Abstract
Extracts of red clover (Trifolium pratense L.), containing estrogenic isoflavones like genistein and daidzein and the proestrogenic isoflavones formononetin and biochanin A, are used by women as dietary supplements for the management of menopausal symptoms. Although marketed as a safer alternative to hormone therapy, red clover isoflavones have been reported to inhibit some cytochrome P450 (CYP) enzymes involved in drug metabolism. To evaluate the potential for clinically relevant drug-red clover interactions, we tested a standardized red clover dietary supplement (120 mg isoflavones per day) for interactions with the pharmacokinetics of four FDA-approved drugs (caffeine, tolbutamide, dextromethorphan, and alprazolam) as probe substrates for the enzymes CYP1A2, CYP2C9; CYP2D6, and CYP3A4/5, respectively. Fifteen peri- and post-menopausal women completed pharmacokinetic studies at baseline and 2 weeks after consuming red clover. The averaged pharmacokinetic profiles of probe substrates in serum showed no significant alterations and no changes in the areas under the curve (AUC) over 96 h. Subgroup analysis based on the demographic characteristics (BMI, menopausal status, race, and age) also showed no differences in AUC for each probe substrate. Analysis of red clover isoflavones in serum showed primarily conjugated metabolites that explain, at least in part, the red clover pharmacokinetic safety profile.
Keywords: red clover, clinical trial, pharmacokinetics, drug interactions, botanical dietary supplements
Graphical Abstract
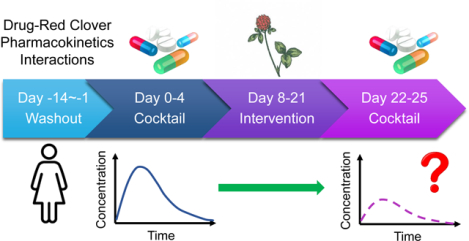
Introduction
Reports from the Women’s Health Initiative have indicated that prolonged use of conventional hormone therapy by menopausal women increases the risk of breast cancer, heart attack, stroke, and blood clots,1,2 and many women are consuming botanical dietary supplements as potentially safer alternatives. Extracts of red clover (Trifolium pratense L.) are becoming popular botanical dietary supplements for women’s health and particularly for the management of menopausal symptoms. Red clover extracts are rich in estrogenic and pro-estrogenic isoflavones including formononetin, biochanin A, genistein, and daidzein (Figure 1). Furthermore, red clover has potential beneficial activity in preventing menopausal symptoms such as hot flashes and postmenopausal osteoporosis and demonstrates a favorable safety profile.3–6
Figure 1.
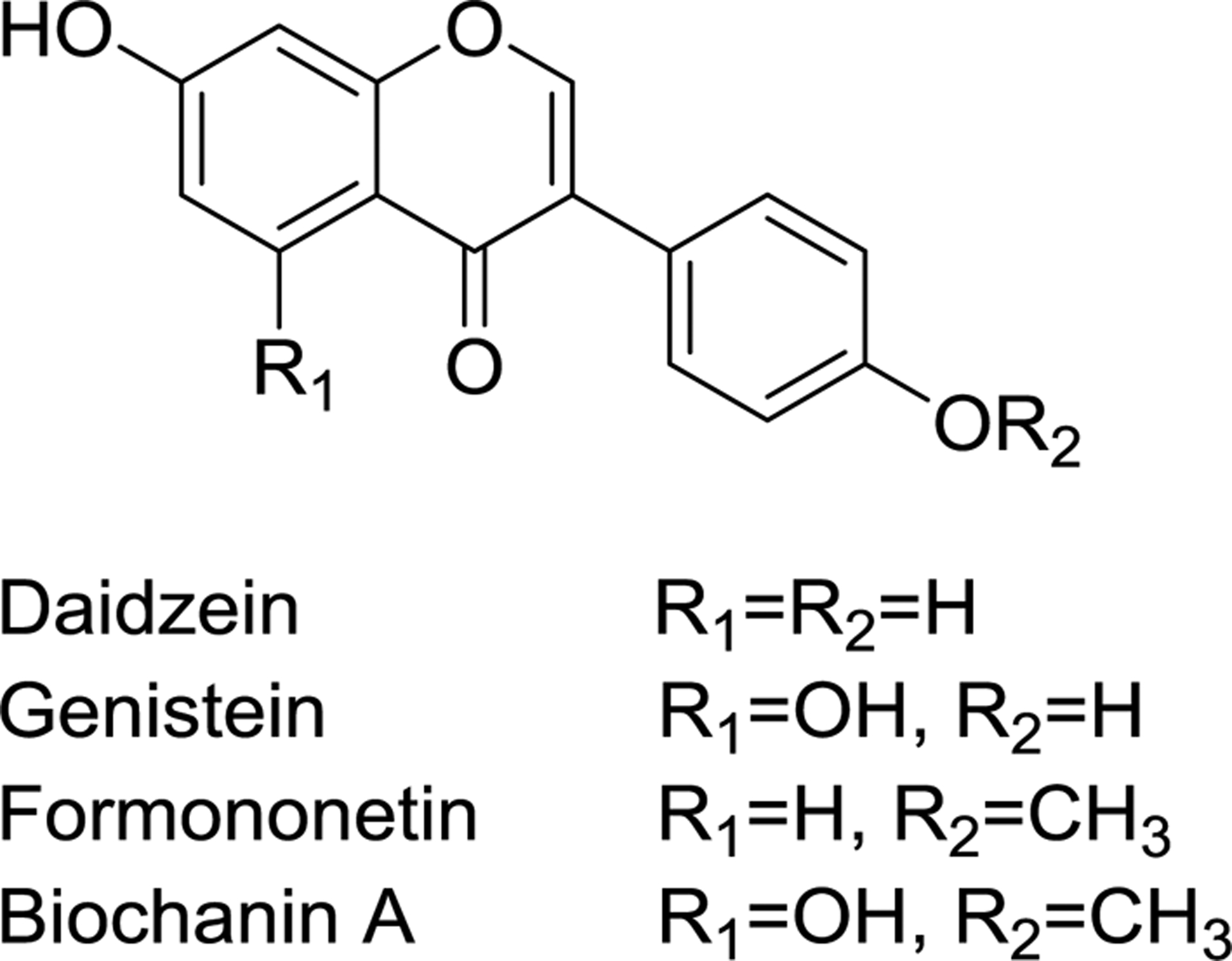
Structures of red clover isoflavones
Red clover dietary supplements contain multiple isoflavones with levels of up to 1350 mg/100 g biochanin A, 1000 mg/100 g formononetin, 724 mg/100 g irilone, 707 mg/100 g prunetin, 236 mg/100 g genistein, and 79 mg/100 g daidzein.7 Various foods also contain substantial quantities of isoflavones. For example, soybeans contain 46.6 mg/100 g daidzein and 73.8 mg/100 g genistein.8 Garbanzo beans9 and blackeyed beans8 contain biochanin A at 1.78 mg/100 g and 1.73 mg/100 g, respectively, and alfalfa sprouts contain formononetin at 261 mg/100 g.10 Whether consumed as botanical dietary supplements or conventional foods, humans are routinely exposured to considerable quantities of isoflavones.
Many women consume botanical dietary supplements concurrently with prescription medications and do not disclose this use to their healthcare providers.11 Unlike drug-drug interaction safety studies for new prescription medications, investigation of possible drug-botanical interactions is not required before marketing botanical dietary supplements.12 Mechanisms of drug-botanical interactions can include inhibition and/or induction of drug transporters13,14 and/or drug metabolizing enzymes.15 The popularity of botanical dietary supplements marketed for women’s health combined with lack of knowledge regarding their possible drug pharmacokinetic interactions is a medical concern.
Drug metabolizing enzymes are grouped into Phase I, catalyzing chemical reactions such as oxidation, reduction and hydrolysis, and Phase II, involving conjugation reactions. The most important Phase I enzymes are a family of heme-containing proteins known as the cytochromes P450 (CYP). Expressed in many organs, but at the highest levels in human liver, the CYP enzymes involved in the metabolism of most clinically used drugs include CYP3A4, CYP2D6, CYP2C9, CYP1A2, CYP2B6, and CYP2C19. Inhibition of CYP enzymes can prolong the half-lives of drugs, leading to increased drug exposure and possibly toxicity. Alternatively, induction of CYP enzymes can shorten drug half-lives thereby decreasing drug exposure and possibly causing loss of efficacy.
The U.S. Food and Drug Administration has established guidelines for the clinical evaluation of drugs for potential drug interactions16 and recommends clinical evaluation when inhibition or induction is suspected for CYP3A4, CYP2D6, CYP2C9, CYP2C19, and CYP1A2. This approach has been extended to the evaluation of the interactions between CYP enzymes and botanical dietary supplements, including hops (Humulus lupulus L.),17 black cohosh (Actaea racemosa),18 echinacea (Echinacea purpurea),19,20 gingko (Ginkgo biloba),21 milk thistle (Silybum marianum),22 and St. John’s wort (Hypericum perforatum L.).23 The approach uses one or a cocktail of probe substrates, each of which is mainly metabolized by a single CYP enzyme. The probe substrates are administered once before and again after intervention with the test agent, and serial blood samples are obtained for quantitative analysis of the probe substrate each time for pharmacokinetic modeling. Changes in the pharmacokinetics of a probe substrate due to the consumption of a test drug or botanical dietary supplement would indicate a pharmacokinetic interaction such as inhibition or induction of the relevant CYP enzyme.
Red clover isoflavones have been reported to inhibit CYP1A1, CYP1A2, CYP1B1, CYP2C9, CYP2D6, CYP19A1, and CYP3A4 in vitro.24–28 For example, Arora, et al.,24 reported that the isoflavone formononetin inhibits CYP2D6 and that both formononetin and biochanin A competitively inhibit CYP1A2 with IC50 values of 13.42 μM and 24.98 μM, respectively. Arora, et al.,24 also reported inhibition of CYP2C9 by biochanin A, and Kopečná-Zapletalová, et al.,25 reported noncompetitive inhibition of CYP2C9 by the isoflavones genistein and daidzein (Ki 35.96 μM and 60.56 μM, respectively). Sato, et al.,26 reported inhibition of CYP3A4 by red clover extracts, and Kopečná-Zapletalová, et al.,25 found that the isoflavones genistein and biochanin A inhibit CYP3A4 with Ki values of 23.25 μM and Ki 57.69 μM, respectively. Roberts, et al.,27 reported that extrahepatic CYP1A1 and CYP1B1 catalyze the O-demethylation of formononetin and biochanin A to form the more estrogenic isoflavones daidzein and genistein, respectively, and that daidzein and genistein inhibitor these enzymes. Wang, et al.,28 found that biochanin A inhibits extrahepatic CYP19A1 (aromatase) and suppressed its expression.
Red clover isoflavones have long half-lives (13–23 h),29 caused in part by enterohepatic recirculation. After Phase II enzymatic sulfation or glucuronidation, isoflavone conjugates can be excreted into the bile, deconjugated by intestinal microbiota, and then reabsorbed.30,31 In vitro evidence of CYP inhibition and clinical studies showing long half-lives of red clover isoflavones suggest the potential for drug pharmacokinetic interactions in humans. In addition, exposure to these isoflavones from a variety of food sources including soy, alfalfa sprouts, blackeyed beans, and garbanzo beans enhances the probability of isoflavone-drug pharmacokinetic interactions.
To evaluate the clinical relevance for such drug interactions with red clover, we conducted an open label Phase I clinical trial using a probe substrate cocktail approach in 16 peri- and postmenopausal women to test whether a red clover dietary supplement causes pharmacokinetic interactions with CYP1A2, CYP2C9, CYP2D6, and CYP3A4/5.
Materials and Methods
Chemicals and Reagents
Formononetin, biochanin A, daidzein, genistein, caffeine, [trimethyl-13C3]-caffeine, tolbutamide, dextromethorphan, [methyl-d3]-dextromethorphan, alprazolam, [phenyl-d5]-alprazolam, and Helix pomatia β-glucuronidase and arylsulfatase were purchased from Sigma-Aldrich (St. Louis, MO). [butyl-d9]-4-Hydroxy-tolbutamide was purchased from Toronto Research Chemicals (Toronto, Canada). LC-MS grade acetonitrile and methanol were purchased from VWR (Radnor, PA), and LC-MS grade formic acid was purchased from Thermo Scientific (Rockford, IL). Water was prepared using an Elga Purelab Ultra (Siemens Water Technologies, Woodridge, IL) water purification system. Blank serum obtained from individual donors was purchased from BioIVT (Westbury, NY). The red clover dietary supplement was standardized chemically and biologically32 and had been used in our previous clinical trials.33,34
Study Design
The study design was similar to that of our previously reported hop-drug interaction clinical trial13 and is summarized in Figure 2. Briefly, beginning two weeks prior to the study initiation and throughout the study, participants avoided consuming food, beverages or other dietary supplements that could interfere with the study such as citrus, chocolate, cocoa, caffeinated drinks, or soy-containing products (tofu, soymilk, etc.). On Day 0, after fasting overnight, a baseline blood sample (7 mL) was obtained, and a probe substrate cocktail was administered consisting of 100 mg caffeine (CYP1A2 substrate), 250 mg tolbutamide (CYP2C9 substrate), 30 mg dextromethorphan (CYP2D6 substrate), and 2 mg alprazolam (CYP3A4/5 substrate). Serial blood samples were obtained at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12 h, 24 h (Day 1), 48 h (Day 2), 72 h (Day 3), and 96 h (Day 4) post-dosing with the cocktail. Serum was isolated from the blood samples and frozen at −80 °C until analysis.
Figure 2.
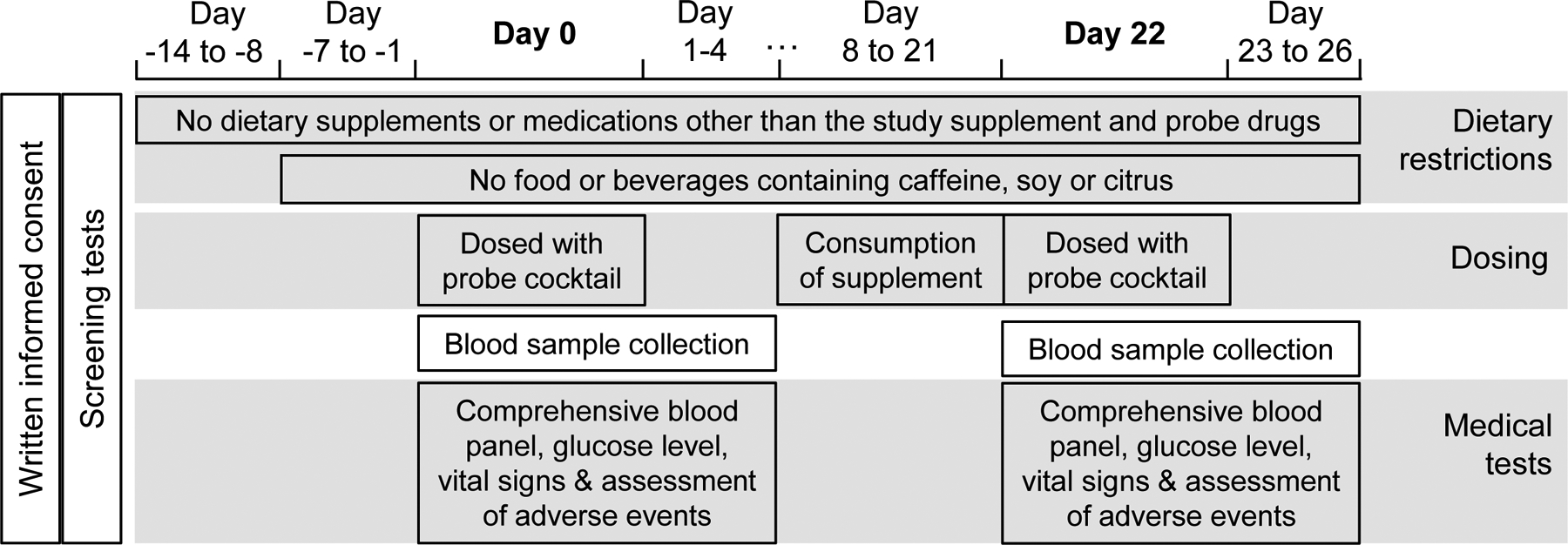
Schedule of clinical study procedures
Additional safety blood samples were obtained at 0 h and 0.5 h for measurement of glucose levels, and at 2 h for comprehensive blood panel measurements. Vital signs were recorded at 0, 1, 4, 8, 12 h on Day 0, and any adverse reactions toward the drug cocktail were closely monitored. Vital signs and possible adverse events were also assessed and recorded on Days 1 through 4 when participants reported for their pharmacokinetic blood draws.
The red clover dietary supplement was administered in capsules standardized to contain ~60 mg isoflavones (including 28.3 mg formononetin, 28.8 mg biochanin A, 0.8 mg genistein, and 0.5 mg daidzein) (Figure 1).34 Participants took one capsule in the morning and one in the evening daily for 14 days (Day 8 through Day 21; Figure 2), for a total of 120 mg isoflavones per day. Participants returned to the clinic on Day 22 after an overnight fast. The cocktail administration, blood sampling and medical tests were repeated as described for Days 0–4. Compliance with the red clover supplementation protocol was evaluated by participant self-reporting, a count of returned red clover capsules, and detection of red clover isoflavones and metabolites in participant serum.
Participant Criteria
This clinical study was reviewed by the National Center for Complementary and Integrative Health (NCCIH), granted an exemption from FDA Investigational New Drug requirements, and registered on Clinicaltrials.gov (NCT03205787). Participants were recruited and gave informed consent following the human participant protocol approved by the University of Illinois at Chicago Institutional Review Board (#2015-0651). Inclusion and exclusion criteria were as described in our previous publication.17 Briefly, peri-menopausal and post-menopausal women were included between 40 to 79 years of age with a body mass index (BMI) ≤40 and in good health (based on physical examination including blood and urine screens). Individuals were excluded if they had any chronic diseases or significant medical conditions; smoking; alcohol or drug abuse; known allergy or hypersensitivity to red clover or any probe substrates used in the study; positive pregnancy test; or hormone therapy within 8 (for oral agents) or 4 weeks (for transdermal or other topical agents). Women were also excluded from the study if phenotype screening indicated significant deficiency in CYP2D6 activity.
Mass Spectrometry
To compare the pharmacokinetics of each probe substrate before and after dosing with the red clover supplement, serum concentrations of caffeine, tolbutamide, dextromethorphan, and alprazolam were measured using ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) on a Shimadzu (Kyoto, Japan) LCMS-8060 triple quadruple mass spectrometer and Nexera UHPLC system as reported previously.35 As an additional confirmation of compliance, UHPLC-MS/MS on a Shimadzu LCMS-9030 Q-ToF high-resolution mass spectrometer was used to profile the red clover isoflavones formononetin, biochanin A, genistein, daidzein, and their Phase II conjugated metabolites in serum obtained on Day 22. Briefly, serum proteins were precipitated by mixing 150 μL serum from Day 22 with 3 volumes of acetonitrile/methanol (9:1; v/v), followed by vortexing for 0.5 min and centrifuging for 15 min at 13000 g at 4 °C. Alternatively, 150 μL serum was adjusted to pH 6 using 1% formic acid, and isoflavone metabolites were deconjugated using 1000 units of β-glucuronidase and 100 units of arylsulfatase for 2 h at 37 °C. Following protein precipitation, each supernatant was removed, evaporated to dryness and reconstituted in 75 μL of 50% aqueous methanol solution prior to analysis in duplicate using UHPLC-MS/MS.
Chromatographic separations were carried out using a Thermo Scientific (Waltham, MA) Hypersil Gold column (1.9 μm, 2.1 × 50 mm) with a gradient from water to acetonitrile (both containing 0.01% formic acid) as follows: 5–20% acetonitrile 0–1 min, 20–60% acetonitrile 1–5 min, 60–95% acetonitrile from 5–5.1 min, 0.5 min hold at 95% acetonitrile, and then re-equilibration at 5% acetonitrile for 1.5 min. The column oven temperature was 40 °C, and the flow rate was 0.4 mL/min. The injection volumes were 3 μL for measurement of sulfates, glucuronides and sulfate-glucuronic acid diconjugates of the red clover isoflavones, or 6 μL for measuring unconjugated red clover compounds.
Negative ion mass spectra were obtained at a resolving power 25,171 (full width at half-maximum at m/z 426.6935). The nebulizing gas flow was 3.0 L/min, the heating gas flow was 10.0 L/min, the drying gas flow was 10.0 L/min, the interface temperature was 300 °C, the desolvation line temperature was 250 °C, the heat block temperature was 400 °C, and the interface voltage was 3.5 kV. The collision energy for product ion tandem mass spectrometry was 35 eV with a 13 eV spread for unconjugated isoflavones, 40 eV with a 13 eV spread for sulfate conjugates, 45 eV with a 13 eV spread for glucuronic acid conjugates and sulfate-glucuronic acid diconjugates.
As additional confirmation of the integrity of the red clover dietary supplement, red clover capsules used in the clinical trial were emptied, weighed, mixed with 5 mL methanol per capsule, sonicated for 40 min, and centrifuged at 4000 g for 15 min at 4 °C. Each supernatant was removed and diluted to 250 ng/mL with 50% aqueous methanol for analysis using UHPLC-MS as described above.
Pharmacokinetics Modeling and Statistics
The pharmacokinetics parameters, including the area under the concentration-time curve (AUC), the peak serum concentration (Cmax), time to reach peak concentration (Tmax), elimination half-life (T1/2), and oral clearance rate (CL/F), were calculated using noncompartmental analysis and the log-linear trapezoidal method with Phoenix WinNonlin software (Certara, Princeton, NJ; Version 8.0). Following the FDA guidance for clinical drug interaction studies,16 the geometric mean ratio before and after the supplement intervention and associated 90% confidence interval were obtained. P-values of <0.05 were considered statistically significant. Statistical analyses were carried out using R36 and GraphPad Prism 7 (GraphPad Software; San Diego, CA). Comparison of AUC values for individual participants before and after red clover supplement intervention was also carried out using GraphPad Prism 7.
Results
Out of the 18 applicants, 2 failed screening and 16 completed the study. The reasons for screening failure were elevated fasting glucose (1 applicant) and use of prescription medications for asthma (1 applicant). During the study, one participant experienced mild nausea and vomiting which resolved at the beginning of Day 0 and on Day 22 and stomachache and vomiting on Day 23 that were probably related to the probe substrate cocktail, and missed the blood draws at 0–0.75 h on Day 0 and on Days 23–25. Therefore, this participant was excluded from the pharmacokinetic analysis. One participant reported an aphthous ulcer on her upper lip, moderate headache on Day 2, and irritated throat-post-nasal drip. This participant had a history of such ulcers, so that the events were probably unrelated to the drug cocktail or the red clover supplement. Another participant reported a moderate headache on Day 2, and another reported post-nasal drip/throat irritation on Day 2, both of which were unlikely to be related to the drug cocktail or the red clover supplement intervention. The other 12 participants reported no adverse events. Counting the returned red clover supplement capsules indicated that compliance ranged from 92.8% to 100% with 14 participants exhibiting 100% compliance.
The mean age of the participants (N=15) was 56.20 ± 8.56 years with a range of 41 – 73 years, and the mean BMI was 28.37 ± 6.02 kg/m2 (Table 1). Eight participants were African American and 7 were Caucasian. Three participants were ethnically Hispanic, and 11 were post-menopausal. Metabolic panels of serum (Table 1) were obtained before and after the red clover supplement intervention, and the data were compared using paired two-way t-test for each parameter. Most serum metabolic parameters such as liver enzymes, bilirubin, creatinine, and BUN showed no changes following red clover supplementation. Although serum sodium decreased 0.85%, CO2 concentration decreased 3.15%, and fasting glucose increased 15.46% from Day 0 to Day 22, these values remained within normal limits.
Table 1.
Demographic and clinical characteristics of evaluated participants (N=15) before (Day 0) and after (Day 22) red clover dietary supplement intervention. Data are expressed as mean ± standard deviation (SD). BMI: body mass index; BUN: blood urea nitrogen; ALK: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase.
| Parameter | Day 0 | Day 22 | %Change in mean | p-value |
|---|---|---|---|---|
| Age (years) | 56.20 ± 8.56 | |||
| Race (N) | ||||
| White | 7 (46.7%) | |||
| African American | 8 (53.3%) | |||
| Ethnicity (N) | ||||
| Hispanic | 3 (20.0%) | |||
| Non-Hispanic | 12 (80.0%) | |||
| Menopausal status (N) | ||||
| Post-menopausal | 11 (73.3%) | |||
| Peri-menopausal | 4 (26.7%) | |||
| BMI (kg/m2) | 28.37 ± 6.02 | |||
| Sodium (mmol/L) | 141.27 ± 1.62 | 140.07 ± 1.49 | −0.85 | 0.0086 |
| Potassium (mmol/L) | 4.05 ± 0.34 | 4.01 ± 0.34 | −0.82 | 0.781 |
| Chloride (mmol/L) | 106.53 ± 1.96 | 106.27 ± 1.71 | −0.25 | 0.658 |
| Anion gap (mmol/L) | 7.20 ± 1.82 | 7.00 ± 1.20 | −2.78 | 0.638 |
| Glucose (fasting; mg/dL) | 93.13 ± 17.41 | 107.53 ± 19.86 | 15.46 | 0.0467 |
| Glucose (0.5 h; mg/dL) | 107.00 ± 18.29 | 103.07 ± 4.19 | −3.67 | 0.374 |
| BUN (mg/dL) | 14.47 ± 3.8 | 14.93 ± 3.45 | 3.23 | 0.509 |
| Creatinine (mg/dL) | 0.78 ± 0.18 | 0.77 ± 0.17 | −1.03 | 0.517 |
| BUN/Creatinine ratio | 19.53 ± 6.18 | 20.05 ± 5.59 | 2.63 | 0.589 |
| Bilirubin (mg/dL) | 0.48 ± 0.23 | 0.47 ± 0.21 | −1.39 | 0.849 |
| ALK (U/L) | 71.60 ± 13.68 | 73.40 ± 14.17 | 2.51 | 0.329 |
| ALT (U/L) | 11.80 ± 3.63 | 11.53 ± 4.09 | −2.26 | 0.628 |
| AST (U/L) | 15.80 ± 3.78 | 15.40 ± 3.38 | −2.53 | 0.680 |
| Albumin (g/dL) | 3.75 ± 0.17 | 3.72 ± 0.22 | −0.89 | 0.465 |
| Protein (g/dL) | 6.33 ± 0.43 | 6.29 ± 0.44 | −0.63 | 0.585 |
| Calcium (mg/dL) | 8.93 ± 0.27 | 9.05 ± 0.36 | 1.34 | 0.0634 |
| CO2 (mmol/L) | 27.53 ± 2.00 | 26.67 ± 2.26 | −3.15 | 0.0484 |
The concentration-time curves averaged from 15 participants (Figure 3) and individual participant AUC values (Figure 4) indicated that red clover supplementation for 2 weeks produced no clinically relevant changes in the pharmacokinetics profiles of any of the 4 probe substrates. Similar to the AUC values, none of the CYP probe substrates showed significant changes in T1/2, Tmax or CL/F (Table 2). However, the Cmax values of caffeine and alprazolam increased 13.9% from 3045 to 3469 μg/L and 24.4% from 29.64 to 36.88 μg/L, respectively.
Figure 3.
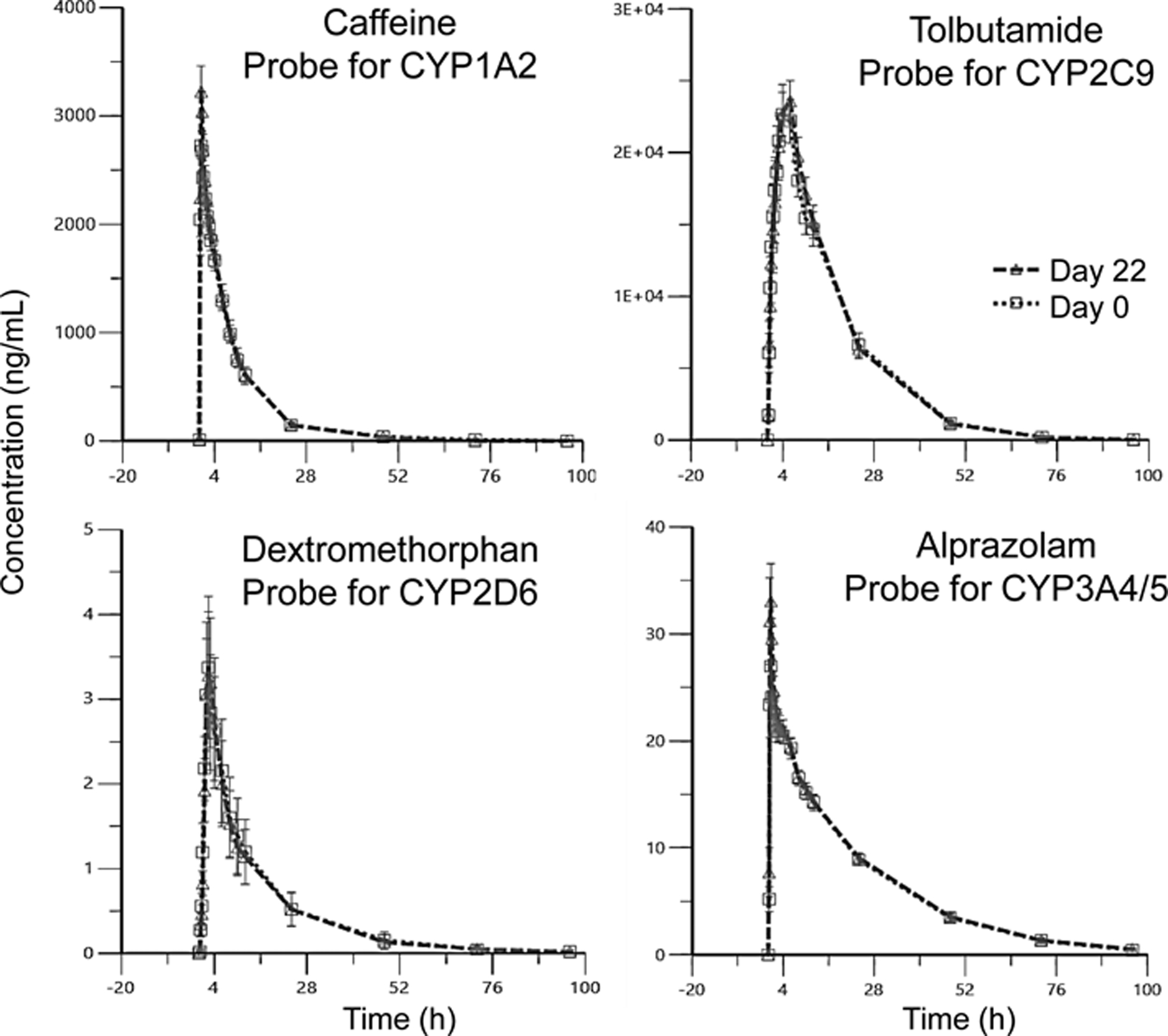
Average concentration-time curves for each CYP probe substrate for 15 participants. (Day 0/Day 22 = before/after intervention with red clover dietary supplement). Error bars denote ± standard error (SE).
Figure 4.
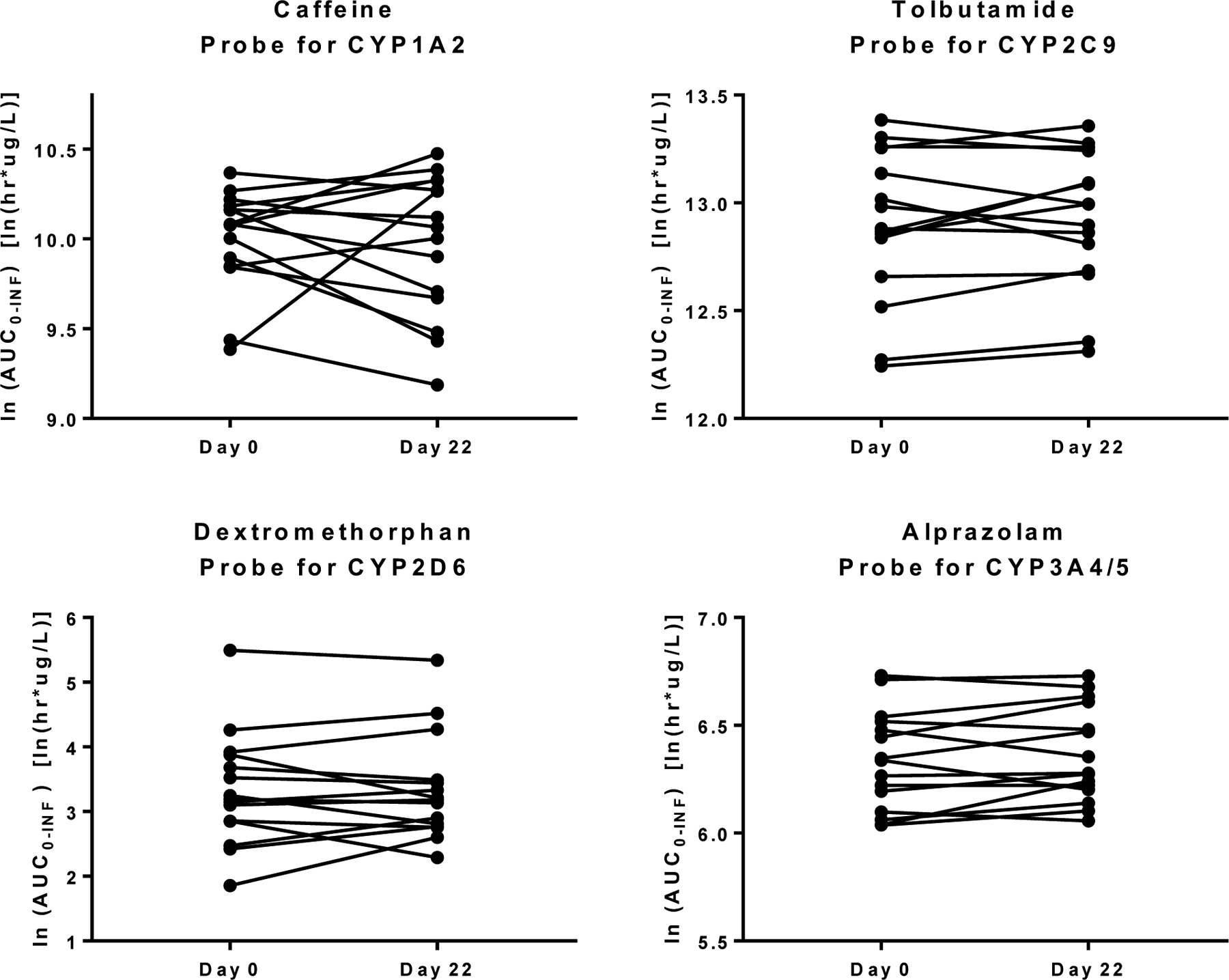
Comparison of individual AUC values for each probe substrate before (Day 0) and after (Day 22) consuming a red clover dietary supplement for 2 weeks.
Table 2.
Pharmacokinetic parameters of each probe substrate before (Day 0) and after (Day 22) red clover dietary supplementation for 2 weeks in perimenopausal and postmenopausal women (N=15). Data are expressed as [ln(mean) ± ln(standard error)]. GR (22:0) = geometric means ratio of Day 22 to Day 0; 90% CI = 90% confidence interval for the GR (22:0).
| Substrate (Enzyme) | PK parameter | Day 0 | Day 22 | %Change in mean | GR (22:0) | 90% CI | p-value |
|---|---|---|---|---|---|---|---|
|
Caffeine (CYP1A2) |
AUC (h*μg/L) | 10.00 ± 0.07 | 9.97 ± 0.10 | 0.74 | 0.97 | (0.82; 1.15) | 0.932 |
| T1/2 (h) | 1.65 ± 0.07 | 1.56 ± 0.08 | −7.59 | 0.91 | (0.80; 1.05) | 0.310 | |
| Tmax (h) | −0.57 ± 0.16 | −0.70 ± 0.10 | −23.81 | 0.87 | (0.62; 1.24) | 0.324 | |
| Cmax (μg/L) | 7.98 ± 0.07 | 8.12 ± 0.07 | 13.92 | 1.15 | (1.04; 1.27) | 0.0355 | |
| CL/F (L/h) | 1.51 ± 0.07 | 1.54 ± 0.10 | 6.51 | 1.03 | (0.87; 1.21) | 0.566 | |
|
Tolbutamide (CYP2C9) |
AUC (h*μg/L) | 12.90 ± 0.09 | 12.93 ± 0.08 | 1.74 | 1.03 | (0.97; 1.09) | 0.637 |
| T1/2 (h) | 2.16 ± 0.05 | 2.20 ± 0.05 | 4.12 | 1.04 | (0.99; 1.10) | 0.195 | |
| Tmax (h) | 1.24 ± 0.16 | 1.37 ± 0.13 | 9.09 | 1.14 | (0.91; 1.42) | 0.433 | |
| Cmax (μg/L) | 10.15 ± 0.06 | 10.17 ± 0.06 | 1.91 | 1.01 | (0.92; 1.12) | 0.735 | |
| CL/F (L/h) | −0.47 ± 0.09 | −0.50 ± 0.08 | −3.91 | 0.97 | (0.91; 1.03) | 0.252 | |
|
Dextromethorphan (CYP2D6) |
AUC (h*μg/L) | 3.33 ± 0.22 | 3.34 ± 0.20 | −3.10 | 1.01 | (0.85; 1.21) | 0.726 |
| T1/2 (h) | 2.20 ± 0.08 | 2.24 ± 0.08 | 3.62 | 1.04 | (0.97; 1.12) | 0.322 | |
| Tmax (h) | 0.76 ± 0.11 | 0.91 ± 0.10 | 14.89 | 1.17 | (0.95; 1.44) | 0.366 | |
| Cmax (μg/L) | 1.13 ± 0.19 | 1.11 ± 0.19 | 3.41 | 0.98 | (0.75; 1.27) | 0.815 | |
| CL/F (L/h) | 6.98 ± 0.22 | 6.97 ± 0.20 | −7.69 | 0.99 | (0.83; 1.18) | 0.615 | |
|
Alprazolam (CYP3A4/5) |
AUC (h*μg/L) | 6.34 ± 0.06 | 6.36 ± 0.06 | 2.77 | 1.03 | (0.98; 1.08) | 0.295 |
| T1/2 (h) | 2.79 ± 0.05 | 2.80 ± 0.06 | 0.43 | 1.00 | (0.95; 1.06) | 0.880 | |
| Tmax (h) | −0.03 ± 0.20 | −0.40 ± 0.08 | −48.78 | 0.69 | (0.46; 1.04) | 0.0723 | |
| Cmax (μg/L) | 3.35 ± 0.08 | 3.54 ± 0.09 | 24.45 | 1.22 | (1.08; 1.36) | 0.0121 | |
| CL/F (L/h) | 1.27 ± 0.06 | 1.24 ± 0.06 | −3.15 | 0.97 | (0.93; 1.02) | 0.241 |
The AUC values for each probe substrate were reanalyzed based on the demographic characteristics of the participants including BMI, menopausal status, race, and age (Table 3). There were no differences in AUC based on BMI, race or age. However, the AUC of dextromethorphan (probe of CYP2D6) was significantly different between peri-menopausal (−26.7 ± 9.6%) and post-menopausal (21.1 ± 12.5%) women.
Table 3.
Effects of red clover dietary supplementation on the areas under the concentration-time curve (AUC) for each probe substrate based on participant demographics. Data are expressed as mean ± standard error (SE).
| Demographics | % Change in AUC (mean ± SE) | ||||
|---|---|---|---|---|---|
| Caffeine (CYP1A2) |
Tolbutamide (CYP2C9) |
Dextromethorphan (CYP2D6) | Alprazolam (CYP3A4/5) |
||
| BMI | |||||
| 26.1–40 (N=7) | 11.07 ± 24.99 | 1.36 ± 5.48 | −4.18 ± 12.51 | 4.99 ± 3.75 | |
| p-value | 0.616 | 0.562 | 0.302 | 0.608 | |
| Meno-pausal status | |||||
| Post-menopausal (N=11) | 7.75 ± 15.65 | 3.93 ± 4.48 | 21.07 ± 12.54 | 4.10 ± 3.19 | |
| p-value | 0.658 | 0.928 | 0.0485 | 0.709 | |
| Race | |||||
| African American (N=8) | −1.32 ± 11.53 | 5.31 ± 4.46 | 16.97 ± 17.66 | 6.13 ± 2.36 | |
| p-value | 0.620 | 0.656 | 0.418 | 0.296 | |
| Age | |||||
| 56–73 (N=8) | −9.46 ± 10.39 | 3.02 ± 4.33 | 12.59 ± 11.72 | 1.67 ± 3.25 | |
| p-value | 0.219 | 0.845 | 0.692 | 0.482 | |
The adherence of participants to taking the red clover dietary supplement was additionally confirmed by measuring red clover isoflavones (Figure 5) and their metabolites (Figure 6) in serum from Day 22 of the protocol using UHPLC with high-resolution tandem mass spectrometry. Isoflavones were identified based on comparison with standards. In the administered red clover extract, the most abundant isoflavones were formononetin and biochanin A, which produced UHPLC-MS peak areas that were 12-fold and 11-fold larger, respectively, than the peaks for genistein and daidzein (Figure 5A). However, in the deconjugated participant serum (Figure 5B), daidzein and genistein predominated over formononetin and daidzein with peak area ratios of 22:16:2:1, respectively. These results are consistent with previous publications that formononetin and biochanin A form daidzein and genistein, respectively, through enzymatic O-demethylation.27,29,33 Therefore, these serum analyses confirmed that participants had consumed the red clover supplement.
Figure 5.
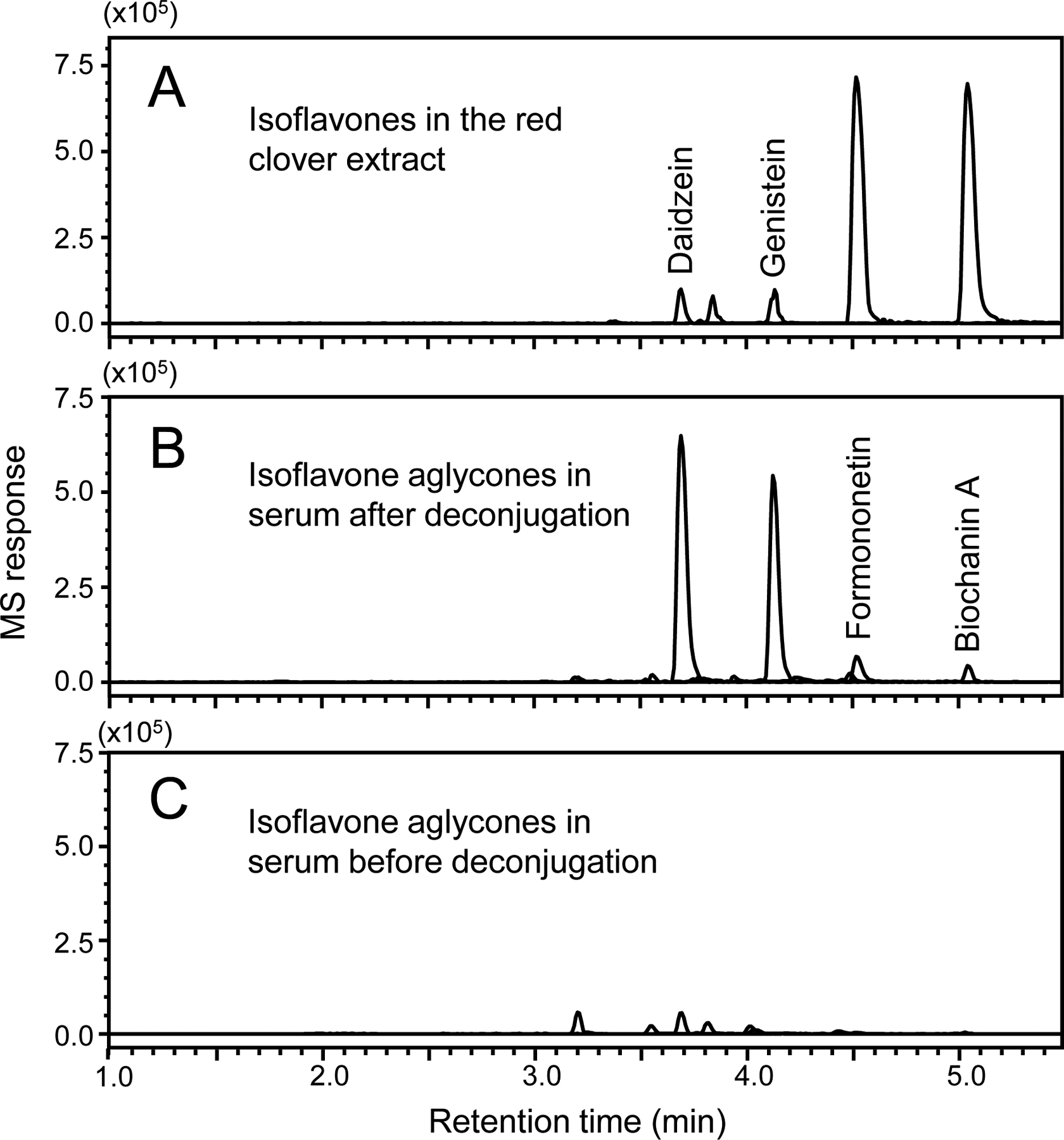
Negative ion electrospray UHPLC-MS mass chromatograms of the deprotonated red clover constituents daidzein, genistein, formononetin, and biochanin A in A) the red clover extract used in this study; B) deconjugated serum from a participant after consuming the red clover supplement for 2 weeks; and C) serum from the same participant drawn at the same time point but without deconjugation.
Figure 6.
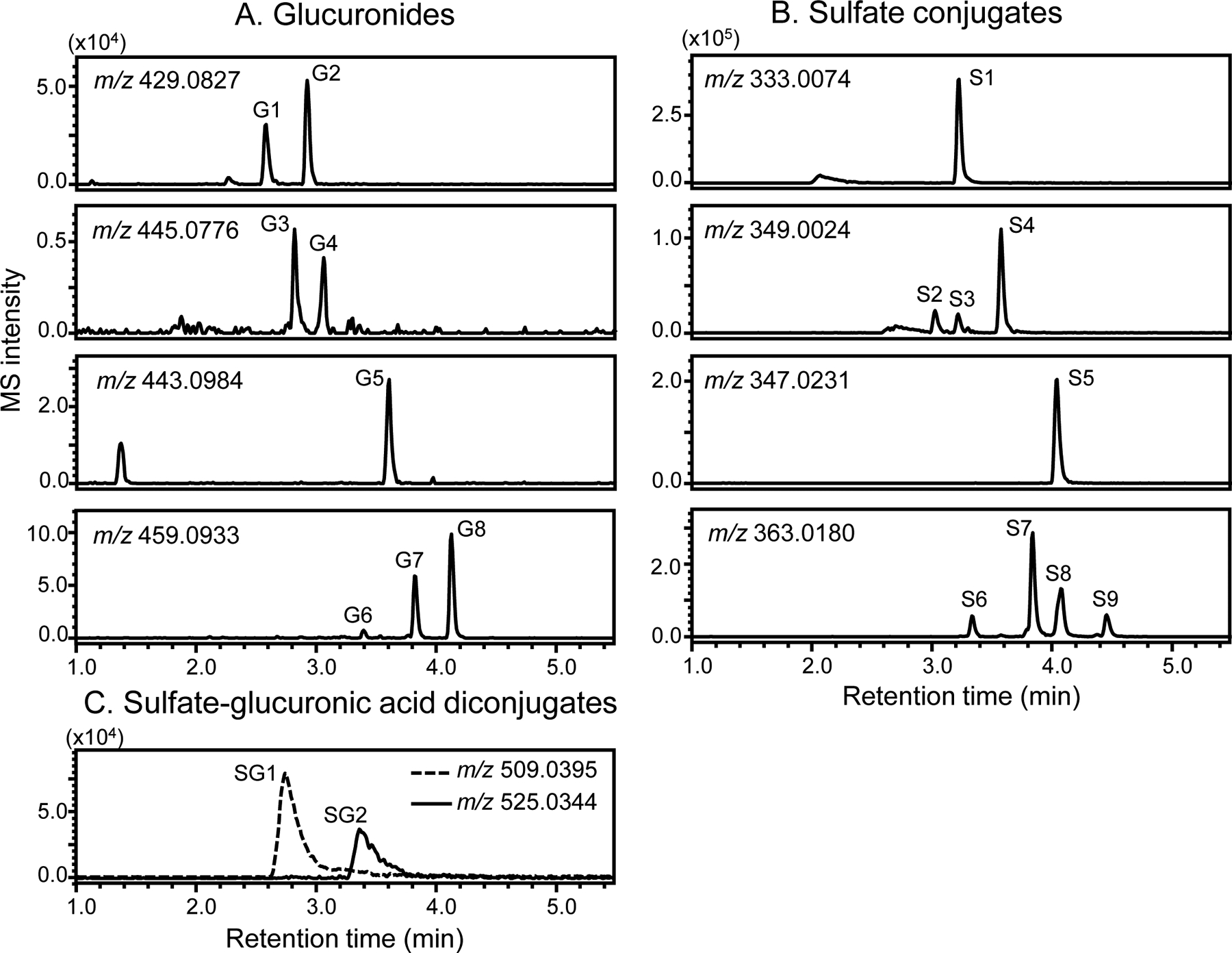
Negative ion electrospray UHPLC-MS high resolution mass chromatograms of the deprotonated molecules of Phase II metabolites of red clover constituents in human serum after consuming the red clover dietary supplement for 2 weeks.
By comparing the levels of red clover isoflavones in human serum with and without enzymatic deconjugation (Figure 5B and 5C), the vast majority of isoflavones were determined to be conjugated. These Phase II metabolites were profiled using high resolution UHPLC-MS (Figure 6) and UHPLC-MS/MS (Table 4). Characteristic fragmentation pathways of isoflavones including retro-Diels-Alder reaction and neutral losses of formaldehyde and ketene were used to facilitate the characterization and identification of these conjugated metabolites.37,38 Eight isoflavone monoglucuronides, 11 monosulfate conjugates and 2 monosulfate-monoglucuronic acid diconjugates were observed (Table 4).
Table 4.
High resolution negative ion electrospray LC-MS and LC-MS/MS analyses of red clover isoflavones and their metabolites in human serum after consumption of a red clover extract for 2 weeks (see chromatograms in Figures 5 and 6).
| Red clover compound | Retention time (min) | Observed m/z; [M-H]− | Mass error; ppm | MS/MS fragment ions (Relative abundance) | Peak identification |
|---|---|---|---|---|---|
| Daidzein | 3.70 | 253.0511 C15H9O4 |
3.95 | 91.0197 (23); 132.0213 (26); 133.0292 (16); 135.0084 (13); 180.0581 (14); 195.0453 (22); 196.0528 (11); 208.0536 (32); 209.0614 (12); 223.0404 (33); 224.0485 (24); 253.0509 (100) | Parent compound |
| Genistein | 4.14 | 269.0459 C15H9O5 |
3.34 | 91.0192 (7); 117.0346 (12); 132.0216 (23); 133.0294 (50); 159.0449 (19); 169.0657 (6); 180.0584 (12); 196.0535 (8); 201.0562 (10); 224.0483 (10); 269.0457 (100) | Parent compound |
| Formononetin | 4.52 | 267.0658 C16H11O4 |
0.37 | 91.0183 (15); 104.0262 (6); 132.0207 (27); 135.0078 (14); 167.0492 (13); 195.0441 (77); 208.0519 (12); 223.0391 (95); 224.0467 (15); 251.0338 (59); 252.0418 (100); 267.0649 (5) | Parent compound |
| biochanin A | 5.05 | 283.0606 C16H11O5 |
0.00 | 104.0262 (3); 132.0207 (17); 135.0076 (4); 167.0492 (12); 183.0437 (7); 184.0516 (3); 195.0439 (15); 211.0389 (45); 223.0389 (14); 239.0338 (49); 268.0366 (100); 283.0598 (8) | Parent compound |
| G1 | 2.58 | 429.0815 C21H17O10 |
−1.63 | 91.0180 (4); 107.0492 (6); 135.0074 (4); 169.0644 (3); 180.0570 (4); 195.0450 (5); 208.0530 (12); 209.0598 (5); 223.0386 (12); 224.0465 (11); 253.0493 (100) | Daidzein-7-O- glucuronide |
| G2 | 2.92 | 429.0817 C21H17O10 |
−1.16 | 91.0183 (8); 117.0337 (100); 133.0285 (10); 180.0568 (8); 195.0436 (4); 208.0521 (11); 209.0595 (12); 211.0386 (14); 224.0465 (17); 225.0558 (7); 253.0491 (100) | Daidzein-4’-O- glucuronide |
| G3 | 2.82 | 445.0771 C21H17O11 |
0.00 | 91.0192 (4); 133.0287 (30); 160.0163 (9); 195.0435 (5); 213.0536 (6); 224.0483 (5); 225.0548 (4); 239.0402 (5); 241.0494 (5); 269.0438 (100) | Genistein-7-O- glucuronide |
| G4 | 3.06 | 445.0772 C21H17O11 |
0.22 | 93.0339 (6); 113.0241 (3); 133.0284 (15); 159.0448 (6); 197.0833 (3); 201.0549 (6); 204.0656 (3); 225.0544 (5); 269.0438 (100) | Genistein-4’-O-glucuronide |
| G5 | 3.61 | 443.0981 C22H19O10 |
0.68 | 132.0207 (5); 165.0355 (6); 195.0442 (7); 208.0533 (8); 223.0387 (20); 252.0415 (100); 267.0647 (20) | Formononetin glucuronide |
| G6 | 3.42 | 459.0922 C22H19O11 |
−1.09 | 131.0147 (4); 167.0488 (5); 183.0449 (3); 195.0447 (12); 212.0474 (15); 223.0395 (21); 224.0470 (62); 239.0342 (25); 267.0277 (22); 268.0361 (100); 283.0602 (55) | Biochanin A or prunetin glucuronide |
| G7 | 3.85 | 459.0924 C22H19O11 |
−0.65 | 109.0301 (8); 133.0283 (16); 167.0486 (10); 183.0439 (7); 195.0437 (8); 211.0383 (13); 225.0546 (9); 239.0334 (20); 254.0571 (100); 268.0369 (41); 283.0596 (48) | Biochanin A or prunetin glucuronide |
| G8 | 4.15 | 459.0924 C22H19O11 |
−0.65 | 132.0205 (2); 195.0440 (2); 211.0389 (7); 224.0466 (3); 239.0338 (18); 267.0279 (21); 268.0360 (100); 283.059 (21) | Biochanin A or prunetin glucuronide |
| S1 | 3.22 | 333.0082 C15H9O7S |
3.90 | 91.0188 (7); 132.0214 (10); 135.0085 (6); 180.0579 (7); 195.0454 (8); 196.053 (6); 208.0533 (17); 209.0613 (8); 223.0403 (15); 224.0483 (16); 253.0509 (100) | Daidzein-4’-O-sulfate |
| S2 | 3.03 | 349.0014 C15H9O8S |
−1.15 | 117.0338 (54); 167.0489 (18); 169.0642 (87); 185.0597 (36); 195.0436 (13); 201.1115 (16); 213.0547 (43); 224.0464 (33); 225.0543 (53); 241.0488 (32); 269.0434 (100) | Genistein sulfate |
| S3 | 3.21 | 349.0030 C15H9O8S |
3.44 | 91.0187 (10); 119.0498 (10); 133.0291 (100); 135.0087 (15); 167.0499 (5); 183.0441 (11); 195.0462 (15); 213.0559 (15); 223.0408 (11); 224.0479 (13); 239.0347 (12); 251.0343 (10); 269.0453 (64) | Genistein sulfate |
| S4 | 3.57 | 349.0032 C15H9O8S |
4.01 | 133.0288 (25); 159.0441 (11); 169.0638 (4); 183.0437 (6); 196.0520 (4); 201.0545 (6); 213.0540 (2); 223.0394 (3); 224.0468 (7); 269.0440 (100) | Genistein sulfate |
| S5 | 4.03 | 347.0222 C16H11O7S |
−0.86 | 91.0182 (2); 132.0209 (8); 135.0077 (4); 167.0492 (1); 195.0440 (15); 208.0518 (5); 223.0389 (29); 251.0335 (21); 252.0414 (100); 267.0647 (24) | Formononetin sulfate |
| S6 | 3.33 | 363.0170 C16H11O8S |
−1.38 | 184.0523 (1); 195.0442 (2); 196.0514 (2); 211.0392 (13); 224.0467 (5); 239.0332 (18); 251.0353 (3); 267.0265 (6); 268.0361 (100); 283.0599 (3) | Biochanin A or prunetin sulfate |
| S7 | 3.84 | 363.0173 C16H11O8S |
−0.55 | 167.0490 (11); 175.0024 (5); 183.0436 (5); 195.0439 (21); 211.039 (9); 212.0468 (15); 223.0388 (19); 224.0468 (59); 239.0335 (21); 268.0362 (100); 283.0595 (56) | Biochanin A or prunetin sulfate |
| S8 | 4.07 | 363.0170 C16H11O8S |
−1.38 | 133.0282 (25); 167.0489 (7); 185.0593 (26); 195.0446 (11); 211.0391 (22); 224.0471 (21); 239.0332 (27); 254.0569 (94); 268.0354 (94); 283.06 (100) | Biochanin A or prunetin sulfate |
| S9 | 4.45 | 363.0172 C16H11O8S |
−0.82 | 135.0078 (2); 154.0414 (1); 167.0491 (2); 195.0437 (3); 211.0388 (11); 224.0465 (3); 239.0335 (18); 240.0413 (5); 267.0282 (23); 268.0361 (100); 283.0596 (33) | Biochanin A or prunetin sulfate |
| SG1 | 2.75 | 509.0403 C21H17O13S |
2.55 | 133.0279 (1); 169.0648 (1); 197.0598 (1); 213.0554 (1); 224.0464 (1); 225.0544 (1); 241.0489 (3); 268.0357 (2); 269.0439 (100); 349.0009 (5) | Daidzein sulfate-glucuronide |
| SG2 | 3.38 | 525.0333 C21H17O14S |
−1.14 | 91.0184 (1); 117.0343 (4); 135.0082 (4); 180.0581 (1); 195.0453 (3); 209.061 (3); 224.0479 (4); 225.0561 (5); 253.0507 (100); 333.0077 (3) | Genistein sulfate-glucuronide |
Metabolites G1 and G2 (Figure 6A) formed deprotonated molecules of m/z 429.0815 and 429.0817 (Table 4), respectively, which correspond to an elemental composition of C21H18O10. The base peak of the tandem mass spectra of G1 and G2 was detected at m/z 253, corresponding to loss of dehydrated glucuronic acid (Table 4). Based on elemental composition, loss of glucuronic acid and fragment ions characteristic of isoflavones, G1 and G2 were determined to be monoglucuronides of daidzein. Based on previous reports that daidzein-7-O-glucuronide eluted earlier than daidzein-4’-O-glucuronide during reverse phase HPLC,39,40 G1 and G2 were determined to be daidzein-7-O-glucuronide and daidzein-4’-O-glucuronide (predominant isomer), respectively. In a similar manner, G3 and G4, which produced deprotonated molecules of m/z 445.0771 and 445.0772 (corresponding to a molecular formula of C21H18O11) (Table 4), were identified as genistein-7-O-glucuronide and genistein-4’-O-glucuronide, respectively.
Metabolite G5 was identified as formononetin glucuronide based on a deprotonated molecule of m/z 443.0981 (C22H20O10) and a product ion tandem mass spectrum which included elimination of dehydrated glucuronic acid at m/z 267 and other fragment ions characteristic of formononetin (m/z 132.0207, m/z 195.0442, m/z 208.0533, m/z 223.0387, m/z 252.0415, m/z 267.0647) (Table 4). The exact mass measurements of G6-G8 indicated the elemental composition of these metabolites to be C22H20O11. Based on the elemental composition and elimination of dehydrated glucuronic acid, [M-H-176]− at m/z 283, G6-G8 were probably biochanin A 7-O-glucuronide, biochanin A 5-O-glucuronide, and prunetin glucuronide.32,33
Monosulfate and sulfate-glucuronic acid diconjugates were also observed in human serum from Day 22 based on UHPLC with high-resolution tandem mass spectrometry. One sulfate conjugate of daidzein was detected, S1, which was probably daidzein-4’-O-sulfate. S2-S4 were sulfates of genistein, S5 was formononetin sulfate, S6-S11 were sulfates of biochanin A and prunetin. SG1 and SG2 were monosulfate-monoglucuronic acid diconjugates of daidzein and genistein, respectively.
Discussion
Except for the Cmax values of caffeine (probe substrate for CYP1A2) and alprazolam (probe of CYP3A4/5), supplementation with the red clover extract did not alter any pharmacokinetic parameters for the probe substrates of CYP1A2, CYP2D6, CYP3A4/5 (Table 2). According to the FDA Guidance for Clinical Drug Interaction Studies, clinically relevant pharmacokinetic interactions are defined as a decrease of at least 20% in AUC for induction and an increase of at least 1.25-fold in AUC for inhibition.16 Therefore, the changes in Cmax did not meet the criteria for clinically relevant CYP1A2 and CYP3A4/5 interactions.
Although the number of participants was small, possible effects of demographic characteristics on red clover-drug pharmacokinetic interactions were evaluated. The AUC changes for each probe substrate were determined for each participant, participants were grouped with respect to BMI, race, menopausal status, and age, and the AUC changes due to red clover supplementation were compared using unpaired two-way t-test (Table 3). Only menopausal status showed a difference, and this was specifically for AUC changes for dextromethorphan (probe substrate for CYP2D6). Because there were only 4 peri-menopausal women out of 15 participants, this result is probably not conclusive. Also, there was considerable variation between subject in dextromethorphan metabolism due to inclusion of both intermediate and extensive metabolizing phenotypes for CYP2D6 (Figure 3). Similar phenotype variations in dextromethorphan CYP2D6 pharmacokinetics have been reported previously.17
Although fasting serum glucose levels increased 15.41% from Day 0 to Day 22, both glucose concentrations (93.13 mg/dL and 107.53 mg/dL; Table 1) were within the normal range. Instead of raising glucose levels, red clover and formononetin have been reported to produce antidiabetic effects in mice and rats,41–44 and Cheng et al.45 reported that daidzein and genistein lower blood glucose and insulin levels in postmenopausal women. Small decreases in serum sodium and CO2 (0.85% and 3.15%, respectively) were also observed following consumption of the red clover supplement. Nevertheless, both sodium and CO2 concentrations remained within healthy limits. There are no published reports of increases in serum glucose or decreases in sodium or CO2 levels that has been associated with consumption of red clover supplements or red clover isoflavones.
Red clover, soy, garbanzo beans, blackeyed beans, alfalfa sprouts, and many other botanicals consumed either as dietary supplements or conventional foods contain isoflavones such as formononetin, biochanin A, genistein, prunetin, irilone, and daidzein.7–10 These isoflavones undergo rapid and extensive Phase II metabolism, forming conjugates with glucuronic acid and sulfate. The prevalence of these polar metabolites and the low serum concentrations of unconjugated isoflavones might contribute to the discrepancy between the in vitro models predicting red clover-drug pharmacokinetic interactions, isoflavone-drug pharmacokinetic interactions, and the present trial clinical results. Another reason for this discrepancy might be the high serum binding of isoflavones,46 which would lower the concentrations of unconjugated isoflavones available for pharmacokinetic interactions. Therefore, although in vitro assays predicted in vivo drug-botanical interactions, the present clinical trial safety data suggest no clinically relevant interactions of red clover dietary supplements with the important cytochrome P450 enzymes CYP1A2, CYP2C9, CYP2D6, or CYP3A4/5.
Acknowledgment
We thank Shimadzu Scientific Instruments for providing the Nexera LCMS-9030 Q-ToF and LCMS-8060 triple quadrupole mass spectrometer systems used during this investigation.
Funding Sources
This research was supported by grant P50 AT000155 from the Office of Dietary Supplements and the National Center for Complementary and Integrative Health of the National Institutes of Health. Clinical services were provided by the UIC Center for Clinical and Translational Science supported by grant UL1TR002003 from the National Center for Advancing Translational Sciences.
Abbreviations used
- AUC
area under concentration-time curve
- CL/F
oral clearance rate
- CI
confidence interval
- CYP
cytochrome P450
- T1/2
elimination half-life
- Cmax
peak serum concentration
- Q-ToF
quadrupole time-of-flight
- SE
standard error
- SD
standard deviation
- Tmax
time to reach peak concentration
- UHPLC-MS/MS
ultra-high pressure liquid chromatography-tandem mass spectrometry
- BMI
body mass index
- BUN
blood urea nitrogen
- ALK
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
Footnotes
Clinical Trial Registration
This study was registered on ClinicalTrials.gov (NCT03205787).
Conflict of Interest/Disclosure
The authors have no conflicts of interest to disclose.
References
- 1.The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2018, 25, 1362–1387. [DOI] [PubMed] [Google Scholar]
- 2.Rossouw JE; Anderson GL; Prentice RL; LaCroix AZ; Kooperberg C; Stefanick ML; Jackson RD; Beresford SA; Howard BV; Johnson KC; Kotchen JM; Ockene J Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. J. Am. Med. Assoc 2002, 288, 321–333. [DOI] [PubMed] [Google Scholar]
- 3.Lambert MNT; Thybo CB; Lykkeboe S, Rasmussen LM; Frette X; Christensen LP; Jeppesen PB Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: a randomized controlled trial. Am J Clin Nutr. 2017, 106, 909–920. [DOI] [PubMed] [Google Scholar]
- 4.Dietz BM; Hajirahimkhan A; Dunlap TL; Bolton JL Botanicals and their bioactive phytochemicals for women’s health. Pharmacol. Rev 2016, 68, 1026–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fritz H; Seely D; Flower G; Skidmore B; Fernandes R; Vadeboncoeur S; Kennedy D; Cooley K; Wong R; Sagar S; Sabri E; Fergusson D Soy, red clover, and isoflavones and breast cancer: a systematic review. PLoS One. 2013, 8(11);e81968. doi: 10.1371/journal.pone.0081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mainini G; Torella M; Di Donna MC; Esposito E; Ercolano S; Correa R; Cucinella G; Stradella L; Luisi A; Basso A; Cerreto FV; Cicatiello R; Matteo M; De Franciscis P Nonhormonal management of postmenopausal women: effects of a red clover based isoflavones supplementation on climacteric syndrome and cardiovascular risk serum profile. Clin. Exp. Obstet. Gynecol 2013, 40, 337–341. [PubMed] [Google Scholar]
- 7.Muchiri RN; van Breemen RB Single laboratory validation of UHPLC-MS/MS assays for red clover isoflavones in human serum and dietary supplements. J. AOAC Int 2020, 103, 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franke AA; Custer LJ; Cerna CM; Narala K Rapid HPLC analysis of dietary phytoestrogens from legumes and from human urine. Proc. Soc. Exp. Biol. Med 1995, 208, 18–26. [DOI] [PubMed] [Google Scholar]
- 9.Mazur WM; Duke JA; Wähälä K; Rasku S; Aldercreutz H Isoflavononoids and lignans in legumes: Nutritional and health aspects in humans. Nutr. Biochem 1998, 9, 193–200. [Google Scholar]
- 10.Murphy PA; Song T; Buseman G; Barua K,; Beecher GR; Trainer D; Holden J Isoflavones in retail and institutional soy foods. J. Agric. Food Chem 1999, 47, 2697–2704. [DOI] [PubMed] [Google Scholar]
- 11.Gardiner P; Graham RE; Legedza ATR; Eisenberg DM; Phillips RS Factors associated with dietary supplement use among prescription medication users. Arch. Intern. Med 2006, 166, 1968–1974. [DOI] [PubMed] [Google Scholar]
- 12.Sprouse AA; van Breemen RB Pharmacokinetic interactions between drugs and botanical dietary supplements. Drug Metab. Dispos 2016, 44, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shugarts S; Benet LZ The role of transporters in the pharmacokinetics of orally administered drugs. Pharm. Res 2009, 26, 2039–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shitara Y; Horie T; Sugiyama Y Transporters as a determinant of drug clearance and tissue distribution. Eur. J. Pharm. Sci 2006, 27, 425–446. [DOI] [PubMed] [Google Scholar]
- 15.Zanger UM; Schwab M Cytochrome P450 enzymes in drug metabolism: regulation of gene expression; enzyme activities, and impact of genetic variation. Pharmacol. Ther 2013, 138, 103–141. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Food & Drug Administration. Clinical Drug Interaction Studies — Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions Guidance for Industry January 2020. URL https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions (accessed 22 October 2020)
- 17.van Breemen RB; Chen L; Tonsing-Carter A; Banuvar S; Barengolts E; Viana M; Chen SN; Pauli GF; Bolton JB Pharmacokinetic interactions of a hop dietary supplement with drug metabolism in perimenopausal and postmenopausal women. J. Agric. Food Chem 2020, 68, 5212–5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurley BJ; Gardner SF; Hubbard MA; Williams DK; Gentry WB; Khan IA; Shah A In vivo effects of goldenseal; kava kava; black cohosh; and valerian on human cytochrome P450 1A2, 2D6, 2E1, and 3A4/5 phenotypes. Clin. Pharmacol. Ther 2005, 77, 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurley BJ; Gardner SF; Hubbard MA; Williams DK; Gentry WB; Carrier J; Khan IA; Edwards DJ; Shah A In vivo assessment of botanical supplementation on human cytochrome P450 phenotypes: Citrus aurantium, Echinacea purpurea, milk thistle, and saw palmetto. Clin. Pharmacol. Ther 2004, 76, 428–440. [DOI] [PubMed] [Google Scholar]
- 20.Gorski JC; Huang SM; Pinto A; Hamman MA; Hilligoss JK; Zaheer NA; Desai M; Miller M; Hall SD The effect of echinacea (Echinacea purpurea root) on cytochrome P450 activity in vivo. Clin. Pharmacol. Ther 2004, 75, 89–100. [DOI] [PubMed] [Google Scholar]
- 21.Zadoyan G; Rokitta D; Klement S; Dienel A; Hoerr R; Gramatté T; Fuhr U Effect of Ginkgo biloba special extract EGb 761® on human cytochrome P450 activity: a cocktail interaction study in healthy volunteers. Eur. J. Clin. Pharmacol 2012, 68, 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawaguchi-Suzuki M; Frye RF; Zhu HJ; Brinda BJ; Chavin KD; Bernstein HJ; Markowitz JS The effects of milk thistle (Silybum marianum) on human cytochrome P450 activity. Drug Metab. Dispos 2014, 42, 1611–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zahner C; Kruttschnitt E; Uricher J; Lissy M; Hirsch M; Nicolussi S; Krähenbühl S; Drewe J No clinically relevant interactions of St. John’s wort extract Ze 117 low in hyperforin with cytochrome P450 enzymes and P-glycoprotein. Clin. Pharmacol. Ther 2019, 106, 432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arora S; Taneja I; Challagundla M; Raju KS; Singh SP; Wahajuddin M In vivo prediction of CYP-mediated metabolic interaction potential of formononetin and biochanin A using in vitro human and rat CYP450 inhibition data. Toxicol. Lett 2015, 239, 1–8. [DOI] [PubMed] [Google Scholar]
- 25.Kopečná-Zapletalová M; Krasulová K; Anzenbacher P; Hodek P; Anzenbacherová E Interaction of isoflavonoids with human liver microsomal cytochromes P450: inhibition of CYP enzyme activities. Xenobiotica. 2017, 47, 324–331. [DOI] [PubMed] [Google Scholar]
- 26.Sato Y; Sasaki T; Takahashi S; Kumagai T; Nagata K Development of a highly reproducible system to evaluate inhibition of cytochrome P450 3A4 activity by natural medicines. J. Pharm. Pharm. Sci 2015, 18, 316–327. [DOI] [PubMed] [Google Scholar]
- 27.Roberts DW; Doerge DR; Churchwell MI; Gamboa da Costa G; Marques MM; Tolleson WH Inhibition of extrahepatic human cytochromes P450 1A1 and 1B1 by metabolism of isoflavones found in Trifolium pratense (red clover). J. Agric. Food Chem 2004, 52, 6623–6632. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y; Gho WM; Chan FL; Chen S; Leung LK The red clover (Trifolium pratense) isoflavone biochanin A inhibits aromatase activity and expression. Br. J. Nutr 2008, 99, 303–310. [DOI] [PubMed] [Google Scholar]
- 29.Howes J; Waring M; Huang L; Howes LG Long-term pharmacokinetics of an extract of isoflavones from red clover (Trifolium pratense). J. Altern. Complement. Med 2002, 8, 135–142. [DOI] [PubMed] [Google Scholar]
- 30.Rodríguez-Morató J; Farré M; Pérez-Mañá C; Papaseit E; Martínez-Riera R; de la Torre R; Pizarro N Pharmacokinetic comparison of soy isoflavone extracts in human plasma. J. Agric. Food Chem 2015, 63, 6946–6953. [DOI] [PubMed] [Google Scholar]
- 31.Yuan JP; Wang JH; Liu X Metabolism of dietary soy isoflavones to equol by human intestinal microflora--implications for health. Mol. Nutr. Food Res 2007, 51, 765–781. [DOI] [PubMed] [Google Scholar]
- 32.Booth NL; Overk CR; Yao P; Burdette JE; Nikolic D; Chen SN; Bolton JL; van Breemen RB; Pauli GF; Farnsworth NR The chemical and biologic profile of a red clover (Trifolium pratense L.) phase II clinical extract. J. Altern. Complement. Med 2006, 12, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piersen CE; Booth NL; Sun Y; Liang W; Burdette JE; van Breemen RB; Geller SE; Gu C; Banuvar S; Shulman LP; Bolton JL; Farnsworth NR Chemical and biological characterization and clinical evaluation of botanical dietary supplements: a phase I red clover extract as a model. Curr. Med. Chem 2004, 11, 1361–1374. [DOI] [PubMed] [Google Scholar]
- 34.Geller SE; Shulman LP; van Breemen RB; Banuvar S; Zhou Y; Epstein G; Hedayat S; Nikolic D; Krause EC; Piersen CE; Bolton JL; Pauli GF; Farnsworth NR Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: a randomized controlled trial. Menopause. 2009, 16, 1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen L; van Breemen RB Validation of a sensitive UHPLC-MS/MS method for cytochrome P450 probe substrates caffeine; tolbutamide; dextromethorphan; and alprazolam in human serum reveals drug contamination of serum used for research. J. Pharm. Biomed. Anal 2020, 179, 112983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria, 2013, URL (https://www.r-project.org/) (accessed 23 April 2020) [Google Scholar]
- 37.Kang J; Hick LA; Price WE A fragmentation study of isoflavones in negative electrospray ionization by MSn ion trap mass spectrometry and triple quadrupole mass spectrometry. Rapid Commun. Mass Spectrom 2007, 21, 857–868. [DOI] [PubMed] [Google Scholar]
- 38.March RE; Miao X; Metcalfe CD; Stobiecki M; Marczak L A fragmentation study of an isoflavone glycoside; genistein-7-O-glucoside; using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. Int. J. Mass Spectrom 2004, 232, 171–183. [Google Scholar]
- 39.Hosoda K; Furuta T; Yokokawa A; Ogura K; Hiratsuka A; Ishii K Plasma profiling of intact isoflavone metabolites by high-performance liquid chromatography and mass spectrometric identification of flavone glycosides daidzin and genistin in human plasma after administration of kinako. Drug Metab. Dispos 2008, 36, 1485–1495. [DOI] [PubMed] [Google Scholar]
- 40.Yang Z; Zhu W; Gao S; Xu H; Wu B; Kulkarni K; Singh R; Tang L; Hu M Simultaneous determination of genistein and its four phase II metabolites in blood by a sensitive and robust UPLC-MS/MS method: Application to an oral bioavailability study of genistein in mice. J. Pharm. Biomed. Anal 2010, 53, 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oza MJ; Kulkarni YA Trifolium pratense (red clover) Improve SIRT1 expression and glycogen content in high fat diet-streptozotocin induced type 2 diabetes in rats. Chem. Biodivers 2020, 17, e2000019. [DOI] [PubMed] [Google Scholar]
- 42.Qiu L; Chen T; Zhong F; Hong Y; Chen L; Ye H Red clover extract exerts antidiabetic and hypolipidemic effects in db/db mice. Exp. Ther. Med 2012, 4, 699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiu G; Tian W; Huan M; Chen J; Fu H Formononetin exhibits anti-hyperglycemic activity in alloxan-induced type 1 diabetic mice. Exp. Biol. Med. (Maywood) 2017, 242, 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oza MJ; Kulkarni YA Formononetin treatment in type 2 diabetic rats reduces insulin resistance and hyperglycemia. Front. Pharmacol 2018, 18, 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng SY; Shaw NS; Tsai KS; Chen CY The hypoglycemic effects of soy isoflavones on postmenopausal women. J. Womens Health (Larchmt) 2004, 13, 1080–1086. [DOI] [PubMed] [Google Scholar]
- 46.Bolli A; Marino M; Rimbach G; Fanali G; Fasano M; Ascenzi P Flavonoid binding to human serum albumin. Biochem. Biophys. Res. Commun 2010, 398, 444–449 [DOI] [PubMed] [Google Scholar]


