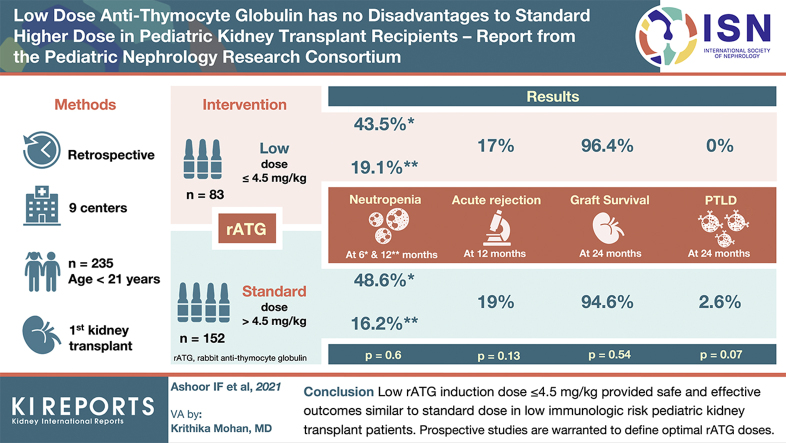
Abstract
Introduction
Rabbit antithymocyte globulin (rATG) dosing strategies for induction in pediatric kidney transplantation vary between centers. It is not known whether a lower rATG induction dose provides safe and effective immunosuppression compared with a “standard” higher dose.
Methods
We performed a retrospective multicenter study of all isolated first-time kidney transplant recipients <21 years old who received rATG induction between 1 January 2010 and 31 December 2014 at 9 pediatric centers. An a priori cutoff of a 4.5-mg/kg cumulative rATG dose was used to identify low (≤ 4.5 mg/kg) and standard (> 4.5 mg/kg) exposure groups. Outcomes examined included 12 months posttransplant graft function (estimated glomerular filtration rate [eGFR]); the occurrence of acute rejection, donor-specific antibody (DSA), neutropenia, and viral infection (cytomegalovirus [CMV], Epstein-Barr virus [EBV], and BK virus); and 24-month outcomes of posttransplant lymphoproliferative disorder (PTLD) occurrence and patient and graft survival.
Results
Two hundred thirty-five patients were included. Baseline features of the low and standard rATG dose groups were similar. By 12 months, the rATG dose group had no significant impact on the occurrence of neutropenia, positive DSA, or viral polymerase chain reaction (PCR). Graft function was similar. Acute rejection rates were similar at 17% (low dose) versus 19% (standard dose) (P = 0.13). By 24 months, graft survival (96.4% vs. 94.6%) and patient survival (100% vs. 99.3%) were similar between the low- and standard-dose groups (P = 0.54 and 0.46), whereas the occurrence of PTLD trended higher in the standard-dose group (0% vs. 2.6%, P = 0.07).
Conclusion
A low rATG induction dose ≤ 4.5 mg/kg provided safe and effective outcomes in this multicenter low immunologic risk pediatric cohort. Prospective studies are warranted to define the optimal rATG induction dose in pediatric kidney transplantation.
Keywords: induction immunosuppression, kidney transplantation, pediatric, rabbit antithymocyte globulin
Graphical abstract
Potent immunosuppression in the form of depleting antibody induction therapy has been credited with a significant reduction in first-year acute kidney transplant rejection rates compared with nondepleting or no induction therapy.1,2 The majority of US pediatric kidney transplant programs use induction therapy at the time of kidney transplantation,3 with rATG being the most commonly used lymphocyte-depleting induction agent in the United States.4 Up until 2017, the use of rATG for that indication was considered off-label because its original US Food and Drug Administration approval was limited to the treatment of established acute kidney transplant rejection in a wide range of 7 to 14 days at a 1.5-mg/kg/dose.5 As such, a variety of dosing protocols existed for induction, leading to a wide range of center-specific cumulative dosing targets in both pediatric and adult kidney transplant recipients.4 Given the expense associated with rATG use and the well-documented enhanced risk for infections,6 there has been a movement toward a limited exposure approach in dosing and administration.7,8 The current US Food and Drug Administration dosing guidance recommends a minimum of 4 doses of rATG at 1.5 mg/kg for a cumulative dose exposure minimum of 6 mg/kg for induction purposes,5 whereas prior studies that established the efficacy of rATG as an induction agent to prevent acute rejection have used a minimum of 5 doses for a cumulative exposure of 7.5 mg/kg of body weight.1 In this study, we sought to determine whether a lower rATG induction dosing regimen is effective and safe in a multicenter US cohort of pediatric kidney transplant recipients.
Methods
Study Design
This is a retrospective multicenter study that collected data from 9 member institutions within the Pediatric Nephrology Research Consortium (PNRC). The PNRC is a collaborative group of North American pediatric nephrology centers that aims to facilitate collaborative clinical and translational research, define best practices, and promote career development of their members. The study was approved by the local institutional review board of each participating institution with a waiver of informed consent. All clinical research described in this article adheres to the Declaration of Helsinki. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.9
The inclusion criteria were as follows: all isolated first-time pediatric recipients <21 years old at the time of kidney transplant who received rATG induction between 1 January 2010, and 31 December 2014. Recipients of repeat kidney transplants or multiorgan transplants and recipients who received induction other than rATG or no induction therapy were excluded from the study.
Exposure Variable
Based on a single rATG dose of 1.5 mg/kg of body weight, the rATG cumulative exposure threshold was set a priori at 3 doses or less (≤ 4.5 mg/kg) for the low-dose exposure group and at greater than 3 doses (> 4.5 mg/kg) for the standard-dose exposure group. Each center followed its individual center-specific immunosuppression protocol and rATG induction dosing strategy at the discretion of the treating physician. The assignment of subjects to the low-dose and standard-dose exposure groups was based on the actual delivered rATG dose at the time of induction.
Outcomes
We compared 12-month outcome measures of graft function (eGFR), acute rejection, DSA development, neutropenia, and the occurrence of viral infection (CMV, EBV, and BK virus), as well as 24-month outcome measures of PTLD occurrence and patient and graft survival.
Data Collection
Baseline demographic and clinical data were collected at the time of admission and discharge from index kidney transplant hospitalization and subsequently at 6, 12, and 24 months after kidney transplantation. eGFR was calculated using the modified Schwartz formula.10 Acute rejection episodes captured all biopsy-proven acute rejection events, including borderline cellular rejection, acute cellular rejection, and antibody-mediated rejection. Neutropenia was defined as an absolute neutrophil count < 1500/mm3. Viral infections included both symptomatic infections and asymptomatic viremia on surveillance monitoring as measured by PCR testing at each individual center. Additional information regarding participating centers’ practice patterns with regard to surveillance biopsy, DSA, and viral testing as well as immunosuppression drug level targets is summarized in Supplementary Table S1.
Statistical Analysis
Continuous variables were summarized as means with SD and medians with interquartile ranges. The cumulative rATG induction dose was summarized numerically by exposure group; t-tests based on linear models were used to test for group differences for continuous outcomes. Categoric variables were summarized as frequencies, and tests of association between them were conducted using chi-square tests. Graft survival was calculated using Kaplan-Meier estimates. A generalized logistic regression model was used to test the odds of an event occurring over time including patient survival, acute rejection, occurrence of DSA, neutropenia, or positive viral PCR testing. Potential covariates considered for the model included baseline characteristics (age, sex, race, end-stage kidney disease etiology, transplant type, panel-reactive antibody, and CMV and EBV risk category), center effect, and immunosuppression at discharge. Sensitivity analysis at the 5.0-mg/kg, 5.5-mg/kg, and 6.0-mg/kg rATG cumulative dose thresholds was completed for the outcomes of acute rejection, neutropenia occurrence, and graft survival.
Results
rATG Dosing Trends and Baseline Characteristics of Low-dose and Standard-dose rATG Exposure Groups
Two-hundred eighty-two kidney transplant recipients were included from 9 member centers of the PNRC. Complete data on rATG dosing were available for 235 recipients who were included in the final analysis (Supplementary Table S2). Using an a priori cutoff of 3 doses of rATG induction at 1.5 mg/kg per dose, we defined the low-dose exposure group (≤ 4.5-mg/kg cumulative rATG induction dose), which included 83 recipients with a median cumulative exposure dose of 4.11 mg/kg, and the standard dose exposure group (> 4.5-mg/kg cumulative rATG induction dose), which included 152 recipients with a median cumulative exposure dose of 5.96 mg/kg. Baseline characteristics including age, sex, race, etiology of end-stage kidney disease, transplant type, panel-reactive antibody, and CMV/EBV risk status category at the time of transplant admission were similar between both groups (Table 1). Overall, recipients were predominantly white, male, nonsensitized deceased donor recipients with intermediate risk for both CMV and EBV reactivation. At the time of hospital discharge from index kidney transplant admission (Table 2), recipients in both rATG dose exposure groups had similar graft function (creatinine-based modified Schwartz mean eGFR of 79 ml/min/1.73 m2 in the low-dose exposure group vs. 75 ml/min/1.73 m2 in the standard-dose exposure group) and similar rates of antiviral prophylaxis (98.8% in the low-dose group vs. 98% in the standard-dose group). Approximately 64% of the study cohort received a steroid avoidance or wean protocol in which steroids were discontinued within 3 to 14 days after kidney transplant. Both groups were similar in terms of tacrolimus adoption as a long-term immunosuppressant agent at the time of discharge (98.8% vs. 96.7%); however, the low-dose group recipients were less likely to be on mycophenolate (92.8% vs. 98%, P = 0.05) and prednisone (25.3% vs. 41.4%, P = 0.01). Because no significant differences were noted in baseline characteristics between rATG dose groups, the final logistic regression model used for the following analyses only included rATG dose, center effects, and whether the subject received prednisone therapy at discharge from kidney transplant admission as covariates. The limited availability of subjects not receiving mycophenolate mofetil at the time of discharge from kidney transplant admission precluded this variable from being used in the final model.
Table 1.
Baseline characteristics at the time of transplant admission
| Low-dose rATG induction ≤ 4.5 mg/kg (n = 83) | Standard-dose rATG induction > 4.5 mg/kg (n = 152) | P value | |
|---|---|---|---|
| Age (mean), yr | 13 | 12.1 | 0.2 |
| Sex (male), % | 62.7 | 61.2 | 0.83 |
| Race, % | 0.18 | ||
| White | 57 | 47 | |
| Black | 26 | 38 | |
| Other | 17 | 15 | |
| Etiology of ESKD, % | 0.65 | ||
| Obstructive uropathy/dysplasia | 38 | 39.5 | |
| Focal segmental glomerulosclerosis | 16 | 14.5 | |
| Other glomerular diseases | 7 | 13 | |
| Unknown | 4 | 4 | |
| Other | 35 | 29 | |
| Transplant type (deceased), % | 56.8 | 66.2 | 0.36 |
| PRA (mean ± standard error), % | |||
| Class I | 4.5 ± 1.6 | 2.3 ± 1 | 0.26 |
| % Subjects with class I PRA > 80% | 0 | 0 | |
| Class II | 5.9 ± 1.7 | 2.1 ± 1.1 | 0.07 |
| % Subjects with class II PRA > 80% | 0 | 0 | |
| CMV risk, % | 0.52 | ||
| High | 33 | 35 | |
| Intermediate | 39 | 45 | |
| Low | 28 | 20 | |
| EBV risk, % | 0.29 | ||
| High | 19 | 31 | |
| Intermediate | 78 | 65 | |
| Low | 3 | 4 |
CMV, cytomegalovirus; EBV, Epstein-Barr virus; ESKD, end-stage kidney disease; PRA, panel reactive antibody; rATG, rabbit antithymocyte globulin.
Table 2.
Baseline characteristics at the time of discharge from index kidney transplant admission
| Low-dose rATG induction ≤ 4.5 mg/kg (n = 83) | Standard-dose rATG induction > 4.5 mg/kg (n = 152) | P value | |
|---|---|---|---|
| Graft function, mean eGFR (ml/min/1.73 m2) | 79 | 75 | 0.49 |
| Immunosuppression at discharge, % | |||
| Tacrolimus | 98.8 | 96.7 | 0.33 |
| Mycophenolate | 92.8 | 98 | 0.05 |
| Prednisone | 25.3 | 41.4 | 0.01 |
| Other | 4 | 3 | 0.9 |
| Antiviral prophylaxis (yes), % | 98.8 | 98 | 0.66 |
eGFR, estimate glomerular filtration rate; rAGT, rabbit antithymocyte globulin.
The Effect of rATG Exposure Group on Patient and Graft Outcomes Through 12 Months of Follow-up
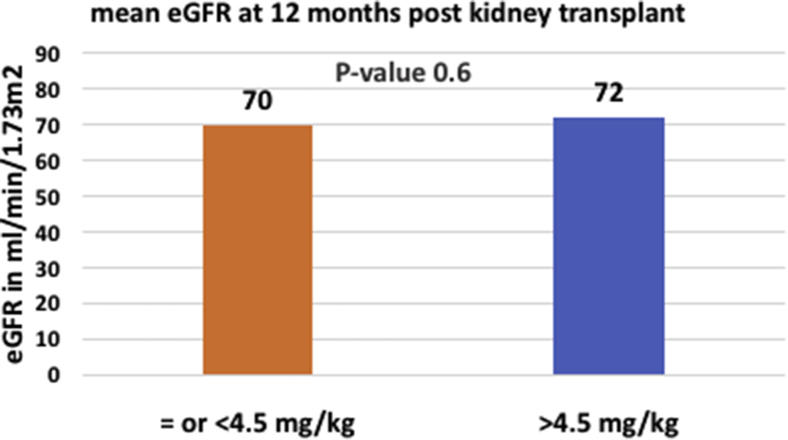
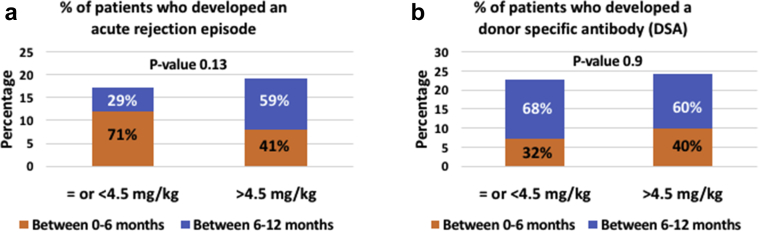
Graft function at 12 months posttransplant as measured by eGFR was similar in both groups at a mean eGFR of 70 ml/min/1.73 m2 in the low-dose exposure group versus 72 ml/min/1.73 m2 in the standard-dose exposure group (P = 0.6, Figure 1). Acute rejection rates and DSA development rates were similar in both groups (Figure 2a and b). Seventeen percent of recipients in the low-dose exposure group experienced an acute rejection episode in the first year posttransplantation with 71% of these episodes occurring in the first 6 months posttransplant, whereas 19% experienced an acute rejection episode in the first year posttransplantation in the standard-dose group with 41% occurring in the first 6 months posttransplantation. In the low rATG dose exposure group, data on rejection histology were available for 93% of all cases. Of those, 21% had borderline cell-mediated rejection, 43% had acute cell-mediated rejection (Banff IA or greater severity), 14% had antibody-mediated rejection, and 22% had mixed acute cellular and antibody-mediated rejection. In the standard rATG dose exposure group, data on rejection histology were available for 97% of all cases. Of those, 16% had borderline cell-mediated rejection, 55% had acute cell-mediated rejection (Banff IA or greater severity), 6% had antibody-mediated rejection, and 23% had mixed acute cellular and antibody-mediated rejection. Ninety percent of low-dose rATG induction subjects with early rejection in the first 6 months after kidney transplant were maintained on steroid-free regimens as opposed to 47% of the standard-dose rATG induction subjects experiencing an acute rejection in the same time frame.
Figure 1.
Graft function comparison between low-dose rabbit antithymocyte globulin and standard-dose rabbit antithymocyte globulin exposure groups at 12 months after kidney transplant. eGFR, estimated glomerular filtration rate.
Figure 2.
(a) Acute rejection comparison between low-dose rabbit antithymocyte globulin (rATG) and standard-dose rATG exposure groups at 12 months after kidney transplant. (b) The development of de novo donor-specific antibody comparison between low-dose rATG and standard-dose rATG exposure groups at 12 months after kidney transplant.
With regard to DSA development, 22.8% and 24.4% developed a de novo DSA over the first year in the low-dose and standard-dose groups, respectively, with most DSAs developing between 6 to 12 months posttransplantation at 68% and 60% for the low-dose and standard-dose groups. DSA class was not specified in 33% of the low rATG dose group and 13% of the standard rATG dose group. In subjects with complete data on DSA specificity, class I DSAs accounted for only 5% of all positive DSAs in the standard rATG dose group with the remainder having either class II (60%) or combined class I and II DSAs (35%). Subjects in the low rATG dose group had either class II (67%) or combined class I and II DSAs (33%).
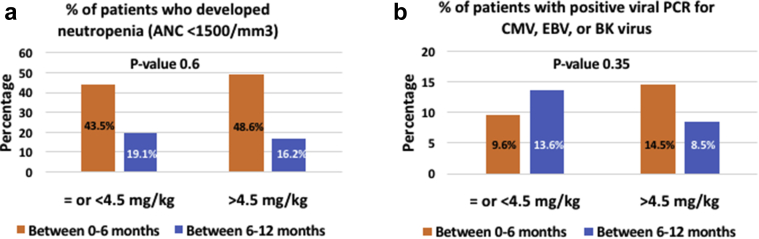
The rates of neutropenia were similar between both groups as well, with the majority of patients developing neutropenia in the first 6 months after kidney transplantation (Figure 3a). Approximately 43.5% of recipients in the low-dose group developed neutropenia in the first 6 months compared with 48.6% in the standard-dose group. The percentage of recipients with neutropenia dropped to 19% between 6 and 12 months posttransplant in the low-dose group and to 16% in the standard-dose group. In the low-dose exposure group, 24% of neutropenic subjects experienced severe neutropenia with an absolute neutrophil count < 500/mm3 as opposed to 27% of neutropenic subjects in the standard-dose exposure group. Twenty-four percent of neutropenia episodes in the low-dose group were associated with a serious infection requiring hospitalization, and 15% received treatment with granulocyte colony-stimulating factor. This compares to 17% with serious infections and 5% who received treatment with granulocyte colony-stimulating factor in the standard-dose group. Serious infections were similar in both groups and included viral infections (CMV disease, adenovirus infection, and EBV disease), bacterial pneumonia, and urinary tract infections with and without sepsis. The rate of positive viral PCRs for CMV, EBV, or BK virus infections was similar in both groups (Figure 3b).
Figure 3.
(a) Neutropenia occurrence comparison between low-dose rabbit antithymocyte globulin (rATG) and standard-dose rATG exposure groups at 12 months after kidney transplant. (b) The occurrence of positive viral polymerase chain reaction testing for either cytomegalovirus (CMV), Epstein-Barr virus (EBV), or BK virus (BKV) infection comparison between low-dose rATG and standard-dose rATG exposure groups at 12 months after kidney transplant. ANC, absolute neutrophil count.
The Effect of rATG Exposure Group on Patient and Graft Outcomes Through 24 Months of Follow-up
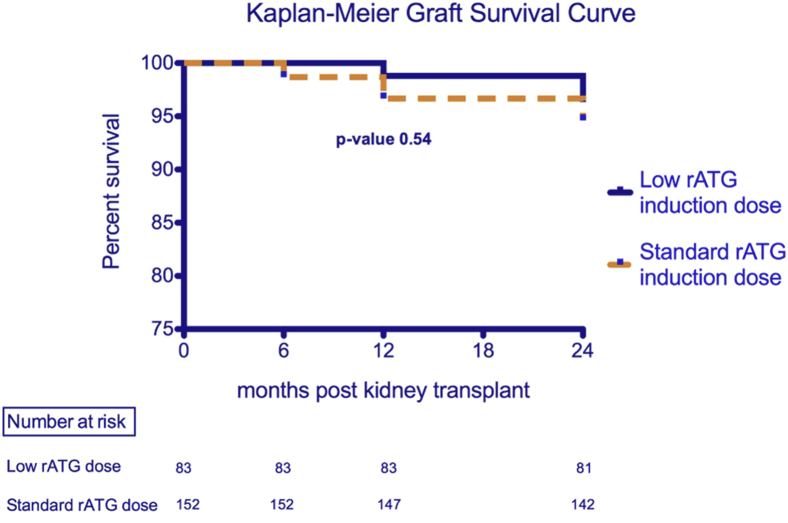
Graft survival was similar between both groups through 24 months of follow-up after kidney transplantation (Figure 4). In the low-dose rATG exposure group, graft survival measured at 100%, 98.8%, and 96.4% through 6, 12, and 24 months of follow-up, respectively, whereas graft survival in the standard-dose rATG exposure group measured at 98.7%, 96.7%, and 94.6% at the same time points (P = 0.54). There were no deaths or PTLD cases reported in the low-dose group, whereas 1 patient died secondary to urosepsis (P = 0.46) and 4 patients developed PTLD (P = 0.07) in the standard-dose group. Three patients who developed PTLD were diagnosed between 6 and 12 months posttransplantation, whereas the fourth patient was diagnosed between 12 and 24 months. Three of these PTLD patients were in the high-risk EBV mismatch category (EBV-negative recipients with an EBV-positive donor), and 1 was of intermediate risk (both recipient and donor were EBV positive). All patients were alive and in remission at 24 months posttransplantation with functioning grafts. Treatment involved immunosuppression reduction in all patients, rituximab in 2 patients, and chemotherapy in 1 patient.
Figure 4.
Graft survival comparison between low-dose rabbit antithymocyte globulin (rATG) and standard-dose rATG exposure groups through 24 months after kidney transplant.
Finally, to address the issue of unequal group sizes and improve the balance of participants within each group, a sensitivity analysis for the outcomes of acute rejection, neutropenia occurrence, and graft survival was performed. Using the rATG cumulative dose cutoffs of 5.0 mg/kg (n = 118 vs. 117), 5.5 mg/kg (n = 137 vs. 98), and 6.0 mg/kg (n = 162 vs. 73) produced similar results as 4.5 mg/kg (n = 83 vs. 152), all showing no significant differences between dosage and outcome.
Discussion
This is the largest and first multicenter cohort study to examine the relationship between rATG induction dose exposure and a wide variety of safety and efficacy outcomes in first-time pediatric kidney transplant recipients. rATG use has increased over time, and it is currently the most commonly used induction therapy for kidney transplantation.3,4 However, the lack of specific dosing recommendations until very recently has led to many immunosuppressive protocols in the adult transplant literature, with an overall trend toward a lower cumulative exposure in the most recent era.7,11, 12, 13 We have previously demonstrated a down trend in median cumulative rATG induction dose from 7.9 mg/kg (1998–2008) to 6.3 mg/kg (2009–2016) in the North American Pediatric Renal Trials and Collaborative Studies registry.14 In that analysis, we did not identify a difference in patient and graft outcomes using a threshold of 5 rATG induction doses (7.5 mg/kg). Although data from Tsapepas et al.15 have demonstrated a significantly higher incidence of acute rejection in adults receiving a 5- to 6-mg/kg cumulative rATG induction dose compared with those receiving 6 mg/kg or more (essentially a 4-dose threshold), several other studies demonstrated satisfactory outcomes with even smaller cumulative rATG induction doses.7,12,13 Because the majority of pediatric recipients are low immunologic risk, first-time kidney transplant recipients in whom rATG induction is primarily used to allow the adoption of a steroid avoidance immunosuppression protocol, we were most interested in examining the lower end of the rATG cumulative dose spectrum (3 doses or less) compared with higher doses to identify the lowest safe and effective rATG dose threshold. By leveraging the collaborative nature of the PNRC, we were able to recruit a large cohort of pediatric kidney transplant recipients with granular rATG dosing information and detailed outcome variables including DSA development and viral infection PCR positivity that are not typically captured in large national registries such as the North American Pediatric Renal Trials and Collaborative Studies registry and the Scientific Registry of Transplant Recipients database. Our findings mirror the recent trend toward lower rATG dosing induction strategies.7,12,13,16 Approximately one-third of recipients in our cohort received ≤ 4.5 mg/kg in a cumulative rATG induction dose, which falls below the recent US Food and Drug Administration label recommendation of 4 doses at 1.5 mg/kg per dose or a cumulative exposure target of 6 mg/kg. The “standard” dosing group in our cohort fell more in line with the recent US Food and Drug Administration label recommendation with a median cumulative exposure of 5.96 mg/kg, which is generally equivalent to 4 daily doses at 1.5 mg/kg each and represents a lower exposure relative to the original clinical trials that popularized rATG use for induction immunosuppression in kidney transplantation using a 5-day treatment course.1,11,17
One concern related to lower rATG dose exposure is decreased efficacy leading to possible increased risk of acute rejection and how that may impact both graft function and graft survival. Our findings suggest that low-dose rATG induction is comparable with standard-dose rATG induction from short-term acute rejection prophylaxis, graft function, and graft survival standpoints. Our acute rejection rate in the first 12 months posttransplant was similar in both the low and standard rATG dose groups at 17% and 19%, respectively, although there was a trend for a higher proportion of acute rejections in the low-dose group occurring in the first 6 months posttransplantation. Graft function at 12 months was satisfactory in both groups, with eGFR at 70 and 72 ml/min/1.73 m2. Graft survival was similar as well through 24 months of follow-up at 96.4% and 94.6% for the low- and standard-dose groups, respectively. Although our study design did not allow for a 3-year longitudinal follow-up, our 2-year graft survival data fall between 1- and 3-year graft survival data reported in the most recent North American Pediatric Renal Trials and Collaborative Studies cohort.2 In the absence of overt clinical rejection or graft failure, we were interested in assessing the risk of DSA development in relation to a lower rATG induction dose, which may lead to subclinical rejection and subsequent late acute antibody-mediated rejection not captured in our follow-up period. Our findings suggest no significant difference in de novo DSA development through 12 months of follow-up after kidney transplantation based on the rATG induction dose. The proportion of recipients who developed a de novo DSA (either class I or class II) in our cohort was 22.8% and 24.4% in the low and standard rATG dose groups, which is in line with reported rates in other pediatric studies.18,19 Overall, those findings are consistent with what has been shown in smaller single-center, adult studies in which low rATG dosing protocols demonstrated excellent 1-year graft outcomes without a significant increase in acute rejection rates. For example, in comparison with conventional rATG exposure targets of 6 to 10 mg/kg, Singh et al.7 reviewed their outcomes using a tailored rATG cumulative exposure target of 3 to 6 mg/kg over a 5-year period and found comparable outcomes with those reported in the annual Scientific Registry of Transplant Recipients reports.7 Another study by Grafals et al.13 using a much lower rATG cumulative exposure target of either 2.25 mg/kg or 3.75 mg/kg demonstrated a biopsy-proven acute rejection rate of 10% and 17%, respectively, at 1 year follow-up and similar T-cell subpopulation depletion and repopulation kinetics in both groups.
Posttransplant infections are the leading cause of death in pediatric kidney transplant recipients and have surpassed acute rejection as a cause for hospitalization.2 Therefore, a major driver for induction immunosuppression protocols using low-dose rATG is the concern for a higher risk of opportunistic infections that may be observed with higher rATG dose exposure.6,8 Paradoxically, a low-dose rATG induction protocol that inadvertently leads to increased incidence of early acute rejection requiring further intensification of immunosuppression can further compound that infectious risk and negate any protective benefit derived from the low-dose protocol. Similarly, posttransplant neutropenia, which is observed more frequently in association with rATG induction, can have detrimental patient and graft outcomes should it lead to serious life-threatening infections or graft rejection resulting from mitigation efforts that lower maintenance immunosuppression.20 The frequency of posttransplant neutropenia in our cohort was high but no different in relation to rATG exposure dose. Most of the cases occurred in the first 6 months posttransplant, with 43.5% and 48.6% of recipients in the low-dose and standard-dose group developing neutropenia in that time frame. Similarly, we found no difference in the occurrence of viral reactivation for CMV, EBV, or BK virus infection in relation to rATG dose exposure. Although it is reassuring that there was no significant difference in patient survival or the occurrence of PTLD between groups, it is worth noting that the only death, which was secondary to an infection, and all 4 PTLD cases noted in our cohort belonged to the standard rATG dose exposure group. In a large analysis involving 25,127 recipients in the United States Renal Data System database specifically designed to address PTLD occurrence in relation to immunosuppression, the authors found an increased PTLD risk in relation to a composite of all formulations of antithymocyte globulin used for induction in patients transplanted between 1996 and 2000. However, the use of the rATG formulation (also known as Thymoglobulin), which was used for induction therapy during our study time frame, was not specifically linked to an increased risk in that report.21 In another pediatric-focused analysis, the increased risk only applied to the equine formulation of antithymocyte globulin.22 Additionally, the cumulative dose of rATG was not associated with the development of PTLD in a systematic review of 2246 kidney and heart transplant recipients who received rATG induction, further suggesting that the antithymocyte globulin formulation and other recipient- and transplant-related factors may be more relevant to the development of PTLD.23
Our study has several limitations; as with any other chart review retrospective analysis, data completeness is a major limiting factor. In our study, 47 patients of the enrolled 282 (17%) had received rATG induction but lacked dosing information to facilitate their classification to either the low-dose or standard-dose rATG group and as such were excluded from further analysis. Data regarding dialysis vintage was lacking, which limited our ability to control for that variable as a confounding factor. Although the graft failure rate was small in our study, causes of graft failure were not collected, limiting our ability to extrapolate whether graft failures could be attributed to rATG-relevant adverse events such as rejection or infection. Data regarding long-term maintenance immunosuppression details were limited to discharge medication records from the index transplant admission, thus limiting the ability to adjust for changes in maintenance immunosuppression in our model. Thus, our study design was specifically limited to capturing primarily 12-month and a smaller subset of 24-month outcomes because longer-term outcomes are more likely to be influenced by multiple unmeasured confounding factors such as long-term changes in immunosuppression, whereas the shorter-term outcomes would more reliably reflect the induction dosing effect. Another limitation relates to each center using its own center-specific surveillance biopsy, DSA, and viral monitoring protocols, which may have affected the frequency at which borderline rejections, positive DSAs, or viral PCRs were detected depending on the testing frequency and positive cutoff thresholds. We attempted to account for that by adjusting for center effect in our regression model. The retrospective study design also limits our ability to draw a causal relationship between the rATG dose and the outcomes examined; however, the data are compelling and lay the groundwork for future prospective cohort analysis and randomized clinical trials addressing the same question. Also, despite the large number of patients for a pediatric-focused study, our study was not sufficiently powered to examine all the outcome measures described, and our findings should be viewed as an exploratory analysis laying the groundwork for future studies. In addition, although the PNRC offers a platform to conduct large-scale collaborative research across its member institutions, we were limited to data from 9 participating member sites, which can limit the generalizability of our findings to the larger pediatric transplant community. However, our findings complement the growing body of literature available from larger adult-focused or database-only studies with a wide range of outcomes linked to granular dosing information and exclusively focused on a pediatric population.
In conclusion, we have demonstrated that a low rATG cumulative induction dose ≤ 4.5 mg/kg provides safe and effective short-term patient and graft outcomes in this multicenter, low immunologic risk, pediatric kidney transplant cohort. Prospective longitudinal cohort or randomized controlled trial studies are warranted to define the optimal rATG dosing strategy in pediatric kidney transplantation.
Disclosure
VRD received honoraria from Bristol-Myers-Squibb, Atara Bio, and CareDx and grant support from Atara Bio and CareDx. AM received grant research support from Leadiant. DJW received speaker bureaus membership support from Alexion. All the other authors declared no competing interests.
Acknowledgments
This study was supported in part by U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health which funds the Louisiana Clinical and Translational Science Center. VRD is supported in part by NIH grant R01DK102981. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions
IFA, RAB, and VRD participated in research design, performance of the research, data analysis and interpretation, writing of the paper, and approval of the final manuscript draft for publication. CG, AJ, SGK, AM, HP, JS, DJW, and RSZ participated in performance of the research, writing of the paper, and approval of the final manuscript draft for publication.
Footnotes
Table S1. Participating centers’ practice patterns
Table S2. Rabbit antithymocyte globulin dosing range in study participants stratified by exposure group
STROBE Checklist
Supplementary Material
Table S1. Participating centers’ practice patterns
Table S2. Rabbit antithymocyte globulin dosing range in study participants stratified by exposure group
STROBE Checklist
References
- 1.Brennan D.C., Daller J.A., Lake K.D., Cibrik D., Del Castillo D., Thymoglobulin Induction Study G Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355:1967–1977. doi: 10.1056/NEJMoa060068. [DOI] [PubMed] [Google Scholar]
- 2.Chua A., Cramer C., Moudgil A. Kidney transplant practice patterns and outcome benchmarks over 30 years: the 2018 report of the NAPRTCS. Pediatr Transplant. 2019;23 doi: 10.1111/petr.13597. [DOI] [PubMed] [Google Scholar]
- 3.Hart A., Smith J.M., Skeans M.A. OPTN/SRTR 2018 annual data report: kidney. Am J Transplant. 2020;20(suppl s1):20–130. doi: 10.1111/ajt.15672. [DOI] [PubMed] [Google Scholar]
- 4.Dharnidharka V.R., Naik A.S., Axelrod D.A. Center practice drives variation in choice of US kidney transplant induction therapy: a retrospective analysis of contemporary practice. Transpl Int. 2018;31:198–211. doi: 10.1111/tri.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration April 21, 2017 approval letter - thymoglubin. Updated April 21, 2017. https://www.fda.gov/media/104907/download Available at:
- 6.Puliyanda D.P., Stablein D.M., Dharnidharka V.R. Younger age and antibody induction increase the risk for infection in pediatric renal transplantation: a NAPRTCS report. Am J Transplant. 2007;7:662–666. doi: 10.1111/j.1600-6143.2006.01675.x. [DOI] [PubMed] [Google Scholar]
- 7.Singh N., Rossi A.P., Savic M., Rubocki R.J., Parker M.G., Vella J.P. Tailored rabbit antithymocyte globulin induction dosing for kidney transplantation. Transplant Direct. 2018;4:e343. doi: 10.1097/TXD.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill P., Cross N.B., Barnett A.N., Palmer S.C., Webster A.C. Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients. Cochrane Database Syst Rev. 2017;1:CD004759. doi: 10.1002/14651858.CD004759.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Participants in the International Summit on Transplant Tourism and Organ Trafficking Convened by the Transplantation Society and International Society of Nephrology in Istanbul, Turkey, April 30-May 2, 2008. The Declaration of Istanbul on organ trafficking and transplant tourism. Transplantation. 2008;86:1013–1018. [Google Scholar]
- 10.Alkandari O., Hebert D., Langlois V., Robinson L.A., Parekh R.S. Validation of serum creatinine-based formulae in pediatric renal transplant recipients. Pediatr Res. 2017;82:1000–1006. doi: 10.1038/pr.2017.209. [DOI] [PubMed] [Google Scholar]
- 11.Mohty M., Bacigalupo A., Saliba F., Zuckermann A., Morelon E., Lebranchu Y. New directions for rabbit antithymocyte globulin (Thymoglobulin((R))) in solid organ transplants, stem cell transplants and autoimmunity. Drugs. 2014;74:1605–1634. doi: 10.1007/s40265-014-0277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laftavi M.R., Alnimri M., Weber-Shrikant E. Low-dose rabbit antithymocyte globulin versus basiliximab induction therapy in low-risk renal transplant recipients: 8-year follow-up. Transplant Proc. 2011;43:458–461. doi: 10.1016/j.transproceed.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 13.Grafals M., Smith B., Murakami N. Immunophenotyping and efficacy of low dose ATG in non-sensitized kidney recipients undergoing early steroid withdrawal: a randomized pilot study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashoor I.F., Martz K., Galbiati S., Beyl R.A., Dharnidharka V.R. Reassessing Rabbit Antithymocyte Globulin Induction in Kidney Transplantation (RETHINK): an analysis of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Registry. Transplant Direct. 2020;6:e598. doi: 10.1097/TXD.0000000000001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsapepas D.S., Mohan S., Tanriover B. Impact of small variations in the delivered dose of rabbit antithymocyte induction therapy in kidney transplantation with early corticosteroid withdrawal. Transplantation. 2012;94:325–330. doi: 10.1097/TP.0b013e318257ad1a. [DOI] [PubMed] [Google Scholar]
- 16.Klem P., Cooper J.E., Weiss A.S. Reduced dose rabbit anti-thymocyte globulin induction for prevention of acute rejection in high-risk kidney transplant recipients. Transplantation. 2009;88:891–896. doi: 10.1097/TP.0b013e3181b6f38c. [DOI] [PubMed] [Google Scholar]
- 17.Hardinger K.L., Rhee S., Buchanan P. A prospective, randomized, double-blinded comparison of thymoglobulin versus Atgam for induction immunosuppressive therapy: 10-year results. Transplantation. 2008;86:947–952. doi: 10.1097/TP.0b013e318187bc67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demirok A., Ranzijn C., Lardy J., Florquin S., Bouts A. Evaluation of the current post-transplantation human leukocyte antigen antibody screening in pediatric renal transplant recipients. Pediatr Transplant. 2019;23:e13338. doi: 10.1111/petr.13338. [DOI] [PubMed] [Google Scholar]
- 19.Engen R.M., Park G.E., Schumacher C.S. Donor-specific antibody surveillance and graft outcomes in pediatric kidney transplant recipients. Transplantation. 2018;102:2072–2079. doi: 10.1097/TP.0000000000002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurst F.P., Belur P., Nee R. Poor outcomes associated with neutropenia after kidney transplantation: analysis of United States Renal Data System. Transplantation. 2011;92:36–40. doi: 10.1097/TP.0b013e31821c1e70. [DOI] [PubMed] [Google Scholar]
- 21.Caillard S., Dharnidharka V., Agodoa L., Bohen E., Abbott K. Posttransplant lymphoproliferative disorders after renal transplantation in the United States in era of modern immunosuppression. Transplantation. 2005;80:1233–1243. doi: 10.1097/01.tp.0000179639.98338.39. [DOI] [PubMed] [Google Scholar]
- 22.Dharnidharka V.R., Stevens G. Risk for post-transplant lymphoproliferative disorder after polyclonal antibody induction in kidney transplantation. Pediatr Transplant. 2005;9:622–626. doi: 10.1111/j.1399-3046.2005.00361.x. [DOI] [PubMed] [Google Scholar]
- 23.Marks W.H., Ilsley J.N., Dharnidharka V.R. Posttransplantation lymphoproliferative disorder in kidney and heart transplant recipients receiving thymoglobulin: a systematic review. Transplant Proc. 2011;43:1395–1404. doi: 10.1016/j.transproceed.2011.03.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.