Abstract
Purpose
Predicting the risk of early distant brain failure (DBF) is in demand for management decisions in patients who are candidates for local treatment of brain metastases. This study aimed to analyze the association between circulating tumor cells (CTCs) and brain disease control after stereotactic radiation therapy/radiosurgery (SRT) for breast cancer brain metastasis (BCBM).
Methods and Materials
We prospectively assessed CTCs before (CTC1) and 4 to 5 weeks after (CTC2) SRT and their relationship with the number of new lesions (NL) suggestive of BCBM before SRT. CTC were quantified and analyzed by immunocytochemistry to evaluate the expression of the proteins COX2, EGFR, ST6GALNAC5, NOTCH1, and HER2. Distant brain failure-free survival (DBFFS), the primary endpoint, diffuse DBFFS (D-DBFFS), and overall survival were estimated. Analysis for DBF within 6 months, with death as competing risk, was performed.
Results
Patients were included between 2016 and 2018. CTCs were detected in all 39 patients before and in 34 of 35 patients after SRT. After median follow-up of 16.6 months, median DBFFS, D-DBFFS, and overall survival were 15.3, 14.1, and 19.5 months, respectively. DBF at 6 months was 40% with CTC1 ≤0.5 and 8.82% with CTC1 >0.5 CTC/mL (P = .007), and D-DBF at 6 months was 40% with CTC1 ≤0.5 and 0 with CTC1 >0.5 CTC/mL (P = .005) and 25% with NL/CTC1 >6.8 and 2.65% with NL/CTC1 ≤6.8 (P = .063). On multivariate analysis, DBFFS was inferior with CTC1 ≤0.5 (hazard ratio, 8.27; 95% confidence interval, 2.12-32.3; P = .002), and D-DBFFS was inferior with CTC1 ≤0.5 (hazard ratio, 10.22; 95% confidence interval, 1.99-52.41; P = .005). Protein expression was not associated with outcomes.
Conclusions
These data suggest that CTC1 and NL/CTC1 may have a role as a biomarker of early diffuse DBF and as a subsequent guide between focal or whole-brain radiation therapy in patients with BCBM.
Introduction
Breast cancer brain metastases (BCBM) have been reported in 18% to 30% of patients with metastatic disease,1 with an increasing incidence related to the evolution of brain imaging and more effective control of extracranial disease as a consequence of improvements in systemic therapy and the resulting decrease in overall mortality.2 The advances in the management of BCBM have led to a median overall survival (OS) of 16 months for all patients and 36 months in the best prognostic group from a large contemporary cohort.3
In this context, predicting the risk of early distant brain failure (DBF) is a useful and in demand resource for management decisions in patients who are candidates for local treatment of BCBM. Selecting focal stereotactic radiation therapy (SRT) or whole-brain radiation therapy (WBRT) is a clinical conundrum between optimizing intracranial tumor control and avoiding potential deterioration of cognitive function and quality of life.4,5
Risk score, nomogram, and prognostic metrics for DBF after initial treatment with upfront SRT have been recently developed.6, 7, 8 However, despite the large patient population and multi-institutional validation, none are disease specific, and all of them are retrospective and based on noncontemporary cohorts. Within this context, we hypothesized whether the evaluation of a biological marker of micrometastatic disease could predict DBF and help clinicians to decide between SRT or WBRT for BCBM. This study aims to analyze the association between circulating tumor cells (CTCs) and control of brain disease after SRT for BM.
Methods and Materials
Study design and participants
We prospectively assessed CTCs before (CTC1) and 4 to 5 weeks after (CTC2) SRT for BCBM and their relationship with the number of new lesions (NL) suggestive of BCBM before SRT. Those eligible included adult patients (≥18 years of age) with BCBM who were candidates for SRT. Priority patients were those who had oligometastatic disease (<4 lesions) and no more than 10 lesions, expected survival >6 months defined by the diagnosis-specific graded prognostic assessment (DS-GPA), or those with prior WBRT. SRT or resection of BCBM before the CTC1 was allowed. Patients were excluded if they were pregnant, had undergone WBRT less than 30 days before a blood sample was collected, or if they received any systemic therapy less than 7 days before a blood sample was collected.
This study was approved by the institutional review board and all participants provided written informed consent, and the Reporting of Tumor Marker Studies guidelines were followed.
Procedures
Participants underwent magnetic resonance imaging (MRI) and computed tomography-based simulation and were immobilized using a stereotactic mask. SRT was performed with single stereotactic radiosurgery (SRS) or stereotactic fractionated radiation therapy (SFRT) depending on the size and location of the target volume following published evidence-based experience.9,10 All patients were treated within 7 days after MRI simulation for SRT planning with a Varian TrueBeam linear accelerator with micromultileaf colimator, cone beam computed tomography, and robotic couch.
Assessments
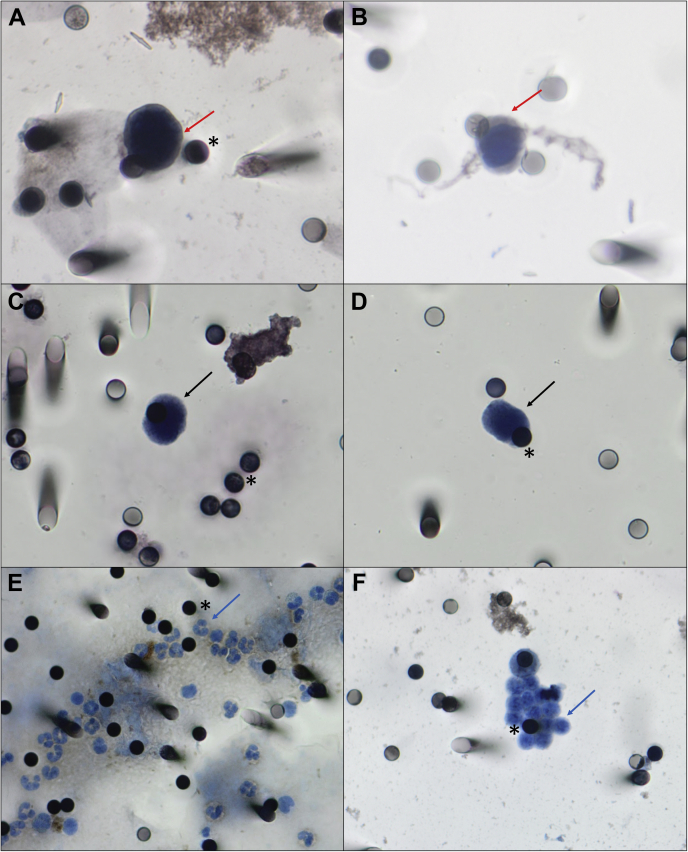
Venous blood samples for CTC1 and CTC2 analysis were timely collected on the same day of the simulation MRI and the first follow-up MRI, respectively. The ISET (Isolation by SizE of Tumors, Rarecells, France) was used to quantify and evaluate CTC as described.11 Briefly, 10 mL of blood was collected in ethylenediaminetetraacetic acid tubes and kept under homogenization for up to 4 hours at room temperature to avoid blood coagulation. Then, the blood was diluted 1:10 with the ISET filtration buffer, transferred to the ISET block, and filtered through a polycarbonate membrane with calibrated, 8-μm diameter, cylindrical pores. The ISET system is based on the principle that white blood cells are the smallest cells of the body and that CTCs are larger than 8 μm. After the filtration, membranes were washed once with phosphate-buffered saline, decoupled of the block, and stored at –20°C until time of analysis. CTCs were counted per 1 mL of blood and characterized according to 5 criteria: negativity for CD45 staining, nucleus size >12 μm, hyperchromatic and irregular nuclei, visible cytoplasm, and a nuclear to cytoplasm ratio >80%. Immunocytochemistry was performed to evaluate the expression of the proteins COX2, EGFR, and ST6GALNAC5, which are mediators of CTC passage through the blood-brain barrier,12 and NOTCH1 and HER2, which are associated with a metastatic competency to the brain (Fig 1).13
Figure 1.
Pictures of circulating tumor cells (CTCs) isolated from immunostaining of CTC from patients with metastatic breast cancer and their relationship with the 8 μm pores (asterisks) of the Isolation by SizE of Tumors (ISET) membranes. (A,B) CTC stained with HER2 (arrows), visualized by DAB (diaminobenzidine). (C,D) CTC visualized with haematoxylin (arrows). (E,F) Leukocytes from patients visualized with haematoxylin (arrows). Images were taken at x400 magnification using a light microscope (Research System Microscope BX61; Olympus, Tokyo, Japan) coupled to a digital camera (SC100; Olympus).
Follow-up acquired-volumetric postcontrast MRI was obtained 4 to 5 weeks after SRT, then every 3 months during the first year and every 4 months in the second year, unless an earlier time point was clinically indicated. Imaging evaluators were blinded to CTC analysis and CTC evaluators were blinded to imaging analysis.
Endpoints
DBF was defined as any new lesion suggestive of BCBM that developed outside the previous planning target volume, not present on prior scans and visible in a minimum of 2 projections on MRI, following the Response Assessment in Neuro-Oncology Brain Metastases working group.14 Diffuse DBF (D-DBF) was defined as progression with more than 4 new BCBM or meningeal carcinomatosis, a more representative endpoint of the potential indication for salvage WBRT. OS was defined as time from date of SRT to date of death. DBF-free survival (DBFFS) and diffuse DBFFS (D-DBFFS) were defined from date of SRT to date of either DBF, D-DBF, or death.
Statistical analysis
The required sample size was defined following the prediction of 15 events (DBF) per variable (CTC) for time-to-event endpoint (DBFFS).15 Estimating that the events would occur in half of the participants and taking into account the eventual dropout and possible loss of follow-up, the target enrollment was 40 patients.
The baseline characteristics were expressed as absolute and relative frequencies for qualitative variables and as the median, minimum, and maximum for quantitative variables. DBFFS, the primary endpoint, D-DBFFS, and OS were estimated by Kaplan-Meier estimator.16 Log-rank tests were applied to compare the survival curves and the optimal cut-off values were determined following Lausen and Schumacher.17 The Cox semiparametric proportional hazards model was fitted to assess which variables would be associated with the endpoints.18 Variables that achieved significance level of 0.2 in a single regression were used at the multiple regression models. The final model was obtained using the stepwise backward method (likelihood ratio) with criteria for entry P < .05 and removal P > .10.
The assumption of proportional hazards was assessed based on the so-called “Schoenfeld residuals.” There was evidence that covariates had a constant effect over time in all cases. In addition, competing risk analysis for DBF in the presence of death was applied. The cumulative incidence function was estimated and the Gray’s test was considered to compare the curves. We fitted univariate subdistribution hazards of an event for different variables according to the Fine-Gray model, which is a Cox type proportional subdistribution hazards model.19
The significance level was fixed at 5% for all tests. Statistical analyses were performed using R software version 3.5 (R Foundation for Statistical Computing, Austria). The study closed in February 2018 and the data set was locked on October 30, 2018.
Results
Participants
Between November 2016 and February 2018, 40 women were enrolled and 39 accrued (1 withdrew from the study). Baseline characteristics are listed in Table 1.
Table 1.
Baseline characteristics
| Median age, years (range) | 54 (34-70) |
| Immunophenotype (%) | |
| HER2-positive | 20 (51) |
| Luminal B | 12 (31) |
| Triple negative | 7 (18) |
| DS-GPA (%) | |
| 0-1 | 1 (2.5) |
| 1.5-2 | 6 (15.5) |
| 2.5-3 | 6 (15.5) |
| 3.5-4 | 26 (66.5) |
| KPS (%) | |
| 70-80 | 7 (18) |
| 90-100 | 32 (82) |
| ECM (%) | |
| Absent | 6 (15.5) |
| Present | 33 (84.5) |
| ECM status (%) | |
| Absent | 6 (15.5) |
| Progressive | 17 (43.5) |
| Stable | 16 (41) |
| Number of ECM sites (%) | |
| None | 6 (15.5) |
| 1 | 29 (74.5) |
| 2 | 2 (5) |
| 3 | 2 (5) |
| Previous treatment to the brain (%) | |
| None | 18 (46) |
| SRT | 9 (23) |
| Surgery | 5 (13) |
| WBRT | 4 (10) |
| Surgery and SRT or WBRT | 3 (8) |
| Systemic therapy before CTC1 (%) | |
| None | 3 (8) |
| Hormonal therapy | 9 (23) |
| Chemotherapy | 12 (31) |
| HER2-targeted therapy | 15 (38) |
Abbreviations: CTC = circulating tumor cells; DS-GPA = diagnosis-specific graded prognostic assessment; ECM = extracranial metastases; KPS = Karnofsky performance score; SRT = focal stereotactic radiation therapy; WBRT = whole-brain radiation therapy.
Radiation therapy
A total of 119 BCBM were irradiated, and the median number of BCBM per patient was 2 (1-15), with a median volume of 0.9 cc (0.027-39.18). SRT was performed as SRS or SFRT in 27 (69%) and 12 (31%) patients, respectively. The median prescribed dose was 20 (15-22) Gy and 27.5 (25-30) Gy with SRS and SFRT, respectively. Adjuvant SRT was performed in 4 surgical cavities in 4 patients, all of them underwent SFRT with a dose of 25 Gy in 5 fractions. Only 1 patient, with 3 previous SRS in contiguous areas, evolved with a lesion suggestive of radionecrosis 3 months after SFRT. The actuarial brain local control at 6 and 12 months after SRT was 100% and 97.93%, respectively.
Circulating tumor cells
The detection rate of CTC1 and CTC2 was, respectively, 100% in the 39 patients before SRT and 97% (34/35) in the 35 patients after SRT (4 deaths between CTC1 and CTC2). The median CTC1 and CTC2 was 2 CTC/mL and 2.33 CTC/mL, respectively (P = .357). The expressions of the proteins in CTC1 and CTC2 are listed in Table 2.
Table 2.
Frequencies of the expression of the proteins in CTC1 and CTC2
| Proteins | CTC1 |
CTC2 |
||||
|---|---|---|---|---|---|---|
| Category | n | % | Category | n | % | |
| Negative | 1 | 3.6 | Negative | 1 | 4 | |
| COX2 | Positive | 27 | 96.4 | Positive | 24 | 96 |
| Total | 28 | 100 | Total | 25 | 100 | |
| Negative | 22 | 78.6 | Negative | 9 | 50 | |
| EGFR | Positive | 6 | 21.4 | Positive | 9 | 50 |
| Total | 28 | 100 | Total | 18 | 100 | |
| Negative | 12 | 46.2 | Negative | 6 | 33.3 | |
| ST6GALNAC5 | Positive | 14 | 53.8 | Positive | 12 | 66.7 |
| Total | 26 | 100 | Total | 18 | 100 | |
| NOTCH1 | Negative | 13 | 40.6 | Negative | 11 | 40.7 |
| Positive | 19 | 59.4 | Positive | 16 | 59.3 | |
| Total | 32 | 100 | Total | 27 | 100 | |
| Negative | 29 | 90.6 | Negative | 19 | 70.4 | |
| HER2 | Positive | 3 | 9.4 | Positive | 8 | 29.6 |
| Total | 32 | 100 | Total | 27 | 100 | |
Abbreviation: CTC = circulating tumor cells.
Regarding the expression of HER2, there was a discrepancy between the immunophenotype of the primary tumor and CTC1 in 15 of the 32 tested patients: 14 of the 15 patients with HER2-positive immunophenotype had negative expression in the CTC1, and 1 of the 17 without HER2-positive immunophenotype had positive expression of HER2 in the CTC1. Among the 27 patients tested in the CTC2, there was disagreement in 14: 12 of the 14 patients with HER2-positive immunophenotype had negative expression in CTC2, and 2 of the 13 without HER2-positive immunophenotype had positive expression of HER2 in CTC2.
Among the 15 and 14 patients with HER2-positive immunophenotype on primary tumor who were tested for the expression of the proteins in, respectively, CTC1 and CTC2, 10 patients had negative expression of HER2 both in CTC1 and CTC2.
DBF
After a median follow-up of 14.6 months (95% confidence interval [CI], 11.1-18.1) in the 36 evaluable patients, there were 15 patients with DBF and 6 with D-DBF (3 with progression with more than 4 new BCBM and 3 with leptomeningeal carcinomatosis). The median DBFFS and D-DBFFS was 15.3 months (95% CI, 12.2-not reached) and not reached, respectively.
The mean time to D-DBF in the 6 patients was 6.2 (1-12) months and the salvage treatment was performed in 4 patients: SRS in 1 patient with more than 4 new BCBM, WBRT in 2 patients with more than 4 new BCBM, and 1 patient with leptomeningeal carcinomatosis.
The median DBFFS was 6 months in patients with CTC1 ≤0.5 CTC/mL and not reached in patients with CTC1 >0.5 CTC/mL (hazard ratio [HR], 4.97; 95% CI, 1.48-16.69; P = .0041), and the median D-DBFFS was 6 months in patients with CTC1 ≤0.5 CTC/mL and not reached in patients with CTC1 >0.5 CTC/mL (HR, 10.22; 95% CI, 1.99-52.4; P = .005).
The median DBFFS was 7 months in patients with immunophenotype triple negative and not reached in patients with immunophenotypes luminal B and HER2-positive (HR, 0.25; 95% CI, 0.07-0.89; P = .03) and it was 7.47 months in patients with DS-GPA ≤3 and not reached in patients with DS-GPA >3 (HR, 0.34; 95% CI, 0.12-0.95; P = .04).
The median DBFFS was not reached in patients with NL ≤5 and 10.6 months in patients with NL >5 (HR, 3.60; 95% CI, 1.08-12.05; P = .037), and the median D-DBFFS was not reached in patients with NL ≤6 and 10.6 months in patients with NL >6 (HR, 10.72; 95% CI, 2.13-53.82; P = .004). The median D-DBFFS was 12.1 months in patients with NL/CTC1 >6.8 and not reached in patients with NL/CTC1 ≤6.8 (HR, 7.37; 95% CI, 1.34-40.5; P = .022).
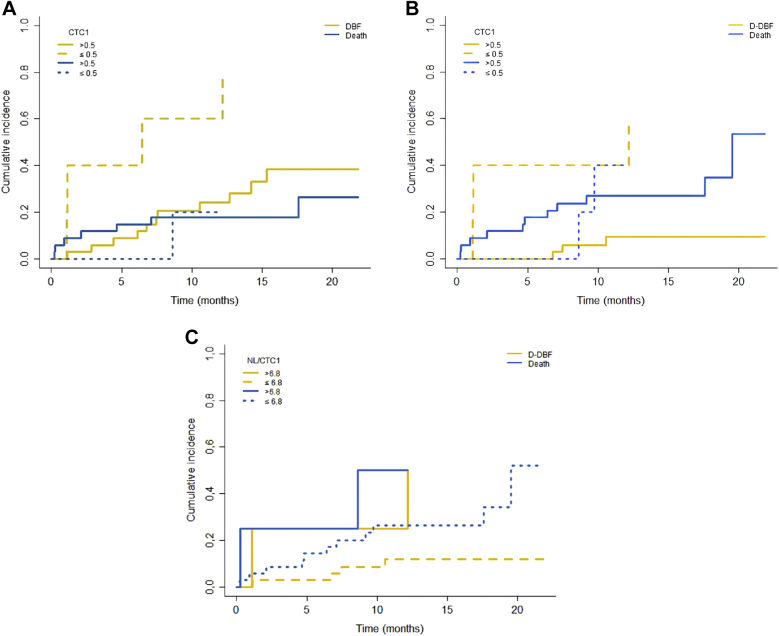
The cumulative incidence of DBF at 6 months, with death as a competing risk factor, was 40% in patients with ≤0.5 CTC/mL and 8.82% in patients with CTC1 >0.5 CTC/mL (P = .007; Fig 1A). That of D-DBF at 6 months was 40% in patients with ≤0.5 CTC/mL and 0 in patients with CTC1 >0.5 CTC/mL (P = .005; Fig 1B) and 25% in patients with NL/CTC1 >6.8 and 2.65% with NL/CTC1 ≤6.8 (P = .063; Fig 2).
Figure 2.
Cumulative incidence of (A) distant brain failure (DBF), (B) diffuse DBF (D-DBF) stratified by circulating tumor cells (CTC) 1 (≤ .5 or >0.5 CTC/mL), and D-DBF stratified by new lesions (NL)/CTC1 (≤6.8 or >6.8) based on the Fine-Gray model.
On multivariate analysis, after the Cox proportional selection and stepwise regression, DBFFS was inferior in patients with CTC1 ≤0.5 CTC/mL (HR, 8.27; 95% CI, 2.12-32.3; P = .002) and superior in patients with immunophenotype HER2-positive (HR, 0.128; 95% CI, 0.025-0.534; P = .013), and D-DBFFS was inferior in patients with CTC1 ≤ 0.5 CTC/mL (HR, 10.22; 95% CI, 1.99-52.41; P = .005).
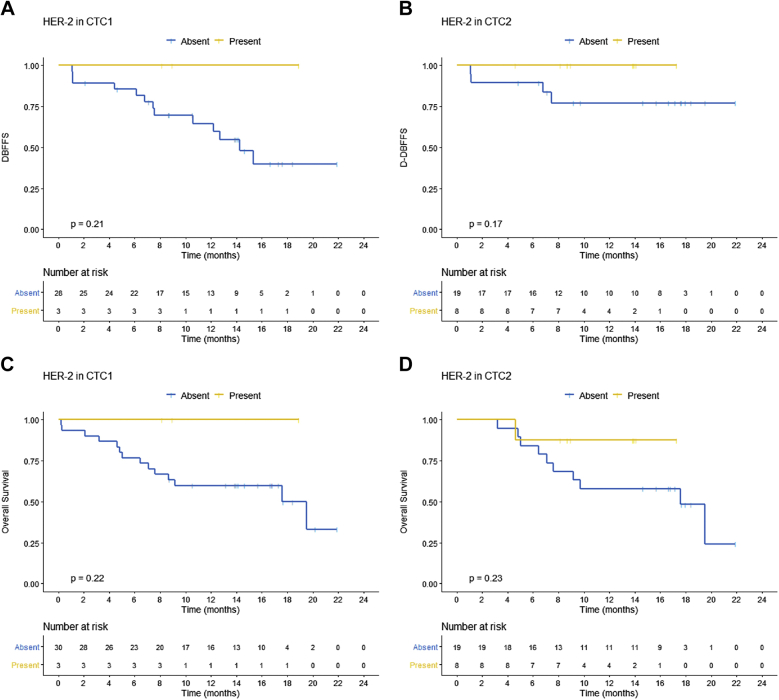
There was no significant association between DBFFS/D-DBFFS and CTC2, number of extracranial metastases (ECM) sites (1 vs ≥2), or kinetics of CTC (CTC2/CTC1). The expression of the proteins COX2, EGFR, ST6GALNAC5, and NOTCH1 in CTC1 and CTC2 was not associated with DBFFS and D-DBFFS. However, there was a trend to longer DBFFS in patients who expressed HER2 in CTC1 and CTC2 (Fig 3A and 3B).
Figure 3.
Kaplan-Meier plot for distant brain failure-free survival (DBFFS) stratified by HER2 expression in (A) circulating tumor cells (CTC) 1 and (B) CTC2 and for overall survival (OS) stratified by HER2 expression in (C) CTC1 and (D) CTC2.
OS
After a median follow-up of 16.6 months (95% CI, 14.8-18.4) in the 39 evaluable patients, there were 16 deaths, 11 (68%) due to extracranial progression, mainly in the lung (9 out of 11). The median OS was 19.5 months (95% CI, 16.1-22.9).
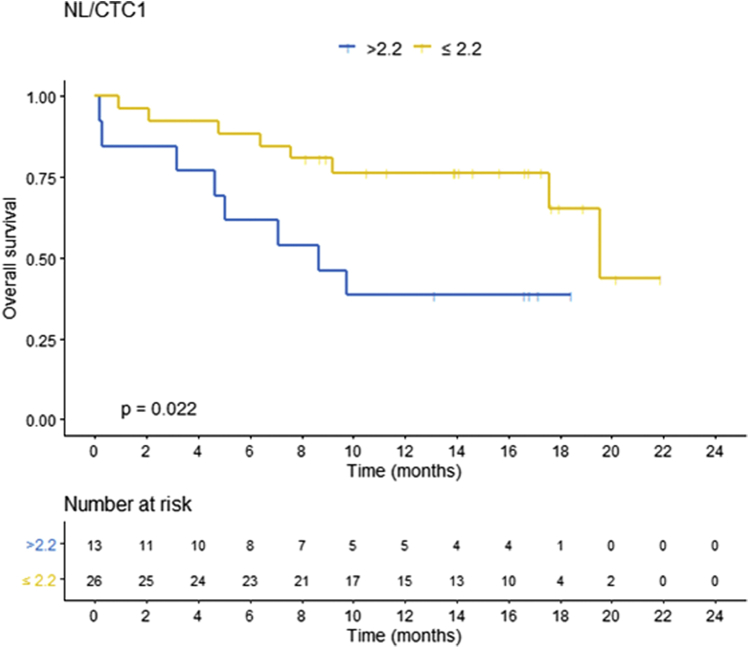
The median OS was 8.6 months in patients with CTC1 ≤0.5 CTC/mL and 19.5 months in patients with CTC1 >0.5 CTC/mL (HR, 3.07; 95% CI, 0.95-9.82; P = .047). It was 4.8 months in patients with immunophenotype triple negative and not reached in patients with immunophenotypes luminal B and HER2-positive (HR, 0.15; 95% CI, 0.04-0.5; P = .002) and 19.5 months in patients with DS-GPA >2, 7.6 months in patients with DS-GPA ≤2 (HR, 0.23; 95% CI, 0.08-0.65; P = .006), 8.6 months in patients with NL/CTC1 >2.2, and 19.5 months in patients with CTC1 ≤2.2 (HR, 3.32; 95% CI, 1.19-9.26; P = .02; Fig 4)
Figure 4.
Kaplan-Meier plot for overall survival (OS) stratified by new lesions (NL)/circulating tumor cells (CTC) 1.
On multivariate analysis, after the Cox proportional selection and stepwise regression, OS was superior in patients with NL/CTC1 ≤2.2 CTC/mL (HR, 0.159, 95% CI, 0.050-0.505; P = .002) and superior in patients with immunophenotype HER2-positive (HR, 0.073; 95% CI, 0.018-0.288; P < .0001) and luminal B (HR, 0.224; 95% CI, 0.062-0.816; P = .023).
There was no significant association between OS and CTC2, number of ECM sites (1 vs ≥2), or kinetics of CTC (CTC2/CTC1). The expression of the proteins COX2, EGFR, ST6GALNAC5, and NOTCH1 in CTC1 and CTC2 was not associated with OS. However, there was also a trend to longer OS in patients who expressed HER2 in CTC1 and CTC2 (Fig 3C,D).
Discussion
This translational study showed that CTCs were detectable in almost all patients and that among women with BCBM, those with a lower number of CTCs (≤0.5 CTC/mL) before SRT were significantly more likely to develop early DBF and D-DBF. Additionally, the ratio of NL to CTC before SRT was a potential prognostic factor of D-DBF and an independent prognostic factor of OS. These results are promising and may be applicable in a recurrent clinical dilemma, which is the decision between SRT or WBRT to optimize the control of BCBM and mitigate toxicity.20,21
The high observed rates of CTC detection may be related to the ISET method of isolation by filtration compared with the CellSearch system (Veridex), which is the most used and based on the separation of cells expressing epithelial markers. During cancer cell dissemination, especially in the epithelial to mesenchymal transition, the epithelial surface markers can be downregulated. Therefore, a lower detection rate may be observed in a method in which the CTC detection and isolation rely only on the positivity of epithelial markers.22,23 The ISET method has been validated in several published studies with different types of cancer, providing high sensitivity (1 CTC/mL) and specificity (100%).24
A significant association between the number of CTCs before treatment and survival results has already been established; it is an independent predictor of progression-free survival and OS, with an inverse relation in patients with metastatic breast cancer.25,26 Still, there is a unique clinical study that evaluated the effect of CTC on BCBM outcome. In a preplanned analysis of the LANDSCAPE phase II trial, patients with HER2-positive metastatic breast cancer with BCBM without previous WBRT who received first-line combination of lapatinib and capecitabine had CTC detected (CellSearch) at baseline and day 21. The central nervous system objective response and 1-year OS rate were significantly higher in patients with no CTC at day 21, but there was no difference in time to progression, an outcome that involved the evaluation of new brain metastases.27
Despite being counterintuitive, our finding of significant association of the direct relationship between the number of CTC and DBFFS has been reported in exploratory analysis of a few retrospective studies. Undetectable CTC status was positively correlated with presence of BCBM and OS in a series of patients with metastatic breast cancer.28 Likewise, in a cohort of patients with brain metastases of non-small cell lung cancer, patients with isolated metastases to the brain were less frequently identified as CTC-positive compared with patients with multiple metastatic sites, including the brain, although CTC were still predictive for OS.29 More recently, an update of the breast DS-GPA revealed that time from primary diagnosis to BCBM was shorter in patients without ECM compared with those with ECM, suggesting that some patients may have occult BCBM at presentation of early-stage breast cancer and/or a more brain-metastatic tumor phenotype.3 Besides that, there was no relationship between the burden of extracranial disease, represented by the number of ECM sites, with DBF and OS in our study, which is in contrast with recent findings of higher incidence of BCBM in patients with a greater number of metastatic sites.30
Therefore, beyond quantity, a qualitative analysis of CTC may refine the prediction of brain disease control. For this purpose, we evaluated the ratio of NL to CTC, which embodies a qualitative indicator, as the greater the ratio, the more we can infer that a smaller number of CTCs is associated with a greater number of BCBM, and therefore these CTCs probably generate more brain metastasis. In fact, NL/CTC1 was a potential prognostic factor of D-DBF and an independent prognostic factor of OS. Regarding the expressions of proteins that eventually could characterize BCBM-associated CTC in this study, HER2 was the only one associated with a trend to longer DBFFS and OS, both in CTC1 and CTC2. Interestingly, HER2 was 1 of 4 markers that composed a BCBM signature of CTC that was highly invasive and capable of generating brain and lung metastases in a patient-derived xenograft mouse model,13 and 9 out of 16 deaths in our cohort were due to ECM progression in the lung. This is coherent with a shorter OS in patients with a NL/CTC1 >2.2, suggesting that this sort of CTC is prone to brain and lung progression.
In this context, the possibility of spontaneous interconversion of HER2 phenotypes in the CTC, irrespective of the HER2 status of the primary breast cancer,31 highlights the potential predictive and prognostic effect of phenotypic characterization of CTC. Additionally, in patients with HER2-positive immunophenotype on primary tumor that had negative expression of HER2 in CTC, the conversion of the phenotype may be associated with a response to the anti-HER2 targeted therapies. Of note, among the 20 patients with HER2-positive immunophenotype on primary tumor in our study, 15 were on anti-HER2 targeted therapies before SRT, with 8 of them on dual blockade.
After evaluating the multiple factors associated with DBF and reviewing our results and clinical and experimental evidence from the recent literature, we hypothesize that, among multiple other possibilities, in patients with BCBM there is a development of a premetastatic brain environment that involves the infiltration of immunosuppressive neutrophils and the reduction of cytotoxic T cells,32 in addition to the infiltration of myeloid cells that produce chemokines and attract other myeloid cells and CTC, with consequent proliferation of brain metastasis.33 Myeloid cells are stimulated by COX2 from primary tumors, and a high expression of COX2 was observed in CTC1 and CTC2 in our data. Considering that COX2 is associated with intercompartmental migration between the brain, cerebrospinal fluid, and blood,34 it is plausible that the brain environment is already amenable to the formation of metastases and tends to attract CTC to the central nervous system and reduce their amount in the bloodstream. Thus, patients with BCBM and a lower CTC count in the blood would have a higher risk of new brain metastasis, because it is likely that a greater number of CTC is in the brain compartment. On the other hand, patients with a higher number of CTC in the blood would have a mechanism of evasion from the brain attraction. The way forward to continue this investigation and test our hypothesis is to carry out a study to evaluate and compare CTC in the blood and cerebrospinal fluid of patients with BCBM.
These results should be considered in the circumstances of the limitations of the research. The small sample size may have led to a biased overestimated analysis, and the inclusion of multiple immunophenotypes of breast cancer with different propensity to develop brain metastases was a causal factor of the heterogeneity of the results. The immunocytochemistry performed to evaluate the expression of multiple proteins in CTC is a challenging process, with a sensitivity variability and risk of cross-reactivity with the distinct antibodies. Additionally, the different systemic therapies used may have influenced both the number of CTC and brain disease control, although the latter is unlikely. From another perspective, this was a prospective and pragmatic study that accrued only patients with breast cancer, without the inherent biases from retrospective analysis with different primary tumors that developed risk score, nomogram, or prognostic metrics to predict DBF after SRT.6, 7, 8 Although adding some evidence in a seldom-explored topic, the data presented herein are hypothesis-generating, and further prospective validation is required.
In conclusion, our findings indicate that CTCs were detectable in almost all patients with BCBM. CTC before SRT was an independent prognostic factor of DBFFS, and D-DBFFS and NL/CTC before SRT was an independent prognostic factor of OS and a potential prognostic factor of D-DBF at 6 months. These data suggest that CTC and NL/CTC1 may have a role as a biomarker of early D-DBF and as a subsequent guide between focal or WBRT in patients with BCBM.
Footnotes
Sources of support: This study was supported by a grant from International Atomic Energy Agency (contract 20541/R0).
Disclosures: none.
Patient level data for this study are available at Castro, Douglas (2020), “DataCTC,” Mendeley Data, V1, https://doi.org/10.17632/4rwvkddj9v.1.
References
- 1.Ostrom Q.T., Wright C.H., Barnholtz-Sloan J.S. Brain metastases: Epidemiology. Hand Clin Neurol. 2018;149:27–42. doi: 10.1016/B978-0-12-811161-1.00002-5. [DOI] [PubMed] [Google Scholar]
- 2.Moravan M.J., Fecci P.E., Anders C.K. Current multisdiscipinary management of brain metastases. Cancer. 2020;126:1390–1406. doi: 10.1002/cncr.32714. [DOI] [PubMed] [Google Scholar]
- 3.Sperduto P.W., Mesko S., Li J. Beyond an updated graded prognostic assessment (breast GPA): A prognostic index and trends in treatment and survival in breast cancer brain metastases from 1985 to today. Int J Radiat Oncol Biol Phys. 2020;107:334–343. doi: 10.1016/j.ijrobp.2020.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kocher M., Soffieti R., Abacioglu U. Adjuvant whole-brain radiotherapy vs observation after radiosurgery or surgical resection of 1 to 3 cerebral metastases: Results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown P.D., Jaeckle K., Ballman K.V. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Press R.H., Prabhu R.S., Nickleach D.C. Novel risk stratification score for predicting early distant brain failure and salvage whole-brain radiotherapy after stereotactic radiosurgery for brain metastases. Cancer. 2015;121:3836–3843. doi: 10.1002/cncr.29590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayala-Peacock D.N., Attia A., Braunstein S.E. Prediction of new brain metastases after radiosurgery: Validation and analysis of performance of a multi-institutional nomogram. J Neurooncol. 2017;135:403–411. doi: 10.1007/s11060-017-2588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McTyre E.R., Soike M.H., Farris M. Multi-institutional validation of brain metastasis velocity, a recently defined predictor of outcomes following stereotactic radiosurgery. Radiother Oncol. 2020;142:168–174. doi: 10.1016/j.radonc.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 9.De Azevedo Santos T.R., Tundisi C.F., Ramos H. Local control after radiosurgery for brain metastases: Predictive factors and implications for clinical decision. Radiat Oncol. 2015;10:63. doi: 10.1186/s13014-015-0367-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkpatrick J.P., Soltys S.G., Lo S.S. The radiosurgery fractionation quandary: Single fraction or hypofractionation? Neuro Oncol. 2017;19:ii38–ii49. doi: 10.1093/neuonc/now301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flores B.C.T., Silva V.S., Abdallah E.A. Molecular and kinetic analyses of circulating tumor cells as predictive markers of treatment response in locally advanced rectal cancer patients. Cells. 2019;8:641. doi: 10.3390/cells8070641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bos P.D., Zhang X.H.-F., Nadal C. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L., Ridgway L.D., Wetzel M.D. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin N.U., Lee E.Q., Aoyama H. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015;16:e270–e278. doi: 10.1016/S1470-2045(15)70057-4. [DOI] [PubMed] [Google Scholar]
- 15.Halabi S., Owzar K. The importance of identifying and validating prognostic factors in oncology. Semin Oncol. 2010;37:e9–e18. doi: 10.1053/j.seminoncol.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assoc. 1958;53:457–481. [Google Scholar]
- 17.Lausen B., Schumacher M. Maximally selected rank statistics. Biometrics. 1992;48:73–85. [Google Scholar]
- 18.Cox DR. Regression models and life tables (with discussion). J Royal Stat Soc. 1972;Series B, 34:187-220.
- 19.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Amer Statist Assoc. 1999;94:496–509. [Google Scholar]
- 20.Yamamoto M., Serizawa T., Shuto T. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 21.Brown P.D., Gondi V., Pugh S. Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: Phase III trial NRG Oncology CC001. J Clin Oncol. 2020;38:1019–1029. doi: 10.1200/JCO.19.02767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farace F., Massard C., Vimond N. A direct comparison of CellSearch and ISET for circulating tumor cell detection in patients with metastatic carcinomas. Br J Cancer. 2011;105:847–853. doi: 10.1038/bjc.2011.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huebner H., Fasching P.A., Gumbrecht W. Filtration based assessment of CTCs and CellSearch® based assessment are both powerful predictors of prognosis for metastatic breast cancer patients. BMC Cancer. 2018;18:204. doi: 10.1186/s12885-018-4115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ried K., Eng P., Sali A. Screening for circulating tumor cells allows early detection of cancer and monitoring of treatment effectiveness: An observational study. Asian Pac J Cancer Prev. 2017;18:2275–2285. doi: 10.22034/APJCP.2017.18.8.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cristofanilli M., Budd G.T., Ellis M.J. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 26.Bidard F.C., Peeters D.J., Fehm T. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: A pooled analysis of individual patient data. Lancet Oncol. 2014;15:406–414. doi: 10.1016/S1470-2045(14)70069-5. [DOI] [PubMed] [Google Scholar]
- 27.Pierga J.Y., Bidard F.C., Cropet C. Circulating tumor cells and brain metastasis outcome in patients with HER2-positive breast cancer: the LANDSCAPE trial. Ann Oncol. 2013;24:2999–3004. doi: 10.1093/annonc/mdt348. [DOI] [PubMed] [Google Scholar]
- 28.Mego M., De Giorgi U., Dawood S. Characterization of metastatic breast cancer patients with nondetectable circulating tumor cells. Int J Cancer. 2011;129:417–423. doi: 10.1002/ijc.25690. [DOI] [PubMed] [Google Scholar]
- 29.Hanssen A., Riebensahm C., Mohme M. Frequency of circulating tumor cells (CTC) in patients with brain metastases: Implications as a risk assessment marker in oligo-metastatic disease. Cancers (Basel) 2018;10.pii:E527. doi: 10.3390/cancers10120527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin A.M., Cagney D.N., Catalano P.J. Brain metastases in newly diagnosed breast cancer: A population-based study. JAMA Oncol. 2017;3:1069–1077. doi: 10.1001/jamaoncol.2017.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jordan N.V., Bardia A., Wittner B.S. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature. 2016;537:102–106. doi: 10.1038/nature19328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L., Yao J., Wei Y. Blocking immunosuppressive neutrophils deters pY696-EZH2-driven brain metastases. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.aaz5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y., Kosaka A., Ikeura M. Premetastatic soil and prevention of breast cancer brain metastasis. Neuro Oncol. 2013;15:891–903. doi: 10.1093/neuonc/not031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen J.E., Patel A.S., Prabhu V.V. COX-2 drives metastatic breast cells from brain lesions into the cerebrospinal fluid and systemic circulation. Cancer Res. 2014;74:2385–2390. doi: 10.1158/0008-5472.CAN-13-2660. [DOI] [PubMed] [Google Scholar]