Abstract
Psychological stress has long-been known to reduce adaptability to inflammatory challenges, although the precise mechanism has remained elusive. In the latest issue of Cell, Qing et al. demonstrate that psychological stress induces secretion of IL-6 from brown adipose tissue, which promotes hepatic gluconeogenesis, and reduces host fitness to inflammatory insults.
Considerable anecdotal evidence points to psychological stress causing “flare-ups” in inflammatory diseases, and these claims have been substantiated in several conditions such as autoimmune disorders (Roussou et al., 2013). This may seem paradoxical given that the most-studied mediators of stress physiology, glucocorticoids and catecholamines, are believed to largely be anti-inflammatory. Indeed, cortisone is routinely administered as an anti-inflammatory agent. It has been known that stress increases circulating levels of interleukin-6 (IL-6) (Frank et al., 2013). Despite this, there has not been an in-depth study examining the relationship between IL-6 and stress-induced decreases in host fitness to inflammatory insult. In the latest issue of Cell, Qing et al. (2020) report that in murine models, psychological induces the release of IL-6 from brown adipose tissue (BAT), which acts to stimulate hepatic gluconeogenesis to promote the “fight or flight” response (Figure 1A). Qing et al. have discovered that this action, unfortunately, has the trade-off of reducing host fitness to inflammatory challenges.
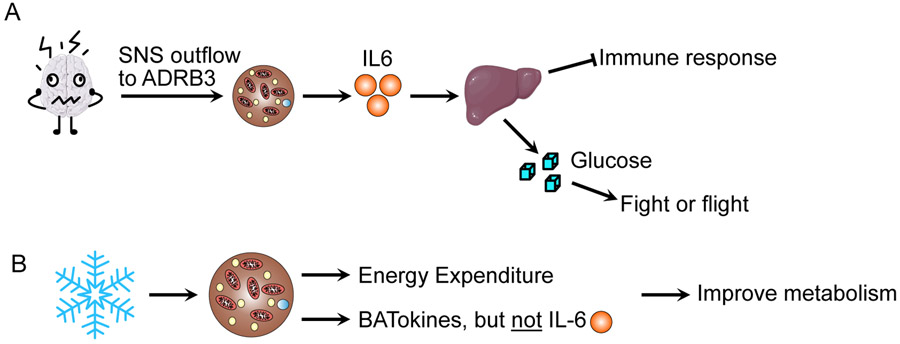
Figure 1. Brown fat secretion of IL-6: It depends on the stimulus.
A. Qing et al. demonstrated that in murine models, psychological stress induces brown fat to release IL-6, which increases hepatic gluconeogenesis. Unfortunately, this results in impaired host tolerance to inflammatory challenges. B. Normal activation of brown adipose tissue through cold exposure causes the release of BATokines that influence metabolism; however, it does not induce the release of IL-6.
To identify inflammatory cytokines induced by stress, Qing et al. use retro-orbital bleeding and cage restraints as stressors and noted that IL-6 was highly induced. Indeed, IL-6 levels in circulation were still detectible 18 hours post-bleed. This is in sharp contrast to corticosterone, which peaked 2 hours post-bleed, suggesting that IL-6 may mediate sustained aspects of the stress response. To identify the source of IL-6 production, Qing et al. screened over ten tissues for IL-6 expression following induced stress and found that BAT had the most robust increase in IL-6. Importantly, a BATectomy abrogated the increase in circulating IL-6 following a retro-orbital bleed. Since uncoupling protein 1 (UCP1) is the major thermogenic protein involved in BAT thermogenesis, the authors assessed stress-induced IL-6 production in Ucp1 knockout mice and found that IL-6 production is independent of UCP1 activity. Moreover, they found that housing mice at varying temperatures (increases or decreases thermogenesis) had no effect on stress-induced IL-6 production. This is a critical point that suggests normal activation of BAT does not increase IL-6 production (Figure 1B). Interestingly, Qing et al. noted that anesthetized mice did not have an increase in IL-6 production following a retro-orbital bleed, suggesting there must be sympathetic outflow to the BAT to stimulate IL-6 production. By administering 6-hydroxydopamine to inhibit sympathetic outflow to BAT, or β-adrenergic receptor agonists (such as CL316-243) and by using β3-adrenergic receptor (ADRB3) knockout mice, the authors determined that sympathetic outflow to ADRB3 in BAT is necessary for stress-induced IL-6 production. Taken together, these findings suggest that stress-induced IL-6 production happens in BAT following sympathetic outflow to ADRB3, and that circulating IL-6 may mediate a persistent response to stressful stimuli.
Normally, stress induces a shift towards catabolic processes, which is thought to provide metabolic fuel to aid the fight or flight response. Qing et al. hypothesized that IL-6 may be mediating this effect since antagonizing IL-6 prior to retro-orbital bleeding caused a shift in energy expenditure. The authors subsequently found that antagonizing IL-6 action reduced post-bleed increases in glycemia, suggesting that IL-6 was mediating stress-induced hyperglycemia. Since animals at post-bleed presented with normal glucose tolerance, the authors hypothesized that gluconeogenesis may be perturbed. Indeed, following acute stress, endogenous glucose production was increased in an IL-6-dependent manner, which was further confirmed by a pyruvate tolerance test using knockout mice for either IL-6 or Adrb3. Moreover, deleting the IL-6 receptor from hepatocytes was sufficient to abrogate hepatic gluconeogenesis in response to acute stress. Collectively, these data demonstrate that the IL-6 secreted from BAT in response to acute psychological stress is directly mediating an increase in glycemia via an upregulation of hepatic gluconeogenesis.
The final piece of this study linked acute stress, IL-6, and an impaired inflammatory response. The authors demonstrated that pretreating mice with stress resulted in increased mortality following lipopolysaccharide (LPS) treatment. Moreover, the authors found that pretreatment with ADRB3 agonists, or stress-dosed IL-6, was sufficient to increase LPS-induced mortality. The authors went on to demonstrate that in wildtype animals, but not in BAT-specific IL-6 knockout mice, acute stress caused renal damage, and trended to increase cardiac damage, suggesting that host tolerance to inflammation may be reduced following acute stress in an IL-6-dependent manner. While the authors note that the mechanism by which host tolerance is decreased remains unknown, there is a clear “trade-off” occurring, where stress-induced IL-6 produces glucose for the fight or flight response at the expense of fitness against inflammatory insult.
Unlike conventional white adipose tissue (WAT), BAT is thermogenic, and has garnered considerable interest as an anti-obesity therapeutic (Nedergaard and Cannon, 2018). Recently, BAT as a secretory organ is gaining considerable interest, and BATokines ranging from proteins to microRNA have been identified that can improve whole-body metabolism (Figure 1B) (Scheele and Wolfrum, 2020). Indeed, one such BATokine is IL-6, which was demonstrated to be responsible for the insulin sensitizing effect of BAT transplants (Stanford et al., 2013), similarly to its effect when secreted from muscle during exercise (Benrick et al., 2012). Importantly, IL-6 in these instances does not seem to have a negative trade-off with inflammatory processes. It is critical to note that natural BAT activators (e.g., cold exposure) do not cause an increase in IL-6 production. However, IL-6 secreted from WAT is typically thought to be pro-inflammatory, and impairs insulin sensitivity (Makki et al., 2013). This underscores the notion that IL-6 can function as both a pro- and anti-inflammatory cytokine (Carey et al., 2006; Castaneda et al., 2019). The mechanism behind why IL-6 can act as both a pro- and anti-inflammatory mediator remains unknown, but the types of stimulus, source of production, and target tissues may provide some clues.
An unresolved issue for individuals with chronic immune-related disorders is how stress worsens their conditions. Qing et al. add to our current knowledge by illustrating a complex brain-BAT-liver axis, where stress triggers the release of IL-6 from BAT, which promotes hepatic gluconeogenesis. Unfortunately, this comes at the expense of host fitness against inflammatory insults. While Qing et al. demonstrated that stress-induced production of IL-6 does occur in humans, its origin and function has not yet been defined, requiring further investigation. These data do, however, point to an exciting new area of research involving stress-produced cytokines, and their effects on immune physiology and beyond.
Acknowledgements
This work was supported in part by U.S. NIH grants R01DK077097 and R01DK102898, and by US Army Medical Research grant W81XWH-17-1-0428 (to Y.-H.T.), and by grant P30DK036836 (to Joslin Diabetes Center’s Diabetes Research Center; DRC) from the National Institute of Diabetes and Digestive and Kidney Diseases. J.D. was supported by NIH grant T32DK007260, and American Heart Association grant 20POST35210497. We apologize to the many scientists whose work we could not cite due to space limitations.
References
- Benrick A, Wallenius V, and Asterholm IW (2012). Interleukin-6 mediates exercise-induced increase in insulin sensitivity in mice. Exp Physiol 97, 1224–1235. [DOI] [PubMed] [Google Scholar]
- Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, et al. (2006). Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 55, 2688–2697. [DOI] [PubMed] [Google Scholar]
- Castaneda S, Remuzgo-Martinez S, Lopez-Mejias R, Genre F, Calvo-Alen J, Llorente I, Aurrecoechea E, Ortiz AM, Triguero A, Blanco R, et al. (2019). Rapid beneficial effect of the IL-6 receptor blockade on insulin resistance and insulin sensitivity in non-diabetic patients with rheumatoid arthritis. Clin Exp Rheumatol 37, 465–473. [PubMed] [Google Scholar]
- Frank MG, Watkins LR, and Maier SF (2013). Stress-induced glucocorticoids as a neuroendocrine alarm signal of danger. Brain Behav Immun 33, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makki K, Froguel P, and Wolowczuk I (2013). Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm 2013, 139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J, and Cannon B (2018). Brown adipose tissue as a heat-producing thermoeffector. Handb Clin Neurol 156, 137–152. [DOI] [PubMed] [Google Scholar]
- Qing H, Desrouleaux R, Israni-Winger K, Mineur YS, Fogelman N, Zhang C, Rashed S, Palm NW, Sinha R, Picciotto MR, et al. (2020). Origin and Function of Stress-Induced IL-6 in Murine Models. Cell. [DOI] [PubMed] [Google Scholar]
- Roussou E, Iacovou C, Weerakoon A, and Ahmed K (2013). Stress as a trigger of disease flares in SLE. Rheumatol Int 33, 1367–1370. [DOI] [PubMed] [Google Scholar]
- Scheele C, and Wolfrum C (2020). Brown Adipose Crosstalk in Tissue Plasticity and Human Metabolism. Endocr Rev 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, et al. (2013). Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123, 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]