Abstract
Simple Summary
Head and neck cancers (HNC) account for approximately 500,000 new cases of tumors annually worldwide and are represented by upper aerodigestive tract malignant neoplasms, which particularly arise in oral cavity, larynx, and pharynx tissues. Thus, due to the biological diversity between the upper aerodigestive organs, and to the heterogeneity of risk factors associated with their malignant transformation, HNC behavior, and prognosis seem to strongly vary according to the tumor site. However, despite to the heterogeneity which characterizes head and neck tumors, squamous cell carcinomas (SCC) represent the predominant histopathologic HNC subtype. In this sense, it has been reported that SCC tumor biology is strongly associated with deregulations within the extracellular matrix compartment. Accordingly, it has been shown that laminin plays a remarkable role in the regulation of crucial events associated with head and neck squamous cell carcinomas (HNSCC) progression, which opens the possibility that laminin may represent a convergence point in HNSCC natural history.
Abstract
Head and neck squamous cell carcinomas (HNSCC) are among the most common and lethal tumors worldwide, occurring mostly in oral cavity, pharynx, and larynx tissues. The squamous epithelia homeostasis is supported by the extracellular matrix (ECM), and alterations in this compartment are crucial for cancer development and progression. Laminin is a fundamental component of ECM, where it represents one of the main components of basement membrane (BM), and data supporting its contribution to HNSCC genesis and progression has been vastly explored in oral cavity squamous cell carcinoma. Laminin subtypes 111 (LN-111) and 332 (LN-332) are the main isoforms associated with malignant transformation, contributing to proliferation, adhesion, migration, invasion, and metastasis, due to its involvement in the regulation of several pathways associated with HNSCC carcinogenesis, including the activation of the EGFR/MAPK signaling pathway. Therefore, it draws attention to the possibility that laminin may represent a convergence point in HNSCC natural history, and an attractive potential therapeutic target for these tumors.
Keywords: head and neck cancer, oral cancer, pharyngeal cancer, laryngeal cancer, extracellular matrix, laminin, Laminin-111, Laminin-332, Laminin γ2, LAMC2
1. Introduction
Head and neck cancer (HNC) are among the most common and lethal tumors worldwide, affecting mostly men and populations from low- and middle-income countries [1,2]. The great majority of HNC originate from the squamous cells lining the mucosal epithelium in head and neck sites, being collectively named head and neck squamous cell carcinomas (HNSCC). The head and neck anatomical sites more frequently affected by the development of HNSCC are the oral cavity, pharynx, and larynx [3,4]. Together, these three anatomical sites congregate the vast majority of HNSCC cases and the major number of deaths, as previously estimated [1,5] and summarized in Table 1.
Table 1.
Estimated numbers of new cases and deaths per year worldwide for the most common types of head and neck squamous cell carcinomas.
| Type of Head and Neck Squamous Cell Carcinoma | New Cases per Year Worldwide [1,5] | Deaths per Year Worldwide [1,5] |
|---|---|---|
| Oral Cavity Squamous Cell Carcinoma | 355,000/male-to-female incidence ratio of 2:1 | 177,000 |
| Pharyngeal Squamous Cell Carcinoma | 302,000 | 159,000 |
| Laryngeal Squamous Cell Carcinoma | 177,000/male-to-female incidence ratio of 7:1 | 95,000 |
The main risk factors associated with these tumors are tobacco smoking and alcohol consumption [6]. Additionally, viral infections caused by Human Papilloma Virus (HPV) and Epstein Barr Virus (EBV) are highly associated with oropharynx and nasopharynx development, respectively [6].
HNSCC presents poor prognosis since most patients are diagnosed presenting advanced-stage tumors [7]. HNSCC treatment protocols can include surgery, radiotherapy, chemotherapy, targeted therapy, or a combination of treatments, depending on the location and stage of the tumor, patient age, and health condition [8]. The high incidence and lethality of these tumors, together with their poor prognosis, draw attention to need for deeper understanding their biology to identify potential biomarkers and therapeutic strategies to improve patient management and survival.
Molecular characterization of HNSCC has shown a great heterogeneity in molecular alterations present in these tumors, not only because of the different sites analyzed together, but also because of the low frequency of specific genetic alterations, even when a specific site is considered [9]. This characteristic hampers the development of target therapies based on the genetic alterations present in the neoplastic cells. However, numerous studies have shown the fundamental role of the extracellular matrix (ECM) during cancer development and progression, as well as its potential as therapeutic targets [10,11,12]. In fact, the relevance of ECM to cancer progression and treatment has long been established and is originated from the observation that an increase in ECM deposition occurs in the evolution of malignant neoplasms and is associated with poor patient prognosis and treatment resistance [13].
ECM includes the interstitial matrix and the basement membrane (BM) [14]. Although the interstitial matrix is composed of polysaccharides and fibrous proteins that fill the spaces between cells and acts as a sort of buffer against mechanical stresses and strains placed on the ECM [14], BM are sheet-like specialized ECM regions that surround most animal tissues [15]. BM functions are quite diverse, not only involving physical roles such as anchoring the epithelium, but also maintaining tissue integrity and acting by storing growth factors and cytokines, functioning as a bridge between physical forces and biochemical signaling [15]. The main components of BM are laminins, collagen IV, nidogens and the proteoglycans perlecan and agrine [15]. Defects in BM assembly or composition result in a multitude of human diseases. Moreover, although traditionally viewed as a protective structure to defend tissues against cancer spread and invasion, BM dysregulation is a hallmark of many cancers [16]. BM degradation by proteases, specifically matrix metalloproteinases (MMP -1, -3, -7, -9, -10, -12, -13 and -19) produced by tumor cells, such as carcinoma, is also followed by the production of their own array of molecules, used as substrates for cancer cell invasion and proliferation. Although tumor-derived BM molecules do not form a similar network as those from normal tissues, there are striking intramolecular interactions followed by dynamic modification occurring in the newly formed BM that are crucial in supporting cancer development [16]. In this sense, laminins are glycoproteins of high molecular weight (approximately 400 to 900 KDa) found in the BM of several epithelial tissues and are presented in the shape of a cross or T, formed by three interlaced chains called α, β and γ. They were first identified in Engelbreth–Holm–Swarm sarcoma, a tumor that produces large amounts of basement membrane [17]. Laminins are known to directly regulate crucial biological events associated with morphogenesis, such as proliferation, adhesion, migration, angiogenesis, and survival. However, alterations in laminin expression pattern as well as activity are associated with pathological events, for instance the carcinogenic process, since they can modulate key cellular processes thus influencing different cellular behaviors [18,19], thus increasing their relevance as a prominent therapeutic target [18]. In addition, several studies have shown that the alterations in laminin expression pattern and activity in tumor tissues are associated with patient outcomes, such as tumor invasiveness and poor prognosis, revealing its potential as prognosis biomarker [20,21].
This review will particularly focus on an important component of the BM, laminin, exploring its role in HNSCC carcinogenesis and offering possibilities for its use as a target therapy for patients with these tumors.
2. Laminin and Squamous Cell Carcinomas
In vertebrates there are five types of α chains (α1–5), three types of β chains (β1–3) and three types of γ chains (γ1–3). The three-dimensional arrangement of these chains forms two to three short arms, in their N-terminal region, a small portion of each of the chains, and a single long arm formed by the intertwining of most part of the three chains. The short arms of each chain are formed by the globular domains in addition to the laminin epidermal-growth factor-like domains [22,23,24]. On the other hand, the long arm forms only a single domain, called laminin coiled-coil domain. The C-terminal region in the long arm has five globular domains originating from the α chain, called the laminin globular domain [24,25,26,27]. In total, 18 different laminin isoforms have been found so far and they are named according to the composition of their chains. For example, the best-studied laminin is laminin-111 (LN-111) composed of the α1, β1, and γ1 chains [24].
Laminins can bind to several proteins; however, its four main transmembrane receptors are integrins, dystroglycan, syndecans, and Lutheran blood group glycoprotein. Laminin binding to these receptors is mediated mainly by the laminin globular domains. Integrins are the most studied laminin receptors. They are heteromeric membrane proteins composed of two subunits, α and β. So far, 24 integrins have been identified in mammals and α1β1, α2β1, α3β1, α6β1, α6β4, α7β1, α9β1, and αvβ3 function as laminin receptors (for a more in-depth review, see reference [28]).
Although the literature shows that all laminin isoforms are associated with carcinogenesis, most of the studies indicates that laminin 332 (LN-332) is the one most associated with malignant transformation of squamous tumors [21]. In fact, in normal stratified squamous mucosa, LN-332 expression is associated with epithelial-stromal connection where it exerts a vital role on the maintenance of the cohesion between the BM and these compartments [29].
LN-332 expression has been shown to correlate well with tumor invasiveness [30,31] and poor patient prognosis in different squamous cell carcinomas [31,32]. Overall, tumors overexpressing LN-332 normally arise from tissue sites where LN-332 was physiologically present, although there are some exceptions, such as decreases in LN-332 expression in basal cell carcinomas [33] or in breast and prostate cancers [34,35], tissues known to express LN-332 in physiological situations. Nonetheless, the molecules which compose this “aberrant” BM can interact with LN-332 and, consequently, contribute to squamous cell carcinomas development [21]. In this sense, the carcinogenic role mediated by LN-332 primarily occurs due to its bind to the integrins α3β1 and α6β4, during focal adhesion and anchoring process, respectively [36,37]. However, besides the relevance of adhesion/migration process to tumorigenesis, which are associated with the well-established interaction between LN-332 and integrins α3β1 and α6β4, it seems that the activation of EGFR and PI3K signaling pathways by LN-332 proteolytic fragments also play a central role in squamous cell carcinomas development [38,39]. Nevertheless, γ2 chain, as a single subunit, may also favor the adhesion of epithelial cells, since γ2 chain secretion is necessary for the incorporation of LN-332 into the BM [40]. Furthermore, Ogawa and colleagues demonstrated that the short arm of γ2 chain can induce cell adhesion by preventing the phosphorylation of β4 integrin, through a mechanism that involves the binding of γ2 chain to Syndecan-1 [41]. Ultimately, the key role played by LN-332 in epithelia homeostasis is also observed in squamous tissue malignization, where its functions are not restricted only to the assembly of BM, but also comprises the regulation of signaling pathways associated with adhesion, migration, and growth of malignant cells.
The role of laminin in genesis and progression of the most frequently HNSCC affected sites—oral cavity, pharynx, and larynx—is the focus of the present review and is discussed below.
3. Oral Cavity Squamous Cell Carcinomas and Laminin
Oral cavity squamous cell carcinomas (OCSCC) comprise tumors arising from different anatomical sites, such as lips, oral tongue, floor of mouth, buccal mucosa, upper and lower gums, retromolar trigone, and hard palate [42]. The main etiological factors associated with OCSCC development are tobacco smoking and alcohol consumption, and in Asian countries, betel nut and tobacco chewing have also been associated with these tumors [2,43,44]. Tumor clinical stage at diagnosis greatly impacts on patient prognosis and, unfortunately, OCSCC are usually detected at late stages, when metastasis is frequently detected, conferring poor overall survival to patients [42].
In this sense, ECM seems to play an important role in oral cavity carcinogenesis [21], and the importance of laminin in OCSCC natural history has been increasingly acknowledged during recent decades. Initially, alterations in the expression pattern of distinct laminin were observed in the malignant transformation, conferring an aberrant laminin pattern in these tumors [45,46,47]. Indeed, this aberrant pattern of laminins expression was associated with the worst OCSCC patient prognosis, with laminin overexpression being associated with increased tumor size, presence of lymph node invasion, and treatment resistance, among other parameters [48,49,50], indicating that the presence and deposition pattern of LN-332 may be a potential prognosis marker [51,52,53,54].
However, due to the heterotrimeric structure of LN-332, its biological relevance in oral cavity cancer progression seems to be more complex, since it is known that each subunit possesses its own intrinsic activity [40]. To this extent, it has been consistently reported that the aberrant levels of LN-332 γ2 chain are associated with the invasive phenotype of oral cavity tumor cells, since early local invasion [55,56,57]. Furthermore, immunohistochemistry and morphological analysis of oral cavity carcinomas have previously revealed that the LN-332 γ2 chain is actively expressed in tumor-budding areas surrounded by myofibroblasts, indicating that the γ2 chain may act as a mediator of the invasion process through stromal compartment [58]. This is in accordance with the positive association between overexpression of the LN-332 γ2 chain and decreased survival rates of OCSCC patients observed in the study of Gasparoni and colleagues [59]. Furthermore, among a panel of 131 OCSCC prognosis biomarker candidates, the gene that encodes the γ2 chain (LAMC2) was the one that presented the best performance, being associated with the worst prognosis subtypes [60].
In addition to its prognosis potential, the LN-332 γ2 chain may also be useful as an early diagnosis marker for OCSCC, since it has been shown that dysplastic cells from the oral cavity display high levels of γ2 subunit [61]. Additionally, by using large-scale gene expression analysis, Chen and colleagues showed that LAMC2 represents one of the most promising genes to predict the onset of oral cavity squamous tumors [62].
The association between the expression of the LN-332 γ2 chain and the invasive and metastatic processes of OCSCC has been reported not only by translational studies, but also by functional analysis that showed that the expression of the γ2 chain is associated with enhanced migration and invasion processes [18]. Contrarily, Yuen and colleagues observed that the abrogation of γ2 chain expression was able to increase the invasive capacity of OCSCC cell lineages. Nevertheless, the mechanisms underlying this phenomenon was not reported by the authors [63]. In accordance with Jourquin and colleagues, Oku et al. further demonstrated that claudin-1 leads to an increase in the expression of matrix metalloproteinase 2 (MMP-2) and membrane type 1 matrix metalloproteinase (MT1-MMP), as well to the cleavage rate of laminin γ2 chain by these proteases, inducing the invasive phenotype of OCSCC cells [64]. Therefore, the role of the γ2 chain during the migration of malignant oral cells seems to result from its different assembly modes: present in BM as part of LN-332 structure or as a single chain [65]. In this way, it is known that LN-332 binding to distinct integrin complexes, such as α2β1, α3β1, and α6β4 during the anchoring of epithelial cells represents a fundamental step in cell migration [66]. For instance, the binding of LN-332 to α3β1 integrin is a central event in cell–cell adhesion mediated by e-cadherin, which culminates in the decrease of epithelial cells motility [67]. This is in accordance with the data produced by Yuen and colleagues showing that the abrogation of γ2 chain expression could weak cell–cell adhesion and, in turn, favor cell motility [63]. In addition, cell–cell adhesion also represents a crucial regulator of γ2 chain activity, since the destabilization of tight junction, because of claudin-1 silencing, can decrease the expression of metalloproteinases, such as MT1-MMP and MMP-2, leading to the decrease of the cleavage and activation of γ2 chain [64]. Furthermore, the decrease of cell–cell adhesion due to the down-regulation of e-cadherin expression during the epithelial–mesenchymal transition (EMT) also induces the up-regulation of the γ2 chain and increases cellular migration [68]. Moreover, it is already known that besides representing a mandatory step during EMT, the activation of the transcription factor Snail is also able to down regulate the expression of claudin-1 [69]. Thus, the adhesion weakness promoted by the activation of Snail/EMT program seems to be a central pathway involved in the regulation and activation of the γ2 chain, since this pathway could play a dual regulation of γ2 chain expression/activation. This is because low cadherin expression associated with the EMT program could induce overexpression of the γ2 chain, while Snail activation during the EMT program may down regulate claudin-1 expression, which, in turn, culminates with a decrease in γ2 chain activation.
Moreover, the γ2 chain binding to EGFR may also represent a critical event during tumor progression [12,70]. In this sense, it was already reported that the release of a fragment called DIII after the degradation of the γ2 chain by MMP2 and MT1-MMP can bind to EGFR, culminating not only in a positive regulation of cell motility, but also in the activation of the MAPK pathway [38]. These data sound interesting since the overexpression of the γ2 chain before the invasion of OCSCC tumors [55,56,57] is also associated with the proliferation of oral malignant cells due to the triggering of the MAPK pathway by the γ2 chain DIII fragment. In addition to confirming the relationship between EGFR/MAPK signaling pathways and γ2 chain expression, an elegant study produced by Degen and colleagues demonstrated that the overexpression of the γ2 chain itself may force the activation of EGFR/MAPK signaling [70]. Moreover, functional analysis involving the inhibition of EGFR, by using siRNA approach or its chemical blockage, reinforced the association between EGF pathway and γ2 chain in tumor progression [20]. Therefore, since it was previously reported that amplification of EGFR gene is associated with the overexpression of the γ2 chain in OCSCC [71], it is reasonable to think that a loop mechanism may be involved in the aberrant expression of the γ2 chain in OCSCC development. In this sense, the amplification of EGFR gene may induce the expression of the γ2 chain that, in turn, would hyperactivate the EGFR/MAPK signaling pathway, besides inducing migration and growth of malignant cells, also in a feedback circuit, by increasing γ2 chain expression.
Lately, several studies have shown that the biology of HNSCC could be strongly influenced by miRNAs [72]. In this sense, after identifying that the down-regulation of miR-29s family represents a molecular signature associated with HNSCC, Kinoshita and colleagues showed that γ2 chain is a target of miR-29s. The induction of miR-29s can repress γ2 chain expression, reverting the invasive phenotype of HNSCC cell lines [73]. Furthermore, in a similar way, upon ectopic expression of miR-134 in OCSCC cell lines, it was observed that the down-regulation of LAMC2 culminates in the inactivation of PI3K/AKT signaling [74], which seems to reinforce the involvement of γ2 chain in the activation of MAPK pathway. Furthermore, it was reported that the long non-coding RNA LINC00511 may act as a decoy to the miR-765 in the tongue squamous cell carcinoma (TSCC), avoiding the negative regulation of LAMC2 expression by miR-765 as well the malignant progression of TSCC cells [75]. Finally, it seems that the influence spectrum of γ2 chain in oral squamous tumor development is not limited to the regulation of malignant cell behavior per se, since the release of extracellular vesicle enriched in the γ2 chain by OCSCC cells is able to stimulate the lymphatic endothelial cells to promote the lymphangiogenesis, which represents an important route during metastasis establishment [76].
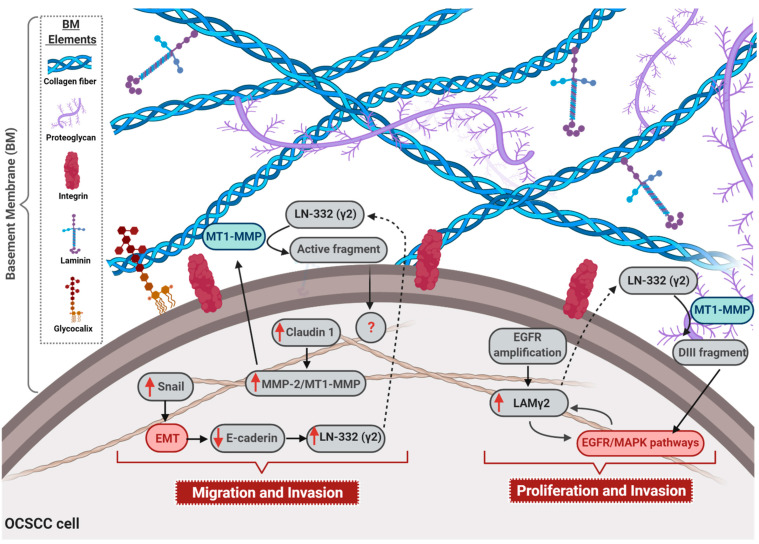
Even though the main evidence points to the γ2 chain as a major player in OCSCC development, some studies have revealed that α3 and β3 chains may also be involved in the carcinogenesis of the oral cavity [77,78]. Moreover, despite the relevance of LN-332, the identification of LN-111-derived peptides has reinforced the importance of laminin “active fragments” in the biology of oral cavity cancers, since it was shown that the LN-111-derived peptide, AG73, positively modulates the migration and invasion of OCSCC cell lines by triggering an axis which involves syndecan-1 and β1 integrin receptors, as well as the secretion of matrix metalloproteinase protein 9 (MMP-9) [79]. In addition, activation of β1 integrin, Src, and ERK 1/2 signaling pathways by LN-111-derived peptide C16 seems to be essential for the invasion of OCSCC cells, due to the regulation of invadopodia activity [80]. The mechanisms through which laminin is involved in the OCSCC malignant phenotype acquisition are summarized in Figure 1.
Figure 1.
Role of laminin in oral cavity squamous cell carcinomas (OCSCC) progression. The figure illustrates the main mechanisms through which laminin is involved in OCSCC malignant phenotype acquisition. Up-regulation of the transcription factor Snail activates epithelial–mesenchymal transition (EMT), and consequently e-cadherin down-regulation, which enhances laminin 332 (LN-332) γ2 chain expression. Additionally, overexpression of claudin-1 leads to an increase in the expression of matrix metalloproteinase 2 (MMP-2) and membrane type 1 (MT1)—MMP, as well as to the cleavage rate of LN-332 (γ2) by these proteases, culminating in the migration and invasion of OCSCC. Also, EGFR gene amplification is associated with overexpression of γ2 chain (LAM γ2) that, in turn, hyperactivates EGFR/MAPK signaling pathway and, in a feedback circuit, increases LAM γ2expression. Moreover, the secreted LN-332 (γ2) is cleaved by MMP2 and MT1-MMP, generating the DIII fragment that can bind to EGFR, ending up in the activation of EGFR/MAPK pathway. Finally, the activation of EGFR/MAPK signaling pathway enhances proliferation and invasion rates of OCSCC cells. Dotted arrows: molecules secreted by OCSCC cells; solid arrows: activation of cellular events or intracellular signaling pathways; red solid arrow: overexpressed molecules; red boxes: activated signaling pathways; blue boxes: cleavage by MT1-MMP. BM = basement membrane.
Thus, the involvement of laminin in the progression of OCSCC, through the modulation of key signaling pathways and its consequent impact on crucial cellular mechanisms such as EMT, migration, and invasion is quite clear and justifies its envisagement as a potential therapeutic target.
4. Pharyngeal Squamous Cell Carcinomas and Laminin
Pharyngeal tumors comprise neoplasms originating from nasopharynx, oropharynx, and hypopharynx. Nasopharyngeal cancers are the most common among them, accounting for approximately 40% of the pharyngeal tumors, whereas oropharyngeal tumors represent around 30% of them, as well as hypopharyngeal cancers [1]. The great majority of oropharyngeal and hypopharyngeal tumors are squamous cell carcinomas whose development is associated with heavy tobacco and alcohol consumption [81,82]. Another important risk factor associated with the development of oropharyngeal squamous cell carcinomas (OPSCC) is the infection caused by HPV. In recent decades, the incidence of OPSCC associated with tobacco and alcohol abuse has declined gradually worldwide, due to anti-tobacco policies, while the incidence of OPSCC associated with HPV infection is increasing [82]. Similarly, nasopharyngeal tumors are mostly represented by squamous cell carcinomas (NPSCC), nevertheless, the main risk factor associated with their development is the infection with EBV [83,84]. Thus, despite the differences in their site of origin and associated risk factors, pharyngeal tumors are mostly squamous cell carcinomas and, thus, associated with aberrant BM deposition and loss of its structural and functional integrity. Alteration in the expression or deposition of laminin has been extensively investigated as markers of loss of integrity of the BM. In NPSCC, as cited previously, most of the tumors are associated with EBV infection [85,86,87], and part of its transforming potential occurs through the expression of LMP1 oncoprotein. This protein induces cell motility and invasion by activating several different signaling pathways and MMPs, among others. In this sense, induced LMP1 infection in a NPSCC cell line triggered LAMC2 overexpression, as well as that of integrin α6 [88], suggesting that the development of NPSCC is linked to laminin presence. Furthermore, the ectopic expression of the viral oncogenes LMP1, LMP2a, and LMP2b in a normal keratinocyte cell line enhanced epithelial thickness and vacuolization. Additionally, upon ectopic expression of LMP1, LMP2a, and LMP2b, LN-332, whose expression is normally restricted to the basal layer of normal keratinocytes, is strongly detected in the suprabasal layers. This phenomenon is also observed for integrin α6, β4, α3, α5, β1 and other cell adhesion molecules [89], suggesting that EBV infection leads to an altered cell adhesion molecule expression in keratinocytes, compatible with nasopharyngeal carcinomas, and that alteration in laminin is tightly involved in this process.
Additionally, epigenetic alterations are also involved in the differential expression of laminin in NPSCC. Sengupta and colleagues reported a significant up-regulation of LN-332 γ1 chain, as well as seven types of collagen, in nasopharyngeal carcinoma, when compared to non-malignant nasopharyngeal epithelia. The mechanism underlying LN-332 γ1 chain induction is the down-regulation of miR-29c, which specifically targets γ1 and different collagens differentially expressed in NPSCC [90].
Alterations in laminin are also present in OPSCC. Ricci and coworkers demonstrated that LN-332 is aberrantly expressed in oropharyngeal carcinomas. Specifically, while in non-tumor oropharyngeal mucosa the expression of LN-111, LN-332 and collagen IV is displayed as linear and continuous in the BM, in OPSCC this pattern shifts to irregular with loss of linear distribution and fragmentation. In addition, integrin distribution pattern is also irregular and diffuse, being this molecule known for binding to LN-332. Importantly, the observed disruption of basal lamina enhances the invasive and metastatic potential of OPSCC [91], suggesting that the differential laminin expression pattern in tumors is associated with patients’ worst prognosis.
Regarding hypopharynx squamous cell carcinoma (HPSCC), Frenette and coworkers analyzed the involvement of laminin in the metastatic process. In this study, laminin expression pattern was assessed in primary HPSCC, in recurrent or metastatic hypopharyngeal carcinomas, and in non-malignant human keratinocytes. The authors did not observe any significant differences in the levels of laminin expression in the different samples evaluated, nevertheless, they observed that non-malignant keratinocytes secreted lower levels of laminin when compared to tumor samples. Furthermore, HPSCC samples secreted and shed most of the laminin produced and this phenomenon was positively associated with the aggressiveness of the tumor [92]. Still in HPSCC, LN-332 expression was evaluated in a set of samples and its presence was detected in over 90% of the HPSCC investigated, being most of them classified as high LN-332 expression. Additionally, LN-332 was predominantly present in the invasive front of the tumor mass and its expression was positively associated with high infiltrative tumors and presence of vascular invasion. Of note, these were poor prognosis parameters, negatively impacting HPSCC patient overall survival. Finally, other tumor markers were evaluated in the same set of samples, such as E-cadherin, β-catenin and IL-6, and none of them presented significant association with patient clinicopathological parameters or prognosis [93].
Therefore, despite the scarce literature, the data produced so far points out to the role of laminin in the development and/or progression of pharyngeal carcinomas, and consequently, the impact on patient prognosis.
5. Laryngeal Squamous Cell Carcinoma and Laminin
Laryngeal tumors represent another very common HNC. Approximately 96% of laryngeal cancers are LSCC [94]. It has been reported that the incidence of LSCC is associated with modern lifestyle factors, including smoking and alcohol consumptions [95]. Furthermore, due to inefficient early diagnosis and prognosis methods, recurrence and metastasis remain the principal causes of death from LSCC [96,97]. Therefore, it is of great importance to find effective early diagnostic indicators and to establish more reliable treatment strategies for LSCC. Among such strategies are the studies that investigate changes in BM. Although early observations had considered BM as a protective structure against cancer spread and metastasis [16], it is now accepted that BM role in cancer invasion and tumor development is much more complex [98].
Modifications in BM structure, particularly those involved with its disorganization or dissolution have been correlated with the biological course of tumors [99], including LSCC, where loss of BM constituents, such as collagen VII in general, has been associated with prognostic factors [100,101,102]. However, tumor cells can secrete their own BM molecules, also used as substrates for invasion and proliferation [98]. One of these molecules is LN-332, which has been shown to be highly expressed in several squamous tumors such as cutaneous, oral and LSCC [21,30,31,32,103]. Particularly for LSCC, Hagedorn and coworkers [102] conducted a study using 26 different carcinomas staged between T1 and T4, with half presenting lymph node metastasis and the majority with diffuse infiltration pattern as poorly differentiated carcinoma. The authors observed the presence of LN-332 in the BM zone of all cases evaluated as well as within tumor cells at the tumor invasion front, indicating a disturbed synthesis and non-polarized distribution of LN-332. These observations agree with Kainulainen et al. [50] and Matsui et al. [104] who also described high expression of LN-332 in oral squamous cell carcinoma, at the tumor-stroma-interface. Although no significant correlation between staining of LN-332 and other clinicopathological parameters and/or prognostic significance could be established. Ricci et al. [91] demonstrated that partial or intense fragmentation of the BM zone are followed by greater loss of the linear distribution of LN-332, which could be considered an additional marker of tumor aggressiveness with increasingly poor prognosis, although they also observed that oropharyngeal carcinomas were found to spread and metastasize more rapidly than laryngeal carcinomas.
High levels of LN-332 could promote the proliferation of tumor cells expressing their interacting integrin and/or non-integrin receptors and should also have a greater metastatic capacity, especially when BM fragmentation exists [105]. It has been suggested that an increase in LN-332 expression (particularly the chain) is associated with tumor invasion. Pyke and collaborators [106] reported similar observations in colon adenocarcinoma, while Nordermar et al. [32] examined 38 different patients with in situ squamous epithelial carcinomas (CIS) of the larynx and observed that 100% of the CIS lesions that progressed into invasive cancer were LN-332 chain positive, while only 37% that did not progress to invasive carcinoma showed positivity. Thus, the LN-332 positive laryngeal CIS lesion indicates a high risk of the progression to invasive cancer.
Apart from LN-332 chain overexpression, other studies have associated the 67-kDa laminin receptor (LR), a nonintegrin receptor, with the metastatic phenotype and poor prognosis in a variety of tumors [107], including LSCC [108]. Zhou and coworkers [108] demonstrated that the expression of LR positively and significantly correlated with the extent of differentiation of LSCC, suggesting the role of this receptor in the proliferative process of this tumor. Moreover, LSCC tumors with cervical metastases expressed higher amounts of LR when compared to those without metastatic features, indicating that LR together with LN-332 could play a critical role in the process of tumor invasion and metastasis. Finally, the authors also speculated that blocking LR expression could be useful to inhibit LSCC tumor aggressiveness at early stage and tested this hypothesis in vitro using a cell lineage derived from a previous LSCC. They showed that the monoclonal antibody against LR inhibited the adhesion of cells to laminin substrates and reduced the invasive capability of the cells to matrigels, suggesting that LR may participate not only in cell migration but also in cell adhesion and matrix dissolution, the three steps involved in tumor invasiveness. Indeed, other studies have shown the crucial role of LR. It has been demonstrated that the binding of tumor cells to laminin induce cells to increase production of LR mRNA [109]. Moreover, binding of LR to laminin induced the secretion of BM degrading enzymes, such as type IV collagenase [110]. In addition, it has been found that LR activated proteolytic enzymes and promoted tumor invasion after ECM degradation [111].
Finally, we must emphasize that this research area still needs to be further investigated. There are still some controversies, such as investigations that did not observe correlations between laminin expression and increases in LSCC metastasis to the neck [112]. It is clear that differences in ECM components and composition have a strong impact on LSCC development and progression. Thus, it is necessary to increase investigations to go ahead unraveling the mechanisms involved to help define possible targets for clinical intervention.
6. Laminin as a Therapeutic Target for HNSCC—Future Perspectives
The rising understanding of the mechanisms through which laminin enhances proliferation, migration, and invasion of HNSCC cells, thus contributing to cancer progression, indicates that its suppression may be an interesting therapeutic target for these tumors.
In this context, different strategies could be envisaged to generate potential laminin-based anticancer therapy. For instance, antagonists/neutralizing antibodies could be a promising strategy to block the “oncogenic” effects of laminin, as well as the development of recombinant laminins able to elicit cellular adhesion, but not motility. Also, the generation of molecules that promote the degradation of laminin in tumors could be an intriguing therapeutic strategy. Clearly, one of the challenges of such approaches would be to direct the inhibition effects over laminin expression and/or activity specifically to tumor cells, but not to the healthy ones. Yet, considering HNSCC, LN-111 and LN-332 should be the preferential targets, since these laminins seem to be the most involved with their progression. Of note, the use of a LN-332 antagonist antibody was already tested in skin squamous cell carcinomas (SSCC) demonstrating encouraging and anti-tumor specific results [113]. Additionally, the inhibition of LN-332 expression and/or its functions in tumors, by using synthetic peptides or pharmaceutical reagents, has been investigated and indicated promising outcomes [114,115,116].
In addition to direct target laminin, other molecules intrinsically involved in the activation and/or function of laminin in HNSCC could also be the target of a potential anti-laminin therapy. In this sense, the 37 kDa laminin receptor precursor (LRP)/67 kDa high-affinity LR also represents an attractive target. LRP/LR is a transmembrane receptor that presents high affinity to LN-111 [117], which is one of the main laminins involved in head and neck carcinogenesis. LRP/LR is overexpressed in various cancer types [118] and associated with enhanced tumor invasive potential [107]. Additionally, LR plays an important role in LSCC carcinogenesis, as discussed above. Currently, there are patented approaches targeting LRP/LR as anti-tumor therapy, such as monoclonal antibodies and small interfering RNAs that have already demonstrated to be effective in preventing cell adhesion, invasion and survival in different tumor types [119].
MMPs are also crucial for laminins activation and could also be suggested as an interesting target for anti-laminin therapy. In fact, in recent decades, MMPs have been suggested and evaluated as potential therapeutic target for distinct diseases due to their crucial role in ECM remodeling, but without high success rate. The main reason for this is the fact that there are 23 different MMPs in humans, sharing high structural homology and substrate specificity overlap, but without functional redundancy. This means that each MMP is unique concerning the effect caused on ECM properties [120]. Thus, MMPs inhibitors usually affect more than one metalloproteinase, due to their structural homology, generating undesired off-target effects. Nevertheless, in recent years, the knowledge of MMPs activity has greatly improved, enabling the development of the next-generating MMP inhibitors that are highly specific and capable of discriminating between homologous MMPs. The next-generating of MMP inhibitors includes small molecules and antibody-based inhibitors that unveil new potential anti-laminin therapeutic opportunities [121].
However, it seems that laminin may influence the therapeutic response to classical and new treatment approaches, since it has been reported that laminin could play a key role in chemotherapy and immunotherapy treatments. It was already reported that the ratio between laminin 411 and laminin 511 may affect the migration and polarization of leukocytes that, in turn, could disturb the shift from the immunotolerant to immunoreactive state, thus indirectly impacting on immunotherapy response [122]. However, as previously discussed, laminin 332 and its γ2 chain represent the main laminin subtype associated with natural history of squamous tumors from head and neck [21]. In this way, the study performed by Ly and colleagues shed some light on the mechanisms involved in the immune responsiveness of anti-PD-1 therapy, since it clarifies the intimal association between TGF-β1 and LN-332 γ2 expression, in lung and esophageal cancer patients anti-PD-1 therapy escape, due to a complex circuit which involve the triggering of c-Jun N-terminal kinase (JNK) and AP1 [123]. Moreover, these data seem to reinforce the relevance of the tumor microenvironment during the therapeutic management, since they reveal that the immunosuppressive cytokine TGF-β1 released by cancer-associated fibroblasts (CAFs), was able to induce the overexpression of LN-332 γ2 that, in turn, blocked the entering of T cells in the tumor tissue. This culminates with poor response to immunotherapy mediated by PD-1 blockage. Finally, alterations in the expression pattern of laminin after chemotherapeutic approach [124,125], may also reveal its influence on this therapy, otherwise, no mechanism has been already described until the present moment.
Finally, the feedback activation loop between LAMγ2 and EGFR signaling pathway in OCSCC (summarized in Figure 1) points out the opportunity of using EGFR anti-tumor target therapy as, or in combination with, an anti-laminin approach. Of note, one of the few currently available molecular target therapies for HNSCC is the EGFR monoclonal antibody, cetuximab. It works as a radiosensitizer and is employed alone or in combination with chemotherapy. Nevertheless, cetuximab, and other EGFR target drugs, still do not reach high efficiency rate for HNSCC treatment [118,126]. Considering the multimodal therapeutic approach preconized for HNSCC, one could suggest that the addition of another molecular target capable of modulating EGFR/MAPK signaling pathway—laminin-based therapy—could improve their efficacy and, thus, patient management and prognosis.
Taken together, this review summarizes the main data that leads to the proposition that laminin is a feasible and potential therapeutic target to be further investigated and explored for HNSCC patient treatment. Laminin has already been suggested as a key therapeutic target for other tumor types [20,119,127], reinforcing its potential as anti-tumor therapy.
7. Conclusions
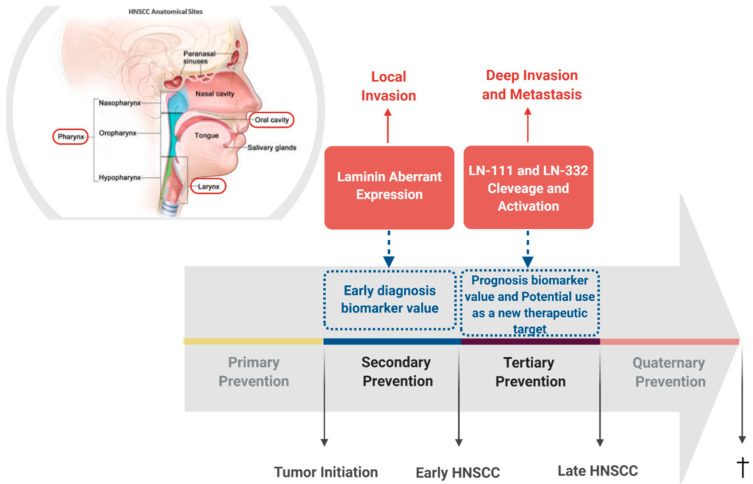
In this review, we discussed the clear contribution of laminin for the progression of HNSCC, particularly for those of oral cavity, pharynx, and larynx. In this sense, the aberrant expression of LN332 seems to represent the main characteristics which connect the distinct head and neck squamous tumors, since several studies have been shown that the LN332, and particularly its γ2 chain overexpression, are associated with tumor progression of oral cavity, pharynx, and larynx tissues. However, in the development of OCSCC, despite the relevance of LN332/γ2 chain expression, the biological fragments which arise from the degradation not only from LN332 but also from LN111 may play a remarkable role in the carcinogenesis of oral tissue. In fact, it was previously reported that the laminin fragments activate EGFR/MAPK as well as PI3K/AKT signaling pathways, consequently triggering crucial cellular mechanisms, such as EMT, migration, and invasion (Figure 1). Although the scarce literature demonstrates that laminin is involved with the progression of pharyngeal and laryngeal squamous cell carcinoma tumors towards a more aggressive and invasive phenotype, the mechanisms underlying this phenomenon are not yet reported, mainly because of limited in vitro and in vivo models currently available for the study of these tumors. Nevertheless, based on tumor similarities, one could suggest that similar signaling pathways may be modulated in pharyngeal and laryngeal squamous cell carcinomas, representing common mechanisms by which laminin plays a role in HNSCC progression. Therefore, the data discussed here draws attention to the possibility that laminin may represent a convergence point in HNSCC natural history, and an attractive potential therapeutic target for these tumors (Figure 2).
Figure 2.
Laminin involvement in head and neck squamous cell carcinomas (HNSCC) progression. Schematic representation of HNSCC most frequent affected anatomical sites and summary of the already identified involvement of laminin in HNSCC, pointing out its intervention potential in tumor’s natural history. Head and neck anatomical sites from where the squamous cell carcinomas discussed in this review arise are highlighted by a red frame. Secondary prevention is highlighted since the aberrant expression pattern of laminin in HNSCC unveils a potential value of its use as early diagnosis biomarker. Likewise, tertiary prevention is also highlighted since cleavage and activation of laminin trigger intracellular signaling pathways that lead to the acquisition of a malignant phenotype and may represent a potential new therapeutic target. Additionally, cleavage and activation of laminin are associated with patient outcome, indicating a potential value of its use as prognosis biomarker.
Acknowledgments
We would like to thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq—Brazil), Fundação de Amparo à Pesquisa Carlos Chagas Filho (FAPERJ—Brazil), Programa de Oncobiologia IBqM, Fundação Para Pesquisa e Estudo do Câncer Ary Frauzino (FAF).
Abbreviations
| HNC | Head and Neck cancers |
| SCC | squamous cell carcinomas |
| HNSCC | Head and neck squamous cell carcinomas |
| ECM | Extracellular matrix |
| LN-111 | Laminin subtype 111 |
| LN-332 | Laminin subtype 332 |
| BM | Basement membrane |
| EGF | Epidermal-growth factor |
| EGFR | Epidermal-growth factor |
| MAPK | Mitogen-activated protein kinase |
| HPV | Human papiloma virus |
| EBV | Epstein Barr Virus |
| PI3K | Phosphoinositide 3-kinase |
| OCSCC | Oral cavity squamous cell carcinomas |
| MMP-2 | matrix metalloproteinase 2 |
| MT1-MMP | membrane type 1 matrix metalloproteinase |
| EMT | epithelial–mesenchymal transition |
| DIII | γ2 chain fragment |
| TSCC | tongue squamous cell carcinoma |
| AKT | Protein kinase B |
| RNA | Ribonucleic acid |
| MMP-9 | matrix metalloproteinase 9 |
| Src | Proto-oncogene tyrosine-protein kinase Src |
| ERK 1/2 | Extracellular signal-regulated kinases 1 and 2 |
| OPSCC | oropharyngeal squamous cell carcinomas |
| NPSCC | nasopharyngeal squamous cell carcinomas |
| LMP1, 2a and 2b | Latent membrane proteins 1, 2a and 2b |
| LAMC2 | gene that encodes the γ2 chain |
| HPSCC | hypopharyngeal squamous cell carcinomas |
| IL-6 | Interleukin 6 |
| LSCC | Laryngeal squamous cell carcinomas |
| CIS | in situ squamous epithelial carcinomas |
| LR | Laminin receptor |
| SSCC | Skin squamous cell carcinomas |
| LRP | Laminin receptor precursor |
Author Contributions
N.M.D.C. and A.P.J. conceived, delineated, discussed and wrote the manuscript. F.A.M. and B.P. discussed, critically reviewed and wrote the manuscript. L.F.R.P. and L.E.N. critically reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that they have no competing interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Anantharaman D., Brennan P., Leemans C.R. Head and Neck Cancers: New Etiological Insights. In: Wild C.P., Weiderpass E., Stewart B.W., editors. World Cancer Report 2020. 1st ed. Volume 1. International Agency for Research on Cancer; Lyon, France: 2020. pp. 310–322. [Google Scholar]
- 3.National Cancer Institute Head and Neck Cancers. [(accessed on 12 September 2020)]; Available online: https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet#what-are-cancers-of-the-head-and-neck.
- 4.Mishra M.S., Upadhyaya N., Dive A.M., Bodhade A.S. Histological patterns of head and neck tumors: An insight to tumor histology. J. Oral Maxillofac. Pathol. 2014;18:58–68. doi: 10.4103/0973-029X.131912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford C.R., Ferlito A., Devaney K.O., Mäkitie A.A., Rinaldo A. Prognostic factors in laryngeal squamous cell carcinoma. Laryngoscope Investig. Otolaryngol. 2020;5:74–81. doi: 10.1002/lio2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pezzuto F., Buonaguro L., Caponigro F., Ionna F., Starita N., Annunziata C., Buonaguro F.M., Tornesello M.L. Update on Head and Neck Cancer: Current Knowledge on Epidemiology, Risk Factors, Molecular Features and Novel Therapies. Oncology. 2015;89:125–136. doi: 10.1159/000381717. [DOI] [PubMed] [Google Scholar]
- 7.Pfister D.G., Spencer S., Brizel D.M., Burtness B., Busse P.M., Caudell J.J., Cmelak A.J., Colevas A.D., Dunphy F., Eisele D.W. Head and neck cancers, version 2. 2014: Clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2014;12:1454–1487. doi: 10.6004/jnccn.2014.0142. [DOI] [PubMed] [Google Scholar]
- 8.Chow L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020;382:60–72. doi: 10.1056/NEJMra1715715. [DOI] [PubMed] [Google Scholar]
- 9.The Cancer Genome Atlas Network Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickup M.W., Mouw J.K., Weaver V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15:1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker C., Mojares E., Hernández A.D.R. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018;19:3028. doi: 10.3390/ijms19103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palumbo A., Jr., Meireles Da Costa N., Pontes B., Oliveira F.L., Codeço M.L., Ribeiro Pinto L.F., Nasciutti L.E. Esophageal Cancer Development: Crucial Clues Arising from the Extracellular Matrix. Cells. 2020;9:455–465. doi: 10.3390/cells9020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henke E., Nandigama R., Ergün S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020;6:160. doi: 10.3389/fmolb.2019.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theocharis A.D., Skandalis S.S., Gialeli C., Karamanos N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Jayadev R., Sherwood D.R. Basement membranes. Curr. Biol. 2017;27:R207–R211. doi: 10.1016/j.cub.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Liotta L.A., Rao C.N., Wewer U.M. Biochemical interactions of tumor cells with the basement membrane. Annu. Rev. Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- 17.Timpl R., Rohde H., Robey P.G., Rennard S.I., Foidart J.M., Martin G.R. Laminin--a glycoprotein from basement membranes. J. Biol. Chem. 1979;254:9933–9937. doi: 10.1016/S0021-9258(19)83607-4. [DOI] [PubMed] [Google Scholar]
- 18.Jourquin J., Tripathi M., Guess C., Quaranta V. Laminins and Cancer Progression. In: Zent R., Pozzi A., editors. Cell-Extracellular Matrix Interactions in Cancer. Volume 1. Springer; New York, NY, USA: 2009. pp. 87–109. [Google Scholar]
- 19.Ramovs V., Molder L.T., Sonnenberg A. The opposing roles of laminin-binding integrins in cancer. Matrix Biol. 2017;57–58:213–243. doi: 10.1016/j.matbio.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Garg M., Kanojia D., Okamoto R., Jain S., Madan V., Chien W., Sampath A., Ding L.-W., Xuan M., Said J.W., et al. Laminin-5 gamma-2 (LAMC2) is highly expressed in anaplastic thyroid carcinoma and is associated with tumor progression, migration and invasion by modulating signaling of EGFR. J. Clin. Endocrinol. Metab. 2014;74:5570. doi: 10.1158/1538-7445.am2014-5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marinkovich M.P. Tumour microenvironment: Laminin 332 in squamous-cell carcinoma. Nat. Rev. Cancer. 2007;7:370–380. doi: 10.1038/nrc2089. [DOI] [PubMed] [Google Scholar]
- 22.Engel J., Odermatt E., Engel A., Madri J.A., Furthmayr H., Rohde H., Timpl R. Shapes, domain organizations and flexibility of laminin and fibronectin, two multifunctional proteins of the extracellular matrix. J. Mol. Biol. 1981;150:97–120. doi: 10.1016/0022-2836(81)90326-0. [DOI] [PubMed] [Google Scholar]
- 23.Odenthal U., Haehn S., Tunggal P., Merkl B., Schomburg D., Frie C., Paulsson M., Smyth N. Molecular Analysis of Laminin N-terminal Domains Mediating Self-interactions. J. Biol. Chem. 2004;279:44504–44512. doi: 10.1074/jbc.M402455200. [DOI] [PubMed] [Google Scholar]
- 24.Aumailley M. The laminin family. Cell Adhes. Migr. 2013;7:48–55. doi: 10.4161/cam.22826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paulsson M., Deutzmann R., Timpl R., Dalzoppo D., Odermatt E., Engel J. Evidence for coiled-coil alpha-helical regions in the long arm of laminin. EMBO J. 1985;4:309–316. doi: 10.1002/j.1460-2075.1985.tb03630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nomizu M., Otaka A., Utani A., Roller P.P., Yamada Y. Assembly of synthetic laminin peptides into a triple-stranded coiled-coil structure. J. Biol. Chem. 1994;269:30386–30392. doi: 10.1016/S0021-9258(18)43825-2. [DOI] [PubMed] [Google Scholar]
- 27.Timpl R., Tisi D., Talts J.F., Andac Z., Sasaki T., Hohenester E. Structure and function of laminin LG modules. Matrix Biol. 2000;19:309–317. doi: 10.1016/S0945-053X(00)00072-X. [DOI] [PubMed] [Google Scholar]
- 28.Yamada M., Sekiguchi K. Molecular Basis of Laminin–Integrin Interactions. Curr. Top. Membr. 2015;76:197–229. doi: 10.1016/bs.ctm.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Ryan M.C., Christiano A.M., Engvall E., Wewer U.M., Miner J.H., Sanes J.R., Burgeson R.E. The functions of laminins: Lessons from in vivo studies. Matrix Biol. 1996;15:369–381. doi: 10.1016/S0945-053X(96)90157-2. [DOI] [PubMed] [Google Scholar]
- 30.Berndt A., Hyckel P., Könneker A., Katenkamp D., Kosmehl H. Oral squamous cell carcinoma invasion is associated with a laminin-5 matrix re-organization but independent of basement membrane and hemidesmosome formation. clues from an in vitro invasion model. Invasion Metastasis. 1997;17:251–258. [PubMed] [Google Scholar]
- 31.Ono Y., Nakanishi Y., Ino Y., Niki T., Yamada T., Yoshimura K., Saikawa M., Nakajima T., Hirohashi S. Clinocopathologic significance of laminin-5 γ2 chain expression in squamous cell carcinoma of the tongue: Immunohistochemical analysis of 67 lesions. Cancer. 1999;85:2315–2321. doi: 10.1002/(SICI)1097-0142(19990601)85:11<2315::AID-CNCR3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 32.Nordemar S., Kronenwett U., Auer G., Högmo A., Lindholm J., Edström S., Tryggvasson K., Linder S., Munck-Wikland E. Laminin-5 as a predictor of invasiveness in cancer in situ lesions of the larynx. Anticancer Res. 2001;21:509–512. [PubMed] [Google Scholar]
- 33.Savoia P., Trusolino L., Pepino E., Cremona O., Marchisio P.C. Expression and Topography of Integrins and Basement Membrane Proteins in Epidermal Carcinomas: Basal but not Squamous Cell Carcinomas Display Loss of α6β4 and BM-600/Nicein. J. Investig. Dermatol. 1993;101:352–358. doi: 10.5555/uri:pii:0022202X9390539T. [DOI] [PubMed] [Google Scholar]
- 34.Holler E. Laminin isoform expression in breast tumors. Breast Cancer Res. 2005;7:166–167. doi: 10.1186/bcr1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hao J., Jackson L., Calaluce R., McDaniel K., Dalkin B.L., Nagle R.B. Investigation into the mechanism of the loss of laminin 5 (α3β3γ2) expression in prostate cancer. Am. J. Pathol. 2001;158:1129–1135. doi: 10.1016/S0002-9440(10)64060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carter W.G., Ryan M.C., Gahr P.J. Epiligrin, a new cell adhesion ligand for integrin α3β1 in epithelial basement membranes. Cell. 1991;65:599–610. doi: 10.1016/0092-8674(91)90092-D. [DOI] [PubMed] [Google Scholar]
- 37.Litjens S.H., de Pereda J.M., Sonnenberg A. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 2006;16:376–383. doi: 10.1016/j.tcb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Schenk S., Hintermann E., Bilban M., Koshikawa N., Hojilla C., Khokha R., Quaranta V. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J. Cell Biol. 2003;161:197–209. doi: 10.1083/jcb.200208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waterman E.A., Sakai N., Nguyen N.T., Horst B.A.J., Veitch D.P., Dey C.N., Ortiz-Urda S., Khavari P.A., Marinkovich M.P. A Laminin-Collagen Complex Drives Human Epidermal Carcinogenesis through Phosphoinositol-3-Kinase Activation. Cancer Res. 2007;67:4264–4270. doi: 10.1158/0008-5472.CAN-06-4141. [DOI] [PubMed] [Google Scholar]
- 40.Guess C.M., Quaranta V. Defining the role of laminin-332 in carcinoma. Matrix Biol. 2009;28:445–455. doi: 10.1016/j.matbio.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogawa T., Tsubota Y., Hashimoto J., Kariya Y., Miyazaki K. The short arm of laminin gamma2 chain of laminin-5 (laminin-332) binds syndecan-1 and regulates cellular adhesion and migration by suppressing phosphorylation of integrin beta4 chain. Mol. Biol. Cell. 2007;18:1621–1633. doi: 10.1091/mbc.e06-09-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montero P.H., Patel S.G. Cancer of the Oral Cavity. Surg. Oncol. Clin. North Am. 2015;24:491–508. doi: 10.1016/j.soc.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blot W.J. Alcohol and cancer. Cancer Res. 1992;52(Suppl. 7):2119s–2123s. [PubMed] [Google Scholar]
- 44.Blot W.J., McLaughlin J.K., Winn D.M., Austin D.F., Greenberg R.S., Preston-Martin S., Bernstein L., Schoenberg J.B., Stemhagen A., Fraumeni J.F., Jr. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–3287. [PubMed] [Google Scholar]
- 45.Meyer J.R., Silverman S., Daniels T.E., Kramer R.H., Greenspan J.S. Distribution of fibronectin and laminin in oral leukoplakia and carcinoma. J. Oral Pathol. Med. 1985;14:247–255. doi: 10.1111/j.1600-0714.1985.tb00488.x. [DOI] [PubMed] [Google Scholar]
- 46.Noguchi M., Kohama G., Hiratsuka H., Sekiguchi T. Clinical significance of laminin deposition and T-cell infiltration in oral cancer. Head Neck. 1993;15:125–132. doi: 10.1002/hed.2880150208. [DOI] [PubMed] [Google Scholar]
- 47.Kumagai S., Kojima S., Imai K., Nakagawa K., Yamamoto E., Kawahara E., Nakanishi I. Immunohistologic distribution of basement membrane in oral squamous cell carcinoma. Head Neck. 1994;16:51–57. doi: 10.1002/hed.2880160111. [DOI] [PubMed] [Google Scholar]
- 48.Harada T., Shinohara M., Nakamura S., Oka M. An immunohistochemical study of the extracellular matrix in oral squamous cell carcinoma and its association with invasive and metastatic potential. Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 1994;424:257–266. doi: 10.1007/BF00194609. [DOI] [PubMed] [Google Scholar]
- 49.Kannan S., Balaram P., Chandran G.J., Pillai M.R., Mathew B., Nalinakumari K.R., Nair M.K. Alterations in expression of basement membrane proteins during tumour progression in oral mucosa. Histopathology. 2007;24:531–537. doi: 10.1111/j.1365-2559.1994.tb00571.x. [DOI] [PubMed] [Google Scholar]
- 50.Kainulainen T., Autio-Harmainen H., Oikarinen A., Salo S., Tryggvason K., Salo T. Altered distribution and synthesis of laminin-5 (kalinin) in oral lichen planus, epithelial dysplasias and squamous cell carcinomas. Br. J. Dermatol. 1997;136:331–336. doi: 10.1111/j.1365-2133.1997.tb14938.x. [DOI] [PubMed] [Google Scholar]
- 51.Kosmehl H., Berndt A., Strassburger S., Borsi L., Rousselle P., Mandel U., Hyckel P., Zardi L., Katenkamp D. Distribution of laminin and fibronectin isoforms in oral mucosa and oral squamous cell carcinoma. Br. J. Cancer. 1999;81:1071–1079. doi: 10.1038/sj.bjc.6690809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berndt A., Borsi L., Hyckel P., Kosmehl H. Fibrillary co-deposition of laminin-5 and large unspliced tenascin-C in the invasive front of oral squamous cell carcinoma in vivo and in vitro. J. Cancer Res. Clin. Oncol. 2001;127:286–292. doi: 10.1007/s004320000205. [DOI] [PubMed] [Google Scholar]
- 53.Haas M.K., Berndt A., Stiller K.J., Hyckel P., Kosmehl H. A Comparative Quantitative Analysis of Laminin-5 in the Basement Membrane of Normal, Hyperplastic, and Malignant Oral Mucosa by Confocal Immunofluorescence Imaging. J. Histochem. Cytochem. 2001;49:1261–1268. doi: 10.1177/002215540104901008. [DOI] [PubMed] [Google Scholar]
- 54.Kawano K., Yanagisawa S. Predictive value of laminin-5 and membrane type 1-matrix metalloproteinase expression for cervical lymph node metastasis in T1 and T2 squamous cell carcinomas of the tongue and floor of the mouth. Head Neck. 2006;28:525–533. doi: 10.1002/hed.20349. [DOI] [PubMed] [Google Scholar]
- 55.Stoltzfus P., Högmo A., Lindholm J., Aspenblad U., Auer G., Munck-Wikland E. The gamma2 chain of laminin-5 as an indicator of increased risk for recurrence in T1 stage tongue cancer. Anticancer Res. 2004;24:3109–3114. [PubMed] [Google Scholar]
- 56.Lindberg P., Larsson A., Nielsen B.S. Expression of plasminogen activator inhibitor-1, urokinase receptor and laminin gamma-2 chain is an early coordinated event in incipient oral squamous cell carcinoma. Int. J. Cancer. 2006;118:2948–2956. doi: 10.1002/ijc.21568. [DOI] [PubMed] [Google Scholar]
- 57.Kuratomi Y., Kumamoto M., Kidera K., Toh S., Masuda M., Nakashima T., Inokuchi A. Diffuse expression of laminin gamma2 chain in disseminating and infiltrating cancer cells indicates a highly malignant state in advanced tongue cancer. Oral Oncol. 2006;42:73–76. doi: 10.1016/j.oraloncology.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 58.Junior H.M., Rocha V.N., Leite C.F., De Aguiar M.C.F., Souza P.E.A., Horta M.C.R. Laminin-5 gamma 2 chain expression is associated with intensity of tumor budding and density of stromal myofibroblasts in oral squamous cell carcinoma. J. Oral Pathol. Med. 2013;43:199–204. doi: 10.1111/jop.12121. [DOI] [PubMed] [Google Scholar]
- 59.Gasparoni A., Della Casa M., Milillo L., Lorenzini G., Rubini C., Urso R., Muzio L.L. Prognostic value of differential expression of Laminin-5 gamma2 in oral squamous cell carcinomas: Correlation with survival. Oncol. Rep. 2007;18:793–800. [PubMed] [Google Scholar]
- 60.Méndez E., Houck J.R., Doody D.R., Fan W., Lohavanichbutr P., Rue T.C., Yueh B., Futran N.D., Upton M.P., Farwell D.G., et al. A Genetic Expression Profile Associated with Oral Cancer Identifies a Group of Patients at High Risk of Poor Survival. Clin. Cancer Res. 2009;15:1353–1361. doi: 10.1158/1078-0432.CCR-08-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Driemel O., Dahse R., Hakim S.G., Tsioutsias T., Pistner H., Reichert T.E., Kosmehl H. Laminin-5 immunocytochemistry: A new tool for identifying dysplastic cells in oral brush biopsies. Cytopathology. 2007;18:348–355. doi: 10.1111/j.1365-2303.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 62.Chen C., Méndez E., Houck J., Fan W., Lohavanichbutr P., Doody D., Yueh B., Futran N.D., Upton M., Farwell D.G., et al. Gene Expression Profiling Identifies Genes Predictive of Oral Squamous Cell Carcinoma. Cancer Epidemiol. Biomark. Prev. 2008;17:2152–2162. doi: 10.1158/1055-9965.EPI-07-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuen H.-W., Ziober A.F., Gopal P., Nasrallah I., Falls E.M., Meneguzzi G., Ang H.-Q., Ziober B.L. Suppression of laminin-5 expression leads to increased motility, tumorigenicity, and invasion. Exp. Cell Res. 2005;309:198–210. doi: 10.1016/j.yexcr.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 64.Oku N., Sasabe E., Ueta E., Yamamoto T., Osaki T. Tight junction protein claudin-1 enhances the invasive activity of oral squamous cell carcinoma cells by promoting cleavage of laminin-5 gamma2 chain via matrix metalloproteinase (MMP)-2 and membrane-type MMP-1. Cancer Res. 2006;66:5251–5257. doi: 10.1158/0008-5472.CAN-05-4478. [DOI] [PubMed] [Google Scholar]
- 65.Yasuda H., Nakagawa M., Kiyokawa H., Yoshida E., Yoshimura T., Koshikawa N., Itoh F., Seiki M. Unique Biological Activity and Potential Role of Monomeric Laminin-γ2 as a Novel Biomarker for Hepatocellular Carcinoma: A Review. Int. J. Mol. Sci. 2019;20:226. doi: 10.3390/ijms20010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ryan M.C., Lee K., Miyashita Y., Carter W.G. Targeted Disruption of the LAMA3 Gene in Mice Reveals Abnormalities in Survival and Late Stage Differentiation of Epithelial Cells. J. Cell Biol. 1999;145:1309–1324. doi: 10.1083/jcb.145.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Z., Symons J.M., Goldstein S.L., McDonald A., Miner J.H., Kreidberg J.A. (Alpha)3(beta)1 integrin regulates epithelial cytoskeletal organization. J. Cell Sci. 1999;112:2925–2935. doi: 10.1242/jcs.112.17.2925. [DOI] [PubMed] [Google Scholar]
- 68.Richter P., Umbreit C., Franz M., Berndt A., Grimm S., Uecker A., Böhmer F.D., Kosmehl H., Berndt A. EGF/TGFβ1 co-stimulation of oral squamous cell carcinoma cells causes an epithelial-mesenchymal transition cell phenotype expressing laminin 332. J. Oral Pathol. Med. 2010;40:46–54. doi: 10.1111/j.1600-0714.2010.00936.x. [DOI] [PubMed] [Google Scholar]
- 69.Ikenouchi J., Matsuda M., Furuse M., Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: Direct repression of the gene expression of claudins/occludin by Snail. J. Cell Sci. 2003;116:1959–1967. doi: 10.1242/jcs.00389. [DOI] [PubMed] [Google Scholar]
- 70.Degen M., Natarajan E., Barron P., Widlund H.R., Rheinwald J.G. MAPK/ERK-Dependent Translation Factor Hyperactivation and Dysregulated Laminin γ2 Expression in Oral Dysplasia and Squamous Cell Carcinoma. Am. J. Pathol. 2012;180:2462–2478. doi: 10.1016/j.ajpath.2012.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ono Y., Nakanishi Y., Gotoh M., Sakamoto M., Hirohashi S. Epidermal growth factor receptor gene amplification is correlated with laminin-5 gamma2 chain expression in oral squamous cell carcinoma cell lines. Cancer Lett. 2002;175:197–204. doi: 10.1016/S0304-3835(01)00682-6. [DOI] [PubMed] [Google Scholar]
- 72.Esquela-Kerscher A., Slack F.J. Oncomirs—MicroRNAs with a role in cancer. Nat. Rev. Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 73.Kinoshita T., Nohata N., Hanazawa T., Kikkawa N., Yamamoto N., Yoshino H., Itesako T., Enokida H., Nakagawa E.M., Okamoto Y., et al. Tumour-suppressive microRNA-29s inhibit cancer cell migration and invasion by targeting laminin–integrin signalling in head and neck squamous cell carcinoma. Br. J. Cancer. 2013;109:2636–2645. doi: 10.1038/bjc.2013.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou Y.-M., Yao Y.-L., Liu W., Shen X.-M., Shi L.-J., Wu L. MicroRNA-134 inhibits tumor stem cell migration and invasion in oral squamous cell carcinomas via downregulation of PI3K-Akt signaling pathway by inhibiting LAMC2 expression. Cancer Biomark. 2020;29:51–67. doi: 10.3233/CBM-191362. [DOI] [PubMed] [Google Scholar]
- 75.Ding J., Yang C., Yang S. LINC00511 interacts with miR-765 and modulates tongue squamous cell carcinoma progression by targeting LAMC2. J. Oral Pathol. Med. 2018;47:468–476. doi: 10.1111/jop.12677. [DOI] [PubMed] [Google Scholar]
- 76.Wang S.H., Liou G.G., Liu S.H., Chang J.S., Hsiao J.R., Yen Y.C., Chen Y.L., Wu W.L., Chang J.Y., Chen Y.W. Laminin γ2-enriched extracellular vesicles of oral squamous cell carcinoma cells enhance in vitro lymphangiogenesis via integrin α3-dependent uptake by lymphatic endothelial cells. Int. J. Cancer. 2019;144:2795–2810. doi: 10.1002/ijc.32027. [DOI] [PubMed] [Google Scholar]
- 77.Snijders A.M., Schmidt B.L., Fridlyand J., Dekker N., Pinkel D., Jordan R.C.K., Albertson D.G. Rare amplicons implicate frequent deregulation of cell fate specification pathways in oral squamous cell carcinoma. Oncogene. 2005;24:4232–4242. doi: 10.1038/sj.onc.1208601. [DOI] [PubMed] [Google Scholar]
- 78.Tanis T., Cincin Z.B., Gökçen-Röhlıg B., Bireller E.S., Ulusan M., Tanyel C.R., Cakmakoglu B., Gokcen-Rohlig B. The role of components of the extracellular matrix and inflammation on oral squamous cell carcinoma metastasis. Arch. Oral Biol. 2014;59:1155–1163. doi: 10.1016/j.archoralbio.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 79.Siqueira A.S., Gama-de-Souza L.N., Arnaud M.C.V., Pinheiro J.J.V., Jaeger R.G. Laminin-derived peptide AG73 regulates migration, invasion, and protease activity of human oral squamous cell carcinoma cells through syndecan-1 and beta1 integrin. Tumour Biol. 2010;31:46–58. doi: 10.1007/s13277-009-0008-x. [DOI] [PubMed] [Google Scholar]
- 80.Siqueira A.S., Pinto M.P., Cruz M.C., Smuczek B., Cruz K.S.P., Barbuto J.M.M., Hoshino D., Weaver A.M., Freitas V.M., Jaeger R.G. Laminin-111 peptide C16 regulates invadopodia activity of malignant cells through β1 integrin, Src and ERK ½. Oncotarget. 2016;7:47904–47917. doi: 10.18632/oncotarget.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garneau J.C., Bakst R.L., Miles B.A. Hypopharyngeal cancer: A state of the art review. Oral Oncol. 2018;86:244–250. doi: 10.1016/j.oraloncology.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 82.Johnson D.E., Burtness B., Leemans R.C., Lui V.W.Y., Bauman J.E., Grandis J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers. 2020;6:92–100. doi: 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Y.-P., Chan A.T.C., Le Q.-T., Blanchard P., Sun Y., Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 84.Sinha S., Gajra A. StatPearls. Volume 1. StatPearls Publishing; Treasure Island, FL, USA: 2020. Nasopharyngeal Cancer; pp. 1–20. [PubMed] [Google Scholar]
- 85.De Schryver A., Friberg S., Jr., Klein G., Henle W., Henle G., De-Thé G., Clifford P., Ho H.C. Epstein–Barr virus-associated antibody patterns in carcinoma of the post-nasal space. Clin. Exp. Immunol. 1969;5:443–459. [PMC free article] [PubMed] [Google Scholar]
- 86.Nakanishi Y., Wakisaka N., Kondo S., Endo K., Sugimoto H., Hatano M., Ueno T., Ishikawa K., Yoshizaki T. Progression of understanding for the role of Epstein-Barr virus and management of nasopharyngeal carcinoma. Cancer Metastasis Rev. 2017;36:435–447. doi: 10.1007/s10555-017-9693-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goldman J.M., Goodman M.L., Miller D. Antibody to Epstein-Barr virus in American patients with carcinoma of the nasopharynx. JAMA. 1971;216:1618–1622. doi: 10.1001/jama.1971.03180360064009. [DOI] [PubMed] [Google Scholar]
- 88.Lo A.K., Liu Y., Wang X., Wong Y.C., Lee C.K.F., Huang D.P., Tsao S.W. Identification of downstream target genes of latent membrane protein 1 in nasopharyngeal carcinoma cells by suppression subtractive hybridization. Biochim. Biophys. Acta Gene Struct. Expr. 2001;1520:131–140. doi: 10.1016/s0167-478100260-3. [DOI] [PubMed] [Google Scholar]
- 89.Farwell D.G., McDougall J.K., Coltrera M.D. Expression of Epstein-Barr Virus Latent Membrane Proteins Leads to Changes in Keratinocyte Cell Adhesion. Ann. Otol. Rhinol. Laryngol. 1999;108:851–859. doi: 10.1177/000348949910800906. [DOI] [PubMed] [Google Scholar]
- 90.Sengupta S., den Boon J.A., Chen I.-H., Newton M.A., Stanhope S.A., Cheng Y.-J., Chen C.-J., Hildesheim A., Sugden B., Ahlquist P. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc. Natl. Acad. Sci. USA. 2008;105:5874–5878. doi: 10.1073/pnas.0801130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ricci E., Cavalot A.L., Sanvito F., Bussi M., Albera R., Staffieri A., Cortesina G., Marchisio P.C. Differential Expression and Topography of Adhesion Molecules in Laryngeal and Oropharyngeal Carcinomas. Acta Oto-Laryngol. 2002;122:234–240. doi: 10.1080/00016480252814298. [DOI] [PubMed] [Google Scholar]
- 92.Frenette G.P., Carey T.E., Varani J., Schwartz D.R., Fligiel S.E., Ruddon R.W., Peters B.P. Biosynthesis and secretion of laminin and laminin-associated glycoproteins by nonmalignant and malignant human keratinocytes: Comparison of cell lines from primary and secondary tumors in the same patient. Cancer Res. 1988;48:5193–5202. [PubMed] [Google Scholar]
- 93.Nakayama M., Sato Y., Okamoto M., Hirohashi S. Increased Expression of Laminin-5 and Its Prognostic Significance in Hypopharyngeal Cancer. Laryngoscope. 2004;114:1259–1263. doi: 10.1097/00005537-200407000-00022. [DOI] [PubMed] [Google Scholar]
- 94.Dündar R., Aslan H., Özbay C., Basoglu S., Güvenç I.A., Ögredik E.A., Öztürkcan S., Tayfun M.A., Katilmis H. The necessity of dissection of level IIb in laryngeal squamous cell carcinoma: A clinical study. Otolaryngol. Head Neck Surg. 2012;146:390–394. doi: 10.1177/0194599811430818. [DOI] [PubMed] [Google Scholar]
- 95.Maurya S.S., Katiyar T., Dhawan A., Singh S., Jain S.K., Pant M.C., Parmar D. Gene-environment interactions in determining differences in genetic susceptibility to cancer in subsites of the head and neck. Environ. Mol. Mutagen. 2014;56:313–321. doi: 10.1002/em.21920. [DOI] [PubMed] [Google Scholar]
- 96.Qiu X., You Y., Huang J., Wang X., Zhu H., Wang Z. LAMP3 and TP53 overexpression predicts poor outcome in laryngeal squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2015;8:5519–5527. [PMC free article] [PubMed] [Google Scholar]
- 97.Rodrigo J.P., Martínez P., Allonca E., Alonso-Durán L., Suárez C., Astudillo A., García-Pedrero J.M. Immunohistochemical markers of distant metastasis in laryngeal and hypopharyngeal squamous cell carcinomas. Clin. Exp. Metastasis. 2014;31:317–325. doi: 10.1007/s10585-013-9630-5. [DOI] [PubMed] [Google Scholar]
- 98.Peltanova B., Raudenska M., Masarik M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: A systematic review. Mol. Cancer. 2019;18:1–24. doi: 10.1186/s12943-019-0983-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nerlich A., Haraida S., Hagedorn H., Wiest I., Schreiner M., Schleicher E. Morphological aspects of basement-membranes and their receptors in benign and malignant neoplasms (review) Int. J. Oncol. 1995;6:1193–1202. doi: 10.3892/ijo.6.6.1193. [DOI] [PubMed] [Google Scholar]
- 100.Hagedorn H.G., Tübel J., Wiest I., Schleicher E.D., Nerlich A.G. Prognostic aspects of the loss of epithelial basement membrane components in preinvasive and invasive laryngeal carcinomas. Anticancer Res. 1998;18:201–207. [PubMed] [Google Scholar]
- 101.Hagedorn H., Schreiner M., Wiest I., Tubel J., Schleicher E.D., Nerlich A.G. Defective basement membrane in laryngeal carcinomas with heterogeneous loss of distinct components. Hum. Pathol. 1998;29:447–454. doi: 10.1016/S0046-8177(98)90059-4. [DOI] [PubMed] [Google Scholar]
- 102.Hagedorn H.G., Sauer U., Schleicher E.D., Nerlich A.G. Divergence in distribution and prognostic significance of major basement components in laryngeal carcinomas. Int. J. Oncol. 2001;18:1045–1051. doi: 10.3892/ijo.18.5.1045. [DOI] [PubMed] [Google Scholar]
- 103.Nordemar S., Högmo A., Lindholm J., Auer G., Munck-Wikland E. Laminin-5 gamma 2: A marker to identify oral mucosal lesions at risk for tumor development? Anticancer Res. 2003;23:4985–4989. [PubMed] [Google Scholar]
- 104.Matsui C., Nelson C.F., Hernandez G.T., Herron G.S., Bauer E.A., Hoeffler W.K. gamma 2 chain of laminin-5 is recognized by monoclonal antibody GB3. J. Investig. Dermatol. 1995;105:648–652. doi: 10.1111/1523-1747.ep12324108. [DOI] [PubMed] [Google Scholar]
- 105.Wewer U.M., Taraboletti G., Sobel M.E., Albrechtsen R.A., Liotta L.A. Role of laminin receptor in tumor cell migration. Cancer Res. 1987;47:5691–5698. [PubMed] [Google Scholar]
- 106.Pyke C., Salo S., Ralfkiaer E., Rømer J., Danø K., Tryggvason K. Laminin-5 is a marker of invading cancer cells in some human carcinomas and is coexpressed with the receptor for urokinase plasminogen activator in budding cancer cells in colon adenocarcinomas. Cancer Res. 1995;55:4132–4139. [PubMed] [Google Scholar]
- 107.Fülöp T., Larbi A. Putative role of 67 kDa elastin-laminin receptor in tumor invasion. Semin. Cancer Biol. 2002;12:219–229. doi: 10.1016/S1044-579X(02)00025-1. [DOI] [PubMed] [Google Scholar]
- 108.Zhou L., Xie M., Zhou J.Q., Tao L. 67-kDa Laminin Receptor in Human Laryngeal Squamous Cell Carcinoma. Laryngoscope. 2006;116:28–32. doi: 10.1097/01.mlg.0000184505.05662.b9. [DOI] [PubMed] [Google Scholar]
- 109.Castronovo V., Sobel M.E. Laminin and fibronectin increase the steady state level of the 67 kD high affinity metastasis-associated laminin receptor mRNA in human cancer cells. Biochem. Biophys. Res. Commun. 1990;168:1110–1117. doi: 10.1016/0006-291X(90)91144-H. [DOI] [PubMed] [Google Scholar]
- 110.Turpeenniemi-Hujanen T., Thorgeirsson U.P., Rao C.N., Liotta L.A. Laminin increases the release of type IV collagenase from malignant cells. J. Biol. Chem. 1986;261:1883–1889. doi: 10.1016/S0021-9258(17)36025-8. [DOI] [PubMed] [Google Scholar]
- 111.Berno V., Porrini D., Castiglioni F., Campiglio M., Casalini P., Pupa S.M., Balsari A., Ménard S., Tagliabue E. The 67 kDa laminin receptor increases tumor aggressiveness by remodeling laminin-1. Endocr. Relat. Cancer. 2005;12:393–406. doi: 10.1677/erc.1.00870. [DOI] [PubMed] [Google Scholar]
- 112.Mielcarek-Kuchta D., Olofsson J., Golusinski W. Laminin expression in advanced laryngeal squamous cell carcinoma does not correlate to neck metastases. Eur. Arch. Oto-Rhino-Laryngol. 2008;265:1257–1261. doi: 10.1007/s00405-008-0666-0. [DOI] [PubMed] [Google Scholar]
- 113.Tran M., Rousselle P., Nokelainen P., Tallapragada S., Nguyen N.T., Fincher E.F., Marinkovich M.P. Targeting a Tumor-Specific Laminin Domain Critical for Human Carcinogenesis. Cancer Res. 2008;68:2885–2894. doi: 10.1158/0008-5472.CAN-07-6160. [DOI] [PubMed] [Google Scholar]
- 114.Seftor R.E., Seftor E.A., Kirschmann D.A., Hendrix M.J. Targeting the tumor microenvironment with chemically modified tetracyclines: Inhibition of laminin 5 gamma2 chain promigratory fragments and vasculogenic mimicry. Mol. Cancer Ther. 2002;1:1173–1179. [PubMed] [Google Scholar]
- 115.Sroka T.C., Pennington M.E., Cress A.E. Synthetic D-amino acid peptide inhibits tumor cell motility on laminin-5. Carcinogenesis. 2006;27:1748–1757. doi: 10.1093/carcin/bgl005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Akita N., Maruta F., Seymour L.W., Kerr D.J., Parker A.L., Asai T., Oku N., Nakayama J., Miyagawa S. Identification of oligopeptides binding to peritoneal tumors of gastric cancer. Cancer Sci. 2006;97:1075–1081. doi: 10.1111/j.1349-7006.2006.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nelson J., McFerran N.V., Pivato G., Chambers E., Doherty C., Steele D., Timson D.J. The 67 kDa laminin receptor: Structure, function and role in disease. Biosci. Rep. 2008;28:33–48. doi: 10.1042/BSR20070004. [DOI] [PubMed] [Google Scholar]
- 118.Hanahan D., Weinberg R.A. The Hallmarks of Cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 119.Vania L., Morris G., Otgaar T.C., Bignoux M.J., Bernert M., Burns J., Gabathuse A., Singh E., Ferreira E., Weiss S.F.T. Patented therapeutic approaches targeting LRP/LR for cancer treatment. Expert Opin. Ther. Patents. 2019;29:987–1009. doi: 10.1080/13543776.2019.1693543. [DOI] [PubMed] [Google Scholar]
- 120.Solomonov I., Zehorai E., Talmi-Frank D., Wolf S.G., Shainskaya A., Zhuravlev A., Kartvelishvily E., Visse R., Levin Y., Kampf N., et al. Distinct biological events generated by ECM proteolysis by two homologous collagenases. Proc. Natl. Acad. Sci. USA. 2016;113:10884–10889. doi: 10.1073/pnas.1519676113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Levin M., Udi Y., Solomonov I., Sagi I. Next generation matrix metalloproteinase inhibitors—Novel strategies bring new prospects. Biochim. Biophys. Acta Bioenerg. 2017;1864:1927–1939. doi: 10.1016/j.bbamcr.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 122.Mushtaq M.U., Papadas A., Pagenkopf A., Flietner E., Morrow Z., Chaudhary S.G., Asimakopoulos F. Tumor matrix remodeling and novel immunotherapies: The promise of matrix-derived immune biomarkers. J. Immunother. Cancer. 2018;6:65. doi: 10.1186/s40425-018-0376-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li L., Wei J.R., Dong J., Lin Q.G., Tang H., Jia X.X., Tan W., Chen Q.Y., Zeng T.T., Xing S., et al. Laminin γ2-mediating T cell exclusion attenuates response to anti-PD-1 therapy. Sci. Adv. 2021;7:58–68. doi: 10.1126/sciadv.abc8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Németh Z., Szigeti K., Máthé M., Szabó G., Velich N., Suba Z. Effect of Induction Chemotherapy on Changes of Laminin and Syndecan Expression in Oral Squamous Cell Carcinomas: A Prospective, Randomized, Clinicopathologic and Immunohistochemical Study. J. Craniofacial Surg. 2005;16:205–212. doi: 10.1097/00001665-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 125.Nakawatari M., Iwakawa M., Ohno T., Katoh S., Tamaki T., Imadome K., Sakai M., Tsujii H., Imai T. Chemoradiation-induced expression of fibroblast growth factor-2 and laminin in patients with cervical cancer. Cancer Biol. Ther. 2007;6:1780–1786. doi: 10.4161/cbt.6.11.4858. [DOI] [PubMed] [Google Scholar]
- 126.Worden F.P., Sacco A.G. Molecularly targeted therapy for the treatment of head and neck cancer: A review of the ErbB family inhibitors. OncoTargets Ther. 2016;9:1927–1943. doi: 10.2147/OTT.S93720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kunitomi H., Kobayashi Y., Wu R.-C., Takeda T., Tominaga E., Banno K., Aoki D. LAMC1 is a prognostic factor and a potential therapeutic target in endometrial cancer. J. Gynecol. Oncol. 2020;31:e11. doi: 10.3802/jgo.2020.31.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]