Abstract
Listeria monocytogenes is one of the most important foodborne pathogens that may be present in food and in food processing environments. In the present study, 91 L. monocytogenes isolates of serogroup IVb from raw meat, ready-to-eat food and food production environments in Poland were characterized by whole genome sequencing (WGS). The strains were also compared, using core genome multi-locus sequence typing (cgMLST) analysis, with 186 genomes of L. monocytogenes recovered worldwide from food, environments, and from humans with listeriosis. The L. monocytogenes examined belonged to three MLST clonal complexes: CC1 (10; 11.0% isolates), CC2 (70; 76.9%), and CC6 (11; 12.1%). CC1 comprised of two STs (ST1 and ST515) which could be divided into five cgMLST, CC2 covered two STs (ST2 and ST145) with a total of 20 cgMLST types, whereas CC6 consisted of only one ST (ST6) classified as one cgMLST. WGS sequences of the tested strains revealed that they had several pathogenic markers making them potentially hazardous for public health. Molecular comparison of L. monocytogenes strains tested in the present study with those isolated from food and human listeriosis showed a relationship between the isolates from Poland, but not from other countries.
Keywords: Listeria monocytogenes, serogroup IVb, WGS, cgMLST, virulence genes
1. Introduction
Listeria monocytogenes is an opportunistic foodborne pathogen responsible for invasive listeriosis, one of the most severe foodborne diseases with a high mortality rate [1]. The infection usually results from the consumption of contaminated food, especially ready-to-eat (RTE) foods of plant and animal origins [2,3]. According to a recent European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC) report, the overall prevalence of L. monocytogenes in RTE food of meat origin was 1.4% [1]. Various RTE food categories have different potential to infect consumers with L. monocytogenes. For example, deli meats and not re-heated frankfurters were established as food that had a very high predicted risk for consumers due to the relatively high rates of bacterial contamination and ability to support the rapid growth of L. monocytogenes under refrigerated storage conditions [4]. Additionally, recent investigations from different countries showed that RTE meat products were a source of epidemics caused by L. monocytogenes, including the largest outbreak in South Africa reported so far [5,6].
L. monocytogenes has the ability to persist in food processing facilities for months and even years, despite the application of sanitation measures [7,8,9]. Therefore, control of L. monocytogenes in the food processing industry is essential to reduce the risk of contamination and to protect consumers [10]. It has also been shown that some L. monocytogenes clones survive better in food production environments than others [11]. This phenomenon usually depends on the ability of the bacteria to form a biofilm and express tolerance to sanitizers and other environmental conditions [12]. Furthermore, such strains may also contain genomic islands: Stress Survival Islet SSI-1, which plays a role in acidic and gastric stress responses and growth of the bacteria in food, and SSI-2, involved in oxidative and alkaline stress responses, respectively [13,14,15,16].
Among 13 recognized serotypes of L. monocytogenes, only four are of significant public concern, with three, 1/2a, 1/2b, and 4b, being responsible for over 95% of invasive listeriosis cases [17,18]. The majority of the sporadic cases and outbreaks are associated with strains of serotype 4b, while isolates classified to serotypes 1/2a and 1/2c are more often isolated from food and environmental samples [19,20,21]. Based on molecular analyses, L. monocytogenes are classified into four lineages, with most isolates belonging to lineages I (serotypes 1/2b, 3b, 3c, and 4b) and II (serotypes 1/2a, 3a, and 1/2c) [17,22]. Furthermore, the serotypes are also distinguished into molecular PCR-based serogroups: IIa (with serotypes 1/2a and 3a), IIb (1/2b and 3b), IIc (1/2c and 3c), IVb (4b, 4d, and 4e) [23].
Classification of L. monocytogenes into a certain lineage or serogroup determines some properties of the isolates [24]. For example, the pathogenicity islands LIPI-3 encoding listerolysin S and LIPI-4, containing six genes responsible for a cellobiose-specific phosphotransferase system, are most frequently found among the isolates classified as lineage I. Strains belonging to the IVb and IIb serogroups typically harbour the full length of the inlA gene encoding a protein critical for attachment of L. monocytogenes to human host cells [17,25]. Additionally, isolates of some serogroups are over-represented and are more often recovered from the same sources, e.g., L. monocytogenes of IVb predominates among clinical isolates, including those responsible for meningitis [24,26]. However, classification of L. monocytogenes into serogroups is not often enough for epidemiological investigation. In this case, next generation sequencing (NGS) to obtain the whole genome sequence (WGS) is used [6,27,28,29,30,31,32]. WGS-based typing is the preferred method for molecular classification and analyses of L. monocytogenes to assess the sources of infection [29]. The broad tools used to analyse the WGS data for the determination of the genetic relationship between isolates are multi-locus sequence typing (MLST) and analysis of core genome (cgMLST), which allow clonal complexes (CCs) with sequence types (STs) and cgMLST complex types (CTs) to be identified, respectively [32,33]. cgMLST for L. monocytogenes has been demonstrated as a highly reproducible method and is widely used for molecular typing of isolates classified to the same serotypes and lineages and to identify hypervirulent clones [5,29,31,34,35,36,37].
The objectives of the present study were: (i) to establish the comprehensive molecular characteristics and establishment of the genetic diversity of L. monocytogenes IVb serogroup isolated from food and food production environments in Poland; (ii) comparison and assessment of the phylogenetic relationship between the current isolates and strains recovered from food and foodborne listeriosis cases in Poland and in other countries.
2. Results
2.1. MLST and cgMLST Typing
Among the 91 L. monocytogenes isolates of molecular serogroup IVb, three MLST clonal complexes were identified: CC1 (10; 11.0% isolates), CC2 (70; 76.9%), and CC6 (11; 12.1%). CC1 comprised of two STs, i.e., ST1 (9; 9.9% isolates) and ST515 (1; 1.1%), which could be divided into 5 cgMLST. CC2 covered two STs: ST2 (44; 48.3%) and ST145 (26; 28.6%) with a total of 20 cgMLST types, whereas CC6 consisted of only one ST (ST6; 1.1%) classified as one cgMLST (Table S1).
Based on the cgMLST analysis, the isolates were further classified into three sublineages (SLs: SL1, SL2, and SL6) and 32 CT types, with the most prevalent being CT375 (26; 28.6% isolates) (Table 1). L. monocytogenes isolated from RTE food (n = 62) belonged to 26 different cgMLST types, mainly to SL2-ST2-CT4325 and SL2-ST2-CT4380 (9 isolates of each; 14.5%). The majority of isolates from raw meat (n = 21; 6 cgMLST types) were classified to SL2-ST145-CT375 (16; 76.2%) whereas isolates from food production environments (meat processing plants) (n = 8) were diverse (7 different CTs) without any predominant cgMLST type (Table 1).
Table 1.
Molecular characteristics of L. monocytogenes serogroup IVb isolates tested.
| Strain Origin | Molecular Type (No. of Isolates) | |||
|---|---|---|---|---|
| Clonal Complex | Sublineage | Sequence Type | cgMLST type | |
| RTE 1
(n = 62) |
CC1 (7) | SL1 (7) | ST1 (7) | CT322 (4), CT5260 (2), CT4326 (1), |
| CC2 (49) | SL2 (49) | ST2 (40) | CT4325 (9), CT4380 (9), CT2416 (4), CT4382 (4), CT5257 (1), CT5259 (1), CT7734 (1), CT7735 (1), CT7739 (1), CT7740 (1), CT7741 (1), CT7742 (1), CT7743 (1), CT7744 (1), CT7745 (1), CT7746 (1), CT7747 (1), CT7748 (1) | |
| ST145 (9) | CT375 (9) | |||
| CC6 (6) | SL6 (6) | ST6 (6) | CT461 (2), CT477 (2), CT434 (1), CT5608 (1) | |
| Raw meat (n = 21) |
CC1 (2) | SL1 (2) | ST1 (2) | CT322 (1), CT7733 (1) |
| CC2 (17) | SL2 (17) | ST2 (1) | CT2416 (1) | |
| ST145 (16) | CT375 (16) | |||
| CC6 (2) | SL6 (2) | ST6 (2) | CT7738 (1), CT5306 (1) | |
| FPE 2
(n = 8) |
CC1 (1) | SL1 (1) | ST515 (1) | CT7737 (1) |
| CC2 (4) | SL2 (4) | ST2 (3) | CT2416 (1), CT4325 (1), CT7736 (1) | |
| ST145 (1) | CT375 (1) | |||
| CC6 (3) | SL6 (3) | ST6 (3) | CT434 (2), CT4402 (1) | |
1 RTE: ready-to-eat food. 2 FPE: food production environments.
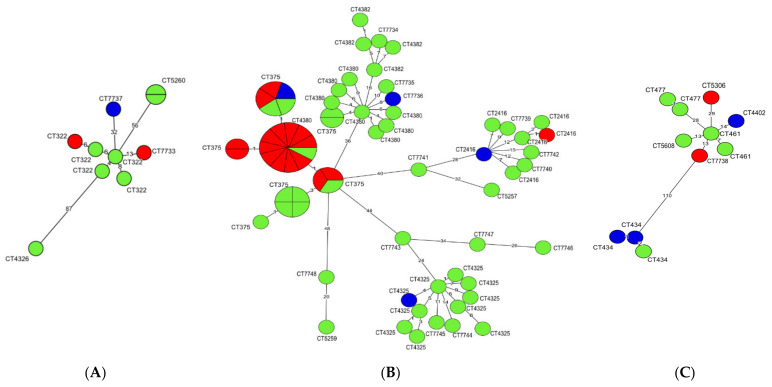
A clonal relationship of L. monocytogenes isolates classified to each of three CCs was determined using a minimum spanning tree (MST) analysis based on cgMLST allelic profiles (Figure 1). It was found that all isolates of CC2, irrespective of origin, were characterized by the lowest number of differences in the allelic variants among the compared sequences, ranging from 0 (no difference = identical isolates) to 48 differences. More heterogenic were L. monocytogenes classified to CC1 which differed from 0 to 87 allelic variants, whereas the isolates of CC6 were the most genotypically diverse, ranging from 1 to 110 allelic differences between CTs (Figure 1).
Figure 1.
Minimum spanning tree (MST) analysis based on cgMLST allelic profiles of 91 L. monocytogenes isolates. Each CC is shown on a separate MST: (A) CC1; (B) CC2; (C) CC6. The circles represent cgMLST types (CTs). The different colors indicate isolate source (green, ready-to-eat food; red, raw meat; blue, food production environments). Numbers on the branches show allele differences between neighboring nodes (CTs).
The isolates belonging to the most numerous cgMLST types, i.e., SL2-ST145-CT375 (n = 26), SL2-ST2-CT4325 (n = 10) and SL2-ST2-CT4380 (n = 9) were further compared using MST analysis (Figure S1). Strains classified to CT375 were recovered from all three sources included in the study, mainly from raw meat (n = 16) and RTE food (n = 9), during the years 2015–2018 (Table S1). Interestingly, five of the SL2-ST145-CT375 isolates (IDs: 47103, 47107, 47124, 47125, and 47140) were identical according to the cgMLST allelic profile and were isolated in the years 2016 and 2018 (Figure 1 and Table S1). More information on the L. monocytogenes SL2-ST145-CT375 cgMLST type and other strains shown in Figure 1 and Figure S1 are described in Table S1.
2.2. Virulence Factor and Resistance Genes
L. monocytogenes isolates were tested towards several virulence marker genes to assess their potential pathogenic hazard to public health (Table S1). All of them harbored the genes of the pathogenic island LIPI-1, which contains six virulence genes regulated by PrfA regulatory protein, a transcriptional activator for more than 140 genes, including the inlA and inlB sequences, responsible for L. monocytogenes internalization into non-phagocytic cells [38]. Analysis of the inlA sequence of all 91 isolates tested revealed that two variants were identified: variant 4 (81; 89.0% strains) and variant 6 (10; 11.0% strains), whereas among the prfA gene, four variants were found, i.e., 3 (77; 84.6% strains), 8 (11; 12.1%), 226 (2; 2.2%), and 251 (1; 1.1%), respectively. None of the tested strains possessed the premature stop codons (PMSCs) in the inlA gene, involved in attenuation of virulence in L. monocytogenes [25,31]. However, the mdrM gene, one of the genes of the multidrug resistance transporter MDR playing a role in various drug and bile resistance, was present in all strains tested [32,39]. On the other hand, none of the 91 L. monocytogenes isolates possessed the LIPI-4 pathogenicity island and inlL internalin gene sequences. WGS data analysis also revealed that some of the isolates harboured only selected virulence genes, e.g., the inlG marker was observed in 11 (12.1%) isolates classified to the SL6-ST6 cgMLST profile, whereas 21 (23.1%) strains of ST1, ST6, and ST515 sequence types had the pathogenicity island LIPI-3.
In silico identification of the antimicrobial resistance genes using the Bacterial Isolate Genome Sequence Database L. monocytogenes (BIGSdb-Lm) revealed that in all 91 L. monocytogenes isolates tested four intrinsic genes were present: fosX (resistance to fosfomycin), lmo0919 (lincosamides), norB (quinolones), and sul (sulfonamides). Furthermore, all strains possessed the phosphatidylglycerol lysyl-transferase (mprF) gene, encoding the multiple peptide resistance factor responsible for bacterial peptide resistance [32].
Analysis of the WGS sequences towards the genetic factors responsible for resistance to quaternary ammonium compounds (QACs), including benzalkonium chloride (BAC), revealed that the Tn6188_qac (ermC) sequence was present in only one of the strains tested, whereas as many as 68 (74.7%) isolates possessed the Listeria Genomic Island 2 (LGI2) marker. These strains were classified to CC2 (59 out of 62; 95.2% strains) and CC1 (9 out of 10; 90.0% isolates) clonal complexes, respectively. On the other hand, none of the investigated strains harboured the bcrABC gene cassette and the emrE marker encoding a putative small multidrug-resistant (SMR) efflux pump, both responsible for tolerance to benzalkonium chloride.
Among the two analyzed gene sequences encoding resistance to cadmium (cadA and cadC), only cadA responsible for cadmium-transporting ATPase was identified among 3 of 91 (3.3%) L. monocytogenes isolates classified to SL6-ST6 cgMLST type (IDs 47078, 47086, 47110) (Table S1). However, none of these three isolates had the cadmium and arsenic resistance genes localized on the LGI2 sequence. Further genomic analysis identified another 68 (74.7%) isolates with both cadmium and arsenic resistance sequences present on the LGI2 Island.
Furthermore, none of the 91 isolates was positive for all five or two genes of Stress Survival Islets 1 or 2 (SSI-1 and SSI-2), respectively, which play a role in bacterial survival under adverse gastric conditions. However, the lmo0447 gene of SSI-1 was identified in all L. monocytogenes strains tested. Additionally, all tested isolates possessed the comK gene, responsible for biofilm formation and virulence. Detailed information on all genes identified in the present study are shown in Table S1.
2.3. Detection of Prophage Regions and Plasmid Sequences
Analysis of WGS data of the 91 L. monocytogenes isolates revealed a total of 299 DNA prophage sequences, including 69 intact sequences found in 51 (56.0%) strains, with the most common sequence being PHAGE_Lister_vB_LmoS_188, identified among 38 (41.8%) isolates. Furthermore, 132 incomplete and 94 questionable sequences were identified in 87 (95.6%) and 91 (100%) isolates, respectively. Strains with IDs 47078 and 47130 (both from RTE food) and with ID 47084, 47086, and 47110 (originating from food production environments) had the highest number (three in each isolate) of intact prophage sequences (Table S4).
Examination of L. monocytogenes identified 15 (16.5%) strains with plasmid sequences, classified to pLM5578 (12 isolates) and J1776 (3 strains). Isolates which harboured the pLM5578 sequences were classified to CC2, ST2, and four cgMLST types, mainly CT4380 (9 strains). All L. monocytogenes with J1776 plasmid belonged to CC6, ST6, and CT434 types and only these strains showed the presence of the cadA gene.
2.4. Molecular Comparison of L. monocytogenes from Different Sources
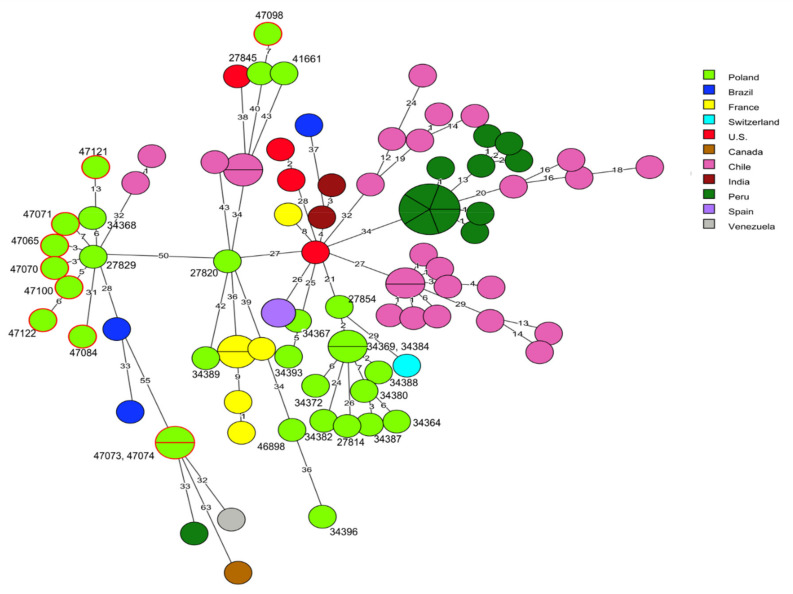
cgMLST analysis was used for comparison of the genome sequences of the 91 L. monocytogenes tested in the present study with the sequences of 186 L. monocytogenes strains available in the BIGSdb-Lm database or in GenBank. Detailed information related to these isolates, including the source of isolation and country of origin, are shown in Table S3. It was found that the current strains classified to clonal complex CC1 did not reveal any genotypic relationship with 59 strains of the corresponding CC, recovered from patients with listeriosis (n = 38), food (n = 19), and food production environments (n = 2) in other countries. Comparison with 20 other L. monocytogenes strains previously isolated in Poland, including 14 isolates from clinical cases, showed that some of these, e.g., the current isolates of cgMLST type SL1-ST1-CT322 (IDs 47065, 47070, 47071, 47100, and 47122) and one strain previously described by Kurpas et al. [40] (ID 27929) displayed a very close molecular relationship with up to 7 allelic differences, although they had been isolated in different regions of Poland and in different years. A similar genetic relationship was noted for L. monocytogenes ID 47098 and the previously isolated strain ID 27845, both classified to CT4326 and recovered from RTE food, which showed 7 allelic difference in the cgMLST analysis (Figure 2, Tables S1 and S3).
Figure 2.
Minimum spanning tree (MST) analysis based on the core genome multi-locus sequence typing (cgMLST) profiles (CTs) of 10 L. monocytogenes CC1 strains tested in the present study together with 79 strains of CC1 available at BIGSdb-Lm and in the literature. cgMLST types are represented by circles with different colors related to countries of the strains’ origin. Numbers on the connecting lines show alleles differences between adjacent nodes (CTs). The numbers next to circles show ID of Polish L. monocytogenes strains. Circles with red rim represent isolates from the present study.
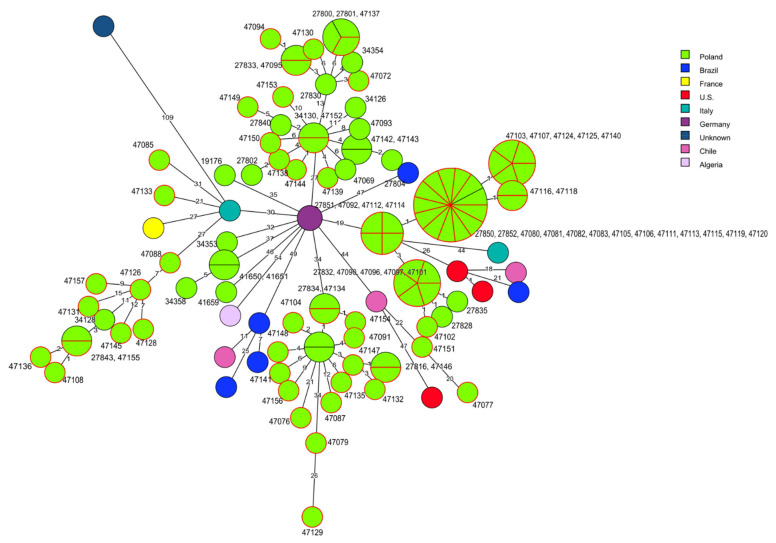
Comparative molecular analysis of the present 70 L. monocytogenes strains belonging to CC2 and the sequences of 45 other isolates of the same clonal complex did not show any close relationships, especially with the isolates identified in other countries. However, some strains of the current study displayed a genetic similarity with the sequences of other Polish L. monocytogenes recovered from food or from clinical cases. For example, four strains of cgMLST type SL1-ST1-CT4382 of RTE food origin revealed a close genetic relationship with the strains of the same origin with IDs 27830, 27800, 27801, 27833, isolated in 2015 and 2016 [40] and with one isolate (ID 34354) responsible for human listeriosis isolated in 2011 [26]. Additionally, 11 strains (IDs: 47080, 47081, 47082, 47083, 47105, 47106, 47111, 47113, 47115, 47119, 47120) from the current study, classified to SL2-ST145-CT375 and mainly recovered from raw meat (10 strains) were identical, based on the cgMLST allelic profile, with strains with ID 27850 and ID 27852 from RTE food previously described in Poland [40]. Similarly, such a close molecular relatedness was also observed among 10 strains classified to SL2-ST2-CT4325, isolated mainly from RTE food and four strains (IDs: 27816, 27834, 27838, 27842) characterized by Kurpas et al. [40] (Figure 3, Tables S1 and S3).
Figure 3.
Minimum spanning tree (MST) analysis based on the cgMLST profiles (CTs) of 70 L. monocytogenes CC2 strains tested in the present study together with 45 strains of CC2 at BIGSdb-Lm and the literature. cgMLST types are represented by circles with different colors related to countries of strains’ origin. Numbers on the connecting lines show allele differences between adjacent nodes (CTs). The numbers next to circles show ID of Polish L. monocytogenes strains. Circles with red rim represent isolates from the present study.
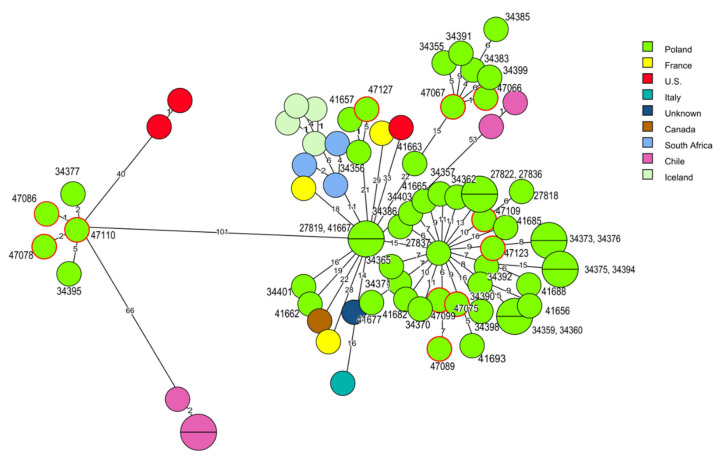
Molecular relationships were also identified among L. monocytogenes of clonal complex CC6 isolated in Poland but not in other countries, e.g., the strain classified to cgMLST type SL6-ST6-CT434 (ID 47110), isolated in 2016 from food production environments, displayed two to five allelic differences with two isolates (ID 34377, ID 34395) of human origin recovered in 2012 and 2013 [26]. A similar genetic relatedness (from four to six allelic differences) was observed between two current strains (ID 47066 and ID 47067) and four isolates (IDs: 34355, 34383, 34385, 34399) from clinical listeriosis cases from the years 2011–2013 and L. monocytogenes SL6-ST6-CT5306 type (ID 47127) of raw meat origin with two human strains recovered in 2011 (ID 34356 and ID 41657) [26] (Figure 4, Tables S1 and S3).
Figure 4.
Minimum spanning tree (MST) analysis based on the cgMLST profiles (CTs) of 11 L. monocytogenes CC6 strains tested in the present study together with 62 strains of CC6 at BIGSdb-Lm and the literature. cgMLST types are represented by circles with different colors related to countries of strains’ origin. Numbers on the connecting lines show allele differences between adjacent nodes (CTs). The numbers next to circles show ID of Polish L. monocytogenes strains. Circles with red rim represent isolates from the present study.
3. Discussion
The current study on the molecular characteristics of L. monocytogenes isolated from food and food production environments are in line with the previous investigations performed in our laboratory [40,41]. However, in contrast to those analyses, the present study focused on the bacteria of serogroup IVb, mainly due to their clinical importance. In Poland, according to the studies of Kuch et al. [26], this serogroup is responsible for more than 55% of invasive listeriosis cases. Additionally, the isolates of food origin usually belong to serogroup IIa or IIb [27,42], thus the information about L. monocytogenes IVb may be important to understand the epidemiological chain of food-borne listeriosis. Among the 91 isolates tested in the present study, three cgMLST variants (CC1, CC2, and CC6) were identified, which were also previously found in strains of serogroup IVb [31,32,43]. Isolates of CC6 were described as the cause of meningitis, whereas L. monocytogenes classified to CC1 were also isolated from other clinical listeriosis cases [31]. Furthermore, it has been suggested that strains of the later clonal complex show an increased virulence potential as compared to other isolates [31]. However, in the present study, L. monocytogenes of CC2 was predominant, which was also found during a previous investigation of food [43]. It seems that this molecular variant may be less virulent than other CCs (e.g., CC4 and CC6) which are mainly responsible for human infections [24,31,43].
Further WGS analysis of the 91 L. monocytogenes sequences revealed that the isolates were classified into five sequence types; among them were ST1, ST2, ST6, and ST145, which were previously identified by us in food of animal origin or in food production environments [40,41]. However, the fifth sequence type detected in the current investigation (ST515; one isolate) has not been identified in Poland before. The strains of the four STs mentioned above were recovered from all currently tested sources, which supports the previous findings that most CCs and STs are not assigned to one origin but may be found among various sources [44]. It was described that L. monocytogenes isolates of the most common ST2 identified during the present analyses, were responsible for food-borne listeriosis outbreaks worldwide and were also commonly identified in food and food processing environments [32]. Furthermore, L. monocytogenes ST6, also commonly detected in the present study, was previously identified as the sequence type involved in food-borne sporadic infections or outbreaks [5,6,30,45].
It has been previously shown that the cgMLST analysis is a very useful molecular tool to assess the L. monocytogenes structure population [5,6,27,30,34,35,36]. In the present study, genetically closely related strains of the SL2-ST145-CT375 type were isolated in nine administrative provinces (voivodeships) of Poland during 2015-2018, mostly from raw meat (Table S1). This fact can be explained by, e.g., the ability of such isolates to persist in food production environments or introduction of the bacteria from outside sources, e.g., from the meat supplying slaughterhouses [35].
Based on the cgMLST results, the current L. monocytogenes isolates were compared with the publicly available sequences of Polish and other strains recovered from food, food production environments, and human listeriosis cases. The results showed that national isolates were more closely related to each other as compared to the strains from other countries. This finding supports the data of Lee et al. [43] who suggested that there is a regional molecular heterogeneity among L. monocytogenes of the same cgMLST types. On the other hand, some strains isolated previously in Poland from food and listeriosis cases were highly genetically related to the strains of the respective CCs identified in the current investigation [26,40].
The analysis of the WGS sequences of the current isolates towards virulence markers revealed that they were potentially pathogenic for humans since they possessed several genes responsible for, e.g., entering the bacteria into host cells, intracellular replication and escaping from phagocytic vacuoles. The LIPI-1 gene cluster and inlA and inlB internalin genes were detected in all strains tested, similarly to the results of Camargo et al. [46]. Additionally, within the inlA marker, the premature stop codons (PMSCs) responsible for reduced invasion of L. monocytogenes were not observed, making the isolate potentially more virulent. The presence of the full-length inlA gene among the genome of IVb serogroup isolates was also demonstrated by other authors [25,31,43]. Another internalin family gene member, inlG, was identified only in some of the isolates, similarly as in our previous study [40].
In the present investigation, the pathogenicity island LIPI-3 was found in several of the strains classified to clonal complexes CC1 and CC6 but not to CC2. This cluster contains genes involved in the production of listeriolysin S (LLS) and is associated with a higher virulence potential of L. monocytogenes due to bactericidal activity and modification of the host microbiota during infection [47]. It was previously shown that such LIPI-3-positive strains, classified to CC6 and ST6, were more often isolated from listeriosis outbreaks [6,30,32,47].
Other molecular markers involved in the pathogenicity of L. monocytogenes were identified in the sequences of the currently tested strains. Among them there were, e.g., the arsenic resistance gene cluster of genomic island 2 (LGI2) and the comK gene, involved in intracellular survival, biofilm formation and persistence of the bacteria [32,48]. Therefore, identification of such L. monocytogenes in food, including RTE food of animal origin, may suggest that they potentially pose a public health risk. It has also been previously shown that strains of IVb serogroup, due to their higher virulence potential, have a reduced ability to survive in food and food production environments compared to other L. monocytogenes serogroups [17,24,28]. Indeed, the genetic elements such as bcrABC and Tn6188 (ermC) markers associated with BAC tolerance were rare or not present at all among the currently tested strains. Similar results were also obtained by other authors [3,49]. However, the Tn6188 transposon is often identified in L. monocytogenes classified to sequence type ST121, which was not detected in the present study [46,50,51].
In the current investigation, the cadA gene, encoding cadmium-transporting ATPase responsible for cadmium resistance, was observed in only few strains classified to SL6-ST6-CT434; all these isolates also harbored the J1776 plasmid sequence. This finding indicates that the cadA gene was present on the above plasmid. Other genes encoding resistance to cadmium and arsenic, localized on the LGI2 Island, were identified among 74.7% isolates tested. According to Parsons et al. [52], heavy metals present in the environment can exert a long-term selective pressure on bacteria, including L. monocytogenes, and allow them to persist in food or food production environments. Other genetic elements responsible for resistance to various stress conditions were sporadically present in the tested strains. The stress survival islet 1, encoding resistance to a wide range of temperatures, pH or salinity, was represented only by one gene (lmo0447) identified in all L. monocytogenes, whereas SSI-2, possessing genes responsible for protecting the bacteria against alkaline pH conditions and oxidative stress, was not found in any of the strains tested [13,14,53].
Analysis of the WGS data toward antimicrobial resistance genes revealed that none of the 91 isolates possesses the penA (penicillin) and tetM and tetS (tetracycline) markers. However, the genes responsible for resistance to fosfomycin, quinolones, sulphonamides, and lincosamides were identified. These molecular markers were often detected also in L. monocytogenes by other authors [26,34,46,54]. It has been previously described that L. monocytogenes and other Listeria species are characterized by a low resistance to antimicrobials [36,41]. However, the presence of the antibiotic and sanitizer resistance traits among isolates originated from food and food production environments should be constantly monitored to assess the potential impact on public health.
Over 500 phage sequences present in the Listeria genome have been identified, including all L. monocytogenes serogroups [55]. In the present study, several intact prophages were detected. Similar results were previously obtained by Matle et al. [54], who found almost the same phages among L. monocytogenes isolated from food in South Africa. Such prophage sequences were also reported in strains associated with survival evolution and persistence of L. monocytogenes in food-processing facilities [55,56,57]. It has been suggested that prophage sequences present in the L. monocytogenes genome were probably one of the main causes of molecular diversity of the isolates classified to the same STs [9,13,58,59]. Furthermore, the presence of some prophages has been connected with the increased virulence and pathogenicity of L. monocytogenes [54]. Therefore, identification of prophage sequences in several currently tested strains may suggest the possibility of these isolates acquiring genetic material that would have an influence on a higher infection potential of such L. monocytogenes for humans.
4. Materials and Methods
4.1. L. monocytogenes Isolates
The strains were isolated between 2013 and 2019 during routine microbiological food and food production environment investigations by veterinary official laboratories located in 13 out of 16 voivodeships (administrative provinces) of Poland using the ISO-11290-1 standard method (ISO 11290-1:1996 and 11290-1:2017) and sent to the National Veterinary Research Institute in Pulawy (Table S1). Then, the isolates were streaked directly on TSYEA (Tryptone Soya Yeast Extract Agar; Bio-Rad, Hercules, CA, USA) and incubated at 37 °C for 24 ± 2 h. The bacteria were stored at −80 °C in a Viabank (BioMaxima, Lublin, Poland). All isolates were then tested toward L. monocytogenes molecular serogroups using PCR as described earlier [23,60]. Briefly, L. monocytogenes from the Viabank were cultured on TSYEA at 37 °C for 18–24 h and a loopful of bacteria was transferred into 100 µL of TRIS (Tris-(hydroxymethyl)-aminomethane) buffer (A&A Biotechnology, Gdynia, Poland). DNA was isolated using the Genomic Mini protocol (A&A Biotechnology) modified by adding 20 µL of lysozyme (10 mg/mL; Sigma-Aldrich, St. Louis, MO, USA) for 30 min at 37 °C. The amplification reactions were carried out in a thermal cycler (Biometra, Jena, Germany) under the following conditions: initial DNA denaturation at 95 °C for 5 min, followed by 30 cycles of 94 °C for 1 min, 55 °C for 1 min, and 72 °C for 2 min. The final cycle was carried out at 55 °C for 2 min and 72 °C for 5 min.
A total of 1439 L. monocytogenes isolates from various sources and voivodeships of Poland were collected. For the purpose of the present study, 91 isolates classified to serogroup IVb and recovered from raw meat (n = 21), ready-to-eat (RTE) food of animal origin (n = 62), and from food production environments (FPE), i.e., meat processing plants (n = 8) were selected and used for further analyzes.
4.2. Whole Genome Sequencing (WGS) Analysis
4.2.1. DNA Isolation, Library Preparation and Sequencing
DNA was extracted as described in point 4.1. DNA quality and concentration were measured by NanoDrop or Qubit 3 (Thermo Fisher Scientific, Waltham, MA, USA). Sequencing libraries were prepared with a Nextera XT DNA Sample Preparation Kit (Illumina, San Diego, CA, USA) and a KAPA HyperPlus Kit (Hoffman-La Roche, Basel, Switzerland) according to the producers’ instructions and sequenced in a MiSeq (Illumina) with a MiSeq Reagent Kit (Illumina) at approximately 50× average coverage. All sequences were trimmed and assembled with Trimmomatic v.0.36 and SPAdes v.3.9.0 [61]. The L. monocytogenes sequence parameters used in the present study are shown in Table S2.
4.2.2. WGS Characteristics of L. monocytogenes
MLST (7 loci) and cgMLST profiles (1,748 loci) were extracted from the assemblies using the tool available on the BIGSdb-Lm platform [32,33]. MLST profiles with the same alleles for seven loci were classified into sequence types (ST) and grouped into clonal complexes (CCs) if at least five out of seven loci were the same as previously described [33]. cgMLST profiles were grouped into cgMLST types (CTs) and sublineages (SLs), using the cut-offs of seven and 150 allelic mismatches, respectively, as previously described [32]. Allele numbers, CTs, and SLs were determined according to the Listeria sequence typing database (BIGSdb-Lm platform) [32]. Minimum spanning trees were constructed using BioNumerics software version 7.6 (Applied Maths, Sint-Martens-Latem, Belgium) based on the categorical differences in the allelic cgMLST profiles for each isolate. Loci with no allele calls were not considered in the pairwise comparison between two genomes. The number of allelic differences between isolates was read from genetic distance matrices computed from the absolute number of categorical differences between the genomes.
4.2.3. Identification of Virulence and Other Genetic Markers
Identification of virulence factor and resistance genes was performed in silico with the Listeria PasteurMLST sequence definition database [32] as described previously by Wieczorek et al. [62]. Detection of particular alleles was based on the virulence, antimicrobial resistance, metal and detergent resistance, stress islands, Listeria Stress Islands, and the sigB and rhamnose operon schemes [14,16,32,63,64,65].
4.2.4. Detection of Prophage and Plasmid Sequences
To identify the putative prophage determinants within the genomes of the L. monocytogenes tested, the WGS sequences were analysed with the PHASTER (PHAge Search Tool Enhanced Release) web server [66,67]. The presence of plasmid sequences was identified using the PlasmidFinder software 2.1 for the specified Gram-positive scheme [68].
4.2.5. Comparison of L. monocytogenes Isolated from Different Sources
A total of 186 L. monocytogenes isolates were used for comparison with the current strains. The isolates were selected based on the same CCs as the present strains, i.e., CC1, CC2, and CC6. These L. monocytogenes were recovered from humans, food, and food production environments in Poland and in other countries (Table S3). All sequences meeting the above criteria present in the BIGSdb-Lm database (n = 168 isolates) were chosen. Additionally, based on the literature [28,69], 18 strains not present in the above data base, but also classified to CC1, CC2, and CC6 and isolated from listeriosis outbreaks, mainly in the U.S. (a total of 10 strains) were selected for the comparison (Table S3). The L. monocytogenes sequences were extracted directly from the BIGSdb-Lm database or from GenBank when such L. monocytogenes strains were described in the literature [28,40,62,69,70]. Detailed information on these isolates, including the source and year of isolation, country of origin, and accession numbers, are shown in Table S3. The cgMLST profiles of all compared strains were created using sequences of the 1748 loci according to the scheme described before [32]. The phylogenetic trees, based on cgMLST profiles, were constructed using the BioNumerics 7.6 software as described in point 4.2.2. Altogether, the sequences of 186 L. monocytogenes strains were used for phylogenetic comparison, i.e., 79 strains classified to CC1, 46 to CC2, and 62 to CC6, respectively (Table S3).
4.2.6. Data Availability
All genome sequences of the L. monocytogenes isolates used in the present study were deposited in the BIGSdb-Lm database under the accession numbers 47065-47116 and 47118-47157.
5. Conclusions
WGS data analysis allows the molecular diversity, genetic relationships and identification of pathogenic, survival, and resistance markers of L. monocytogenes isolates from food and food production environments to be explored. The present investigation contributes towards a broader information on virulence traits and molecular diversity of L. monocytogenes of IVb serogroup isolated from food and food production environments in Poland and in other countries. The cgMLST molecular types with certain virulence gene profiles, especially of the intact inlA gene and LIPI-3 pathogenicity island suggest that at least some of the strains are capable of causing human illness. Identification of isolates harbouring the sequences related to stress associated factors and the presence of prophage and plasmid mobile genetic elements enhances the ability of the bacteria to adapt and survive in adverse environmental conditions as well as to increase their pathogenic potential. The presence of genetically identical L. monocytogenes recovered from different areas in different years may suggest the ability of such strains to persist outside a host for a long time and/or the cross-contamination of different food production plants. Thus, monitoring and molecular characteristics of L. monocytogenes are needed for further improvement of consumers’ safety.
Acknowledgments
The authors wish to thank Alexandra Moura (Pasteur Institute, Paris, France) for assistance with Listeria MLST system (http://bigsdb.pasteur.fr), including assignment of novel CTs with BIGSdb. We also thank the staff of Department of Omics Analyses at NVRI, Pulawy for expert technical support.
Abbreviations
BAC benzalkonium chloride, BIGSdb-Lm bacterial isolate genome sequence database L., monocytogenes, CC clonal complex, cgMLST core genome multi-locus sequence typing, FPE Food Production Environments, CT core genome MLST complex type, LIPIs Listeria pathogenicity islands, LGI Listeria Genomic Island, MLST multi-locus sequence typing, MST minimum spanning tree, PMSC premature stop codon, QAC quarternary ammonium, RTE ready to eat, SSI Survival Stress Islet, SL sublineage, ST sequence type, TRIS Tris-(hydroxymethyl)-Aminomethane, WGS whole-genome sequencing.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/article/10.3390/pathogens10040482/s1. Table S1: Characteristics of L. monocytogenes tested. Table S2. L. monocytogenes contig sequence parameters. Table S3: Characteristics of L. monocytogenes strains selected for comparison with the current isolates. Table S4: Predicted prophage regions and plasmids of Listeria monocytogenes strains.
Author Contributions
Conceptualization, B.L. and K.W.; methodology, B.L.; software, B.L.; formal analysis, B.L.; investigation, B.L.; resources, K.W.; data curation, B.L.; writing—original draft preparation, B.L.; writing—review and editing, J.O. and K.W.; visualization, B.L.; supervision, J.O. and K.W.; funding acquisition, J.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All genome sequences of the L. monocytogenes isolates used in the present study were deposited in the BIGSdb-Lm database under the accession numbers 47065-47116 and 47118-47157.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.EFSA. ECDC (European Food Safety Authority. European Centre for Disease Prevention and Control) The European Union One Health 2018 zoonoses report. EFSA J. 2019;17:5926. doi: 10.2903/j.efsa.2019.5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allerberger F., Wagner M. Listeriosis: A resurgent foodborne infection. Clin. Microbiol. Infect. 2010;16:16–23. doi: 10.1111/j.1469-0691.2009.03109.x. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan R.L., Gorris L.G.M., Hayman M.M., Jackson T.C., Whiting R.C. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control. 2017;75:1–13. doi: 10.1016/j.foodcont.2016.12.016. [DOI] [Google Scholar]
- 4.U.S. Food and Drug Administration Home Page. [(accessed on 15 January 2021)]; Available online: https://www.fda.gov.
- 5.Smith A.M., Tau N.P., Smouse S.L., Allam M., Ismail A., Ramalwa N.R., Disenyeng B., Ngomane M., Thomas J. Outbreak of Listeria monocytogenes in South Africa, 2017–2018: Laboratory activities and experiences associated with whole-genome sequencing analysis of isolates. Foodborne Pathog. Dis. 2019;16:524–530. doi: 10.1089/fpd.2018.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Centre for Disease Prevention and Control. European Food Safety Authority . Multi-Country Outbreak of Listeria monocytogenes Sequence Type 6 Infections Linked to Ready-to-Eat Meat Products—25 November 2019. EFSA; Parma, Italy: 2019. [DOI] [Google Scholar]
- 7.Holch A., Webb K., Lukjancenko O., Ussery D., Rosenthal B.M., Gram L. Genome sequencing identifies two nearly unchanged strains of persistent Listeria monocytogenes isolated at two different fish processing plants sampled 6 years apart. Appl. Environ. Microbiol. 2013;79:2944–2951. doi: 10.1128/AEM.03715-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira V., Wiedmann M., Teixeira P., Stasiewicz M.J. Listeria monocytogenes persistence in food-associated environments: Epidemiology, strain characteristics, and implications for public health. J. Food Prot. 2014;77:150–170. doi: 10.4315/0362-028X.JFP-13-150. [DOI] [PubMed] [Google Scholar]
- 9.Orsi R.H., Borowsky M.L., Lauer P., Young S.K., Nusbaum C., Galagan J.E., Birren B.W., Ivy R.A., Sun Q., Graves L.M., et al. Short-term genome evolution of Listeria monocytogenes in a non-controlled environment. BMC Genom. 2008;9:539. doi: 10.1186/1471-2164-9-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tompkin R.B. Control of Listeria monocytogenes in the food processing environment. J. Food Prot. 2002;65:709–725. doi: 10.4315/0362-028X-65.4.709. [DOI] [PubMed] [Google Scholar]
- 11.Roberts B.N., Chakravarty D., Gardner J.C., Ricke S.C., Donaldson J.R. Listeria monocytogenes response to anaerobic environments. Pathogens. 2020;9:210. doi: 10.3390/pathogens9030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colegiogri A., Bruini I., Di Ciccio P.A., Zanardi E., Ghidini S., Ianeri A. Listeria monocytogenes biofilms in the wonderland of food industry. Pathogens. 2017;6:41. doi: 10.3390/pathogens6030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fagerlund A., Langsrud S., Schirmer B.C., Møretrø T., Heir E. Genome analysis of Listeria monocytogenes sequence type 8 strains persisting in salmon and poultry processing environments and comparison with related strains. PLoS ONE. 2016;11:e0151117. doi: 10.1371/journal.pone.0151117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harter E., Wagner E.M., Zaiser A., Halecker S., Wagner M., Rychli K. Stress Survival Islet 2, predominantly present in Listeria monocytogenes strains of sequence type 121, is involved in the alkaline and oxidative stress responses. Appl. Environ. Microbiol. 2017;83:e00827-17. doi: 10.1128/AEM.00827-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hein I., Klinger S., Dooms M., Flekna G., Stess B., Leclercq A., Hill C., Allerberger F., Wagner M. Stress Survival Islet 1 (SSI-1) survey in Listeria monocytogenes reveals an insert common to Listeria innocua in sequence type 121 L. monocytogenes strains. Appl. Environ. Microbiol. 2011;77:2169–2173. doi: 10.1128/AEM.02159-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan S., Begley M., Hill C., Gahan C.G.M. A five-gene stress survival islet (SSI-1) that contributes to the growth of Listeria monocytogenes in suboptimal conditions. J. Appl. Microbiol. 2010;109:984–995. doi: 10.1111/j.1365-2672.2010.04726.x. [DOI] [PubMed] [Google Scholar]
- 17.Orsi R.H., den Bakker H.C., Wiedmann M. Listeria monocytogenes lineages: Genomics, evolution, ecology, and phenotypic characteristics. Int. J. Med. Microbiol. 2011;301:79–96. doi: 10.1016/j.ijmm.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Vines A., Swaminathan B. Identification and characterization of nucleotide sequence differences in three virulence-associated genes of Listeria monocytogenes strains representing clinically important serotypes. Curr. Microbiol. 1998;36:309–318. doi: 10.1007/s002849900315. [DOI] [PubMed] [Google Scholar]
- 19.Burall L.S., Grim C.J., Datta A.R. A clade of Listeria monocytogenes serotype 4b variant strains linked to recent listeriosis outbreaks associated with produce from a defined geographic region in the US. PLoS ONE. 2017;12:e0176912. doi: 10.1371/journal.pone.0176912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laksanalamai P., Steyert S.R., Burall L.S., Datta A.R. Genome sequences of Listeria monocytogenes serotype 4b variant strains isolated from clinical and environmental sources. Genome Announc. 2013;1:713–771. doi: 10.1128/genomeA.00771-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laksanalamai P., Huang B., Sabo J., Burall L.S., Zhao S., Bates J., Datta A.R. Genomic characterization of novel Listeria monocytogenes serotype 4b variant strains. PLoS ONE. 2014;9:e89024. doi: 10.1371/journal.pone.0089024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiedmann M., Bruce J.L., Keating C., Johnson A.E., McDonough P.L., Batt C.A. Ribotypes and virulence gene polymorphisms suggest three distinct Listeria monocytogenes lineages with differences in pathogenic potential. Infect. Immun. 1997;65:2707–2716. doi: 10.1128/IAI.65.7.2707-2716.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doumith M., Buchrieser C., Glaser P., Jacquet C., Martin P. Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. J. Clin. Microbiol. 2004;42:3819–3822. doi: 10.1128/JCM.42.8.3819-3822.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergholz T.M., Shah M.K., Burall L.S., Rakic-Martinez M., Datta A.R. Genomic and phenotypic diversity of Listeria monocytogenes clonal complexes associated with human listeriosis. Appl. Microbiol. Biotechnol. 2018;102:3475–3485. doi: 10.1007/s00253-018-8852-5. [DOI] [PubMed] [Google Scholar]
- 25.Jacquet C., Doumith M., Gordon J.I., Martin P.M., Cossart P., Lecuit M. A molecular marker for evaluating the pathogenic potential of foodborne Listeria monocytogenes. J. Infect. Dis. 2004;189:2094–2100. doi: 10.1086/420853. [DOI] [PubMed] [Google Scholar]
- 26.Kuch A., Goc A., Belkiewicz K., Filipello V., Ronkiewicz P., Gołębiewska A., Wróbel I., Kiedrowska M., Waśko I., Hryniewicz W., et al. Molecular diversity and antimicrobial susceptibility of Listeria monocytogenes isolates from invasive infections in Poland (1997–2013) Sci. Rep. 2018;8:14562. doi: 10.1038/s41598-018-32574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarez-Molina A., Cobo-Díaz J.F., López M., Prieto M., de Toro M., Alvarez-Ordóñez A. Unraveling the emergence and population diversity of Listeria monocytogenes in a newly built meat facility through whole genome sequencing. Int. J. Food Microbiol. 2021;340:109043. doi: 10.1016/j.ijfoodmicro.2021.109043. [DOI] [PubMed] [Google Scholar]
- 28.Burall L.S., Grim C.J., Mammel M.K., Datta A.R. Whole genome sequence analysis using JSpecies tool establishes clonal relationships between Listeria monocytogenes strains from epidemiologically unrelated listeriosis outbreaks. PLoS ONE. 2016;11:e0150797. doi: 10.1371/journal.pone.0150797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cabal A., Pietzka A., Huhulescu A., Allerberger F., Ruppitsch W., Schmid D. Isolate-based surveillance of Listeria monocytogenes by whole genome sequencing in Austria. Front. Microbiol. 2019;10:2282. doi: 10.3389/fmicb.2019.02282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.European Centre for Disease Prevention and Control. European Food Safety Authority . EFSA; Parma, Italy: 2018. Multicountry Outbreak of Listeria monocytogenes Serogroup Ivb, Multi-Locus Sequence Type 6, Infections Linked to Frozen Corn and Possibly to Other Frozen Vegetables—First Update. [DOI] [Google Scholar]
- 31.Maury M.M., Tsai Y.H., Charlier C., Touchon M., Chenal-Francisque V., Leclercq A., Criscuolo A., Gaultier C., Roussel S., Brisabois A., et al. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat. Genet. 2016;48:308–313. doi: 10.1038/ng.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moura A., Criscuolo A., Pouseele H., Maury M.M., Leclercq A., Tar C., Björkman J.T., Dallman T., Reimer A., Enouf V., et al. Whole genome-based population biology and epidemiological surveillance of Listeria monocytogenes. Nat. Microbiol. 2016;2:16185. doi: 10.1038/nmicrobiol.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ragon M., Wirth T., Hollandt F., Lavenir R., Lecuit M., Le Monnier A., Brisse S. A new perspective on Listeria monocytogenes evolution. PLoS Pathog. 2008;4:e1000146. doi: 10.1371/journal.ppat.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurley D., Luque-Sastre L., Parker C.T., Huynh S., Eshwar A.K., Nguyen S.V., Andrews N., Moura A., Fox E.M., Jordan K., et al. Whole-genome sequencing-based characterization of 100 Listeria monocytogenes isolates collected from food processing environments over a four-year period. mSphere. 2019;4:e00252-19. doi: 10.1128/mSphere.00252-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fagerlund A., Langsrud S., Møretrø T. In-depth longitudinal study of Listeria monocytogenes ST9 isolates from the meat processing industry: Resolving diversity and transmission patterns using whole-genome sequencing. Appl. Environ. Microbiol. 2020;86:e00579-20. doi: 10.1128/AEM.00579-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Møller Nielsen E., Björkman J.T., Kiil K., Grant K., Dallman T., Painset A., Amar C., Roussel S., Guillier L., Félix B., et al. Closing gaps for performing a risk assessment on Listeria monocytogenes in ready-to-eat (RTE) foods: Activity 3, the comparison of isolates from different compartments along the food chain, and from humans using whole genome sequencing (WGS) analysis. EFSA Support. Publ. 2017;14:1151E. doi: 10.2903/sp.efsa.2017.EN-1151. [DOI] [Google Scholar]
- 37.Ruppitsch W., Pietzka A., Prior K., Bletz S., Fernandez H.L., Allerberger F., Harmsen D., Mellmann A. Defining and evaluating a core genome multilocus sequence typing scheme for whole-genome sequence-based typing of Listeria monocytogenes. J. Clin. Microbiol. 2015;53:2869–2876. doi: 10.1128/JCM.01193-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lingnau A., Domann E., Hudel M., Bock M., Nichterlein T., Wehland J., Chakraborty T. Expression of the Listeria monocytogenes EGD inlA and inlB genes, whose products mediate bacterial entry into tissue culture cell lines, by PrfA-dependent and -independent mechanisms. Infect. Immun. 1995;63:3896–3903. doi: 10.1128/IAI.63.10.3896-3903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaplan Zeevi M., Shafir N.S., Shaham S., Friedman S., Sigal N., Nir Paz R., Boneca I.G., Herskovits A.A. Listeria monocytogenes multidrug resistance transporters and cyclic di-AMP, which contribute to type I interferon induction, play a role in cell wall stress. J. Bacteriol. 2013;195:5250–5261. doi: 10.1128/JB.00794-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurpas M., Osek J., Moura A., Leclercq A., Lecuit M., Wieczorek K. Genomic characterization of Listeria monocytogenes isolated from ready-to-eat meat and meat processing environments in Poland. Front. Microbiol. 2020;11:1412. doi: 10.3389/fmicb.2020.01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sosnowski M., Lachtara B., Wieczorek K., Osek J. Antimicrobial resistance and genotypic characteristics of Listeria monocytogenes isolated from food in Poland. Int. J. Food Microbiol. 2019;289:1–6. doi: 10.1016/j.ijfoodmicro.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 42.Henriques A.R., Cristino J.M., Fraqueza M.J. Genetic characterization of Listeria monocytogenes isolates from industrial and retail ready-to-eat meat-based foods and their relationship with clinical strains from human listeriosis in Portugal. J. Food Prot. 2017;80:551–560. doi: 10.4315/0362-028X.JFP-16-310. [DOI] [PubMed] [Google Scholar]
- 43.Lee S., Chen Y., Gorski L., Ward T.J., Osborne J., Kathariou S. Listeria monocytogenes source distribution analysis indicates regional heterogeneity and ecological niche preference among serotype 4b clones. mBio. 2018;9:e00396-18. doi: 10.1128/mBio.00396-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haase J.K., Didelot X., Lecuit M., Korkeala H. L. monocytogenes MLST Study Group; Achtman, M. The ubiquitous nature of Listeria monocytogenes clones: A large-scale Multilocus Sequence Typing study. Environ. Microbiol. 2014;16:405–416. doi: 10.1111/1462-2920.12342. [DOI] [PubMed] [Google Scholar]
- 45.Tomáštíková Z., Gelbíčová T., Karpíšková R. Population structure of Listeria monocytogenes isolated from human listeriosis cases and from ready-to-eat foods in the Czech Republic. J. Food Nutr. Res. 2019;58:99–106. [Google Scholar]
- 46.Camargo A.C., Moura A., Avillan J., Herman N., McFarland A.P., Sreevatsan S., Call D.R., Woodward J.J., Lecuit M., Nero L.A. Whole-genome sequencing reveals Listeria monocytogenes diversity and allows identification of long-term persistent strains in Brazil. Environ. Microbiol. 2019;21:4478–4487. doi: 10.1111/1462-2920.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quereda J.J., Meza-Torres J., Cossart P., Pizarro-Cerda J. Listeriolysin S: A bacteriocin from epidemic Listeria monocytogenes strains that targets the gut microbiota. Gut Microbes. 2017;8:384–391. doi: 10.1080/19490976.2017.1290759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee S., Ward T.J., Jima D.D., Parsons C., Kathariou S. The arsenic resistance-associated Listeria genomic island LGI2 exhibits sequence and integration site diversity and a propensity for three Listeria monocytogenes clones with enhanced virulence. Appl. Environ. Microbiol. 2017;83:e01189-17. doi: 10.1128/AEM.01189-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ortiz S., López-Alonso V., Rodríguez P., Martínez-Suárez J.V. The connection between persistent, disinfectant-resistant Listeria monocytogenes strains from two geographically separate Iberian pork processing plants: Evidence from comparative genome analysis. Appl. Environ. Microbiol. 2016;82:308–317. doi: 10.1128/AEM.02824-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Müller A., Rychli K., Muhterem-Uyar M., Zaiser A., Stessl B., Guinane C.M., Cotter P.D., Wagner M., Schmitz-Esser S. Tn6188—A novel transposon in Listeria monocytogenes responsible for tolerance to benzalkonium chloride. PLoS ONE. 2013;8:e76835. doi: 10.1371/journal.pone.0076835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuber I., Lakicevic B., Pietzka A., Milanov D., Djordjevic V., Karabasil N., Teodorovic V., Ruppitsch W., Dimitrijevic M. Molecular characterization of Listeria monocytogenes isolates from a small-scale meat processor in Montenegro, 2011–2014. Food Microbiol. 2019;79:116–122. doi: 10.1016/j.fm.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Parsons C., Lee S., Jayeola V., Kathariou S. Novel cadmium resistance determinant in Listeria monocytogenes. Appl. Environ. Microbiol. 2017;83:e02580-16. doi: 10.1128/AEM.02580-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hilliard A., Leong D., O’Callaghan A., Culligan E.P., Morgan C.A., DeLappe N., Hill C., Jordan K., Cormican M., Gahan C. Genomic characterization of Listeria monocytogenes isolates associated with clinical listeriosis and the food production environment in Ireland. Genes. 2018;9:171. doi: 10.3390/genes9030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matle I., Pierneef R., Mbatha K.R., Magwedere K., Madoroba E. Genomic diversity of common sequence types of Listeria monocytogenes isolated from ready-to-eat products of animal origin in South Africa. Genes. 2019;10:1007. doi: 10.3390/genes10121007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klumpp J., Loessner M.J. Listeria phages: Genomes, evolution, and application. Bacteriophage. 2013;3:e26861. doi: 10.4161/bact.26861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Casey A., Jordan K., Neve H., Coffey A., McAuliffe O. A tail of two phages: Genomic and functional analysis of Listeria monocytogenes phages vB_LmoS_188 and vB_LmoS_293 reveal the receptor-binding proteins involved in host specificity. Front. Microbiol. 2015;6:1107. doi: 10.3389/fmicb.2015.01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Denes T., Vongkamjan K., Ackermann H.W., Moreno Switt A.I., Wiedmann M., den Bakker H.C. Comparative genomic and morphological analyses of Listeria phages isolated from farm environments. Appl. Environ. Microbiol. 2014;80:4616–4625. doi: 10.1128/AEM.00720-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knudsen G.M., Nielsen J.B., Marvig R.L., Ng Y., Worning P., Westh H., Gram L. Genome-wide-analyses of Listeria monocytogenes from food-processing plants reveal clonal diversity and date the emergence of persisting sequence types. Environ. Microbiol. Rep. 2017;9:428–440. doi: 10.1111/1758-2229.12552. [DOI] [PubMed] [Google Scholar]
- 59.Stasiewicz M.J., Oliver H.F., Wiedmann M., den Bakker H.C. Whole-genome sequencing allows for improved identification of persistent Listeria monocytogenes in food-associated environments. Appl. Environ. Microbiol. 2015;81:6024–6037. doi: 10.1128/AEM.01049-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wieczorek K., Dmowska K., Osek J. Characterization and antimicrobial resistance of Listeria monocytogenes isolated from retail beef meat in Poland. Foodborne Pathog. Dis. 2012;9:681–685. doi: 10.1089/fpd.2012.1137. [DOI] [PubMed] [Google Scholar]
- 61.Bankevich A., Nurk S., Antipov D., Gurevich A., Dvorkin M., Kulikov A.S., Lesin V., Nikolenko S., Pham S., Prjibelski A., et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wieczorek K., Bomba A., Osek J. Whole-genome sequencing-based characterization of Listeria monocytogenes from fish and fish production environments in Poland. Int. J. Mol. Sci. 2020;21:9419. doi: 10.3390/ijms21249419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gilmour M.W., Graham M., Van Domselaar G., Tyler S., Kent H., Trout-Yakel K.M., Larios O., Allen V., Lee B., Nadon C. High-throughput genome sequencing of two Listeria monocytogenes clinical isolates during a large foodborne outbreak. BMC Genom. 2010;11:120. doi: 10.1186/1471-2164-11-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palma F., Brauge T., Radomski N., Mallet L., Felten A., Mistou M.Y., Brisabois A., Guillier L., Midelet-Bourdin G. Dynamics of mobile genetic elements of Listeria monocytogenes persisting in ready-to-eat seafood processing plants in France. BMC Genom. 2020;21:130. doi: 10.1186/s12864-020-6544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J., Cao G., Xu X., Allard M., Li P., Brown E., Yang X., Pan H., Meng J. Evolution and diversity of Listeria monocytogenes from clinical and food samples in Shanghai, China. Front. Microbiol. 2016;7:1138. doi: 10.3389/fmicb.2016.01138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arndt D., Grant J., Marcu A., Sajed T., Pon A., Liang Y., Wishart D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016;44:16–21. doi: 10.1093/nar/gkw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou Y., Liang Y., Lynch K.H., Dennis J.J., Wishart D.S. PHAST: A fast phage search tool. Nucleic Acids Res. 2011;39:W347–W352. doi: 10.1093/nar/gkr485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carattoli A., Zankari E., García-Fernández A., Voldby Larsen M., Lund O., Villa L., Møller Aarestrup F., Hasman H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014;58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Y., Gonzalez-Escalona N., Hammack T.S., Allard M.W., Strain E.A., Brown E.W. Core genome multilocus sequence typing for identification of globally distributed clonal groups and differentiation of outbreak strains of Listeria monocytogenes. Appl. Environ. Microbiol. 2016;82:6258–6272. doi: 10.1128/AEM.01532-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Benson D.A., Cavanaugh M., Clark K., Karsch-Mizrachi I., Lipman D.J., Ostell J., Sayers E.W. GenBank. Nucleic Acids Res. 2013;41:D36–D42. doi: 10.1093/nar/gks1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All genome sequences of the L. monocytogenes isolates used in the present study were deposited in the BIGSdb-Lm database under the accession numbers 47065-47116 and 47118-47157.