Abstract
Purpose
Localized pancreatic cancer is commonly treated with stereotactic body radiation therapy (SBRT), which often requires the placement of fiducial markers. We compared the clinical outcomes of patients with and without fiducial markers.
Methods and Materials
We retrospectively collected data on patients with pancreatic cancer treated with neoadjuvant SBRT at a single institution. Patients were divided into 2 groups based on the placement of a fiducial marker. Local recurrence was the primary outcome. Time to event endpoints were analyzed using COX regression.
Results
We included 96 patients with unresectable pancreatic cancer: 46 patients (47.9%) did not have a fiducial marker, and 50 patients (52.1%) had a fiducial placed. Patients in the fiducial group were older and had more locally advanced pancreatic cancer compared with those who did not have a fiducial placed. Most patients in both groups (92.7%) received chemotherapy before SBRT treatment. SBRT was delivered to a median of 36 Gy over 5 fractions in the no-fiducial group, and 38 Gy over 5 fractions in the fiducial group. At a median follow-up of 20 months, local recurrence was similar irrespective of fiducial placement (adjusted hazard ratio [aHR] 0.6, 95% CI 0.3-1.3, P = .59). Furthermore, no difference in overall survival was noted between the 2 groups (aHR 0.8, 95% CI 0.3-1.9, P = .65). In patients who eventually underwent surgery post-SBRT, no difference in surgical margins (P = .40) or lymphovascular invasion (P = .76) was noted between the 2 groups. No patient developed acute pancreatitis after fiducial placement.
Conclusions
Our data suggest that the use of fiducial markers does not negatively affect clinical outcomes in patients with localized pancreatic cancer. Prospective confirmation of our results is still needed.
Introduction
Pancreatic cancer is the fourth leading cause of cancer death in the United States.1 Despite aggressive treatment methods, the 5-year survival remains dismal at less than 10%. The majority of patients present with unresectable disease at presentation, which highly influences prognosis. Most patients with locally advanced pancreatic cancer experience morbidity from a combination of local progression and metastatic spread to other organs.2 Chemotherapy regimens extrapolated from the metastatic setting have prolonged survival, but without surgery there has been no clear method to improve local control.3
Currently, the standard of care for patients with unresectable tumors includes a combination of chemotherapy and radiation therapy.4 Chemotherapy often consists of induction folinic acid, fluorouracil, irinotecan, oxaliplatin or gemcitabine/nab-paclitaxel.3,5 For patients whose tumors respond but remain unresectable, it is common to consider consolidation with radiation therapy.6 Conventional radiation therapy can take up to 6 weeks and delays patients from resuming full-dose systemic therapy. In contrast, stereotactic body radiation therapy (SBRT) usually takes place over a week and allows for delivery of higher doses of radiation to the tumor, which may contribute to improved local control rates with less toxicity, allowing patients to return to systemic treatment, if needed.7, 8, 9, 10
Delivery of radiation therapy to the pancreas can be challenging owing to the movements of the pancreas during respiration and the proximity of vulnerable organs at risk of radiation toxicity such as the stomach, duodenum, and other parts of the small bowel.11,12 Thus, fiducial markers as an aid for better localization and tracking have gained wide appeal in pancreatic radiation therapy, particularly when onboard imaging systems do not have sufficient resolution to identify interfaces between the bowel and the tumor.13 However, placing a fiducial carries some risk. This additional procedure carries a very small risk of pancreatitis and may result in tumor seeding along the needle track.13,14 Moreover, systems that do not rely on fiducials such as magnetic resonance linear accelerators (MR Linac) or CT-on-rails have shown that high-quality SBRT can be done without fiducial markers.15, 16, 17 We aimed to compare patients with pancreatic cancer who received SBRT with fiducial placement before treatment with patients who did not have fiducials placed to determine whether there are any differences in survival, rates of local and distant disease progression, surgical outcomes, and treatment-related toxicities.
Methods
Study design and population
The following study is a retrospective analysis of patients with pancreatic cancer treated with SBRT. We reviewed the records of patients with nonmetastatic pancreatic cancer treated with neoadjuvant SBRT at our home institution (The University of Texas MD Anderson Cancer Center) between the years 2016 and 2019. Patients presenting with metastatic disease and those who underwent surgery before SBRT treatment were excluded from our analysis. Patients were categorized into 2 groups based on the placement of a fiducial marker before SBRT. The decision to place fiducials was based on a variety of factors such as patient anatomy, predicted reproducibility of the set-up, physician preference, and mutual decision among the patient, endoscopist, and radiation oncologist. All patients were treated with image guided radiation therapy (IGRT) using either computed tomography (CT)-on-rails (69 patients, 71.9%) or cone beam CT (27 patients, 28.1%). Generally, patients who could be treated using CT-on-rails did not receive fiducial markers, and those who were treated with cone beam CT had a fiducial marker placed. Of the 69 patients treated with CT-on-rails, 28 patients (40.6%) had a fiducial placed, and 22 patients (81.5%) treated with cone beam CT had a fiducial placed. Furthermore, fiducial placement was often omitted in cases in which patients could not get off anticoagulation for the procedure, or when fiducial placement was technically challenging due to excessive pancreatic fibrosis and/or poor tumor visualization. Planning tumor volumes (PTV) and margins were calculated similarly irrespective of fiducial placement. Intravenous contrast was used at simulation to better delineate target volumes. Furthermore, patients were placed NPO 3 hours before radiation treatment and were treated using daily breath holds. Patient information regarding demographics and baseline tumor and treatment characteristics were collected. The University of Texas MD Anderson Cancer Center institutional review board approved all protocols in this study.
Outcomes
The primary endpoint of our study was local recurrence, defined by the occurrence of local (tumor) recurrence from time of diagnosis. Secondary endpoints included overall survival (OS), local-regional recurrence-free survival (LRRFS), distant metastasis-free survival (DMFS), progression-free survival (PFS), and surgical clinical outcomes. OS was defined by the occurrence of death due to any cause from time of diagnosis. Local-regional recurrence was defined by the first occurrence of local or regional (regional lymph nodes) recurrence from time of diagnosis. Distant metastasis was defined by the first occurrence of distant metastasis from time of diagnosis. Lastly, PFS was defined by the occurrence of any new pancreatic cancer disease progression (local-regional recurrence/progression or distant metastasis), or the occurrence of death from time of diagnosis. All time-to-event endpoint definitions are in line with the DATECAN (Definition for the Assessment of Time-to-event End-points in CANcer trials) classification.18 Local-regional and distant recurrences were assessed on follow-up CT scans performed approximately every 3 months. The scans were assessed by gastrointestinal specialized radiologists, and recurrences were assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria.19 In cases in which surgery was performed after SBRT, we analyzed pathology reports to identify surgical margins and lymphovascular invasion. Furthermore, we analyzed differences in treatment-related toxicity between the fiducial and no fiducial groups. All toxicity data were physician-reported, and graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.0.20 Acute pancreatitis was considered fiducial related if it happened within 7 days of EUS-guided placement of the fiducials and the patient presented with any of the following 2: new or worsening upper abdominal pain, amylase or lipase levels 3 times higher than the upper normal limit, and/or radiographic evidence of pancreatitis.21
Statistical analysis
We used descriptive statistics to summarize the patients’ demographic, disease, and treatment characteristics. Categorical variables are presented as frequencies and percentages, and continuous variables are presented as medians with interquartile ranges. We used binary logistic regression to calculate unadjusted and adjusted odds ratio for the association between the use of fiducial markers and surgical outcomes and multivariate regression to evaluate the association of fiducial placement with OS and PFS. Cox proportional hazard regression was used to calculate unadjusted and adjusted hazard ratios (aHR) with their corresponding 95% confidence interval (CI). The reported odds and hazard ratios are for the comparison of fiducial placement to no-fiducial placement. Adjusted statistics were adjusted for age, gender, performance status (measured on the Eastern Cooperative Oncology Group scale), chemotherapy, type of IGRT, tumor stage, and tumor site. Survival graphs for OS and PFS were generated using the Kaplan-Meier method. In addition, Pearson chi-squared statistic was used to assess the difference in tumor recurrence sites between the 2 groups. Statistical significance was set a priori at a 2-sided P value of .05. All statistical analyses were conducted using IBM SPSS Statistics version 26.22 Kaplan-Meier graphs were generated using Prism version 8.23
Results
Patient and treatment characteristics
The demographic and tumor characteristics of the 96 patients (42 women and 54 men) with nonmetastatic, unresectable pancreatic cancer who underwent SBRT are presented in Table 1. Forty-six patients (47.9%) did not have a fiducial marker placed before SBRT treatment, and 50 patients (52.1) had a fiducial marker placed. Patients in the fiducial group were slightly older than those in the no-fiducial group (median age, 72.4 vs 68.0, respectively). The fiducial group included more men (32, 64%) compared with the no-fiducial group (22, 47.8%). The no-fiducial group included more patients with Eastern Cooperative Oncology Group performance status of 0 (18, 39.1% vs 9, 18.0% in the fiducial group). By cancer stage, about half of the tumors included in our sample were borderline resectable pancreatic cancer (41, 42.7%). The fiducial group included more patients with locally advanced pancreatic cancer (23, 46.0% vs 14, 30.4% in the no-fiducial group). Lastly, most tumors included were either in the head (64, 66.7%) or body (20, 20.8%) of the pancreas.
Table 1.
Patient and tumor characteristics
| Characteristics | No fiducial (n = 46) | Fiducial (n = 50) |
|---|---|---|
| Age Median (range) | 68 (42.0-81.9) | 72.4 (37.0-87.0) |
| Sex | ||
| Female | 24 (52.2) | 18 (36.0) |
| Male | 22 (47.8) | 32 (64.0) |
| Baseline ECOG performance status | ||
| 0 | 18 (39.1) | 9 (18.0) |
| 1 | 23 (50.0) | 36 (72.0) |
| 2 | 4 (8.7) | 5 (10.0) |
| 3 | 1 (2.2) | 0 (0.0) |
| Pancreatic cancer stage | ||
| Resectable | 11 (23.9) | 7 (14.0) |
| Borderline resectable | 21 (45.7) | 20 (40.0) |
| Locally advanced | 14 (30.4) | 23 (46.0) |
| Tumor location | ||
| Head of the pancreas | 34 (73.9) | 30 (60.0) |
| Body of the pancreas | 8 (17.4) | 12 (24.0) |
| Tail of the pancreas | 1 (2.2) | 0 (0.0) |
| Pancreatic duct | 0 (0.0) | 2 (4.0) |
| Uncinate process | 1 (2.2) | 0 (0.0) |
| Other | 2 (4.3) | 6 (12.0) |
Abbreviation: ECOG = Eastern Cooperative Oncology Group.
The treatment characteristics of the patients included are presented in Table 2. The majority of patients received chemotherapy treatment before SBRT (no-fiducial group: 41, 89.1% vs fiducial group: 48, 96.0%). The most common chemotherapy regimens received before SBRT treatment were folinic acid, fluorouracil, irinotecan, oxaliplatin (45.8%) and a combination of gemcitabine and nab-paclitaxel (29.2%). SBRT treatment was similar between the 2 groups, and given to a median of 36 Gy over 5 fractions in the no-fiducial group, and 38 Gy over 5 fractions in the fiducial group. About two-thirds of patients continued chemotherapy treatment post-SBRT (65.6%).
Table 2.
Chemotherapy, radiation therapy, and surgical treatment characteristics
| Characteristics | No fiducial (n = 46) | Fiducial(n = 50) |
|---|---|---|
| Chemotherapy pre-SBRT | ||
| Yes | 41 (89.1) | 48 (96.0) |
| No | 5 (10.9) | 2 (4.0) |
| Type of chemotherapy pre-SBRT | ||
| FOLFIRINOX | 19 (46.3) | 25 (52.1) |
| Gemcitabine/nab-paclitaxel | 16 (39.0) | 12 (25.0) |
| FOLFIRINOX + Gemcitabine/nab-paclitaxel | 4 (9.8) | 6 (12.5) |
| Other | 2 (4.9) | 5 (10.4) |
| SBRT treatment | ||
| Median dose (range) | 36 (6-55) | 38 (30-55) |
| Median fractions (range) | 5 (1-5) | 5 (5-10) |
| Chemotherapy post-SBRT | ||
| Yes | 29 (63.0) | 34 (69.4) |
| No | 17 (37.0) | 15 (30.6) |
| Type of chemotherapy post-SBRT | ||
| FOLFIRINOX | 4 (13.8) | 9 (26.5) |
| Gemcitabine/nab-paclitaxel | 9 (31.0) | 12 (35.3) |
| FOLFIRINOX + Gemcitabine/nab-paclitaxel | 2 (6.9) | 0 (0.0) |
| Gemcitabine/Capecitabine | 6 (20.7) | 4 (11.8) |
| Capecitabine | 3 (10.3) | 5 (14.7) |
| Other | 5 (17.2) | 4 (11.8) |
| Surgery post-SBRT | ||
| Yes | 23 (50.0) | 18 (36.0) |
| No | 23 (50.0) | 32 (64.0) |
Abbreviations: FOLFIRINOX = folinic acid, fluorouracil, irinotecan, oxaliplatin; SBRT = stereotactic body radiation therapy.
Clinical and surgical outcomes
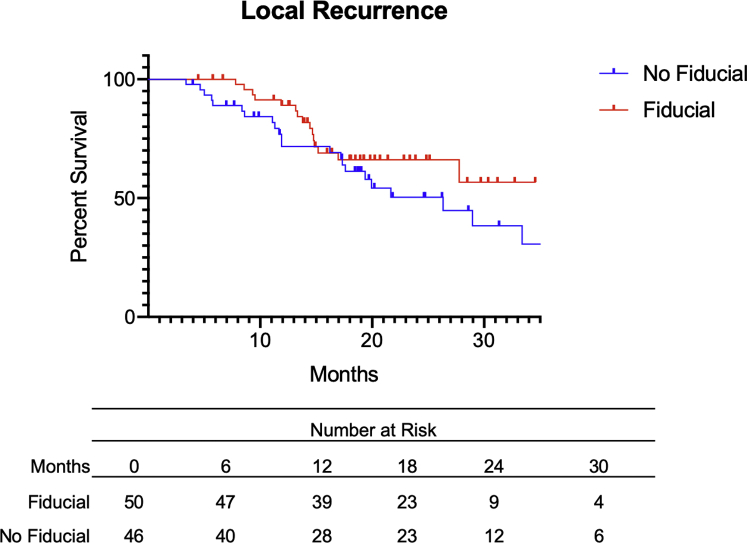
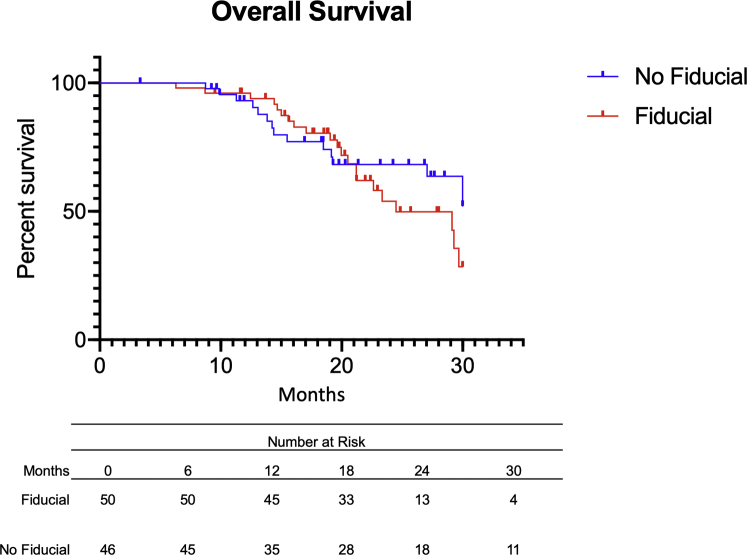
The clinical outcomes of the patients included are presented in Table 3. At a median follow-up of approximately 20 months, 16 patients (32.0%) who had fiducial marker placement developed local recurrence, and 22 patients (47.8%) who did not have fiducials developed local recurrence (Fig 1). Median time to first local recurrence was 30 months in the fiducial group and 25.2 months in patients who did not received fiducial placement. No difference in local recurrence-free survival was noted between the 2 groups (aHR = 0.6, 95% CI 0.3-1.3, P = .59). Moreover, a total of 36 deaths were noted in our population (OS, 62.5%). In particular, 15 patients in the no-fiducial group died (32.6%), versus 21 patients in the fiducial group (42.0%) (Fig 2). Median OS was 30.0 months in the no-fiducial group, versus 25.8 months in the fiducial group. The placement of a fiducial marker before SBRT treatment was not associated with improved OS (aHR = 0.8, 95% CI 0.3-1.9, P = .65).
Table 3.
Clinical outcomes
| Clinical event | No fiducial (n = 46) | Fiducial (n = 50) | uHR/uOR | P | aHR/aOR | P |
|---|---|---|---|---|---|---|
| Local recurrence-free survival | 52.2% | 68.0% | 0.7 (0.4-1.3)∗ | .27 | 0.6 (0.3-1.3) | .59 |
| Overall survival | 67.4% | 58.0% | 1.4 (0.7-2.7)∗ | .34 | 0.8 (0.3-1.9)∗ | .65 |
| LRRFS | 50.0% | 62.0% | 0.8 (0.4-1.5)∗ | .55 | 0.7 (0.3-1.5)∗ | .71 |
| DMFS | 50.0% | 44.0% | 1.4 (0.8-2.5)∗ | .23 | 1.1 (0.5-2.2)∗ | .86 |
| PFS | 34.8% | 34.0% | 0.9 (0.6-1.6)∗ | .86 | 1.0 (0.5-1.9)∗ | .99 |
| Post-SBRT surgery | 50.0% | 36.0% | 0.6 (0.2-1.3)† | .17 | 0.6 (0.3-1.4)† | .26 |
| Positive margins | 17.4% | 29.4% | 2.0 (0.4-8.9)† | .37 | 2.1 (0.4-11.4)† | .40 |
| LVIS | 56.5% | 62.5% | 1.3 (0.3-4.7)† | .71 | 1.2 (0.3-5.0)† | .76 |
Abbreviation: aHR = adjusted hazard ratio; aOR = adjusted odds ratio; DMFS = distant metastasis-free survival; LRRFS = local-regional recurrence-free survival; LVIS = lympho-vascular invasion; PFS = progression-free survival; SBRT = stereotactic body radiation therapy; uHR = unadjusted hazard ratio; uOR = unadjusted odds ratio.
Adjusted hazard ratio and adjusted odds ratio were adjusted for age, gender, Eastern Cooperative Oncology Group performance status, chemotherapy, type of image guided radiation therapy , tumor stage, and tumor site.
Hazard ratio calculated.
Odds ratio calculated.
Figure 1.
Local recurrence (LR) of patients with pancreatic ductal adenocarcinoma with (red) or without (blue) fiducial before stereotactic body radiation therapy. There was no significant difference between both groups with regard to LR (adjusted hazard ratio = 0.6, 95% CI 0.3-1.3, P = .59).
Figure 2.
Overall survival (OS) of patients with pancreatic ductal adenocarcinoma with (red) or without (blue) fiducial before stereotactic body radiation therapy. There was no significant difference between both groups with regard to OS (adjusted hazard ratio = 1.4, 95% CI 0.7-2.8, P = .34).
When analyzing local-regional recurrence, a total of 42 local-regional events occurred (LRRFS, 56.3%). In patients who did not have a fiducial placed, 23 patients developed local-regional recurrence (LRRFS, 50%), and in those who had a fiducial, 19 patients developed local-regional recurrence (LRRFS, 62%). Median time to LRR was 21.7 months in the no-fiducial group and 27.8 months in the fiducial group, and there was no difference in LRRFS between the groups (aHR = 0.7, 95% CI 0.3-1.5, P = .71). Additionally, 51 distant metastasis events occurred in our cohort (DMFS, 46.9%), with 23 events in the no-fiducial group (DMFS, 50.0%), and 28 events in the fiducial group (DMFS, 44.0%). No difference in DMFS between the 2 groups was noted (aHR = 1.1, 95% CI 0.5-2.2, P = .86).
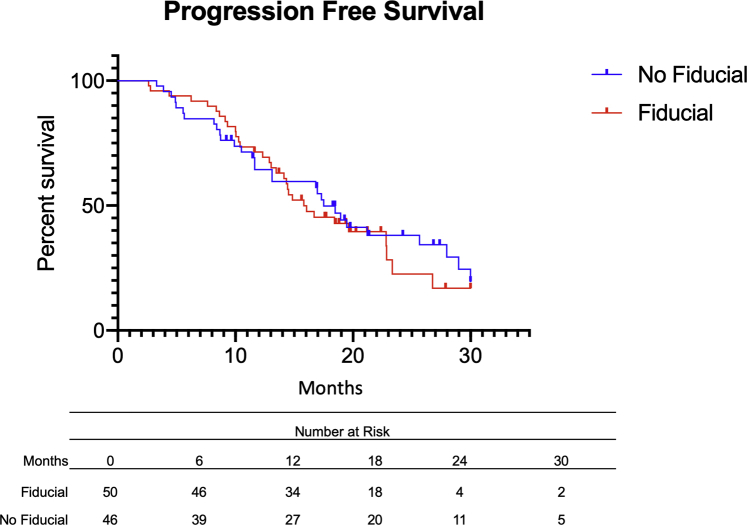
Furthermore, median time to progression was 17.9 months in the no-fiducial group, versus 16.5 months in the fiducial group (Fig 3). In total, 30 PFS events (65.2%) were noted in patients who did not have a fiducial marker, and 33 events (66.0%) were noted in the fiducial group. PFS was also similar irrespective of fiducial placement (no-fiducial group: 34.8% vs fiducial group: 34.0%, aHR = 1.0, 95% CI 0.5-1.9, P = .99). The most common sites of recurrence or metastasis were the liver (29, 30.2%), the lungs (17, 17.7%), and local recurrence in the pancreas (39, 40.6%) (Supplementary Table E1). Similar recurrence sites were noted among patients in both groups.
Figure 3.
Progression-free survival (PFS) of patients with pancreatic ductal adenocarcinoma with (red) or without (blue) fiducial before stereotactic body radiation therapy. There was no significant difference between both groups with regard to PFS (adjusted hazard ratio = 1.0. 95%CI 0.6-1.6, P = .87).
After SBRT treatment, 50% of patients with no fiducial marker underwent surgery versus 36% of patients with fiducial markers (P = .26). When analyzing patients who underwent surgical resection, no difference in OS (HR = 2.1, P = .30) or local recurrence (HR = 0.9, P = .89) was noted between patients with or without fiducial markers. Similar results were also noted among patients who did not undergo surgical resection (OS: P = .76; local recurrence: P = .18). For patients who had surgical resection, there were no differences in surgical margins (P = .40) or lympho-vascular invasion between the 2 groups (P = .76) between the 2 groups.
Toxicity data analysis
Table E2 presents the differences in treatment-related toxicity between the fiducial and no fiducial groups. Patients in the fiducial group had slightly less abdominal pain compared with the no-fiducial group (11, 23.9% vs 14, 30.4%, respectively). On the other hand, patients in the fiducial group had more fatigue (21, 42.0%) and nausea (18, 36.0%) compared with patients who did not receive fiducial placement. Overall, the majority of toxicities were grade 1 in both groups. Lastly, no patient from our population developed acute pancreatitis after SBRT treatment, irrespective of fiducial marker placement.
Discussion
Although patients with pancreatic cancer tend to have poor prognoses, recent advances in treatments have shown improvement clinical outcomes and treatment-related toxicity.24 The use of SBRT, compared with conventional radiation therapy, has been associated with improved local control and PFS, in part by enabling higher and potentially more effective radiation doses with acceptable toxicity profiles.7, 8, 9,25 The placement of a fiducial has long been the accepted method of IGRT (image guided radiation therapy) with Cyberknife or conventional LINAC approaches because onboard imaging usually fails to produce imaging with sufficiently high resolutions for the tight margins needed for high-quality, high-dose SBRT. With wider availability of newer technologies that enable high-quality soft tissue imaging for IGRT, fiducials may not be necessary.
Our findings suggest that the placement of fiducial markers does not negatively affect OS or local recurrence. Our findings also suggest similar surgical outcomes irrespective of fiducial placement. Additionally, the placement of fiducial markers was not associated with an increased risk of local-regional or distant recurrence. There was a slightly higher rate of regional nodal and peritoneal recurrence in patients who had a fiducial marker; however, this association was not statistically significant (P = .88, P = .55, respectively).
Recent reports have suggested that fiducial placement carries some risk of morbidity, such as the potential for needle track seeding by extrapolating cases reported from EUS FNA of the pancreas or pancreatitis.13,14 However, no patient developed acute pancreatitis after fiducial marker placement in our study.21 Moreover, there were similar patterns of failure in the 2 groups, suggesting that the fiducial placement did not affect disease progression. Tumor seeding from fiducial placement or a biopsy would most likely be observed along the needle track or in the peritoneum as has been previously reported.14 However, our data suggest that peritoneal seeding is unlikely when fiducials are placed by experienced endoscopists (in our case, all fiducials were placed by the same expert gastroenterologist, Dr Bhutani), or may be an exceedingly rare event that will not affect clinical outcomes. The following is similar to data from endoscopic ultrasound-guided fine needle aspiration (EUS-guided FNA) of the pancreas.26 Kim et al retrospectively analyzed the effects of EUS-guided FNA on peritoneal recurrence and clinical outcomes in patients with pancreatic cancer and showed that preoperative EUS-guided FNA did not worsen peritoneal recurrence, cancer-free survival, and OS.26
Patients who received SBRT for pancreatic cancer had similar outcomes whether they had fiducials for IGRT. Patients in the fiducial group had a lower rate of local recurrence compared with patients without fiducial placement (fiducial group: 32.0% vs no fiducial group: 47.8%). Despite this association not reaching statistical significance (P = .59), this difference could suggest a slight improvement in local control with the use of fiducial markers. Additionally, approximately 40% of patients were able to eventually undergo surgical resection, regardless of fiducial placement. This is best explained by the use of strong neoadjuvant chemoradiation regimens with SBRT that converted those patients and allowed for subsequent tumor resection.27 One possible explanation for the lack of benefit from fiducial placement might be explained by the higher proportion of patients with locally advanced pancreatic cancer in the fiducial group (46.0% vs 30.4% in the no-fiducial group). The similar efficacy may also stem from the fact that the radiation dosing was not significantly different between the 2 groups (median dose 38 Gy in the fiducial group vs 36 Gy in the no-fiducial group. With the advent of real-time tumor visualization techniques, radiation oncologists may opt to treat their patients without the need for a fiducial marker. For example, the use of CT-on-rails while delivering radiation allows direct visualization of the tumor and overcomes respiratory tumor motion.15 More recently, MR-linac imaging has also been used in patients with pancreatic cancer and is being studied in ongoing clinical trials.16,17 Despite limited data comparing MR-linac clinical outcomes with or without fiducial markers, such imaging techniques could eventually allow proper real-time tumor monitoring while saving patients the potential burden of fiducial placement. However, it is important to stress that if radiation oncologists only have access to cone beam CT, fiducials and breathing control (breath hold or gating) should be strongly considered to ensure accurate SBRT delivery.
Our study has a few limitations worth noting. First of all, it was based on a retrospective cohort at a single institution, which limits the generalizability of our findings to other medical settings. SBRT treatment and planning were not standardized across all patients, and specific information on dosimetry planning and volumes were not collected, and thus were not analyzed in this article. Moreover, there was a mixture of patients with resectable and unresectable disease, which may have confounded our analyses. Lastly, our study may have lacked enough statistical power to show significant differences in clinical outcomes between the 2 groups. Despite these limitations, our study still presents the largest modern comparison of pancreatic cancer patients treated with SBRT both with and without fiducial placement. Importantly, these patients were treated during a similar recent timeframe by the same group of radiation physicists and radiation oncologists, which reduces the confounding that might occur by comparing different institutions or techniques that span different eras of treatment.
In conclusion, our results suggest that the use of fiducial markers before SBRT in patients with pancreatic cancer was not associated with inferior PFS or OS, and was not associated with the development of acute pancreatitis. These findings should reassure physicians that high-quality IGRT, whether with a fiducial or not, is key to enabling SBRT. These data also reassure that fiducial placement does not promote disease progression and should be used if it can ensure the best possible image guidance in the abdomen, where margins for error are slim. Randomized prospective confirmation of our results is still needed to better assess the role of fiducial markers in patients with localized pancreatic cancer treated with SBRT.
Acknowledgments
We would like to thank all the patients and families who were included in this analysis.
Footnotes
Sources of support: The authors report no funding for this work.
Disclosures: All authors report no conflicts of interest related to the current manuscript. Dr Das reports personal fees from Adlai Nortye and personal fees from MD Anderson Cancer Center Madrid Spain. Dr Bhutani has received research support from OncoSil, Galera, Augmenix, and Silenseed. Dr Herman has received research support from OncoSil, Galera, and Augmenix, and works as a consultant at 1440 Foundation. Dr Koong acts as a consultant at Aravive, Inc. Dr Taniguchi is supported by funding from NIH under award R01CA227517-01A1, Cancer Prevention & Research Institute of Texas (CPRIT) grant RR140012, V Foundation (V2015-22), the Kimmel Foundation, Sabin Family Foundation Fellowship, and the McNair Foundation. No other financial disclosures or conflicts of interest to report.
S.M. and J.A.J. contributed equally as first authors.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2020.11.006.
Supplementary Materials
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Yachida S., Iacobuzio-Donahue C.A. The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med. 2009;133:413–422. doi: 10.5858/133.3.413. [DOI] [PubMed] [Google Scholar]
- 3.Suker M., Beumer B.R., Sadot E. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17:801–810. doi: 10.1016/S1470-2045(16)00172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khorana A.A., McKernin S.E., Berlin J. Potentially curable pancreatic adenocarcinoma: ASCO clinical practice guideline update. J Clin Oncol. 2019;37:2082–2088. doi: 10.1200/JCO.19.00946. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff D.D., Ervin T., Arena F.P. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palta M., Godfrey D., Goodman K.A. Radiation therapy for pancreatic cancer: executive summary of an ASTRO clinical practice guideline. Pract Radiat Oncol. 2019;9:322–332. doi: 10.1016/j.prro.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Koong A.C., Le Q.T., Ho A. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017–1021. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Koong A.C., Christofferson E., Le Q.-T. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:320–323. doi: 10.1016/j.ijrobp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Herman J.M., Chang D.T., Goodman K.A. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121:1128–1137. doi: 10.1002/cncr.29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moningi S., Dholakia A.S., Raman S.P. The role of stereotactic body radiation therapy for pancreatic cancer: a single-institution experience. Ann Surg Oncol. 2015;22:2352–2358. doi: 10.1245/s10434-014-4274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy J.D., Christman-Skieller C., Kim J. A dosimetric model of duodenal toxicity after stereotactic body radiotherapy for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78:1420–1426. doi: 10.1016/j.ijrobp.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 12.Colbert L.E., Rebueno N., Moningi S. Dose escalation for locally advanced pancreatic cancer: How high can we go? Adv Radiat Oncol. 2018;3:693–700. doi: 10.1016/j.adro.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coronel E., Cazacu I.M., Sakuraba A. EUS-guided fiducial placement for GI malignancies: a systematic review and meta-analysis. Gastrointest Endosc. 2019;89:659–670.e18. doi: 10.1016/j.gie.2018.10.047. [DOI] [PubMed] [Google Scholar]
- 14.Matsui T., Nishikawa K., Yukimoto H. Needle tract seeding following endoscopic ultrasound-guided fine-needle aspiration for pancreatic cancer: a report of two cases. World J Surg Oncol. 2019;17:134. doi: 10.1186/s12957-019-1681-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reyngold M., Parikh P., Crane C.H. Ablative radiation therapy for locally advanced pancreatic cancer: techniques and results. Radiat Oncol. 2019;14:95. doi: 10.1186/s13014-019-1309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koay E.J., Hanania A.N., Hall W.A. Dose-escalated radiation therapy for pancreatic cancer: a simultaneous integrated boost approach. Pract Radiat Oncol. 2020;10:e495–e507. doi: 10.1016/j.prro.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US National Library of Medicine Stereotactic MRI-guided on-table adaptive radiation therapy (SMART) for locally advanced pancreatic cancer. https://clinicaltrials.gov/ct2/show/NCT03621644 Available at:
- 18.Bonnetain F., Bonsing B., Conroy T. Guidelines for time-to-event end-point definitions in trials for pancreatic cancer. Results of the DATECAN initiative (Definition for the Assessment of Time-to-event End-points in CANcer trials) Eur J Cancer. 2014;50:2983–2993. doi: 10.1016/j.ejca.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.National Institutes of Health National Cancer Institute Common terminology criteria for adverse events (CTCAE) v4.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm Available at:
- 21.Foster B.R., Jensen K.K., Bakis G. Revised Atlanta classification for acute pancreatitis: a pictorial essay. Radiographics. 2016;36:675–687. doi: 10.1148/rg.2016150097. [DOI] [PubMed] [Google Scholar]
- 22.IBM Corp. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY.
- 23.Motulsky H. Prism. GraphPad Prism version 8.0.0, GraphPad Software, San Diego, CA. Available at: www.graphpad.com. Accessed December 17, 2020.
- 24.Abi Jaoude J., Kouzy R., Nguyen N.D. Radiation therapy for patients with locally advanced pancreatic cancer: evolving techniques and treatment strategies. Curr Probl Cancer. 2020;44:100607. doi: 10.1016/j.currproblcancer.2020.100607. [DOI] [PubMed] [Google Scholar]
- 25.Schellenberg D., Kim J., Christman-Skieller C. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81:181–188. doi: 10.1016/j.ijrobp.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Kim S.H., Woo Y.S., Lee K.H. Preoperative EUS-guided FNA: effects on peritoneal recurrence and survival in patients with pancreatic cancer. Gastrointest Endosc. 2018;88:926–934. doi: 10.1016/j.gie.2018.06.024. [DOI] [PubMed] [Google Scholar]
- 27.Blair A.B., Rosati L.M., Rezaee N. Postoperative complications after resection of borderline resectable and locally advanced pancreatic cancer: The impact of neoadjuvant chemotherapy with conventional radiation or stereotactic body radiation therapy. Surgery. 2018;163:1090–1096. doi: 10.1016/j.surg.2017.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.