Abstract
Purpose
To compare ipsilateral breast event (IBE) risks in patients with ductal carcinoma in situ of the breast (DCIS) post-lumpectomy, as estimated by breast radiation oncologists, the Van Nuys Prognostic Index, the Memorial Sloan Kettering Cancer Center (MSKCC) DCIS nomogram, and the 12-gene Oncotype DX DCIS score assay.
Methods and Materials
Consecutive DCIS cases treated with lumpectomy from November 2011 to August 2014 with available DCIS score results were identified. Three radiation oncologists independently estimated the 10-year IBE risk. The Van Nuys Prognostic Index and MSKCC nomogram 10-year IBE risk estimates were generated. Differences and correlations between the IBE estimates and clinicopathologic factors were evaluated.
Results
Ninety-one patients were identified for inclusion. Forty-eight percent would have been ineligible for the E5194 study. The mean risk of IBE from the DCIS score assay was 12.4%, compared with a range of 18.9% to 26.8% from other sources. The mean IBE risk from the DCIS score assay was lower regardless of E5194 eligibility. The MSKCC nomogram and DCIS score assay risk estimates were weakly correlated with each other (P = .23) and were each moderately correlated with the other risk estimates (P = .41-.56). When applying the radiation oncologists’ treatment recommendations based on their proposed risk cutoffs, evaluating risk according to the DCIS score assay led to the highest proportion of patients recommended excision alone.
Conclusions
IBE risk estimates for this general community cohort of DCIS cases vary significantly among commonly available clinical predictive tools and individual radiation oncologist estimates. Surgical margins and tumor size continue to factor prominently in radiation oncologist decision algorithms. The differences found between the IBE risk estimate methods suggests that they are not interchangeable and the methods that rely on clinicopathologic features may tend to overestimate risk.
Introduction
Ductal carcinoma in situ (DCIS) encompasses a biologically and clinically heterogeneous spectrum of noninvasive or preinvasive intraductal lesions. With the widespread implementation of screening mammography, the frequency of DCIS now accounts for greater than 15% of all breast cancer diagnoses.1,2 Thirty percent of untreated DCIS lesions will progress to invasive breast cancer over a 30-year period.3,4 The optimal management of DCIS—a recognized, albeit nonobligate, precursor to invasive carcinoma—has been debated for the past 3 decades.
DCIS management philosophy differs widely among clinicians, with many treating DCIS in the same manner as early-stage invasive disease, including wide surgical excision, often with subsequent radiation therapy or antihormonal therapy, and others favoring surgery alone. Breast-conserving surgery without further treatment yields subsequent ipsilateral breast event (IBE) rates ranging from 15% to 60%, with invasive disease accounting for ~50% of IBEs.5
Determining the least aggressive treatment needed to minimize the risk of subsequent IBE in an individual patient remains the elusive holy grail of DCIS management. The Van Nuys Prognostic Index (VNPI) was developed to aid clinical decision-making by stratifying ipsilateral recurrence risk according to nuclear grade, presence of necrosis, margin width, and later age, to choose between excision alone, excision with radiation therapy, and mastectomy with the goal of <20% local recurrence at 12 years.6,7 The validity of VNPI remains controversial due to conflicting reports from external data sets.8, 9, 10, 11, 12, 13 In 2012, investigators from Memorial Sloan Kettering Cancer Center (MSKCC) published a DCIS nomogram that combines 10 clinicopathologic and treatment factors to predict IBE risk; it too, remains controversial due to variability of results at separate validation centers.14, 15, 16, 17, 18, 19
Molecular tests, including the 12-gene Oncotype DX DCIS score assay and the DCISionRT test, provide individualized assessments of patients’ IBE risk, based on the biology of the tumor.20,21 Although VNPI and the MSKCC DCIS nomogram derive a risk estimate for any IBE (in situ and invasive) from historical populational data, the DCIS score assay has been validated to provide individualized risk estimates for invasive and in situ IBE, based on quantitative RNA levels from formalin-fixed, paraffin-embedded tumor samples.
The radiation oncology community has been slow to adopt the DCIS score assay, owing largely to the extremely favorable characteristics of the clinical validation cohort, a subset from the E5194 cohort.20 E5194 patients had either low-or intermediate-grade DCIS with tumor ≤2.5 cm or high-grade DCIS with tumor ≤1 cm. Additionally, all E5194 patients had a minimum negative surgical margin of 3 mm. There remains uncertain applicability outside the E5194 entry criteria, as well as the sizable uncertainty in the high-risk group. To our knowledge, no study has compared the predictions from the DCIS score assay to the estimates from either the MSKCC nomogram or the VNPI. Although the initial DCIS score assay clinical validation study did not find VNPI to be predictive, that lack of predictive power is not unexpected, given that VNPI is heavily influenced by margin status and 77% of the E5194 validation cohort had margins of 1 to 9 mm.22
We performed an exploratory analysis in an unselected clinical population, comparing 10-year post-lumpectomy IBE risk as estimated by the VNPI and the MSKCC DCIS nomogram as well as estimates generated by the DCIS score assay and 3 experienced breast radiation oncologists, to answer the following questions: Do the VNPI, MSKCC DCIS nomogram, or experienced physician judgment offer similar risk estimates as the DCIS score assay? Do the VNPI and the MSKCC DCIS nomogram offer similar or different clinical advice? How might the physician’s choice of clinical tool or risk-estimation algorithm affect clinical management?
Methods and Materials
Study data set
Patients who underwent lumpectomy between November 2011 and August 2014 with an available 12-gene DCIS Score result and who were consented to one of 2 IRB-approved protocols (NCT01185145 and NCT01185132) were identified in clinical practice; cases with any invasive (including microinvasive) disease were excluded. The IBE risk estimates provided by the DCIS score assay used the patient-specific meta-analysis method that refined risk estimates by incorporating the DCIS score result with the independent risk factors identified both in E5194 and the Ontario validation studies, that is, patient age, tumor size, and year of diagnosis.23
Clinicopathologic factors, excluding DCIS score result, were summarized for blinded review. Three experienced breast radiation oncologists from our practice (A.G.A., C.E.L., and D.L.C.)—representing nonoverlapping multidisciplinary tumor boards and a range of usage experience and confidence in the DCIS score assay—then independently estimated the 10-year risks for any IBE without knowledge of the DCIS score result; they were also surveyed for the effect (on a scale of 1-5) of age and menopausal status at DCIS diagnosis, tumor morphology (nuclear grade and presence of necrosis), tumor span, and margin width on their risk estimates.
Traditional risk estimates were obtained from the MSKCC nomogram and VNPI. The MSKCC 10-year IBE risk estimates were generated with the online tool based on the previously published study.24 The MSKCC risk estimates were independently entered by 2 people and discrepancies were adjudicated by a third person. IBE risk at 10 years after excision alone according to the VNPI was estimated graphically from Figures 8 to 10 from a previous publication of Silverstein et al.6
The 3 radiation oncologists were surveyed for 10-year IBE risk cutoffs that would prompt different modes of treatment based solely on their general practice recommendations. Factors such as comorbidities, life expectancy, and patient or family preference were not included in this assessment. The treatment recommendations associated with the DCIS score risk estimates were categorized using the radiation oncologists’ cutoffs, and the resulting treatment recommendations were assessed for agreement.
Statistical methods
Descriptive statistics were generated for the demographic, clinical, and pathologic variables, both overall and stratified by the patients’ E5194 eligibility status. The mean 10-year risk estimates of any IBE were calculated, along with 2-sided 95% confidence intervals, which were also stratified by E5194 eligibility status.
Spearman’s rank correlation coefficient was calculated to assess the degree of the monotonic relationships between the 10-year risk of any IBE as estimated by each source, stratified by E5194 eligibility status. In addition, each radiation oncologist’s any-IBE risk estimates and the indicated effect of the corresponding subject age, tumor morphology (ie, nuclear grade and presence of necrosis), tumor span, and margin size were evaluated to assess their correlations.
Analyses were performed using SAS software version 9.4 of the SAS System for Windows and R version 3.5.1.25 Graphics were created using the ggplot2 package.26
Results
Ninety-one cases were identified, with demographic and disease characteristics summarized in Table 1. Nearly half of the cases (44 out of 91) would not have been eligible for E5194. The E5194-eligible patients tended to have smaller total DCIS span and larger margin size. Median patient age at diagnosis in the E5194-ineligible cohort was higher, but the interquartile and overall ranges were similar.
Table 1.
Patient characteristics for the entire cohort and by E5194 eligibility
| All (N = 91) | E5194 eligible∗ (n = 47) | E5194 ineligible (n = 44) | |
|---|---|---|---|
| Age at diagnosis | |||
| Median (IQR) | 60 (52-67) | 56 (52-69) | 63 (53-67) |
| Range | 37-85 | 37-85 | 42-81 |
| <50 years | 16 (17.6%) | 8 (17.0%) | 8 (18.2%) |
| ≥50 years | 75 (82.4%) | 39 (83.0%) | 36 (81.8%) |
| Family history | |||
| Yes | 50 (54.9%) | 25 (53.2%) | 25 (56.8%) |
| No | 41 (45.1%) | 22 (46.8%) | 19 (43.2%) |
| Presentation | |||
| Radiologic/imaging | 88 (96.7%) | 47 (100%) | 41 (93.2%) |
| Clinical/palpable | 3 (3.3%) | 0 (0.0%) | 3 (6.8%) |
| Menopausal status at study entry | |||
| Pre/peri | 30 (33.0%) | 17 (36.2%) | 13 (29.5%) |
| Post | 61 (67.0%) | 30 (63.8%) | 31 (70.5%) |
| Highest nuclear grade | |||
| Low | 12 (13.2%) | 9 (19.1%) | 3 (6.8%) |
| Intermediate | 52 (57.1%) | 30 (63.8%) | 22 (50.0%) |
| High | 27 (29.7%) | 8 (17.0%) | 19 (43.2%) |
| Presence of necrosis | |||
| Present | 54 (59.3%) | 27 (57.4%) | 27 (61.4%) |
| Not present | 37 (40.7%) | 20 (42.6%) | 17 (38.6%) |
| Total span of DCIS†, mm | |||
| Median (IQR) | 10 (5-22) | 7 (5-13) | 20 (10-30) |
| Range | 2-115 | 3-25 | 2-115 |
| ≤5 | 24 (26.4%) | 18 (38.3%) | 6 (13.6%) |
| >5-10 | 23 (25.3%) | 17 (36.2%) | 6 (13.6%) |
| > 10-30 | 36 (39.6%) | 12 (25.5%) | 24 (54.5%) |
| > 30 | 8 (8.8%) | 0 (0.0%) | 8 (18.2%) |
| Margin size (mm) | |||
| Median (IQR) | 5 (3 – 10) | 8 (5 – 10) | 2.3 (1 – 6) |
| Range | 0-20 | 3-20 | 0-16 |
| 0 (ie, positive) | 2 (2.2%) | 0 (0.0%) | 2 (4.5%) |
| >0 to <1 | 8 (8.8%) | 0 (0.0%) | 8 (18.2%) |
| 1 to <3 | 13 (14.3%) | 0 (0.0%) | 13 (29.5%) |
| 3 to <5 | 13 (14.3%) | 9 (19.1%) | 4 (9.1%) |
| 5 to <10 | 26 (28.6%) | 17 (36.2%) | 9 (20.5%) |
| ≥10 | 29 (31.9%) | 21 (44.7%) | 8 (18.2%) |
| Estrogen receptor status | |||
| Positive | 83 (91.2%) | 43 (91.5%) | 40 (90.9%) |
| Negative | 7 (7.7%) | 3 (6.4%) | 4 (9.1%) |
| Not performed | 1 (1.1%) | 1 (2.1%) | 0 (0.0%) |
| Number of surgical excisions | |||
| 1 | 60 (65.9%) | 36 (76.6%) | 24 (54.5%) |
| 2 | 30 (33.0%) | 10 (21.3%) | 20 (45.5%) |
| 3 | 1 (1.1%) | 1 (2.1%) | 0 (0.0%) |
Abbreviations: DCIS = ductal carcinoma in situ; IQR = interquartile range.
There were 3 cases of DCIS <3 mm with margin ≥3 mm that presented radiologically. E5194 eligibility criteria specified nonpalpable DCIS 3 mm or larger with margins of at least 3 mm after breast-conserving surgery. Tumor size was limited to 2.5 cm or smaller for tumors of low or intermediate histologic grade, and 1 cm or smaller for tumors of high histologic grade.
In cases of microscopically multifocal disease, total span included all foci and any intervening distance.
Differences and correlations in numerical estimates
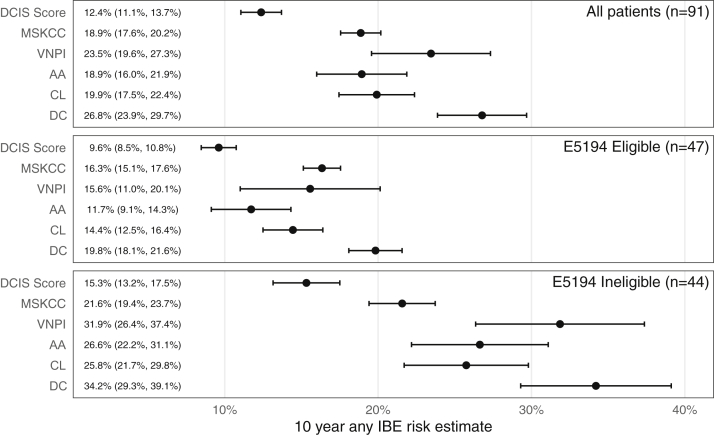
Mean 10-year risk estimates of any IBE from each source are shown in Figure 1. For the overall cohort, the mean of the risk estimates from the DCIS score assay was 12.4%, compared with a range of 18.9% to 26.8% for the other risk estimate sources. For all sources, the mean of the risk estimates was lower among the E5194 eligible subjects and higher among the E5194 ineligible subjects, and the confidence intervals in the E5194 eligible cohort were narrower than the ineligible cohort. The mean risk estimate for the DCIS score assay remained lower than those from the other sources in both the E5194 eligible and ineligible subjects.
Figure 1.
Mean risk of any ipsilateral breast event at 10 years by source of estimate and E5194 eligibility. Sources of estimates are the 12-gene ductal carcinoma in situ score assay, Memorial Sloan Kettering Cancer Center ductal carcinoma in situ nomogram, Van Nuys Prognostic Index, and 3 radiation oncologists (A.G.A., C.E.L., and D.L.C.). Means are represented by points with error bars displaying the 95% confidence interval and are annotated on the left side of the graph.
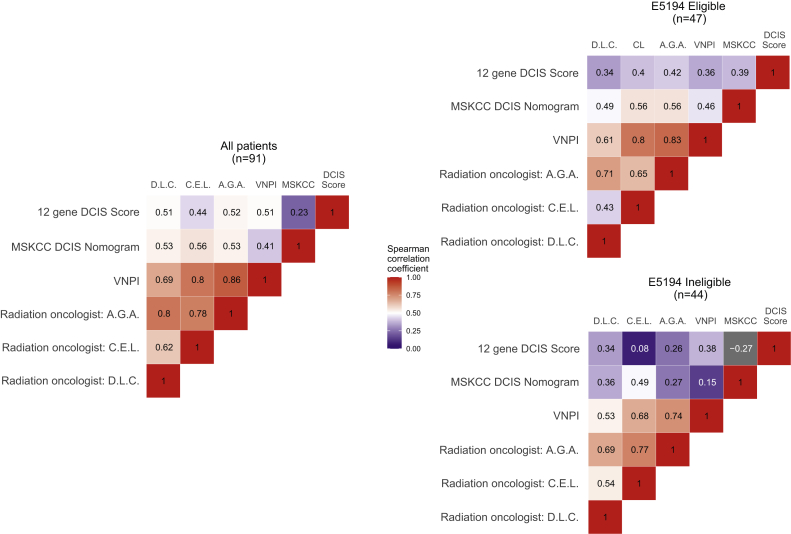
Results of the Spearman correlations of 10-year any-IBE risk estimates are shown as heat maps in Figure 2, both overall and by E5194 eligibility. In the heat maps, strong correlations (P > .7) have darker hues of red, weak correlations (P < .3) have darker shades of blue, and moderate correlations (P between .3 and .7) are lighter shades of red and blue. All correlations were positive, with the exception of the DCIS score assay and the MSKCC nomogram in the E5194 ineligible group, which had a weakly negative correlation (P = –.27).
Figure 2.
Heatmap of Spearman correlations (P coefficients) of risk estimates for any ipsilateral breast event at 10 years, overall, and by E5194 eligibility. For this analysis, strong correlations were classified as P > .7, moderate correlations had P between .3 and .7, and weak had P < .3. Abbreviations: MSKCC = Memorial Sloan Kettering Cancer Center; VNPI = Van Nuys Prognostic Index; A.G.A., C.E.L., and D.L.C.: radiation oncologists.
Factors affecting IBE risk estimates
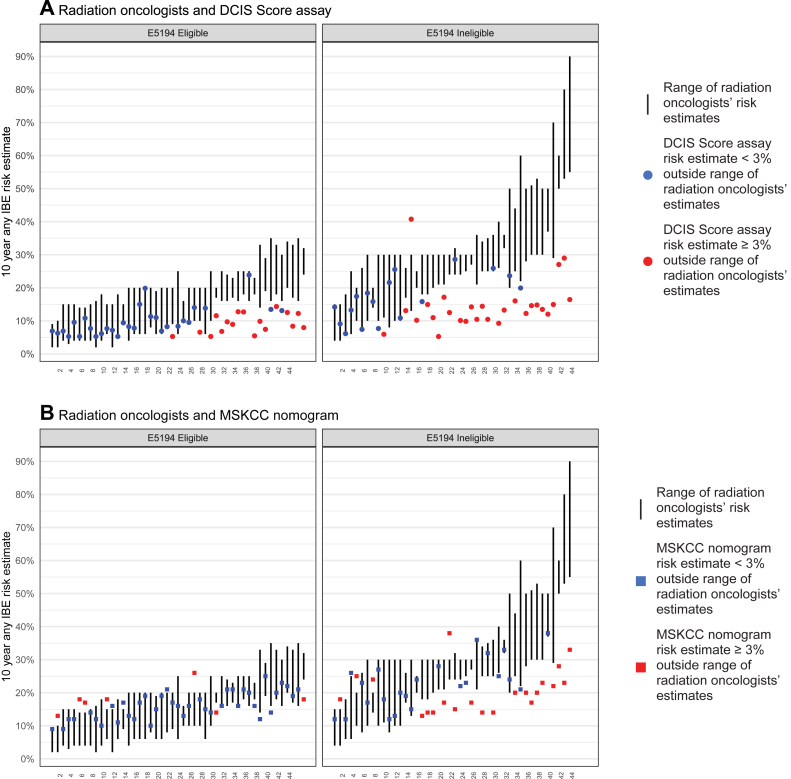
Figure 3 displays a comparison of (A) the 10-year any-IBE risk estimates for the DCIS score assay and (B) the MSKCC nomogram with the range of radiation oncologist risk estimates, by E5194 eligibility. In 44 of 91 cases evaluated, the DCIS score assay 10-year any-IBE risk estimate was outside the range of the corresponding radiation oncologist estimates by at least 3% (eg, the radiation oncologists estimated 25%, 34%, and 35% and the DCIS score assay estimated ≤22% or ≥38%). Within these 44 cases, 27 cases were E5194 ineligible, and the differences were largely in the same direction, with 43 of 44 cases having DCIS score risk estimates below the range of corresponding radiation oncologist estimates.
Figure 3.
10-year any-IBE risk for individual patients by E5194 eligibility as estimated by radiation oncologists and (A) ductal carcinoma in situ score result and (B) Memorial Sloan Kettering Cancer Center nomogram. Individual patients are on the x axis. The vertical lines represent the range of 10-year any-IBE risk estimated by the 3 radiation oncologists. The points represent the 10-year any-IBE risk estimated by the (A) ductal carcinoma in situ score result and (B) Memorial Sloan Kettering Cancer Center nomogram. Red points are ≥3% outside the range of the radiation oncologists’ estimates, blue points are <3% outside the range of the radiation oncologists’ estimates. Abbreviation: IBE = ipsilateral breast event.
The radiation oncologists’ 10-year any-IBE risk estimates were moderately correlated with tumor span (P = .41-.62) and weakly to moderately correlated with presence of necrosis (P = .23-.44) and nuclear grade (P = .29-0.54). The radiation oncologists’ risk estimates had a weak inverse correlation with age at diagnosis (P = −.08 to −.21), but a moderate to high inverse correlation with margin size (P = −.39 to −.75).
The 3 radiation oncologists also self-evaluated the effect (on a scale of 1-5) of age and menopausal status at DCIS diagnosis, tumor morphology (nuclear grade and presence of comedo necrosis), tumor length, and margin width on their IBE risk estimates for each case. The ratings showed similar weight given to these factors, with margin width receiving the highest rating by all 3 radiation oncologists, then tumor morphology, followed closely by tumor length, with age receiving the lowest rating.
Comparison of treatment recommendations
The 3 radiation oncologists (A.G.A., C.E.L., and D.L.C.) were surveyed to propose cutoffs for 10-year estimates of any IBE risk that would prompt various treatment recommendations. The proposed cutoffs are presented in Table 2, by oncologist. Radiation oncologists A.G.A. and C.E.L., created a 3-category set of treatment recommendations. D.L.C. had an additional treatment recommendation category between excision alone and excision with radiation. The radiation oncologists had different risk estimate cutoffs for a treatment recommendation of excision alone with <16%, <13%, and <10% for A.G.A., C.E.L., and D.L.C., respectively, but all 3 recommended re-excision or mastectomy for risk estimates at or above 40%.
Table 2.
10-year any-IBE risk estimate cutoffs for different modes of treatment as recommended by the radiation oncologists (A.G.A., C.E.L., and D.L.C.)
| Radiation oncologist |
|||
|---|---|---|---|
| A.G.A. | C.E.L. | D.L.C. | |
| Treatment recommendation | |||
| Excision alone, % | <16 | <13 | <10 |
| Consider excision alone vs excision with radiation, % | 10-20 | ||
| Excision with radiation, % | 16-39 | 13-39 | 21-39 |
| Re-excision or mastectomy, % | ≥40 | ≥40 | ≥40 |
Abbreviation: IBE = ipsilateral breast event.
Table 3 assigns each patient to a treatment based on the cutoffs proposed in Table 2. Treatment recommendations based on DCIS score assay were categorized separately using the risk estimate cutoffs from A.G.A. and D.L.C. to accommodate the different number of treatment categories. Comparing the 3-category results, the risk of any IBE as estimated by the DCIS score assay led to 62% of patients recommended excision alone, compared with 45% and 40% for A.G.A. and C.E.L. For the 4-category results, 90% of the patients were in the recommended treatment categories “excision alone” or “consider excision alone versus excision with radiation,” based on their DCIS score result risk estimate compared with 46% for D.L.C. In 78% (71 out of 91 cases) there was a consensus treatment recommendation among the radiation oncologists A.G.A. and C.E.L.: 32 for excision alone, 36 for excision with radiation, and 3 for further surgery or mastectomy. In 45% (41 out of 91 cases), the treatment recommendation was consistent across risk estimates based on the DCIS score result and radiation oncologists A.G.A. and C.E.L. In 23% (21 out of 91 cases), the treatment associated with the risk estimate provided by the DCIS score result agreed with that for the estimate by the radiation oncologist D.L.C. Among the 41 cases where the treatment recommendation for A.G.A., C.E.L., and the DCIS score assay was concordant, 25 (61%) were E5194 eligible, and for the 21 concordant recommendations for D.L.C. and the DCIS score assay, 12 (57%) were E5194 eligible.
Table 3.
Number of patients (with column percentages) in each treatment category using the any-IBE risk estimate cutoffs for each radiation oncologist (A.G.A., C.E.L., and D.L.C.) in Table 2.
| Source of estimate |
|||
|---|---|---|---|
| Radiation oncologist: A.G.A. | Radiation oncologist: C.E.L. | DCIS score assay | |
| Treatment recommendation (3-category), n (%) | |||
| Excision alone | 41 (45) | 36 (40) | 56 (62) |
| Excision with radiation | 42 (46) | 50 (55) | 34 (37) |
| Re-excision or mastectomy | 8 (9) | 5 (5) | 1 (1) |
| Source of estimate |
|||
|---|---|---|---|
| Radiation oncologist: D.L.C. | DCIS score assay | ||
| Treatment recommendation (4-category), n (%) | |||
| Excision alone | 1 (1) | 38 (42) | |
| Consider excision alone vs excision with radiation | 41 (45) | 44 (48) | |
| Excision with radiation | 38 (42) | 8 (9) | |
| Re-excision or mastectomy | 11 (12) | 1 (1) | |
Abbreviation: DCIS = ductal carcinoma in situ.
Treatment recommendations based on the 12-gene DCIS score assay were categorized using the A.G.A. risk estimate cutoffs for 3-category recommendations and D.L.C. risk estimate cutoffs for 4-category recommendations.
Discussion
We hypothesized that if different methods of IBE risk assessment result in similar IBE estimates, this would imply similarity or reliable reproducibility between methods. Subsequently, if the risk estimates were highly correlated among methods, it would suggest that the methods are interchangeable. Neither case was observed in this study. Instead, there was a range of estimates across the various methods of calculating risk, and there was varying agreement between the 10-year IBE risk estimates of the MSKCC DCIS calculator, the VNPI nomogram, radiation oncologists, and the DCIS score assay.
Regardless of clinicopathologic factors such as age at diagnosis, extent of surgery, use of tamoxifen, method of DCIS detection, margin status, focality, grade, comedo necrosis, architecture, or disease span, adjuvant radiation therapy consistently reduces the risk of a subsequent IBE (in situ and invasive) by half or more.27, 28, 29, 30, 31 The dilemma at the core of the debate is essentially whether to err on the side of overtreatment or undertreatment. Clinicians who advocate for a less aggressive prophylactic approach cite the lack of survival benefit, coupled with the observation that ~70% of women with small (≤20 mm), low-grade DCIS and negative margins remained disease-free at 10 years after surgery alone, to suggest that radiation therapy for all DCIS cases may represent significant overtreatment.32,33
On the other hand, undertreatment remains a significant concern even for patients with low-grade tumors. One retrospective series that identified DCIS in biopsies originally thought to be benign highlighted this issue. Invasive carcinoma developed in almost 40% of the women (mostly within 10 years), and 5 women (17.9%) eventually died of metastatic breast cancer.34 Whether this cautionary tale from the pre-mammography era is generalizable to the low-grade DCIS tumors detected by imaging today remains a relevant question.
Although there is a general consensus that adjuvant radiation therapy does not confer any survival or mortality advantages, a simulated model integrating clinical events as reported by published studies (including the UK/ANZ study) suggests that the addition of adjuvant radiation and tamoxifen increases overall survival by 12 months (for women aged 45 years at diagnosis) relative to lumpectomy alone, while radiation therapy alone increases survival by only 6 months.35 In addition, Sagara et al have demonstrated that patients with a combination of poor prognostic variables did gain a survival benefit with adjuvant breast radiation therapy.36 The benefit of radiation may also be greater in women older than age 50 than in younger women, although the simulated model suggests the survival benefit remains similar between the age groups, with radiation improving survival by 4 months in women aged 45 years and 5 months in women aged 60 years.33,35 Supporters of the adjuvant treatment approach point to the fact that the majority of IBEs occurring inside the index quadrant reinforces the adjuvant approach of treating residual cancer cells, even in the presence of clear margins, with local treatment. Further, they contend that the use of tamoxifen does not eliminate the need for radiation therapy because studies have found that ~15% of patients who experienced an invasive IBE developed subsequent metastases.5,37, 38, 39, 40, 41, 42
Strengths of this study include the diversity of methods employed and the number of participating radiation oncologists. In addition to traditional methods of calculating risk and radiation oncologist risk estimates, we obtained molecular test results from the DCIS score assay. This tool has been clinically validated as an objective, analytical, and reproducible prognostic tool for estimating 10-year ipsilateral breast recurrence risk in 2 separate studies.20,22 Limitations of this study include the small number of patients and the lack of clinical outcomes.
Despite the results from the 2 clinical validation studies of the DCIS score assay, physicians continue to advocate for the use of the MSKCC DCIS nomogram or VNPI as no-additional-cost methods to replicate the DCIS score risk estimates. The results presented in this study show that these alternative methods and radiation oncologist estimates do not strongly correlate with IBE risk as determined by the DCIS score assay. Our study found that the only strong correlation between methods (P > .7) was observed between radiation oncologist estimates and the VNPI estimates. This may indicate the VNPI was used by the radiation oncologists in this study for their assessment of IBE risk, as they calculated their estimates using the tools in their usual clinical practice without knowledge of the DCIS score result. Importantly, our data show how the traditional and radiation oncologist risk estimates overestimate risk as unfavorable clinicopathologic factors increase compared with the DCIS score estimates, leading to different treatment recommendations (Table 3). This finding may explain how use of the DCIS score results led to a reduction in adjuvant breast radiation therapy recommendations in 2 clinical utility studies.43,44
Conclusions
In conclusion, we have shown how use of the DCIS score assay provides independent IBE risk estimates compared with traditional methods of calculating risk and radiation oncologist risk estimates. When clinicopathologic risk factors increase, traditional methods of calculating risk and radiation oncologist risk assessments overestimate the risk of recurrence compared with DCIS score risk estimates. Traditional methods of calculating risk and radiation oncologist risk estimates are not highly associated with the DCIS score assay risk estimates and are not interchangeable.
Footnotes
Sources of support: This work was supported by the Department of Radiation Oncology, Rocky Mountain Cancer Centers, and Genomic Health, Inc (now Exact Sciences Corporation).
Disclosures: Dr Leonard served on the speaker’s bureau with Genomic Health, Inc (now Exact Sciences Corporation). Mr Bennett, Ms Turner, and Dr Baehner report employment and stock ownership, Exact Sciences Corporation. All other authors have no disclosures to declare.
References
- 1.Rosner D., Bedwani R.N., Vana J., Baker H.W., Murphy G.P. Noninvasive breast carcinoma: results of a national survey by the American College of Surgeons. Ann Surg. 1980;192:139–147. doi: 10.1097/00000658-198008000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeSantis C.E., Ma J., Gaudet M.M. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 3.Dupont W.D., Page D.L. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312:146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- 4.Page D.L., Dupont W.D., Rogers L.W., Landenberger M. Intraductal carcinoma of the breast: follow-up after biopsy only. Cancer. 1982;49:751–758. doi: 10.1002/1097-0142(19820215)49:4<751::aid-cncr2820490426>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B., Dignam J., Wolmark N. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998;16:441–452. doi: 10.1200/JCO.1998.16.2.441. [DOI] [PubMed] [Google Scholar]
- 6.Silverstein M.J. The University of Southern California/Van Nuys prognostic index for ductal carcinoma in situ of the breast. Am J Surg. 2003;186:337–343. doi: 10.1016/s0002-9610(03)00265-4. [DOI] [PubMed] [Google Scholar]
- 7.Silverstein M.J., Lagios M.D. Choosing treatment for patients with ductal carcinoma in situ: Fine tuning the University of Southern California/Van Nuys Prognostic Index. J Natl Cancer Inst Monogr. 2010;2010:193–196. doi: 10.1093/jncimonographs/lgq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Mascarel I., Bonichon F., MacGrogan G. Application of the Van Nuys Prognostic Index in a retrospective series of 367 ductal carcinomas in situ of the breast examined by serial macroscopic sectioning: Practical considerations. Breast Cancer Res Treat. 2000;61:151–159. doi: 10.1023/a:1006437902770. [DOI] [PubMed] [Google Scholar]
- 9.Boland G.P., Chan K.C., Knox W.F., Roberts S.A., Bundred N.J. Value of the Van Nuys Prognostic Index in prediction of recurrence of ductal carcinoma in situ after breast-conserving surgery. Br J Surg. 2003;90:426–432. doi: 10.1002/bjs.4051. [DOI] [PubMed] [Google Scholar]
- 10.Asjoe F.T., Altintas S., Huizing M.T. The value of the Van Nuys Prognostic Index in ductal carcinoma in situ of the breast: a retrospective analysis. Breast J. 2007;13:359–367. doi: 10.1111/j.1524-4741.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- 11.MacAusland S.G., Hepel J.T., Chong F.K. An attempt to independently verify the utility of the Van Nuys Prognostic Index for ductal carcinoma in situ. Cancer. 2007;110:2648–2653. doi: 10.1002/cncr.23089. [DOI] [PubMed] [Google Scholar]
- 12.Gilleard O., Goodman A., Cooper M., Davies M., Dunn J. The significance of the Van Nuys prognostic index in the management of ductal carcinoma in situ. World J Surg Oncol. 2008;6:61. doi: 10.1186/1477-7819-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim T., Park H.K., Lee K.H. Is radiotherapy necessary for intermediate risk ductal carcinoma in situ after breast conserving surgery? Springerplus. 2014;3:405. doi: 10.1186/2193-1801-3-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudloff U., Jacks L.M., Goldberg J.I. Nomogram for predicting the risk of local recurrence after breast-conserving surgery for ductal carcinoma in situ. J Clin Oncol. 2010;28:3762–3769. doi: 10.1200/JCO.2009.26.8847. [DOI] [PubMed] [Google Scholar]
- 15.Mazouni C., Delaloge S., Rimareix F., Garbay J.R. Nomogram for risk of relapse after breast-conserving surgery in ductal carcinoma in situ. J Clin Oncol. 2011;29:e44. doi: 10.1200/JCO.2010.32.3717. author reply e45-46. [DOI] [PubMed] [Google Scholar]
- 16.Ballehaninna U.K., Chamberlain R.S. Inclusion of tumor biology molecular markers to improve the ductal carcinoma in situ ipsilateral breast tumor recurrence nomogram predictability. J Clin Oncol. 2011;29:e97–e98. doi: 10.1200/JCO.2010.32.6850. author reply e99. [DOI] [PubMed] [Google Scholar]
- 17.Yi M., Meric-Bernstam F., Kuerer H.M. Evaluation of a breast cancer nomogram for predicting risk of ipsilateral breast tumor recurrences in patients with ductal carcinoma in situ after local excision. J Clin Oncol. 2012;30:600–607. doi: 10.1200/JCO.2011.36.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sweldens C., Peeters S., van Limbergen E. Local relapse after breast-conserving therapy for ductal carcinoma in situ: a European single-center experience and external validation of the Memorial Sloan-Kettering Cancer Center DCIS nomogram. Cancer J. 2014;20:1–7. doi: 10.1097/PPO.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 19.Wang F., Li H., Tan P.H. Validation of a nomogram in the prediction of local recurrence risks after conserving surgery for Asian women with ductal carcinoma in situ of the breast. Clin Oncol (R Coll Radiol) 2014;26:684–691. doi: 10.1016/j.clon.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Solin L.J., Gray R., Baehner F.L. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;105:701–710. doi: 10.1093/jnci/djt067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bremer T., Whitworth P., Patel R. A biologic signature for breast ductal carcinoma in situ to predict radiation therapy (RT) benefit and assess recurrence risk. Clin Cancer Res. 2018;24:5895–5901. doi: 10.1158/1078-0432.CCR-18-0842. [DOI] [PubMed] [Google Scholar]
- 22.Rakovitch E., Nofech-Mozes S., Hanna W. A population-based validation study of the DCIS Score predicting recurrence risk in individuals treated by breast-conserving surgery alone. Breast Cancer Res Treat. 2015;152:389–398. doi: 10.1007/s10549-015-3464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rakovitch E., Gray R., Baehner F.L. Refined estimates of local recurrence risks and the impact of the DCIS score adjusting for clinico-pathological features: Meta-analysis of E5194 and Ontario DCIS cohort studies. 2017 ASCO. Annual Meeting. 2017 doi: 10.1007/s10549-018-4693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Memorial Sloan Kettering Cancer Center Breast Cancer Nomogram: Ductal Carcinoma In Siture (DCIS) Recurrence. http://nomograms.mskcc.org/Breast/DuctalCarcinomaInSituRecurrencePage.aspx Available at:
- 25.R Core Team . 2018. R: A language and environment for statistical computing. [Google Scholar]
- 26.Wickham H. Springer-Verlag; New York: 2016. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- 27.Fisher E.R., Dignam J., Tan-Chiu E. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17: Intraductal carcinoma. Cancer. 1999;86:429–438. doi: 10.1002/(sici)1097-0142(19990801)86:3<429::aid-cncr11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 28.EORTC Breast Cancer Cooperative Group, EORTC Radiotherapy Group. Bijker N. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853—a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24:3381–3387. doi: 10.1200/JCO.2006.06.1366. [DOI] [PubMed] [Google Scholar]
- 29.Holmberg L., Garmo H., Granstrand B. Absolute risk reductions for local recurrence after postoperative radiotherapy after sector resection for ductal carcinoma in situ of the breast. J Clin Oncol. 2008;26:1247–1252. doi: 10.1200/JCO.2007.12.7969. [DOI] [PubMed] [Google Scholar]
- 30.Cuzick J., Sestak I., Pinder S.E. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: Long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12:21–29. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCormick B., Moughan J., Hudis C. Low-risk breast ductal carcinoma in situ (DCIS): Results from the Radiation Therapy Oncology Group 9804 phase 3 trial. Int J Radiat Oncol Biol Phys. 2012;84:S5. [Google Scholar]
- 32.Punglia R.S., Schnitt S.J., Weeks J.C. Treatment of ductal carcinoma in situ after excision: would a prophylactic paradigm be more appropriate? J Natl Cancer Inst. 2013;105:1527–1533. doi: 10.1093/jnci/djt256. [DOI] [PubMed] [Google Scholar]
- 33.Early Breast Cancer Trialists’ Collaborative Group. Correa C., McGale P. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010:162–177. doi: 10.1093/jncimonographs/lgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanders M.E., Schuyler P.A., Dupont W.D., Page D.L. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005;103:2481–2484. doi: 10.1002/cncr.21069. [DOI] [PubMed] [Google Scholar]
- 35.Soeteman D.I., Stout N.K., Ozanne E.M. Modeling the effectiveness of initial management strategies for ductal carcinoma in situ. J Natl Cancer Inst. 2013;105:774–781. doi: 10.1093/jnci/djt096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sagara Y., Freedman R.A., Vaz-Luis I. Patient prognostic score and associations with survival improvement offered by radiotherapy after breast-conserving surgery for ductal carcinoma in situ: A population-based longitudinal cohort study. J Clin Oncol. 2016;34:1190–1196. doi: 10.1200/JCO.2015.65.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liberman L., Van Zee K.J., Dershaw D.D., Morris E.A., Abramson A.F., Samli B. Mammographic features of local recurrence in women who have undergone breast-conserving therapy for ductal carcinoma in situ. AJR Am J Roentgenol. 1997;168:489–493. doi: 10.2214/ajr.168.2.9016233. [DOI] [PubMed] [Google Scholar]
- 38.Pinsky R.W., Rebner M., Pierce L.J. Recurrent cancer after breast-conserving surgery with radiation therapy for ductal carcinoma in situ: mammographic features, method of detection, and stage of recurrence. AJR Am J Roentgenol. 2007;189:140–144. doi: 10.2214/AJR.06.1281. [DOI] [PubMed] [Google Scholar]
- 39.Cutuli B., Bernier J., Poortmans P. Radiotherapy in DCIS, an underestimated benefit? Radiother Oncol. 2014;112:1–8. doi: 10.1016/j.radonc.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Wapnir I.L., Dignam J.J., Fisher B. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103:478–488. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donker M., Litiere S., Werutsky G. Breast-conserving treatment with or without radiotherapy in ductal carcinoma in situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol. 2013;31:4054–4059. doi: 10.1200/JCO.2013.49.5077. [DOI] [PubMed] [Google Scholar]
- 42.Cutuli B., Lemanski C., Le Blanc-Onfroy M. Local recurrence after ductal carcinoma in situ breast conserving treatment. Analysis of 195 cases. Cancer Radiother. 2013;17:196–201. doi: 10.1016/j.canrad.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Alvarado M., Carter D.L., Guenther J.M. The impact of genomic testing on the recommendation for radiation therapy in patients with ductal carcinoma in situ: A prospective clinical utility assessment of the 12-gene DCIS score result. J Surg Oncol. 2015;111:935–940. doi: 10.1002/jso.23933. [DOI] [PubMed] [Google Scholar]
- 44.Manders J.B., Kuerer H.M., Smith B.D. Clinical utility of the 12-gene DCIS Score assay: impact on radiotherapy recommendations for patients with ductal carcinoma in situ. Ann Surg Oncol. 2016;24:660–668. doi: 10.1245/s10434-016-5583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]