of MCP radiological sign, as well as the presence (yes/no), onset (age/years) and duration (years) of tremor and ataxia symptomology are described.
| Ataxia | Tremor | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Group | Age | Sex | CGG | PMI (hrs) | Inclusions | MCP | Yes/No | Onset (age) | Duration (yrs) | Yes/No | Onset (age) | Duration (yrs) |
| FXTAS-1 | FXTAS | 66 | M | 105 | 36.0 | Yes | Yes | Yes | 48 | 18 | Yes | 55 | 11 |
| FXTAS-2 | FXTAS | 69 | M | 58 | 4.6 | Yes | No | Yes | 64 | 5 | No | - | - |
| FXTAS-3 | FXTAS | 69 | M | 98 | 6.5 | Yes | Yes | Yes | NA | NA | Yes | NA | NA |
| FXTAS-4 | FXTAS | 69 | M | 118 | 18.0 | NA | NA | Yes | 55 | 14 | Yes | 59 | 10 |
| FXTAS-5 | FXTAS | 72 | M | 60 | 5.0 | NA | No | Yes | 65 | 7 | Yes | 65 | 7 |
| FXTAS-6 | FXTAS | 77 | M | 95 | 5.3 | NA | NA | Yes | NA | NA | Yes | 65 | 12 |
| FXTAS-7 | FXTAS | 80 | F | 31/73 | 5.0 | Yes | NA | Yes | NA | NA | Yes | NA | NA |
| FXTAS-8 | FXTAS | 81 | M | 75 | 12.0 | Yes | NA | NA | NA | NA | Yes | NA | NA |
| FXTAS-9 | FXTAS | 81 | M | 110 | 11.0 | Yes | NA | Yes | 69 | 12 | Yes | 69 | 12 |
| FXTAS-10 | FXTAS | 86 | F | 29/87 | NA | Yes | NA | Yes | 79 | 6 | Yes | 82 | 3 |
| FXTAS-11 | FXTAS | 87 | M | 65 | NA | Yes | NA | Yes | 86 | 1 | No | - | - |
| CTL-1 | CTL | 53 | M | 20 | 133.5 | No | - | No | - | - | No | - | - |
| CTL-2 | CTL | 53 | M | 30 | 153.0 | No | - | No | - | - | No | - | - |
| CTL-3 | CTL | 53 | M | 30 | 60.9 | No | - | No | - | - | No | - | - |
| CTL-4 | CTL | 62 | M | 30 | 36.8 | No | - | No | - | - | No | - | - |
| CTL-5 | CTL | 65 | M | 30 | 105.0 | No | - | No | - | - | No | - | - |
| CTL-6 | CTL | 65 | M | 24 | 42.2 | No | - | No | - | - | No | - | - |
| CTL-7 | CTL | 69 | M | 23 | 132.5 | No | - | No | - | - | No | - | - |
| CTL-8 | CTL | 74 | M | 30 | 136.1 | No | - | No | - | - | No | - | - |
| CTL-9 | CTL | 77 | M | 29 | 78.0 | No | - | No | - | - | No | - | - |
| CTL-10 | CTL | 78 | F | 29 | NA | No | - | No | - | - | No | - | - |
| CTL-11 | CTL | 81 | F | 22 | NA | No | - | No | - | - | No | - | - |
Cytokine Analysis.
Cerebellar biopsies were harvested from fresh frozen postmortem brain tissue from the subjects described above and homogenized for ELISA analysis. Cytokine levels were assessed using the Multi-Analyte ELISArray (Qiagen), which utilizes a standard sandwich ELISA protocol to quantify alterations in the following cytokines IL-1A, IL-1B, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-17A, IFNγ, TNFα, and GM-CSF, following the manufacturer’s instructions. Optical density measures were read with a plate reader and values for each cytokine were averaged for control subjects, and used as a baseline to calculate fold-changes from average control values for each individual subject. Fold-changes were utilized for all final statistical analyses.
Statistical Analysis.
All statistical analyses were carried out in JMP 14 (SAS Institute Inc., Cary, NC). T-tests were utilized to assess the effect of diagnosis (FXTAS or Control) on fold changes in tissue cytokine levels. Correlation analyses were utilized to assess the effects of PMI and Age on cytokine levels; this was done separately for each diagnosis.
Results
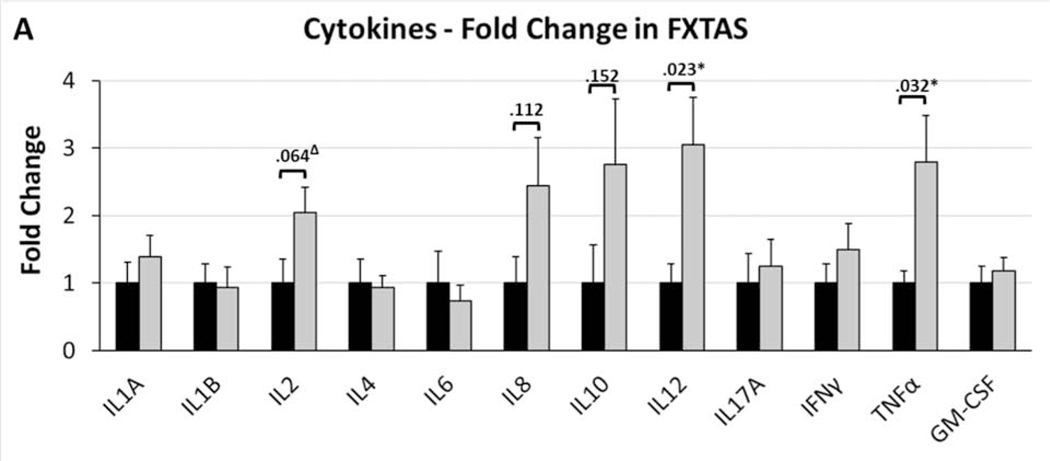
Of the 12 cytokines assessed in postmortem FXTAS and control brain tissue, there were large elevations in five cytokines in FXTAS; however only two of these met statistical significance (Fig. 1). There was a significant 3.0-fold increase in Interleukin-12 (IL-12) in FXTAS (t20=2.57, p=.023) and a significant 2.8-fold increase in Tumor Necrosis Factor Alpha (TNFα) in FXTAS (t20=2.44, p=.032). There was a 2.0-fold increase in Interleukin-2 (IL-2) in FXTAS, although this was only a statistical trend (t20=1.96, p=.064). There were also large elevations in Interleukin 8 (IL-8) and Interleukin 10 (IL-10) that did not meet statistical significance, with FXTAS patients showing a 2.4-fold increase in IL-8 (t18=1.58, p=.112) and a 2.8-fold increase in IL-10 (t20=1.51, p=.152). The rest of the cytokines assessed showed small non-significant differences (p>.10 for all) between groups, with the following fold increases in FXTAS relative to controls: 1.4-fold for Interleukin 1A (IL-1A, p=.398), 0.9-fold for Interleukin 1B (IL-1B, p=.878), 0.9-fold for Interleukin 4 (IL-4, p=.867), 0.7-fold for Interleukin 6 (IL-6, p=.641), 1.2-fold for Interleukin 17A (IL-17A, p=.701), 1.5-fold for Interferon Gamma (IFNγ, p=.339), and 1.2-fold for Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF, p=.581). It is important to note the statistical limitations caused by the small sample size utilized here, in that: 1) The lack of significance in multiple elevated cytokines here, particularly IL-8 and IL-10, may represent false negatives due to low power; and 2) The significant differences (p<.05) detected here for IL-12 and TNFα would likely lose significance if we accounted for multiple comparisons.
Figure 1. Cytokine Levels in Postmortem FXTAS Brain Tissue.
Fold-changes in cytokine levels in FXTAS cases, relative to controls, were assessed individually by t-test, revealing significant (p<.05) increases in Interleukin 12 and Tumor Necrosis Factor Alpha (TNF). Additionally, there was a trend (p=.064) for an increase in Interleukin 2 (IL2). There were large magnitude elevations in Interleukin 8 (IL8) and Interleukin 10 (IL10) that did not meet statistical significance (p-values indicated in graph). For all others, the magnitude of change was low and all were non-significant and non-trending (p>.10). Mean fold-changes indicated +SEM. P-values indicated for all cytokines alterations that were 2-fold or greater (IL2, IL8, IL10, IL12, TNF). *p<.05, Δp<.10
A limitation of the sample utilized in this study was that the Post-Mortem Interval (PMI) for the FXTAS cases were lower than those for the Control cases. This is of concern in that it is theoretically possible that longer PMI could be associated with protein degradation, including cytokines. However, for all 12 cytokines, we found that there was not a significant correlation between PMI and cytokine levels in FXTAS (p>.05 for all) or in Controls (p>.05 for all), demonstrating that prolonged PMI did not lead to a reduction in cytokine values for long PMI cases. There was also a significant difference in age (t20 = 2.52, p=.0213) between the FXTAS cases (Mean: 76.1 ± 2.2 years, Range: 66–87) and Controls (Mean: 66.4 ± 3.1 years, Range: 53–81). As with PMI, we also did not find a significant correlation between age and any cytokine level for FXTAS (p>.05 for all) or Controls (p>.05 for all). Finally, we wished to see if there was a relationship between FMR1 CGG repeat length and cytokine profile, using correlation analysis within FXTAS cases. We found that there was not a significant correlation between CGG repeat length and the magnitude of cytokine fold changes for any cytokine (p>.05 for all).
Discussion
Our results provide evidence that cytokine levels are elevated in the FXTAS brain, consistent with previously demonstrated microglial activation [6] and astrogliosis [5]. While elevations in central cytokine levels are a commonality across neurodegenerative disorders, we found a unique profile in the FXTAS brain (See Supplementary Table 1 for cytokine profile comparison), which includes large and significant elevations in TNFα and IL-12; large but non-significant elevations in IL-2, IL-8, and IL-10; and no alterations in IL-1A, IL1B, IL-4, IL-6, IL-17A, IFNγ, and GM-CSF
TNFα is also elevated in the AD, PD, and MS brain [2, 3, 7]. TNFα is a key pro-inflammatory cytokine that initiates inflammatory cascades, leading to widespread immune cell activation and cytokine release [8]. The effects of TNFα are complex – it can have both neuroprotective and neurotoxic effects, depending on its form (bound vs soluble), specific receptor transduction mechanisms (pro-apoptotic at TNF Receptor 1, anti-apoptotic at TNF Receptor 2), as well input from other molecular signals [Reviewed extensively in 8]. Oligodendrocytes are particularly vulnerable to TNFα induced apoptosis, which is further exacerbated by iron accumulation [9]. Since there are high levels of iron accumulation in the FXTAS brain, particularly in oligodendrocytes [10], it is possible that increased TNFα signaling contributes to FXTAS white matter disease by inducing oligodendrocyte cell death. We also found that the pro-inflammatory cytokine IL-12 is increased in the FXTAS brain. IL-12 is also increased in MS lesions, and elevated peripheral IL-12 levels correlate with disease severity [7]. Astrocytes are key inhibitory regulators of microglial IL-12 release [4]. Since astrocytes show high levels of inclusion burden and gliosis in FXTAS [5] – it is plausible that impaired astrocyte function could contribute to increased IL-12 levels. We also found large but non-significant elevations in IL-2, IL-8, and IL-10 here, which should be re-evaluated in future studies to confirm whether or not they are significantly altered in the FXTAS brain.
It is unclear whether the neuroinflammatory FXTAS cytokine profile presented here reflect a CNS specific immune response, a systemic response involving infiltrating peripheral immune cells, or perhaps both. TNFα and IL-12 are both produced by microglia [4, 8]; thus, an endogenous CNS cytokine response to FXTAS-associated brain toxicity may alone explain our findings. However, in FXTAS, there is evidence of frequent microhemorrhage (under review), which could possibly facilitate an infiltration of cytokine-producing peripheral immune cells. It is also unclear whether the alterations in cytokines detected here reflect a healthy and adaptive immune response to widespread FXTAS-associated neurotoxicity (i.e. increased levels of FXTAS-associated apoptosis driving a concomitant elevation in microglial cytokine response during clearance of cellular debris), or if they instead reflect pathological immune cell function caused by intracellular FXTAS-associated toxicity (i.e. FMR1 RNA toxicity in microglia leads to elevated levels of IL-12 synthesis or release in a cell autonomous manner). While peripheral cytokine measures have never been assessed in FXTAS, the cytokines IL-12, IFNɣ, MCP-1, and GM-CSF are reduced in the periphery in FMR1 premutation cases without FXTAS [11]. Additionally, there is a downregulation in IL-6 and TNFα in Fmr1 knockout mice [12]. Together, these studies suggest that abnormalities in FMR1 gene networks may directly alter cytokine synthesis and release.
This study serves as an initial characterization of cytokine levels in the FXTAS brain. Our finding of increased cytokine levels here raises many questions regarding both the mechanisms that underlie these changes as well as the consequences of these changes in FXTAS symptomology and disease progression. It will important to clarify the specific cell and molecular mechanisms that underlie the elevation in cytokines detected here in future studies, including 1) Whether these cytokine alterations are mediated by microglia or by peripheral immune cells that have infiltrated the FXTAS brain and 2) Whether intracellular FXTAS pathology (i.e. FMR1 mRNA levels, inclusion burden, iron accumulation, senescence in microglia) is causal to the upregulation of IL-12 and TNFα detected here. It is also of particular interest to better understand the consequences of elevated TNFα and IL-12 as possible contributors to FXTAS white matter disease progression. If implicated, these pathways may be important novel therapeutic targets for FXTAS.
Supplementary Material
This table outlines the unique profile of cytokine alterations in FXTAS cases, illustrating differences in profile with other Neurodegenerative disease cases. Only data from postmortem human brain tissue are included here for all diseases. Alterations in cytokine levels listed here for each disease are relative to controls.
Highlights.
The pro-inflammatory cytokines IL-12 and TNFα are elevated in the FXTAS CNS
The cytokines IL-1A, IL-1B, IL-4, IL-6, IL-17A, IFN, and GM-CSF are unaltered in the FXTAS CNS
IL-12 and TNFα contribute to white matter degeneration in MS, and may play a similar role in FXTAS
Elevated TNFα could contribute to white matter degeneration in FXTAS by inducing apoptosis in iron accumulated oligodendrocytes
Acknowledgments
Authors role
BD analyzed data and wrote the manuscript. SA performed experiments. VMC designed study, supervised experiments, analysis and writing of the manuscript.
Funding:
This research was supported by funds from the National Institute of Neurological Disorders and Stroke (NINDS) grant R01 1NS10713, Shriners Hospitals for Children, and the MIND Institute Intellectual and Developmental Disability Research Center U54 HD 079125.
Footnotes
Financial disclosures
None
Declaration of competing interest: The authors declare that they have no conflict of interest.
References
- 1.Hagerman RJ and Hagerman P, Fragile X-associated tremor/ataxia syndrome - features, mechanisms and management. Nat Rev Neurol, 2016. 12(7): p. 403–12. [DOI] [PubMed] [Google Scholar]
- 2.Chitnis T. and Weiner HL, CNS inflammation and neurodegeneration. J Clin Invest, 2017. 127(10): p. 3577–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner MD, et al. , Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta, 2014. 1843(11): p. 2563–2582. [DOI] [PubMed] [Google Scholar]
- 4.Hanisch UK, Microglia as a source and target of cytokines. Glia, 2002. 40(2): p. 140–55. [DOI] [PubMed] [Google Scholar]
- 5.Greco CM, et al. , Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS). Brain, 2006. 129(Pt 1): p. 243–55. [DOI] [PubMed] [Google Scholar]
- 6.Martinez Cerdeno V, et al. , Microglial cell activation and senescence are characteristic of the pathology FXTAS. Mov Disord, 2018. 33(12): p. 1887–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imitola J, Chitnis T, and Khoury SJ, Cytokines in multiple sclerosis: from bench to bedside. Pharmacol Ther, 2005. 106(2): p. 163–77. [DOI] [PubMed] [Google Scholar]
- 8.Probert L, TNF and its receptors in the CNS: The essential, the desirable and the deleterious effects. Neuroscience, 2015. 302: p. 2–22. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, et al. , Cytokine toxicity to oligodendrocyte precursors is mediated by iron. Glia, 2005. 52(3): p. 199–208. [DOI] [PubMed] [Google Scholar]
- 10.Ariza J, et al. , Iron accumulation and dysregulation in the putamen in fragile X-associated tremor/ataxia syndrome. Mov Disord, 2017. 32(4): p. 585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Careaga M, et al. , Immune dysregulation as a cause of autoinflammation in fragile X premutation carriers: link between FMRI CGG repeat number and decreased cytokine responses. PLoS One, 2014. 9(4): p. e94475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodges SL, et al. , Adult Fmr1 knockout mice present with deficiencies in hippocampal interleukin-6 and tumor necrosis factor-alpha expression. Neuroreport, 2017. 28(18): p. 1246–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This table outlines the unique profile of cytokine alterations in FXTAS cases, illustrating differences in profile with other Neurodegenerative disease cases. Only data from postmortem human brain tissue are included here for all diseases. Alterations in cytokine levels listed here for each disease are relative to controls.