Abstract
Fungi represent one of the most diverse and abundant eukaryotes on earth, and their ubiquity and small proteolytically active products make them pervasive allergens that affect humans and other mammals. The immunologic parameters surrounding fungal allergies are still not fully elucidated despite their importance given that a large proportion of severe asthmatics are sensitized to fungal allergens. Herein, we explore fungal allergic asthma with emphasis on mouse models that recapitulate the characteristics of human disease, and the main leukocyte players in the pathogenesis of fungal allergies. The endogenous mycobiome may also contribute to fungal asthma, a phenomenon that we discuss only superficially, as much remains to be discovered.
Keywords: Aspergillus, mouse models, mycobiome, eosinophils
1. Introduction
Symptoms of asthma have been described in ancient literature, with the first detailed description by Sir John Floyer dating back to 1698 [1]. Asthma is a respiratory syndrome with high incidence and economic burden worldwide [2,3]. This heterogenous syndrome has many subtypes and phenotypes, but typical symptoms generally include chest tightness, wheezing, and shortness of breath [4,5] that can result from narrowing of large airways due to heightened inflammation, mucus, and smooth muscle cell constriction (Figure 1). Asthma is generally classified as allergic and nonallergic, wherein allergic asthma is defined by the presence of atopy, i.e., allergen-specific IgE in plasma and positive skin-prick test to common aeroallergens [6,7,8,9]. Genetics, epigenetics, the endogenous microbiome, and environmental factors influence the development, exacerbation, and severity of allergic asthma [9,10,11]. During early development, genetics and epigenetics shape the immune system, thus playing an important foundational role in determining the allergic asthma endotype [12]. Sensitivity to indoor and outdoor aeroallergens results as a secondary trigger in individuals that are genetically prone to developing allergic asthma. Common aeroallergens include house dust mite and cockroach antigens, molds, and pollen [13].
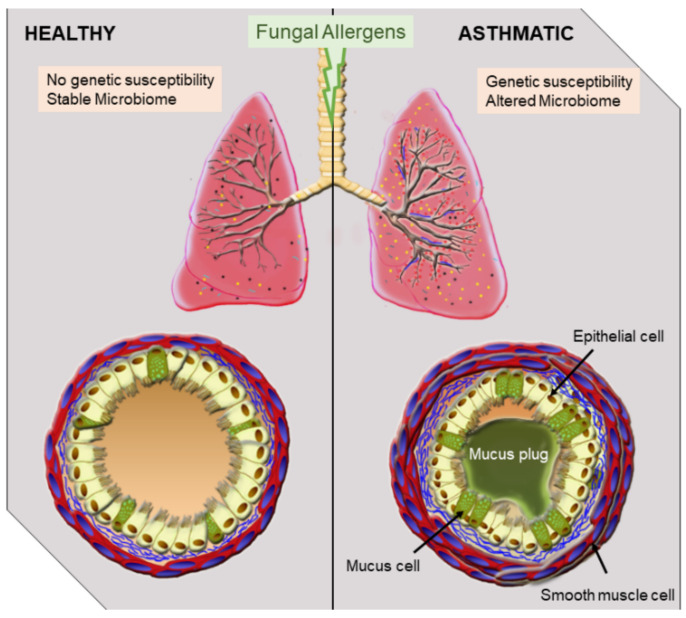
Figure 1.
Overview of airway pathophysiology in health and asthma. Asthma triggers include fungal allergens which are abundant in the environment. While healthy individuals do not respond to inhaled fungal allergens, in the presence of confounders including genetic susceptibility and an altered microbiome as in atopic individuals, fungal exposures can lead to alterations to the mucosa. Typical occurrences after fungal exposure in asthmatics include inflammation of the airways, mucus hyperproduction, smooth muscle thickening, and remodeling events.
Fungi are ubiquitous in both indoor and outdoor environments and widespread worldwide, making up the largest organic component in air particulates [14,15]. Owing to effective antifungal host mechanisms such as antimicrobial peptides, effective clearance by the mucociliary escalator, and macrophage phagocytosis, most often, fungi fail to cause infections in immunosufficient individuals. However, fungal components are important aeroallergens for asthma development, exacerbation, and severity. Alternaria alternata, Aspergillus fumigatus, and Cladosporium herbarum are known triggers of allergic sensitization in humans [16]. Although fungi are predominant triggers of asthma exacerbations, and severe asthma with fungal sensitization (SAFS) is often steroid resistant and difficult to treat/control, the pathophysiology of SAFS and its molecular pathways are yet to be fully elucidated. The possibility that SAFS may protect the host from subsequent respiratory virus infections [17] also elevates the need to understand the immunobiology of SAFS. We focus this review on SAFS and known effector functions of key immune players that may shape the pathogenesis of fungal allergies in the lungs as regulated by a plethora of cytokines and chemokines (Table 1).
Table 1.
Immunological and Inflammatory Events Following Fungal Exposure.
| Source | Mediator | Effect | References |
|---|---|---|---|
| Fungi | Serine proteases | Membrane permeability Disruption of tight junctions Airway smooth muscle constriction |
[18,19,20,21,22,23,24] |
| Epithelial cells | Interleukins -25 and -33 TSLP TGF-β |
Inflammation Leukocyte activation Airway remodeling |
[25,26,27,28,29,30] |
| Dendritic cells | Pattern recognition receptors Interleukin-6 TNF-α |
Fungal recognition Interleukin-17A production Neutrophil recruitment |
[31,32,33,34] |
| TH2 cells | Interleukin-4 Interleukin-5 Interleukin-13 |
Inflammation B cell class switching Eosinophil activation Mucus cell activation |
[26,27,31,35] |
| TH17 cells | Interleukin-17A | Neutrophil recruitment Epithelial cell activation |
[33] |
| Plasma cells | Immunoglobulin E Immunoglobulin A |
Mast cell activation Fungal neutralization |
[26,27,31,35,36,37] |
| Eosinophils | Interleukins -17 and -23 DNA traps |
Inflammation Fungal neutralization |
[38,39] |
2. Overview of Fungal Allergen-Mediated Immune Responses Leading to SAFS
More than 2 million species of fungi exist globally [40], but only a small fraction of these species are considered to be human pathogens [41] as the human immune system is highly efficient at safeguarding the host from environmental fungi that enter [42]. Fungus-sensitized individuals can have pathophysiologic changes in the lungs (Figure 1) resulting in the symptoms that are associated with asthma. At the respiratory interface, epithelial cells act as the primary blockade against environmental fungal colonization. Airway epithelial cells use physical barrier components such as the mucociliary escalator and antimicrobial peptides as a strategy to hinder fungal entry and germination within lung tissue [43,44]. Numerous pattern recognition receptors (PRRs) on the surface of the respiratory epithelia, including the proteinase-activated receptor (PAR), toll-like receptors (TLRs), C-type lectin receptors (CLRs), mannose receptors (MRs), and dectins, recognize a broad range of fungal antigens and are able to trigger epithelial cells to release cytokines to recruit and activate innate leukocytes to counter any invading fungi [42,45,46]. Similarly, dendritic cells (DCs) sampling the airways can become activated by fungal antigens and mucosal cytokines, allowing them to cause a fungal-specific T cell response [42,45,46] (Figure 2). Cumulatively, these immune responses in the airways can augment physiologic changes, thereby eliciting an asthma attack.
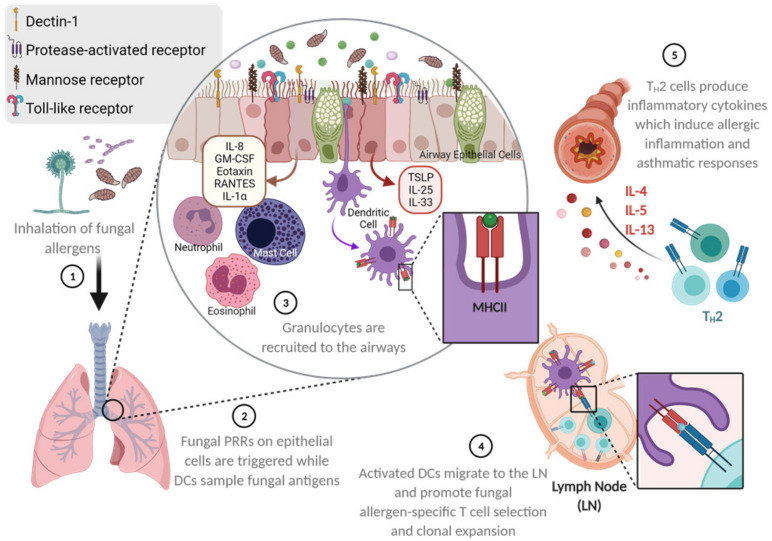
Figure 2.
Fungal allergen-mediated early activation of the respiratory barrier as a trigger for asthma development. Environmentally ubiquitous fungi can be inhaled and travel deep into the lungs owing to their small size and surface properties. Fungal pattern recognition molecules on the respiratory epithelia may be triggered to release cytokines and chemokines that can recruit and activate a number of leukocytes. Intraepithelial dendritic cells that survey the airways may also be activated by fungal antigens and traffic into draining lymph nodes in search of antigen-specific T cells that are subsequently activated. These fungal antigen-specific T cells then accumulate at the respiratory barrier to induce resident and recruited leukocytes and structural cells to become activated and respond culminating in the characteristics of allergic asthma. Illustration drawn with BioRender.
3. Animal Models of Severe Asthma with Fungal Sensitization
Epidemiological data have established the association of fungi with asthma [47,48,49,50,51,52]. Fungal asthma is broadly characterized by the occurrence of fungal sensitization or allergy in patients that present with asthma hallmarks [53,54]. SAFS is mainly differentiated from allergic bronchopulmonary aspergillosis by the absence of bronchiectasis and fungal growth in lungs and sensitivity to antifungal treatments [55]. The severity of SAFS can vary from mild to severe airway inflammation and airway hyperresponsiveness (AHR) [49,51,52]. SAFS is usually TH2 biased and characterized by inflammatory cell (predominately eosinophil) recruitment, elevated serum IgE, peribronchial and perivascular inflammation, increased AHR, mucus hypersecretion, and airway remodeling [56]. Asthmatics with fungal sensitization have similar characteristics to those that are not sensitized to fungi except for lower age of symptom onset and significantly higher levels of IgE and IL-33 in the serum [57].
Animal models that recapitulate characteristics of human asthma are important, as they provide opportunities to dissect the underlying mechanisms of asthma pathology. Ovalbumin (OVA)-induced asthma-like inflammation, the first mouse model for asthma, is more than 100 years old, and replicates a number of features of allergic asthma. However, there are several arguments against this model due to two main shortcomings. Firstly, OVA is not a clinically relevant aeroallergen; secondly, chronic exposure to OVA may lead to immune tolerance and less robust inflammation [58,59]. However, OVA is still a commonly used antigen to induce acute airway inflammation, largely due to the ease of use and the plethora of research reagents (including mouse strains) for immune assays that have been developed using OVA antigen. In the past two decades, however, more clinically relevant antigens, including fungal antigens, have been used effectively to model the characteristics of allergic asthma in mice [60] owing to the ubiquity and clinical relevance of fungi as aeroallergens. Approximately 80% of asthmatics in the United States show positive skin tests for one or more fungal allergens [61,62]. Compared with grass and pollen, fungal conidia have 1000-fold higher exposure and are among the most important clinically relevant allergens for asthma [55,62].
The establishment of a mouse model with a clinically relevant allergen, Aspergillus fumigatus, occurred in 1984. This model uses extract from cultured A. fumigatus to develop the immune response during allergen exposure. Furthermore, researchers developed conidia-based models to recapitulate the pathophysiology of fungal asthma. Various routes of conidia delivery of different fungi, including intranasal (IN), intratracheal (IT), or inhalation (IH) challenge, have been attempted [26,35,63,64]. Havaux et al. performed IN challenge of BALB/c mice with resuspended A. alternata and C. herbarum spores after sensitization. The model showed immune cell infiltration into the airway, perivascular and peribronchial eosinophilic inflammation, and increased AHR and goblet cell metaplasia [64]. As exposure to this airborne fungus nearly doubles the odds of experiencing asthma symptoms [65] Alternaria mouse models are important. Alternaria is independently capable of inducing allergic inflammation in mice and also enhances the strength and TH2-polarization in mice inoculated with other allergens [66,67].
Fungal exposure in humans occurs through the inhalation of airborne dry fungal particles. As fungi are complex, with several stages in their life cycle in which physical changes during growth and germination result in variations in the antigenic signatures [68], the ability to mimic the natural nature of inhaled fungi is important to model SAFS. To date, A. fumigatus is the only clinically relevant fungus utilized in dry inhalation models. Hoselton et al. developed an IH fungal asthma model by exposing fungal extract-sensitized BALB/c mice to dry unmanipulated airborne A. fumigatus conidia for 10 min. This exposure strategy led to AHR, mixed granulocytic airways inflammation, goblet cell metaplasia, increased serum IgE, reversible airway wall fibrosis, and smooth muscle hyperplasia [35]. A second IH exposure administered two weeks following the first led to marked eosinophilia and worsening of the above characteristics in C57BL/6 mice [69], a strain that has a TH1 immune bias and therefore difficult to model AHR in [70,71]. Samarasinghe et al. compared the asthma output in the IH and IT models of fungal asthma in C57BL/6 mice and showed that IH challenge leads to more robust eosinophilic inflammation, serum IgE, and airway wall remodeling events compared to IT challenge with suspended conidia [63]. However, sensitization to whole fungal extract is required for a robust and long-lasting asthma phenotype in Aspergillus models; the use of live conidia for IH elicits a robust immune response compared to irradiated (dead) conidia [72]. Buskirk et al. exposed BALB/c mice to a specific amount of A. fumigatus conidia with an acoustical generator, and showed that inflammation and goblet cell metaplasia peaked two days after the final exposure [73]. In summary, IH exposure to A. fumigatus recapitulates the hallmarks of SAFS observed in patients.
4. Mechanisms of SAFS Induction at the Respiratory Barrier
Major asthma-causing fungal allergens belong to genera Alternaria and Aspergillus [55,74]. Aspergillus conidia are roughly <3 μm [75], i.e., small enough to penetrate the deep bronchoalveolar spaces in the lower airway. Fungal conidia can interact with the airway epithelial barrier, triggering inflammatory signals in response. The major pathogen associated molecular patterns (PAMPs) of fungi include chitin, β-glucans, proteases, glycosidases, and fungal nucleic acids. Receptors for fungal products include TLRs, CLRs, PARs, and receptor for glycation end products (RAGE). Figure 3 illustrates some inflammatory cascades that can be initiated during fungal interaction at the lung epithelial cell (LEC) interface.
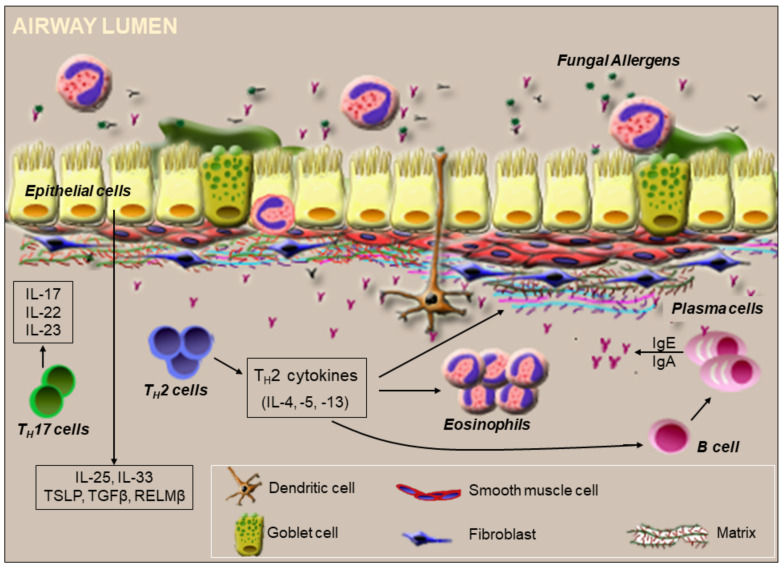
Figure 3.
Immunologic events orchestrated by fungal exposure at the airway surface. Fungal conidia and products bind to receptors present on epithelial cells. Activated epithelial cells release cytokines and growth factors that are responsible for TH2 cell recruitment. Fungal proteases may also disrupt the tight junctions of the epithelial barrier, thus inducing membrane permeability. Newly differentiated TH2 or TH17 cells induced by dendritic cells activated at the barrier arrive at the respiratory barrier to regulate local immune responses to the fungal exposure. TH2 cells promote differentiation of B cells into plasma cells, which secrete IgE in the presence of IL-4 and IL-13, while IL-5 supports the survival of recruited eosinophils. TH2 cytokines also induce airway remodeling by altering the extracellular matrix. Conversion of hyaluronan from high to low molecular weight forms can further promote B cell recruitment and activation to secrete neutralizing antibodies that also activate leukocytes like mast cells at the respiratory barrier. IL-17 and IL-22 produced by TH17 cells also enhance inflammation. On the luminal end, mucus and eosinophil extracellular nets may cause fungal entrapment while eosinophil degranulation may neutralize fungal antigens.
Fungal components such as β-glucan can be recognized by PRRs on DCs independently or in conjunction [76,77], and fungal-antigen activated DCs can go on to trigger both TH2 and TH17 cells [55,78]. Fungal β-glucans also induce dectin-1-dependent IL-6 production in murine LECs [79]. Conidia surface constituent chitin induces TLR-2-dependent IL-17A production by murine macrophages in vitro [80]. Moreover, exposure of murine LECs to fungal chitin induces production of CCL2 [81,82]. LECs also produce IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) in response to fungal allergens [45,46], which regulate downstream immune responses, as the deletion of one or more of these cytokine genes in mice leads to reduced inflammation in response to chitin [83]. Eosinophils, mast cells, and T-cells also secrete IL-25 [84], which is important to perpetuate airway eosinophilia [85], while IL-33 plays a significant role in eosinophilia, goblet cell hyperplasia, and airway remodeling in SAFS [86]. Fungal allergens induce IL-33 secretion in LECs through oxidative stress responses and NADPH oxidase DUOX1 mediated activation of calpain-2 and EGFR signaling [87]. IL-33 promotes CD11b, β-glucan, and ICAM-1 expression on eosinophils [88], which are important for eosinophil activation, and also promote eosinophil survival [89]. While the presence of TH2 and TH17 cytokines in the airways is prominent, early pro-inflammatory cytokines such as IL-1 and TNF-α are also produced in response to fungal sensitization and challenge [90,91]. Resistin-like molecule (RELM)-β is a secreted protein that is abundant in the gut, but is also produced by LECs [92,93]. Architectural changes in the lungs including goblet cell metaplasia and peribronchial fibrosis increase in the absence of RELM-β in A. fumigatus allergen-sensitized and challenged mice [94]. Therefore, LEC products may also impede immunopathologic changes triggered by fungal allergens and therefore be beneficial to mucosal immunity.
Fungal spores and conidia produce a variety of proteases during their life cycle, and secreted proteases are often immunogenic. These proteases are recognized by PARs [95] and can be pro-inflammatory and lead to tight junction disruption between LECs [53,96,97,98], thereby compromising the physical barrier. The deletion of lung tight junction protein claudin-18 in mice causes elevated serum IgE levels and increased AHR after Aspergillus sensitization [98]. Fungal components also induce ion secretion by stimulating the CFTR and Ca2+ channels on epithelial cells thereby affecting mucociliary clearance in the airways [99]. Due to protease-induced irritation, LECs in injured lungs release IL-33, which interacts with its receptor (ST2) on recruited leukocytes including type 2 innate lymphoid cells, resulting in further enhancement of the TH2-type cytokine production and immune responses [100,101]. A TH2-biased immune response and airway remodeling can be triggered by IN delivery of A. fumigatus matrix metalloprotease Asp f 5 and serine protease Asp f 13 [102]. Typically, fungus-epithelial barrier interaction activates LECs to relay a TH2 bias, which includes a specific type of cytokine/chemokine response and immune cell profile.
5. T Cell Response to Fungal Allergen Exposure in the Airways
Immune responses that occur in the lungs in response to fungal allergens involve mast cells, basophils, eosinophils, innate lymphoid cells (ILCs), M2-polarized macrophages, and TH2 cells, all of which can produce TH2-type cytokines like IL-4, IL-5, and IL-13 [103,104,105,106,107]. Inhaled fungal allergens can be endocytosed by DCs, which process and present fungal antigens using major histocompatibility complex II (MHC II). Activated DCs can then migrate to draining lymphoid organs, where they control the differentiation of CD4+ T cells into TH2 cells [107]. The differentiation of CD4+ T cells into TH2 cells depends upon TSLP, CCL17, and CCL22. Once activated, TH2 cells produce inflammatory cytokines IL-4, IL-5, IL-9, and IL-13 (Figure 2). TH2 cells also interact with allergen-specific B-cells, and IL-4 and IL-13 produced by TH2 cells cause B cell class switching to IgE production [108,109]. Major TH2-type cytokine, IL-13, induces goblet cell metaplasia, fibrosis, and AHR [110,111], and, in conjunction with IL-5, promotes the proliferation and survival of eosinophils in the airway [112]. In addition to these cytokines, chemokines CCL5 and CCL11 stimulate eosinophil recruitment into the airway [108]. Cumulatively, these immune responses culminate pathophysiologically as airway constriction, a major hallmark of asthma symptoms (Figure 2).
Recent research implicates IL-17 production by TH17 cells in SAFS (Figure 3). In addition to TH17 cells, ILCs, B-cells, neutrophils, γδ T cells, and natural killer T cells secrete IL-17 [113,114,115,116]. IL-17 orchestrates asthma pathophysiology including inflammation (primarily neutrophil recruitment), smooth muscle proliferation, and fibrosis. These functions of IL-17 are regulated through the induction of type 2 cytokines, proallergic chemokines, and proinflammatory cytokines [117]. Regulatory T cells are activated by TLR-2 signaling in response to fungal antigens [118], and Tregs can elevate the functions of TH17 cells or help suppress the functions of TH2 cells depending on the level of fungal antigens at the mucosa [119]. Intriguingly, TLR-6 upregulated during A. fumigatus exposure [120] contributes to IL-17A and IL-23 production by TH17 cells in response to fungal allergens during asthma [32] (Figure 3).
6. Eosinophils in SAFS
Eosinophils are granulocytes with distinct, acentric bilobed nuclei and cytoplasmic granules with cytotoxic properties. Since its first description by Paul Ehrlich in 1879, eosinophils have been shown to have immunoregulatory and homeostatic functions [121]. During SAFS, TH2 cytokines IL-4, IL-5, and IL-13, as well as fungal antigens, stimulate the production of eosinophil chemoattractant, CCL11 by LECs [122] (Figure 2). The most potent growth factor and chemoattractant for eosinophils is IL-5, which is sensed by the IL-5R complex expressed on both eosinophils and basophils [123,124]. Therefore, IL-5 produced by TH2 cells induces differentiation, proliferation, and maturation of eosinophils during fungal allergies. As eosinophilia is a common manifestation in SAFS patients, biologics that inhibit the effects of IL-5 (mepolizumab, reslizumab, and benralizumab) may be efficacious at alleviating asthma symptoms [125]. Once recruited, eosinophils may perform several functions in situ in response to fungal antigens, as they have been shown to directly bind Alternaria [126], release granule proteins in response [127], and kill A. fumigatus [128]. Eosinophils also produce extracellular DNA traps in response to A. fumigatus, which are not fungicidal [39], but may be immunoregulatory in the context of the allergic airways.
Eosinophils may contribute to fungal asthma pathophysiology by increasing AHR, activating TH2 cells, and inducing airway remodeling. Eosinophils induce AHR by releasing cytokines such as IL-13 [129] and inducing mast cell and basophil degranulation [130,131]. Intriguingly, β-integrin CD11b on the surface of human eosinophils specifically binds to fungal wall β-glucan, causing eosinophil activation and degranulation which may promote fungal killing but could potentially contribute to asthma pathophysiology [126]. Eosinophils induce airway remodeling by releasing profibrotic mediators such as TGF-β in the presence of IL-4 [132]. TGF-β induces extracellular protein production, fibroblast proliferation, and smooth muscle cell proliferation [133,134]. However, eosinophil depletion by anti-IL-5 treatment does not completely suspend airway remodeling [135,136], as a number of cell types in the lungs, such as LECs and macrophages, produce TGF-β, thereby limiting our understanding of the exact role of TGF-β produced by eosinophils during fungal asthma. As RELM-β is important in lung fibrosis in response to A. fumigatus [94], and LECs [137] and to a lesser degree leukocytes (including eosinophils) [138] produce RELM-β, it is highly likely that additional mechanisms may be activated to induce subepithelial fibrosis in response to fungi. Eosinophils are also a significant source of IL-17 and IL-23 after fungal exposure [38]. As one of the most highly recruited leukocytes in fungal asthma [139], eosinophils can indirectly contribute to asthma pathophysiology through crosstalk with TH2 cells and other leukocytes in situ by releasing a plethora of cytokines [140].
According to the current paradigm, peripheral eosinophils are considered end-stage effector cells that play an active role in the initiation and prolongation of allergic asthma pathology. Additionally, many organs in healthy individuals have resident eosinophils [141]. This evidence questions the deleterious end-stage effector role of eosinophils. A large number of studies have demonstrated the tissue repair/remodeling, tissue homeostasis, and developmental functions of eosinophils [141], and their role in regulating antiviral defense mechanisms during fungal asthma [91,142,143]. Due to the multiple beneficial roles of eosinophils, Lee et al. proposed the new hypothesis “local immunity and/or remodeling/repair in both health and diseases” (LIAR), wherein eosinophil recruitment during both healthy and pathological conditions, like fungal asthma, occurs to maintain tissue homeostasis [121]. More recently, eosinophils have been shown to play host protective functions against respiratory pathogens [144] that further support a role for eosinophils as an ally to respiratory health. As significant eosinophil reduction through biologics does not always result in the total ablation of asthma pathophysiology in patients [145], it may be necessary to consider the impact of long-term eosinophil depletion on human health.
7. B Cells in SAFS
The discovery of B cells occurred in the mid 1960s. B cells significantly increase in the blood stream and bronchial mucosa of asthmatic patients [146,147,148]. Mice rendered allergic to A. fumigatus also show markedly increased B cells in the airways [103,117] and mediastinal lymph nodes [37]. TH2 cytokines induce antibody class switching from IgG to IgE [108,109]. IgE produced by B cells in fungal asthma bind to Fcε receptors on mast cells and cross link upon contact with allergenic antigens, causing mast cell degranulation and release of histamine and prostaglandins [149,150]. In our A. fumigatus-based murine fungal asthma model, B cells produce high amounts of IgA and IgE, which are localized to peribronchial and perivascular spaces in the lungs [37]. This finding indicates local production of antibodies by mucosal B cells during fungal asthma. Ghosh et al. determined that B cells play a significant role in regulating inflammation during fungal asthma, as mice deficient in mature B cells (JH−/− strain) have elevated pro-inflammatory cytokines (IL-6 and IL-17A) and reduced canonical TH2 cytokines [117]. Using a combination of allergens including OVA, house dust mite, Aspergillus and Alternaria, Drake et al. showed that JH−/− mice had reduced lung eosinophilia, increased AHR, and elevated cytokines and chemokines compared to their wild-type counterparts [104,151]. Fungal allergic inflammation promotes the enzymatic cleavage of hyaluronan to its low molecular mass form which attracts B cells through CD44 engagement [103]. Together, these findings highlight the possibility that B cells may play an immunomodulatory role during fungal allergic asthma that surpasses that of antibody production.
8. Commensals and Allergic Asthma
The human body harbors a vast amount of diverse microbial communities. The complex interactions between the microbiome and host in the gastrointestinal tract, skin, and respiratory system is pivotal in development and health [152,153,154,155]. Historically, the lungs were considered sterile in healthy individuals. However, recent research revealed that the lungs of healthy individuals harbor low levels of diverse microbiota, mainly comprised of bacteria. To date, data on the fungal population or mycobiome of the lungs are scarce. Charlson et al. performed a comparative study on bronchoalveolar lavage samples from healthy individuals and lung transplant recipients, and found that healthy individuals showed minimal fungal ITS amplification compared to lung transplant recipients, surmising that antibiotics and immunosuppressants prescribed to lung transplant recipients likely caused the increased abundance of Candida spp., Aspergillus spp. and Cryptococcus spp. [155]. An individual’s microbiome contributes to immune system development, while dysbiosis in the lung may contribute to the etiology of allergic diseases like asthma [156,157]. The unique fungal population in the airway is also associated with an increased risk of allergic asthma. In a human cohort study, the sputum of asthmatic patients showed 90 common fungal species including Psathyrella candolleana, Malassezia pachydermatis, Termitomyces clypeatus and Grifola sordulenta, whereas the sputum of control subjects showed 46 common species [57,158]. However, sputum samples do not truly represent the lung microbiome because of possible contamination from gut and oral microbiomes. Therefore, the lung mycobiome with respect to allergic asthma requires further exploration.
Due to the gut–lung axis, gut microbiota can have a peripheral impact on the development and regulation of the lung immune system. For example, dysbiosis in the gut may increase the risk of allergic diseases such as asthma [152]. At present, most reports, including ours [159], have characterized the bacterial microbiome in the context of allergic asthma, and very few have characterized the mycobiome. In a recent mouse study, dysbiosis in the gut mycobiome induced by fluconazole, an antifungal drug, reduced the number of Candida spp. and expanded commensal fungi Aspergillus spp., Wallemia spp., and Epicoccum spp. These antifungal-treated mice demonstrated severe allergic asthma in response to intratracheally delivered house dust mite antigen with elevated eosinophil infiltration, serum IgE, and cytokines IL-4, IL-5 and IL-10 [152]. Intriguingly, oral supplementation with commensal fungus Wallemia mellicola was sufficient to recapitulate characteristics of allergic asthma in mice [160]. These studies confirm that the gut mycobiome plays an important role in peripheral immune responses including those in the lungs. The contribution of the mycobiome may help protect the barrier from invading environmental fungi and other infectious agents. Mannan derived from Saccharomyces cerevisiae, a component of the human gut mycobiome [161], can promote airway epithelial cell spreading and wound healing [162]. However, the use of mannan therapeutically did not reduce the pathogenesis of A. fumigatus-induced fungal asthma [163]. Our understanding is limited regarding the complex interplay between the gut mycobiome and development/regulation of the peripheral immune system during fungal asthma.
The reduction in parasitic infections has been considered to, at least partially, contribute to the increase in allergic diseases in the Western world. While both positive and negative impacts of parasites on the development of allergies have been demonstrated [164,165], information that is specific to the relationship between parasitic infections and the development of fungal allergies is limited. Mice pre-infected with gut parasite Heligmosomoides polygyrus had altered responses to A. fumigatus sensitization and challenge based on the age of parasite infection [166], suggesting that the age-related maturity of the immune system has a direct impact on the development of fungal allergies during an active parasite infection. As gut parasites alter the gut microbiota which, in turn, affect the pathogenesis of allergic asthma [167], the interrelationship between parasites and the development of fungal asthma may be multifaceted and remain to be fully elucidated.
9. Conclusions
Fungal allergies are a growing concern, as fungi are found both indoors and outdoors in abundance in rural and city environments, making avoidance strategies difficult. Despite the millions of fungal species found in the global environment, only a small fraction is known cause human infections. Even fewer species are known allergens even with the high incidence of fungal allergies in atopic individuals [168]. Why some patients are susceptible to fungal sensitization while others are not remains to be determined. As fine classifications of immune responses at the airway barrier, and differentiation of immune responses at the initiation and perpetuation of fungal sensitization are not well understood in humans, animal models that can recapitulate the hallmarks of SAFS in humans are of immense importance. Elucidating the correlation between environmental fungal load and sensitization may also be beneficial to determine if immune tolerance may be induced in the clinical setting to offset sensitization.
Most SAFS patients have heightened eosinophilic inflammation [137]. While SAFS is generally nonresponsive to corticosteroids, the advent of anti-eosinophilic biologics has led to better management of asthma severity in SAFS patients [169]. The knowledge that eosinophils play a host-protective role during virus infections [142,170], including IAV infections [91,141,171] and possibly SARS-CoV-2 infection [172], raises the question of whether fungal allergen-induced eosinophilia has some benefit, at least seasonally, and if so, what the possible long-term complications may be of eliminating eosinophils in SAFS patients [173]. Much remains to be discovered on the immune pathogenesis of fungal allergies and the consequences of fungal allergic disease on immune responses to subsequent or concomitant infectious stimuli.
Author Contributions
Conceptualization, M.T. and A.E.S.; writing—original draft preparation, M.T.; writing—review and editing, A.E.S.; visualization, M.T. and A.E.S.; funding acquisition, A.E.S. All authors have read and agreed to the published version of the manuscript.
Funding
The work in this article was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI125481 to A.E.S. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sakula A. Sir John Floyer’s A Treatise of the Asthma (1698) Thorax. 1984;39:248–254. doi: 10.1136/thx.39.4.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braman S.S. The global burden of asthma. Chest. 2006;130:4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 3.Nurmagambetov T., Kuwahara R., Garbe P. The Economic Burden of Asthma in the United States, 2008–2013. Ann. Am. Thorac. Soc. 2018;15:348–356. doi: 10.1513/AnnalsATS.201703-259OC. [DOI] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services . National Heart, Lung and Blood Institute: Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3 2007) US Department of Health and Human Services; Washington, DC, USA: 2007. NIH Item No. 08-4051. [Google Scholar]
- 5.Lötvall J., Akdis C.A., Bacharier L.B., Bjermer L., Casale T.B., Custovic A., Lemanske R.F., Jr., Wardlaw A.J., Wenzel S.E., Greenberger P.A. Asthma endotypes: A new approach to classification of disease entities within the asthma syndrome. J. Allergy Clin. Immunol. 2011;127:355–360. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 6.Romanet-Manent S., Charpin D., Magnan A., Lanteaume A., Vervloet D., Group E.C. Allergic vs nonallergic asthma: What makes the difference? Allergy. 2002;57:607–613. doi: 10.1034/j.1398-9995.2002.23504.x. [DOI] [PubMed] [Google Scholar]
- 7.Munthe-Kaas M.C., Carlsen K.H., Håland G., Devulapalli C.S., Gervin K., Egeland T., Carlsen K.L., Undlien D. Immunology, C. T cell–specific T-box transcription factor haplotype is associated with allergic asthma in children. J. Allergy Clin. Immunol. 2008;121:51–56. doi: 10.1016/j.jaci.2007.07.068. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen K.D., Vanichsarn C., Nadeau K.C. Impaired IL-10–dependent Induction of Tolerogenic Dendritic Cells by CD4+ CD25hiCD127lo/− Natural Regulatory T Cells in Human Allergic Asthma. Am. J. Respir. Crit. Care Med. 2009;180:823–833. doi: 10.1164/rccm.200905-0761OC. [DOI] [PubMed] [Google Scholar]
- 9.Sears M.R., Greene J.M., Willan A.R., Wiecek E.M., Taylor D.R., Flannery E.M., Cowan J.O., Herbison G.P., Silva P.A., Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N. Engl. J. Med. 2003;349:1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 10.DeVries A., Wlasiuk G., Miller S.J., Bosco A., Stern D.A., Nicodemus-Johnson J., Jones A.C., Rothers J., Lohman I.C., Wright A.L., et al. Neonatal epigenetic predictors of childhood asthma map to immunoregulatory and pro-inflammatory pathways. Am. J. Respir. Crit. Care Med. 2015;191:A3524. [Google Scholar]
- 11.Lockett G.A., Soto-Ramírez N., Ray M.A., Everson T.M., Xu C.J., Patil V.K., Terry W., Kaushal A., Rezwan F.I., Ewart S.L.J.A. Association of season of birth with DNA methylation and allergic disease. Allergy. 2016;71:1314–1324. doi: 10.1111/all.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeVries A., Vercelli D. Early predictors of asthma and allergy in children: The role of epigenetics. Curr. Opin. Allergy Clin. Immunol. 2015;15:435. doi: 10.1097/ACI.0000000000000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson H.S. The importance of allergens in the development of asthma and the persistence of symptoms. J. Allergy Clin. Immunol. 2000;105:S628–S632. doi: 10.1067/mai.2000.106154. [DOI] [PubMed] [Google Scholar]
- 14.Frohlich-Nowoisky J., Pickersgill D.A., Despres V.R., Poschl U. High diversity of fungi in air particulate matter. Proc. Natl. Acad. Sci. USA. 2009;106:12814–12819. doi: 10.1073/pnas.0811003106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Womble S.E., Burton L.E., Kolb L., Girman J.R., Carpenter M., McCarthy J.F. Prevalence and concentrations of culturable airborne fungal spores in 86 office buildings from the Building Assessment Survey and Evaluation (BASE) study; Proceedings of the 8th Inernational Conference on Indoor Air and Climate; Ediburgh, Scotland. 8–13 August 1999; pp. 261–266. [Google Scholar]
- 16.Bozek A., Pyrkosz K. Immunotherapy of mold allergy: A review. Hum. Vaccin. Immunother. 2017;13:2397–2401. doi: 10.1080/21645515.2017.1314404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samarasinghe A.E., Woolard S.N., Boyd K.L., Hoselton S.A., Schuh J.M., McCullers J.A. The immune profile associated with acute allergic asthma accelerates clearance of influenza virus. Immunol. Cell. Biol. 2014;92:449–459. doi: 10.1038/icb.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leino M.S., Loxham M., Blume C., Swindle E.J., Jayasekera N.P., Dennison P.W., Shamji B.W., Edwards M.J., Holgate S.T., Howarth P.H., et al. Barrier disrupting effects of alternaria alternata extract on bronchial epithelium from asthmatic donors. PLoS ONE. 2013;8:e71278. doi: 10.1371/journal.pone.0071278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomee J.F., Wierenga A.T., Hiemstra P.S., Kauffman H.K. Proteases from Aspergillus fumigatus induce release of proinflammatory cytokines and cell detachment in airway epithelial cell lines. J. Infect. Dis. 1997;176:300–303. doi: 10.1086/517272. [DOI] [PubMed] [Google Scholar]
- 20.Kogan T.V., Jadoun J., Mittelman L., Hirschberg K., Osherov N. Involvement of secreted Aspergillus fumigatus proteases in disruption of the actin fiber cytoskeleton and loss of focal adhesion sites in infected A549 lung pneumocytes. J. Infect. Dis. 2004;189:1965–1973. doi: 10.1086/420850. [DOI] [PubMed] [Google Scholar]
- 21.Tai H.Y., Tam M.F., Chou H., Peng H.J., Su S.N., Perng D.W., Shen H.D. Pen ch 13 allergen induces secretion of mediators and degradation of occludin protein of human lung epithelial cells. Allergy. 2006;61:382–388. doi: 10.1111/j.1398-9995.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen J.C., Chuang J.G., Su Y.Y., Chiang B.L., Lin Y.S., Chow L.P. The protease allergen Pen c 13 induces allergic airway inflammation and changes in epithelial barrier integrity and function in a murine model. J. Biol. Chem. 2011;286:26667–26679. doi: 10.1074/jbc.M110.193987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redes J.L., Basu T., Ram-Mohan S., Ghosh C.C., Chan E.C., Sek A.C., Zhao M., Krishnan R., Rosenberg H.F., Druey K.M. Aspergillus fumigatus-Secreted Alkaline Protease 1 Mediates Airways Hyperresponsiveness in Severe Asthma. Immunohorizons. 2019;3:368–377. doi: 10.4049/immunohorizons.1900046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balenga N.A., Klichinsky M., Xie Z., Chan E.C., Zhao M., Jude J., Laviolette M., Panettieri R.A., Jr., Druey K.M. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat. Commun. 2015;6:6763. doi: 10.1038/ncomms7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurst S.D., Muchamuel T., Gorman D.M., Gilbert J.M., Clifford T., Kwan S., Menon S., Seymour B., Jackson C., Kung T.T., et al. New IL-17 family members promote Th1 or Th2 responses in the lung: In vivo function of the novel cytokine IL-25. J. Immunol. 2002;169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 26.Hogaboam C.M., Blease K., Mehrad B., Steinhauser M.L., Standiford T.J., Kunkel S.L., Lukacs N.W. Chronic airway hyperreactivity, goblet cell hyperplasia, and peribronchial fibrosis during allergic airway disease induced by Aspergillus fumigatus. Am. J. Pathol. 2000;156:723–732. doi: 10.1016/S0002-9440(10)64775-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent M., Percier P., De Prins S., Huygen K., Potemberg G., Muraille E., Romano M., Michel O., Denis O. Investigation of inflammatory and allergic responses to common mold species: Results from in vitro experiments, from a mouse model of asthma, and from a group of asthmatic patients. Indoor Air. 2017;27:933–945. doi: 10.1111/ina.12385. [DOI] [PubMed] [Google Scholar]
- 28.Toki S., Goleniewska K., Zhang J., Zhou W., Newcomb D.C., Zhou B., Kita H., Boyd K.L., Peebles R.S., Jr. TSLP and IL-33 reciprocally promote each other’s lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy. 2020;75:1606–1617. doi: 10.1111/all.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lv J., Yu Q., Lv J., Di C., Lin X., Su W., Wu M., Xia Z. Airway epithelial TSLP production of TLR2 drives type 2 immunity in allergic airway inflammation. Eur. J. Immunol. 2018;48:1838–1850. doi: 10.1002/eji.201847663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim Y.H., Lee S.H. TGF-beta/SMAD4 mediated UCP2 downregulation contributes to Aspergillus protease-induced inflammation in primary bronchial epithelial cells. Redox Biol. 2018;18:104–113. doi: 10.1016/j.redox.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi T., Iijima K., Dent A.L., Kita H. Follicular helper T cells mediate IgE antibody response to airborne allergens. J. Allergy Clin. Immunol. 2017;139:300–313.e7. doi: 10.1016/j.jaci.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreira A.P., Cavassani K.A., Ismailoglu U.B., Hullinger R., Dunleavy M.P., Knight D.A., Kunkel S.L., Uematsu S., Akira S., Hogaboam C.M. The protective role of TLR6 in a mouse model of asthma is mediated by IL-23 and IL-17A. J. Clin. Investig. 2011;121:4420–4432. doi: 10.1172/JCI44999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fei M., Bhatia S., Oriss T.B., Yarlagadda M., Khare A., Akira S., Saijo S., Iwakura Y., Fallert Junecko B.A., Reinhart T.A., et al. TNF-alpha from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proc. Natl. Acad. Sci. USA. 2011;108:5360–5365. doi: 10.1073/pnas.1015476108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramirez-Ortiz Z.G., Means T.K. The role of dendritic cells in the innate recognition of pathogenic fungi (A. fumigatus, C. neoformans and C. albicans) Virulence. 2012;3:635–646. doi: 10.4161/viru.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoselton S.A., Samarasinghe A.E., Seydel J.M., Schuh J.M. An inhalation model of airway allergic response to inhalation of environmental Aspergillus fumigatus conidia in sensitized BALB/c mice. Med. Mycol. 2010;48:1056–1065. doi: 10.3109/13693786.2010.485582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghosh S., Hoselton S.A., Schuh J.M. mu-chain-deficient mice possess B-1 cells and produce IgG and IgE, but not IgA, following systemic sensitization and inhalational challenge in a fungal asthma model. J. Immunol. 2012;189:1322–1329. doi: 10.4049/jimmunol.1200138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doorley L.A., LeMessurier K.S., Iverson A.R., Palipane M., Samarasinghe A.E. Humoral immune responses during asthma and influenza co-morbidity in mice. Immunobiology. 2017;222:1064–1073. doi: 10.1016/j.imbio.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guerra E.S., Lee C.K., Specht C.A., Yadav B., Huang H., Akalin A., Huh J.R., Mueller C., Levitz S.M. Central Role of IL-23 and IL-17 Producing Eosinophils as Immunomodulatory Effector Cells in Acute Pulmonary Aspergillosis and Allergic Asthma. PLoS Pathog. 2017;13:e1006175. doi: 10.1371/journal.ppat.1006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muniz V.S., Silva J.C., Braga Y.A.V., Melo R.C.N., Ueki S., Takeda M., Hebisawa A., Asano K., Figueiredo R.T., Neves J.S. Eosinophils release extracellular DNA traps in response to Aspergillus fumigatus. J. Allergy Clin. Immunol. 2018;141:571–585.e7. doi: 10.1016/j.jaci.2017.07.048. [DOI] [PubMed] [Google Scholar]
- 40.Hawksworth D.L., Lucking R. Fungal Diversity Revisited: 2.2 to 3.8 Million Species. Microbiol. Spectr. 2017;5 doi: 10.1128/microbiolspec.FUNK-0052-2016. [DOI] [PubMed] [Google Scholar]
- 41.Kohler J.R., Casadevall A., Perfect J. The spectrum of fungi that infects humans. Cold Spring Harb. Perspect. Med. 2014;5:a019273. doi: 10.1101/cshperspect.a019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Templeton S.P., Rivera A., Hube B., Jacobsen I.D. Editorial: Immunity to Human Fungal Pathogens: Mechanisms of Host Recognition, Protection, Pathology, and Fungal Interference. Front. Immunol. 2018;9:2337. doi: 10.3389/fimmu.2018.02337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuek L.E., Lee R.J. First contact: The role of respiratory cilia in host-pathogen interactions in the airways. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020;319:L603–L619. doi: 10.1152/ajplung.00283.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silva P.M., Goncalves S., Santos N.C. Defensins: Antifungal lessons from eukaryotes. Front. Microbiol. 2014;5:97. doi: 10.3389/fmicb.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salazar F., Ghaemmaghami A.M. Allergen recognition by innate immune cells: Critical role of dendritic and epithelial cells. Front. Immunol. 2013;4:356. doi: 10.3389/fimmu.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gandhi V.D., Vliagoftis H. Airway epithelium interactions with aeroallergens: Role of secreted cytokines and chemokines in innate immunity. Front. Immunol. 2015;6:147. doi: 10.3389/fimmu.2015.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bernton H.S. Asthma due to a mold—Aspergillus fumigatus. J. Am. Med. Assoc. 1930;95:189–191. doi: 10.1001/jama.1930.02720030019004. [DOI] [Google Scholar]
- 48.Brown G.T. Hypersensitiveness to fungi. J. Allergy. 1936;7:455–470. doi: 10.1016/S0021-8707(36)80004-5. [DOI] [Google Scholar]
- 49.Hopkins J.G., Benham R.W., Kesten B.M. Asthma due to a fungus—Alternaria. J. Am. Med. Assoc. 1930;94:6–10. doi: 10.1001/jama.1930.02710270008002. [DOI] [Google Scholar]
- 50.Black P.N., Udy A.A., Brodie S.M. Sensitivity to fungal allergens is a risk factor for life-threatening asthma. Allergy. 2000;55:501–504. doi: 10.1034/j.1398-9995.2000.00293.x. [DOI] [PubMed] [Google Scholar]
- 51.Targonski P.V., Persky V.W., Ramekrishnan V. Effect of environmental molds on risk of death from asthma during the pollen season. J. Allergy Clin. Immunol. 1995;95:955–961. doi: 10.1016/S0091-6749(95)70095-1. [DOI] [PubMed] [Google Scholar]
- 52.Zureik M., Neukirch C., Leynaert B., Liard R., Bousquet J., Neukirch F. Sensitisation to airborne moulds and severity of asthma: Cross sectional study from European Community respiratory health survey. BMJ. 2002;325:411–414. doi: 10.1136/bmj.325.7361.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agarwal R., Gupta D. Severe asthma and fungi: Current evidence. Med. Mycol. 2011;49:S150–S157. doi: 10.3109/13693786.2010.504752. [DOI] [PubMed] [Google Scholar]
- 54.Denning D.W., Pashley C., Hartl D., Wardlaw A., Godet C., Del Giacco S., Delhaes L., Sergejeva S. Fungal allergy in asthma-state of the art and research needs. Clin. Transl. Allergy. 2014;4:14. doi: 10.1186/2045-7022-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Denning D.W., O’Driscoll B.R., Hogaboam C.M., Bowyer P., Niven R.M. The link between fungi and severe asthma: A summary of the evidence. Eur. Respir. J. 2006;27:615–626. doi: 10.1183/09031936.06.00074705. [DOI] [PubMed] [Google Scholar]
- 56.Croisant S. Heterogeneity in Asthma. Humana Press; Boston, MA, USA: 2014. Epidemiology of asthma: Prevalence and burden of disease; pp. 17–29. [DOI] [PubMed] [Google Scholar]
- 57.Masaki K., Fukunaga K., Matsusaka M., Kabata H., Tanosaki T., Mochimaru T., Kamatani T., Ohtsuka K., Baba R., Ueda S., et al. Characteristics of severe asthma with fungal sensitization. Ann. Allergy Asthma Immunol. 2017;119:253–257. doi: 10.1016/j.anai.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 58.Swirski F.K., Sajic D., Robbins C.S., Gajewska B.U., Jordana M., Stampfli M.R. Chronic exposure to innocuous antigen in sensitized mice leads to suppressed airway eosinophilia that is reversed by granulocyte macrophage colony-stimulating factor. J. Immunol. 2002;169:3499–3506. doi: 10.4049/jimmunol.169.7.3499. [DOI] [PubMed] [Google Scholar]
- 59.Van Hove C.L., Maes T., Joos G.F., Tournoy K.G. Prolonged inhaled allergen exposure can induce persistent tolerance. Am. J. Respir. Cell Mol. Biol. 2007;36:573–584. doi: 10.1165/rcmb.2006-0385OC. [DOI] [PubMed] [Google Scholar]
- 60.Kumar R.K., Herbert C., Foster P.S. Mouse models of acute exacerbations of allergic asthma. Respirology. 2016;21:842–849. doi: 10.1111/resp.12760. [DOI] [PubMed] [Google Scholar]
- 61.Beezhold D.H., Green B.J., Blachere F.M., Schmechel D., Weissman D.N., Velickoff D., Hogan M.B., Wilson N.W. Prevalence of allergic sensitization to indoor fungi in West Virginia. Allergy Asthma Proc. 2008;29:29–34. doi: 10.2500/aap2008.29.3076. [DOI] [PubMed] [Google Scholar]
- 62.Knutsen A.P., Bush R.K., Demain J.G., Denning D.W., Dixit A., Fairs A., Greenberger P.A., Kariuki B., Kita H., Kurup V.P., et al. Fungi and allergic lower respiratory tract diseases. J. Allergy Clin. Immunol. 2012;129 doi: 10.1016/j.jaci.2011.12.970. [DOI] [PubMed] [Google Scholar]
- 63.Samarasinghe A.E., Hoselton S.A., Schuh J.M. A comparison between intratracheal and inhalation delivery of Aspergillus fumigatus conidia in the development of fungal allergic asthma in C57BL/6 mice. Fungal Biol. 2011;115:21–29. doi: 10.1016/j.funbio.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Havaux X., Zeine A., Dits A., Denis O. A new mouse model of lung allergy induced by the spores of Alternaria alternata and Cladosporium herbarum molds. Clin. Exp. Immunol. 2005;139:179–188. doi: 10.1111/j.1365-2249.2004.02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salo P.M., Arbes S.J., Jr., Sever M., Jaramillo R., Cohn R.D., London S.J., Zeldin D.C. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J. Allergy Clin. Immunol. 2006;118:892–898. doi: 10.1016/j.jaci.2006.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kobayashi T., Iijima K., Radhakrishnan S., Mehta V., Vassallo R., Lawrence C.B., Cyong J.C., Pease L.R., Oguchi K., Kita H. Asthma-related environmental fungus, Alternaria, activates dendritic cells and produces potent Th2 adjuvant activity. J. Immunol. 2009;182:2502–2510. doi: 10.4049/jimmunol.0802773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Snelgrove R.J., Gregory L.G., Peiro T., Akthar S., Campbell G.A., Walker S.A., Lloyd C.M. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J. Allergy Clin. Immunol. 2014;134:583–592.e6. doi: 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Green B.J., Tovey E.R., Sercombe J.K., Blachere F.M., Beezhold D.H., Schmechel D. Airborne fungal fragments and allergenicity. Med. Mycol. 2006;44:S245–S255. doi: 10.1080/13693780600776308. [DOI] [PubMed] [Google Scholar]
- 69.Samarasinghe A.E., Hoselton S.A., Schuh J.M. The absence of VPAC2 leads to aberrant antibody production in Aspergillus fumigatus sensitized and challenged mice. Peptides. 2011;32:131–137. doi: 10.1016/j.peptides.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watanabe H., Numata K., Ito T., Takagi K., Matsukawa A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock. 2004;22:460–466. doi: 10.1097/01.shk.0000142249.08135.e9. [DOI] [PubMed] [Google Scholar]
- 71.Atochina E.N., Beers M.F., Tomer Y., Scanlon S.T., Russo S.J., Panettieri R.A., Jr., Haczku A. Attenuated allergic airway hyperresponsiveness in C57BL/6 mice is associated with enhanced surfactant protein (SP)-D production following allergic sensitization. Respir. Res. 2003;4:15. doi: 10.1186/1465-9921-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pandey S., Hoselton S.A., Schuh J.M. The impact of Aspergillus fumigatus viability and sensitization to its allergens on the murine allergic asthma phenotype. Biomed Res. Int. 2013;2013:619614. doi: 10.1155/2013/619614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buskirk A.D., Green B.J., Lemons A.R., Nayak A.P., Goldsmith W.T., Kashon M.L., Anderson S.E., Hettick J.M., Templeton S.P., Germolec D.R., et al. A murine inhalation model to characterize pulmonary exposure to dry Aspergillus fumigatus conidia. PLoS ONE. 2014;9:e109855. doi: 10.1371/journal.pone.0109855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roy R.M., Klein B.S. Fungal glycan interactions with epithelial cells in allergic airway disease. Curr. Opin. Microbiol. 2013;16:404–408. doi: 10.1016/j.mib.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kwon-Chung K.J., Sugui J.A. Aspergillus fumigatus--what makes the species a ubiquitous human fungal pathogen? PLoS Pathog. 2013;9:e1003743. doi: 10.1371/journal.ppat.1003743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown G.D. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 77.Taylor P.R., Tsoni S.V., Willment J.A., Dennehy K.M., Rosas M., Findon H., Haynes K., Steele C., Botto M., Gordon S., et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Speakman E.A., Dambuza I.M., Salazar F., Brown G.D. T Cell Antifungal Immunity and the Role of C-Type Lectin Receptors. Trends Immunol. 2020;41:61–76. doi: 10.1016/j.it.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chung Y.J., Copeland L.B., Doerfler D.L., Ward M.D. The relative allergenicity of Stachybotrys chartarum compared to house dust mite extracts in a mouse model. Inhal. Toxicol. 2010;22:460–468. doi: 10.3109/08958370903380712. [DOI] [PubMed] [Google Scholar]
- 80.Da Silva C.A., Hartl D., Liu W., Lee C.G., Elias J.A. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J. Immunol. 2008;181:4279–4286. doi: 10.4049/jimmunol.181.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roy R.M., Wuthrich M., Klein B.S. Chitin elicits CCL2 from airway epithelial cells and induces CCR2-dependent innate allergic inflammation in the lung. J. Immunol. 2012;189:2545–2552. doi: 10.4049/jimmunol.1200689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levy M., Kolodziejczyk A.A., Thaiss C.A., Elinav E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017;17:219–232. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- 83.Van Dyken S.J., Mohapatra A., Nussbaum J.C., Molofsky A.B., Thornton E.E., Ziegler S.F., McKenzie A.N., Krummel M.F., Liang H.E., Locksley R.M. Chitin activates parallel immune modules that direct distinct inflammatory responses via innate lymphoid type 2 and gammadelta T cells. Immunity. 2014;40:414–424. doi: 10.1016/j.immuni.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hammad H., Lambrecht B.N. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 85.Andreakos E., Papadopoulos N.G. IL-25: The Missing Link between Allergy, Viral Infection, and Asthma? Sci. Transl. Med. 2014;6:256fs238. doi: 10.1126/scitranslmed.3010273. [DOI] [PubMed] [Google Scholar]
- 86.Stolarski B., Kurowska-Stolarska M., Kewin P., Xu D., Liew F.Y. IL-33 Exacerbates Eosinophil-Mediated Airway Inflammation. J. Immunol. 2010;185:3472–3480. doi: 10.4049/jimmunol.1000730. [DOI] [PubMed] [Google Scholar]
- 87.Hristova M., Habibovic A., Veith C., Janssen-Heininger Y.M., Dixon A.E., Geiszt M., van der Vliet A. Airway epithelial dual oxidase 1 mediates allergen-induced IL-33 secretion and activation of type 2 immune responses. J. Allergy Clin. Immunol. 2016;137:1545–1556.e11. doi: 10.1016/j.jaci.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suzukawa M., Koketsu R., Iikura M., Nakae S., Matsumoto K., Nagase H., Saito H., Matsushima K., Ohta K., Yamamoto K., et al. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab. Investig. 2008;88:1245–1253. doi: 10.1038/labinvest.2008.82. [DOI] [PubMed] [Google Scholar]
- 89.Holmes D.A., Yeh J.H., Yan D., Xu M., Chan A.C. Dusp5 negatively regulates IL-33-mediated eosinophil survival and function. EMBO J. 2015;34:218–235. doi: 10.15252/embj.201489456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Daines M., Zhu L., Pereira R., Zhou X., Bondy C., Pryor B.M., Zhou J., Chen Y. Alternaria induces airway epithelial cytokine expression independent of protease-activated receptor. Respirology. 2020;25:502–510. doi: 10.1111/resp.13675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.LeMessurier K.S., Rooney R., Ghoneim H.E., Liu B., Li K., Smallwood H.S., Samarasinghe A.E. Influenza A virus directly modulates mouse eosinophil responses. J. Leukoc. Biol. 2020;108:151–168. doi: 10.1002/JLB.4MA0320-343R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Artis D., Wang M.L., Keilbaugh S.A., He W., Brenes M., Swain G.P., Knight P.A., Donaldson D.D., Lazar M.A., Miller H.R., et al. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc. Natl. Acad. Sci. USA. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mishra A., Wang M., Schlotman J., Nikolaidis N.M., DeBrosse C.W., Karow M.L., Rothenberg M.E. Resistin-like molecule-beta is an allergen-induced cytokine with inflammatory and remodeling activity in the murine lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L305–L313. doi: 10.1152/ajplung.00147.2007. [DOI] [PubMed] [Google Scholar]
- 94.LeMessurier K.S., Palipane M., Tiwary M., Gavin B., Samarasinghe A.E. Chronic features of allergic asthma are enhanced in the absence of resistin-like molecule-beta. Sci. Rep. 2018;8:7061. doi: 10.1038/s41598-018-25321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brown G.D., Denning D.W., Levitz S.M. Tackling human fungal infections. Science. 2012;336:647. doi: 10.1126/science.1222236. [DOI] [PubMed] [Google Scholar]
- 96.Paris S., Boisvieux-Ulrich E., Crestani B., Houcine O., Taramelli D., Lombardi L., Latge J.P. Internalization of Aspergillus fumigatus conidia by epithelial and endothelial cells. Infect. Immun. 1997;65:1510–1514. doi: 10.1128/IAI.65.4.1510-1514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hamelmann E., Schwarze J., Takeda K., Oshiba A., Larsen G.L., Irvin C.G., Gelfand E.W. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 1997;156:766–775. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 98.Sweerus K., Lachowicz-Scroggins M., Gordon E., LaFemina M., Huang X., Parikh M., Kanegai C., Fahy J.V., Frank J.A. Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J. Allergy Clin. Immunol. 2017;139:72–81.e1. doi: 10.1016/j.jaci.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zaidman N.A., O’Grady K.E., Patil N., Milavetz F., Maniak P.J., Kita H., O’Grady S.M. Airway epithelial anion secretion and barrier function following exposure to fungal aeroallergens: Role of oxidative stress. Am. J. Physiol. Cell. Physiol. 2017;313:C68–C79. doi: 10.1152/ajpcell.00043.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martin N.T., Martin M.U. Interleukin 33 is a guardian of barriers and a local alarmin. Nat. Immunol. 2016;17:122–131. doi: 10.1038/ni.3370. [DOI] [PubMed] [Google Scholar]
- 101.Piehler D., Eschke M., Schulze B., Protschka M., Muller U., Grahnert A., Richter T., Heyen L., Kohler G., Brombacher F., et al. The IL-33 receptor (ST2) regulates early IL-13 production in fungus-induced allergic airway inflammation. Mucosal Immunol. 2016;9:937–949. doi: 10.1038/mi.2015.106. [DOI] [PubMed] [Google Scholar]
- 102.Namvar S., Warn P., Farnell E., Bromley M., Fraczek M., Bowyer P., Herrick S. Aspergillus fumigatus proteases, Asp f 5 and Asp f 13, are essential for airway inflammation and remodelling in a murine inhalation model. Clin. Exp. Allergy. 2015;45:982–993. doi: 10.1111/cea.12426. [DOI] [PubMed] [Google Scholar]
- 103.Ghosh S., Hoselton S.A., Wanjara S.B., Carlson J., McCarthy J.B., Dorsam G.P., Schuh J.M. Hyaluronan stimulates ex vivo B lymphocyte chemotaxis and cytokine production in a murine model of fungal allergic asthma. Immunobiology. 2015;220:899–909. doi: 10.1016/j.imbio.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Drake L.Y., Iijima K., Hara K., Kobayashi T., Kephart G.M., Kita H. B cells play key roles in th2-type airway immune responses in mice exposed to natural airborne allergens. PLoS ONE. 2015;10:e0121660. doi: 10.1371/journal.pone.0121660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Albacker L.A., Chaudhary V., Chang Y.J., Kim H.Y., Chuang Y.T., Pichavant M., DeKruyff R.H., Savage P.B., Umetsu D.T. Invariant natural killer T cells recognize a fungal glycosphingolipid that can induce airway hyperreactivity. Nat. Med. 2013;19:1297–1304. doi: 10.1038/nm.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Voehringer D. Basophils in allergic immune responses. Curr. Opin. Immunol. 2011;23:789–793. doi: 10.1016/j.coi.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 107.Halim T.Y., Steer C.A., Matha L., Gold M.J., Martinez-Gonzalez I., McNagny K.M., McKenzie A.N., Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barnes P.J. The cytokine network in asthma and chronic obstructive pulmonary disease. J. Clin. Investig. 2008;118:3546–3556. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wills-Karp M., Finkelman F.D. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci. Signal. 2008;1:pe55. doi: 10.1126/scisignal.1.51.pe55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kuperman D.A., Huang X., Koth L.L., Chang G.H., Dolganov G.M., Zhu Z., Elias J.A., Sheppard D., Erle D.J. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat. Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 111.Mishina K., Shinkai M., Shimokawaji T., Nagashima A., Hashimoto Y., Inoue Y., Inayama Y., Rubin B.K., Ishigatsubo Y., Kaneko T. HO-1 inhibits IL-13-induced goblet cell hyperplasia associated with CLCA1 suppression in normal human bronchial epithelial cells. Int. Immunopharmacol. 2015;29:448–453. doi: 10.1016/j.intimp.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 112.Blanchard C., Rothenberg M.E. Biology of the eosinophil. Adv. Immunol. 2009;101:81–121. doi: 10.1016/s0065-2776(08)01003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu S., Kim H.Y., Chang Y.J., DeKruyff R.H., Umetsu D.T. Innate lymphoid cells and asthma. J. Allergy Clin. Immunol. 2014;133 doi: 10.1016/j.jaci.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 114.Vazquez-Tello A., Halwani R., Li R., Nadigel J., Bar-Or A., Mazer B.D., Eidelman D.H., Al-Muhsen S., Hamid Q. IL-17A and IL-17F expression in B lymphocytes. Int. Arch. Allergy Immunol. 2012;157:406–416. doi: 10.1159/000329527. [DOI] [PubMed] [Google Scholar]
- 115.Taylor P.R., Roy S., Leal S.M., Jr., Sun Y., Howell S.J., Cobb B.A., Li X., Pearlman E. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nat. Immunol. 2014;15:143–151. doi: 10.1038/ni.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Murdoch J.R., Lloyd C.M. Resolution of allergic airway inflammation and airway hyperreactivity is mediated by IL-17-producing {gamma}{delta}T cells. Am. J. Respir. Crit. Care Med. 2010;182:464–476. doi: 10.1164/rccm.200911-1775OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ghosh S., Hoselton S.A., Asbach S.V., Steffan B.N., Wanjara S.B., Dorsam G.P., Schuh J.M. B lymphocytes regulate airway granulocytic inflammation and cytokine production in a murine model of fungal allergic asthma. Cell. Mol. Immunol. 2015;12:202–212. doi: 10.1038/cmi.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sutmuller R.P., den Brok M.H., Kramer M., Bennink E.J., Toonen L.W., Kullberg B.J., Joosten L.A., Akira S., Netea M.G., Adema G.J. Toll-like receptor 2 controls expansion and function of regulatory T cells. J. Clin. Investig. 2006;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Van de Veerdonk F.L., Netea M.G. T-cell Subsets and Antifungal Host Defenses. Curr. Fungal Infect. Rep. 2010;4:238–243. doi: 10.1007/s12281-010-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kurup V.P., Raju R., Manickam P. Profile of gene expression in a murine model of allergic bronchopulmonary aspergillosis. Infect. Immun. 2005;73:4381–4384. doi: 10.1128/IAI.73.7.4381-4384.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee J.J., Jacobsen E.A., McGarry M.P., Schleimer R.P., Lee N.A. Eosinophils in health and disease: The LIAR hypothesis. Clin. Exp. Allergy. 2010;40:563–575. doi: 10.1111/j.1365-2222.2010.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Conroy D.M., Williams T.J. Eotaxin and the attraction of eosinophils to the asthmatic lung. Respir. Res. 2001;2:150–156. doi: 10.1186/rr52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Robinson D.S., Damia R., Zeibecoglou K., Molet S., North J., Yamada T., Kay A.B., Hamid Q. CD34(+)/interleukin-5Ralpha messenger RNA+ cells in the bronchial mucosa in asthma: Potential airway eosinophil progenitors. Am. J. Respir. Cell. Mol. Biol. 1999;20:9–13. doi: 10.1165/ajrcmb.20.1.3449. [DOI] [PubMed] [Google Scholar]
- 124.Menzies-Gow A., Flood-Page P., Sehmi R., Burman J., Hamid Q., Robinson D.S., Kay A.B., Denburg J. Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J. Allergy Clin. Immunol. 2003;111:714–719. doi: 10.1067/mai.2003.1382. [DOI] [PubMed] [Google Scholar]
- 125.Dhariwal J., Hearn A.P., Kavanagh J.E., d’Ancona G., Green L., Fernandes M., Thomson L., Roxas C., Kent B.D., Nanzer A.M., et al. Real world effectiveness of anti-IL5/5R therapy in severe atopic eosinophilic asthma with fungal sensitisation. J. Allergy Clin. Immunol. Pract. 2021 doi: 10.1016/j.jaip.2021.02.048. [DOI] [PubMed] [Google Scholar]
- 126.Yoon J., Ponikau J.U., Lawrence C.B., Kita H. Innate antifungal immunity of human eosinophils mediated by a beta 2 integrin, CD11b. J. Immunol. 2008;181:2907–2915. doi: 10.4049/jimmunol.181.4.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Inoue Y., Matsuwaki Y., Shin S.H., Ponikau J.U., Kita H. Nonpathogenic, environmental fungi induce activation and degranulation of human eosinophils. J. Immunol. 2005;175:5439–5447. doi: 10.4049/jimmunol.175.8.5439. [DOI] [PubMed] [Google Scholar]
- 128.Lilly L.M., Scopel M., Nelson M.P., Burg A.R., Dunaway C.W., Steele C. Eosinophil deficiency compromises lung defense against Aspergillus fumigatus. Infect. Immun. 2014;82:1315–1325. doi: 10.1128/IAI.01172-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schmid-Grendelmeier P., Altznauer F., Fischer B., Bizer C., Straumann A., Menz G., Blaser K., Wuthrich B., Simon H.U. Eosinophils express functional IL-13 in eosinophilic inflammatory diseases. J. Immunol. 2002;169:1021–1027. doi: 10.4049/jimmunol.169.2.1021. [DOI] [PubMed] [Google Scholar]
- 130.Gibson P.G. Inflammatory phenotypes in adult asthma: Clinical applications. Clin. Respir. J. 2009;3:198–206. doi: 10.1111/j.1752-699X.2009.00162.x. [DOI] [PubMed] [Google Scholar]
- 131.Kay A.B. Mediators of hypersensitivity and inflammatory cells in the pathogenesis of bronchial asthma. Eur. J. Respir. Dis. 1983;129:1–44. [PubMed] [Google Scholar]
- 132.Elovic A.E., Ohyama H., Sauty A., McBride J., Tsuji T., Nagai M., Weller P.F., Wong D.T. IL-4-dependent regulation of TGF-α and TGF-β1 expression in human eosinophils. J. Immunol. 1998;160:6121–6127. [PubMed] [Google Scholar]
- 133.Fine A., Goldstein R.J. Regulation of type I collagen mRNA translation by TGF-beta. Reg. Immunol. 1993;5:218–224. [PubMed] [Google Scholar]
- 134.Wicks J., Haitchi H.M., Holgate S.T., Davies D.E., Powell R.M. Enhanced upregulation of smooth muscle related transcripts by TGFβ2 in asthmatic (myo) fibroblasts. Thorax. 2006;61:313–319. doi: 10.1136/thx.2005.050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Flood-Page P., Menzies-Gow A., Phipps S., Ying S., Wangoo A., Ludwig M., Barnes N., Robinson D., Kay A.J. Anti-IL-5 treatment (mepolizumab) reduces deposition of extracellular matrix proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics: Evidence for a role for eosinophils in airways remodeling. J. Clin. Investig. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Boxall C., Holgate S., Davies D.J. The contribution of transforming growth factor-β and epidermal growth factor signalling to airway remodelling in chronic asthma. Eur. Respir. J. 2006;27:208–229. doi: 10.1183/09031936.06.00130004. [DOI] [PubMed] [Google Scholar]
- 137.Liu T., Jin H., Ullenbruch M., Hu B., Hashimoto N., Moore B., McKenzie A., Lukacs N.W., Phan S.H. Regulation of found in inflammatory zone 1 expression in bleomycin-induced lung fibrosis: Role of IL-4/IL-13 and mediation via STAT-6. J. Immunol. 2004;173:3425–3431. doi: 10.4049/jimmunol.173.5.3425. [DOI] [PubMed] [Google Scholar]
- 138.Fang C., Meng Q., Wu H., Eid G., Zhang G., Zhang X., Yang S., Huang K., Lee T.H., Corrigan C.J., et al. Resistin-like molecule-beta is a human airway remodelling mediator. Eur. Respir. J. 2012;39:458–466. doi: 10.1183/09031936.00107811. [DOI] [PubMed] [Google Scholar]
- 139.Figueiredo R.T., Neves J.S. Eosinophils in fungal diseases: An overview. J. Leukoc. Biol. 2018;104:49–60. doi: 10.1002/JLB.4MR1117-473R. [DOI] [PubMed] [Google Scholar]
- 140.Melo R.C., Liu L., Xenakis J.J., Spencer L.A. Eosinophil-derived cytokines in health and disease: Unraveling novel mechanisms of selective secretion. Allergy. 2013;68:274–284. doi: 10.1111/all.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jacobsen E.A., Helmers R.A., Lee J.J., Lee N.A. The expanding role(s) of eosinophils in health and disease. Blood. 2012;120:3882–3890. doi: 10.1182/blood-2012-06-330845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Percopo C.M., Dyer K.D., Ochkur S.I., Luo J.L., Fischer E.R., Lee J.J., Lee N.A., Domachowske J.B., Rosenberg H.F. Activated mouse eosinophils protect against lethal respiratory virus infection. Blood. 2014;123:743–752. doi: 10.1182/blood-2013-05-502443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Samarasinghe A.E., Melo R.C., Duan S., LeMessurier K.S., Liedmann S., Surman S.L., Lee J.J., Hurwitz J.L., Thomas P.G., McCullers J.A. Eosinophils Promote Antiviral Immunity in Mice Infected with Influenza A Virus. J. Immunol. 2017;198:3214–3226. doi: 10.4049/jimmunol.1600787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.LeMessurier K.S., Samarasinghe A.E. Eosinophils: Nemeses of Pulmonary Pathogens? Curr. Allergy Asthma Rep. 2019;19:36. doi: 10.1007/s11882-019-0867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Agache I., Beltran J., Akdis C., Akdis M., Canelo-Aybar C., Canonica G.W., Casale T., Chivato T., Corren J., Del Giacco S., et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines—Recommendations on the use of biologicals in severe asthma. Allergy. 2020;75:1023–1042. doi: 10.1111/all.14221. [DOI] [PubMed] [Google Scholar]
- 146.Lindell D.M., Berlin A.A., Schaller M.A., Lukacs N.W. B cell antigen presentation promotes Th2 responses and immunopathology during chronic allergic lung disease. PLoS ONE. 2008;3:e3129. doi: 10.1371/journal.pone.0003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bice D.E., Gray R.H., Evans M.J., Muggenburg B.A. Identification of plasma cells in lung alveoli and interstitial tissues after localized lung immunization. J. Leukoc. Biol. 1987;41:1–7. doi: 10.1002/jlb.41.1.1. [DOI] [PubMed] [Google Scholar]
- 148.Takhar P., Corrigan C.J., Smurthwaite L., O’connor B.J., Durham S.R., Lee T.H., Gould H.J. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma. J. Allergy Clin. Immunol. 2007;119:213–218. doi: 10.1016/j.jaci.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 149.Gould H.J., Sutton B.J. IgE in allergy and asthma today. Nat. Rev. Immunol. 2008;8:205. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 150.Zhang M., Murphy R.F., Agrawal D.K. Decoding IgE Fc receptors. Immunol. Res. 2007;37:1–16. doi: 10.1007/BF02686092. [DOI] [PubMed] [Google Scholar]
- 151.Oh S., McCaffery J.M., Eichelberger M.C. Dose-dependent changes in influenza virus-infected dendritic cells result in increased allogeneic T-cell proliferation at low, but not high, doses of virus. J. Virol. 2000;74:5460–5469. doi: 10.1128/JVI.74.12.5460-5469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wheeler M.L., Limon J.J., Bar A.S., Leal C.A., Gargus M., Tang J., Brown J., Funari V.A., Wang H.L., Crother T.R., et al. Immunological Consequences of Intestinal Fungal Dysbiosis. Cell Host Microbe. 2016;19:865–873. doi: 10.1016/j.chom.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Singanayagam A., Ritchie A.I., Johnston S.L. Role of microbiome in the pathophysiology and disease course of asthma. Curr. Opin. Pulm. Med. 2017;23:41–47. doi: 10.1097/MCP.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 154.Huang Y.J., Charlson E.S., Collman R.G., Colombini-Hatch S., Martinez F.D., Senior R.M. The role of the lung microbiome in health and disease. A National Heart, Lung, and Blood Institute workshop report. Am. J. Respir. Crit. Care Med. 2013;187:1382–1387. doi: 10.1164/rccm.201303-0488WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Charlson E.S., Diamond J.M., Bittinger K., Fitzgerald A.S., Yadav A., Haas A.R., Bushman F.D., Collman R.G. Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. Am. J. Respir. Crit. Care Med. 2012;186:536–545. doi: 10.1164/rccm.201204-0693OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hilty M., Burke C., Pedro H., Cardenas P., Bush A., Bossley C., Davies J., Ervine A., Poulter L., Pachter L., et al. Disordered microbial communities in asthmatic airways. PLoS ONE. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huffnagle G.B., Dickson R.P. The bacterial microbiota in inflammatory lung diseases. Clin. Immunol. 2015;159:177–182. doi: 10.1016/j.clim.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Van Woerden H.C., Gregory C., Brown R., Marchesi J.R., Hoogendoorn B., Matthews I.P. Differences in fungi present in induced sputum samples from asthma patients and non-atopic controls: A community based case control study. BMC Infect. Dis. 2013;13:69. doi: 10.1186/1471-2334-13-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.LeMessurier K.S., Iverson A.R., Chang T.C., Palipane M., Vogel P., Rosch J.W., Samarasinghe A.E. Allergic inflammation alters the lung microbiome and hinders synergistic co-infection with H1N1 influenza virus and Streptococcus pneumoniae in C57BL/6 mice. Sci. Rep. 2019;9:19360. doi: 10.1038/s41598-019-55712-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Skalski J.H., Limon J.J., Sharma P., Gargus M.D., Nguyen C., Tang J., Coelho A.L., Hogaboam C.M., Crother T.R., Underhill D.M. Expansion of commensal fungus Wallemia mellicola in the gastrointestinal mycobiota enhances the severity of allergic airway disease in mice. PLoS Pathog. 2018;14:e1007260. doi: 10.1371/journal.ppat.1007260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hoffmann C., Dollive S., Grunberg S., Chen J., Li H., Wu G.D., Lewis J.D., Bushman F.D. Archaea and fungi of the human gut microbiome: Correlations with diet and bacterial residents. PLoS ONE. 2013;8:e66019. doi: 10.1371/journal.pone.0066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Michael C.F., Waters C.M., LeMessurier K.S., Samarasinghe A.E., Song C.Y., Malik K.U., Lew D.B. Airway Epithelial Repair by a Prebiotic Mannan Derived from Saccharomyces cerevisiae. J. Immunol. Res. 2017;2017:8903982. doi: 10.1155/2017/8903982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lew D.B., LeMessurier K.S., Palipane M., Lin Y., Samarasinghe A.E. Saccharomyces cerevisiae-Derived Mannan Does Not Alter Immune Responses to Aspergillus Allergens. Biomed Res. Int. 2018;2018:3298378. doi: 10.1155/2018/3298378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cooper P.J., Chico M.E., Vaca M.G., Moncayo A.L., Bland J.M., Mafla E., Sanchez F., Rodrigues L.C., Strachan D.P., Griffin G.E. Effect of albendazole treatments on the prevalence of atopy in children living in communities endemic for geohelminth parasites: A cluster-randomised trial. Lancet. 2006;367:1598–1603. doi: 10.1016/S0140-6736(06)68697-2. [DOI] [PubMed] [Google Scholar]
- 165.Van den Biggelaar A.H., Rodrigues L.C., van Ree R., van der Zee J.S., Hoeksma-Kruize Y.C., Souverijn J.H., Missinou M.A., Borrmann S., Kremsner P.G., Yazdanbakhsh M. Long-term treatment of intestinal helminths increases mite skin-test reactivity in Gabonese schoolchildren. J. Infect. Dis. 2004;189:892–900. doi: 10.1086/381767. [DOI] [PubMed] [Google Scholar]
- 166.Apiwattanakul N., Palipane M., Samarasinghe A.E. Immune responses to fungal aeroallergen in Heligmosomoides polygyrus-infected mice vary by age. Cell Immunol. 2017;317:26–36. doi: 10.1016/j.cellimm.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 167.Zaiss M.M., Rapin A., Lebon L., Dubey L.K., Mosconi I., Sarter K., Piersigilli A., Menin L., Walker A.W., Rougemont J., et al. The Intestinal Microbiota Contributes to the Ability of Helminths to Modulate Allergic Inflammation. Immunity. 2015;43:998–1010. doi: 10.1016/j.immuni.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Simon-Nobbe B., Denk U., Poll V., Rid R., Breitenbach M. The spectrum of fungal allergy. Int. Arch. Allergy Immunol. 2008;145:58–86. doi: 10.1159/000107578. [DOI] [PubMed] [Google Scholar]
- 169.Caminati M., Menzella F., Guidolin L., Senna G. Targeting eosinophils: Severe asthma and beyond. Drugs Context. 2019;8:212587. doi: 10.7573/dic.212587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rosenberg H.F., Dyer K.D., Domachowske J.B. Respiratory viruses and eosinophils: Exploring the connections. Antiviral Res. 2009;83:1–9. doi: 10.1016/j.antiviral.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tiwary M., Rooney R.J., Liedmann S., LeMessurier K.S., Samarasinghe A.E. Eosinophil Responses at the Airway Epithelial Barrier during the Early Phase of Influenza A Virus Infection in C57BL/6 Mice. Cells. 2021;10:509. doi: 10.3390/cells10030509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ferastraoaru D., Hudes G., Jerschow E., Jariwala S., Karagic M., de Vos G., Rosenstreich D., Ramesh M. Eosinophilia in Asthma Patients Is Protective Against Severe COVID-19 Illness. J. Allergy Clin. Immunol. Pract. 2021;9:1152–1162.e3. doi: 10.1016/j.jaip.2020.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jacobsen E.A., Jackson D.J., Heffler E., Mathur S.K., Bredenoord A.J., Pavord I.D., Akuthota P., Roufosse F., Rothenberg M.E. Eosinophil Knockout Humans: Uncovering the Role of Eosinophils Through Eosinophil-Directed Biological Therapies. Annu. Rev. Immunol. 2021 doi: 10.1146/annurev-immunol-093019-125918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.