Abstract
Purpose
Basal cell and cutaneous squamous cell carcinoma are common malignancies (keratinocyte carcinomas [KCs]). Surgical resection is the standard of care. Radiation using high-dose rate brachytherapy (HDR-BT) may serve as a superior alternative where surgical scars may be of cosmetic concern or in elderly patients with significant comorbidity. We aim to describe the clinical and cosmetic outcomes as well as posttreatment radiation toxicities associated with HDR-BT in patients who were treated for KCs of the face.
Methods and Materials
Patients with KCs treated with HDR-BT from 2015 to 2018 were included in the study. Patient medical records and clinical photos were reviewed at multiple time points: start of treatment, end of treatment, short-term (2 week) follow-up, 3-month follow-up, and if needed at 6 months. Radiation toxicity was graded using the Radiation Therapy Oncology Grading (RTOG) acute toxicity scale. Median (range) toxicity grades at follow-up intervals were calculated. Clinical outcomes including local recurrence were evaluated for all patients.
Results
The study included 19 patients and 20 KCs. The median radiation dose was 42 Gy (39-42 Gy) over 6 fractions. The median toxicity at completion of treatment was RTOG grade 2 (85% of patients). At short-term follow-up, 50% of patients (n = 10) improved to RTOG grade 1 (0-2). At 3 months, 70% of patients (n = 14) had RTOG grade 0, and by 6 months, 100% of patients (n = 18) had RTOG grade 0. No RTOG grade 3 or higher skin toxicity was observed. With a median follow-up of 7.2 months (range, 1.3-54.4 months), the local recurrence-free survival was 95%.
Conclusions
We demonstrate that HDR-BT can be used as definitive treatment of KCs of the face with excellent cosmetic outcomes and local control. Acute and subacute skin toxicities were most commonly RTOG grade 2 or less with resolution of patient’s skin toxicity by 3 months.
Introduction
Skin cancers, including basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (SCC), are the most common human malignancies and combined account for approximately 5.4 million new cases annually in the United States.1,2 Although BCC and SCC, also known as keratinocyte carcinomas (KCs), have low metastatic potential and mortality rates compared with other malignancies, they can be locally invasive and destructive and have deleterious effects on quality of life (QOL) if left untreated.3,4 The standard of care for KC is a diagnostic biopsy followed by surgery with excision or Mohs micrographic surgery. Although surgery is considered the definitive treatment by many due to low local recurrence of <5%, it has limitations, such as being invasive and costly, projected at 8 billion dollars annually.5, 6, 7 Overall, in the United States, treatment for skin cancers grew by >50% from 1996 to 2008, but the use of Mohs micrographic surgery increased by 400% over this approximate same period.8 However, in functionally limiting or sensitive cosmetic sites including the tip of the nose, ear, finger knuckles, or large tumors on the scalp, surgery can introduce morbidities, including nerve damage, movement limiting scars, or less desirable cosmetic outcomes.9,10 Moreover, given the aging population, many patients who will require treatment for KCs are elderly with multiple medical comorbidities, including venous insufficiency, compromised blood circulation, and aging skin, all of which can contribute to slow or nonhealing surgical wounds, and thus a noninvasive approach in this patient population may be ideal.11, 12, 13 Finally, in addition to limitations caused by cosmetically limiting sites and aging patients, cases involving markedly larger areas of the face, patients who are poor surgical candidates, recurrences after previous surgical attempts, or patient’s wishes to avoid any line of scar, other treatment alternatives to surgery exist and should be discussed with patients in selecting an optimal treatment strategy.
Specifically, radiation treatment using high-dose rate brachytherapy (HDR-BT) has become increasingly used over the past decade for the treatment of early-stage KC, with high local control rates with series reporting local control between 90% to 98% and good-excellent cosmetic outcomes.14, 15, 16, 17, 18 HDR-BT is a growing treatment option for KC due to its less invasive nature and higher tolerability profile in elderly patients who may not tolerate the required time on the operating table or whose comorbidities will contribute to poor wound healing and diminished cosmetic outcome. In addition to excellent local control, HDR-BT also has an appealing cosmetic outcome, with some series reporting excellent cosmetic outcomes in 98% of patients treated.14,16,17 Traditional radiation using daily external beam radiation therapy (EBRT) takes between 3 to 6 weeks, with patients receiving between 15 to 20 fractions. There are alternative EBRT fractionation schedules that use hypofractionation, including a 5-fraction course that is supported by the National Comprehensive Cancer Center Network guidelines for KCs <20 mm in size.19 However, in clinical practice, this fractionation is generally limited to small lesions <5 mm in size, and a 5 fraction schedule is not supported by guidelines from the American Society of Radiation Oncology.20 For small facial KCs, HDR-BT allows for an expedited treatment timeline and greater patient convenience, with definitive treatment in 6 to 7 fractions, and it can be used in lesions >5 mm.
In this study, we aimed to describe the clinical outcomes and posttreatment radiation toxicity associated with HDR-BT in patients treated for KCs of the face at our institution as well as our procedure for prescribing and using HDR-BT in our clinic. We believe this will provide valuable information for patients, caregivers, and clinicians when discussing possible treatment options and outcomes regarding facial KCs in patients unable to have surgery or who are seeking alternative approaches.
Methods and Materials
After institutional review board approval, consecutive patients treated with HDR-BT at our institution with pathologically confirmed KC, primarily of the nose, lip, or scalp, were included in the current study.
Procedure details and radiation prescription
All lesions for selected cases were limited to a maximum depth of 4 mm and a diameter equal to or less than 25 mm. The depth of invasion was assessed by both a radiation oncologist and dermatologist. In scenarios where there were significant concerns about depth of invasion, ultrasound or computed tomography was used to determine depth. The maximal diameter of 25 mm is a limitation of the Leipzig skin applicator and reflects the largest diameter used in our clinic. All patients were immobilized with thermoplastic cranial masks with face and nose exposed. The gross tumor volume was assessed visually and outlined by the treating physician. The planning target volume consisted of the gross tumor volume with a 3-mm margin and was outlined on the surface of the skin with a marker pen. Appropriately sized Leipzig applicators were chosen based on the planning target volume size. All patients were treated with the Leipzig skin applicator (Nucletron, Elekta AB, Stockholm, Sweden). Immobilization of the applicator was achieved using a department manufactured articulated arm device (Figs 1 and 2). All treatments were delivered under the direct supervision of the radiation oncologist, an authorized user, for accurate applicator positioning and the authorized medical physicist. The treatment dose was prescribed to a depth of 3 mm or less. The prescription dose was 42 Gy in 6 or 7 fractions, delivered 2 to 3 times per week, with a minimum interval of 48 hours between fractions and not exceeding 3 weeks.
Figure 1.
Leipzig applicator set. Images courtesy of Elekta.
Figure 2.
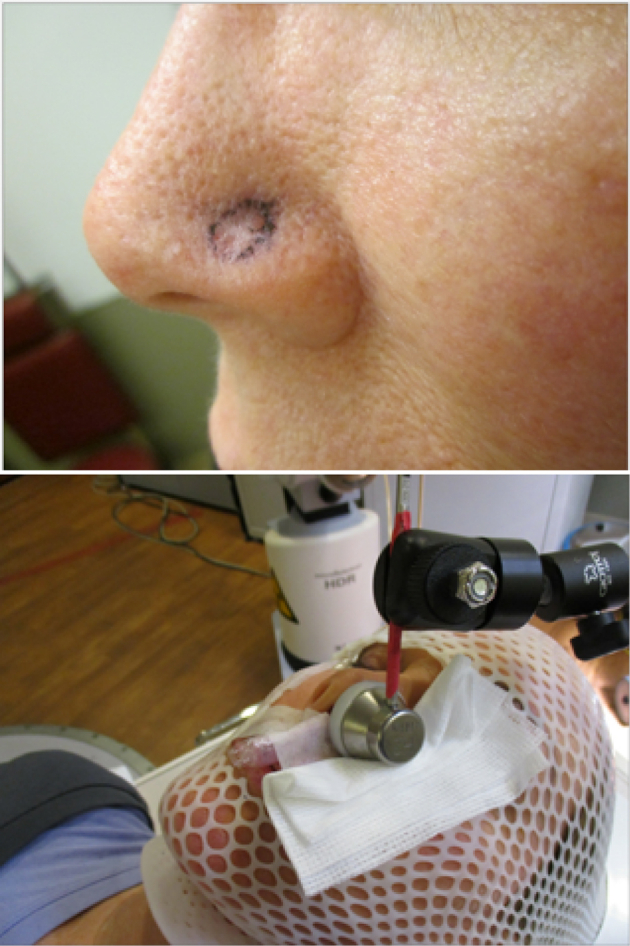
KC on the left nasal ala (top). KC treated using Leipzig applicator and articulated arm device (bottom). Abbreviation: KC = keratinocyte carcinoma.
Toxicity assessment
Patient notes and treatment images were collected retrospectively for all patients to determine type of KC, follow-up dates, and grading for toxicity at the lesion site. Patient images were examined at 4-time points for all patients (5-time points for some) including (1) at the start of treatment, (2) end of treatment, (3) short-term (2-week) follow-up, and (4) 3-month follow-up. Therefore, all patients had images reviewed a minimum of 4 times. Six-month follow-up pictures were taken for patients who had any unresolved skin toxicities at the 3-month follow-up. However, all patients in our series had long-term follow-up visits around 6 months. Follow-up dates were calculated from time of treatment until last follow-up. Images were graded by both an attending dermatologist and radiation oncologist using the Radiation Therapy Oncology Grading (RTOG) acute toxicity scale for skin from 0 to 5 based on cosmetic features. Cosmetic features include erythema, dry or wet desquamation, ulceration, edema, and decreased sweating at the site. Data values for toxicity grades according to RTOG strata were reported as median and range toxicity grades. Ranges were determined by independent t-tests. Chi-square analysis was used to determine the significance of changes between different follow-up dates and toxicity grading of patient lesions as categorical variables.
Clinical outcome assessment
To assess clinical outcomes, electronic medical records of all patients were searched for last follow-up with clinical documentation by either the treating radiation oncologist or referring dermatologist. In addition, all available patients were contacted and interviewed by a radiation oncologist using telehealth to assess for recurrences. Telehealth was only used to assess for disease recurrence and was not intended to grade toxicity. All toxicity assessments were conducted at regular in-person clinical appointments. Median follow-up for recurrence was calculated from time of last HDR-BT until the patient was contacted. In the event the patient was unable to be contacted due to death, other illness, or other causes, the last documentation in the electronic medical records by the radiation oncologist or dermatologist was used for the calculation.
Results
Between 2015 to 2018, 19 consecutive patients with a total of 20 KCs were treated with HDR-BT at our institution and included for analysis. The median age of patients was 75.5 years (44-93). Of the patients, 16 presented with BCC, and 3 had SCC (Table 1). The most common location of malignancy was the nose (12 KCs, 60%), followed by scalp (4 KCs, 20%), lip/labial fold (2 KCs, 10%), temporal region (1 KC, 5%), and eyelid (1 KC, 5%) (Table 2). Moreover, 1 patient had KCs at more than 1 location at presentation (5%). Of the lesions, the most common subtype was nodular BCC (56%), followed by SCC in situ (for additional details on subtype please refer to Table 2). Median treatment dose was 42 Gy in 6 fractions. Median (standard deviation) treatment duration in days was 18 (4), whereas median short-term follow-up was 14 days (15), and 3-month follow-up was 63 (24). If needed, the median time for 6-month follow-up was 160 days (92). Of the 19 patients in the series, 19 had clinical follow-up at 2 weeks, 19 had clinical follow-up at 3 months, and 18 of 19 (95%) had long-term follow-up at or around 6 months, with 5 patients requiring toxicity assessments to include photographs at 6 months due to unresolved toxicity at 3 months.
Table 1.
Clinical and treatment characteristics
| Number of patients with KC | n = 19 |
| BCC | 17 (85%) |
| SCC | 3 (20%) |
| Patient demographics | |
| Age (years) | 75.5 (44-93) |
| Sex | |
| Male | 8 (44%) |
| Female | 10 (56%) |
| Location of malignancy | |
| Nose | 12 (63%) |
| Eyelid | 1 (5%) |
| Scalp | 4 (21%) |
| Lips/labial fold | 2 (11%) |
| Temporal region | 1 (5%) |
| Multiple locations | 2 (11%) |
| Local recurrence | |
| No | 19 (95%) |
| Yes | 1 (5%) |
| Sex | |
| Male | 8 (44%) |
| Female | 10 (56%) |
| Location of malignancy | |
| Nose | 12 (63%) |
| Eyelid | 1 (5%) |
| Scalp | 4 (21%) |
| Lips/labial fold | 2 (11%) |
| Temporal region | 1 (5%) |
| Multiple locations | 2 (11%) |
| Clinical follow-up for toxicity | |
| 2 wk | 19 patients (100%) |
| 3 mo | 19 patients (100%) |
| 6 mo | 18 patients (95%) |
| Local recurrence | |
| No | 19 (95%) |
| Yes | 1 (5%) |
Abbreviations: BCC = basal cell carcinoma; KC = keratinocyte carcinoma; SCC = squamous cell carcinoma.
Table 2.
Tumor location and subtype for all patients
| Patient number | Age/sex | Type of skin cancer | Location | Subtype |
|---|---|---|---|---|
| 1 | 86/M | SCC | Scalp | In situ |
| 2 | 88/M | BCC | Scalp | Nodular |
| 3 | 58/F | BCC | Nose | Nodular |
| 4 | 75 | SCC | Nose | In situ |
| 5 | 70/F | BCC | Nose | Nodular |
| 6 | 80/M | SCC | Left superior parietal scalp | Focally pseudoglandular |
| 7 | 74/F | BCC | Nose | Nodular |
| 8 | 52/F | BCC | Left nasal sidewall | Nodular |
| 9 | 51/F | BCC | Left nasal ala | Nodular |
| 10 | 67/F | BCC | Nasolabial fold | Nodular |
| 11 | 76/M | BCC | Nasal supratip | Nodular |
| 12 | 91/M | BCC | Right inferior vermillion lip | Nodular |
| 13 | 44/F | BCC | Left upper nasal bridge | Nodular |
| 14 | 77/M | BCC | Right forehead, Left nose |
Nodular ulcerated Ulcerated |
| 15 | 92/F | BCC | Left central parietal scalp | Pigmented nodular |
| 16 | 93/M | BCC | Left nasal ala | Nodular |
| 17 | 92/M | BCC | Right upper lip | Nodular |
| 18 | 62/F | BCC | Nasal tip | Nodular and ulcerated |
| 19 | 60/F | BCC | Left nose | Nodular |
Abbreviations: BCC = basal cell carcinoma; SCC = squamous cell carcinoma.
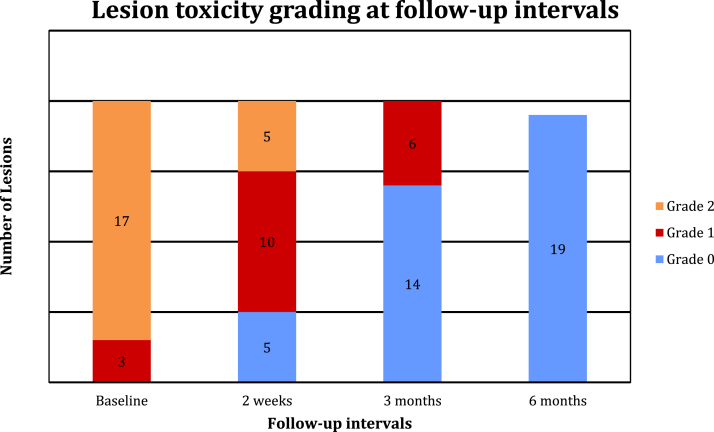
The median toxicity (range) reported immediately posttreatment was RTOG grade 2 (1-2). At the end of treatment, 85% of treated lesions (n = 17) exhibited RTOG grade 2 toxicity and 15% of treated lesions (n = 3) had grade 1. No grade 3 toxicities were reported at the end of treatment. The most common grade 2 toxicities after treatment were moderate erythema or edema at the lesion site (Fig 3). At short-term follow-up (2 weeks posttreatment), 50% of treated KCs (n = 10) had RTOG grade 1 toxicity, and 25% of treated KCs (n = 5) had grade 2 toxicity. By 3-month follow-up, 70% of treated KCs (n = 14) had RTOG grade 0 toxicity, and the other 30% of KCs (n = 6) had grade 1 toxicity. The most common RTOG grade 1 toxicity at 3 months of follow-up was due to faint erythema and dryness at the treatment site. By 6 months, all patients (n = 5) who had unresolved toxicity at 3 months had RTOG grade 0 toxicity (n = 18), with no visible scarring or lesion. One patient did not have follow-up for postradiation toxicity and is inconclusive. Given the lack of data for this patient at long-term follow-up, our toxicity analysis reports on 18 patients at the final toxicity assessment. The RTOG toxicity grade distribution is presented in Figure 4. No RTOG grade 3 toxicity was observed at any time in the current study. With a median follow-up of 7.2 months (range, 1.3-54.4 months), the local recurrence-free survival was 94.7%, with only a single recurrence in our series.
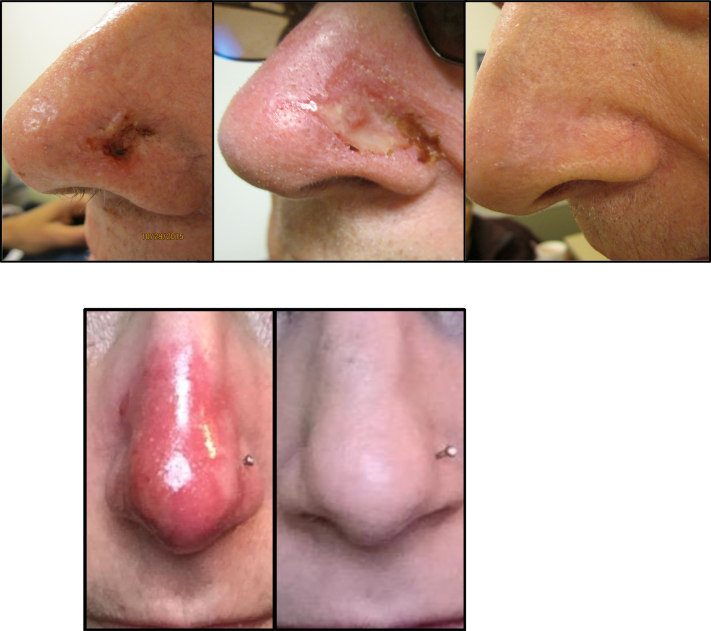
Figure 3.
Toxicity course- top image of a treated KC at 2 weeks, 3 months, and 6 months post-treatment. Bottom image- treated KC at 2 weeks and 3 months post-treatment.
Figure 4.
Lesion grading at different time intervals. Data are expressed in numbers representing patients at different intervals. One patient was lost to follow-up at 6 months, so the final toxicity assessment includes 19 treated lesions.
Discussion
The incidence of KCs has been rising over the last several decades, as has the percentage of patients treated for these malignancies.21,22 As the population ages the proportion of elderly patients with KCs will also increase, as 80% of KCs occur in people over the age of 60.18 Given the aging population, it is estimated that the number of KCs presenting to dermatologists could increase by 50%. Surgical approaches with either excision or Mohs micrographic surgery (MMS) are considered the standard of care treatment for KCs. However, the increasing proportion of elderly patients with multiple medical comorbidities and advancements in radiation delivery have led to the justification for the increasing use of HDR-BT.
HDR-BT is an effective and noninvasive alternative for the treatment of KCs in settings where surgical excision may be limited by select patient factors. Further, HDR-BT is a more convenient option for patients as it requires fewer numbers of treatments compared with traditional EBRT. Although hypofractionated regimens consisting of 5 or 6 fractions are sometimes employed, they are more commonly used for lesions <5 mm and are not supported by the American Society of Radiation Oncology guidelines.20,23 In addition to a lack of consensus regarding hypofractionation regimens, 5-fraction courses are often avoided for lesions >5 mm given concerns for lower biological effective doses compared with HDR-BT.20 However, a 5-fraction course is supported by the National Comprehensive Cancer Center Network guidelines for KCs <20 mm, and this fractionation schedule may be used in certain clinical scenarios.19 Further, recent studies have shown that HDR-BT leads to better cosmesis and local tumor control compared with EBRT.24,25 Moreover, the increasing evidence of the utility of HDR-BT for the treatment of KCs has led to the development and publication of skin brachytherapy recommendations by Groupe Européen de Curiethérapie - European Society for Therapeutic Radiology and Oncology and Advisory Committee on Radiation Oncology Practice.16 In addition to providing treatment recommendations, the conclusion of this report specifies that brachytherapy (BT) is an efficient and well-tolerated treatment that offers excellent cosmesis and low toxicity for patients with skin cancer. Further, the report concludes that carefully tailored BT is a good alternative, if not the treatment of choice for those lesions that cannot be safely removed by surgery.
Although KCs have low metastatic potential, their potential for local tissue destruction has been associated with detriments in QOL if left untreated.26, 27, 28 Specifically, in elderly populations, studies have demonstrated that healthy skin leads to better mental and emotional health as well as improvements in social engagement.17,29 Other studies have found that, independent of age, QOL impairments are more pronounced in patients with tumors located in exposed areas such as the face that may pose significant cosmetic challenges for surgical resection.3
In our single-institution series of patients treated with HDR-BT, we report high rates of local control (95%) with good to excellent cosmetic outcomes. Our patient population, with a median treated age of 75.5 years, serves as a representative population of patients who are routinely referred for noninvasive treatment strategies. As such, we believe that our study can provide meaningful information in counseling elderly patients regarding the outcomes and potential immediate, acute, and subacute toxicities of radiation treatment with HDR-BT, should such an approach be taken for their KCs.
A common criticism of HDR-BT compared with surgical approaches has been that radiation therapy offers inferior local control. Series of surgical resection show recurrence rates at 5 years <5% even in sensitive areas.7,30,31 In our study, we demonstrate a local control rate of 95% with a median follow-up of 7.2 months (range, 1.3-54.3 months). During our study period, only 1 lesion treated with HDR-BT had a local recurrence. The local control we report is in accordance with prior data and reflects the high fidelity and efficacy of HDR-BT in this patient population. Several studies have shown excellent rates of local control that approach those reported in series of surgery in patients treated with radiation therapy alone. In a review, Delishaj and colleagues14 report a median local control rate of 97% in studies of HDR-BT. Several studies included in this review show local control rates between 95% to 100%.32,33 Other studies report excellent outcomes at 1 year both in terms of local control (96.2%) and cosmetic outcomes.8 In a systematic review and meta-analysis published in Cancer, Lee and colleagues24 reported on cosmetic outcomes and recurrence rates among 18,095 patients with KCs treated with either conventional excision, MMS, EBRT, or BT. In this report, the authors demonstrate 1-year recurrence rates of 0.2% and 0.0% for MMS and BT, respectively.24 Although data are limited, HDR-BT seems to provide excellent long-term outcomes with respect to tumor control, and we have confirmed this in our patient population.
Specifically, in older populations, surgical resection has been associated with higher rates of wound complications and poor healing.34 In a series of 241 patients over the age of 75 undergoing surgical resection for skin cancer, 20% of treated patients had 1 or more significant surgical complications.35 In our study, with a median patient age of 75 years, we demonstrated a 15% rate of grade 2 toxicity at the completion of treatment. On further follow-up, at 6 months we demonstrated a 0% rate of toxicity as measured using the RTOG scale, thus providing evidence that HDR-BT may be a desired option or even superior in this population compared with more invasive approaches.
Another common criticism of radiation therapy is the paucity of data detailing the long-term cosmetic outcomes in patients treated with HDR-BT. However, in a recently published series, the authors reported excellent cosmetic outcomes in 98% of patients treated with HDR-BT at 5 years.17 In a study by Gauden et al,32 the authors reported that 88% of patients treated with HDR-BT had good or excellent cosmetic outcomes, with a follow-up of 66 months. Additionally, in the systematic review by Lee et al,24 the summary effect size for “good” cosmetic outcomes was 81%, 74.6%, and 97.6% for conventional excision, EBRT, and BT, respectively. The only study included in this review reporting on cosmetic outcomes for MMS reported a 96% rate of “good” cosmesis.
Another limiting factor in the use of HDR-BT for skin lesions is the limitation of the Leipzig skin applicator. The size of the applicator limits its use to similarly small lesions. In addition, the applicator requires treatment en face to achieve appropriate dose distributions to ensure tumor coverage. Given these limitations, it can sometimes be difficult to apply the skin applicator to areas of the face where an en face technique is not achievable, as on the bridge of the nose. However, with proper set-up and meticulous planning, HDR-BT can still be applied safely and effectively to many lesions on the face and scalp.
Long-term data are a known limiting factor of the RTOG scale, as cosmesis recorded at 3 and 6 months of follow-up may deteriorate over time, and extended follow-ups are needed to better characterize changes over time. However, given that most series of HDR-BT are in elderly patients with multiple medical comorbidities who likely have underlying disease processes that lead to impaired wound healing, the results on cosmetic outcomes reported in the literature are reassuring. Further, with high-dose radiation delivery, acute toxicities are often more exaggerated than when using lower dose or traditionally fractionated external beam radiation, and the acute toxicities we report also provide evidence that HDR-BT in elderly patients is a safe alternative to surgical resection. In our current analysis, as well as in practice, we have noticed a favorable cosmetic outcome in patients who opt for noninvasive treatment strategies in areas of cosmetic importance, such as the tip of their nose (Fig 1).
To ensure high quality of care, it is important for radiation oncologists, dermatologists, and geriatricians to identify patients who are ideal candidates for HDR-BT based on tumor characteristics, location, and patient risk assessment. This should be balanced with other approaches such as surgery and in the context of any associated medical comorbidity and risk of surgery or postoperative complications. Our study intended to evaluate the immediate, acute, and subacute adverse events of HDR-BT as well as local control for the treatment of KCs in our clinical practice. Our results indicate a promising outcome and indication for the use of radiation in the treatment of KCs and provides additional evidence and support for the continued use of HDR-BT as an alternative to surgery in high-risk populations or in patients who decline surgery for fear of decreased cosmetic outcomes.
To further investigate, we plan to conduct a large prospective study assessing the outcome of HDR-BT in the treatment of KCs of the skin. Moreover, proper indications for enhanced cosmetic and functional outcomes in certain anatomic sites, like the tip of the nose, helix of the ear, or finger knuckles, will be studied and compared with reported surgical results in the literature. The follow-up prospective study also aims to report the efficacy and recurrence rate over a 2-year period of HDR-BT versus surgical treatment.
The current analysis has several strengths, including a multidisciplinary treatment approach at a high volume academic center for patients with KCs, a detailed follow-up posttreatment for cosmetic outcomes (using patient photos), and a uniform and reproducible brachytherapy procedure. Limitations of the current study include the small sample size, the lack of long-term cosmetic and oncologic outcomes, and the retrospective nature of the study. However, despite these limitations, we believe our study provides evidence of the role, safety, and efficacy of an HDR-BT approach for the treatment of KCs in a predominantly elderly population.
Conclusions
HDR-BT demonstrates an expected course of toxicity in patients being treated for KCs of appropriate type and size with similar efficacy to surgical excision during the follow-up period and desired cosmetic results. A peak RTOG grade 2 toxicity and cosmetic features including dryness, erythema, and decreased sweating can be expected from the end of treatment to short-term follow-up. The toxicity typically resolves within 3 months, and by 6 months, almost all toxicity resolves. As such, we demonstrate the safety and efficacy of HDR-BT for the treatment of KCs in sensitive areas among an elderly population.
Footnotes
Sources of support: This work had no specific funding.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Disclosures: none.
Contributor Information
Bahar Dasgeb, Email: bahar.dasgeb@jefferson.edu.
Wenyin Shi, Email: wenyin.shi@jefferson.edu.
References
- 1.Rogers H.W., Weinstock M.A., Feldman S.R., Coldiron B.M. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the us population, 2012. JAMA Dermatol. 2015;151:1081–1086. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 2.Bhatnagar A. Nonmelanoma skin cancer treated with electronic brachytherapy: Results at 1 year. Brachytherapy. 2013;12:134–140. doi: 10.1016/j.brachy.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Abedini R., Nasimi M., Noormohammad Pour P., Moghtadaie A., Tohidinik H.R. Quality of life in patients with non-melanoma skin cancer: Implications for healthcare education services and supports. J Cancer Educ. 2019;34:755–759. doi: 10.1007/s13187-018-1368-y. [DOI] [PubMed] [Google Scholar]
- 4.Gaulin C., Sebaratnam D.F., Fernández-Peñas P. Quality of life in non-melanoma skin cancer. Australas J Dermatol. 2015;56:70–76. doi: 10.1111/ajd.12205. [DOI] [PubMed] [Google Scholar]
- 5.Chen J.T., Kempton S.J., Rao V.K. The economics of skin cancer: An analysis of medicare payment data. Plast Reconstr Surg - Glob Open. 2016;4:1–7. doi: 10.1097/GOX.0000000000000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalaghchi B., Esmati E., Ghalehtaki R. High-dose-rate brachytherapy in treatment of non-melanoma skin cancer of head and neck region: Preliminary results of a prospective single institution study. J Contemp Brachytherapy. 2018;10:115–122. doi: 10.5114/jcb.2018.75596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chren M.M., Torres J.S., Stuart S.E., Bertenthal D., Labrador R.J., Boscardin W.J. Recurrence after treatment of nonmelanoma skin cancer: A prospective cohort study. Arch Dermatol. 2011;147:540–546. doi: 10.1001/archdermatol.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delishaj D., Laliscia C., Manfredi B. Non-melanoma skin cancer treated with high-doserate brachytherapy and Valencia applicator in elderly patients: A retrospective case series. J Contemp Brachyther. 2015;7:437–444. doi: 10.5114/jcb.2015.55746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tormo A., Celada F., Rodriguez S. Non-melanoma skin cancer treated with HDR valencia applicator: Clinical outcomes. J Contemp Brachyther. 2014;6:167–172. doi: 10.5114/jcb.2014.43247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paravati A.J., Hawkins P.G., Martin A.N. Clinical and cosmetic outcomes in patients treated with high-dose-rate electronic brachytherapy for nonmelanoma skin cancer. Pract Radiat Oncol. 2015;5:e659–e664. doi: 10.1016/j.prro.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Garcovich S., Colloca G., Sollena P. Skin cancer epidemics in the elderly as an emerging issue in geriatric oncology. Aging Dis. 2017;8:643–661. doi: 10.14336/AD.2017.0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peris K., Fargnoli M.C., Garbe C. Diagnosis and treatment of basal cell carcinoma: European consensus–based interdisciplinary guidelines. Eur J Cancer. 2019;118:10–34. doi: 10.1016/j.ejca.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Valentina L., Gyoergy K., Luca T. The role of personalized interventional radiotherapy (brachytherapy)in the management of older patients with non-melanoma skin cancer. J Geriatr Oncol. 2019;10:514–517. doi: 10.1016/j.jgo.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Delishaj D., Rembielak A., Manfredi B. Non-melanoma skin cancer treated with high-dose-rate brachytherapy: A review of literature. J Contemp Brachytherapy. 2016;8:533–540. doi: 10.5114/jcb.2016.64112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballester-Sánchez R., Pons-Llanas O., Candela-Juan C. Electronic brachytherapy for superficial and nodular basal cell carcinoma: A report of two prospective pilot trials using different doses. J Contemp Brachyther. 2016;8:48–55. doi: 10.5114/jcb.2016.57531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guinot J.L., Rembielak A., Perez-Calatayud J. GEC-ESTRO ACROP recommendations in skin brachytherapy. Radiother Oncol. 2018;126:377–385. doi: 10.1016/j.radonc.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Guix B., Finestres F., Tello J.I. Treatment of skin carcinomas of the face by high-dose-rate brachytherapy and custom-made surface molds. Int J Radiat Oncol Biol Phys. 2000;47:95–102. doi: 10.1016/s0360-3016(99)00547-7. [DOI] [PubMed] [Google Scholar]
- 18.Zaorsky N.G., Lee C.T., Zhang E., Keith S.W., Galloway T.J. Hypofractionated radiation therapy for basal and squamous cell skin cancer: A meta-analysis. Radiother Oncol. 2017;125:13–20. doi: 10.1016/j.radonc.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bichakjian C.K., Olencki T., Aasi S.Z. Basal cell skin cancer, version 1.2016: Clinical practice guidelines in oncology. JNCCN J Natl Compr Cancer Netw. 2016;14:574–597. doi: 10.6004/jnccn.2016.0065. [DOI] [PubMed] [Google Scholar]
- 20.Likhacheva A., Awan M., Barker C.A. Definitive and postoperative radiation therapy for basal and squamous cell cancers of the skin: Executive summary of an American Society for Radiation Oncology clinical practice guideline. Pract Radiat Oncol. 2020;10:8–20. doi: 10.1016/j.prro.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Lomas A., Leonardi-Bee J., Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166:1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 22.Rogers H.W., Coldiron B.M. Analysis of skin cancer treatment and costs in the United States Medicare population, 1996-2008. Dermatol Surg. 2013;39:35–42. doi: 10.1111/dsu.12024. [DOI] [PubMed] [Google Scholar]
- 23.Likhacheva A.O., Devlin P.M., Shirvani S.M. Skin surface brachytherapy: A survey of contemporary practice patterns. Brachytherapy. 2017;16:223–229. doi: 10.1016/j.brachy.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee C.T., Lehrer E.J., Aphale A., Lango M., Galloway T.J., Zaorsky N.G. Surgical excision, Mohs micrographic surgery, external-beam radiotherapy, or brachytherapy for indolent skin cancer: An international meta-analysis of 58 studies with 21,000 patients. Cancer. 2019;125:3582–3594. doi: 10.1002/cncr.32371. [DOI] [PubMed] [Google Scholar]
- 25.Zaorsky N.G., Lee C.T., Zhang E., Galloway T.J. Skin CanceR Brachytherapy vs External beam radiation therapy (SCRiBE) meta-analysis. Radiother Oncol. 2018;126:386–393. doi: 10.1016/j.radonc.2017.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steenrod A.W., Nash E., Elizabeth S., von Hoff D.D., Brail L.H., Coyne K.S. Original research a qualitative comparison of symptoms and impact of varying stages of basal cell carcinoma. Dermatol Ther (Heidelb) 2015;5:183–199. doi: 10.1007/s13555-015-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen T., Bertenthal D., Sahay A., Sen S., Chren M. Predictors of skin-related quality of life after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. JAMA Dermatol. 2007;143:1386–1392. doi: 10.1001/archderm.143.11.1386. [DOI] [PubMed] [Google Scholar]
- 28.Association A.M. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: A systematic review and meta-analysis. JAMA Dermatol. 2020;55905:419–428. doi: 10.1001/jamadermatol.2015.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blume-Peytavi U., Kottner J., Sterry W. Age-associated skin conditions and diseases: Current perspectives and future options. Gerontologist. 2016;56:S230–S242. doi: 10.1093/geront/gnw003. [DOI] [PubMed] [Google Scholar]
- 30.Parvataneni R., Boscardin W.J. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013;133:1188–1196. doi: 10.1038/jid.2012.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weesie F.D., Naus N.C., Vasilic D. Recurrence of periocular basal cell carcinoma and squamous cell carcinoma after Mohs micrographic surgery: A retrospective cohort study. 5. British Journal of Dermatology. 2019;180:1176–1182. doi: 10.1111/bjd.17516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gauden R., Pracy M., Avery A.M., Hodgetts I., Gauden S. HDR brachytherapy for superficial non-melanoma skin cancers. J Med Imaging Radiat Oncol. 2013;57:212–217. doi: 10.1111/j.1754-9485.2012.02466.x. [DOI] [PubMed] [Google Scholar]
- 33.Arenas M., Arguís M., Díez-Presa L. Hypofractionated high-dose-rate plesiotherapy in nonmelanoma skin cancer treatment. Brachytherapy. 2015;14:859–865. doi: 10.1016/j.brachy.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Lee W.W., Ashley W., Cotliar J., Jung J. Management of elderly patients with skin cancer. J Geriatr Oncol. 2016;7:7–9. doi: 10.1016/j.jgo.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Bouhassira J., Bosc R., Greta L. Factors associated with postoperative complications in elderly patients with skin cancer: A retrospective study of 241 patients. J Geriatr Oncol. 2016;7:10–14. doi: 10.1016/j.jgo.2015.11.004. [DOI] [PubMed] [Google Scholar]