Abstract
Purpose
Selective internal radiation therapy (SIRT) is administered to treat tumors of the liver and is generally well tolerated. Although widely adopted for its therapeutic benefits, SIRT is rarely combined with external beam radiation therapy (EBRT) owing to the complexity of the dosimetry resulting from the combination of treatments with distinct radiobiological effects. The purpose of this study was to establish a dosimetric framework for combining SIRT and EBRT using clinical experience derived from representative patients with hepatocellular carcinoma (HCC) who received both therapies.
Methods and Materials
Treatments from 10 patients with HCC given EBRT either before or after SIRT were analyzed. The dosimetry framework used here considered differences in the radiobiological effects and fractionation schemes of SIRT versus EBRT, making use of the concepts of biological effective dose (BED) and equivalent dose (EQD). Absorbed dose from SIRT was calculated, converted to BED, and summed with BED from EBRT dose plans. Two of these patients were used in a virtual planning exercise to investigate the feasibility of combining stereotactic body radiation therapy and SIRT.
Results
The combination of EBRT and SIRT in 10 patients with HCC showed no major toxicity. No Child-Pugh scores went above 8 and albumin-bilirubin scores from only 1 patient worsened to grade 3 (> -1.39) from treatment through 3-months follow-up. A framework with radiobiological modeling was developed to manage the combined treatments in terms of their sum BED. The exploratory SIRT plus SABR inverse dose plans for 2 patients, incorporating radiobiologically informed 90Y SIRT dosimetry, achieved dose distributions comparable to SBRT alone.
Conclusions
Treatment with both EBRT and SIRT can be given safely to patients with HCC. The BED and EQD concepts should be used in combined dosimetry to account for the differing radiobiological effects of EBRT and SIRT. Inverse dose planning of EBRT after SIRT could provide improved dose distributions and flexibility to the clinical workflow. Further research into combination therapy is needed through prospective trials.
Introduction
Absorbed radiation dose, the energy deposited in a mass of tissue, is the cornerstone of radiation therapy treatment. For external beam radiation therapy (EBRT), treatment planning has been undertaken for the past several decades considering absorbed physical dose together with the biological effects on exposed tissues. In this way, information about the radiobiological responses of different tissues informs EBRT protocols worldwide.
In recent years, the importance of tailoring selective internal radiation therapy (SIRT) dosimetry to optimize the radiation absorbed dose for individual patients has been gaining traction. For example, a European Union directive now requires radionuclide therapy treatments to have dose quantification performed to improve efficacy.1 To the benefit of the patient, these developments offer clinicians an opportunity to compare doses achieved with yttrium-90 (90Y) SIRT to treatment outcomes.
It has been shown in a number of recent studies that radiation absorbed dose after 90Y SIRT predicts outcomes. These studies have assessed efficacy and toxicity in relation to radiation absorbed dose.2,3 A strong correlation was identified that gives credibility to the dose-response relationship of 90Y SIRT. The consensus has been that 90Y SIRT is well tolerated owing to its low dose rate and, therefore, capacity for damage repair during the exposure period.
In general, modeling to illuminate the radiobiological effects of 90Y has used the Lea-Catcheside protraction factor (G) applied to the β-term of the linear quadratic model.4 This assumes that the α-term of the linear quadratic model is equivalent for EBRT and 90Y SIRT, meaning radiobiologically informed doses were comparable to physical doses from EBRT. However, experimental evidence from 2 in vitro studies has shown that this is not the case. In these studies, it was found that a physical dose of 90Y requires 2 to 3 times the dose of EBRT to achieve the same level of cell-kill.4,5
Few studies have investigated the possible combination of EBRT with radionuclide therapy from a dosimetric approach.6,7 Bodey et al8 considered combination therapy of EBRT with iodine-131-metaiodobenzylguanidine or -NaI and found improvement in the dose distribution compared with either single therapy. Wang et al9 reported for the first time 90Y dosimetry and outcomes for 22 patients with hepatocellular carcinoma (HCC) treated with SIRT and EBRT. The main finding of this important study was that, with careful patient selection, the combination was feasible and safe. As acknowledged by the authors, the potential limitations of this study include the use of single-photon emission computed tomography (SPECT) imaging as the basis for dose calculation, as it has inferior spatial resolution compared with positron emission tomography (PET). Also in the Wang et al study, the inclusion of voxels outside the body may have led to the underestimation of dose, and thus the reliance on nondeformable image registration would fail to account for changes in the geometry of abdominal organs. Also, inverse dose planning, which can improve the sum doses through precision targeting of EBRT, was omitted. Although this study did address biological effective dose (BED), the radiobiological parameters used were extrapolated from EBRT and not based on experimental evidence. BED is the dose of 1 source of ionizing radiation that is required to achieve the same effect as a reference irradiation. Equivalent dose (EQD) is the BED scaled in terms of the physical dose of the reference irradiation so equivalent biological effects can be compared in the same dose units.10
Based on a rare cohort of patients treated with both 90Y SIRT and EBRT, the aim of this study was to develop a workflow using radiobiologically informed dosimetry to facilitate the future combination of stereotactic body radiation therapy (SBRT) and 90Y SIRT. Combined treatments would be particularly useful for patients who do not need acute pain palliation but in whom downsizing of large tumors would enable further directed therapy. The hypothesis investigated was that 90Y SIRT followed by SBRT may be used to increase dose to targeted tumor tissue while retaining the benefit of low normal tissue complication rate. Treatment plans of 10 patients with HCC given both SIRT and EBRT (palliative or SBRT) were analyzed. Absorbed dose distributions were calculated from SIRT imaging, converted to BED, and summed with BED from EBRT dose plans. Two of these patients were investigated further in a virtual inverse dose planning exercise incorporating radiobiological information from 90Y SIRT.
Methods and Materials
Patient selection
Ten patients out of a total of 95 patients with HCC treated with 90Y SIRT were also treated with EBRT (palliative or SBRT) at the Sarah Cannon Cancer Center (Nashville, TN) between June 2014 and July 2018. Eight patients received palliative EBRT followed by 90Y SIRT. These patients were ineligible for SBRT before 90Y SIRT as institutional guidelines disqualified patients with tumors larger than 6 cm. Two patients received 90Y SIRT followed by EBRT. All patients granted informed consent and were included in the institutional review board ethics master agreement of Sarah Cannon.
Simulation and treatment
External beam planning involved supine positioning within an indexed (to the table) custom immobilization Vac-Lok bag (Civco Medical Instruments Co, Inc, Coralville, IA) with both arms raised in a wing board. TrueBeam (Varian Medical Systems, Palo Alto, CA), Trilogy (Varian), or CyberKnife (Accuray Incorporated, Sunnyvale, CA) systems delivered photons at energies of 6-23 MV. Apart from 1 SBRT case, palliative EBRT regimens of 3 Gy per fraction were used to irradiate large tumors. Smaller satellite tumors were not targeted by the EBRT plan. A free-breathing 4-dimensional computed tomography (CT) scan of the abdomen and chest without intravenous or oral contrast was acquired in 1.25-mm thick image slices. Daily image guidance was performed using cone beam CT before each treatment. The intent was to allow 4 weeks post-EBRT for recovery of acute side effects, then to proceed with assessment for 90Y SIRT. For the patients who were given SIRT first, the intent of EBRT was to treat residual tumor that could not be adequately accessed via the hepatic artery due to poor flow or occlusion of tumor-feeding vessels, as identified by hepatic angiogram and CT angiography. SIR-Spheres (Sirtex Medical Ltd, North Sydney, Australia) resin microspheres with embedded 90Y (physical half-life 64.1 h) delivered 0.93 MeV electrons. All patients were evaluated and treated according to the REBOC consensus guidelines.11 Post-SIRT follow-up included physical examination, laboratory tests, and multiphasic abdominal CT or fat-saturated magnetic resonance imaging with Eovist (Bayer HealthCare Pharmaceuticals Inc, Whippany, NJ) contrast at 6 weeks, 12 weeks, and every 3 months thereafter in clinic. Common Criteria for Adverse Events 4.0 was used to score acute and delayed side effects of radiation therapy.12 Alpha-fetoprotein, Child-Pugh categories, and albumin-bilirubin (ALBI) scores were collected for each patient, before and after each treatment.
Dosimetry
The liver and any intrahepatic tumors were contoured on all available images to determine change in volumes. Normal liver was defined as the nontumoral liver. 90Y SIRT dosimetry was performed using a custom workflow in MIM (version 6.9.4; MIM Software, Inc, Cleveland, OH). The 90Y SIRT doses were determined using the total PET signal inside the body contour, without lung-shunt correction. Dosimetry using the body-based (MIM SurePlan default) and liver-based (common for research) methods differed in that the administered activity was equated to total signal from 90Y imaging in the body versus liver contours. Mean absorbed tumor doses from the body-based method were compared with those from the common liver-based method. EBRT dosimetry was performed using the Eclipse (Varian) treatment planning system.
Radiobiology
MIM software was used to compare radiation doses from SIRT and EBRT. BED was calculated in terms of 6 MV LINAC photons using the model adapted from equation 2 of Abbott et al.13 The assumed radiobiologic model parameters for HCC informed by the findings from Lee et al4 and Tai et al14 are listed in Table 1.
Table 1.
Assumed HCC radiobiologic model parameters respective of Abbott et al13
| Parameter (units) | SBRT |
90Y SIRT |
Description | ||
|---|---|---|---|---|---|
| HCC tumor | Normal liver | HCC tumor | Normal liver | ||
| (Gy) | Variable | Variable | Variable | Variable | Physical absorbed dose |
| 1 | 1 | 0.6 | 0.6 | Maximum relative biological effectiveness to reference radiation | |
| 1 | 1 | 0 | 0 | Lea-Catcheside protraction factor | |
| (Gy) | 17 | 3 | 17 | 3 | Radiosensitivity |
| Variable | Variable | 1 | 1 | Number of fractions | |
Abbreviations: HCC = hepatocellular carcinoma; SBRT = stereotactic body radiation therapy; SIRT = selective internal radiation therapy.
The custom workflow also used MIM software to calculate EQD of 90Y SIRT to EBRT for different fractionation schedules. EQD was calculated at each voxel using the equation where is the BED expression for the desired fractionation scheme.
Treatment planning
To assess whether there is a dosimetric advantage to inverse-dose planning, dose and fractionation regimens were adapted from the Radiation Therapy Oncology Group randomized phase III trial (RTOG 1112) that compared sorafenib versus SBRT followed by sorafenib in HCC.15 Dosimetric parameters were taken from the SCRI treatment protocols, which recommend a maximum of 40 Gy average dose to normal liver from 90Y SIRT to protect against liver decompensation. This threshold was considered in comparison to the accepted limit for EBRT of 30 Gy uniform dose at 2 Gy per fraction.16 The 90Y SIRT dosimetry was converted to equivalent SBRT dose through use of EQD. SBRT was planned with curative intent, treating the tumor uniformly with a total EQD of 50 Gy delivered over 5 fractions of 10 Gy from the combined therapies.
Two exemplar patients were selected to capture variable tumor presentations. For each patient, 2 different radiation therapy plans were generated to compare the differences of the combined therapy. The first plan incorporated SBRT followed by 90Y SIRT. The second plan was 90Y SIRT followed by SBRT using the isodose method. Corresponding voxels in the CTs associated with each treatment were determined in MIM through a proprietary contour and intensity-based deformation algorithm. The BED maps calculated from EBRT and 90Y SIRT were accumulated in MIM using a voxel-wise sum.
Statistical analysis
Statistical analysis was performed using OriginPro (version 2019b; OriginLab Corporation, Northampton, MA). Kaplan-Meier estimation for overall survival (OS) used the date of the first treatment and known survival censor dates. Linear regression was used to assess dose-response relationships based on mean voxel doses or BEDs from contours of interest. The paired t test was used to compare the difference in mean tissue doses.
Results
Patient selection
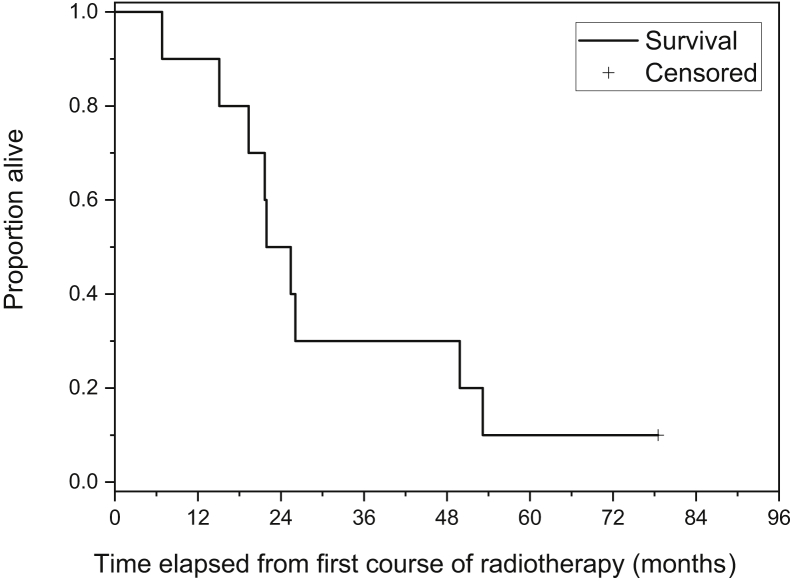
Characteristics of the 10 patients with HCC at the time of initial presentation are included in Table 2. No patient had prior chemotherapy, ablation, chemoembolization, or vascular procedures. Apart from patient 8, who received sorafenib, no other therapies were rendered to patients after SIRT and EBRT. OS was calculated from the date of first radiation therapy (SIRT or SBRT) to the liver. Median OS was 21.9 months (Fig 1).
Table 2.
Patient characteristics at initial presentation (n = 10)
| Characteristics | n (%) or mean ± standard deviation |
|---|---|
| Age (y) | 61 ± 10 |
| Sex | |
| Male | 8 (80%) |
| Female | 2 (20%) |
| Weight (kg) | 82.8 ± 24.3 |
| Height (m) | 1.8 ± 0.12 |
| ∗Individual tumor volume (mL) | 965 ± 1242 |
| †Tumor burden (%) | 46 ± 21 |
| <25% | 1 (10%) |
| 25%-50% | 4 (40%) |
| >50% | 5 (50%) |
| ‡Total tumor volume (mL) | 1737 ± 1226 |
| §Extrahepatic metastases | |
| Lung and lymph node | 1 (10%) |
| Prostate | 1 (10%) |
| Rectum | 1 (10%) |
| Prior treatment | |
| Primary HCC resection | 1 (10%) |
| Chemotherapy | 0 (0%) |
| Radiation therapy | 0 (0%) |
| Ablation | 0 (0%) |
| Concurrent treatment | 0 (0%) |
| Therapies after EBRT + SIRT (sorafenib) | 1 (10%) |
| Portal vein thrombosis | 3 (30%) |
| Hepatitis | |
| Type B | 0 (0%) |
| Type C | 6 (60%) |
| Number of tumors per patient | 1.8 ± 0.8 |
| 1 | 4 (40%) |
| 2 | 4 (40%) |
| 3 | 2 (20%) |
| Targeted liver lobe | |
| Right | 3 (30%) |
| Left | 1 (10%) |
| Both | 6 (60%) |
| SIRT treatment delivered before EBRT | 2 (20%) |
| Body surface area (m2) | 1.86 ± 0.32 |
| ‖Lung-shunt (%) | 7.3 ± 4.7 |
| Administered 90Y activity (MBq) | 1934 ± 293 |
| EBRT dose schedule | |
| 7 fractions of 3 Gy | 8 (80%) |
| 6 fractions of 3 Gy | 1 (10%) |
| 5 fractions of 10 Gy | 1 (10%) |
| EBRT photon beam energy (MV) | 11 ± 6 |
| 6 MV | 4 (40%) |
| 10 MV | 3 (30%) |
| 18 MV | 1 (10%) |
| 23 MV | 1 (10%) |
| 6 MV + 23 MV | 1 (10%) |
| EBRT treatment time (d) | 8.6 ± 1.1 |
Abbreviations: EBRT = external beam radiation therapy; HCC = hepatocellular carcinoma; SIRT = selective internal radiation therapy.
All tumors considered independently before any radiation therapy
Percent (%) of whole liver occupied by tumor before any radiation therapy
Cumulative volume of tumors from the last computed tomography (CT) before any radiation therapy
Three distinct patients
Missing data for 1 patient
Figure 1.
Overall survival in patients with hepatocellular carcinoma (HCC) who received external beam radiation therapy (EBRT) and 90Y selective internal radiation therapy (SIRT). The median survival was 8.64 months and the mean survival was 13.2 months (95% confidence interval, 7.2-19.2 months), determined by the Kaplan-Meier estimator. Survival analysis disregarded whether SIRT or EBRT was delivered first.
For 8 patients with large hepatic tumors, palliative EBRT was delivered before 90Y SIRT for pain relief. SIRT was not used for palliation as it does not provide immediate pain relief as workup takes 7 to 10 days. No patient had sufficiently small tumors to be a candidate for SBRT. Because 90Y SIRT is generally well-tolerated, it was used following the palliative EBRT treatments, which did not achieve cure by itself in any of the patients. For 2 patients, SIRT was given before SBRT because they initially met the REBOC criteria for liver-directed therapy.11 SIRT was preferred over other liver-directed therapies such as transarterial chemoembolization due to the lower risk of postembolization syndrome, which requires hospital admission of patients for pain control. Avoidance of toxicity was particularly important in these patients because of their low performance status, and most were already experiencing some abdominal symptoms at presentation. For each patient, Child-Pugh scores, ALBI scores, and laboratory tests (alanine aminotransferase, aspartate aminotransferase, total bilirubin, albumin, international normalized ratio, and alpha-fetoprotein) were recorded before and after treatment (Tables E1-9). No Child-Pugh scores went above 8 and ALBI scores from only 1 patient worsened to grade 3 (> –1.39) from treatment through 3-months follow-up. Although both patients who received 90Y SIRT before EBRT showed clinical response in areas that accumulated 90Y microspheres well, hypovascular areas of liver tumor had poor microsphere uptake. Subsequently, SBRT deposited additional dose to undertreated areas to maximize therapeutic effects. EBRT and 90Y SIRT dosimetry were considered separately owing to the variable timing of the patient treatment schedules (Figs E1-4).
Volumetry
Volume was measured relative to baseline imaging: the EBRT planning CT or the last CT or magnetic resonance scan acquired before 90Y SIRT. Bias in the measured percent volume change at the first follow-up time point is possible owing to inflammation immediately after 90Y SIRT administration. Most of the EBRT-treated tumors did not change in volume, likely owing to the modest doses delivered for palliation. The few tumors that increased in volume were smaller lesions assumed as asymptomatic and which had intentionally not been included in the EBRT treatment volume. Among all 18 tumors treated with 90Y SIRT, only 2 progressed. A noteworthy example was the progression of 1 tumor in patient 1, which also had the largest mean and standard deviation dose of 39.56 ± 11.23 Gy from EBRT. Differential response was observed within the same patient where the 2 other tumors reduced in volume despite lower doses from EBRT of 6.38 ± 3.37 and 11.41 ± 1.48 Gy.
Dosimetry
Absorbed doses from 90Y SIRT were calculated using the body- and liver-based methods (Fig E5). EBRT dosimetry was retained from the original plans, but new contours were made for this study to ensure consistency, for example, tumor indexing across time points, and to eliminate interoperator variability. All tumor contours from baseline and follow-up images were reviewed centrally by an experienced hepatobiliary radiologist (RSY) to include all pre- and posttreatment scans. The images were anonymized and no information on treatment history was provided to the radiologist. Differences between the original EBRT planning contours and new contours were observed; for example, patient 2 had part of the new tumor contour outside of the EBRT fields based on the original planning contours.
Combined treatments
The time interval between EBRT and subsequent 90Y SIRT was a median of 7.4 weeks (range, 1.9-20.7 weeks), and the intervals between SIRT with subsequent EBRT were 20.7 and 15.9 weeks. Patients treated with EBRT before SIRT typically had a dominant large mass contributing to tumor burden (Table 2). No patient experienced Common Criteria for Adverse Events 4.0 grade 3 or higher toxicities. Three patients had mild acute side effects from SIRT, 2 of whom also had mild side effects from EBRT (Table 3). For the cases where 90Y SIRT was delivered first, the tumor burden was lower compared with those where EBRT was the first treatment. Small tumors that did not contribute greatly to the total tumor burden were intentionally not targeted by EBRT. Change in individual tumor volumes, illustrated by bar charts, were used in subsequent regression analysis.
Table 3.
Summary of CTCAE 4.0 toxicity scores of delivered radiation therapy treatments
| Follow-up timepoint | EBRT toxicity |
90Y SIRT toxicity |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 wk | 2 wk | 6 wk | 12 wk | 6 mo | 9 mo | 12 mo | 15 mo | 18 mo | 21 mo | 24 mo | |
| Patient 1 | 0 | 0 | 0 | 0 | 0 | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. |
| Patient 2 | 0 | 0 | 0 | 0 | 0 | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. |
| Patient 3 | 0 | 0 | 0 | 0 | 0 | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. |
| Patient 4 | 0 | 0 | 0 | 0 | 0 | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. |
| Patient 5 | Fatigue grade 2 | Fatigue grade 2 | 0 | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. |
| Patient 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | n.c. |
| Patient 7 | Fatigue grade 2 | Fatigue grade 2 | 0 | 0 | 0 | 0 | n.c. | n.c. | n.c. | n.c. | n.c. |
| Patient 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Patient 9 | 0 | Pain grade 2 | 0 | 0 | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. |
| Patient 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | n.c. | n.c. | n.c. | n.c. |
Abbreviations: CTCAE = Common Criteria for Adverse Events; EBRT = external beam radiation therapy; n.c. = not collected; SIRT = selective internal radiation therapy.
Detailed measures of transaminases, albumin-bilirubin, and Child-Pugh scores can be found in Tables E1 to E4.
Although dosimetry was shown to predict 90Y SIRT response in several recent studies, the patients described here also received EBRT, which potentially affects outcomes. However, there were no complications from procedure administration. All tumors were targeted to achieve ablative doses by the SIRT treatment. However, 2 treatments (patients 1 and 2) were delivered without prior EBRT, and those patients had a lower baseline tumor burden than did the group with prior EBRT. Because these cases would not be comparable with the other 8 patients in the cohort, they were considered separately. The normal liver typically absorbed lower doses from SIRT than tumor (15.6 Gy difference in mean dose, P = .0044), which is necessary to achieve a therapeutic effect (Fig E6). Radiologic response and changes in individual tumor volumes were used in subsequent analysis.
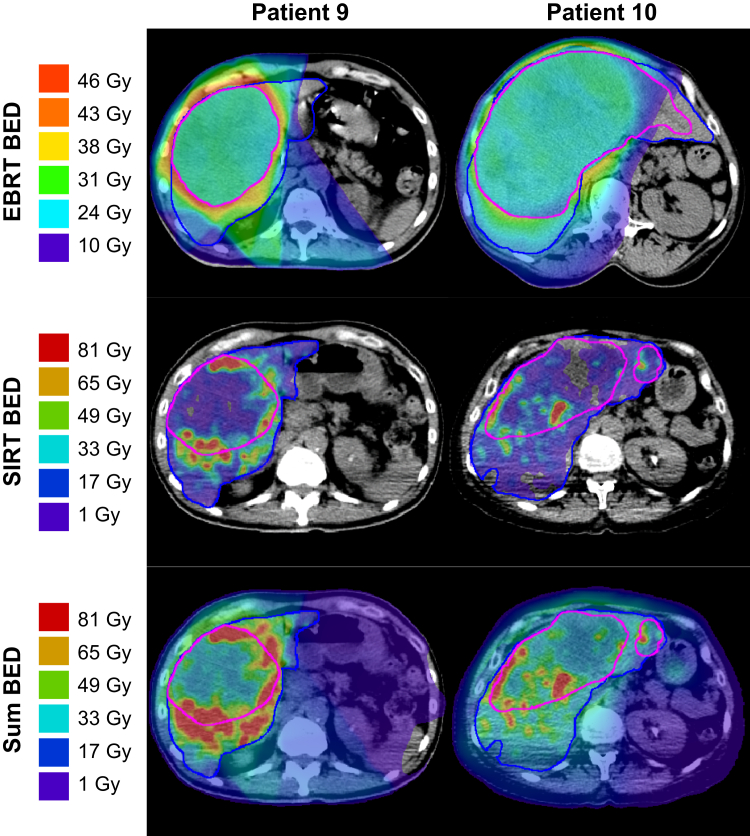
The SIRT and EBRT doses were combined using sum BED. The BED for each treatment was calculated independently using the same “BED units.” Due to a lack of data describing repopulation dynamics, no repopulation term was adopted in the BED models. For the 2 patients who received EBRT after SIRT, 40 Gy of physical dose from 90Y was selected as the maximum dose tolerated by normal tissue based on the planning constraint adopted from clinical experience at the SCRI and guided by consensus opinion.17 The BEDs were accumulated on a voxel-wise basis where the voxel doses from each treatment were assigned to their respective location in the patient as defined using registration. Not all images were collected in the same manner, for example, a breath hold was not always feasible during SPECT/CT scans, which required a longer exposure than PET/CT. The MIM workflow used the liver and tumor contour- and intensity-based deformable registration algorithm. Calculated BEDs and their summation are exemplified in Figure 2 with 2 sample patients. The histograms from each treatment are shown in Figure E7 for all patients.
Figure 2.
Biological effective dose (BED) maps for representative patients (9 and 10). The rows illustrate the BED calculated from external beam radiation therapy (EBRT), 90Y selective internal radiation therapy (SIRT), and their voxel-wise sum determined through a contour and intensity-based deformation using MIM. To aid in visualizing treated areas exceeding the 40 Gy physical dose constraint to normal liver, the SIRT and sum BED rows are displayed using differing scaling. In addition, tumor tissues had different BED scaling than the normal tissue due to the differing radiosensitivity parameters of each tissue. Liver is contoured in blue and tumor 1 in magenta.
External beam planning after 90Y microsphere radiation therapy
A separate exercise aimed to assess the feasibility of SBRT dose planning after 90Y SIRT treatment was performed (Fig 3). The selected patients did not have highly specific uptake of 90Y to tumor, but rather included 1 case (patient 9) with only peripheral tumor uptake and moderate uptake to normal tissue and 1 case (patient 10) with multiple lesions with differing uptake. Although many tumors shrunk after prior palliative EBRT, tumors from patients 9 and 10 remained larger than those typically targeted by SBRT.
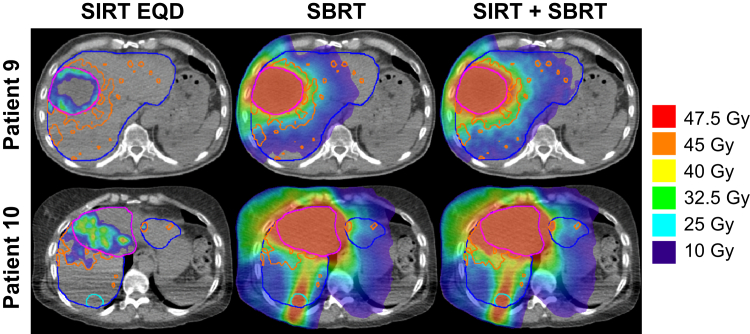
Figure 3.
Stereotactic body radiation therapy (SBRT) plans considering 90Y selective internal radiation therapy (SIRT) for patients 9 and 10. Liver (blue), tumor 1 (magenta), tumor 2 (cyan, patient 10 only), and dose-constraining 7 Gy equivalent dose (EQD) isodose (orange, corresponding to ≈40 Gy to normal liver from 90Y SIRT) contours were used for SBRT planning. The left column illustrates the 90Y SIRT EQD derived from radiobiological modeling. The center column represents the SBRT plan with a single planning target volume (PTV) of the collective tumor contours. The right column shows the accumulated doses of the left and center columns.
The EBRT plans were normalized to deliver the prescription dose to 95% of the target volume while having at least 700 mL of normal liver receiving no more than 21 Gy. However, physical doses from 90Y SIRT and EBRT are not directly comparable owing to differences in the radiobiological effect of the 2 modalities. To account for this, the doses derived from 90Y SIRT were converted to EQD based on 5 × 10 Gy fractions for the EBRT plans. EQD doses of 90Y SIRT were converted from the MIM software body-based dosimetry method using the radiobiology modeling parameters shown in Table 1. The 90Y SIRT 40 Gy isodose curve, EQD of approximately 7 Gy (see next calculation), was imported into Eclipse as a dose constraint to normal liver (α/β = 3 Gy). Dose constraints to tumor were not necessary because improved tumor control is anticipated beyond the prescribed EBRT dose.
The choice of parameter values in the denominator was challenging owing to the need to use a 90Y isodose contour to threshold dose. Rather than using a voxelized base dose approach, where each voxel has a different yet well-defined dose, 40 Gy LINAC dose was selected as the denominator for a 5 × 10 Gy plan. There was some subjectivity involved in selecting the threshold value in the denominator of the EQD equation. The resulting approximated 90Y EQD threshold of 7 Gy was large enough to allow EBRT to be conformally added in some areas of normal liver. A less cautious estimate with 30 Gy total dose to normal liver in the denominator created a 90Y EQD threshold of 8 Gy, which allowed for more EBRT dose in the normal liver. A more cautious estimate using 50 Gy in the denominator, which is an unrealistic choice given the observed average dose of about 25 Gy, created a 90Y EQD threshold of approximately 6 Gy, which allowed less dose and covered a larger portion of the normal liver. To compare, the standard 15 fractions of 2 Gy EBRT normal liver uniform dose threshold has a BED of 50 Gy and corresponding EQDs of 11.5 or 16.7 Gy, erring to the more conservative side. These parameters assume uniform irradiation, but normal liver tolerates uniform liver dose from EBRT less well than the heterogenous SIRT dose.18 Therefore, to best balance the advantages and disadvantages of the varied approaches, 40 Gy was used to achieve a satisfactory compromise.
Lastly, several SBRT plans were generated using the constraints defined previously by an experienced stereotactic dosimetrist (KM). For patient 9, directly accumulating 90Y EQD maps to the standard SBRT plan resulted in definitively inferior dosimetry for all tissues. However, consideration of only normal liver 90Y doses while disregarding targeted tumor 90Y dose improved the plan because it reduced the number of constraints for the optimization algorithm. This algorithm minimizes normal liver doses regardless of any doses it previously received, so resulting normal liver doses were comparable. As is typical when attempting to reduce hot areas using this approach, lower doses were given to more of the normal liver volume while higher doses were given to less volume. Not taking 90Y dose into account resulted in better uniformity and was the superior plan. For the hypothesis that planning EBRT around 90Y dose accomplishes superior dose distributions, this negative finding may explain why such an approach is not usually taken. Subsequently, SBRT was planned for patient 9 with a lower 90Y isodose constraint, which resulted in a larger volume and greater freedom for the optimization algorithm to run.
Doses from 90Y were considered only in the normal liver because it meant that, irrespective of previously delivered tumor doses, the optimization algorithm better minimized toxicity-constraining total normal liver dose. Including constraints on tumor tissue was found to decrease total tumor dose, which is unfavorable for achieving tumor control. The ideal method going forward would be to prescribe SBRT dose using 90Y EQD as a base dose in the optimization calculations. Although this is more challenging to implement due to not being a standard software solution, for example, the aforementioned beam sequence error, the voxelized information effectively reduces the algorithm constraints and allows for a more robust approach. In addition, planning SBRT for patients with smaller tumors might result in superior treatment plans as there would be more spatial degrees of freedom to conform the beam to the planning target volume (PTV). Although the main risk of SIRT toxicity is from 90Y uptake in the normal liver, the observed doses were low (mean, 14.6 Gy; standard deviation, 8.2 Gy; range, 4.3-26.8 Gy). This cohort demonstrated that combined dosimetry of 90Y SIRT and SBRT, taking radiobiological considerations into account, can generate safe plans.
Discussion
This study shows that 90Y SIRT can be delivered in combination with EBRT regardless of the sequencing of the 2 treatments. SBRT alone offered sparing of normal tissue doses, whereas SBRT followed by SIRT delivered unnecessarily high doses to tumor. However, SIRT followed by SBRT offered similar sparing of dose to normal liver while achieving the desired tumor dose.
The images used for inverse dose planning were acquired after the patients received palliative EBRT. When given their initial palliative EBRT treatment, the patients were not eligible for SBRT due to large tumor size. Yet, larger tumors are more difficult to plan using SBRT, so this imperfect approach may still have been conservative. Inverse planning should be applied to SBRT-eligible patients. Patients with large tumors may become eligible after downsizing with 90Y SIRT.
Clinical evidence has suggested that repeated administration of 90Y SIRT to the same patient give similar distributions.6 Accordingly, there exists an opportunity to reduce 90Y SIRT doses to determine whether the 90Y adequately accumulates in tumors while avoiding normal liver. Reduced initial doses allow for repeat treatment with 90Y SIRT if the initial treatment was well tolerated. If there is no objective tumor response, the option remains to top up undertreated tumors with SBRT while controlling doses to limit toxicity. The flexibility of an SIRT-first approach offers several advantages for the clinic.
The time intervals between SIRT and EBRT were not included in the analysis due to the small number of cases. However, these varied substantially from the 4-week guideline with intervals exceeding 20 weeks, which may influence the frequency of liver toxicities. If there is combined toxicity, the long interval would allow for damage repair versus the shorter intervals of 2 weeks. This could make a substantial difference for intervals exceeding 20 weeks as combined acute effects are much less likely after 3 months.
This small cohort of patients with HCC provided an opportunity to better understand the steps required for a combined 90Y SIRT plus EBRT treatment plan. Separate consideration of 90Y SIRT and EBRT treatment doses helped to simplify the analysis. Separate consideration of each patient’s history highlighted the complexity of their cases. To illustrate, patient 1 had a remote history of lung cancer and new diagnosis of intermediate stage HCC caused by hepatitis C. Resection of liver segments 4 and 8 was followed by sorafenib for 1-year postop. Recurrent HCC was not resectable and was treated with 90Y SIRT (1.7 GBq total administered activity). When residual HCC progressed, retreatment with SIRT was not selected due to insufficient arterial perfusion to the tumor. SBRT was delivered in 5 fractions of 10 Gy over 9 days to an HCC tumor in the inferior right hepatic lobe.
The patients who received 90Y SIRT before EBRT (patients 1 and 2) had superior sum BED distributions (Fig E7). This was likely due to the superior conformity of the EBRT plans, which took 90Y SIRT dose distribution into account. Alternatively, the sequencing of the therapies might matter if, for example, the EBRT modifies perfusion, which affects the distribution of 90Y microspheres.
It is currently problematic to seamlessly import 90Y SIRT doses from MIM SurePlan directly into Eclipse. To circumvent the problem of using 2 software platforms, a dose constraint for normal tissue from the isodose curve was used. The isodose curve dose constraint approach makes use of readily available clinical tools, which may be useful when 90Y SIRT doses cannot be imported as a base dose for EBRT planning.
Of the 2 patients with SBRT planned around 90Y EQD, the plans of patient 10 were less homogeneous due to targeting multiple locations in the liver (Fig 3). In addition, because tumor 2 of patient 10 was an isolated target in the greater PTV, the planned dose achieved was less than the prescribed 50 Gy minimum to 95% of the volume. To meet the minimum planned tumor dose in isolated tumor targets, multiple PTVs must be defined. Although it could still be convenient to retain a single PTV for multiple tumors, for example, if they were spaced closely, their separation should be considered when planning 90Y SIRT, as combining them was shown here to have deleterious dosimetric implications.8
Clinical guidelines for 90Y absorbed dose measurement have not yet been universally adopted across institutions. Problematically, several methods exist—including the local deposition and voxel kernel convolution methods—which can result in variation in the calculated absorbed dose. For example, a misplaced assumption that the total signal from 90Y uptake imaging is the same in the body contour as it is in the liver contour demonstrated an average dose deviation of 30% (Fig E7). Even when reconstructing the images to calibrate the measured signal, a small background noise in the voxels outside the liver where 90Y dose was not deposited outweighed the signal of interest in the liver voxels. This random error, possibly due to background shot noise of the PET scanner photomultiplier tubes or inadequate image reconstruction, could bias the measurement of total signal. Another caveat is that MIM calculations of absorbed dose omitted dose calculations outside of the liver. Although it is known that nearly zero dose from 90Y SIRT deposits outside the liver, MIM communicated that the dosimetry methodology depends on tissue density parameter. The soft tissue of the liver assumes a density of 1.04 g mL-1, yet dosimetry in organs with differing density, such as the lungs or bowel, would be inaccurate. To account for differing densities, density mapping of tissues to signal from the corresponding SPECT or PET images is currently under development. Although software solutions have endured rigorous testing to meet legal requirements, for example, 510 k clearance, it is necessary to consider that minor deviations in the dosimetry methodology can cause different results. Although these issues are actively being investigated, the wider vital need is for 90Y microsphere product manufacturers and clinical guidelines to adopt a single robust measurement approach.
Although the SPECT/CT reconstruction study by Siman et al19 indicated consensus across methodologies, good agreement was not demonstrated for all tumors considered here. This could be explained by the noisy images even after adopting best-practice reconstruction parameters or by variation among treatment centers.
This work exposed shortcomings of combination 90Y SIRT and SBRT dosimetry methodology and aspects demanding further development. Radiosensitivity characterization of 90Y SIRT in additional tumor types including HCC, breast, and intrahepatic cholangiocarcinoma would contribute valuable evidence to improve the BED model.4,13 Although the available evidence remains limited, further exploration of the proposed treatment approach, combining the reduced toxicity of 90Y SIRT with the precision and prospective planning capabilities of SBRT, has merit, as this work demonstrated that combination 90Y SIRT and SBRT could produce superior dose distributions compared with 90Y SIRT alone. A larger prospective clinical study to characterize efficacy and toxicity in relation to dose and volume constraints is needed to inform best practice. Bearing in mind liver toxicity, a new trial could address appropriate increases of dose given the advancements in conformity from SBRT. We propose a prospective clinical study in patients with HCC or colorectal cancer liver metastases to test the combination of 90Y SIRT with SBRT against SIRT alone.
Conclusions
This work demonstrated a method for 90Y SIRT and EBRT dose combination in a small cohort of patients with HCC. It also illustrated steps for planning SBRT treatment in patients who had received prior 90Y SIRT. Future studies are necessary to validate safety and efficacy and demonstrate clinical utility of prospectively combining EBRT and 90Y SIRT. Improved dosimetry through inverse dose planning adds flexibility to the clinical workflow, which could further benefit the patient.
Acknowledgments
The authors thank Justin Roper, PhD, at the Sarah Cannon Research Institute for external beam dosimetry planning support and patient data collation; Pamela Purcell at TriStar Centennial Medical Center for patient data collation; Jessica Heritage at the Sarah Cannon Research Institute for patient data access, ethics guidance, and research agreements; Adam Neff at MIM Software, Inc, for in-kind access to SurePlan LiverY90; Daniel Parvin at MIM Software, Inc, for custom workflow support; and Aaron Nelson, MD, and Dennis Nelson, PhD, at MIM Software, Inc, for dosimetry support.
Footnotes
Sources of support: K.A.V., N.F., and E.A. acknowledge financial support from CRUK (grant C5255/A23755).
Disclosures: Outside the submitted work, K.A.V. reports consultant fees from Blue Earth Diagnostics and A.K. reports phase II clinical trial funding to the Sarah Cannon Research Institute through grants from Sirtex Medical with no personal reimbursements to any author.
Data sharing statement: Research data are not available at this time.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2020.11.002.
Supplementary Materials
References
- 1.Council directive 2013/59/EURATOM of 5 December 2013 laying down basic safety standards for protection against the dangers arising from exposure to ionizing radiation, and repealing Directives 89/618/Euratom, 90/641/Euratom, 96/29/Euratom, 97/43/Euratom and 2003/122/Euratom. OJEU. 2014;(December 2003):1-73.
- 2.Strigari L., Sciuto R., Rea S. Efficacy and toxicity related to treatment of hepatocellular carcinoma with 90Y-SIR spheres: Radiobiologic considerations. J Nucl Med. 2010;51:1377–1385. doi: 10.2967/jnumed.110.075861. [DOI] [PubMed] [Google Scholar]
- 3.van den Hoven A.F., Rosenbaum CENMNM, Elias S.G. Insights into the dose-response relationship of radioembolization with resin 90Y-microspheres: A prospective cohort study in patients with colorectal cancer liver metastases. J Nucl Med. 2016;57:1014–1019. doi: 10.2967/jnumed.115.166942. [DOI] [PubMed] [Google Scholar]
- 4.Lee B.Q., Abbott E.M., Able S. Radiosensitivity of colorectal cancer to 90Y and the radiobiological implications for radioembolisation therapy. Phys Med Biol. 2019;64:135018. doi: 10.1088/1361-6560/ab23c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gholami Y.H., Willowson K.P., Forwood N.J. Comparison of radiobiological parameters for 90Y radionuclide therapy (RNT) and external beam radiotherapy (EBRT) in vitro. EJNMMI Physics. 2018;5:18. doi: 10.1186/s40658-018-0217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cremonesi M., Ferrari M., Bartolomei M. Radioembolisation with 90Y-microspheres: Dosimetric and radiobiological investigation for multi-cycle treatment. Eur J Nucl Med Mol Imaging. 2008;35:2088–2096. doi: 10.1007/s00259-008-0857-3. [DOI] [PubMed] [Google Scholar]
- 7.Cremonesi M., Ferrari M., Botta F. Planning combined treatments of external beam radiation therapy and molecular radiotherapy. Cancer Biother Radiopharm. 2014;29:227–237. doi: 10.1089/cbr.2014.1607. [DOI] [PubMed] [Google Scholar]
- 8.Bodey R.K., Flux G.D., Evans P.M. Combining dosimetry for targeted radionuclide and external beam therapies using the biologically effective dose. Cancer Biother Radiopharm. 2003;18:89–97. doi: 10.1089/108497803321269368. [DOI] [PubMed] [Google Scholar]
- 9.Wang T.-H., Huang P.-I., Hu Y.-W. Combined yttrium-90 microsphere selective internal radiation therapy and external beam radiotherapy in patients with hepatocellular carcinoma: From clinical aspects to dosimetry. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowler J.F. 21 years of biologically effective dose. Br J Radiol. 2010;83:554–568. doi: 10.1259/bjr/31372149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy A., Nag S., Salem R. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: A consensus panel report from the Radioembolization Brachytherapy Oncology Consortium. Int J Radiat Oncol Biol Phys. 2007;68:13–23. doi: 10.1016/j.ijrobp.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 12.Common terminology criteria for adverse events (CTCAE) version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf Available at: Accessed January 1, 2020.
- 13.Abbott E.M., Falzone N., Lee B.Q. Impact of radiobiologically informed dose prescription on maximising the clinical benefit of 90Y SIRT in colorectal cancer patients. J Nucl Med. 2020 doi: 10.2967/jnumed.119.233650. [DOI] [PubMed] [Google Scholar]
- 14.Tai A., Erickson B., Khater K.A., Li X.A. Estimate of radiobiologic parameters from clinical data for biologically based treatment planning for liver irradiation. Int J Radiat Oncol Biol Phys. 2008;70:900–907. doi: 10.1016/j.ijrobp.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 15.Dawson L.A., Zhu A., Knox J. Radiation Therapy Oncology Group RTOG 1112 randomized phase III study of sorafenib versus stereotactic body radiation therapy followed by sorafenib in hepatocellular carcinoma. https://www.rtog.org/Portals/0/RTOG%20Broadcasts/Attachments/1112_master_w_update_5.7.13.pdf Available at: Accessed January 1, 2020.
- 16.Pan C.C., Kavanagh B.D., Dawson L.A. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S94–S100. doi: 10.1016/j.ijrobp.2009.06.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau W.Y., Kennedy A.S., Kim Y.H. Patient selection and activity planning guide for selective internal radiotherapy with yttrium-90 resin microspheres. Int J Radiat Oncol Biol Phys. 2012;82:401–407. doi: 10.1016/j.ijrobp.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Cremonesi M., Chiesa C., Strigari L. Radioembolization of hepatic lesions from a radiobiology and dosimetric perspective. Front Oncol. 2014;4:1–20. doi: 10.3389/fonc.2014.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siman W., Mikell J.K., Kappadath S.C. Practical reconstruction protocol for quantitative 90Y bremsstrahlung SPECT/CT. Med Phys. 2016;43:5093–5103. doi: 10.1118/1.4960629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.