Abstract
Immune checkpoint inhibitors (ICIs), including anti-CTLA-4 (cytotoxic T lymphocyte antigen-4) and anti-PD-1/PD-L1 (programmed death-1/programmed death-ligand 1), represent a turning point in the cancer immunotherapy. However, only a minor fraction of patients could derive benefit from such therapy. Therefore, new strategies targeting additional immune regulatory mechanisms are urgently needed. CD4+Foxp3+ regulatory T cells (Tregs) represent a major cellular mechanism in cancer immune evasion. There is compelling evidence that tumor necrosis factor (TNF) receptor type II (TNFR2) plays a decisive role in the activation and expansion of Tregs and other types of immunosuppressive cells such as myeloid-derived suppressor cells (MDSCs). Furthermore, TNFR2 is also expressed by some tumor cells. Emerging experimental evidence indicates that TNFR2 may be a therapeutic target to enhance naturally occurring or immunotherapeutic-triggered anti-tumor immune responses. In this article, we discuss recent advances in the understanding of the mechanistic basis underlying the Treg-boosting effect of TNFR2. The role of TNFR2-expressing highly suppressive Tregs in tumor immune evasion and their possible contribution to the non-responsiveness to checkpoint treatment are analyzed. Moreover, the role of TNFR2 expression on tumor cells and the impact of TNFR2 signaling on other types of cells that shape the immunological landscape in the tumor microenvironment, such as MDSCs, MSCs, ECs, EPCs, CD8+ CTLs, and NK cells, are also discussed. The reports revealing the effect of TNFR2-targeting pharmacological agents in the experimental cancer immunotherapy are summarized. We also discuss the potential opportunities and challenges for TNFR2-targeting immunotherapy.
Keywords: TNF, TNFR2, cancer immunology and immunotherapy, CD4+Foxp3+ regulatory T cells, tumor immune microenvironment
Introduction
Cancer immunotherapy, broadly defined as a treatment that can enhance anti-cancer immune responses by targeting certain immune cells or molecules, has achieved significant advances in the past decades and currently becomes a mainstream therapy in cancer treatment, alongside surgery, chemotherapy, and radiotherapy.1 The successful clinical trials of immune checkpoint inhibitors (ICIs), with blockade antibodies such as ipilimumab and nivolumab/pembrolizumab against CTLA-4 or PD-1 respectively, have transformed the ICIs into major therapy in the advanced non-small cell lung cancer (NSCLC) and melanoma (FDA-approved ICIs are summarized in Table 1).2,3 However, only a minor fraction of patients can obtain a complete response after such treatment.4 Moreover, by stimulating the activation of immune cells, treatment with ICIs could result in inflammatory side effects known as immune-related adverse events (irAEs).5 Treatment with these agents could also upregulate the expression of additional immune checkpoint molecules and consequently induce resistance to immunotherapy.6
Table 1.
FDA-Approved Immune Checkpoint Inhibitors (ICIs) for Cancer Treatment
| Drug (Trade Name and Company Name) | Target | Approval Date | Approved Indication | Immune-Related Adverse Events (irAEs) | Reference |
|---|---|---|---|---|---|
| Ipilimumab (Yervoy®, Bristol-Myers Squibb Co.) | CTLA-4 | 28 March 2011 |
|
|
193–195 |
| Pembrolizumab (Keytruda®, Merck & Co.) | PD-1 | 4 September 2014 |
|
|
193,194,196 |
| Nivolumab (Opdivo®; Bristol-Myers Squibb Co.) | PD-1 | 22 December 2014 |
|
|
193,196 |
| Atezolizumab (Tecentriq®, Roche) | PD-L1 | 18 May 2016 |
|
|
193,196 |
| Avelumab (Bavencio®, Merck KGaA & Pfizer) | PD-L1 | 23 March 2017 |
|
|
193,196 |
| Durvalumab (Imfinzi®, AstraZeneca) | PD-L1 | 01 May 2017 |
|
|
193,196 |
| Cemiplimab (Libtayo®, Sanofi/Regeneron) | PD-1 | 28 September 2018 |
|
|
193,197 |
The fact that TNF expression is elevated in the tumor environment and this cytokine can be further enhanced by immunotherapy has recently spurred the extensive discussion about the exact role of TNF in cancer immunology and cancer immunotherapy.7–10 TNF levels are increased in patients with irAEs, raising the possibility that TNF may play a major role in the inflammatory side effect of ICIs.11 Indeed, it was reported that the treatment with anti-TNF antibody is effective in the control of irAEs in patients.12 TNF is an extremely pleiotropic cytokine and its biological functions are mediated by one of its two surface receptors, TNFR1 (TNF receptor type I) and TNFR2. Unlike TNFR1, TNFR2 has no death domain, and its activation can lead to the activation of NF-κB (Nuclear Factor Kappa B) and cell growth.13–16 TNFR1 is ubiquitously expressed, while the expression of TNFR2 is limited to a certain type of cells, including activated T lymphocytes especially CD4+Foxp3+ Tregs, endothelial cells, and neural cells.17–21 Our previous study indicated that TNF-TNFR2 interaction plays a decisive role in the activation, expansion, function, and phenotypical stability of Treg cells.22,23 As shown by studies based on various human and murine cancer, highly suppressive TNFR2-expressing Tregs represent a major cellular mechanism in cancer immune evasion.24–27
We and others have proposed that targeting TNFR2 may be a novel strategy to enhance anti-tumor immune responses.28 In this article, we review and analyze the recent progress in the study that has advanced the understanding of the molecular mechanism underlying the Treg-boosting effect of TNF-TNFR2 signaling. Latest studies on the roles of TNF and TNFR2 in tumor immunology are introduced. The emerging concept of TNFR2-targeting treatment as a novel strategy in cancer immunotherapy and as an adjuvant to enhance the efficacy of checkpoint inhibitors are discussed.
The Decisive Role of TNFR2 Signaling in the Biology of Tregs: New Mechanistic Insight
Tregs are a specialized subset of CD4+ T cells that play a fundamental role in the regulation of immune responses and the maintenance of immune homeostasis.29–31 Impaired function or reduced number of Tregs leads to autoimmune inflammatory responses. In contrast, an increase in the number of highly suppressive Tregs promotes immune evasion of the tumor.32–35 Thus, modulation of Treg activity has important therapeutic implications in the control of physiological and pathological immune responses in clinical settings.36 For example, there is ample evidence that the expansion of antigen-specific Tregs or strengthening their suppressive activity can be useful in the treatment of autoimmune inflammatory diseases and undesirable immune responses such as rejection of transplanted organs.29,35 In contrast, decreased Treg number or suppressive activity can evoke or enhance anti-tumor immune responses or enhance anti-microbial immunity in chronic infection.34,35 Transcription factor Foxp3 (Forkhead Box P3) is a master gene that controls the functionalities of Tregs.37,38 Immunosuppressive function of Treg cells is believed to be mediated by co-inhibitory receptors such as CTLA-4, PD-1, TIM3 (T cell Immunoglobulin and Mucin Domain-containing Protein 3), LAG3 (lymphocyte-Activation Gene 3), or TIGIT (T cell Immunoglobulin (Ig) and Immunoreceptor Tyrosine-based Inhibition Motif (ITIM) domain), or immunosuppressive cytokines such as IL-10 (Interleukin-10), TGFβ (Transforming Growth Factor-beta), granzymes, and IL-35 (Interleukin-35).39–41 Nevertheless, they likely represent only part of effector molecules or functionality of Tregs, and the exact mechanism remains elusive.
We (Xin Chen and Joost J. Oppenheim) found and published the first clear evidence that TNF-TNFR2 signaling is fundamentally important for the biology of Tregs.22 We reported that TNF can enhance the expression of CD25 and Foxp3, promote the proliferation, and enhance the suppressive function of Tregs.22 Intriguingly, TNF selectively upregulates the expression of TNFR2 and other members of the TNF receptor superfamily (OX40, 4-1BB, and FAS) on Tregs.42 Tregs express markedly higher levels of TNFR2 than CD4+CD25− effector T cells (Teffs).22 TNFR1 has a stronger affinity for soluble TNF (sTNF), while membrane-bound TNF (mTNF) preferentially binds to and activates TNFR2.43,44 There is compelling evidence that mTNF-TNFR2 signaling axis favors immune tolerance, which is presumably mainly based on the activation and expansion of Treg cells.45–47 It was reported that vitamin D3, adalimumab (a humanized anti-TNF antibody), and methotrexate up-regulate the expression of mTNF by dendritic cells (DCs) and monocytes. As a consequence, upregulation of mTNF by these compounds promoted the activation and expansion of Tregs through TNFR2.45,46,48 Recently, we have reported that inhibition of two-pore channels with tetrandrine or siRNAs increases the number of CD4+Foxp3+ Tregs in an mTNF-TNFR2-dependent manner in mice. Using the mice colitis model, we showed that the expanded Tregs are attributable to the reduced colon inflammation after tetrandrine treatment.49–51 Furthermore, TNFR2 is closely associated with the immunosuppressive function of Treg cells, and the expression of TNFR2 can define the highly suppressive subset of Tregs.22,25,46,52 Moreover, the number of Tregs in TNFR2−/− mice is markedly lower than in wild-type (WT) mice, and when stimulated with the septic challenge, they fail to expand.22,23 Therefore, TNFR2 plays a role in the homeostasis of Tregs in both steady state and inflammatory responses. This biological function of TNFR2 is at least partially transduced by IκB kinase alpha (IKKα), as shown by the phenotype of mice with conditional knockout (KO) of IKKα in CD4+ T cells.53
It was shown that in vitro treatment with antagonist leads to contraction of Tregs, while treatment with TNFR2 agonist can expand Tregs and promote the phenotypical stability and enhance the function of Tregs.54–56 Mechanistically, TNFR2 agonist promotes the stabilization of Foxp3 expression through mTOR (Mammalian Target of Rapamycin) and NF-κB signal pathways.57 Previously, it was shown that activation of TNFR2 in human CD4+ and CD8+ T cells results in the proliferative expansion through the IKK/NF-κB pathway, including the activation of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT), and these pathways of TNFR2 signaling are likely applicable to Tregs.54,58–62 Further, it was also reported that mitogen-activated protein kinase (MAPK) (Extracellular Signal-Regulated Protein Kinase (Erk1/2), p38, Jun N-Terminal Kinases (JNK)) signaling pathway attributable to the activation and proliferation of naturally occurring Treg cells (nTreg) by TNF-TNFR2 interaction.53,63–67 Moreover, a recent study indicates that the stimulatory effect of TNF on Tregs is partially through an epigenetic mechanism that demethylates the foxp3 gene.68 Ex vivo activation of Treg cells by the stimulation with anti-CD3/CD28 represses the mTOR pathway and disfavors glycolysis, which contrasts with conventional T (Tconvs) cells.69–71 The suppression of glycolysis in Treg cells promotes fatty acid oxidation-fueled oxidative phosphorylation and impairs the stability and function of Treg cells.72–74 Recently, it was shown that co-stimulation of human thymus-derived Treg (tTreg) cells via CD3/TNFR2 switches to PI3K-mTOR drives glycolysis, and this helps to maintain the identity and suppressive function of tTreg.75 In contrast to glycolytic Tconvs, Tregs use a diverse metabolic program downstream of glycolysis upon TNFR2 co-stimulation. Glycolytic tTreg cells produce lactate from glucose and participate in the complete glycolytic pathway upon TNFR2 co-stimulation, while the net lactate secretion remains unaltered.75 Moreover, in contrast to glycolytic Tconvs, upon stimulation with CD3/TNFR2, the glycolytic tTreg cells markedly augment the levels of the labeled tricarboxylic acid cycle (TCA)-cycle intermediates.75 This study also shows that human blood-derived TNFR2hi effector tTreg cells exhibited high glycolytic activity.75
The role of TNF-TNFR2 interaction in the activation of nTreg is well defined, while its role in TGFβ-induced Treg cells (iTregs) remains controversial. It has been reported that TNFR2 contributes to the development of Treg cells in the thymus.76 A recent study shows that the exogenous TNF boosts the differentiation and function of iTreg via TNFR2 signaling.77 Further, this study shows that TNFR2 deficiency impairs the differentiation, proliferation, and function of iTreg cells in both in vitro and in vivo settings. In comparison, TNFR1 deficiency leads to the reduction of the differentiation of inflammatory T cells such as Th1 (T Helper Type 1) and Th17 (T Helper Type 17) cells, while the iTregs function remains intact.77 However, an earlier in vivo study showed that the suppressive function of nTreg is dependent on TNF-TNFR2 signaling, while the activation of iTreg cells does not need the TNFR2 signal.78 In the experimental mouse model of autoimmune encephalomyelitis (EAE), it was found that TNF-TNFR2 signaling impairs the function of iTregs through the activation of Akt-Smad3 (Protein Kinase B-Recombinant Human Mother Against Decapentaplegic Homolog) signaling pathways, which inhibits TGFβ-induced Smad3 phosphorylation and reduces the transcription of foxp3 genes.79 Therefore, more definitive evidence is needed to clarify the exact role of the TNFR2 signal in iTregs.
Taken together, recent studies have greatly advanced our understanding of signaling pathways required for TNFR2 in the activation of Tregs and its mechanism, as well as the molecular basis underlying the immunosuppressive function of Tregs. However, the key signaling events of TNFR2 in Tregs and transcriptional regulation of Foxp3 expression by TNFR2 signals remain elusive. These unanswered questions merit further investigation. Since TNFR2 expression identifies the highly suppressive subset of Tregs and the expression of TNFR2 by Tregs can be preferentially upregulated by TNF stimulation, this system represents an ideal model for understanding the molecular basis and definitive suppressor mechanism of Tregs.
TNFR2-Expressing Tregs: A Major Cellular Mechanism in Tumor Immune Evasion
A large number of Treg cells accumulate in the tumor microenvironment, presumably resulting from the conversion of iTregs from naïve CD4+ T cells, or metabolic adaptation, or stabilized Foxp3 expression, or response to neoantigen and self-antigen.34 The prevalence of Treg cells promotes immune evasion of cancer by suppressing tumor-reactive Teff cells.80 In tumor microenvironment (TME), Treg cells are reprogrammed and consequently acquire an activated phenotype and enhanced suppressor function.81 Elimination of Tregs activity can enhance anti-tumor immune responses by targeting several cell surface proteins, including TNFR2.22,28 TNFR2 is expressed preferentially by highly suppressive Treg cells including those in TME, and expression of TNFR2 is considered as a master control switch for the activation and expansion of Treg population.82,83 There is some experimental evidence that the TNF-TNFR2 interaction is responsible for the activation and expansion of Tregs in TME, and TNFR2-expressing Tregs represents a major cellular mechanism in immune evasion of the tumor.25,80 Therefore, we (Xin Chen and Joost J. Oppenheim) and Dr. Faustman proposed that TNFR2 may be harnessed as a druggable target to enhance anti-tumor immune responses.28,61,82,84–89 This idea is supported by a number of studies, as summarized in Table 2.
Table 2.
Effect of TNFR2-Targeting Treatment on Experimental Tumor Models
| Therapeutics Agents | Types of Tumor Model | Studies Outcomes | Reference |
|---|---|---|---|
| Anti-mouse TNFR2 antagonistic antibody (TY101) (Mouse IgG1) |
Mice colorectal cancer (CT26 & MC-38) |
|
84 |
| Anti-mouse TNFR2 agonist antibody (Y9) (Mouse IgG1) |
Various syngeneic mice tumor model |
|
159 |
| Ab1 (chimeric antibody) and Ab2 (Humanized form) (Human IgG1) |
Humanized mouse models (HT-29, MDA-MB-231, and LG1306) |
|
|
| TNFR2 antagonistic antibody | Sézary syndrome (SS) |
|
85 |
| TNFR2 blocking antibody (M861) (Mouse IgG) |
Mice colorectal cancer (CT26) |
|
88 |
| TNFR2 antagonistic antibody (IgG) |
Human ovarian cancer (OVCAR3) |
|
99 |
| Anti-mouse TNFR2 agonists (TR75-54.7 and TR75-89) (Hamster IgG) |
Mice colorectal cancer (CT26) |
|
97 |
| Azacitidine + panobinostat/azacitidine + lenalidomide | Acute myeloid leukemia |
|
178,198 |
Notes: aCure Rate (complete tumor regression and elimination): In CT26‐tumor implanted animals, TY101 alone: 55%, Anti-PD-1: 25%, and TY101+Anti-PD-1: 62%. In MC38‐tumor implanted animals, TY101 alone: 20%, Anti-PD-1: 10%, and TY101+Anti-PD-1: 70%. bComplete Response (CR): Tumor below 60 mm3 and continued to regress until the end of the study. NR: No Response. cMedian survival: Control: 22 days; Hamster IgG control mAbs: 25 days; TR75-89: 30.5 days; TR75-54.7: 36 days.
We for the first time reported that highly suppressive TNFR2+ Treg cells accumulate in the TME of mouse Lewis lung carcinoma (LLC) and 4T1 breast carcinoma.25,90 Similar observation was also made in mouse hepatocellular carcinoma and CT26 colon cancer.91 In TNFR2-deficient mice, hepatic metastases of the colon (MC-38) and lung (H-59) carcinoma are markedly reduced, accompanied by the decrease in the number of intrahepatic MDSCs as well as Tregs in sites of metastases.92 Using a luciferase-expressing variant of the syngeneic B16-F10 melanoma mouse model of lung metastasis, Chopra and colleagues reported that exogenous TNF could enhance metastasis by promoting the expansion of Treg cells through TNFR2, and deficiency of either TNF or TNFR2 on immune cells results in the reduction of lung metastasis and decrease in the numbers of Treg cells in the lungs.93
Certain human tumor cells can aberrantly express TNFR2, and tumor infiltrates are dominated by highly suppressive TNFR2+ Tregs.94,95 It was reported that Tregs in the peripheral blood of lung cancer patients and ascites in ovarian cancer patients express high levels of TNFR2, and the levels of TNFR2 expression are positively related to a more advanced clinical stage, immune invasion, and progressive metastasis.24,27,96 Tumor-infiltrating Treg cells express higher levels of TNFR2 as compared with Teff cells in surgical resection samples from three lung cancer patients.97 In patients with cervical cancer and cervical intraepithelial neoplasia, TNFR2+ Tregs are increased in both peripheral blood and tumor environment.98 Sézary syndrome (SS), a rare form of cutaneous T-cell lymphomas, is also characterized by high expression of TNFR2 on both tumor cells and Tregs.95,99 A recent study shows that the proportion of TNFR2+ cells was markedly higher in CD4+ T cells than CD19+ B cells or CD8+ T cells in the tumor-draining lymph nodes derived from breast cancer patients.100 Further, it was reported that TNFR2 expression is much higher on Tregs than Tconvs isolated from the peripheral blood of gastric cancer patients and healthy individuals.101 Moreover, the proportion of total Foxp3+ Tregs in the CD4+ T cells and the percentage of TNFR2+ Tregs increased with tumor progression and lymphatic metastasis.101
Taken together, high expression of TNFR2 is a characteristic of tumor-infiltrating Tregs that may represent a major cellular mechanism of cancer immune evasion. Since levels of TNFR2 expression on Tregs are related to the clinical pathology and metastasis status of patients with cancer, TNFR2 expression could be a promising molecular marker for the prognosis of cancer.
Expression of TNFR2 on Tumor Cells
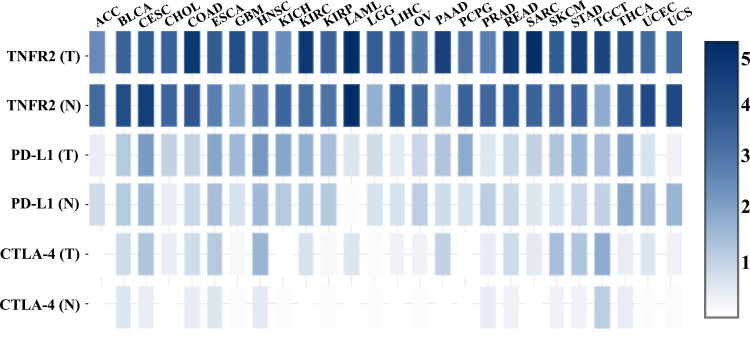
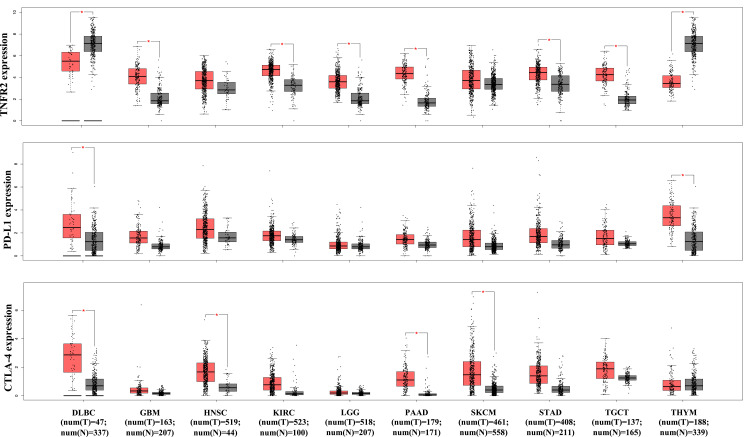
It was reported that TNFR2 is expressed by at least 25 types of tumors, including human renal cell carcinoma, multiple myeloma, colon cancer, ovarian cancer, and cutaneous T-cell lymphomas.82 A recent study of 788 commercially available human cancer cell lines from diverse cancer tissues indicates that various levels of TNFR2 are expressed, while hematopoietic and lymphoid cell lines express the highest levels of TNFR2.86 An immunohistochemistry (IHC) study of 431 tissue specimens from esophageal squamous cell carcinoma (ESCC) patients found that TNFR2 expression is higher in malignant tissue than non-tumor esophageal tissues, and the expression of TNFR2 is positively correlated with invasion depth, advanced clinical stage, low differentiation degree, and poor overall survival.102 Moreover, TNFR2 expression is strongly correlated with the prognosis of middle thoracic ESCC patients than of lower thoracic ESCC patients.102 Another IHC study from 71 primary human non-small cell lung carcinoma (NSCLC) patients and 25 normal tissue samples found that tumor tissues express markedly higher TNFR2 than normal tissues, and the rate of TNFR2hi expression in NSCLC tissue is 35%.103 Moreover, human NSCLC cell lines (A549, H1299, and H1975) also express markedly higher TNFR2 in both mRNA and protein levels than those in the normal human lung epithelial cell line (BEAS-2B).103 We analyzed TCGA (The Cancer Genome Atlas) and GTEx (Genotype-Tissue Expression) database with web-based tool Gene Expression Profiling Interactive Analysis (GEPIA, http://gepia2.cancer-pku.cn/), and found that the overall abundance of TNFR2 gene expression is markedly higher than that of PD-L1 and CTLA-4 in the most of tumor tissues (Figure 1).199 More specifically, TNFR2 gene expression is markedly higher in six human cancer types including glioblastoma multiforme, kidney renal clear cell carcinoma, brain lower grade glioma, pancreatic adenocarcinoma, stomach adenocarcinoma, and testicular germ cell tumors (Figure 2, p<0.01). In contrast, CTLA-4 gene expression is upregulated in four types of human cancer (lymphoid neoplasm diffuse large B-cell lymphoma, head and neck squamous cell carcinoma, pancreatic adenocarcinoma, and skin cutaneous melanoma), and PD-L1 gene expression is upregulated in only two cancers (lymphoid neoplasm diffuse large B-cell lymphoma and thymoma, Figure 2. P<0.01). Nevertheless, TNFR2 gene expression is lower in DLBC (lymphoid neoplasm diffuse large B-cell lymphoma) and THYM (thymoma) than in the normal control tissue (p<0.01). High expression of TNFR2 by tumor tissues is a premise to explore the possibility of tumor treatment with TNFR2-targeting agents. Future studies may need to focus on those types of human cancers with high TNFR2 gene expression.
Figure 1.
TNFR2, PD-L1, and CTLA-4 gene expression profiles across diverse human cancer and normal tissues. The transcriptomic analyses of indicated gene expression by human cancers and paired normal tissues were performed with GEPIA (Gene Expression Profiling Interactive Analysis) online database (http://gepia2.cancer-pku.cn/); Figure 1 is drawn according to specific data in the GEPIA database.199 Log-scale was set to log 2 (TPM+1) in the analysis.
Abbreviations: T, tumor; N, normal; ACC, adrenocortical carcinoma; BLCA, bladder urothelial carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL, cholangiocarcinoma; COAD, colon adenocarcinoma; ESCA, esophageal carcinoma; GBM, glioblastoma multiforme; HNSC, head and neck squamous cell carcinoma; KICH, kidney chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LAML, acute myeloid leukemia; LGG, brain lower grade glioma; LIHC, hepatocellular carcinoma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; TGCT, testicular germ cell tumors; THCA, thyroid carcinoma; UCEC, uterine corpus endometrial carcinoma; UCS, uterine carcinosarcoma.
Figure 2.
Comparison of TNFR2, PD-L1, CTLA-4 gene expression levels by human cancers and paired normal tissues. The transcriptomic analyses of indicated gene expression by human cancers and paired normal tissues were performed with GEPIA (Gene Expression Profiling Interactive Analysis) online database (http://gepia2.cancer-pku.cn/); Figure 2 is drawn according to specific data in the GEPIA database.199 Log-scale was set to log 2 (TPM+1) in the analysis. The transcriptomic analyses were performed as described in Y-axis: transcript per million. X-axis: tumor (T, red) and paired normal tissues (N, grey). The number (num) of samples is indicated. The solid black line represents medium value. The box is the upper and lower quartiles and the two lines outside the box stand for the highest and lowest expression levels. Comparison between tumor and paired normal tissue: *p<0.01 (analyzed by one-way ANOVA).
Abbreviations: DLBC, lymphoid neoplasm diffuse large B-cell lymphoma; GBM, glioblastoma multiforme; HNSC, head and neck squamous cell carcinoma; KIRC, kidney renal clear cell carcinoma; LGG, brain lower grade glioma; N, normal; PAAD, pancreatic adenocarcinoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; TGCT, testicular germ cell tumors; THYM, thymoma; T, tumor.
The functional consequence of TNFR2 expression on cancer cells has also been studied. In colorectal cancer tissue from human patients, the expression of TNFR2 is positively correlated with Ki-67 expression.104 Further, Ki-67 expression is up-regulated by overexpression of TNFR2 in SW1116 cells and inhibited by TNFR2 silence in HT29 cells.104 Importantly, TNFR2 expression is found to promote the growth and clone formation of these two lines via the PI3K/AKT signaling pathway.104 TNFR2 expression levels are also associated with drug-resistant to adriamycin in MDA-MB-231 and MCF-7 breast cancer cells by regulating the PARP (Poly (ADP-ribose) polymerase) via the AKT signaling pathway.105 It was reported that allelic polymorphisms of TNFR2 (196 M/R (methionine/arginine)-TNFR2 variation) are associated with the development of breast carcinoma: 196 M allelic variant is associated with late-onset of breast cancer in post-menopausal patients, while 196R-TNFR2 in patients with breast carcinoma is associated with increased overall disease-free survival as compared with those absences of 196R allele.106 Furthermore, a recent study found that the loss of one TNFR2 allele enhances the incidence of breast cancer in MMTV (mouse mammary tumor virus)-Wnt1 mouse model and results in a more aggressive phenotype and metastasis by activating the canonical NF-κB signaling pathway and autocrine production of TNF.107 The exact role of allelic polymorphisms of TNFR2 in tumorigenesis and progression should be further studied.
The levels of soluble TNFR2 (sTNFR2) in the malignant ovarian neoplasms are markedly higher than that in the benign ovarian neoplasms, and high levels of sTNFR2 were associated with tumor grades differentiation.108 It would be interesting to ask which types of cells are the source of sTNFR2: is it mainly released by tumor cells or other TNFR2-expressing cells including Tregs?
Other Types of TNFR2-Expressing Cells in the Tumor Environment
MDSCs
Myeloid-derived suppressor cells (MDSCs) are another type of major immune suppressors that promote immune evasion of cancer by cooperating with Tregs in TME.109–111 Interestingly, MDSCs also express TNFR2, and the expression of TNFR2 promotes survival and suppressive activity of MDSCs.18,92,112–116 In tumor-bearing TNFR2-/-mice, the development of MDSCs is impaired, and this contributes to the growth inhibition of tumor in mice deficient in TNFR2.114 It was reported that TNFR2 mediates the stimulation of mTNF in the upregulation of CXCR4 (C-X-C chemokine receptor type 4) expression on MDSCs, that is required for the infiltration of MDSCs to the tumor tissue.112 Deficiency of TNFR2 in MDSCs fails to accumulate in pre-metastatic lesions and results in the down-regulation of arginase-1 expression and reduces liver metastasis of lung cancer in mice.92 In mouse 4T1 breast cancer model, the immunosuppressive function and accumulation of MDSCs in TME as well as up-regulation of arginase-1 expression by MDSCs are dependent on TNFR2, through the activation of NF-κB and phosphorylation of p38.113
MSCs
Mesenchymal stem cells (MSCs) are another type of immunosuppressive cells that represent an important component in TME with the capacity to promote tumor growth by suppressing the activation of tumor reactive immune cells.117,118 For example, several in vivo and in vitro studies have shown that MSCs are attributable to the immunosuppressive environment in TME by inhibiting the activation and maturation of DCs, reducing the killing ability of natural killer (NK) cells, promoting Tregs expansion, and suppressing functions of Teff cells.119–121 TNF can promote the expression of immunosuppressive proteins on MSCs.122–124 MSCs-boosting effect of TNF appears to be mediated by TNFR2, since it was reported that signal of TNFR2 plays a key role in the immunosuppressive function of MSCs, including inhibition of T cell activation and pro-inflammatory cytokine production, induction of more suppressive Tregs.125 A recent study showed that the expression of TNFR2 by T cells is crucial for the induction of Tregs by MSCs.126 Moreover, TNFR2-KO MSCs also reduce their proliferation capacity, major characteristics markers (Sca1, CD90, CD105, CD44, and CD73), and functional properties (tissue/cell regeneration functions and endothelial pro-angiogenic support).127
ECs and EPCs
Endothelial cells (ECs) and their precursor endothelial progenitor cells (EPCs) play an important role in cancer-associated angiogenesis and neo-vascularization, thus promoting tumor progression and metastasis.128,129 Both ECs and EPCs express TNFR2 with a dominance of TNFR1 expression.130,131 It has been reported that TNF could increase the expression of pro-angiogenic mediators such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor, and IL-8 expression by ECs.132 There is in vivo evidence that the survival, mobilization, differentiation, and function of endothelial cells also depend on the TNF-TNFR2 signaling pathway.133,134 Intriguingly, a recent study has shown that priming EPCs with TNF markedly up-regulates TNFR2 expression on EPCs and the interaction of TNF with TNFR2 enhances the immunosuppressive function of EPCs.135,136 Blockade of TNFR2 with anti-TNFR2 monoclonal antibodies (mAbs) leads to the polarization of EPCs towards pro-inflammatory and immunogenic phenotype via TNF-TNFR1 pathway.135 Since the expression of TNFR2 is higher in human liver cancer ECs than human normal liver sinusoidal ECs,137 suggesting that TNFR2 signaling may play an important role in the pro-angiogenetic and immunosuppressive function of EPCs or ECs in the TME. This possibility should be further studied.
CD8+ Tregs
Similar to CD4+ Tregs, Foxp3-expressing CD8+ T cells with immunosuppressive activity are considered as CD8+ regulatory T cells (CD8+ Tregs).138,139 Phenotypically, CD8+CD122+ cells, CD8+CD28− cells, and CD8+CD103+ cells were reported to be CD8+ Tregs in humans or mice.140–142 It has been reported that CD8+Foxp3+ Tregs can also express TNFR2 and TNF can induce the activation, proliferation, and suppressive function of CD8+Foxp3+ Tregs in a TNF-TNFR2 dependent manner.143,144 A number of studies indicate that CD8+ Tregs could be found in the tumor microenvironment and contribute to tumor immune escape. For example, CD8+CD28− Tregs are detected in tumor tissues of patients with various cancers.145 They potently inhibit the proliferation and cytotoxicity of Teff cells in IL-10 dependent manner.145 In patients with colorectal cancer, CD8+CD25+Foxp3+ Tregs directly isolated from the tumor can potently inhibit the proliferation of CD8+CD25− Teff cells from tumor tissue.146 It is possible that TNF-TNFR2 interaction also plays a role in the activation of tumor-infiltrating CD8+ Tregs, while the exact role of TNFR2 signal in cancer immune evasion mediated by CD8+ Tregs remains to be further clarified.139,145–148
Bregs
IL-10-producing B cells with immunosuppressive function found in the experimental models of autoimmunity, parasitic infection, cancer, and transplantation indicates that regulatory B cells (Bregs) are also a player in the maintenance of immune tolerance.149–152 It was reported that TNFR2 is expressed on Bregs as well, and the activation of TNFR2 with its agonist TNC-scTNF (143N/145R) increases their production of IL-10.153 It was shown that splenic IL-10-producing CD19+CD21+ Bregs promote the development of papilloma and growth of cancer in skin carcinogenesis induced by 7, 12-dimethylbenzanthracene (DMBA) and 12-O-tetradecanoylphorbol-13-acetate (TPA).154 Interestingly, TNF secreted by tumor cells inhibits anti-tumor immune responses and promotes tumor growth by stimulating the differentiation of Breg cells in the tumor microenvironment.154 The requirement of TNFR2 signal in this process should be further studied in the future.
CD8+ CTLs
In addition, similar to immunosuppressive cells (MSCs, MDSCs, and MSCs); TNFR2 can also be expressed by tumor responsive Teff cells. For example, several studies reported that TNFR2 expression plays a crucial role in the activation, proliferation, and function of CD8+ T cells.60,155–157 The proportion of tumor-specific CD8+ T cells are markedly reduced in tumor-draining lymph nodes of TNFR2-/- mice, suggesting that the sustainability of early proliferation of CD8+ T cells depends on the activation of TNFR2.158 Furthermore, the production of IL-2 and IFNγ (interferon-gamma) by tumor-specific CD8+ T cells are reduced in TNFR2-/- mice, indicating that TNFR2 plays a role in the optimal production of effector cytokines crucial for mount anti-tumor immune responses.158 In sharp contrast, it was shown that the treatment with the antagonist of TNFR2 in tumor-bearing mice results in the activation of CD8+ T cells and long-term tumor-free survival.88,159 This may be explained by the increased survival of CD8+ CTLs, as TNF-TNFR2 signaling in CD8+CTLs can result in activation-induced cell death (AICD).157,160,161 Therefore, the TNFR2 signal in tumor responsive CD8+ CTLs is very complicated and should be further investigated.
NK Cells
Natural Killer (NK) cells, an important component of the innate system, play a pivotal role in TME due to their natural ability of cytotoxicity.162,163 It has been reported that TNFR2 is expressed on NK cells and the TNFR2 signal can induce the differentiation of NK cells.97,164 It was reported that, in response to TNF-TNFR2 stimulation, NK cells promote metastatic dissemination of B16-F10 melanoma in the mouse model.165 In contrast, it was shown that the crosstalk between DCs and NK cells in mice required TNF-TNFR2 interaction: TNFR2 expressed on the surface of NK cells is required for DC-mediated NK-cells proliferation and amplification of NK cell’s cytotoxic activity.166 Moreover, there is compelling in vitro and in vivo evidence that TNF increases NK cell-mediated IFNγ production via TNFR2 dependent manner.167
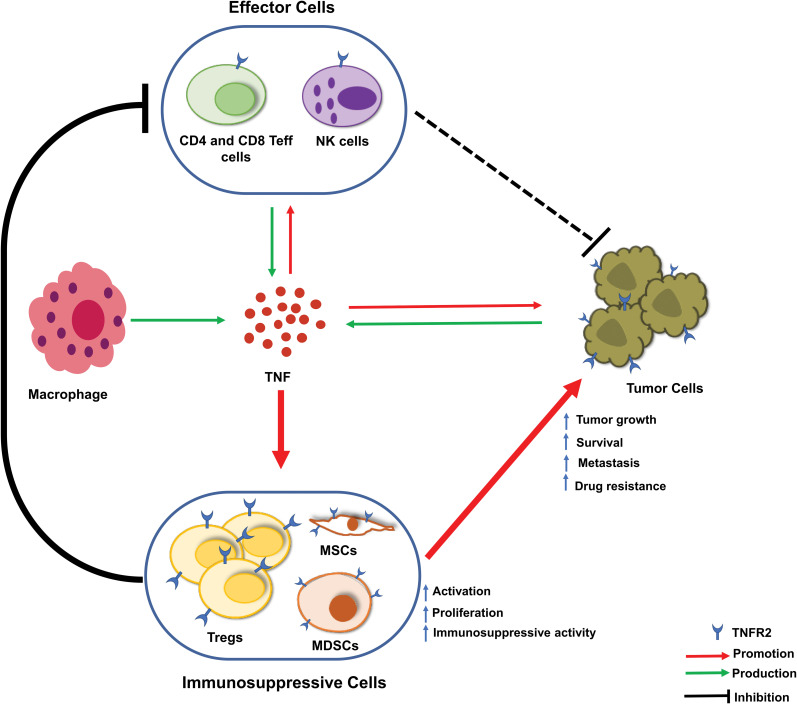
Taken together, in TME, TNFR2 is expressed on certain tumor cells and immune cells including CD4+ Treg cells, CD8+ Treg cells, CD8+ CTLs, Breg cells, NK cells, MDSCs, and other types of cells such ECs, EPCs, and MSCs. As a consequence, these cells play important roles in tumor immune evasion, tumor growth, and tumor progression (Figure 3). However, how TNF-TNFR2 signaling regulates the biological function of these cells in TME and key signaling events with mechanistic details remain elusive and should be further studied.
Figure 3.
Current understanding of the role of TNF-TNFR2 signaling in the tumor microenvironment. In the tumor microenvironment (TME), tumor-associated macrophages, effector cells (CD4+ and CD8+ T effector (Teff) cells and natural killer (NK) cells), and tumor cells are the major source of TNF. In response to TNF stimulation, the number of CD4+Foxp3+TNFR2+ Treg cells are increased. These expanded Treg cells in TME are more stable in phenotype and more immunosuppressive. Moreover, TNF activates TNFR2+ myeloid-derived suppressor cells (MDSCs) and TNFR2+ mesenchymal stem cells (MSCs). Tregs, MDSCs, and MSCs likely operate collaboratively in the inhibition of the anti-tumor immune response and the promotion of tumor evasion. Further, TNFR2 signaling also promotes the survival, metastasis, and growth of the tumor.
TNFR2-Targeting Agents in Cancer Immunotherapy
Anti-TNF Biologics
Melanoma is a prominent type of cancer that benefits most from the treatment with ICIs.8 However, approximately 1% of patients with advanced melanoma treated with ICIs develop severe colitis.8 Anti-TNF therapy is widely used to treat autoimmune diseases, such as rheumatoid arthritis (RA), Crohn’s disease, and ulcerative colitis168 and thus has been used in the treatment of irAEs. For example, infusion of infliximab, the first-generation chimeric TNF blocking monoclonal antibody, was found to effectively cure colitis induced by ICIs in most patients without affecting melanoma prognosis.169 Interestingly, TNF blockade can inhibit the AICD and consequently increase CD8+ tumor-infiltrating lymphocytes (TILs) and decrease PD-L1 and TIM-3 expression.170,171 Recently, it was reported that anti-TNF therapy through inhibition of AICD of CD8+ TILs could enhance the efficacy of combination therapy with anti-PD-1 and anti-CTLA-4 in mouse tumor models.172 To date, the exact role of TNF in cancer progression and TNF inhibitors in cancer treatment is still a matter of debate.7 The observed benefit of anti-TNF antibodies on the efficacy of ICIs in treating tumors may be at least partially attributable to the blockade of TNFR2 and this possibility should be experimentally addressed in future studies.
Thalidomide Analogs
Thalidomide and its analog lenalidomide and pomalidomide are small molecule glutamic acid derivatives and are now classified as immunomodulatory drugs (IMiDs) to treat several inflammatory diseases and tumors.173,174 Thalidomide and its analogs all belong to “class I” IMiDs, characterized as broad inhibitors of LPS-induced inflammatory cytokines like TNF, IL-6, and IL-12.175 Thalidomide and its analogs are effective in the treatment of hematological and solid malignant diseases.176 It was reported that the combination therapy with thalidomide and fludarabine is effective in the treatment of patients with chronic lymphocytic leukemia (CLL). This treatment can reduce the number of CLL and Treg cells simultaneously.177 Moreover, in acute myeloid leukemia patients, the treatment with lenalidomide reduces the number of TNFR2+ Tregs, and it is likely attributable to the reduction of TNFR2 expression on Tregs by lenalidomide.178
TNFR2 Antagonistic Antibody
Antagonistic antibodies specific to TNFR2 may be the most straightforward approach to inactivate TNFR2-positive Tregs.99 It was shown that human TNFR2-specific antagonistic antibodies that can inhibit the proliferation of Tregs while promoting the expansion of effector T cells (Teffs). The capacity of these TNFR2 antibodies in the killing of Tregs isolated from ovarian cancer ascites is stronger than that from healthy donor samples, indicating that these antibodies may preferentially act on tumor-infiltrating Tregs, which characteristically express high levels of TNFR2.99 Moreover, antagonistic antibodies can also reduce TNFR2+CD26− cells and TNFR2+ Treg cells, and expand the Teff cells in Sézary syndrome patients to corrected Treg/Teff ratios in the tumor microenvironment.85 Recently, this team also designed several new variants of human TNFR2-specific antagonistic antibody to achieve high TME specificity by killing TNFR2-expressing tumor cells and Tregs. The optimized version of anti-TNFR2 with IgG2 isoforms stabilize hinge region (disulfide double mutations at C232S and C233S) and the wide separation of antibody arms have demonstrated better TME specificity.86 Besides, they also reported that the TNFR2 expression pattern provided the rationale for antagonistic treatment effectiveness. The TNFR2 antagonistic killing activity is more potent in the cancer cell line with high TNFR2 expression than the cancer cell line with low TNFR2 expression.86 Furthermore, the combination treatment with murine‐directed anti-TNFR2 antibody (TY101) and anti-PD-1 has a greater rate of tumor regression and elimination over single treatment with either anti-TNFR2 or anti-PD-1. Even anti-TNFR2 antibody alone possesses a better anti-tumor effect than anti-PD-1 alone in the murine model of CT26 and MC38. Mechanistically, the treatment restores the Treg/Teff ratio by inducing the death of immunosuppressive Treg cells in the TME.84 We found that TNFR2 blocking antibody M861 combined with a sub-optimal dose of CpG oligodeoxynucleotide (CpG ODN) can synergistically inhibit the growth of subcutaneously transplanted mouse CT26 colon tumor by eliminating TNFR2+ Treg cells and increasing tumor-infiltrating IFNγ+CD8+ CTLs. Moreover, the combination treatment with anti-TNFR2 blocking antibody and anti-CD25 antibody also results in an enhanced inhibition of tumor growth in mouse 4T1 breast cancer model.88
TNFR2 Agonistic Antibody
“TNFR2 agonist” has been repeatedly shown to enhance the ex vivo proliferative expansion of Tregs, maintain the stability of the Treg cell phenotype, and preserve their suppressive function.55 Therefore, the restoration of the function or increase in the number of Tregs via “TNFR2 agonist” provided a rationale as a therapeutic strategy for the treatment of Type 1 diabetes (T1D), graft versus host disease (GvHD), neurodegenerative disease, and other autoimmune diseases.56,89,179–182 However, agonistic TNFR2 antibodies (Y9, and MM-401) were potent anti-tumor activity in different humanized mouse models.159,183–186 Furthermore, Y9 treatment alone elicits more potent anti-tumor activity than the treatment with anti-PD-1 alone in the WEHI-164 model, while a combination of both (Y9+anti-PD-1) showed greater survival in CT26 and EMT6 syngeneic mice tumor models.159 Interestingly, the activity of Y9 did not depend on TNFR2 expression on tumor cells or the depletion of Treg cells.159,183 MM-401, a humanized agonistic anti-TNFR2 antibody that promoted anti-tumor immunity in vitro via induction antibody-dependent cellular cytotoxicity (ADCC).184–186 It is reported that after the treatment with MM-401, the number of Treg cells in human ovarian cancer ascites is reduced.184,186 As TNFR2 agonists appear to have both anti-tumor and anti-inflammatory effects, future studies can reconcile these seemingly opposite effects.
Concluding Remarks
Recent pre-clinical studies indicate that targeting TNFR2 with either antagonistic or agonistic antibodies results in tumor inhibition by mobilizing anti-tumor immune responses, eliminating Treg activity, or stimulating the activation of CD8+ CTLs, respectively.85,99,159 A number of cell types in the tumor microenvironment can express TNFR2, such as Tregs, MDSCs, MSCs, ECs, EPCs, CD8+ CTLs, NK cells, and tumor cells themselves. The responses of these cells to TNF-TNFR2 interaction are different and could even result in the opposite effect on immune responses to the tumor (Figure 3). Thus, the cellular target and mechanism of action of TNFR2-targeting agents could be very complicated. Moreover, the in vivo effect of antibodies could be different from that observed in the in vitro experiment. For example, the biological activity of IL-2 and IL-15 can be enhanced by in vivo treatment with anti-IL-2 mAbs and IL-15Rα-Fc chimera, respectively.187 The unpredictable in vivo effect of antibodies could add an additional layer of complicity to analyze the exact action and underlying mechanism of TNFR2 specific antibodies in cancer treatment. A more carefully designed study may help clarify this issue.
TNFR2 is well known for its function in the promotion of the survival and proliferation of cells and this feature of TNFR2 is likely applicable to tumor cells that express TNFR2.188,189 This provides a strong rationale to pursue further study on TNFR2 antagonists in the treatment of tumors. Indeed, a wide spectrum of tumor cells express higher levels of TNFR2 (as discussed in “Expression of TNFR2 on tumor cells”) than paired normal tissues (Figure 1). Nevertheless, some tumors express even lower levels of TNFR2 than normal tissues (Figure 1). Thus, the levels of TNFR2 expressed by tumors should be considered in the design of the future clinical study if the TNFR2-targeting agent is designed to act directly on tumors. Furthermore, high expression levels of TNFR2 on tumor tissues may be a biomarker for TNFR2-targeting therapy, and this possibility should be assessed in future studies.
Preferentially elimination of TNFR2hi Tregs in TME is a scientific premise for the development of TNFR2-targeting agents. This is mainly based on the fact that only 30~40% of peripheral Tregs are TNFR2-expressing cells in normal mice, while the majority of mouse tumor-infiltrating Tregs are TNFR2hi cells; thus, it is reasonable to assume that inactivation or even depletion of TNFR2-expressing Tregs would not compromise the peripheral tolerance to self-antigen.25 However, TNFR2 is more broadly expressed by human Tregs in the periphery than mouse Tregs,52 therefore, the risk to trigger autoimmune inflammatory responses by TNFR2-targeting treatment in human patients should be considered in future study. In contrast, several recent studies clearly show that the treatment with TNFR2 agonistic antibodies can inhibit autoimmune inflammatory responses by promoting the activation and expansion of Tregs.181,190–192 Therefore, application of TNFR2 agonistic antibodies in cancer treatment may boost Treg activity and result in more profound immune suppression. This possibility should also be carefully assessed in the future.
Despite these unanswered questions, the current pre-clinical data encourage and support the idea that targeting TNFR2 may represent a new avenue and novel strategy in cancer immunotherapy.
Acknowledgements
This project was funded by The Science and Technology Development Fund, Macau SAR (FDCT, File No. 201/2017/A3 and 0056/2019/AFJ) and University of Macau (File No. MYRG2016-00023-ICMS-QRCM, MYRG2017-00120-ICMS, MYRG2019-00169-ICMS and CPG202-00007-ICMS).
Abbreviations
CTLA-4, Cytotoxic T Lymphocyte Antigen-4; PD-1, Programmed Death-1; PD-L1, Programmed Death-Ligand 1; TNF,Tumor Necrosis Factor; TNFR1, Tumor Necrosis Factor Receptor Type I; TNFR2, Tumor Necrosis Factor Receptor Type II; Foxp3, Forkhead Box P3; Tregs, Regulatory T cells; Teffs, Effector T cells; MDSCs, Myeloid-Derived Suppressor Cells; MSCs, Mesenchymal Stem Cells; ECs, Endothelial Cells; EPCs, Endothelial Progenitor Cells; NK cells, Natural Killer Cells; Bregs, Regulatory B cells; CTL, Cytotoxic T Cells; TIL, Tumor-Infiltrating Lymphocytes; ICIs, Immune Checkpoint Inhibitors; FDA, The United States Food and Drug Administration; irAEs, Immune-Related Adverse Events; TIM3,T cell Immunoglobulin and Mucin Domain-containing Protein 3; LAG3, Lymphocyte-Activation Gene 3; TIGIT, T cell Immunoglobulin (Ig) and Immunoreceptor Tyrosine-based Inhibition Motif (ITIM) domain; IL-10, Interleukin-10; IL-35, Interleukin-35; TGFβ, Transforming Growth Factor-beta; NF-κB, Nuclear Factor Kappa B; sTNF, Soluble TNF; mTNF, Transmembrane TNF; DCs, Dendritic Cells; WT, Wild-Type; KO, Knock-Out; IKKα, IκB Kinase alpha; Akt, Protein Kinase B; Smad3, Mothers Against Decapentaplegic Homolog 3; Th1, T Helper Type 1; Th17, T Helper Type 17; mTOR, Mammalian Target of Rapamycin; PI3K, Phosphatidylinositol 3-Kinase; Erk1/2, Extracellular Signal-Regulated Protein Kinase 1/2; MAPK, Mitogen-Activated Protein Kinase; JNK, Jun N-Terminal Kinases; Tconvs, Conventional T cells; tTregs, Thymus-derived Treg cells; TCA, Tricarboxylic Acid Cycle; nTregs, Naturally-occurring Treg cells; iTregs, Induced Treg cells; EAE, Experimental Autoimmune Encephalomyelitis; TME, Tumor Microenvironment; LLC, Lewis Lung Carcinoma; TCGA, The Cancer Genome Atlas; GTEx, Genotype-Tissue Expression; GEPIA, Gene Expression Profiling Interactive Analysis; SS, Sézary Syndrome; IHC, Immunohistochemistry; NSCLC, Non-Small Cell Lung Carcinoma; ESCC, Esophageal Squamous Cell Carcinoma; DLBC, Lymphoid Neoplasm Diffuse Large B-Cell Lymphoma; THYM, Thymoma; M/R, Methionine or Arginine; PARP, Poly (ADP-ribose) Polymerase; sTNFR2, Soluble TNFR2; CXCR4, C-X-C Chemokine Receptor Type 4; VEGF, Vascular Endothelial Growth Factor; IL-8, Interleukin-8; mAbs, Monoclonal Antibodies; AICD, Activation-Induced Cell Death; IFNγ, Interferon Gamma; IL-2, Interleukin-2; RA, Rheumatoid Arthritis; IMiDs, Immunomodulatory Drugs; IL-6, Interleukin-6; IL-12, Interleukin-12; CLL, Chronic Lymphocytic Leukemia; CpG ODN, CpG Oligodeoxynucleotide; T1D, Type 1 Diabetes; GvHD, Graft Versus Host Disease; ADCC, Antibody-Dependent Cellular Cytotoxicity; IL-15, Interleukin-15; IL-15Rα, Interleukin-15 Receptor alpha; T, Tumor; N, Normal; ACC, Adrenocortical Carcinoma; BLCA, Bladder Urothelial Carcinoma; CESC, Cervical Squamous Cell Carcinoma and Endocervical Adenocarcinoma; CHOL, Cholangiocarcinoma; COAD, Colon Adenocarcinoma; ESCA, Esophageal Carcinoma; GBM, Glioblastoma Multiforme; HNSC, Head and Neck Squamous Cell Carcinoma; KICH, Kidney Chromophobe; KIRC, Kidney Renal Clear Cell Carcinoma; KIRP, Kidney Renal Papillary Cell Carcinoma; LAML, Acute Myeloid Leukemia; LGG, Brain Lower Grade Glioma; LIHC, Hepatocellular Carcinoma; OV, Ovarian Serous Cystadenocarcinoma; PAAD, Pancreatic Adenocarcinoma; PCPG, Pheochromocytoma and Paraganglioma; PRAD, Prostate Adenocarcinoma; READ, Rectum Adenocarcinoma; SARC, Sarcoma; SKCM, Skin Cutaneous Melanoma; STAD, Stomach Adenocarcinoma; TGCT, Testicular Germ Cell Tumors; THCA, Thyroid Carcinoma; UCEC, Uterine Corpus Endometrial Carcinoma; UCS, Uterine Carcinosarcoma; RCC, Renal Cell Carcinoma; SCLC, Small Cell Lung Cancer; IgG, Immunoglobulin G; Sca1, Stem Cell Antigen-1; DMBA, 7, 12-dimethylbenzanthracene; TPA, 12-O-tetradecanoylphorbol-13-acetate.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Burugu S, Dancsok AR, Nielsen TO. Emerging targets in cancer immunotherapy. Semin Cancer Biol. 2018;52(Pt 2):39–52. [DOI] [PubMed] [Google Scholar]
- 2.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 3.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marin-Acevedo JA, Dholaria B, Soyano AE, Knutson KL, Chumsri S, Lou Y. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol. 2018;11(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. [DOI] [PubMed] [Google Scholar]
- 6.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montfort A, Colacios C, Levade T, Andrieu-Abadie N, Meyer N, Ségui B. The TNF Paradox in Cancer progression and immunotherapy. Front Immunol. 2019;10:1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montfort A, Dufau C, Colacios C, et al. Anti-TNF, a magic bullet in cancer immunotherapy? J Immunother Cancer. 2019;7(1):303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Lin Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol Sin. 2008;29(11):1275–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9(5):361–371. doi: 10.1038/nrc2628 [DOI] [PubMed] [Google Scholar]
- 11.Murakami NBT, Yamashita M, Riella LV. Severe acute interstitial nephritis after combination immune-checkpoint inhibitor therapy for metastatic melanoma. Clin Kidney J. 2016;9(3):411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ST TJ K, Trinh VA, Suarez-Almazor M, et al. Successful treatment of arthritis induced by checkpoint inhibitors with tocilizumab: a case series. Ann Rheum Dis. 2017;76(12):2061–2064. [DOI] [PubMed] [Google Scholar]
- 13.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10(1):45–65. [DOI] [PubMed] [Google Scholar]
- 14.Wajant H, Scheurich P. TNFR1‐induced activation of the classical NF‐κB pathway. FEBS J. 2011;278(6):862–876. [DOI] [PubMed] [Google Scholar]
- 15.Xie P. TRAF molecules in cell signaling and in human diseases. J Mol Signal. 2013;8(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tartaglia LA, Ayres TM, Wong GH, Goeddel DV. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993;74(5):845–853. [DOI] [PubMed] [Google Scholar]
- 17.Heinrich M, Burger D, Wang L, et al. TNFR1 and TNFR2 Expression and induction on human treg cells from Type 1 diabetic subjects. Antibodies. 2015;4(1):34–47. [Google Scholar]
- 18.Polz J, Remke A, Weber S, et al. Myeloid suppressor cells require membrane TNFR2 expression for suppressive activity. Immun Inflamm Dis. 2014;2(2):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shamdani S, Uzan G, Naserian S. TNFα-TNFR2 signaling pathway in control of the neural stem/progenitor cell immunosuppressive effect: different experimental approaches to assess this hypothetical mechanism behind their immunological function. Stem Cell Res Ther. 2020;11(1):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dopp JM, Sarafian TA, Spinella FM, Kahn MA, Shau H, De Vellis J. Expression of the p75 TNF receptor is linked to TNF-induced NFκB translocation and oxyradical neutralization in glial cells. Neurochem Res. 2002;27(11):1535–1542. [DOI] [PubMed] [Google Scholar]
- 21.Ware CF, Crowe PD, Vanarsdale TL, et al. Tumor necrosis factor (TNF) receptor expression in T lymphocytes. Differential regulation of the type I TNF receptor during activation of resting and effector T cells. J Immunol. 1991;147(12):4229–4238. [PubMed] [Google Scholar]
- 22.Chen X, Bäumel M, Männel DN, Howard OMZ, Oppenheim JJ. Interaction of TNF with TNF Receptor Type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J Immunol. 2007;179(1):154–161. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Wu X, Zhou Q, Howard OMZ, Netea MG, Oppenheim JJ. TNFR2 is critical for the stabilization of the CD4+Foxp3+ regulatory T. cell phenotype in the inflammatory environment. J Immunol. 2013;190(3):1076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Govindaraj C, Scalzo-Inguanti K, Madondo M, et al. Impaired Th1 immunity in ovarian cancer patients is mediated by TNFR2+ Tregs within the tumor microenvironment. Clin Immunol. 2013;149(1):97–110. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Subleski JJ, Kopf H, Howard OMZ, Männel DN, Oppenheim JJ. Cutting Edge: expression of TNFR2 Defines a Maximally Suppressive Subset of Mouse CD4+CD25+FoxP3+ T Regulatory Cells: applicability to Tumor-Infiltrating T Regulatory Cells. J Immunol. 2008;180(10):6467–6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Hamano R, Subleski JJ, Hurwitz AA, Howard OM, Oppenheim JJ. Expression of costimulatory TNFR2 induces resistance of CD4+FoxP3- conventional T cells to suppression by CD4+FoxP3+ regulatory T cells. J Immunol. 2010;185(1):174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan F, Du R, Wei F, et al. Expression of TNFR2 by regulatory T cells in peripheral blood is correlated with clinical pathology of lung cancer patients. Cancer Immunol Immunother. 2015;64(11):1475–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Oppenheim JJ. Targeting TNFR2, an immune checkpoint stimulator and oncoprotein, is a promising treatment for cancer. Sci Sig. 2017;10:462. [DOI] [PubMed] [Google Scholar]
- 29.Shevach EM. Regulatory T cells in autoimmmunity. Annu Rev Immunol. 2000;18(1):423–449. [DOI] [PubMed] [Google Scholar]
- 30.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. [DOI] [PubMed] [Google Scholar]
- 31.Josefowicz SZ, Lu L-F, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T Cells and Immune Tolerance. Cell. 2008;133(5):775–787. [DOI] [PubMed] [Google Scholar]
- 33.Panduro M, Benoist C, Mathis D. Tissue Tregs. Annu Rev Immunol. 2016;34(1):609–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plitas G, Rudensky AY. Regulatory T cells in cancer. Annu Rev Cancer Biol. 2020;4(1):459–477. [Google Scholar]
- 35.Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T cells and human disease. Annu Rev Immunol. 2020;38(1):541–566. [DOI] [PubMed] [Google Scholar]
- 36.Taams LS, Palmer DB, Akbar AN, Robinson DS, Brown Z, Hawrylowicz CM. Regulatory T cells in human disease and their potential for therapeutic manipulation. Immunology. 2006;118(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hori S, Nomura T, Sakaguchi S. Control of Regulatory T Cell development by the transcription Factor Foxp3. Science. 2003;299(5609):1057–1061. [DOI] [PubMed] [Google Scholar]
- 38.Hill JA, Feuerer M, Tash K, et al. Foxp3 Transcription-factor-dependent and -independent regulation of the regulatory T Cell transcriptional signature. Immunity. 2007;27(5):786–800. [DOI] [PubMed] [Google Scholar]
- 39.Azizi E, Carr AJ, Plitas G, et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell. 2018;174(5):1293–1308.e1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Simone M, Arrigoni A, Rossetti G, et al. Transcriptional landscape of human tissue lymphocytes unveils uniqueness of tumor-infiltrating T regulatory cells. Immunity. 2016;45(5):1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plitas G, Konopacki C, Wu K, et al. Regulatory T cells exhibit distinct features in human breast cancer. Immunity. 2016;45(5):1122–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamano R, Huang J, Yoshimura T, Oppenheim JJ, Chen X. TNF optimally activatives regulatory T cells by inducing TNF receptor superfamily members TNFR2, 4-1BB and OX40. Eur J Immunol. 2011;41(7):2010–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grell M, Douni E, Wajant H, et al. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83(5):793–802. [DOI] [PubMed] [Google Scholar]
- 44.Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci U S A. 1998;95(2):570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen DX, Cotton A, Attipoe L, Ciurtin C, Doré CJ, Ehrenstein MR. Regulatory T cells as a biomarker for response to adalimumab in rheumatoid arthritis. J Allergy Clin Immunol. 2018;142(3):978–980.e979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen DX, Ehrenstein MR. Anti-TNF drives regulatory T cell expansion by paradoxically promoting membrane TNF–TNF-RII binding in rheumatoid arthritis. J Exp Med. 2016;213(7):1241–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X, Oppenheim JJ. Paradoxical effects of targeting TNF signalling in the treatment of autoimmunity. Nat Rev Rheumatol. 2016;12(11):625–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kleijwegt FS, Laban S, Duinkerken G, et al. Critical role for TNF in the induction of human antigen-specific regulatory T cells by tolerogenic dendritic cells. J Immunol. 2010;185(3):1412–1418. [DOI] [PubMed] [Google Scholar]
- 49.Chen X, Oppenheim JJ. Tetrandrine (TET), an immunosuppressive component of Chinese herb, induces tolerogenic dendritic cells and consequently expands regulatory T cells. J Immunol. 2016;196(1Supplement):70.78. [Google Scholar]
- 50.He T, Yang D, Chen X, Oppenheim JJ. Tetrandrine (TET) up-regulated mTNF expression on dendritic cells and consequently induces TNFR2 mediated proliferation of Tregs. J Immunol. 2018;200(1Supplement):47.19. [Google Scholar]
- 51.He T, Yang D, Li X-Q, et al. Inhibition of two-pore channels in antigen-presenting cells promotes the expansion of TNFR2-expressing CD4+Foxp3+ regulatory T cells. Sci Adv. 2020;6(40):eaba6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen X, Subleski JJ, Hamano R, Howard OMZ, Wiltrout RH, Oppenheim JJ. Co-expression of TNFR2 and CD25 identifies more of the functional CD4+FOXP3+ regulatory T cells in human peripheral blood. Eur J Immunol. 2010;40(4):1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X, Willette‐Brown J, Wu X, et al. IKKα is required for the homeostasis of regulatory T cells and for the expansion of both regulatory and effector CD4 T cells. FASEB J. 2015;29(2):443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okubo Y, Mera T, Wang L, Faustman DL. Homogeneous expansion of human T-regulatory cells via tumor necrosis factor receptor 2. Sci Rep. 2013;3:3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He X, Landman S, Bauland SC, van den Dolder J, Koenen HJ, Joosten I. A TNFR2-agonist facilitates high purity expansion of human low purity Treg cells. PLoS One. 2016;11(5):e0156311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torrey H, Kühtreiber WM, Okubo Y, et al. A novel TNFR2 agonist antibody expands highly potent regulatory T cells. Sci Sig. 2020;13:661. [DOI] [PubMed] [Google Scholar]
- 57.Miller PG, Bonn MB, McKarns SC. Transmembrane TNF-TNFR2 Impairs Th17 differentiation by promoting Il2 expression. J Immunol. 2015;195(6):2633–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rothe M, Sarma V, Dixit VM, Goeddel DV. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 1995;269(5229):1424–1427. [DOI] [PubMed] [Google Scholar]
- 59.Carpentier I, Coornaert B, Beyaert R. Function and regulation of tumor necrosis factor receptor type 2. Curr Med Chem. 2004;11(16):2205–2212. [DOI] [PubMed] [Google Scholar]
- 60.Kim EY, Teh HS. Critical role of TNF receptor type-2 (p75) as a costimulator for IL-2 induction and T cell survival: a functional link to CD28. J Immunol. 2004;173(7):4500–4509. [DOI] [PubMed] [Google Scholar]
- 61.Chen X, Li P, Yang X, Miao X, Luo H. Tumor necrosis factor receptor II (TNFR2) promotes the growth of mouse CT26 colon cancer. J Immunol. 2018;200(1Supplement):178.177. [Google Scholar]
- 62.So T, Croft M. Regulation of PI-3-Kinase and Akt Signaling in T Lymphocytes and Other Cells by TNFR Family Molecules. Front Immunol. 2013;4:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He T, Liu S, Chen S, et al. The p38 MAPK Inhibitor SB203580 Abrogates tumor necrosis factor-induced proliferative expansion of mouse CD4+Foxp3+ Regulatory T cells. Front Immunol. 2018;9:1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Faustman D, Davis M. TNF receptor 2 pathway: drug target for autoimmune diseases. Nat Rev Drug Discov. 2010;9(6):482–493. [DOI] [PubMed] [Google Scholar]
- 65.Adler HS, Kubsch S, Graulich E, Ludwig S, Knop J, Steinbrink K. Activation of MAP kinase p38 is critical for the cell-cycle–controlled suppressor function of regulatory T cells. Blood. 2007;109(10):4351–4359. [DOI] [PubMed] [Google Scholar]
- 66.Adler HS, Steinbrink K. MAP kinase p38 and its relation to T cell anergy and suppressor function of regulatory T cells. Cell Cycle. 2008;7(2):169–175. [DOI] [PubMed] [Google Scholar]
- 67.Ohkusu‐Tsukada K, Toda M, Udono H, Kawakami Y, Takahashi K. Targeted inhibition of IL‐10‐secreting CD25− Treg via p38 MAPK suppression in cancer immunotherapy. Eur J Immunol. 2010;40(4):1011–1021. [DOI] [PubMed] [Google Scholar]
- 68.Urbano PCM, Koenen HJPM, Joosten I, He X. An Autocrine TNFα–tumor necrosis factor receptor 2 loop promotes epigenetic effects inducing human treg stability in vitro. Front Immunol. 2018;9:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Basu S, Hubbard B, Shevach EM. Foxp3-mediated inhibition of Akt inhibits Glut1 (glucose transporter 1) expression in human T regulatory cells. J Leukoc Biol. 2015;97(2):279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Menk AV, Scharping NE, Moreci RS, et al. Early TCR signaling induces rapid aerobic glycolysis enabling distinct acute T cell effector functions. Cell Rep. 2018;22(6):1509–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frauwirth KA, Riley JL, Harris MH, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16(6):769–777. [DOI] [PubMed] [Google Scholar]
- 72.Michalek RD, Gerriets VA, Jacobs SR, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186(6):3299–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gerriets VA, Kishton RJ, Johnson MO, et al. Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nat Immunol. 2016;17(12):1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Howie D, Cobbold SP, Adams E, et al. Foxp3 drives oxidative phosphorylation and protection from lipotoxicity. JCI Insight. 2017;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Kivit S, Mensink M, Hoekstra AT, et al. Stable human regulatory T cells switch to glycolysis following TNF receptor 2 costimulation. Nat Metab. 2020;1–16. [DOI] [PubMed] [Google Scholar]
- 76.Mahmud SA, Manlove LS, Schmitz HM, et al. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat Immunol. 2014;15(5):473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang S, Xie C, Chen Y, et al. Differential roles of TNFα-TNFR1 and TNFα-TNFR2 in the differentiation and function of CD4+Foxp3+ induced Treg cells in vitro and in vivo periphery in autoimmune diseases. Cell Death Dis. 2019;10(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Housley WJ, Adams CO, Nichols FC, et al. Natural but not inducible regulatory T Cells Require TNF-α Signaling for In Vivo Function. J Immunol. 2011;186(12):6779–6787. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Q, Cui F, Fang L, Hong J, Zheng B, Zhang JZ. TNF-α impairs differentiation and function of TGF-β-induced Treg cells in autoimmune diseases through Akt and Smad3 signaling pathway. J Mol Cell Biol. 2012;5(2):85–98. [DOI] [PubMed] [Google Scholar]
- 80.Woo EY, Chu CS, Goletz TJ, et al. Regulatory CD4+CD25+ T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61(12):4766–4772. [PubMed] [Google Scholar]
- 81.Whiteside TL. FOXP3+ Treg as a therapeutic target for promoting anti-tumor immunity. Expert Opin Ther Targets. 2018;22(4):353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vanamee ES, Faustman DL. TNFR2: a Novel Target for Cancer Immunotherapy. Trends Mol Med. 2017;23(11):1037–1046. [DOI] [PubMed] [Google Scholar]
- 83.Cohen JL, Wood KJ. TNFR2: the new Treg switch? OncoImmunology. 2018;7(1):e1373236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Case K, Tran L, Yang M, Zheng H, Kuhtreiber WM, Faustman DL. TNFR2 blockade alone or in combination with PD-1 blockade shows therapeutic efficacy in murine cancer models. J Leukoc Biol. 2020;107(6):981–991. [DOI] [PubMed] [Google Scholar]
- 85.Torrey H, Khodadoust M, Tran L, et al. Targeted killing of TNFR2-expressing tumor cells and T regs by TNFR2 antagonistic antibodies in advanced Sézary syndrome. Leukemia. 2019;33(5):1206–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang M, Tran L, Torrey H, et al. Optimizing TNFR2 antagonism for immunotherapy with tumor microenvironment specificity. J Leukoc Biol. 2020;107(6):971–980. [DOI] [PubMed] [Google Scholar]
- 87.He J, Li R, Chen Y, Hu Y, Chen X. TNFR2-expressing CD4+ Foxp3+ regulatory T cells in cancer immunology and immunotherapy. Prog Mol Biol Transl Sci. 2019;164:101–117. [DOI] [PubMed] [Google Scholar]
- 88.Nie Y, He J, Shirota H, et al. Blockade of TNFR2 signaling enhances the immunotherapeutic effect of CpG ODN in a mouse model of colon cancer. Sci Sig. 2018;11(511):eaan0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zou H, Li R, Hu H, Hu Y, Chen X. Modulation of Regulatory T Cell Activity by TNF Receptor Type II-Targeting Pharmacological Agents. Front Immunol. 2018;9:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen X, Yang Y, Zhou Q, et al. Effective Chemoimmunotherapy with Anti-TGFβ Antibody and cyclophosphamide in a mouse model of Breast Cancer. PLoS One. 2014;9(1):e85398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chang L-Y, Lin Y-C, Chiang J-M, et al. Blockade of TNF-α signaling benefits cancer therapy by suppressing effector regulatory T cell expansion. OncoImmunology. 2015;4(10):e1040215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ham B, Wang N, D’Costa Z, et al. TNF Receptor-2 Facilitates an immunosuppressive microenvironment in the liver to promote the colonization and growth of hepatic metastases. Cancer Res. 2015;75(24):5235–5247. [DOI] [PubMed] [Google Scholar]
- 93.Chopra M, Riedel SS, Biehl M, et al. Tumor necrosis factor receptor 2-dependent homeostasis of regulatory T cells as a player in TNF-induced experimental metastasis. Carcinogenesis. 2013;34(6):1296–1303. [DOI] [PubMed] [Google Scholar]
- 94.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+ CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163(10):5211–5218. [PubMed] [Google Scholar]
- 95.Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sezary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47(9):1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kampan NC, Madondo MT, McNally OM, Stephens AN, Quinn MA, Plebanski M. Interleukin 6 present in inflammatory ascites from advanced epithelial ovarian cancer patients promotes tumor necrosis factor receptor 2-expressing regulatory T cells. Front Immunol. 2017;8:1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Williams GS, Mistry B, Guillard S, et al. Phenotypic screening reveals TNFR2 as a promising target for cancer immunotherapy. Oncotarget. 2016;7(42):68278–68291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang T, Jiao J, Jiao X, et al. Aberrant frequency of TNFR2(+) Treg and related cytokines in patients with CIN and cervical cancer. Oncotarget. 2017;9(4):5073–5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Torrey H, Butterworth J, Mera T, et al. Targeting TNFR2 with antagonistic antibodies inhibits proliferation of ovarian cancer cells and tumor-associated Tregs. Sci Sig. 2017;10:462. [DOI] [PubMed] [Google Scholar]
- 100.Ghods A, Ghaderi A, Shariat M, Talei A-R A-R, Mehdipour F. TNFR2 but not TNFR1 is the main TNFR expressed by B and T lymphocytes in breast cancer draining lymph nodes. Immunol Lett. 2019;209:36–44. doi: 10.1016/j.imlet.2019.03.013 [DOI] [PubMed] [Google Scholar]
- 101.Niu L. Abstract 1603: TNFR2+ regulatory T cells are key players in immune escape in gastric tumor microenvironment. Cancer Res. 2020;80(16 Supplement):1603. [Google Scholar]
- 102.Yang D, Li R, Wang H, et al. Clinical significance of tumor necrosis factor receptor 2 in middle and lower thoracic esophageal squamous cell carcinoma. Oncol Lett. 2018;16(3):2971–2978. doi: 10.3892/ol.2018.8998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang YW, Chen QQ, Cao J, et al. Expression of tumor necrosis factor receptor 2 in human non-small cell lung cancer and its role as a potential prognostic biomarker. Thorac Cancer. 2019;10(3):437–444. doi: 10.1111/1759-7714.12948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao T, Li H, Liu Z. Tumor necrosis factor receptor 2 promotes growth of colorectal cancer via the PI3K/AKT signaling pathway. Oncol Lett. 2017;13(1):342–346. doi: 10.3892/ol.2016.5403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang F, Zhao N, Wu N. TNFR2 promotes Adriamycin resistance in breast cancer cells by repairing DNA damage. Mol Med Rep. 2017;16(3):2962–2968. doi: 10.3892/mmr.2017.6898 [DOI] [PubMed] [Google Scholar]
- 106.Mestiri S, Bouaouina N, Ahmed SB, Chouchane L. A functional polymorphism of the tumor necrosis factor receptor-II gene associated with the survival and relapse prediction of breast carcinoma. Cytokine. 2005;30(4):182–187. doi: 10.1016/j.cyto.2005.01.007 [DOI] [PubMed] [Google Scholar]
- 107.He L, Bhat K, Duhacheck-Muggy S, et al. Tumor necrosis factor receptor signaling modulates carcinogenesis in a mouse model of breast cancer. Neoplasia. 2021;23(2):197–209. doi: 10.1016/j.neo.2020.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nomelini RS, Borges Júnior LE, de Lima CA, et al. TNF-R2 in tumor microenvironment as prognostic factor in epithelial ovarian cancer. Clin Exp Med. 2018;18(4):547–554. doi: 10.1007/s10238-018-0508-3 [DOI] [PubMed] [Google Scholar]
- 109.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jiang J, Wang Z, Li Z, et al. Early exposure of high-dose interleukin-4 to tumor stroma reverses myeloid cell-mediated T-cell suppression. Gene Ther. 2010;17(8):991–999. [DOI] [PubMed] [Google Scholar]
- 111.Youn JI, Gabrilovich DI. The biology of myeloid‐derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40(11):2969–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ba H, Li B, Li X, et al. Transmembrane tumor necrosis factor-α promotes the recruitment of MDSCs to tumor tissue by upregulating CXCR4 expression via TNFR2. Int Immunopharmacol. 2017;44:143–152. [DOI] [PubMed] [Google Scholar]
- 113.Hu X, Li B, Li X, et al. Transmembrane TNF-α promotes suppressive activities of myeloid-derived suppressor cells via TNFR2. J Immunol. 2014;192(3):1320–1331. [DOI] [PubMed] [Google Scholar]
- 114.Zhao X, Rong L, Zhao X, et al. TNF signaling drives myeloid-derived suppressor cell accumulation. J Clin Invest. 2012;122(11):4094–4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chavez-Galan L, Vesin D, Uysal H, et al. Transmembrane tumor necrosis factor controls myeloid-derived suppressor cell activity via TNF receptor 2 and protects from excessive inflammation during BCG-induced pleurisy. Front Immunol. 2017;8:999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sade-Feldman M, Kanterman J, Ish-Shalom E, Elnekave M, Horwitz E, Baniyash M. Tumor necrosis factor-α blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity. 2013;38(3):541–554. [DOI] [PubMed] [Google Scholar]
- 117.Han Z, Jing Y, Zhang S, Liu Y, Shi Y, Wei L. The role of immunosuppression of mesenchymal stem cells in tissue repair and tumor growth. Cell Biosci. 2012;2(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Trivanović D, Krstić J, Djordjević IO, et al. The roles of mesenchymal stromal/stem cells in tumor microenvironment associated with inflammation. Mediators Inflamm. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383–396. [DOI] [PubMed] [Google Scholar]
- 120.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer–cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2, 3-dioxygenase and prostaglandin E2. Blood. 2008;111(3):1327–1333. [DOI] [PubMed] [Google Scholar]
- 121.Maccario R, Podestà M, Moretta A, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90(4):516–525. [PubMed] [Google Scholar]
- 122.Djouad F, Fritz V, Apparailly F, et al. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor α in collagen‐induced arthritis. Arthritis Rheum. 2005;52(5):1595–1603. [DOI] [PubMed] [Google Scholar]
- 123.Yan L, Zheng D, Xu R-H. Critical role of tumor necrosis factor signaling in mesenchymal stem cell-based therapy for autoimmune and inflammatory diseases. Front Immunol. 2018;9:1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miettinen JA, Pietilä M, Salonen RJ, et al. Tumor necrosis factor alpha promotes the expression of immunosuppressive proteins and enhances the cell growth in a human bone marrow-derived stem cell culture. Exp Cell Res. 2011;317(6):791–801. [DOI] [PubMed] [Google Scholar]
- 125.Beldi G, Khosravi M, Abdelgawad ME, et al. TNFα/TNFR2 signaling pathway: an active immune checkpoint for mesenchymal stem cell immunoregulatory function. Stem Cell Res Ther. 2020;11(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Naserian S, Shamdani S, Arouche N, Uzan G. Regulatory T cell induction by mesenchymal stem cells depends on the expression of TNFR2 by T cells. Stem Cell Res Ther. 2020;11(1):534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Beldi G, Bahiraii S, Lezin C, et al. TNFR2 is a crucial hub controlling mesenchymal stem cell biological and functional properties. Front Cell Dev Biol. 2020;8:1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.de la Puente P, Muz B, Azab F, Azab AK. Cell trafficking of endothelial progenitor Cells in tumor progression. Clin Cancer Res. 2013;19(13):3360–3368. [DOI] [PubMed] [Google Scholar]
- 129.Lyden D, Hattori K, Dias S, et al. Impaired recruitment of bone-marrow–derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7(11):1194–1201. [DOI] [PubMed] [Google Scholar]
- 130.Bertok S, Wilson MR, Dorr AD, et al. Characterization of TNF receptor subtype expression and signaling on pulmonary endothelial cells in mice. Am J Physiol Lung Cell Mol Physiol. 2011;300(5):L781–L789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brockhaus M, Schoenfeld H-J, Schlaeger E-J, Hunziker W, Lesslauer W, Loetscher H. Identification of two types of tumor necrosis factor receptors on human cell lines by monoclonal antibodies. Proc Natl Acad Sci U S A. 1990;87(8):3127–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yoshida S, Ono M, Shono T, et al. Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol Cell Biol. 1997;17(7):4015–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Goukassian DA, Qin G, Dolan C, et al. Tumor necrosis factor-alpha receptor p75 is required in ischemia-induced neovascularization. Circulation. 2007;115(6):752–762. [DOI] [PubMed] [Google Scholar]
- 134.Luo Y, Xu Z, Wan T, et al. Endothelial-Specific Transgenesis of TNFR2 promotes adaptive arteriogenesis and angiogenesis. Arterioscler Thromb Vasc Biol. 2010;30(7):1307–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nouri Barkestani M, Shamdani S, Afshar Bakshloo M, et al. TNFα priming through its interaction with TNFR2 enhances endothelial progenitor cell immunosuppressive effect: new hope for their widespread clinical application. Cell Commun Signal. 2021;19(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Naserian S, Abdelgawad ME, Bakshloo MA, et al. The TNF/TNFR2 signaling pathway is a key regulatory factor in endothelial progenitor cell immunosuppressive effect. Cell Commun Signal. 2020;18(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu LQ, Zhang WJ, Niu JX, et al. Phenotypic and functional differences between human liver cancer endothelial cells and liver sinusoidal endothelial cells. J Vasc Res. 2008;45(1):78–86. [DOI] [PubMed] [Google Scholar]
- 138.Cosmi L, Liotta F, Lazzeri E, et al. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood. 2003;102(12):4107–4114. [DOI] [PubMed] [Google Scholar]
- 139.Chakraborty S, Panda AK, Bose S, et al. Transcriptional regulation of FOXP3 requires integrated activation of both promoter and CNS regions in tumor-induced CD8+ Treg cells. Sci Rep. 2017;7(1):1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rifa’i M, Shi Z, Zhang S-Y, et al. CD8+ CD122+ regulatory T cells recognize activated T cells via conventional MHC class I–αβTCR interaction and become IL-10-producing active regulatory cells. Int Immunol. 2008;20(7):937–947. [DOI] [PubMed] [Google Scholar]
- 141.Colovai AI, Liu Z, Ciubotariu R, Lederman S, Cortesini R, Suciu-Foca N. Induction of xenoreactive CD4+ T-cell anergy by suppressor CD8+CD28- T cells. Transplantation. 2000;69(7):1304–1310. [DOI] [PubMed] [Google Scholar]
- 142.Lu L, Yu Y, Li G, et al. CD8+ CD103+ regulatory T cells in spontaneous tolerance of liver allografts. Int Immunopharmacol. 2009;9(5):546–548. [DOI] [PubMed] [Google Scholar]
- 143.Ablamunits V, Bisikirska B, Herold KC. Acquisition of regulatory function by human CD8+ T cells treated with anti-CD3 antibody requires TNF. Eur J Immunol. 2010;40(10):2891–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Horwitz DA, Pan S, Ou JN, et al. Therapeutic polyclonal human CD8+ CD25+ Fox3+ TNFR2+ PD-L1+ regulatory cells induced ex-vivo. Clinical Immunology (Orlando, Fla). 2013;149(3):450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Filaci G, Fenoglio D, Fravega M, et al. CD8+ CD28− T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol. 2007;179(7):4323–4334. [DOI] [PubMed] [Google Scholar]
- 146.Chen C, Chen D, Zhang Y, et al. Changes of CD4+ CD25+ FOXP3+ and CD8+ CD28− regulatory T cells in non-small cell lung cancer patients undergoing surgery. Int Immunopharmacol. 2014;18(2):255–261. [DOI] [PubMed] [Google Scholar]
- 147.Wu M, Chen X, Lou J, et al. TGF-β1 contributes to CD8+ Treg induction through p38 MAPK signaling in ovarian cancer microenvironment. Oncotarget. 2016;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Alvarez Arias DA, Kim H-J, Zhou P, et al. Disruption of CD8+ treg activity results in expansion of T follicular helper cells and enhanced antitumor immunity. Cancer Immunol Res. 2014;2(3):207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3(10):944–950. [DOI] [PubMed] [Google Scholar]
- 150.Mangan NE, Fallon RE, Smith P, van Rooijen N, McKenzie AN, Fallon PG. Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J Immunol. 2004;173(10):6346–6356. [DOI] [PubMed] [Google Scholar]
- 151.Rowe V, Banovic T, MacDonald KP, et al. Host B cells produce IL-10 following TBI and attenuate acute GVHD after allogeneic bone marrow transplantation. Blood. 2006;108(7):2485–2492. [DOI] [PubMed] [Google Scholar]
- 152.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16(2):219–230. [DOI] [PubMed] [Google Scholar]
- 153.Ticha O, Moos L, Wajant H, Bekeredjian-Ding I. Expression of Tumor necrosis factor Receptor 2 Characterizes TLR9-Driven formation of interleukin-10-producing B cells. Front Immunol. 2017;8:1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schioppa T, Moore R, Thompson RG, et al. B regulatory cells and the tumor-promoting actions of TNF-α during squamous carcinogenesis. Proc Natl Acad Sci U S A. 2011;108(26):10662–10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim EY, Priatel JJ, Teh S-J, Teh H-S. TNF receptor type 2 (p75) functions as a costimulator for antigen-driven T cell responses in vivo. J Immunol. 2006;176(2):1026–1035. [DOI] [PubMed] [Google Scholar]
- 156.Kim EY, Teh H-S. TNF type 2 receptor (p75) lowers the threshold of T cell activation. J Immunol. 2001;167(12):6812–6820. [DOI] [PubMed] [Google Scholar]
- 157.Kim EY, Teh S-J, Yang J, Chow MT, Teh H-S. TNFR2-deficient memory CD8 T cells provide superior protection against tumor cell growth. J Immunol. 2009;183(10):6051–6057. [DOI] [PubMed] [Google Scholar]
- 158.Calzascia T, Pellegrini M, Hall H, et al. TNF-α is critical for antitumor but not antiviral T cell immunity in mice. J Clin Invest. 2007;117(12):3833–3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tam EM, Fulton RB, Sampson JF, et al. Antibody-mediated targeting of TNFR2 activates CD8+ T cells in mice and promotes antitumor immunity. Sci Transl Med. 2019;11(512):eaax0720. [DOI] [PubMed] [Google Scholar]
- 160.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377(6547):348–351. [DOI] [PubMed] [Google Scholar]
- 161.Otano I, Alvarez M, Minute L, et al. Human CD8 T cells are susceptible to TNF-mediated activation-induced cell death. Theranostics. 2020;10(10):4481–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu S-Y, Fu T, Jiang Y-Z, Shao Z-M. Natural killer cells in cancer biology and therapy. Mol Cancer. 2020;19(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Salagianni M, Baxevanis CN, Papamichail M, Perez SA. New insights into the role of NK cells in cancer immunotherapy. Oncoimmunology. 2012;1(2):205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mason AT, McVicar DW, Smith CA, Young HA, Ware CF, Ortaldo JR. Regulation of NK cells through the 80-kDa TNFR (CD120b). J Leukoc Biol. 1995;58(2):249–255. [DOI] [PubMed] [Google Scholar]
- 165.Ivagnès A, Messaoudene M, Stoll G, et al. TNFR2/BIRC3-TRAF1 signaling pathway as a novel NK cell immune checkpoint in cancer. Oncoimmunology. 2018;7(12):e1386826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xu J, Chakrabarti AK, Tan JL, Ge L, Gambotto A, Vujanovic NL. Essential role of the TNF-TNFR2 cognate interaction in mouse dendritic cell–natural killer cell crosstalk. Blood. 2006;109(8):3333–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Almishri W, Santodomingo-Garzon T, Le T, Stack D, Mody CH, Swain MG. TNFα augments cytokine-induced NK Cell IFNγ production through TNFR2. J Innate Immun. 2016;8(6):617–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Monaco C, Nanchahal J, Taylor P, Feldmann M. Anti-TNF therapy: past, present and future. Int Immunol. 2015;27(1):55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lesage C, Longvert C, Prey S, et al. Incidence and clinical impact of Anti-TNFα treatment of severe immune checkpoint inhibitor-induced colitis in advanced melanoma: the Mecolit survey. J Immunother. 2019;42(5):175–179. [DOI] [PubMed] [Google Scholar]
- 170.Bertrand F, Montfort A, Marcheteau E, et al. TNFα blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat Commun. 2017;8(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bertrand F, Rochotte J, Colacios C, et al. Blocking Tumor Necrosis Factor α Enhances CD8 T-cell–dependent immunity in experimental melanoma. Cancer Res. 2015;75(13):2619–2628. [DOI] [PubMed] [Google Scholar]
- 172.Perez-Ruiz E, Minute L, Otano I, et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature. 2019;569(7756):428–432. [DOI] [PubMed] [Google Scholar]
- 173.Shortt J, Hsu AK, Johnstone RW. Thalidomide-analogue biology: immunological, molecular and epigenetic targets in cancer therapy. Oncogene. 2013;32(36):4191–4202. [DOI] [PubMed] [Google Scholar]
- 174.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4(4):314–322. [DOI] [PubMed] [Google Scholar]
- 175.Corral LG, Haslett PA, Muller GW, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-α. J Immunol. 1999;163(1):380–386. [PubMed] [Google Scholar]
- 176.Franks ME, Macpherson GR, Figg WD. Thalidomide. Lancet. 2004;363(9423):1802–1811. [DOI] [PubMed] [Google Scholar]
- 177.Giannopoulos K, Dmoszynska A, Kowal M, et al. Thalidomide exerts distinct molecular antileukemic effects and combined thalidomide/fludarabine therapy is clinically effective in high-risk chronic lymphocytic leukemia. Leukemia. 2009;23(10):1771–1778. [DOI] [PubMed] [Google Scholar]
- 178.Govindaraj C, Madondo M, Kong YY, Tan P, Wei A, Plebanski M. Lenalidomide‐based maintenance therapy reduces TNF receptor 2 on CD4 T cells and enhances immune effector function in acute myeloid leukemia patients. Am J Hematol. 2014;89(8):795–802. [DOI] [PubMed] [Google Scholar]
- 179.Atretkhany K-SN, Mufazalov IA, Dunst J, et al. Intrinsic TNFR2 signaling in T regulatory cells provides protection in CNS autoimmunity. Proc Natl Acad Sci U S A. 2018;115(51):13051–13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Faustman DL. TNF inducers, and TNFR2 agonists: a new path to type 1 diabetes treatment. Diabetes Metab Res Rev. 2018;34(1):e2941. [DOI] [PubMed] [Google Scholar]
- 181.Chopra M, Biehl M, Steinfatt T, et al. Exogenous TNFR2 activation protects from acute GvHD via host T reg cell expansion. J Exp Med. 2016;213(9):1881–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Medler J, Wajant H. Tumor necrosis factor receptor-2 (TNFR2): an overview of an emerging drug target. Expert Opin Ther Targets. 2019;23(4):295–307. [DOI] [PubMed] [Google Scholar]
- 183.Fulton RB, Camblin A, Sampson JF, et al. Abstract 3270: mechanism of action of a novel agonist TNFR2 antibody that induces co-stimulation of T cells and promotes robust anti-tumor immunity. Cancer Res. 2019;79(13 Supplement):3270. [Google Scholar]
- 184.Sampson JF, Fulton RB, Kurella VB, et al. A novel TNFR2 antibody induces T cell co-stimulation and promotes durable anti-tumor immunity. J Immunol. 2019;202(1 Supplement):195.111. [Google Scholar]
- 185.Richards J, Wong C, Koshkaryev A, et al. Abstract 4846: MM-401, a novel anti-TNFR2 antibody that induces T cell co-stimulation, robust anti-tumor activity and immune memory. Cancer Res. 2019;79(13 Supplement):4846. [Google Scholar]
- 186.Sampson JF, Kurella VB, Paragas V, et al. Abstract 555: a novel human TNFR2 antibody (MM-401) modulates T cell responses in anti-cancer immunity. Cancer Res. 2019;79(13 Supplement):555. [Google Scholar]
- 187.Votavova P, Tomala J, Kovar M. Increasing the biological activity of IL-2 and IL-15 through complexing with anti-IL-2 mAbs and IL-15Rα-Fc chimera. Immunol Lett. 2014;159(1–2):1–10. [DOI] [PubMed] [Google Scholar]
- 188.Grell M, Becke FM, Wajant H, Männel DN, Scheurich P. Tumor necrosis factor (TNF) receptor type 2 mediates thymocyte proliferation independently of TNF receptor type 1. Eur J Immunol. 1998;28(1):257–263. [DOI] [PubMed] [Google Scholar]
- 189.Naudé PJW, den Boer JA, Luiten PGM, Eisel ULM. Tumor necrosis factor receptor cross-talk. The FEBS Journal. 2011;278(6):888–898. [DOI] [PubMed] [Google Scholar]
- 190.Ban L, Zhang J, Wang L, Kuhtreiber W, Burger D, Faustman DL. Selective death of autoreactive T cells in human diabetes by TNF or TNF receptor 2 agonism. Proc Natl Acad Sci U S A. 2008;105(36):13644–13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Okubo Y, Torrey H, Butterworth J, Zheng H, Faustman DL. Treg activation defect in type 1 diabetes: correction with TNFR2 agonism. Clin Transl Immunol. 2016;5(1):e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lamontain V, Schmid T, Weber-Steffens D, et al. Stimulation of TNF receptor type 2 expands regulatory T cells and ameliorates established collagen-induced arthritis in mice. Cellular & Molecular Immunology. 2019;16(1):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers. 2020;12(3):738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–2532. [DOI] [PubMed] [Google Scholar]
- 195.Khoja L, Atenafu EG, Ye Q, et al. Real‑world efficacy, toxicity and clinical management of ipilimumab treatment in metastatic melanoma. Oncol Lett. 2016;11(2):1581–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hahn AW, Gill DM, Agarwal N, Maughan BL. PD-1 checkpoint inhibition: toxicities and management. Urol Oncol. 2017;35(12):701–707. [DOI] [PubMed] [Google Scholar]
- 197.Migden MR, Rischin D, Schmults CD, et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N Engl J Med. 2018;379(4):341–351. [DOI] [PubMed] [Google Scholar]
- 198.Govindaraj C, Tan P, Walker P, Wei A, Spencer A, Plebanski M. Reducing TNF Receptor 2+Regulatory T Cells via the Combined Action of Azacitidine and the HDAC Inhibitor, Panobinostat for Clinical Benefit in Acute Myeloid Leukemia Patients. Clin Cancer Res. 2014;20(3):724–735. [DOI] [PubMed] [Google Scholar]
- 199.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi: 10.1093/nar/gkx247 [DOI] [PMC free article] [PubMed] [Google Scholar]