Abstract
Background:
Although frailty status is dynamic, whether changes in frailty predict mortality is unknown.
Objective:
Describe 1-year changes in a frailty index (FI) and association with mortality
Design:
Secondary analysis of the National Health in Aging Trends Study
Setting:
Community
Participants:
5672 Medicare Beneficiaries 65 and older
Measurements:
A deficit accumulation frailty index was measured in 2011 and 2012, based on multidomain assessment including comorbidities, activities of daily living, physical tasks, cognition, and performance testing. We categorized 2011 FI into robust (FI<0.15), pre-frail (FI 0.15–0.24), mild frailty (FI 0.25–0.34), and moderate to severe frailty (FI ≥0.35). Change in frailty was calculated as the FI change from 2011 to 2012, then categorized as either absolute (>0.045 decrease, 0.015–0.045 decrease, ±0.015 change, 0.015–0.045 increase, >0.045 increase) or proportional change (>20% decrease, 5–20% decrease, ±5% change, 5–20% increase, 20% increase). We measured the association of FI change with 48-month mortality using Cox regression.
Results:
From 2011 to 2012, mean FI increased by 0.02 (standard deviation 0.07), with 58.6% having an increase. After adjusting for age and sex, compared to stable frailty, both absolute (>0.045) and proportional (>20%) increases in frailty were associated with higher 48-month mortality among pre-frail participants (hazard ratio [HR] 2.35, 95% confidence interval [1.45–3.79] and HR 2.57 [1.38–4.78], respectively), and participants with mild frailty (HR 1.96 [1.35–2.85] and 2.10 [1.41–3.14]) and moderate or severe frailty (HR 1.99 [1.48–2.67] and 1.94 [1.43–2.63]) but not robust participants (HR 1.48 [0.86–2.54], HR 1.62[0.80–3.28]). However, absolute (>0.045) and proportional (>20%) decrease in frailty was not associated with decrease in mortality compared to stable frailty (large decrease among moderate to severe frailty, HR 1.10 [0.78–1.56] and 0.65 [0.37–1.15], respectively).
Conclusions:
Increasing deficit accumulation FI over one year is associated with increased mortality risk. While decreasing FI occurs, its association with reduced mortality risk was inconclusive.
Keywords: Frailty, Change, Mortality
INTRODUCTION
Frailty is characterized by diminished physiologic reserve and decreased ability to tolerate and recover from stressors.1 Although the relationship between a one-time assessment of frailty and mortality has been demonstrated extensively,2,3 how changes in frailty are associated with mortality remains less well described. Frailty status is dynamic and can worsen,4,5 although this work largely used phenotypic frailty,6 and thus did not account for cognitive or psychosocial factors. Previous studies on longitudinal changes in a deficit-accumulation frailty index (FI) have used diagnosis codes from electronic medical records to measure deficits.7 These remain imperfect measures due to limited clinical details. For example, it is difficult to capture improvements from diagnosis codes in a sensitive way, as diagnoses will generally accumulate. Yet some markers of frailty, such as gait speed or functional status, can improve with interventions such as physical therapy8 or joint replacement. Understanding how an FI changes over time and the impact of these changes may inform measurement and applications of frailty in clinical practice. In this context, we examined the 1-year change in frailty and 4-year mortality in a sample of community-dwelling older adults.
METHODS
Population
We used data from the National Health in Aging Trends Study (NHATS) from 2011 through 2017. NHATS is sponsored by the National Institute on Aging (grant number NIA U01AG032947) through a cooperative agreement with the Johns Hopkins Bloomberg School of Public Health. It is a nationally-representative sample of Medicare beneficiaries over the age of 65 surveyed annually. Participants and proxies are interviewed at home by trained surveyors. Informed consent was obtained from all participants by NHATS. The Hebrew SeniorLife Institutional Review Board approved this study.
We included all NHATS participants from the Round 1 (2011) wave. Participants were excluded if they were not community-dwelling in Round 1 (n=1048), did not have enough data to calculate frailty at Round 1 (n=28), were lost to follow up (n=964) or died (n=533) within a year, thus precluding assessment of FI change from Round 1 to Round 2.
Clinical Assessment
Demographic characteristics including race and gender were self-reported. Age is reported categorically (65–69, 70–74, 75–79, 80–84, 85–89, ≥90 years). Medical history (including dementia) was self-reported, as were activities of daily living ([ADLs] - feeding, dressing, grooming, bed mobility, bathing, toileting), instrumental activities of daily living ([IADLs] - laundry, transport, shopping, meal preparation, managing money, managing medications), and physical tasks (pushing/pulling heavy objects, stooping or kneeling, lifting 10 lbs., reaching arms above shoulder, handling small objects, walking up a flight of stairs, walking half a mile, heavy housework). Depression and anxiety were measured using Patient Health Questionnaire-2 (PHQ score ≥2) and Generalized Anxiety Disorder 2-item (GAD score ≥2), respectively.10 We defined hearing impairment and visual impairment based on previously used definitions in NHATS.11 Gait speed and grip strength were calculated as the average of two attempts. The time needed to complete 5 chair stands on a single attempt was measured. Cognitive measures included tests of orientation, 10-word recall, and clock draw.12
Estimation of FI and Change in FI
We used a previously described deficit accumulation FI,13,14 which includes 40 deficit variables: 13 self-reported comorbidities, 6 ADLs, 6 IADLs, 7 self-reported physical tasks, 3 performance measures (gait speed, grip strength, chair stands), 3 neuropsychiatric measures (cognitive testing, PHQ-2, GAD-2), and 2 nutritional measures (body mass index [BMI] <21 kg/m2, self-reported unintentional weight loss >10 lbs.). The FI was calculated for each year, as the proportion of deficits present divided by the number of deficits considered (range: 0–1, higher numbers indicating higher frailty). Frailty was categorized as robust (FI<0.15), pre-frail (0.15–0.24), mild frailty (0.25–0.34), and moderate-to-severe frailty (≥0.35).
Change in frailty was calculated as the difference in FI from Round 1 to Round 2; thus, a positive change indicates increasing deficits and worsening frailty. Prior literature on change in FI has suggested that the minimally clinically important difference in FI is 0.03.15 However, some studies have also examined change as a percentage of baseline frailty level.16 Given lack of consensus to quantify FI change, we categorized change by both absolute change (>0.045 decrease, 0.015–0.045 decrease, ±0.015 change, 0.015–0.45 increase, >0.045 increase) and proportional change (>20% decrease, 5–20% decrease, ±5% change, 5–20% increase, 20% increase).
Assessment of Mortality
Month and year of death were obtained from follow-up interviews with caregivers. Observations were censored at the time of last known follow-up interview or 48 months from the Round 2 interview, whichever came first.
Statistical Analysis
Demographic and clinical characteristics were summarized using descriptive statistics in Round 1 and Round 2. To understand the overall change in FI in one year, we calculated the mean and standard deviation of the change in FI and examined histogram for the overall sample and by FI category at Round 1. We calculated the number of deaths per 100 person-years and estimated 48-month mortality risk stratified by Round 1 frailty category and 1-year FI change using Kaplan-Meier estimators. We used log-rank test to compare mortality among FI change categories. Multivariable Cox proportional hazards regression was used to determine the hazard ratio (HR) and 95% confidence interval (CI) between 1-year change in FI and mortality, adjusting for age and sex within each Round 1 frailty status. Additionally, we tested the interaction between the Round 1 frailty category and FI change. All analyses were performed using Stata version 16 (StataCorp, Texas) survey procedures to account for the complex sampling design of NHATS, and findings were weighted to reflect national estimates of the Medicare population. A 2-sided p-value <0.05 was considered statistically significant.
RESULTS
Our final sample included 5672 participants, who were 3267 (55.8%) female and 3883 (80.7%) white. After 4 years of follow-up from the Round 2 survey assessment, 1039 (13.6%) participants had died. The most common comorbidity in 2011 was hypertension (n=3817, 63.7%), while all comorbidity prevalence increased modestly over 1 year (Table 1). Mean FI at Round 1 was 0.24 (standard deviation [SD] 0.13), and 0.25 (SD 0.14) one year later, with a mean FI of 0.02 (SD 0.07). Overall, those with higher baseline frailty showed less mean change in FI, but a greater SD (Supplemental Figure 1): the mean FI change (SD) for robust, pre-frail, mild frailty and moderate-to-severe frailty was 0.025 (0.05), 0.02 (0.06), 0.013 (0.07), and 0.001 (0.11), respectively.
Table 1:
Characteristics of the sample at baseline (2011) and after 1 year (n=5672)
| Round 1 (2011) | Round 2 (2012) | |
|---|---|---|
| Age | ||
| 65–69 | 1132 (30.1) | 885 (23.5) |
| 70–74 | 1229 (25.8) | 1207 (26.9) |
| 75–79 | 1172 (19.5) | 1207 (21.2) |
| 80–84 | 1127 (14.1) | 1148 (15.4) |
| 85–89 | 642 (7.6) | 772 (9.2) |
| ≥90 | 370 (2.9) | 453 (3.9) |
| Female | 3267 (55.8) | 3267 (55.8) |
| Race | ||
| White | 3883 (80.7) | 3883 (80.7) |
| Black | 1251 (8.4) | 1251 (8.4) |
| Other | 538 (10.9) | 538 (10.9) |
| Medical Comorbidities | ||
| Heart Attack | 841 (13.4) | 930 (14.9) |
| Heart Disease | 1026 (17.0) | 1196 (19.6) |
| Hypertension | 3817 (63.7) | 3952 (66.0) |
| Arthritis | 3150 (53.5) | 3408 (57.8) |
| Osteoporosis | 1147 (20.8) | 1305 (23.5) |
| Diabetes | 1429 (23.6) | 1513 (25.0) |
| Lung disease | 832 (14.6) | 956 (16.7) |
| Stroke | 630 (9.3) | 710 (10.4) |
| Dementia | 249 (3.0) | 365 (4.5) |
| Cancer | 1458 (25.6) | 1584 (27.9) |
| Number of ADLs that need help (mean, SD) | 0.3 ± 0.9 | 0.3 ± 1.0 |
| Number of IADLs that need help (mean, SD) | 2.3 ± 1.7 | 2.4 ± 1.8 |
| *Gait Speed (mean ± SD) | 0.80 ± 0.24 | 0.78 ± 0.23 |
| Frailty Index (mean ± SD) | 0.24 ± 0.13 | 0.25 ± 0.14 |
| BMI < | ||
Note: Percentages reflect weighted estimates. Abbreviations: ADL – Activities of Daily Living, IADLs – Instrumental Activities of Daily Living, SD – standard deviation
n for gait speed 4753 and 4650 for Round 1 and 2, respectively.
Absolute Change in Frailty and Mortality
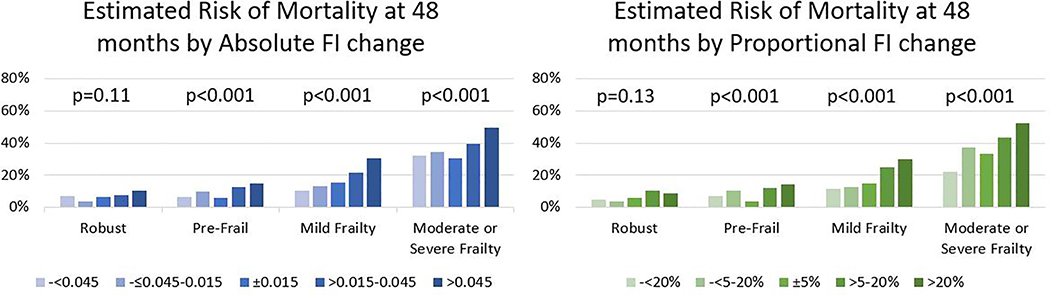
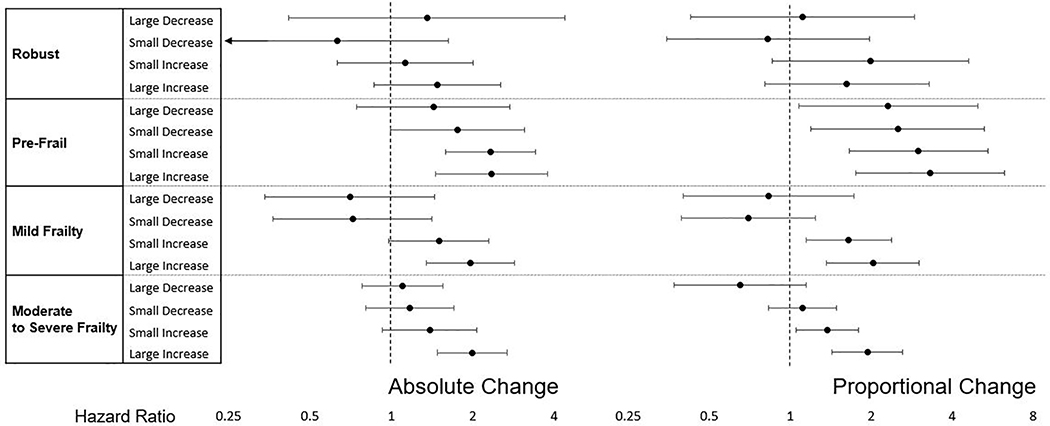
Generally, an increase in FI over 1 year was associated with increased mortality at 48 months across all frailty levels (Figure 1). An increase in FI by >0.045 was associated with a higher mortality rate than those with stable FI, except for those who were robust at Round 1 (Table 2). After adjusting for age and sex, a large increase (>0.045) in FI was not significantly associated with mortality in those who were robust at Round 1 (HR [95% CI], 1.45 [0.86–2.54]). However large increases in FI were associated with mortality in those with pre-frailty (HR 2.35 [1.45–3.79]), mild frailty (HR 1.96 [1.35–2.85]), and moderate-to-severe frailty (HR 1.99 [1.48–2.67]).
Figure 1. Risk of mortality by 1-year change in Frailty Index (FI) and Baseline frailty status.
Risk of mortality was estimated from Kaplan Meier curves and p-values were from the log rank test to compare mortality among FI change categories. Frailty categories are based on 2011 Frailty Index, separated into robust (FI<0.15), pre-frail (FI 0.15–0.24), mild frailty (FI 0.25–0.34) and moderate to severe frailty (FI ≥0.35).
Table 2.
Association between change in Frailty Index (FI) and mortality.
| Absolute Change | Proportional Change | |||||
|---|---|---|---|---|---|---|
| n | Mortality Rate per 100 pys) | Hazard Ratio [95% CI] | n | Mortality Rate per 100 pys) | Hazard Ratio [95% CI] | |
|
Robust
FI<0.15 (n=1338) |
||||||
| Large Decrease | 59 | 1.83 [0.59 – 8.37] | 1.35 [0.42 – 4.40] | 177 | 1.25 [0.63 – 2.83] | 1.11 [0.42 – 2.90] |
| Small Decrease | 187 | 0.99 [0.48 – 2.33] | 0.63 [0.24 – 1.63] | 154 | 1.07 [0.50 – 2.71] | 0.83 [0.35 – 1.97] |
| Stable Frailty | 374 | 1.55 [1.01 – 2.51] | Reference | 172 | 1.25 [0.67 – 2.58] | Reference |
| Small Increase | 343 | 1.88 [1.23 – 3.01] | 1.12 [0.63 – 2.00] | 184 | 2.48 [1.45 – 4.61] | 1.99 [0.86 – 4.61] |
| Large Increase | 375 | 2.71 [1.90 – 3.98] | 1.48 [0.86 – 2.54] | 651 | 2.22 [1.67 – 3.03] | 1.62 [0.80 – 3.28] |
|
Pre-Frail
FI 0.15–0.24 (n=1894) |
||||||
| Large Decrease | 223 | 1.69 [1.01 – 3.02] | 1.43 [0.74 – 2.75] | 275 | 1.94 [1.27 – 3.09] | 1.94 [0.93 – 4.05] |
| Small Decrease | 317 | 2.40 [1.62 – 3.68] | 1.76 [0.99 – 3.11] | 341 | 2.48 [1.71 – 3.73] | 1.92 [0.91 – 4.06] |
| Stable Frailty | 501 | 1.42 [1.00 – 2.08] | Reference | 338 | 0.97 [0.58 – 1.72] | Reference |
| Small Increase | 384 | 3.29 [2.40 – 4.62] | 2.33 [1.58 – 3.42] | 417 | 3.06 [2.26 – 4.25] | 2.18 [1.21 – 3.94] |
| Large Increase | 469 | 3.99 [3.12 – 5.16] | 2.35 [1.45 – 3.79] | 523 | 3.82 [3.01 – 4.91] | 2.57 [1.38 – 4.78] |
|
Mild Frailty
FI 0.25–0.34 (n=1147) |
||||||
| Large Decrease | 206 | 2.50 [1.55 – 4.30] | 0.70 [0.34 – 1.45] | 157 | 2.82 [1.68 – 5.12] | 0.76 [0.33 – 1.75] |
| Small Decrease | 173 | 3.13 [1.99 – 5.20] | 0.72 [0.36 – 1.42] | 224 | 2.87 [1.89 – 4.53] | 0.89 [0.52 – 1.50] |
| Stable Frailty | 245 | 4.04 [2.87 – 5.83] | Reference | 241 | 3.94 [2.78 – 5.72] | Reference |
| Small Increase | 165 | 6.57 [4.60 – 9.61] | 1.50 [0.98 – 2.30] | 244 | 7.21 [5.46 – 9.67] | 1.72 [1.16 – 2.55] |
| Large Increase | 358 | 8.89 [7.20 – 11.06] | 1.96 [1.35 – 2.85] | 281 | 8.91 [7.00 – 11.44] | 2.10 [1.41 – 3.14] |
|
Moderate-to-Severe Frailty
FI>=0.35 (n=1293) |
||||||
| Large Decrease | 380 | 8.78 [7.13 – 10.89] | 1.10 [0.78 – 1.56] | 164 | 5.23 [3.59 – 7.87] | 0.65 [0.37 – 1.15] |
| Small Decrease | 158 | 10.23 [7.53 – 14.11] | 1.17 [0.81 – 1.70] | 325 | 11.00 [8.91 – 13.68] | 1.11 [0.83 – 1.49] |
| Stable Frailty | 193 | 8.95 [6.68 – 12.12] | Reference | 285 | 10.30 [8.17 – 13.07] | Reference |
| Small Increase | 182 | 13.06 [9.93 – 17.34] | 1.39 [0.93 – 2.07] | 304 | 14.68 [11.93 – 18.14] | 1.37 [1.05 – 1.79] |
| Large Increase | 380 | 18.42 [15.41 – 22.08] | 1.99 [1.48 – 2.67] | 215 | 20.41 [16.19 – 25.83] | 1.94 [1.43 – 2.63] |
Note: Mortality rates per 100 person- years are unadjusted. Cox regression models are adjusted for age and sex. Groups defined as follows for absolute vs proportional change, respectively: Stable Frailty (±0.015 or ±5%), Large Decrease (<−0.045 or <−20%), Small Decrease (−0.015–0.045 or −5–20%), Small Increase (0.015–0.045 or 5–20%), Large Increase (>0.045 or >20%)
A reduction in FI over 1 year was not significantly associated with 48-month mortality risk (Figure 1). A decrease in FI by >0.045 was not significantly associated with mortality (Table 2). After adjusting for age and sex, a large decrease (>0.045) in FI was not significantly associated with mortality in any frailty category at Round 1 (robust HR 1.35 [0.42–4.40], pre-frail HR 1.43 [0.74–2.75], mild frailty HR 0.70 [0.34–1.45], moderate-to-severe frailty HR 1.10 [0.78–1.56]). The interaction between the Round 1 frailty category and FI change category was not significant in either absolute or proportional change in FI (p=0.53 and p=0.10 respectively).
Proportional Change in Frailty with Mortality
After adjusting for age and sex, a large increase (>20%) in FI was associated with mortality among those with pre-frailty (HR 2.57 [1.38–4.78]), mild frailty (HR 2.10 [1.41–3.14]), and moderate-to-severe frailty (HR 1.94 [1.43–2.63]), but not robust participants at Round 1 (HR 2.22 [1.67 – 3.03]). After adjustment for age and sex a large decrease (>20%) in FI was not significantly associated with mortality in any frailty category (robust HR 1.25 [0.63 – 2.83], pre-frail HR 1.94 [0.93–4.05], mild frailty HR 0.76 [0.33–1.75], moderate-to-severe frailty HR 0.65 [0.37–1.15]).
DISCUSSION
To our knowledge, this is the first analysis of annual FI change and mortality in a nationally representative survey of older adults in the United States. Our main findings can be summarized as follows: 1) nearly half of the population had a decrease in FI over 1 year; 2) mean FI changed less among those with higher baseline frailty, although they had larger variation in FI change; 3) increases in FI were associated with greater mortality among pre-frail or frail individuals, but not among robust individuals, and 4) decreases in FI were not consistently associated with lower mortality. These findings inform applications of FI in clinical practice.
Previous work using cross-sectional data estimated that deficit accumulation occurs at a rate of 3% per year on a logarithmic scale.17 A recent study suggested that a change in FI of 0.03 was sensitive to detect small changes in EuroQol-5D, a global measure of health status.15 Another study compared frailty measurements from different time points, concluding that the most recent frailty measurement was a better predictor of mortality.16 Our study builds upon prior work by assessing FI longitudinally and examining associations between FI change and mortality. While changes in FI are associated with future mortality risk, this pattern seems to vary by baseline status. Among robust individuals, large changes in FI did not consistently predict mortality. Although this could be due to low mortality rates in this group, robust individuals may have adequate reserve and resilience to deficit accumulation.
Our work provides insights into repeated FI measurements. In our population, the prevalence of chronic conditions increased over 1 year, but the ability to perform ADLs, IADLs, and physical tasks can change more frequently. As a result, FI models that heavily rely on diagnoses may not be sensitive to change (in particular, improvement), unless they are updated frequently.4,18 While the optimal proportion of diagnosis-based deficits versus functional status deficits in FI calculation remains unknown, diagnosis-heavy FI may not be useful for tracking frailty over time, as chronic comorbidities tend to only increase. Moreover, a lack of association between decreases in FI and mortality raises a question about the utility of FI as an outcome to evaluate the benefit of an intervention. Further research is warranted to investigate the selection of deficit items to maximize the responsiveness of FI to improvement in health status.
This work has limitations. Because our study population only included community-dwelling Medicare beneficiaries 65 years or older, our conclusions may not generalize to younger populations or those who are institutionalized. To calculate the change in FI, we required that participants be interviewed for two consecutive years. The exclusion of the frailest individuals who died or dropped out between Round 1 and Round 2 may have affected our results towards the null and reduce precision. Lastly, changes in frailty, particularly decreases, may still be associated with improved quality of life and other meaningful clinical outcomes, which were not explored in this study.
CONCLUSION
In a nationally representative sample of older adults, we found marked variation in changes in FI over 1 year by baseline frailty status. Increasing FI was associated with increased mortality risk among pre-frail or frail individuals, but not among robust individuals. Decreasing FI was observed, but it was not consistently associated with lower mortality risk.
Supplementary Material
Supplementary Figure 1: Distribution of 1 year change in FI by Baseline Frailty level
Note: Abbreviations: FI – Frailty Index.
Figure 2:
Frailty categories are based on 2011 Frailty Index, separated into robust (FI<0.15), pre-frail (FI 0.15–0.24), mild frailty (FI 0.25–0.34) and moderate to severe frailty (FI ≥0.35). Cox regression models are adjusted for age and sex. Groups defined as follows for absolute vs proportional change, respectively: Stable Frailty (±0.015 or ±5%), Large Decrease (<−0.045 or <−20%), Small Decrease (−0.015–0.045 or −5–20%), Small Increase (0.015–0.045 or 5–20%), Large Increase (>0.045 or >20%).
IMPACT STATEMENT.
We certify that this work is novel of recent novel clinical research. To our knowledge, this is the first analysis of annual FI change and mortality in a nationally representative survey of older adults in the United States.
ACKNOWLEDGEMENTS
Funding:
Dr. Shi receives sponsorship from the Harvard Translational Research in Aging Training Program, T32 AG023480.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose
Sponsor’s Role: None
REFERENCES
- 1.Rockwood K, Mitnitski A . Frailty in relation to the accumulation of deficits. Journals Gerontol - Ser A Biol Sci Med Sci. 2007;62(7):722–727. doi:62/7/722 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Song X, Mitnitski A, Rockwood K. Prevalence and 10-Year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58(4):681–687. doi: 10.1111/j.1532-5415.2010.02764.x [DOI] [PubMed] [Google Scholar]
- 3.Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45(3):353–360. doi: 10.1093/ageing/afw039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendonça N, Kingston A, Yadegarfar M, et al. Transitions between frailty states in the very old: the influence of socioeconomic status and multi-morbidity in the Newcastle 85+ cohort study. Age Ageing. 2020:1–8. doi: 10.1093/ageing/afaa054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fallah Nader; Mitnikski Arnold; Searle Samuel D.; Gahbauer Evelyne A.; Gill Thomas M.; Rockwood K Transitions in Frailty Status in Older Adults in Relation to Mobility. J Am Geriatr Soc. 2011;59(3):524–529. doi: 10.1016/j.biotechadv.2011.08.021.Secreted [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kojima G, Taniguchi Y, Iliffe S, Jivraj S, Walters K. Transitions between frailty states among community-dwelling older people: A systematic review and meta-analysis. Ageing Res Rev. 2019;50(January):81–88. doi: 10.1016/j.arr.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 7.Stow D, Matthews FE, Hanratty B. Frailty trajectories to identify end of life: A longitudinal population-based study. BMC Med. 2018;16(1):1–7. doi: 10.1186/s12916-018-1148-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peel NM, Navanathan S, Hubbard RE. Gait speed as a predictor of outcomes in post-acute transitional care for older people. Geriatr Gerontol Int. 2014;14(4):906–910. doi: 10.1111/ggi.12191 [DOI] [PubMed] [Google Scholar]
- 9.Yourman L, Lee SJ, Schonberg MA, Widera E, Smith AK. Prognostic Indices for Older Adults A Systematic Review. JAMA. 2012;307(2):182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montaquila J, Freedman V, Edwards B, Kasper J. National Health and Aging Trends Study Round 1 Sample Design and Selection. NHATS Technical Paper #1. 2012:1–8. Available at www.NHATS.org.
- 11.Simning A, Fox ML, Barnett SL, Sorensen S, Conwell Y. Depressive and Anxiety Symptoms in Older Adults With Auditory, Vision, and Dual Sensory Impairment. J Aging Health. 2018:1–23. doi: 10.1177/0898264318781123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasper JD, Freedman VA, Spillman B. Classification of Persons by Dementia Status in the National Health and Aging Trends Study. Balt Johns Hopkins Univ Sch Public Heal Available wwwNHATS.org. 2013;(July):14. doi: 10.1007/s00129-002-1155-y [DOI]
- 13.Shi SM, McCarthy EP, Mitchell SL, Kim DH. Predicting Mortality and Adverse Outcomes: Comparing the Frailty Index to General Prognostic Indices. J Gen Intern Med. 2020. doi: 10.1007/s11606-020-05700-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi SM, McCarthy EP, Mitchell S, Kim DH. Changes in Predictive Performance of a Frailty Index with Availability of Clinical Domains. J Am Geriatr Soc. 2020:1–7. doi: 10.1111/jgs.16436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang IY, Jung HW, Lee HY, Park H, Lee E, Kim DH. Evaluation of Clinically Meaningful Changes in Measures of Frailty. J Gerontol A Biol Sci Med Sci. 2020;75(6):1143–1147. doi: 10.1093/gerona/glaa003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson MQ, Theou O, Tucker GR, Adams RJ, Visvanathan R. Recurrent Measurement of Frailty Is Important for Mortality Prediction: Findings from the North West Adelaide Health Study. J Am Geriatr Soc. 2019:2311–2317. doi: 10.1111/jgs.16066 [DOI] [PubMed] [Google Scholar]
- 17.Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53(12):2184–2189. doi: 10.1111/j.1532-5415.2005.00506.x [DOI] [PubMed] [Google Scholar]
- 18.Stow D, Matthews FE, Barclay S, et al. Evaluating frailty scores to predict mortality in older adults using data from population based electronic health records: Case control study. Age Ageing. 2018;47(4):564–569. doi: 10.1093/ageing/afy022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Distribution of 1 year change in FI by Baseline Frailty level
Note: Abbreviations: FI – Frailty Index.