Abstract
Thrombotic microangiopathy (TMA) is characterized by systemic microvascular thrombosis, target organ injury, anemia and thrombocytopenia. Thrombotic thrombocytopenic purpura, atypical hemolytic uremic syndrome and Shiga toxin E-coli-related hemolytic uremic syndrome are the three common forms of TMAs. Traditionally, TMA is encountered during pregnancy/postpartum period, malignant hypertension, systemic infections, malignancies, autoimmune disorders, etc. Recently, the patients presenting with trauma have been reported to suffer from TMA. TMA carries a high morbidity and mortality, and demands a prompt recognition and early intervention to limit the target organ injury. Because trauma surgeons are the first line of defense for patients presenting with trauma, the prompt recognition of TMA for these experts is critically important. Early treatment of post-traumatic TMA can help improve the patient outcomes, if the diagnosis is made early. The treatment of TMA is also different from acute blood loss anemia namely in that plasmapheresis is recommended rather than platelet transfusion. This article familiarizes trauma surgeons with TMA encountered in the context of trauma. Besides, it provides a simplified approach to establishing the diagnosis of TMA. Because trauma patients can require multiple transfusions, the development of disseminated intravascular coagulation must be considered. Therefore, the article also provides different features of disseminated intravascular coagulation and TMA. Finally, the article suggests practical points that can be readily applied to the management of these patients.
Keywords: Trauma, Microangiopathy, Complement amplifying condition
Introduction
Thrombotic microangiopathy (TMA) is a pathologic condition characterized by systemic microvascular thrombosis, target organ injury, anemia and thrombocytopenia.1,2 Traditionally, TMA is encountered during pregnancy or in a postpartum period. Also, malignant hypertension, systemic infections, malignancies, autoimmune disorders, stem cell or solid organ transplantation are all known to be associated with TMA.1,2 However, the surgical and trauma patients recently have been reported to have suffered from TMA.3 Regardless of the initiating event, once established, TMA carries a high morbidity and mortality, and demands a prompt recognition and early intervention to limit the target organ damage. The diagnostic considerations and treatments for TMA are different from acute blood loss anemia, and an early diagnosis is a key to improving patient outcomes. Platelet transfusions are not recommended in the treatment of trauma-related TMA, which are different from acute blood loss anemia, and instead a standard of care is plasmapheresis or other immunosuppressive therapy.4 Trauma-related TMA carries a high morbidity and mortality if untreated, but it usually responds a good outcome if recognized promptly.4 Trauma-related TMA is a rule-out diagnosis, so the clinical acumen is paramount. Additionally, the confirmatory laboratory test, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) is not immediately available, which further highlights the importance for awareness among clinicians.2 There are increasing cases of postoperative thrombotic thrombocytopenic purpura (TTP), but clinicians need to be cognizant that trauma, similar to surgery, can be a trigger for the development of TMA.
Globally, trauma is considered as one of the leading causes of death in the young population.4 Frequently, trauma surgeons are focused on prompt diagnosis of contusion, concussion and fracture with or without vascular injury to limit the morbidity and mortality. These experts utilize physical examinations, CT and CT angiography to achieve the optimal injury assessment. In contrast, TMA is often absent at the time of presentation in these patients and develops subsequently during the admission.5, 6, 7 TMA has specific recognizable laboratory features and must be suspected in every patient with microangiopathic hemolytic anemia (MAHA), thrombocytopenia and target organ injury (frequently the kidney).8 In this article, we described a simplified approach that can be adopted by the surgeons to readily establish a diagnosis of TMA in their patients. The article also provides features that TMA is distinguished from disseminated intravascular coagulation (DIC), and highlights interventions that can be employed to manage TMA.
Pathogenesis of TMA
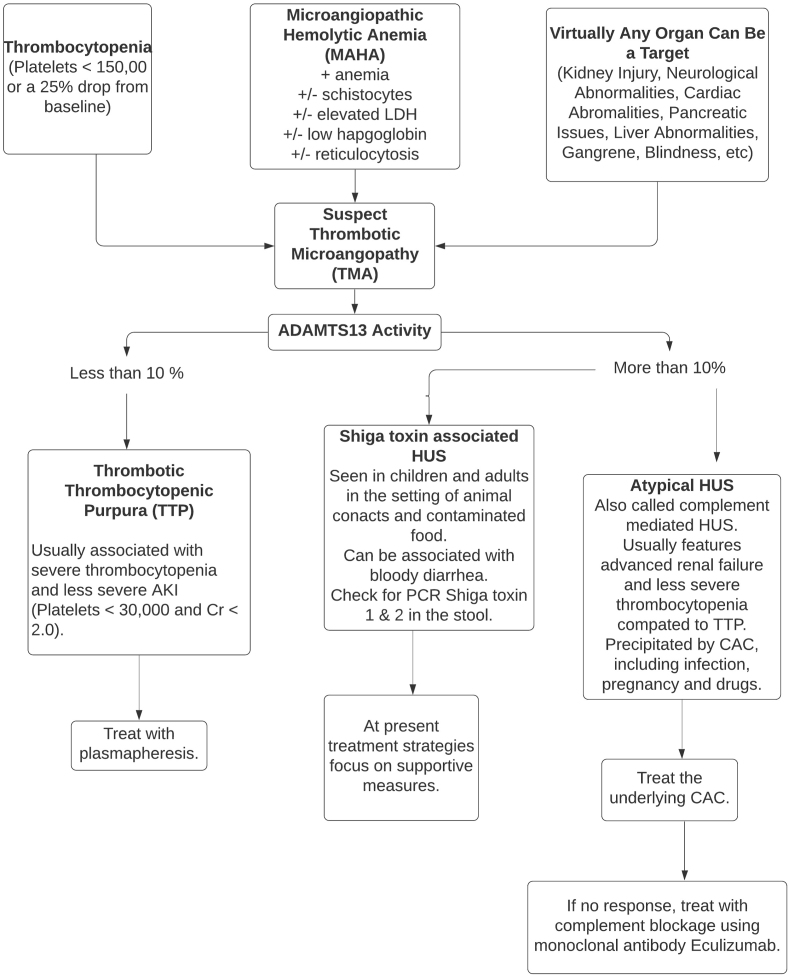
TMA is an umbrella term that includes three separate entities: TTP, atypical hemolytic uremic syndrome (aHUS) and Shiga toxin-related hemolytic uremic syndrome (STEC-HUS).1,2,8, 9, 10 All of these TMAs are characterized by a triad of MAHA, thrombocytopenia and target organ injury.5 Virtually, any organ can be affected. While STEC-HUS is caused by Shiga toxin-producing E-coli, recent data have emphasized that the underlying mechanisms for the development of TTP and aHUS are distinctly different. Antibody-mediated depletion of ADAMTS13 is the common underlying pathology for TTP, whereas uncontrolled activation of the complement system is the chief pathogenesis for the development of aHUS.10, 11, 12
ADAMTS13 plays a pivotal role in cleaving von-Willebrand factor multimers.8 In TTP, this process of cleavage of multimers is disrupted. This leads to the accumulation of large number of von-Willebrand factor multimers causing platelet-rich thrombi in small vessels, explaining both thrombocytopenia and MAHA due to high shear stress resulting in the formation of schistocytes.10,12 Severe deficiency of ADAMTS13 activity (<10%) is usually the cut-off value used for confirmation of TTP in the appropriate settings.1 Activity levels above 10% are considered normal, and otherwise should prompt to look for etiologies other than TTP. Plasmapheresis should not be delayed in cases of high suspicion of TTP, while waiting for the ADAMTS13 results.12, 13, 14
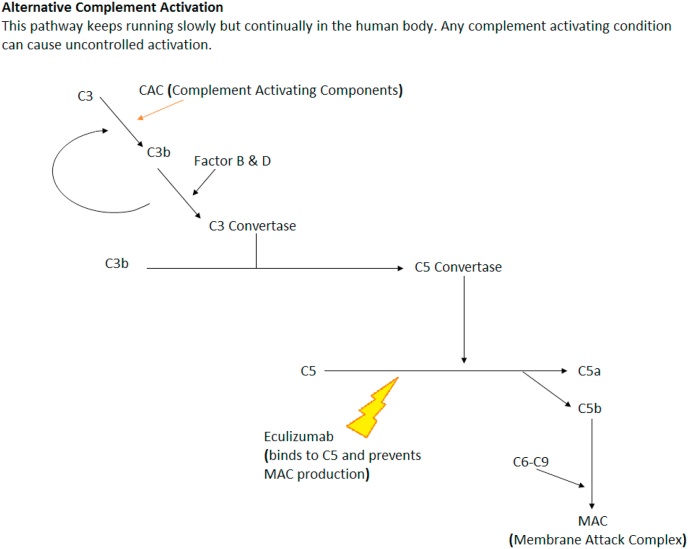
In contrast to the TTP, aHUS results from complement system dysfunction. The complement system is the oldest and most critical component of our innate immunity. The complement system comprises of vast number and variety of plasma proteins, membrane-bound proteins and regulatory receptors.11, 12, 13 In brief, the complement system can be activated via three different pathways: classical pathway, lectin pathway, alternative pathway. Uncontrolled activation of the alternative pathways is the key pathology responsible for the development of aHUS.12,13 Fig. 1 explains the normal function of the complement system.
Fig. 1.
Alternate complement pathway.
Multi-hit theory has emphasized that aHUS is a consequence of at least two components. A genetic predisposition to alternative complement pathway dysregulation and occurrence of events or conditions that can precipitate TMA.14, 15, 16 These events/conditions are referred to as complement amplifying conditions (CAC). There are complement regulatory proteins to play an essential role in protecting the autologous cells from inappropriate complement activity. A few examples of these include complement factor H and membrane cofactor protein and thrombomodulin. Inhibitory antibodies to the complement factor H have been documented as one of the major mechanisms in aHUS patients. Many patients with these mutations may remain asymptomatic before the manifestation of TMA, which could be triggered with any event leading to complement activation. These triggers could be infection, pregnancy and in rare cases trauma-related endothelial injury or inflammation.1,2,16
Multiple reports have confirmed that complement activation can occur in the setting of trauma.17,18 However, the mechanism is still poorly understood. A recent prospective study of Beth Israel Deaconess Medical Center examined the complement activation and platelet activity and aggregation in trauma patients.19 The study documented that complement activation is increased in trauma patients as measured by increased C3a levels.19 They also documented the deposition of complement C4d and C3a on platelets in trauma patients. These deposits altered the ability of platelets to aggregate. The study pointed out that complement activation can trigger platelet aggregation.19
CAC and TMA
Various etiologies and conditions have been associated with a state of complement activation and/or dysregulation. These conditions are usually referred to as CAC. The Oklahoma thrombotic thrombocytopenic purpura-hemolytic uremic syndrome registry provides us with a variety of these conditions and their incidence.14, 15, 16 CAC as an inciting event for the development of aHUS is increasingly being reported.6 Some of the common examples of CACs include pregnancy and its complications, covering pre-eclampsia and eclampsia, malignant hypertension, autoimmune diseases including systemic lupus erythematosus, scleroderma, ulcerative colitis, intravenous drug abuse, infections and trauma. Pregnancy-related aHUS accounts for approximately 7% of total aHUS cases.13, 14, 15, 16 Complement activation can also occur post-partum due to maternal circulation of fetal cells, infection and hemorrhage. Hemolysis, elevated liver enzymes, low platelet count syndrome is another fearsome complication of the increased complement activation. Malignant hypertension causes shear stress on endothelial cells and subsequent vascular injury further leading to complement activation and the development of TMA.1,2,6
Autoimmune conditions such as scleroderma, anti-phospholipid antibody syndrome and medications have been reported to be associated with TMA.20 It has been documented that a wide variety of systemic infections, including bacterial endocarditis, fungal infections, viral etiologies such as HIV, cytomegalovirus and even rickettsial diseases can also precipitate TMA.13,14,16,20
Trauma as a CAC
Surgical trauma has long been implicated as a precipitating event in the subsequent development of TMA, specifically TTP.17,18 As an example, patients undergoing cardiothoracic surgery and abdominal surgery are particularly vulnerable.4,18,19
Trauma per se as a precipitating event for the development of TMA has not been traditionally reported. However, recently, two important reports implicated trauma as the precipitating factor for the development of TMA.17,18 With these recent data, it appears that trauma may be a previously under-detected trigger of TMA, and is something physicians should monitor for in these patients. These patients suffered from TTP were treated with plasmapheresis and recovered, eventually being discharged home. Ikegami et al.17 reported TMA in an 80-year-old woman who sustained a pelvic hematoma and fracture. Transcatheter coil embolization was performed to control the bleeding. The patient was given 560 mL of packed red cells along with 480 mL of fresh frozen plasma, and also received 200 mL of platelets. She made a good recovery and hemodynamic stability was achieved. However, on day three she demonstrated hematuria and became anuric with associated delirium and fever. The patient developed anemia, thrombocytopenia and severe renal failure that did not respond to volume replacement and diuretics. Hemodialysis therapy was initiated to treat severe renal failure. A peripheral smear revealed numerous schistocytes indicating the presence of MAHA. Coomb’s test was negative. Lactate dehydrogenase was elevated. However, complement factors, prothrombin time (PT), partial PT and international normalized ratio were all within the normal range and the ADAMTS13 level was not reduced. Because of anemia, thrombocytopenia, renal failure, fever and mental status change, the clinicians contemplated the diagnosis of TTP and daily plasmapheresis was delivered for five days. The patient showed a dramatic recovery of the clinical picture and laboratory parameters. Anemia and thrombocytopenia resolved and hemodialysis was discontinued on day 15. The authors concluded that the development of TMA post-trauma in her case was due to the production of unusually large von-Willebrand factor multimers. The authors hypothesized that these multimers could not be processed even with normal ADAMTS13 level.17
In a separate report, Lim and Park18 reported TMA in a 23-year-old man after suffering a blunt traumatic liver injury from a motorcycle accident. Mechanism of development of TTP was the release of unusually large multimers of von-Willebrand factor after the liver injury. This patient presented with mental status change, fever, hemolytic anemia, thrombocytopenia and renal failure after the admission. Because of the clinical presentation, anemia, thrombocytopenia and renal failure, TTP was considered. Hemodialysis was initiated for renal failure. On hospital day 23, plasmapheresis was initiated. The patient improved after 18 days of plasma therapy. Thrombocytopenia, anemia and renal failure resolved and hemodialysis was discontinued. While ADAMTS13 was not obtained in this case, a positive response to plasma therapy confirmed the diagnosis of TTP.18
Diagnosis of TMA in the trauma patients
Diagnosing TMA after trauma can be challenging. The occurrence of DIC in trauma patients can complicate the diagnostic dilemmas (Table 1). Undoubtedly, thrombocytopenia and anemia encountered in the context of trauma should firstly raise the suspicion of extravascular blood loss. Indeed, in their report, Ikegami et al.17 considered extravasation as the cause of anemia and thrombocytopenia. However, despite using coil embolization, administering packed red blood cells and transfusing platelet, thrombocytopenia persisted and renal failure ensued.17 Trauma patients often receive judicious intravenous fluids. In this context, dilutional thrombocytopenia and anemia must also be considered. Because trauma patients may require a significant amount of blood products, which may easily arise DIC. Both TMA and DIC can cause thrombocytopenia, anemia and target organ injury (Table 1, Table 2).3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 However, normal PT, activated partial thromboplastin time and fibrin degradation products and normal fibrinogen levels help to differentiate TMA from DIC. Post-traumatic TMA should be considered if the patient in the setting of a traumatic event develops progressive thrombocytopenia, MAHA, renal failure, mental changes and fever.3,7 Patients are expected to improve to a certain extent even if not completely, after timely initiation of plasma exchange. Schistocytes are an important feature of MAHA (shear stress). Hence, peripheral smear must be reviewed to ascertain the presence of Schistocytes. It is important to mention that this type of hemolysis is not due to the development of autoantibodies. Therefore, Coomb’s test is negative in patients with TMA.
Table 1.
Differentiating features and laboratory parameters for aHUS, TTP and DIC.
| Parameter | aHUS | TTP | DIC |
|---|---|---|---|
| Etiology/mechanism | Uncontrolled activation of the alternative pathway of the complement system | Deficiency of ADAMTS13 | Sepsis/infection/multiple transfusions |
| Anemia | Yes | Yes | Yes |
| Thrombocytopenia | Yes | Yes | Yes |
| Renal injury | Usually severe | Usually mild to moderate | Uncommon |
| Schistocytes | Yes | Yes | Yes |
| Reticulocyte count | Elevated | Elevated | Elevated |
| PT | Normal | Normal | Elevated |
| aPTT | Normal | Normal | Elevated |
| Fibrinogen | Normal | Normal | Low/normal |
| FDP | Normal | Normal | Elevated |
| ADAMTS13 activity | Normal (>5%) | Low (<5%) | Normal (>5%) |
| Treatment | Complement blockade by monoclonal antibody | Plasmapheresis | Treat the underlying etiology |
aHUS: atypical hemolytic uremic syndrome; TTP: thrombotic thrombocytopenic purpura; DIC: disseminated intravascular coagulation; PT: prothrombin time; aPTT: activated partial thromboplastin time; FDP: fibrin degradation products.
Table 2.
TMA must be suspected with the triad of anemia, thrombocytopenia and target organ injury.
| TMA characters | Description |
|---|---|
| Microangiopathic hemolytic anemia | Anemia, decreased haptoglobin, elevated lactate dehydrogenase, schistocytes on peripheral blood smear. |
| Thrombocytopenia | Platelets <150,000 or 25% decline from baseline. |
| Target organ injury | Azotemia, hematuria, proteinuria or elevated liver enzymes or elevated amylase and lipase. |
TMA: thrombotic microangiopathy.
Recently, investigators have provided the information to distinguish various forms of TMAs from one and another (Table 3).10,11 All three forms of TMAs present with anemia, thrombocytopenia and target organ injury. For reasons not entirely known, TTP does not usually cause severe renal failure while aHUS typically does. Similarly, TTP traditionally (not always) has been associated with mental status change and fever. From the laboratory standpoint, identification of Shiga toxin is required to make the diagnosis of STEC-HUS.13,14 On the other hand, TTP is diagnosed when the activity of ADAMTS13 is found to be <5%–10%.6,8 In contrast, aHUS is a diagnosis of exclusion. Normal activity of ADAMTS13 (>10%) and absence of Shiga toxin, in the setting of TMA, helps to establish the diagnosis of aHUS.13,16 Because the pathophysiology of aHUS is driven by the uncontrolled activation of the alternative pathway of the complement system, low C3 and normal C4 concentrations can be observed in patients with aHUS. However, a decreased serum C3 level is not specific for the diagnosis of aHUS.13 At the same time, the demonstration of normal C3 and C4 concentrations does not exclude aHUS.13,16 A limitation of the diagnostic approach of a patient with TMA is that the activity of ADAMTS13 result may take a few days to be available. A close follow-up and aggressive approach to obtaining the results of ADAMTS13 are needed to promptly establish the correct diagnosis. In addition, ADAMTS13 should be drawn before the institution of plasma therapy. It is important to reiterate that at present a single confirmatory test to establish the diagnosis of aHUS does not exist. Normal activity of ADAMTS13 and absence of Shiga toxin help establish the diagnosis of aHUS by excluding TTP and STEC-HUS.8,10,13,16
Table 3.
Differentiating features between the three types of TMA.
| Laboratory parameter | TTP | STEC-HUS | aHUS |
|---|---|---|---|
| Anemia | + | + | + |
| Thrombocytopenia | + | + | + |
| Schistocytes | + | + | + |
| Elevated creatinine | +/− | + | + |
| Activity of ADAMTS13 | <10% | >10% | >10% |
| Shiga toxin | – | + | – |
| Complement level | Normal | Normal | Low/Normal |
+: positive; −: negative.
TMA: thrombotic microangiopathy; TTP: thrombotic thrombocytopenic purpura; STEC-HUS: Shiga toxin-related hemolytic uremic syndrome; aHUS: atypical hemolytic uremic syndrome.
It is worth mentioning that novel biomarkers of cellular processes are emerging and may serve in the diagnosis and overall management of patients with aHUS. In this regard, investigators have reported that urinary C5a and C5b-9 are significantly elevated in patients with aHUS.21, 22, 23 In addition, the markers of endothelial damage (thrombomodulin), as well as endothelial activation (VCAM-1), were also found to be significantly increased in aHUS patients.21, 22, 23 At present, these laboratory tests are not readily available. Tissue analysis (renal biopsy) can help to establish the diagnosis of aHUS. However, the performance of renal biopsy in the trauma patient with severe anemia, thrombocytopenia must be carefully evaluated in consultation with a nephrologist. Finally, genetic testing in identifying patients with complement abnormalities is gaining popularity.24 Because all of the genetic abnormalities have not been identified, its role in confirming/making the diagnosis of aHUS has not been conclusively established.
Management of TMA in the setting of trauma
Advances in our understanding of the complement system and the widespread availability of ADAMTS13 have revolutionized our approach to the management of TTP and aHUS. Because the pathophysiology of the two entities is driven by two distinctly different mechanisms, an appropriate diagnosis is critically needed to provide a targeted therapy. Autoantibodies against ADAMTS13 limit the cleavage of the von-Willebrand factor, which results in widespread microvascular thrombosis, MAHA, anemia, thrombocytopenia and target organ injury.15,26 Plasmapheresis removes the autoantibodies and allows ADAMTS13 to cleave the large multimers of the von-Willebrand factor. In contrast, aHUS is driven by uncontrolled activation of the alternative pathway of the complement system. This syndrome requires a complement blockade with a monoclonal antibody (eculizumab) to manage aHUS.21, 22, 23
Two patients having trauma-related TMA were found to have clinical features of TMA, both of who had normal ADAMTS13 activity (which was consistent with aHUS) and improved after plasmapheresis (which was consistent with TTP).17,18 Why might this be the case in the two reported cases? There are a few possibilities. Transient improvement of thrombocytopenia and anemia post plasma exchange has been reported in aHUS.7 However, a great majority of these patients have been reported to progress to end-stage renal disease.7 We have no records for the long-term follow-up of the two reported patients. As suggested by the authors, it is conceivable that TMA was due to the production of unusually large von-Willebrand factor multimers that could not be processed even with normal ADAMTS13 levels. Perhaps plasma therapy provided augmentation to the existing ADAMTS13 activity by possibly removing the autoantibodies and fortifying existing ADAMTS13 activity by administering additional ADAMTS13 contained in the plasma that was being delivered to the patient. The fact remains that both cases demonstrated clear improvement with plasmapheresis despite normal ADAMTS13.26,27
Overall, the management of TMA in the setting of trauma is similar to treating TMA in any other setting, which aims at the treatment of the underlying CAC. A multidisciplinary approach involving a nephrologist and/or hematologist is crucial in preventing any irreversible events such as end-stage renal disease. In a patient suspected of having TMA, the following principles should be considered.21,22
-
1.
Promptly establish a team that includes a nephrologist and a hematologist in a trauma patient presenting with TMA, and establish effective communication.
-
2.
Order an ADAMTS13 test before starting the plasma therapy.
-
3.
Establish an aggressive follow-up in obtaining the results of ADAMTS13.
-
4.
In consultation with the nephrologist and hematologist, plasmapheresis as an empiric treatment is contemplated until TTP is ruled out. TTP carries a very high mortality (as high as 90% in the absence of plasmapheresis).21 In this context, plasmapheresis is lifesaving. Waiting for laboratory results can be detrimental. Plasmapheresis is the fastest way to remove ADAMTS13 autoantibodies.
-
5.
Monitor the response to plasmapheresis and share the progress with the team.
-
6.
Aim at preventing and treating other CACs which can co-exist in settings of trauma, the biggest among these is the sepsis. It can act as CAC on its own and it can also accelerate the progression of TMA in the setting of trauma.
-
7.
Corticosteroids are used in patients with TTP. However, the decision regarding their use should be made in consultation with the nephrologist and hematologist (as there can be limiting factors).22,23
-
8.
Rituximab is a monoclonal antibody targeting CD20 antigen on B lymphocytes. Its current use has been limited to the refractory TTP.27 There have been studies where rituximab has been used as a part of initial therapy with plasmapheresis but its role is still debatable.27
-
9.
Once TTP has been ruled out and the diagnosis of aHUS has been established, eculizumab should be considered to block the complement system. Eculizumab is a humanized monoclonal antibody that effectively binds to complement protein C5 and blocks the generation of C5a and the membrane attack complex (C5b-9), in which the results in prompt improvement of thrombocytopenia, anemia, and target organ injury. At present, to prevent ongoing damage to the target organs, early diagnosis and prompt treatment with eculizumab administered indefinitely are recommended. Multiple studies have conclusively established the efficacy and safety of eculizumab in the treatment of aHUS. Recombinant ADAMTS13 and anti-von-Willebrand therapies are some of the new emerging therapies.20,21,23, 24, 25
-
10.
At present supportive therapy is administered to patients with STEC-HUS. No date, no specific agent or intervention are available to combat this form of TMA.25
Conclusion
TMA can occur in patients presenting with trauma. In these patients, trauma surgeons stand on the first line of deference. Because TMA (TTP and aHUS) carries a high morbidity and mortality, its prompt identification and rapid treatment are needed to avoid target organ injury and reduce the death rate. Both nephrologists and hematologists should be a part of the trauma team addressing TMA patients. ADAMTS13 is critically important to establish an accurate diagnosis and must be ordered earlier on. Plasmapheresis and eculizumab are important therapies that have a major impact on limiting the target organ injury. The decision to choose these therapies and the timing of their intervention must be made after discussion with the entire team including the patient. Our algorithm for approaching TMA is detailed in Fig. 2 below.
Fig. 2.
Thrombotic Microangiopathy and its types.
Funding
Nil.
Ethics statement
The authors have no ethical conflicts to disclose.
Declaration of competing interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.George J.N., Nester C.M. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654–666. doi: 10.1056/NEJMra1312353. [DOI] [PubMed] [Google Scholar]
- 2.Moake J.L. Thrombotic microangiopathies. N Engl J Med. 2002;347:589–600. doi: 10.1056/NEJMra020528. [DOI] [PubMed] [Google Scholar]
- 3.Eskazan A.E., Buyuktas D., Soysal T. Postoperative thrombotic thrombocytopenic purpura. Surg Today. 2015;45:8–16. doi: 10.1007/s00595-013-0823-y. [DOI] [PubMed] [Google Scholar]
- 4.The American association for the surgery of trauma. Trauma facts. 2019 http://www.aast.org/trauma-facts [Google Scholar]
- 5.Choi E.J., Lee S. A postoperative thrombotic thrombocytopenic purpura in a cardiac surgery patient: a case report. Korean J Thorac Cardiovasc Surg. 2013;46:220–222. doi: 10.5090/kjtcs.2013.46.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold D.M., Patriquin C.J., Nazy I. Thrombotic microangiopathies: a general approach to diagnosis and management. CMAJ (Can Med Assoc J) 2017;189:153–159. doi: 10.1503/cmaj.160142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naqvi T.A., Baumann M.A., Chang J.C. Post-operative thrombotic thrombocytopenic purpura: a review. Int J Clin Pract. 2004;58:169–172. doi: 10.1111/j.1368-5031.2004.0080.x. [DOI] [PubMed] [Google Scholar]
- 8.Hassan S., Westwood J.P., Ellis D. The utility of ADAMTS13 in differentiating TTP from other acute thrombotic microangiopathies: results from the UK TTP Registry. Br J Haematol. 2015;171:830–835. doi: 10.1111/bjh.13654. [DOI] [PubMed] [Google Scholar]
- 9.Asif A., Nayer A., Haas C.S. Atypical hemolytic uremic syndrome in the setting of complement-amplifying conditions: case reports and a review of the evidence for treatment with eculizumab. J Nephrol. 2017;30:347–362. doi: 10.1007/s40620-016-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riedl M., Fakhouri F., Quintrec M.L. Spectrum of complement-mediated thrombotic microangiopathies: pathogenetic insights identifying novel treatment approaches. Semin Thromb Hemost. 2014;40:444–464. doi: 10.1055/s-0034-1376153. [DOI] [PubMed] [Google Scholar]
- 11.Asif A., Vaccharajani T., Salman L. A simplified approach to the diagnosis of atypical HUS: clinical considerations and practical implications. Open Urol Nephrol J. 2014;7:91–94. [Google Scholar]
- 12.Legendre C.M., Licht C., Muus P. Terminal complement inhibitor eculizumab in atypical hemolytic–uremic syndrome. N Engl J Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 13.Noris M., Mescia F., Remuzzi G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol. 2012;8:622–633. doi: 10.1038/nrneph.2012.195. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg R.J., Nakagawa T., Johnson R.J. The role of endothelial cell injury in thrombotic microangiopathy. Am J Kidney Dis. 2010;56:1168–1174. doi: 10.1053/j.ajkd.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crowther M.A., George J.N. Thrombotic thrombocytopenic purpura: 2008 update. Cleve Clin J Med. 2008;75:369–375. doi: 10.3949/ccjm.75.5.369. [DOI] [PubMed] [Google Scholar]
- 16.Scully M., Hunt B.J., Benjamin S. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. 2012;158:323–335. doi: 10.1111/j.1365-2141.2012.09167.x. [DOI] [PubMed] [Google Scholar]
- 17.Ikegami K., Yamagishi T., Tajima J. Post-traumatic thrombotic microangiopathy following pelvic fracture treated with transcatheter arterial embolization: a case report. J Med Case Rep. 2018;12:216. doi: 10.1186/s13256-018-1757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim K.H., Park J. Thrombotic thrombocytopenic purpura after blunt traumatic liver injury. Am J Emerg Med. 2016;34:939. doi: 10.1016/j.ajem.2015.09.040. [DOI] [PubMed] [Google Scholar]
- 19.Atefi G., Aisiku O., Shapiro N. Complement activation in trauma patients alters platelet function. Shock. 2016;46:83–88. doi: 10.1097/SHK.0000000000000675. [DOI] [PubMed] [Google Scholar]
- 20.Kapila A., Chhabra L., Chaubey V.K. Opana ER abuse and thrombotic thrombocytopenic purpura (TTP)-like illness: a rising risk factor in illicit drug users. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2013-203122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cofiell R., Kukreja A., Bedard K. Eculizumab reduces complement activation, inflammation, endothelial damage, thrombosis, and renal injury markers in aHUS. Blood. 2015;125:3253–3262. doi: 10.1182/blood-2014-09-600411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Legendre C.M., Muss C.L., Greenbaum L.A. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 23.Bomback A.S., Smith R.J., Barile G.R. Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin J Am Soc Nephrol. 2012;7:748–756. doi: 10.2215/CJN.12901211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noris M., Caprioli J., Bresin E. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5:1844–1859. doi: 10.2215/CJN.02210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nayer A., Asif A. Atypical hemolytic-uremic syndrome: the interplay between complements and the coagulation system. Iran J Kidney Dis. 2013;7:340–345. [PubMed] [Google Scholar]
- 26.Bresin E., Rurali E., Caprioli J. Combined complement gene mutations in atypical hemolytic uremic syndrome influence clinical phenotype. J Am Soc Nephrol. 2013;24:475–486. doi: 10.1681/ASN.2012090884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai Han-Mou. Thrombotic thrombocytopenic purpura: beyond empiricism and plasma exchange. Am J Med. 2019;132:1032–1037. doi: 10.1016/j.amjmed.2019.03.009. [DOI] [PubMed] [Google Scholar]