Abstract
Variants in the TUBB3 gene, one of the tubulin-encoding genes, are known to cause congenital fibrosis of the extraocular muscles type 3 and/or malformations of cortical development. Herein, we report a case of a 6-month-old infant with c.967A>G:p.(M323V) variant in the TUBB3 gene, who had only infantile nystagmus without other ophthalmological abnormalities. Subsequent brain magnetic resonance imaging (MRI) revealed cortical dysplasia. Neurological examinations did not reveal gross or fine motor delay, which are inconsistent with the clinical characteristics of patients with the M323V syndrome reported so far. A protein modeling showed that the M323V mutation in the TUBB3 gene interferes with αβ heterodimer formation with the TUBA1A gene. This report emphasizes the importance of considering TUBB3 and TUBA1A tubulinopathy in infantile nystagmus. A brain MRI should also be considered for these patients, although in the absence of other neurologic signs or symptoms.
Keywords: infantile nystagmus, TUBB3, congenital fibrosis of the extraocular muscle, CFEOM3, tubulinopathy
1. Introduction
Infantile nystagmus syndrome is a genetically heterogeneous disorder in which an involuntary oscillation of the eyes begins within the first 6 months of life [1]. The oscillations usually start at 2 to 3 months of age when motor and visual functions develop and persist throughout life [2]. The prevalence of infantile nystagmus syndrome was estimated from 1 in 3000 to 1 in 1000 [3,4]. Infantile nystagmus can be idiopathic or associated with other ocular diseases, such as retinal disease, albinism, low vision, or loss of vision [5,6,7,8,9,10,11,12]. It can also occur as a common presenting sign of many neurologic and systemic diseases. It is noteworthy that nystagmus has psychological and social effects on children and their parents [13].
An ophthalmic examination involves careful observation of the nystagmus waveform, frequency, amplitude, direction, and the plane of oscillation, and the presence or absence of a null point [14]. Clinical workups, including optical coherence tomography, visual evoked potential, electroretinography (ERG), and genetic testing, are used to differentiate underlying causes of infantile nystagmus. As next-generation sequencing (NGS) technology has enabled us to examine multiple causative genes simultaneously, it is now used as a front-line diagnostic tool in infantile nystagmus patients [1,15].
Tubulin is a basic structural protein of microtubules, which play many instrumental roles, such as mitosis, axonal guidance, and neuronal migration during the development of the nervous system [16,17]. Therefore, mutations in tubulin genes can alter the normal function and structure of microtubules, leading to severe brain malformations, which are called tubulinopathies [18]. Among tubulin encoding genes, mutations in the TUBB3 gene (OMIM #602661), encoding a class III β-tubulin, have been reported to cause two distinct congenital neuro-developmental pathologies: isolated or syndromic congenital fibrosis of the extraocular muscles type 3 (CFEOM3), or malformations of cortical development (MCD) [19]. CFEOM3 is a congenital, nonprogressive, oculomotor disorder that is characterized by variable deficits of vertical or horizontal eye movements and variable ptosis [20]. The limitation of eye movement in CFEOM3 patients is so various that they range from no or mild to severe ophthalmoplegia and may also show a unilateral or asymmetric presentation [21]. Interestingly, a recent study showed that the TUBB3 gene variant could cause congenital monocular elevation deficiency [22]. MCD includes lissencephaly (agyria–pachygyria), polymicrogyria or polymicrogyria-like cortical dysplasia, and cortical gyral simplification. TUBB3 mutations also affect the subcortical regions, generating dysplasia in the corpus callosum, cerebellar vermis, brainstem, basal ganglia, and cerebellum.
Herein, we report a case of c.967A>G:p.(M323V) variant in the TUBB3 gene found in a male infant who had only infantile nystagmus without CFEOM.
2. Case Report
A 6-month-old male infant presented to our clinic with infantile nystagmus. The patient was born at full term (37 weeks), weighing 3.18 kg at the time of birth, after a normal pregnancy and delivery. He was the only child between non-consanguineous Korean parents, and neonatal and perinatal insults were not noted. His family history was also unremarkable.
On initial examination, he could not fix his eyes on an object and follow, and 1–2-Hz pendular nystagmus was noted. Cycloplegic refraction showed +sph1.50 in the right eye and +sph2.00 in the left eye. He had neither eye-poking signs nor photoaversion. Dilated fundus examination showed normal foveal reflex and normal optic disc at the posterior pole. An extraocular motility test showed a full range of motion. The neurological examination was also unremarkable. Targeted NGS revealed a heterozygous missense c.967A>G:p.(M323V) variant [Chr16(GRCh37):g.90001826A>G] in the TUBB3 gene (NM_006086.4). This variant is absent in the population databases, such as the Genome Aggregation Database (gnomAD), the 1000 Genomes Project, and the Korean Reference Genome Database. This genomic position is highly conserved (phastCons: 1.00 and phyloP: 4.64). The M323 residue is located in exon 4 and conserved across β-tubulin isotypes from chicken to humans. Multiple lines of computational evidence support a deleterious effect of this variant (CADD: 25.0, FATHMM: 0.992). It was previously reported as pathogenic in ClinVar (RCV000023202.4). A de novo mutation was confirmed through segregation analysis (Figure 1A). This variant was classified as pathogenic (PS2, PM1, PM2, PP3, and PP5) according to the guideline of the American College of Medical Genetics [23].
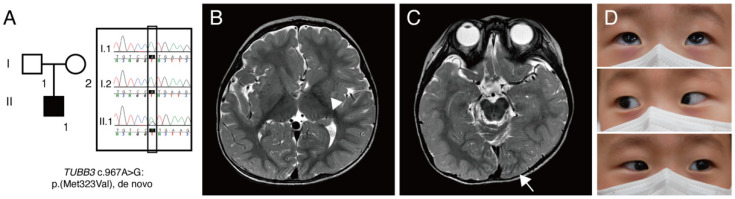
Figure 1.
(A) A pedigree of patient reported in this study. Square, male; round, female; black coloring, affected individual. Targeted next-generation sequencing showed TUBB3 c.967A>G:p.(M323V) variant. Sanger sequencing confirmed that this variant is a de novo mutation. (B) Brain magnetic resonance imaging showing cortical dysplasia. T2-weighted images without contrast revealed an asymmetric caudate nucleus (arrowhead) and globular shape of both basal ganglia and thalamus. (C) Axial T2-weighted image showing an asymmetric configuration of an occipital lobe (arrow) and abnormal cerebellar vermian foldings. (D) Pictures of an extraocular motility examination showing a full range of motion.
Subsequently, brain magnetic resonance imaging (MRI) was performed, and it revealed an asymmetric configuration and size of caudate nuclei and asymmetric configurations of lateral ventricles, occipital lobes, and corpus callosum, which are consistent with cortical dysplasia (Figure 1B,C). Repeated examination of extraocular motility had shown full duction and version until the age of 23 months (Figure 1D), and 2-Hz left-beating jerk nystagmus and intermittent head nodding were observed. A non-sedated hand-held ERG test (RETeval, LKC Technologies, Gaithersburg, MD, USA) using skin electrodes was performed for the diagnosis of retinal dysfunctions associated with nystagmus. The scotopic response was normal, but the result was inconclusive due to poor patient cooperation (Figure 2). Neurological examinations showed no gross or fine motor delays. He could sit, walk, and even run without support, but mild intellectual disability and mild language delay were noted.
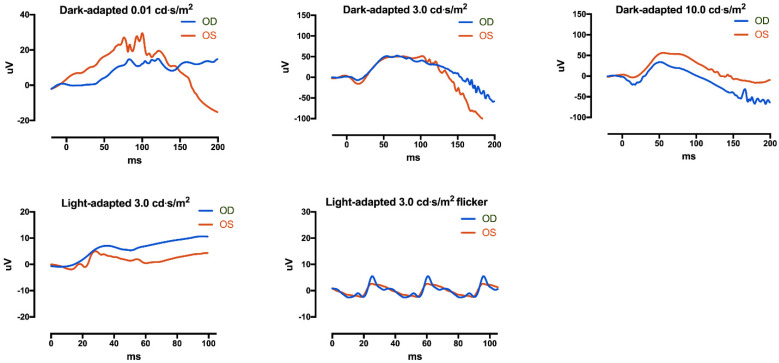
Figure 2.
Electroretinography (ERG) was performed with skin electrodes. (Top) Dark-adapted 0.01 ERG, dark-adapted 3.0 ERG, and dark-adapted 10.0 ERG showed relatively normal waveforms, but the patient was very uncooperative during examinations. (Bottom) Light-adapted 3.0 ERG 3.0 flicker was obtained. Photopic ERG responses seemed to be reduced, but the result was inconclusive due to poor cooperation. The flash strength unit is cd·s/m2.
3. Discussion
Our case demonstrates that the TUBB3 M323V syndrome causes infantile nystagmus without CFEOM. In a previous study, the heterozygous c.967A>G:p.(M323V) TUBB3 variant caused nystagmus phenotypes without CFEOM in two patients in the same family (father and son) [24]. Table 1 summarizes the clinical findings in previous cases of infantile nystagmus with the TUBB3 variants, as well as the case described in this report. Previously reported patients with the M323V syndrome had no CFEOM phenotype, but patients with G71R and G98S syndrome showed both CFEOM and infantile nystagmus [19,24]. Among seven patients, including our case, five patients had horizontal nystagmus, and the other two patients showed multidirectional and rotary nystagmus, respectively.
Table 1.
Literature review of clinical characteristics in infantile nystagmus patients with the TUBB3 variants.
| Clinical Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | |
|---|---|---|---|---|---|---|---|---|
| TUBB3 variant | p.M323V | p.M323V | p.A302V (homo) | p.G71R | p.G71R | p.G98S | p.M323V | |
| Inheritance pattern | AD | AD | Isolated (homo) | Isolated | Isolated | Isolated | Isolated | |
| Age | 36 years | 2 years | 1 year | 5 years | 9 years | 2 years | 6 months | |
| Gender | Male | Male | Female | Female | Male | Female | Male | |
| Ethnicity | NA | NA | NA | European | European | European | Korean | |
| OFC | 3rd p | 25th p | 3rd p | NA | NA | NA | 3-50th p | |
| Motor delay | Hypotonia | Hypotonia | Hypotonia | Hypotonia | Hypotonia | Hypotonia | Absent | |
| Cognitive function | Severe ID | LD | NA | ID | ID | ID | ID, LD | |
| Epilepsy | Absent | Absent | Absent | NA | NA | NA | Absent | |
| CFEOM | No | No | No | Yes | Yes | Yes | No | |
| Nystagmus | Horizontal nystagmus | Horizontal nystagmus | Multidirectional nystagmus | Rotary nystagmus | Horizontal nystagmus | Horizontal nystagmus | Horizontal nystagmus | |
| Cortical dysgenesis | Gyral disorganization | Gyral disorganization | Gyral disorganization | Gyral disorganization | Gyral disorganization | Gyral disorganization | Gyral disorganization | |
| Cerebellum | vermis | Dysplastic | Dysplastic | Dysplastic | Dysplastic | Dysplastic | Dysplastic | Dysplastic |
| Hemisphere | Dysplastic | Normal | Normal | Normal | Normal | Normal | Normal | |
| Brainstem | Hypoplastic | Hypoplastic | Hypoplastic | Hypoplastic | Hypoplastic | Hypoplastic | Normal | |
| Corpus callosum | Thin | Thin | Thin | Thin | Thin | Thin | Asymmetric | |
| Basal ganglia | Hypertrophic/mild fusion | Hypertrophic/mild fusion | Fusion caudate/ putamen |
Hypertrophic/ fusion |
Hypertrophic/ fusion |
Hypertrophic/ fusion |
Asymmetric | |
| Literatures | Poirier et al. Hum Mol Genet (2010) | Poirier et al. Hum Mol Genet (2010) | Poirier et al. Hum Mol Genet (2010) | Whitman et al. Am J Med Genet A. (2016) | Whitman et al. Am J Med Genet A. (2016) | Whitman et al. Am J Med Genet A. (2016) | This study | |
Abbreviations: AD, autosomal dominant; CFEOM, congenital fibrosis of the extraocular muscle; homo, homozygous; ID, intellectual disability; LD, language delay; NA, not available; OFC, occipitofrontal circumference; p, percentile.
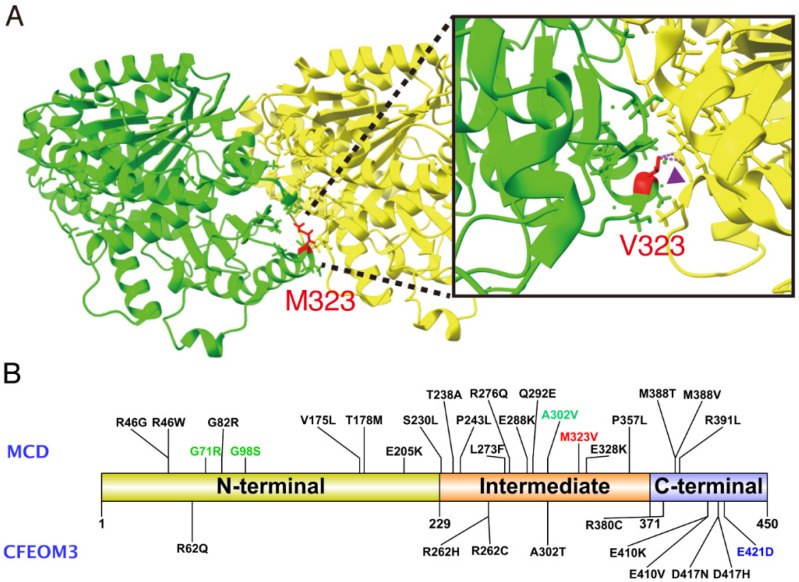
Our report demonstrates that the TUBB3 gene should be considered as a causative gene for infantile nystagmus. The heterozygous missense mutation c.967A>G:p.(M323V) is located at the intermediate domain (residues 230–371) in a class III β-tubulin (Figure 3B), which engages in heterodimer stability and longitudinal and lateral interactions [25]. In a protein model using UCSF ChimeraX [26], a p.M323V is predicted to cause a clash between TUBB3:p.V323 and TUBA1A:p.Y210, which may affect the stability of the heterodimer (Figure 3A). The TUBB3:M323 residue is the interaction site with TUBA1A when forming heterodimers. The proposed mechanism of nystagmus phenotype in M323V syndrome is an impaired capacity to form αβ tubulin heterodimers, not through the independent mechanism of GTP-binding. The present case resembles TUBA1A-associated tubulinopathy, rather than classic TUBB3 CFEOM3, where nystagmus was present in 3/29 (10.3%) cases, and no CFEOM phenotypes were observed [27].
Figure 3.
(A) A protein model with UCSF ChimeraX, showing the unstable formation of αβ tubulin heterodimer in M323V syndrome. Green, class III β-tubulin encoded by TUBB3; Yellow, α tubulin encoded by TUB1A1. A clash occurred between TUBB3:p.V323 and TUBA1A:p.Y210 (arrowhead). (B) Schematic diagram of deleterious variants in TUBB3 functional domains. A total of thirty-two missense variants, including p.M323V, have been reported until recently. Variants associated with malformations of cortical development (MCD) with or without congenital fibrosis of the extraocular muscles type 3 (CFEOM3) are marked above, and variants only representing CFEOM3 are marked at the bottom. Most variants in the N-terminal and the intermediate domain cause MCD, and missense variants in the C-terminal cause either MCD or CFEOM3 phenotypes. A red word indicates the variant in this study. Green words denote previously reported variants associated with infantile nystagmus, and a blue word indicates a variant associated with monocular elevation deficiency.
Optic nerve hypoplasia has been reported with TUBA1A, TUBB2B, and TUBA8 mutations, suggesting that tubulin gene mutations, in general, can cause optic nerve hypoplasia [28,29,30]. The possibility that optic nerve hypoplasia is the cause of nystagmus cannot be excluded. Although optic nerve hypoplasia has been reported in tubulinopathies, there were no reports of infantile nystagmus in patients with optic nerve hypoplasia who had TUBB3 mutations. Moreover, a dilated fundus examination and brain MRI did not reveal optic nerve hypoplasia in our case. TUBB3 has widespread expression in the retinal ganglion cells, amacrine cells, horizontal process, and cone photoreceptors [31]. The neuronal circuit of direction-selective retinal cells may be disrupted due to TUBB3 mutation [32]. Because we could not obtain optical coherence tomography, it is possible that a mild degree of foveal hypoplasia or retinal dystrophy co-exists. However, our targeted panel included 429 genes associated with inherited retinal diseases and infantile nystagmus syndrome, so we can exclude those possibilities. Further research is needed to determine whether the cause of nystagmus is due to cortical, cerebellar, or retinal origin [33].
In conclusion, our case report shows that infantile nystagmus can arise without CFEOM owing to the TUBB3 variant. Therefore, pediatric ophthalmologists should keep in mind that the clinical features of the TUBB3 syndrome are so diverse that only nystagmus could appear as the main presenting sign. We also thought that TUBB3 and TUBA1A genes should be included in the targeted panel of infantile nystagmus. In general, a brain MRI has a low diagnostic yield for patients with infantile nystagmus in the absence of other neurologic signs or symptoms [34]. However, as in this case, an accurate molecular diagnosis will enable clinicians to determine whether a brain MRI is necessary or not. Additionally, collaborations with multiple specialties may facilitate the appropriate management in such cases.
Author Contributions
S.J. and J.H. contributed to writing of the manuscript. S.J., S.-E.P., D.W., S.-T.L., S.-H.H. and J.H. contributed to review and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the Research of Korea Centers for Disease Control (#2018-ER6902–02) and the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2020R1C1C1007965).
Informed Consent Statement
Informed consent was obtained from the parents of the patient involved in the study. Written informed consent has been obtained from the parents of the patient to publish this paper.
Data Availability Statement
The data presented in this study is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rim J.H., Lee S.-T., Gee H.Y., Lee B.J., Choi J.R., Park H.W., Han S.-H., Han J. Accuracy of next-generation sequencing for molecular diagnosis in patients with infantile nystagmus syndrome. JAMA Ophthalmol. 2017;135:1376–1385. doi: 10.1001/jamaophthalmol.2017.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khanna S., Dell’Osso L.F. The diagnosis and treatment of infantile nystagmus syndrome (INS) Sci. World J. 2006;6:1385–1397. doi: 10.1100/tsw.2006.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forssman B., Ringner B. Prevalence and inheritance of congenital nystagmus in a swedish population. Ann. Hum. Genet. 1971;35:139–147. doi: 10.1111/j.1469-1809.1956.tb01386.x. [DOI] [PubMed] [Google Scholar]
- 4.Stewart-Brown S., Haslum M. Partial sight and blindness in children of the 1970 birth cohort at 10 years of age. J. Epidemiol. Community Health. 1988;42:17–23. doi: 10.1136/jech.42.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papageorgiou E., McLean R.J., Gottlob I. Nystagmus in childhood. Pediatr. Neonatol. 2014;55:341–351. doi: 10.1016/j.pedneo.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Kuht H.J., Han J., Maconachie G.D.E., Park S.E., Lee S.T., McLean R., Sheth V., Hisaund M., Dawar B., Sylvius N., et al. Slc38a8 mutations result in arrested retinal development with loss of cone photoreceptor specialization. Hum. Mol. Genet. 2020;29:2989–3002. doi: 10.1093/hmg/ddaa166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han J., Lee T., Lee J.B., Han S.H. Retinal microstructures are altered in patients with idiopathic infantile nystagmus. Graefes Arch. Clin. Exp. Ophthalmol. 2017;255:1661–1668. doi: 10.1007/s00417-017-3713-y. [DOI] [PubMed] [Google Scholar]
- 8.Aamir A., Kuht H.J., Grønskov K., Brooks B.P., Thomas M.G. Clinical utility gene card for oculocutaneous (oca) and ocular albinism (oa)-an update. Eur. J. Hum. Genet. 2021 doi: 10.1038/s41431-021-00809-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J., Lee H., Lee Y.M., Kuht H.J., Thomas M.G., Kim S.J., Lee S.T., Han J. Dync2h1 variants cause leber congenital amaurosis without syndromic features. Clin. Genet. 2021 doi: 10.1111/cge.13958. [DOI] [PubMed] [Google Scholar]
- 10.Yoo T.K., Han S.H., Han J. Rp2 rod-cone dystrophy causes spasmus nutans-like nystagmus. J. Neuroophthalmol. 2021;41:e91–e93. doi: 10.1097/WNO.0000000000000880. [DOI] [PubMed] [Google Scholar]
- 11.Surl D., Shin S., Lee S.T., Choi J.R., Lee J., Byeon S.H., Han S.H., Lim H.T., Han J. Copy number variations and multiallelic variants in korean patients with leber congenital amaurosis. Mol. Vis. 2020;26:26–35. [PMC free article] [PubMed] [Google Scholar]
- 12.Dawar B., Kuht H.J., Han J., Maconachie G.D.E., Thomas M.G. Clinical utility gene card for frmd7-related infantile nystagmus. Eur J. Hum. Genet. 2021 doi: 10.1038/s41431-021-00826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilling R., Thompson J., Gottlob I. Social and visual function in nystagmus. Br. J. Ophthalmol. 2005;89:1278–1281. doi: 10.1136/bjo.2005.070045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards M.D., Wong A. Infantile nystagmus syndrome: Clinical characteristics, current theories of pathogenesis, diagnosis, and management. Can. J. Ophthalmol. 2015;50:400–408. doi: 10.1016/j.jcjo.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Thomas M.G., Maconachie G.D., Sheth V., McLean R.J., Gottlob I. Development and clinical utility of a novel diagnostic nystagmus gene panel using targeted next-generation sequencing. Eur. J. Hum. Genet. 2017;25:725–734. doi: 10.1038/ejhg.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lasser M., Tiber J., Lowery L.A. The role of the microtubule cytoskeleton in neurodevelopmental disorders. Front. Cell. Neurosci. 2018;12:165. doi: 10.3389/fncel.2018.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker A.L., Kavallaris M., McCarroll J.A. Microtubules and their role in cellular stress in cancer. Front. Oncol. 2014;4:153. doi: 10.3389/fonc.2014.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arrigoni F., Romaniello R., Peruzzo D., Poretti A., Bassi M.T., Pierpaoli C., Valente E.M., Nuovo S., Boltshauser E., Huisman T.A.G.M. The spectrum of brainstem malformations associated to mutations of the tubulin genes family: MRI and DTI analysis. Eur. Radiol. 2019;29:770–782. doi: 10.1007/s00330-018-5610-0. [DOI] [PubMed] [Google Scholar]
- 19.Whitman M.C., Andrews C., Chan W.M., Tischfield M.A., Stasheff S.F., Brancati F., Ortiz-Gonzalez X., Nuovo S., Garaci F., MacKinnon S.E. Two unique tubb3 mutations cause both cfeom3 and malformations of cortical development. Am. J. Med. Genet. Part A. 2016;170:297–305. doi: 10.1002/ajmg.a.37362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J.H., Hwang J.M. Imaging of cranial nerves iii, iv, vi in congenital cranial dysinnervation disorders. Korean J. Ophthalmol. 2017;31:183–193. doi: 10.3341/kjo.2017.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tischfield M.A., Baris H.N., Wu C., Rudolph G., Van Maldergem L., He W., Chan W.-M., Andrews C., Demer J.L., Robertson R.L. Human tubb3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 2010;140:74–87. doi: 10.1016/j.cell.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas M.G., Maconachie G.D., Constantinescu C.S., Chan W.-M., Barry B., Hisaund M., Sheth V., Kuht H.J., Dineen R.A., Harieaswar S. Congenital monocular elevation deficiency associated with a novel tubb3 gene variant. Br. J. Ophthalmol. 2020;104:547–550. doi: 10.1136/bjophthalmol-2019-314293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the american college of medical genetics and genomics and the association for molecular pathology. Genet. Med. 2015;17:405–423. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poirier K., Saillour Y., Bahi-Buisson N., Jaglin X.H., Fallet-Bianco C., Nabbout R., Castelnau-Ptakhine L., Roubertie A., Attie-Bitach T., Desguerre I. Mutations in the neuronal β-tubulin subunit tubb3 result in malformation of cortical development and neuronal migration defects. Hum. Mol. Genet. 2010;19:4462–4473. doi: 10.1093/hmg/ddq377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tischfield M.A., Cederquist G.Y., Gupta Jr M.L., Engle E.C. Phenotypic spectrum of the tubulin-related disorders and functional implications of disease-causing mutations. Curr. Opin. Genet. Dev. 2011;21:286–294. doi: 10.1016/j.gde.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pettersen E.F., Goddard T.D., Huang C.C., Meng E.C., Couch G.S., Croll T.I., Morris J.H., Ferrin T.E. Ucsf chimerax: Structure visualization for researchers, educators, and developers. Protein Sci. 2021;30:70–82. doi: 10.1002/pro.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hebebrand M., Hüffmeier U., Trollmann R., Hehr U., Uebe S., Ekici A.B., Kraus C., Krumbiegel M., Reis A., Thiel C.T. The mutational and phenotypic spectrum of tuba1a-associated tubulinopathy. Orphanet. J. Rare Dis. 2019;14:1–13. doi: 10.1186/s13023-019-1020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdollahi M.R., Morrison E., Sirey T., Molnar Z., Hayward B.E., Carr I.M., Springell K., Woods C.G., Ahmed M., Hattingh L., et al. Mutation of the variant alpha-tubulin tuba8 results in polymicrogyria with optic nerve hypoplasia. Am. J. Hum. Genet. 2009;85:737–744. doi: 10.1016/j.ajhg.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aiken J., Buscaglia G., Bates E.A., Moore J.K. The α-tubulin gene tuba1a in brain development: A key ingredient in the neuronal isotype blend. J. Dev. Biol. 2017;5:8. doi: 10.3390/jdb5030008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas M.G., Maconachie G.D.E., Kuht H.J., Chan W.M., Sheth V., Hisaund M., McLean R.J., Barry B., Al-Diri B., Proudlock F.A., et al. Optic nerve head and retinal abnormalities associated with congenital fibrosis of the extraocular muscles. Int. J. Mol. Sci. 2021;22:2575. doi: 10.3390/ijms22052575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma R.K., Netland P.A. Early born lineage of retinal neurons express class III beta-tubulin isotype. Brain Res. 2007;1176:11–17. doi: 10.1016/j.brainres.2007.07.090. [DOI] [PubMed] [Google Scholar]
- 32.Yonehara K., Fiscella M., Drinnenberg A., Esposti F., Trenholm S., Krol J., Franke F., Scherf B.G., Kusnyerik A., Müller J., et al. Congenital nystagmus gene frmd7 is necessary for establishing a neuronal circuit asymmetry for direction selectivity. Neuron. 2016;89:177–193. doi: 10.1016/j.neuron.2015.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konus I., Ozsoy E., Turkcuoglu P., Emre S., Duman F. Evaluation of metabolite changes in the occipital cortex of patients with idiopathic infantile nystagmus or bilateral ametropic amblyopia by magnetic resonance spectroscopy. Korean J. Ophthalmol. 2019;33:406–413. doi: 10.3341/kjo.2019.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertsch M., Floyd M., Kehoe T., Pfeifer W., Drack A.V. The clinical evaluation of infantile nystagmus: What to do first and why. Ophthalmic Genet. 2017;38:22–33. doi: 10.1080/13816810.2016.1266667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study is contained within the article.