Abstract
Background
Arbuscular mycorrhizal (AM) fungi associate with most plants and can increase nutrient uptake. As a result, commercial inoculants called “biofertilizers” containing AM fungi have been developed and marketed to increase plant performance. However, successful establishment of these inoculants remains a challenge, and may be negatively impacted by competition with fungi already present (priority effects). Perennial agriculture may be more amenable if inoculants can be successfully established on crops prior to field planting.
Methods
Here, we inoculate grapevine (Vitis vinifera) with a commercial inoculant in three treatments designed to manipulate the strength and direction of priority effects and quantified the abundance of the fungal strain before and after introduction using droplet digital PCR (ddPCR).
Results
We found that the introduced strain did not establish in any treatment, even with priority advantage, and inoculated vines did not differ in performance from non-inoculated vines. Fungal abundance was not greater than in pre-inoculation soil samples during any of the five years sampled and may have been impaired by high available phosphorus levels in the soil. This study highlights the need to understand and evaluate how the management of the agricultural system will affect establishment before introduction of “biofertilizers”, which is often unpredictable.
Keywords: Biofertilizer, Arbuscular mycorrhizal fungi, Inoculants, Priority effect, Soilcolonization, Root colonization
Introduction
Currently, there is a need to improve agricultural phosphorus use efficiency and sustainability (Cordell & White, 2013), which could be accomplished through optimizing microbially mediated nutrient cycling to promote maximum crop uptake (Vance, 2001; Hamel & Strullu, 2006; Thirkell et al., 2017). In response, inoculants called “biofertilizers” have been developed which aim to improve plant growth through the addition of beneficial soil microbes, of which arbuscular mycorrhizal (AM) fungi are commonly used (Thirkell et al., 2017).
AM fungi are obligate root symbionts that associate with the vast majority of terrestrial plants, and transfer nutrients, primarily phosphorus, to their host plant in exchange for carbon (Smith & Read, 2008). Mycorrhizal plants rely on these fungal partners for nutrients, primarily nitrogen and phosphorus, and, among other benefits, they can provide a significant percentage of a plant’s phosphorus requirements, as well as potentially access phosphorus that the host cannot access (Smith & Read, 2008). It is possible that optimizing the interactions between AM fungi and crops, through either increasing total percent colonization or the relative abundance of more mutualistic partners, could increase crop nutrient uptake. Inoculation with a commercial “biofertilizer” could do so if the abundance of AM fungi in the field is low and limiting the total root colonization of the crops, or if the commercial isolate (strain) is superior to the native isolates and disproportionately increases plant growth. If this could be accomplished, it could reduce the amount of fertilizer required, and could allow crops to access reserves of “legacy” phosphorus leftover in the soil from past fertilization which is not easily accessed by plant roots (Condron et al., 2013; Cordell & White, 2013).
Challenges for practical applications of AM fungal biofertilizers
At last estimate, the market for these inoculants had grown to include over thirty producers worldwide, and they are available to home gardeners as well as large-scale growers. Despite their use, there are many unanswered questions regarding their efficacy and best practices. In some cases, these inoculants have been successful at increasing plant growth and/or yield (Pellegrino et al., 2011; Pellegrino, Öpik & Bonari, 2015; Hernádi et al., 2012). However, in other cases, fungal inoculation did not affect plant performance (Ortas et al., 2011; Janoušková et al., 2013; Emam, 2016). While the cause of these failures is not clear, field applications are typically less successful than in greenhouse applications (Lekberg & Koide, 2005).
The majority of studies on the effect of commercial AM fungal inoculants on plant growth and/or yield have been conducted in greenhouse (Berruti et al., 2016), but this approach does not capture the complexities of field conditions. Often, these studies are conducted in the absence of or with a simplified AM fungal community. Such interactions could potentially inhibit the establishment and/or performance of the inoculant through priority effects (Verbruggen et al., 2013). Priority effects occur when the first species to colonize an area (in this case, host roots) experiences an advantage and ultimately affects the composition of the assembled community. This effect has been observed in many taxa (Alford & Wilbur, 1985; Ladd & Facelli, 2008; Fukami et al., 2010; Peay, Belisle & Fukami, 2012) including AM fungi (Werner & Kiers, 2015). While many field collected roots will show a diversity of AM fungi (Vandenkoornhuyse et al., 2002), it is possible that subsequent establishment of introduced AM fungi may become progressively less likely as host resources become increasingly monopolized. This phenomenon presents a direct challenge for biofertilizers, which must compete with fungi already present in the field.
Quantifying biofertilizer establishment
Establishment and persistence of commercial AM fungal inoculants is crucial for successful application of the biofertilizer; if the inoculant does not survive, it is unlikely to result in any benefit. However, even under the most benign conditions in sterile greenhouse pots, establishment failed in 75% of the commercial inoculants tested (Faye et al., 2013). Field establishment may be even less successful for the reasons above, and reduced establishment could contribute to the reduced benefit observed relative to greenhouse studies. In a meta-analysis, Lekberg & Koide (2005) showed a lower average mycorrhizal response in field studies compared to studies conducted in greenhouse. However, few studies have used specific molecular techniques to monitor the establishment and persistence of a single commercial inoculation event for two or more years.
It is possible that improved inoculation practices may be able to increase the likelihood of successful establishment. With perennial crops, such as fruit trees and grapevines, young plants can be “pre-inoculated” in the greenhouse before being transferred to the field. By establishing the commercial inoculant in the absence of a native AM fungal community before transplanting, the priority advantage could be given to the commercial inoculant. In this experiment, we tested three inoculation strategies on grapevine that differ in the strength and direction of priority effects. We monitored the vines over five years using isolate-specific molecular techniques to determine the abundance of the introduced commercial isolate and recorded the response of economically important measures of vine performance. To the best of our knowledge, this study represents the longest study tracking inoculum persistence in the field.
Materials & Methods
Study site and vine characteristics
This experiment was conducted at Kalala Organic Estate Winery located at 49.84 N, 119.64 W in West Kelowna, British Columbia, Canada (photos of the site are included in Supplementary Materials). West Kelowna is located in the Ponderosa Pine Very Dry Hot Biogeoclimatic Zone (Ministry of Forests, Lands, Natural Resource Operations and Rural Development, 2018), with an annual average temperature of 14.7 °C and total precipitation of 344.5 mm (Environment Canada, 2018). The soil is primarily sandy loam with rapid drainage and 42% coarse fragment material (Wittneben, 1986). This site has been under the same owners for the last 15 years and was not inoculated with AM fungi during that time.
All vines used were Pinot Noir varietals grafted onto 3309C rootstocks purchased from Sunridge Nurseries (Bakersfield, CA). Experimental vines were established in 2013 in a section of the vineyard approximately 50 m × 200 m and planted within empty positions in the vineyard among non-experimental Pinot Noir vines. After planting, vines were maintained according to standard organic practice by the vineyard.
Experimental factors and timing strategies
For this experiment, a factorial design was established with two factors, inoculation and timing strategy, and vines from each treatment were randomly distributed among the available planting locations in the vineyard. Within each timing strategy, half of the vines were inoculated with MYKE Pro Greenhouse G granular inoculum (Premier Tech Ltd., Rivière-du-Loup, Quebec, Canada) containing carrier (peat moss and perlite), and hyphal fragments and spores of Rhizoglomus irregulare DAOM 197198 (∼20 propagules/g), and the remaining half were non-inoculated and exposed to sterile carrier only. Although this product is labelled “Greenhouse”, the company states that it can also be used when transplanting into soil and is suitable for perennial plants. The company information still states that the product contains Glomus intraradices DAOM 197198, however this isolate was reclassified under Glomus irregulare (Stockinger, Walker & Schüzler, 2009), then the species was renamed Rhizophagus irregularis (Schüzler & Walker, 2010; Formey et al., 2012), and most recently Rhizoglomus irregulare (Sieverding et al., 2014).
Three timing strategies were used as follows:
-
1.
Pre-inoculation (Pre): Grafted new vines were inoculated on May 24th 2013 with 60 mL of MYKE Pro Greenhouse G inoculum and grown in a greenhouse in a 2-gallon pot containing sterile Sunshine Mix #4 soil (SunGro Horticulture, Bellevue, WA). Vines were watered daily and grown in a randomized arrangement at 16-hour days and 25 °C for 4 weeks before transplanting into the field with trimmed roots.
-
2.
Co-inoculation (Co): Grafted new vines were planted between May 30th and June 4th 2013 with 60 mL of MYKE Pro Greenhouse G inoculum in the hole at the time of planting. Roots were trimmed and planted vines received 3L water after planting.
-
3.
Established (Est): One-year-old vines growing in the vineyard were randomly selected and inoculated in May 2013 by taking 3 soil cores 10 cm from the vine (left, right, and centre). 20 mL of MYKE Pro Greenhouse G inoculum were added to each hole for a total of 60 mL and watered well.
A total of 120 vines were initially established in the vineyard, and the final sample size used in each test is reported in Tables 1 and 2. All plants had their roots trimmed at the time of planting.
Table 1. Samples sizes of each of the inoculation strategies used in analyses of inoculant establishment and persistence.
Plants were either inoculated prior to planting (Pre), inoculate at planting (Co.) or inoculated onto an existing vine (Est.). Equal numbers of vines in each category were inoculated with a live fungus (Inoc.) or inoculated with an inert carrier (Cont.).
| Pre. | Co. | Est. | |||||
|---|---|---|---|---|---|---|---|
| Sampling Period | Inoc. | Cont. | Inoc. | Cont. | Inoc. | Cont. | Total |
| May 2013 | 0 | 0 | 7 | 9 | 14 | 8 | 38 |
| October 2013 | 8 | 8 | 7 | 7 | 13 | 7 | 50 |
| October 2014 | 7 | 8 | 7 | 9 | 14 | 8 | 53 |
| October 2015 | 7 | 8 | 7 | 5 | 14 | 8 | 49 |
| October 2016 | 8 | 8 | 7 | 8 | 13 | 7 | 51 |
| October 2017 | 7 | 8 | 7 | 9 | 14 | 8 | 53 |
Table 2. Summary of the number of observations from each inoculation strategy used in analyses of grapevine performance.
Note that for tests that include multiple years (i.e., 2013–2015 for shoot length), total n pooled from all years is presented.
| Pre. | Co. | Est. | ||||||
|---|---|---|---|---|---|---|---|---|
| Test | Inoc. | Cont. | Inoc. | Cont. | Inoc. | Cont. | Years | Total |
| Survival | 17 | 16 | 18 | 17 | 20 | 16 | 1 | 104 |
| Shoot length | 45 | 47 | 53 | 49 | 42 | 34 | 3 | 270 |
| Shoot diameter | 29 | 34 | 36 | 32 | 34 | 27 | 2 | 192 |
| Cluster production | 14 | 15 | 18 | 14 | 14 | 13 | 1 | 88 |
| Cluster number | 29 | 34 | 36 | 32 | 34 | 27 | 2 | 192 |
Soil chemistry
Three soil cores were taken at each experimental vine in October 2013 and pooled, dried at 60 °C for 24 h, and sieved in a 2 mm sieve. Sieves were sterilized by thoroughly flaming between samples. Each of the 52 samples were analyzed for pH, phosphorus, and nitrogen by Zenalytics Laboratories in Kelowna, BC. Soil pH in H2O was tested using a pH meter, and total inorganic and organic nitrogen was tested with the Kjeldahl method (Rutherford et al., 2007). Phosphorus was extracted using the Mehlich III extractant (Mehlich, 1984) and tested with the ICP-MS method, which primarily detects orthophosphate (Cade-Menun et al., 2018).
Fungal characteristics in the soil
Soil from cores, collected and processed as described above, and fresh roots collected from vines in October 2017 were sent to the University of California Riverside for analysis. Soil hyphal length was measured following Miller, Jastrow & Reinhardt (1995), and soil spore density was determined following Klironomos et al. (1993). Percent root colonization by hyphae, arbuscules, and vesicles was determined using the McGonigle method (McGonigle et al., 1990). Samples were stored at −20 °C and shipped frozen.
Sampling and DNA extraction
To determine establishment of the commercial isolate on experimental vines, soil cores were from vines in May 2013 prior to inoculation, and October 2013-2017. Three cores were taken from each vine, 10 cm away from the trunk left, right, and centre. The soil was pooled, dried at 60 °C for 24 h, homogenized, and sifted in a sterile 2 mm sieve. Sieves were thoroughly flame-sterilized between each sample. Processed soil was stored at −20 °C until DNA extraction.
Establishment of the isolate on off-target vegetation was determined by taking 199 total root samples in August 2017 from 10 common species found in the interrow vegetation and which represented a range of rooting and life history strategies (Table S1). 50 cm × 50 cm quadrats were placed in front of each experimental vine, and if any of the 10 focal species were present, one representative of each species was randomly selected and sampled by harvesting the plant and removing a portion of the root system. A single soil core was also taken from the center of each of 102 quadrats. To assess the spread from the inoculated block, soil was collected and extracted in July 2017 using the same protocol from three soil cores taken from 80 random locations in interrows north and east of the inoculated block.
All DNA was extracted using the PowerSoils DNA kit (MoBio, Inc., acquired by Qiagen in 2017), and stored at −80 °C, or −20 °C when in frequent use. Root samples were frozen in liquid nitrogen and pulverized by mechanically shaking with ceramic beads prior to extraction.
Droplet digital PCR (ddPCR) analysis
DNA extracted from soil and root samples was tested for the presence of R. irregulare DAOM 197198 using a probe-based ddPCR assay on the Bio-Rad QX100 Droplet Digital PCR System (Bio-Rad Laboratories, Inc., Hercules, California). This technique allows accurate detection and quantification of target DNA by partitioning the sample into >10,000 droplets prior to the PCR reaction. Primers and probe for this assay were designed to target the cox3-rnl intergenic region (Kokkoris et al., 2019), and the sequences are as follows (see Table S2 for further details):
Forward: 5′-AGCAAATCTAAGTTCCTCAGAG-3′
Reverse: 5′-ACTTCTATGGCTTTGTACAGG-3′
Probe: 5′-FAM/CCCACCAGG/ZEN/GCAGATTAATCTTCCTT/3IABKFQ-3′
These primers amplified only DAOM 197198 of 23 R. irregulare strains and 12 AM fungal species tested and have no predicted specificity to other AM fungal isolates. They also did not amplify in environmental soil samples taken from natural areas in nearby Merritt, BC, that should contain a similar background AM fungal community with a minimal risk of agriculturally introduced AM fungi.
The reaction contains 10 µl Bio-Rad ddPCR Supermix for Probes, 2 µl of Integrated DNA Technologies PrimeTime qPCR Assay mixture with a final concentration of 500 nM each forward and reverse primer and 250 nM probe, 1 µl of template (extracted DNA from soil), and 7 µl of dH20 for a total of 20 µl. Positive controls contained amplicon of the target sequence which was produced from DNA extracted from onion roots colonized by the biofertilizer used, and negative controls received sterile water. Three negative control wells were included on each plate, and all three wells must have zero positive droplets for the results of the plate to be considered valid. The total volume was transferred to the Bio-Rad QX100 Droplet Generator with 70 µl of Bio-Rad Droplet Generator Oil for Probes to produce 40 µl of droplets. We required a minimum of 10,000 droplets in an individual well during measurement for the well to be considered valid. The droplets were cycled at: 95 °C for 10 min, 50 cycles of 94 °C for 30 s and 57.8 °C for 2 min, 98 °C for 10 min, and 4 °C hold. After cycling, the amount of fluorescence in the droplets was measured by the Bio-Rad QX100 Droplet Reader. The number of positive (fluorescent) droplets in the sample is converted to the estimated number of target copies per µl of template using QuantaSoft (Version 1.7.4.0917, Bio-Rad Laboratories Inc.). The threshold for a positive call was manually set just below the bottom of the cloud produced in the positive control well on each plate (see Fig. S1). A high threshold was chosen because droplets that do not reach the fluorescence of this level are more likely false positives, as droplets that contain at least one copy of target sequence should reach roughly the same final fluorescence. Further, the primer set alone does amplify non-target sequence from the site, and although the probe-binding region is not a good match on these non-target amplicons, it may bind occasionally and produce some fluorescence (Fig. S2). However, if this does occur, the fluorescence should be much lower than true positives and thus excluded by our high threshold.
GPS coordinates and distance calculations
GPS coordinates for each of the samples taken outside the inoculated block, as well as the corners of the inoculated block, were taken using the app “GPS Status” version 1.2 on an iPhone 6S. Each recording was taken after the location accuracy had stabilized to within 5 m. The distance between the sample site and the nearest edge of the inoculated block was calculated in Microsoft Excel version 14.7.2 using the Haversine formula, as follows:
This formula requires the latitude and longitude input to be in decimal degree format and provides the Great Circle Distance in metres.
Vine measurements
For each vine, length of the longest new shoot (cm), shoot diameter (mm), and the number of grape clusters were recorded to assess vine response to the inoculant. The number of grape clusters was chosen to get a measure of vine yield, and the other variables measured were measured as they are used in determining vine vigor and balance. Vine survival was determined by visiting all vines on May 31st, 2017, well after vines had begun leafing out. Any vine that did not have any fresh growth, had been removed, or had been replaced was recorded as “dead”. Only vines with new growth that season were recorded as “alive”.
Statistical methods
All statistical analyses were conducted using RStudio version 1.1.383 (R Core Team, 2018), and α = 0.05 was used for all analyses. Sample sizes for each test are shown in Tables 1 and 2.
First, spatial autocorrelation of isolate abundance was determined using the function “Moran.I” from the package ape version 5.0 (Paradis & Schliep, 2018). A row-normalized matrix of inverse pair-wise distances between vines was used as the weight matrix, generated using “dist” from the package stats version 3.6.1 (R Core Team, 2018) and taking the inverse and normalizing. Converting the matrix to this format provides greater weight to nearby vines, as the influence of a neighbouring vine is inversely proportional to its distance and ensures the influence of neighbours add to one for each vine, so that the weights are expressed as proportions and thus comparable. The distance between rows in the vineyard is 3.05 m (10 ft) and the distance between vines is 1.22 m (4 ft). This scale was maintained in the distance matrix by multiplying the row numbers by ten and the within-row vine position by four. Establishment of the isolate was then tested by comparing whether inoculated vines were more likely to test positive for the isolate than non-inoculated vines using a Fisher’s Exact test through the “fisher.test” function from the stats package, version 3.6.1 (R Core Team, 2018). Limited positive data prevented statistical analysis of effects of inoculation and strategy on abundance and spread from the inoculated block.
Differences in grapevine survival and the percent of vines producing fruit between inoculated and non-inoculated vines were tested using a Fisher’s Exact test. A global Fisher’s test was performed first on all of the data to test whether inoculation alone reduced mortality, and then the data were subset by strategy and the test was repeated to determine whether there was an interaction with strategy. Analysis of the percent of vines fruiting was restricted to 2015 because this was the most recent year for which we had complete data.
The effect of inoculation and the interaction with strategy on shoot length, diameter, and the number of grapes produced by fruiting vines was tested using a linear mixed-effects model (LMM) approach through the function “lmer” from the package lme4 1.1.21 (Bates et al., 2015). Inoculation and its interaction with strategy were coded as fixed factors and the year the measurement was taken was coded as a random factor. Log-transformations were used to normalize data as needed. The model was assessed using the function “Anova” from the package car version 3.0.5 (Fox & Weisberg, 2019).
Results
Soil characteristics
Chemical
The soil pH at the experimental site was neutral, with a median pH of 7.03 (Table S3). Phosphorus content as measured by Mehlich III/ICP-MS ranged from 184.7–548.6 mg/kg with a median of 320.4 mg/kg (Table S3). Median total nitrogen from the Kjeldahl method was 0.3%, ranging from 0-0.47% (Table S3).
Fungal
Soil collected from the study site had a median hyphal length of 0.93 m/g of soil and a median of 18 spores/g of soil (Table S4). Root fragments from experimental vines showed a median colonization of 35.5% (Table S5), consisting of 5% arbuscules (range = 0–14%), 10% vesicles (range = 5–30%), and 25.5% hyphae (range = 10–39%).
Establishment and spread of the introduced isolate
Overall, there was no evidence that the isolate established over the course of our experiment. The abundance of the isolate in the soil was very low, both in terms of the percentage of samples that were positive and the average abundance in each sample (Table 3). Importantly, the target isolate was also detected in 10.4% of samples taken prior to inoculation, indicating that the isolate was already present in the soil before our experiment began. In comparison, the percentage of positive soil samples post inoculation ranged from 3.8% to 20.4% and fluctuated between years rather than showing a consistent trend. Soil samples taken from vines that had been inoculated were not more likely to be positive for the isolate than vines that had not been inoculated, in any year (Fisher’s Exact test, P: 0.28-1, Table 3).
Table 3. Number of samples positive for R. irregulare DAOM 197198, and the results of the Fisher’s Exact test for the effect of inoculation on the likelihood of detecting the isolate.
Note that Pre-inoculated samples were not available from the May 2013 samp.
| May 2013 | October 2013 | October 2014 | October 2015 | October 2016 | October 2017 | |
|---|---|---|---|---|---|---|
| Positive samples | 4 (10.5%) | 8 (16%) | 2 (3.8%) | 10 (20.4%) | 7 (13.7%) | 4 (7.5%) |
| Positive samples which were inoculated | 2 (50%) | 5 (62.5%) | 2 (100%) | 4 (40%) | 4 (57%) | 3 (75%) |
| Pre-inoculated | n/a | 0 | 0 | 1 | 0 | 1 |
| Co-inoculated | 0 | 1 | 0 | 0 | 1 | 0 |
| Established | 2 | 4 | 2 | 3 | 3 | 2 |
| Total samples | 38 | 50 | 53 | 49 | 51 | 53 |
| P-value (Fisher’s Exact) | 1 | 1 | 0.49 | 0.28 | 1 | 0.62 |
| Median copy number | 9.8 | 13.0 | 3.8 | 6.2 | 6.6 | 5 |
| Mean copy number | 22.5 | 1939.7 | 3.8 | 9.7 | 7.6 | 5 |
| Range copy number | 2.6-68 | 1.6-13440 | 1.6-6 | 2.4-126 | 2.8-10.8 | 2.4-8.8 |
The low number of positive soil samples precluded statistical analysis, but there were no apparent effects. Soil samples from Established vines were marginally more likely to be positive (2–4 positive vines per sampling period) than vines of the Pre- and Co-inoculation strategies (0–1 vines). However, two of the positive Established vines were positive before inoculation and continued to test positive in subsequent years.
Low abundance of the target isolate was also reflected in interrow vegetation, where 3 of 199 root samples and 3 of 102 soil samples were positive (Table 4). Positive root samples came from roots of Bromus carinatus, Trifolium pratense, and Medicago lupulina, which represent both fibrous and taproot species as well as annual and perennial strategies.
Table 4. ddPCR results from the positive samples of interrow soil and vegetation roots from within the inoculated block in August 2017.
These positive samples represented 2.9% of soil samples and 1.5% of root samples. Only 1.43 TP and 1.43 were taken from the same.
| Sample ID | Sample description | Input material weight (g) | Copies/uL | Copies/g input |
|---|---|---|---|---|
| 2.24 BC | B. carinatus | 0.08 | 1.4 | 1750 |
| 1.43 TP | T. pratense | 0.27 | 1.4 | 518.5 |
| 1.20 BM | M. lupulina | 0.1 | 1.4 | 1400 |
| 1.3 | soil | 0.25 | 1.8 | 720 |
| 1.43 | soil | 0.25 | 1.4 | 2560 |
| 2.15 | soil | 0.25 | 6.4 | 560 |
All soil samples taken beyond the inoculated block to determine the spread of the isolate were negative.
Lack of spatial auto-correlation
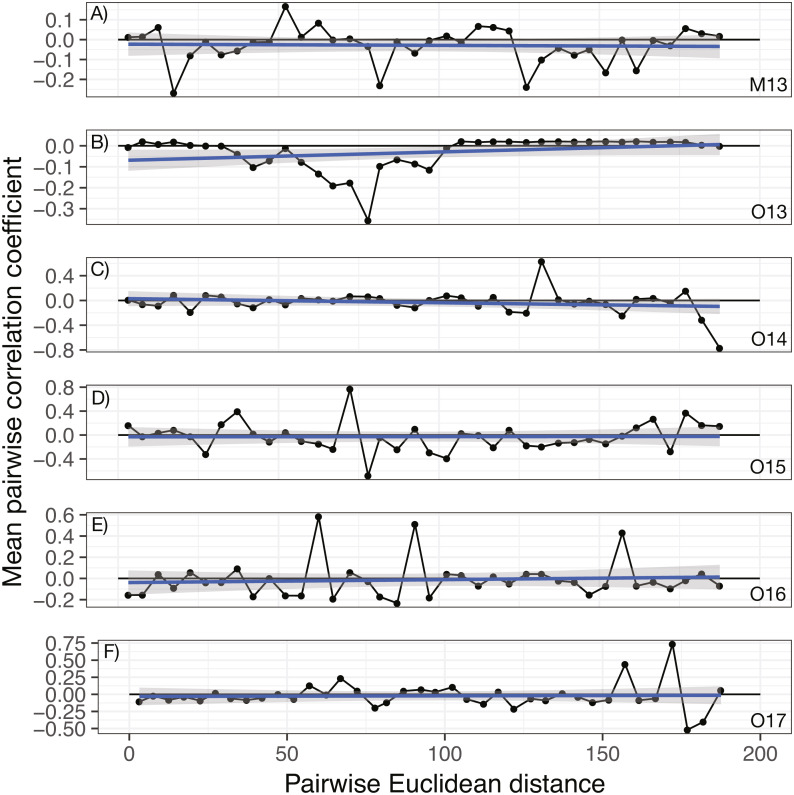
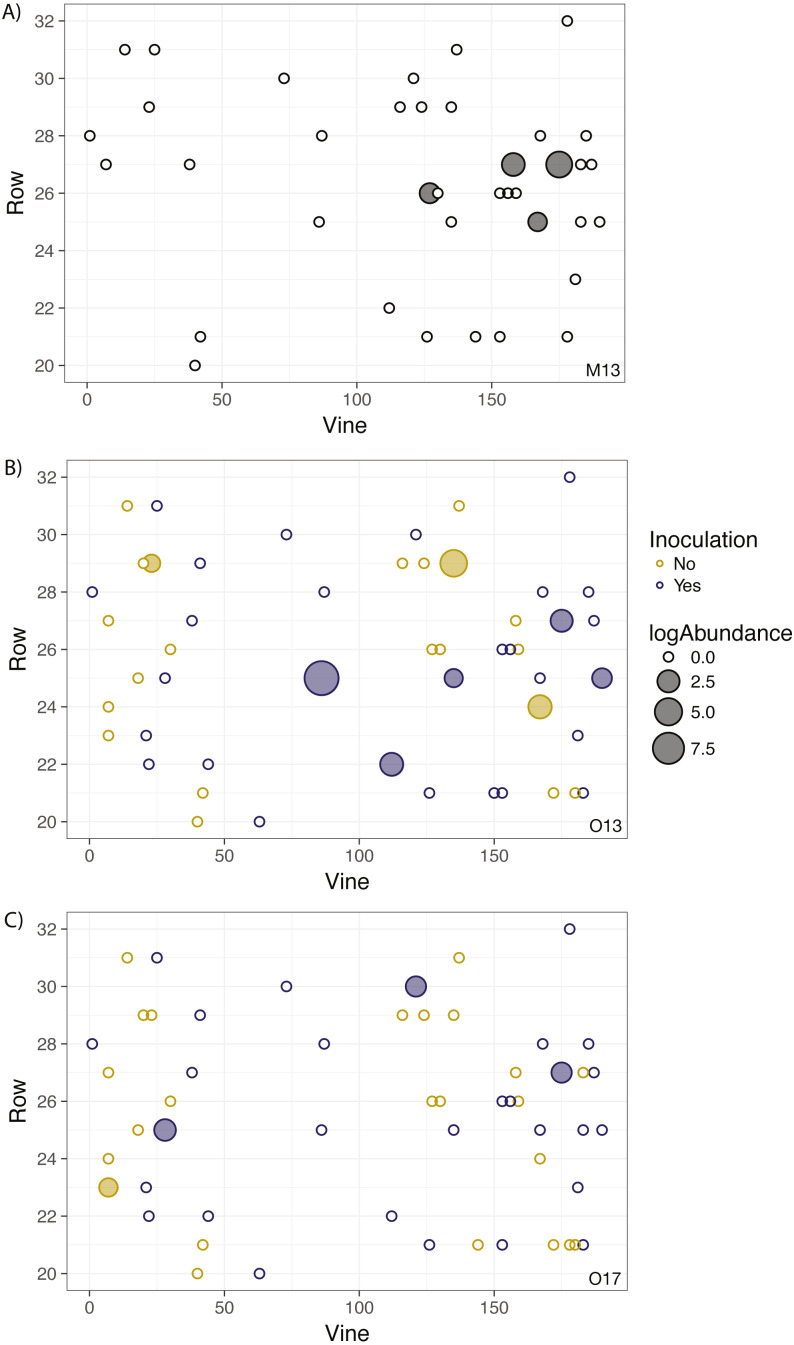
No significant spatial auto-correlation was detected during any sampling period (See Table S6 for statistical results). The correlation coefficients were small, ranging from 0.01 to -0.05, with P- values ranging from 0.27–0.82. The absence of spatial auto-correlation can also be seen in the lack of consistent, positive correlation coefficients between nearby vines in Fig. 1 and the absence of defined spatial structure in abundance in Figs. 2A–2C (See Figs. S3–S8 for all years).
Figure 1. Annual correlograms of target isolate abundance by vine distance.
Correlograms for samples taken in (A) May 2013, (B) October 2013, (C) October 2014, (D) October 2015, (E) October 2016, and (F) October 2017. No spatial autocorrelation was detected in any time frame (see Table S1 for statistics), which would appear as consistent, positive correlation at shorter distances. Each point represents the mean correlation coefficient for all pairwise vine combinations within the range of distance represented by that point. The black line represents the zero correlation line, the blue line shows the regression line, and the shading depicts the 95% confidence interval on the regression line.
Figure 2. Geographic distribution and abundance of the target isolate, R. irregulare DAOM 197198, in soil samples taken in May 2013 (A) and October 2013 (B), and October 2017 (C).
Each point represents an individual experimental vine, and the point is filled if the sample tested positive for the presence of the isolate or left unfilled if the sample tested negative. The size of the point is proportional to the log abundance of the isolate. Yellow points represent non-inoculated vines, and purple points represent inoculated vines (colours omitted in A due to being pre-inoculation). No statistically significant spatial autocorrelation is present.
Grapevine survival
Twenty-seven of 104 vines had died or had been replaced by May 2017. Overall, non-inoculated vines experienced higher mortality (30.6%) than inoculated vines (21.8%), but this difference was not statistically significant (Fig. S9; Fisher’s Exact, odds ratio = 1.574, n = 104, P = 0.38). When subset by strategy, inoculation did not decrease the mortality rate in any strategy (Fisher’s Exact, Pre—odds ratio = 1.081, n = 33, P = 1; Co—odds ratio = 2.040, n = 35, P = 0.44; Est—odds ratio = 1.770, n = 36, P = 0.48).
Shoot length
Median shoot length during 2013–2015 measured 86.5 cm (range = 14–182 cm) for non-inoculated vines, and 85.25 cm (range = 11–259 cm) for inoculated vines, and was not significantly different between these two groups (Fig. S10; LMM, X2 = 0.830, n = 270, P- =0.362). There was also no interaction between inoculation and strategy (LMM, X2 = 2.999, n = 270, P- = 0.223).
Shoot base diameter
Median shoot diameter was very similar between inoculated (6.2 mm; range 2.9–9.0 mm) and non-inoculated vines (6.0 mm; range 2.4–9.9 mm), and was not significantly different (Fig. S11; LMM, X2 = 1.070, n = 190, P = 0.301). Again, there was no interaction between inoculation and strategy (LMM, X2 = 0.424, n = 190, P- =0.810).
Grape cluster production
Binary cluster production
Overall, inoculated vines were not more likely to produce grapes (60.8%) compared to non-inoculated vines (69.0%; Fig. S12; Fisher’s Exact, odds ratio = 0.700, n = 88, P- =0.505). This remained consistent within different strategies (Fisher’s Exact; Pre—odds ratio = 1.241, n = 29, P = 1; Co—odds ratio = 0.440, n = 32, P = 0.442; Est—odds ratio = 0.636, n = 27, P = 0.636).
Cluster number
Within vines that produced fruit, inoculated vines did not produce more grape clusters (median = 3, range = 1–24) than did non-inoculated vines (median = 3, range = 1–8; Fig. S13; LMM, X2 = 0.427, n = 70, P- =0.514). The interaction between inoculation and strategy did not have a significant effect (LMM, X2 = 2.720, n = 70, P- = 0.257).
Discussion
Contrary to our expectations, pre-inoculation did not ensure successful establishment of the commercial inoculant in the field. There were few positive samples overall, and inoculated samples were no more likely to be positive than non-inoculated samples. Root and soil samples from interrow vegetation also showed very low abundance of the inoculant. Thus, inoculation of plants with the commercial inoculant was unsuccessful regardless of whether it was conducted pre-transplant in the greenhouse, during transplant in the field, or on one-year-old vines in the field.
Failure of AM fungal inoculants establishment in the field is not unusual. In one study, 9 out of 12 inoculants failed to establish in sterile soil in greenhouse, and 5 of 12 failed to establish in non-sterile soil (Faye et al., 2013). Establishment failure has been observed in other greenhouse trials using both sterile and non-sterile soil (Corkidi et al., 2004; Tarbell & Koske, 2007; Köhl, Lukasiewicz & Van der Heijden, 2016), and also in field trials of inoculants (Berruti, Lumini & Bianciotto, 2017).
The equal likelihood of detection in both inoculated and non-inoculated samples and detection of the isolate pre-inoculation indicate that positive samples could have been from a pre-existing background population. However, it is not clear whether this is due to deliberate introduction through commercial inoculants simply the natural range of this isolate. The ranges of AM fungal species are very difficult to determine, although some AM fungal species have been found across widespread distributions (Davison et al., 2015; Oehl et al., 2017; Savary et al., 2018). Therefore, the range of R. irregulare DAOM 197198 could conceivably extend from its original collection location in Quebec to our site in British Columbia. Nonetheless, we know that this isolate was not introduced in our site within the last 15 years and we were unable to find other records from natural ecosystems in British Columbia.
Possible causes of establishment failure
The goal of this experiment was to test the influence of priority effects on AM fungal inoculant establishment; however, the inoculant failed to establish in the field in any treatment. One factor that may have contributed to poor establishment in our experiment is the soil chemistry at the site. Soil phosphorus at the site was roughly ten times more abundant than the 20–50 mg/kg recommended for organic grape production in the region (Gough, 1996; Landers et al., 2011). If plants are grown under surplus phosphorus conditions, the nutritional benefit of the partnership can be outweighed by the carbon cost (Johnson, 2010). Elevated soil phosphorus may inhibit root colonization altogether (Balzergue et al., 2011; Balzergue et al., 2013; Nouri et al., 2014). Median root fungal colonization in our grapevines was 35.5%, which is roughly half of the typical 60–80% root colonization for the rootstock used here (Schreiner, 2003).
Additionally, this experiment was conducted in an organic vineyard, which often has higher microbial biomass than conventional viticulture (Karimi et al., 2020) and can also increase mycorrhizal root colonization (De Freitas et al., 2011). This increased microbial biomass and activity may exacerbate priority effects and contribute to establishment failure in organic systems in general, although in our system root fungal colonization was relatively low and pre-inoculation did not improve inoculation success over inoculation in the field. Additionally, the amount and method of inoculum application can affect the strength of colonization (Van Jaarsveld et al., 2021).
Although the roots were trimmed during planting, it has been shown that this practice does not remove all of the active mycorrhiza and colonization can be re-established from mycorrhizas remaining in the older portion of the root system (Holland et al., 2019).
Implications for practical applications of biofertilizers
Successful establishment of AM fungal inoculants is often unreliable, and our results confirm other studies showing high rates of inoculant failure (Corkidi et al., 2004; Tarbell & Koske, 2007; Faye et al., 2013; Berruti, Lumini & Bianciotto, 2017; Köhl, Lukasiewicz & Van der Heijden, 2016). To assess the value of using an inoculant, accurate isolate-specific tracking is essential, because in this case the lack of growth benefits was due to poor inoculant establishment rather than a well-established but poorly beneficial inoculant. It is possible that R. irregulare DAOM 197198 may be effective in producing increased growth, yield, or survival in grapevine if it does establish; however, for growers using these inoculants, uncertainty in the likelihood of establishment remains a significant barrier. Without successful establishment of the isolate, no direct effects of the biofertilizer on the grapevine were possible (although sometimes indirect effects may occur through changes to the soil microbial community, see Berruti, Lumini & Bianciotto, 2017), which is ultimately what growers using these inoculants would like to achieve.
In addition to the financial costs to the producers with inconsistent and unreliable return on investment, these inoculants may also present an ecological cost through the risk of invasion (Schwartz et al., 2006; Hart, Antunes & Abbott, 2017; Ricciardi et al., 2017). When this isolate is introduced to an established AM fungal community, it can significantly reduce the species richness by displacing native AM fungal species (Koch et al., 2011; Symanczik et al., 2015). Aboveground plant communities are closely linked to the identities and diversity of AM fungal communities (Van der Heijden et al., 1998; Van der Heijden, Wiemken & Sanders, 2003; Stampe & Daehler, 2003; Bever et al., 2010), which the introduction of a commercial isolate has the potential to disrupt.
Conclusions
In this study, we examined whether a commercial biofertilizer was beneficial in grapevine inoculated in three different strategies; however, the fungus in the biofertilizer did not successfully establish in any treatment. These results highlight the fact that achieving benefits with biofertilizers will require careful site by site evaluation. These products often claim to guarantee results, which is deceptive as it is not enough to simply introduce the isolate and expect changes to plant performance. Many management practices will affect AM fungi both positively and negatively, which should be evaluated prior to use of AM fungal biofertilizers. For example, our results show that it is important for users to know and regularly test their soil fertility, to avoid inoculating over-fertilized soils. Also, other management practices such as reduced tillage and shortened fallow have been shown to be beneficial to AM fungi (Lekberg & Koide, 2005). Therefore, while the use of AM fungal inoculants would likely be effective under certain conditions where the colonization potential would otherwise be very low, the potential of these inoculants to mitigate the impacts of declining phosphorus availability should be viewed critically in the light of the costs associated with introduction.
Supplemental Information
All the code for the statistical analyses conducted for this research.
Fungal hyphal length and number of spores per gram quantified in root and soil samples.
Results of a grapevine survival survey conducted at the end of the experiment.
Columns:
id: unique vine identifier
treatment: J = juvenile, renamed “established”, N = new, renamed “co-inoculated”, G = greenhouse, renamed “pre-inoculated”
inoc: 0 = control, 1 = inoculated
status: 0 = dead or replaced, 1 = alive.
Size and fruiting measurements made on vines.
Columns: plant.id: the identifier given to each vine
treatment: J = juvenile, renamed “established”, N = new, renamed “co-inoculated”, G = greenhouse, renamed “pre-inoculated” inoc: 0 = control, 1 = inoculated
Year: year measurement was taken.
The results of our ddPCR assay on soil samples from grapevines in our experiment and the results of soil chemistry tests as conducted by Zenalytics Laboratories (Kelowna, BC).
Columns: plant.id: the identifier given to each vine.
treatment: J = juvenile, renamed “established”, N = new, renamed “co-inoculated”, G = greenhouse, renamed “pre-inoculated”
inoc: 0 = control, 1 = inoculated
ddM13S: ddPCR copy number from May 2013 soil sample
ddO13S: ddPCR copy number from October 2013 soil sample
ddO14S: ddPCR copy number from October 2014 soil sample
ddO15S: ddPCR copy number from October 2015 soil sample
ddO16S: ddPCR copy number from October 2016 soil sample
ddO17S: ddPCR copy number from October 2017 soil sample
P: Soil phosphorus result (Mehlich III extraction)
N: Soil nitrogen result (Kjedahl method)
pH: Soil pH.
GPS coordinates for each vine in the experiment. Rows were 10 ft (3.05 m) apart and vines were 4 ft (1.2 m) apart.
Columns:
ID: the identifier given to each vine
Treatment: J = juvenile, renamed “established”, N = new, renamed “co-inoculated”, G = greenhouse, renamed “pre-inoculated”
Inoculation: 0 = control, 1 = inoculated
Row: The vineyard row the vine was located in
VinePos: The number of vines down the row the focal vine was (ie. first vine in the row is 1, next is 2…)
PropRow: The distance in feet from Row 20 (the first row with experimental vines) to the row that the vine is in
PropVine: The distance in feet from the first vine in the row (ie, vine 1 is 0 feet, vine 25 is 96 feet down the row).
Acknowledgments
Thank you for Kalala Organic Estate Winery for their support.
Funding Statement
This work was supported by an NSERC Engage Grant and NSERC Discovery Grant to Miranda Hart and an NSERC CGS-M to Corrina Thomsen. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Corrina Thomsen, Email: corrina.thomsen@ubc.ca.
Miranda Hart, Email: miranda.hart@ubc.ca.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Corrina Thomsen performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Laura Loverock and Vasilis Kokkoris performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Taylor Holland performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Patricia A. Bowen and Miranda Hart conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Field site permission was provided by Ashish Dave, Research Manager Kalala Estate Winery, West Kelowna, BC and Karnail Sidhu, Owner Kalala Estate Winery, West Kelowna, BC.
Data Availability
The following information was supplied regarding data availability:
Raw data are available in the Supplemental Files.
References
- Alford & Wilbur (1985).Alford RA, Wilbur HM. Priority effects in experimental pond communities: competition between Bufo and Rana. Ecological Society of America. 1985;66:1097–1105. [Google Scholar]
- Balzergue et al. (2013).Balzergue C, Chabaud M, Barker DG, Bécard G, Rochange SF. High phosphate reduces host ability to develop arbuscular mycorrhizal symbiosis without affecting root calcium spiking responses to the fungus. Frontiers in Plant Science. 2013;4:426. doi: 10.3389/fpls.2013.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzergue et al. (2011).Balzergue C, Puech-Pags V, Bécard G, Rochange SF. The regulation of arbuscular mycorrhizal symbiosis by phosphate in pea involves early and systemic signalling events. Journal of Experimental Botany. 2011;62:1049–1060. doi: 10.1093/jxb/erq335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates et al. (2015).Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67:1–48. [Google Scholar]
- Berruti et al. (2016).Berruti A, Lumini E, Balestrini R, Bianciotto V. Arbuscular mycorrhizal fungi as natural biofertilizers: let’s benefit from past successes. Frontiers in Microbiology. 2016;6:1–13. doi: 10.3389/fmicb.2015.01559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berruti, Lumini & Bianciotto (2017).Berruti A, Lumini E, Bianciotto V. AMF components from a microbial inoculum fail to colonize roots and lack soil persistence in an arable maize field. Symbiosis. 2017;72:73–80. doi: 10.1007/s13199-016-0442-7. [DOI] [Google Scholar]
- Bever et al. (2010).Bever JD, Dickie IA, Facelli E, Facelli JM, Klironomos J, Moora M, Rillig MC, Stock WD, Tibbett M, Zobel M. Rooting theories of plant community ecology in microbial interactions. Trends in Ecology & Evolution. 2010;25:468–478. doi: 10.1016/j.tree.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cade-Menun et al. (2018).Cade-Menun BJ, Elkin KR, Liu CW, Bryant RB, Kleinman PJA, Moore PA. Characterizing the phosphorus forms extracted from soil by the Mehlich III soil test. Geochemical Transactions. 2018;19:7. doi: 10.1186/s12932-018-0052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condron et al. (2013).Condron LM, Spears BM, Haygarth PM, Turner BL, Richardson AE. Role of legacy phosphorus in improving global phosphorus-use efficiency. Environment, Development and Sustainability. 2013;8:147–148. doi: 10.1016/j.envdev.2013.09.003. [DOI] [Google Scholar]
- Cordell & White (2013).Cordell D, White S. Sustainable phosphorus measures: strategies and technologies for achieving phosphorus security. Agronomy. 2013;3:86–116. doi: 10.3390/agronomy3010086. [DOI] [Google Scholar]
- Corkidi et al. (2004).Corkidi L, Allen EB, Merhaut D, Allen MF, Downer J, Bohn J, Evans M. Assessing the infectivity of commercial mycorrhizal inoculants in plant nursery conditions. Journal of Environmental Horticulture. 2004;22:149–154. [Google Scholar]
- Davison et al. (2015).Davison J, Moora M, Öpik M, Adholeya A, Ainsaar L, Bâ A, Burla S, Diedhiou AG, Hiiesalu I, Jairus T, Johnson NC, Kane A, Koorem K, Kochar M, Ndiaye C, Pärtel M, Reier Ü, Saks Ü, Singh R, Vasar M, Zobel M. Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science. 2015;349:970–973. doi: 10.5061/dryad.2m15n. [DOI] [PubMed] [Google Scholar]
- De Freitas et al. (2011).De Freitas NO, Yano-Melo AM, Da Silva FSB, De Melo NF, Maia LC. Soil biochemistry and microbial activity in vineyards under conventional and organic management at Northeast Brazil. Scientia Agricola. 2011;68:223–229. [Google Scholar]
- Emam (2016).Emam T. Local soil, but not commercial AMF inoculum, increases native and non-native grass growth at a mine restoration site. Restoration Ecology. 2016;24:35–44. doi: 10.1111/rec.12287. [DOI] [Google Scholar]
- Environment Canada (2018).Environment Canada . Canadian climate normals 1981-2010. Kelowna PC Burnett’s Nursery; 2018. [04 January 2018]. [Google Scholar]
- Faye et al. (2013).Faye A, Dalpé Y, Ndung’u-Magiroi K, Jefwa J, Ndoye I, Diouf M, Lesueur D. Evaluation of commercial arbuscular mycorrhizal inoculants. Canadian Journal of Plant Science. 2013;93:1201–1208. doi: 10.4141/cjps2013-326. [DOI] [Google Scholar]
- Formey et al. (2012).Formey D, Molès M, Haouy A, Savelli B, Bouchez O, Bécard G, Roux C. Comparative analysis of mitochondrial genomes of Rhizophagus irregularis—syn. Glomus irregulare—reveals a polymorphism induced by variability generating elements. New Phytologist. 2012;196:1217–1227. doi: 10.1111/j.1469-8137.2012.04283.x. [DOI] [PubMed] [Google Scholar]
- Fox & Weisberg (2019).Fox J, Weisberg S. An R companion to applied regression. 3rd edition Sage; Thousand Oaks: 2019. [Google Scholar]
- Fukami et al. (2010).Fukami T, Dickie IA, Paula Wilkie J, Paulus BC, Park D, Roberts A, Buchanan PK, Allen RB. Assembly history dictates ecosystem functioning: evidence from wood decomposer communities. Ecology Letters. 2010;13:675–684. doi: 10.1111/j.1461-0248.2010.01465.x. [DOI] [PubMed] [Google Scholar]
- Gough (1996).Gough N. Soil and plant tissue testing methods and their interpretation for British Columbia agricultural soils. Report, British Columbia Ministry of Agriculture and Food. https://www2.gov.bc.ca/assets/gov/farming-natural-resources-and-industry/agriculture-and-seafood/agricultural-land-and-environment/soil-nutrients/600-series/634200-3_soil_and_plant_tissue_testing_methods.pdf 1996
- Hamel & Strullu (2006).Hamel C, Strullu D. Arbuscular mycorrhizal fungi in field crop production: potential and new direction. Canadian Journal of Plant Science. 2006;86:941–950. [Google Scholar]
- Hart, Antunes & Abbott (2017).Hart MM, Antunes PM, Abbott LK. Unknown risks to soil biodiversity from commercial fungal inoculants. Nature Ecology & Evolution. 2017;1:0115. doi: 10.1038/s41559-017-0115. [DOI] [PubMed] [Google Scholar]
- Hernádi et al. (2012).Hernádi I, Sasvári Z, Albrechtová J, Vosatka M, Posta K. Arbuscular mycorrhizal inoculant increases yield of spice pepper and affects the indigenous fungal community in the field. HortScience. 2012;47:603–606. [Google Scholar]
- Holland et al. (2019).Holland T, Bowen P, Kokkoris V, Richards A, Rosa D, Hart M. The effect of root pruning on the arbuscular mycorrhizal symbiosis in grapevine rootstocks. Chemical and Biological Technologies in Agriculture. 2019;6:1–6. doi: 10.1186/s40538-019-0159-y. [DOI] [Google Scholar]
- Janoušková et al. (2013).Janoušková M, Krak K, Wagg C, Štorchová H, Caklová P, Vosátka M. Effects of inoculum additions in the presence of a preestablished arbuscular mycorrhizal fungal community. Applied and Environmental Microbiology. 2013;79:6507–6515. doi: 10.1128/AEM.02135-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson (2010).Johnson NC. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytologist. 2010;185:631–647. doi: 10.1111/j.1469-8137.2009.03110.x. [DOI] [PubMed] [Google Scholar]
- Karimi et al. (2020).Karimi B, Cahurel J-Y, Gontier L, Charlier L, Chovelon M, Mahé H, Ranjard L. A meta-analysis of the ecotoxicological impact of viticultural practices on soil biodiversity. Environmental Chemistry Letters. 2020;18:1947–1966. [Google Scholar]
- Klironomos et al. (1993).Klironomos JN, Moutoglis P, Kendrick B, Widden P. A comparison of spatial heterogeneity of vesicular–arbuscular mycorrhizal fungi in two maple-forest soils. Canadian Journal of Botany. 1993;71:1472–1480. doi: 10.1139/b93-178. [DOI] [Google Scholar]
- Koch et al. (2011).Koch AM, Antunes PM, Barto EK, Cipollini D, Mummey DL, Klironomos JN. The effects of arbuscular mycorrhizal (AM) fungal and garlic mustard introductions on native AM fungal diversity. Biological Invasions. 2011;13:1627–1639. doi: 10.1007/s10530-010-9920-7. [DOI] [Google Scholar]
- Köhl, Lukasiewicz & Van der Heijden (2016).Köhl L, Lukasiewicz CE, Van der Heijden MGA. Establishment and effectiveness of inoculated arbuscular mycorrhizal fungi in agricultural soils. Plant, Cell and Environment. 2016;39:136–146. doi: 10.1111/pce.12600. [DOI] [PubMed] [Google Scholar]
- Kokkoris et al. (2019).Kokkoris V, Li Y, Hamel C, Hanson K, Hart M. Site specificity in establishment of a commercial arbuscular mycorrhizal fungal inoculant. Science of the Total Environment. 2019;660:1135–1143. doi: 10.1016/j.scitotenv.2019.01.100. [DOI] [PubMed] [Google Scholar]
- Ladd & Facelli (2008).Ladd B, Facelli JM. Priority effects produced by plant litter result in non-additive competitive effects. Oecologia. 2008;157:687–696. doi: 10.1007/s00442-008-1110-2. [DOI] [PubMed] [Google Scholar]
- Landers et al. (2011).Landers A, Muza A, Bates T, Cousins P, Curtis P, Dunst R, Helms M, Loeb G, Hed B, Martinson T, Reisch B, Senesac A, Timer J, Vanden Heuvel J, Peterson HW, Wilcox W, Wise A. 2011 production guide for organic grapes. Report, New York State Department of Agriculture and Markets 2011
- Lekberg & Koide (2005).Lekberg Y, Koide RT. Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta analysis of studies published between 1988 and 2003. New Phytologist. 2005;168:189–204. doi: 10.1111/j.1469-8137.2005.01490.x. [DOI] [PubMed] [Google Scholar]
- McGonigle et al. (1990).McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytologist. 1990;115:495–501. doi: 10.1111/j.1469-8137.1990.tb00476.x. [DOI] [PubMed] [Google Scholar]
- Mehlich (1984).Mehlich A. Mehlich 3 soil test extractant: a modification of Mehlich 2 extractant. Communications in Soil Science and Plant Analysis. 1984;15:1409–1416. [Google Scholar]
- Miller, Jastrow & Reinhardt (1995).Miller RM, Jastrow JD, Reinhardt DR. External hyphal production of vesicular-arbuscular mycorrhizal fungi in pasture and tallgrass prairie communities. Oecologia. 1995;103:17–23. doi: 10.1007/BF00328420. [DOI] [PubMed] [Google Scholar]
- Ministry of Forests, Lands, Natural Resource Operations and Rural Development (2018).Ministry of Forests, Lands, Natural Resource Operations and Rural Development Biogeographic Ecosystem Classification Subzone/Variant Map for the Penticton Subunit. ftp://ftp.for.gov.bc.ca/HRE/external/!publish/becmaps/PaperMaps/wall/DME_MerrittSubunit_CascadesResourceDistrict_ThompsonOkanaganRegion__wall.pdf. [10 April 2021];2018
- Nouri et al. (2014).Nouri E, Breuillin-sessoms F, Feller U, Reinhardt D. Phosphorus and nitrogen regulate arbuscular mycorrhizal symbiosis in Petunia hybrida. PLOS ONE. 2014;9:e90841. doi: 10.1371/journal.pone.0090841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehl et al. (2017).Oehl F, Laczko E, Oberholzer H, Jansa J, Egli S. Diversity and biogeography of arbuscular mycorrhizal fungi in agricultural soils. Biology and Fertility of Soils. 2017;53:777–797. doi: 10.1007/s00374-017-1217-x. [DOI] [Google Scholar]
- Ortas et al. (2011).Ortas I, Sari N, Akpinar Ç, Yetisir H. Screening mycorrhiza species for plant growth, P and Zn uptake in pepper seedling grown under greenhouse conditions. Scientia Horticulturae. 2011;128:92–98. doi: 10.1016/j.scienta.2010.12.014. [DOI] [Google Scholar]
- Paradis & Schliep (2018).Paradis E, Schliep K. Ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics. 2018;35:526–528. doi: 10.1093/bioinformatics/bty633. [DOI] [PubMed] [Google Scholar]
- Peay, Belisle & Fukami (2012).Peay KG, Belisle M, Fukami T. Phylogenetic relatedness predicts priority effects in nectar yeast communities. Proceedings of the Royal Society B: Biological Sciences. 2012;279:749–758. doi: 10.1098/rspb.2011.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino et al. (2011).Pellegrino E, Bedini S, Avio L, Bonaria E, Giovannetti M. Field inoculation effectiveness of native and exotic arbuscular mycorrhizal fungi in a Mediterranean agricultural soil. Soil Biology and Biochemistry. 2011;43:367–376. doi: 10.1016/j.soilbio.2010.11.002. [DOI] [Google Scholar]
- Pellegrino, Öpik & Bonari (2015).Pellegrino E, Öpik M, Bonari E. Responses of wheat to arbuscular mycorrhizal fungi: a meta-analysis of field studies from 1975 to 2013. Soil Biology and Biochemistry. 2015;84:210–217. doi: 10.1016/j.soilbio.2015.02.020. [DOI] [Google Scholar]
- R Core Team (2018).R Core Team R: a language and environment for statistical computing 2018
- Ricciardi et al. (2017).Ricciardi A, Blackburn TM, Carlton JT, Dick JTA, Hulme PE, Iacarella JC, Jeschke JM, Liebhold AM, Lockwood JL, MacIsaac HJ, Pyšek P, Richardson DM, Ruiz GM, Simberloff D, Sutherland WJ, Wardle DA, Aldridge DC. Invasion science: a horizon scan of emerging challenges and opportunities. Trends in Ecology & Evolution. 2017;32(6):464–474. doi: 10.1016/j.tree.2017.03.007]. [DOI] [PubMed] [Google Scholar]
- Rutherford et al. (2007).Rutherford P, McGill W, Arocena JM, Figuerido C. Total Nitrogen. In: Carter M, Gregorich E, editors. Soil sampling and methods of analysis. 2nd edition CRC Press; Boca Raton, FL: 2007. pp. 239–250. [Google Scholar]
- Savary et al. (2018).Savary R, Masclaux FG, Wyss T, Droh G, Cruz Corella J, Paula Machado A, Morton JB, Sanders IR. A population genomics approach shows widespread geographical distribution of cryptic genomic forms of the symbiotic fungus Rhizophagus irregularis. ISME Journal. 2018;12:17–30. doi: 10.1038/ismej.2017.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoor et al. (2011).Schnoor TK, Lekberg Y, Rosendahl S, Olsson PA. Mechanical soil disturbance as a determinant of arbuscular mycorrhizal fungal communities in semi-natural grassland. Mycorrhiza. 2011;21:211–220. doi: 10.1007/s00572-010-0325-3. [DOI] [PubMed] [Google Scholar]
- Schreiner (2003).Schreiner RP. Mycorrhizal colonization of grapevine rootstocks under field conditions. The American Journal of Enology and Viticulture. 2003;54:143–149. [Google Scholar]
- Schüzler & Walker (2010).Schüzler A, Walker C. The Glomeromycota: a species list with new families. Gloucester: Published in libraries at The Royal Botanic Garden Edinburgh, The Royal Botanic Garden Kew, Botanische Staatssammlung Munich, and Oregon State University; 2010. [Google Scholar]
- Schwartz et al. (2006).Schwartz MW, Hoeksema JD, Gehring CA, Johnson NC, Klironomos JN, Abbott LK, Pringle A. The promise and the potential consequences of the global transport of mycorrhizal fungal inoculum. Ecology Letters. 2006;9:501–515. doi: 10.1111/j.1461-0248.2006.00910.x. [DOI] [PubMed] [Google Scholar]
- Sieverding et al. (2014).Sieverding E, Da Silva GA, Berndt R, Fritz O. Rhizoglomus, a new genus of the Glomeraceae. Mycotaxon. 2014;129:373–386. [Google Scholar]
- Smith & Read (2008).Smith SE, Read DJ. Mycorrhizal symbiosis. 3rd edn Academic Press, Inc; New York: 2008. [Google Scholar]
- Stampe & Daehler (2003).Stampe ED, Daehler CC. Mycorrhizal species identity affects plant community structure and invasion: a microcosm study. Oikos. 2003;100:362–372. [Google Scholar]
- Stockinger, Walker & Schüzler (2009).Stockinger H, Walker C, Schüzler A. ‘Glomus intraradices DAOM197198’, a model fungus in arbuscular mycorrhiza research, is not Glomus intraradices. New Phytologist. 2009;183:1176–1187. doi: 10.1111/j.1469-8137.2009.02874.x. [DOI] [PubMed] [Google Scholar]
- Symanczik et al. (2015).Symanczik S, Courty PE, Boller T, Wiemken A, Al-Yahya’ei MN. Impact of water regimes on an experimental community of four desert arbuscular mycorrhizal fungal (AMF) species, as affected by the introduction of a non-native AMF species. Mycorrhiza. 2015;25:639–647. doi: 10.1007/s00572-015-0638-3. [DOI] [PubMed] [Google Scholar]
- Tarbell & Koske (2007).Tarbell TJ, Koske RE. Evaluation of commercial arbuscular mycorrhizal inocula in a sand/peat medium. Mycorrhiza. 2007;18:51–56. doi: 10.1007/s00572-007-0152-3. [DOI] [PubMed] [Google Scholar]
- Thirkell et al. (2017).Thirkell TJ, Charters MD, Elliott AJ, Sait SM, Field KJ. Are mycorrhizal fungi our sustainable saviours? Considerations for achieving food security. Journal of Ecology. 2017;105:921–929. doi: 10.1111/1365-2745.12788. [DOI] [Google Scholar]
- Van der Heijden et al. (1998).Van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature. 1998;396:69–72. doi: 10.1038/23932. [DOI] [Google Scholar]
- Van der Heijden, Wiemken & Sanders (2003).Van der Heijden MGA, Wiemken A, Sanders IR. Different arbuscular mycorrhizal fungi alter coexistence and resource distribution between co-occurring plants. New Phytologist. 2003;157:569–578. doi: 10.1046/j.1469-8137.2003.00688.x. [DOI] [PubMed] [Google Scholar]
- Van Jaarsveld et al. (2021).Van Jaarsveld WJ, Halleen F, Bester MC, Pierron RJG, Stempien E, Mostert L. Investigation of Trichoderma species colonization of nursery grapevines for improved management of black foot disease. Pest Management Science. 2021;77:397–405. doi: 10.1002/ps.6030. [DOI] [PubMed] [Google Scholar]
- Vance (2001).Vance CP. Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiology. 2001;127:390–397. doi: 10.1104/pp.010331.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenkoornhuyse et al. (2002).Vandenkoornhuyse P, Husband R, Daniell TJ, Watson IJ, Duck JM, Fitter AH, Young JP. Arbuscular mycorrhizal community composition associated with two plant species in a grassland ecosystem. Molecular Ecology. 2002;11:1555–1564. doi: 10.1046/j.1365-294X.2002.01538.x. [DOI] [PubMed] [Google Scholar]
- Verbruggen et al. (2013).Verbruggen E, Van der Heijden MGA, Rillig MC, Kiers ET. Mycorrhizal fungal establishment in agricultural soils: factors determining inoculation success. New Phytologist. 2013;197:1104–1109. doi: 10.1111/j.1469-8137.2012.04348.x. [DOI] [PubMed] [Google Scholar]
- Werner & Kiers (2015).Werner GDA, Kiers ET. Order of arrival structures arbuscular mycorrhizal colonization of plants. New Phytologist. 2015;205:1515–1524. doi: 10.1111/nph.13092. [DOI] [PubMed] [Google Scholar]
- Wittneben (1986).Wittneben U. Soils of the Okanagan and Similkameen Valleys. Victoria, BC: BC Ministry of Environment; 1986. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All the code for the statistical analyses conducted for this research.
Fungal hyphal length and number of spores per gram quantified in root and soil samples.
Results of a grapevine survival survey conducted at the end of the experiment.
Columns:
id: unique vine identifier
treatment: J = juvenile, renamed “established”, N = new, renamed “co-inoculated”, G = greenhouse, renamed “pre-inoculated”
inoc: 0 = control, 1 = inoculated
status: 0 = dead or replaced, 1 = alive.
Size and fruiting measurements made on vines.
Columns: plant.id: the identifier given to each vine
treatment: J = juvenile, renamed “established”, N = new, renamed “co-inoculated”, G = greenhouse, renamed “pre-inoculated” inoc: 0 = control, 1 = inoculated
Year: year measurement was taken.
The results of our ddPCR assay on soil samples from grapevines in our experiment and the results of soil chemistry tests as conducted by Zenalytics Laboratories (Kelowna, BC).
Columns: plant.id: the identifier given to each vine.
treatment: J = juvenile, renamed “established”, N = new, renamed “co-inoculated”, G = greenhouse, renamed “pre-inoculated”
inoc: 0 = control, 1 = inoculated
ddM13S: ddPCR copy number from May 2013 soil sample
ddO13S: ddPCR copy number from October 2013 soil sample
ddO14S: ddPCR copy number from October 2014 soil sample
ddO15S: ddPCR copy number from October 2015 soil sample
ddO16S: ddPCR copy number from October 2016 soil sample
ddO17S: ddPCR copy number from October 2017 soil sample
P: Soil phosphorus result (Mehlich III extraction)
N: Soil nitrogen result (Kjedahl method)
pH: Soil pH.
GPS coordinates for each vine in the experiment. Rows were 10 ft (3.05 m) apart and vines were 4 ft (1.2 m) apart.
Columns:
ID: the identifier given to each vine
Treatment: J = juvenile, renamed “established”, N = new, renamed “co-inoculated”, G = greenhouse, renamed “pre-inoculated”
Inoculation: 0 = control, 1 = inoculated
Row: The vineyard row the vine was located in
VinePos: The number of vines down the row the focal vine was (ie. first vine in the row is 1, next is 2…)
PropRow: The distance in feet from Row 20 (the first row with experimental vines) to the row that the vine is in
PropVine: The distance in feet from the first vine in the row (ie, vine 1 is 0 feet, vine 25 is 96 feet down the row).
Data Availability Statement
The following information was supplied regarding data availability:
Raw data are available in the Supplemental Files.