Abstract
It is already well known that castration improves marbling quality but exact timing of castration is still highly debated in beef cattle production industry. After castration, blood hormonal changes occur in steer and objective of this study was to investigate the effects of growth hormone (GH) levels on adipocyte differentiation in stromal vascular cells (SVCs) and transdifferentiation into adipocytes in C2C12 myoblasts. Total GH concentrations were measured via enzyme-linked immunosorbent assay (ELISA) in 24 male calves and 4 female calves. Cell proliferation, cellular triglyceride (TG) accumulation, and the cell’s lipolytic capability were measured in C2C12 myoblasts and SVCs. Myogenic, adipogenic, and brown adipocyte-specific gene expression was measured via real-time polymerase chain reaction (PCR) using SYBR green. Serum GH levels were the highest in late-castrated calves. Treatment with 5 ng/mL GH resulted in greater TG accumulation as well as increased CCAAT-enhancer-binding protein (C/EBP)α and peroxisome proliferator-activated receptor (PPAR)γ expression compared to that after treatment with 15 ng/mL GH. Treatment with 5 ng/mL GH also resulted in lower myogenin (myo)G and myoD expression compared to that after treatment with 15 ng/mL GH. The expression of bone morphogenetic protein (BMP) 7 after treatment with 5 ng/mL GH was higher than that after treatment with 15 ng/mL GH. But carcass characteristics data showed no significant difference between early and late castrated steers. Therefore, our results indicate that castration timing does not seem to be inevitable determinate of carcass qualities, particularly carcass weight and marbling score in Hanwoo beef cattle.
Keywords: Adipogenesis, Stromal vascular cells, C2C12 myoblast, Growth hormone, Trans-differentiation, Castration timing
INTRODUCTION
The challenge of the beef cattle industry is to produce the high marbled beef that is safe and possesses high sensory qualities, including tenderness, juiciness, presence of intramuscular fat, low fat melting point, and flavor [1]. Marbling, in particular, is known to affect meat palatability, tenderness, juiciness, and flavor. Marbling is formed in several external and internal anatomical locations, including around and within the muscle, together with inter- and intramuscular fat [2].
Intramuscular fat is an unstable depot in which progenitor cells may either become preadipocytes or myoblasts. Accurate adipogenic control is essential for the formation of adipocytes between muscle bundles to create marbling [3]. Adipocytes may form in muscle fibers not only by stimulating existing preadipocytes to create adipocytes between muscle bundles via adipogenic differentiation but also by transdifferentiating myogenic progenitors, such as satellite cells and myoblasts, into adipocytes. Myoblast cells exhibit some flexibility even after the determination stage: they may accumulate lipid droplets and can be transdifferentiated into adipocyte-like cells when exposed to adipogenic conditions or in the absence of myogenic regulatory factors (MRFs) [4]. In the beef production industry, to improve intramuscular fat deposition around muscle bundles, male calves are castrated at various ages via surgery. Early castration is usually undertaken 4–5 months of age, but, late castration is made in 7–8 months of age [5]. After surgical castration, the concentrations of various blood hormones change; among these, the levels of growth hormone (GH) may decrease [6].
GH is a well-known peptide hormone that is produced by somatotropic cells in the pituitary gland. GH plays an important role during the growing stage by promoting growth and renewal of cells in animal body [7]. Blood GH levels peak during the puberty so if calves are castrated at 7–8 months of age, their GH secretion level may be higher than calves castrated at 4–5 months of age. So, the main concern of farmers is that growth performance of early castrated steers may be lower than the steers castrated at later ages. Additionally, GH promotes muscle development while stimulating lipolysis in adipocytes and GH may have negative effect on the intramuscular fat deposition. In GH deficiency, fat cells are low in number but high in volume [8]. However, it is unknown if early castration at 4–5 months of age will result in better carcass quality especially in meat production and marbling score. Therefore, the present study was designed to investigate the effects of different castration timing on carcass quality by evaluating the effect of GH concentrations on the differentiation of stromal vascular cells (SVCs) and the carcass characteristics.
MATERIALS AND METHODS
Animals
A total of 20 animals were used in this study, 16 male cattle for castration and 4 female cattle for the comparison of serum GH. The 16 male cattle were divided into two group, early castration group that were castrated at 4–5 months of age (n=8) and late castration group that were castrated at 7–8 months of age (n=8). After recovery from the surgery of castration of late castration group animal, blood sampling was undertaken against early castrated animals and 4 female cattle at the same age of experimental animals. Animals were then followed same feeding program to the slaughtering at 30 months of age. After slaughter, carcass yield and quality traits of early and late castration group were measured. Animals were fed the commercial diet following with the feeding program for growing and fattening cattle in Hoengseong Hanwoo experimental farm. This study was undertaken in accordance with the Guideline for the Care and Use of Experimental Animals of Hankyong National University Animal Welfare Committee (Hankyong 2020-1).
Growth hormone analysis
Blood sampling was undertaken from jugular vein for 16 male calves and female calves (n = 4). Total GH concentrations were measured using a bovine enzyme-linked immunosorbent assay (ELISA) kit (Cusabio, Houston, TX, USA) according to the manufacturer’s instructions. Briefly, 50 µL of sample was put into the well and 50 µL of horseradish peroxidase (HRP)-conjugate mixed solution was added to each well. After gentle mixing and incubating for 1 h at 37°C, the wells were washed with washing buffer. Then 50 µL of each substrate A and B was added to each well. After incubation at 37°C for 15 min, 50 µL of stop solution was added, and optical density (OD) was measured at 450 nm wavelength using ELISA plate reader (Tecan, Männedorf, Zurich, Switzerland). Concentration of samples were normalized to the standards.
Cell culture
C2C12 myoblast cells were obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (FBS, Gibco, Thermo Fisher Scientific) and 100 U/mL penicillin/streptomycin (Gibco, Thermo Fisher Scientific) at 37°C under 5% CO2. In order to obtain enough SVC cells that can be used in vitro, SVCs were isolated from the longissimus muscle of three castrated Hanwoo beef cattle. Briefly, muscles were collected individually at a slaughterhouse in Anseong (Korea). Adipose tissue (10 g) was removed, cut into small pieces, and digested at 37°C in a shaking water-bath (60 cycles/min). After digestion, the cell suspension was filtered and the SVCs were collected via centrifugation at 700 ×g for 10 min. Finally, the pellet was suspended in DMEM containing 10% bovine calf serum (BCS, Gibco, Thermo Fisher Scientific), 100 U/mL penicillin, and 100 μg/mL streptomycin. Cell cultures were maintained at 37°C under 5% CO2 with medium changes every 2 d until 80% confluence was reached.
CCK-8 assay
To determine the effect of treatment with various concentrations of GH on bovine SVCs and mouse C2C12 myoblasts, we measured cell proliferation using a cell counting kit (CCK)-8 kit (Dojindo, Tokyo, Japan) following the manufacturer’s protocol. In total, 1 × 105 cells/mL were seeded in a 96-well micro-plate and incubated with various concentrations of GH (0, 5, and 15 ng/mL) for 24 h. The medium was then replaced with fresh medium containing CCK-8 reagent, and the OD was measured at a wavelength of 450 nm using ELISA plate reader (Tecan). These tests were carried out independently in triplication (n=3). Cell viability was expressed as a percentage of that of the control cells.
Induction of adipocyte differentiation
SVCs and C2C12 myoblasts were cultured to confluency in proliferation medium (DMEM containing 10% BCS and 0, 5, or 15 ng/mL GH), which was changed every 2 d. To induce differentiation, we replaced the proliferation medium with differentiation medium supplemented with 10% FBS (Gibco, Thermo Fisher Scientific), 10 µg/mL rosiglitazone (ROS, Sigma-Aldrich, St Louis, MO, USA), and methylene diphenyl diisocyanate (MDI) mixture comprising 1 µM dexamethasone (DEX, Sigma-Aldrich), 0.5 mM isobutylmethylxanthine (IBMX, Sigma-Aldrich), and 10 µg/mL insulin (INS, Sigma-Aldrich). The differentiation medium was changed every 2 d. Additionally, C2C12 myoblasts and SVCs were differentiated by treating with INS only out of MDI for 1 and 2 weeks, respectively.
Oil Red O staining and triglyceride (TG) analysis
Adipocyte differentiation was determined by assessing TG accumulation in the cells. Oil Red O reagent (Sigma-Aldrich) was used to stain the TG in the cells. After differentiation, the cells were fixed with 10% formaldehyde and stained with 0.5% Oil red O solution. Cell differentiation was observed under a light microscope (Olympus CK40, Tokyo, Japan) and quantified by eluting the stained cells with isopropanol and measuring the OD at 490 nm. The rate of cell differentiation was expressed as a percentage of that in the control cells.
Crystal violet staining
Differentiated C2C12 myoblast cells were counterstained with 0.2% crystal violet in 2% ethanol to visualize myotube formation. Crystal violet was extracted using 10% acetic acid for 5 min, and the OD was measured at 595 nm.
Lipolysis assay
The cells’ lipolytic capability of the cells was assessed using an adipolysis assay kit (Millipore, Burlington, MA, USA) according to the manufacturer’s instructions. Briefly, differentiated SVC cells were incubated with different concentrations of GH (0, 5, or 15 ng/mL) with/without 0.5 μM isoproterenol (Sigma-Aldrich, a non-selective beta-adrenoceptor agonist) for 24 h, and the amount of glycerol in the medium was determined by measuring the OD at 540 nm. The extent of lipolysis was expressed relative to that in a standard glycerol curve. Isoproterenol- and MDI-treated cells were used as positive controls.
RNA isolation and real-time polymerase chain reaction (PCR) using SYBR green
Total RNA was isolated from differentiated cells using RNAiso Plus (Takara Bio, Kusatsu, Japan) following the manufacturer’s instructions. cDNA was synthesized from 1 µg RNA, and related gene expression was computed via real-time PCR using SYBR Green PCR Master Mix (Thermo Fisher Scientific) according to the manufacturer’s protocol. In brief, 1 µL of cDNA was used to amplify the genes with 1 µL each of primers, 10 µL of SYBR Green PCR Master Mix and 7 µL of diethylpyrocarbonate (DEPC) treated water in a Bio-Rad C1000TM Thermal Cycler. The results were analyzed by comparing the quantification cycle (Cq) value of genes to that of β -actin. The primers shown in Table 1 were used to quantify the following genes in the SVCs: hormone-sensitive lipase (HSL), myogenin (myo)D, myoG, proliferator-activated receptor (PPAR γ), and CCAAT-enhancer-binding protein (C/EBP α), and the following genes in C2C12 cells: myoD, myoG, uncoupling protein (UCP)1, bone morphogenetic protein (BMP7), PPAR γ, and C/EBP β. These primers were purchased from Cell Signaling Technology (Danvers, MA, USA). Expression levels were normalized to that of β -actin (Cell Signaling Technology).
Table 1. Brown adipocyte- and myoblast differentiation-related primer sequences.
| Name | Forward primer (5′ → 3′) | Reverse primer (5′ → 3′) |
|---|---|---|
| β-Actin | CACCCCAGCCATGTACGT | GTCCAGACGCAGGATGGC |
| myoG | TGGGCGTGTAAGGTGTGTAA | TGCAGGCGCTCTATGTACTG |
| myoD | GATGACCCGTGTTTCGACTC | TAGTCGTCTTGCGTTTGCAC |
| HSL | CTTTCGCACCAGCCACAAC | CTCGTCGCCCTCAAAGAAGA |
| UCP1 | AGGGACTACTCCCAATCTGACA | GTTGGGCACACTTGTGTACTGT |
| BMP7 | TGCCACTAGCTCTTCCTGGAA | TGAGAGACCCAGGATCCAGAA |
| C/EBPα | TGGACAAGAACAGCAACGAG | TCACTGGTCAACTCCAGCAC |
| C/EBPβ | CAAGCTGAGCGACGACGAGTACA | AGCTGCTCCACCTTCTTCTG |
| PPARγ | GATGGAAGACCACTCCGATT | AACCATTGGGTCAGCTCTTG |
myo, myogenin; HSL, hormone-sensitive lipase; UCP1, uncoupling protein 1; BMP7, bone morphogenetic protein 7; C/EBP, CCAAT-enhancer-binding proteins; PPARγ; peroxisome proliferator-activated receptor-γ.
Statistical analysis
The data are expressed as the means ± SD. Differences between means were calculated using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test and Student t-test for comparison of means. The differences were considered significant at p < 0.05. The statistical software package SPSS Statistics 21.0 (IBM, Armonk, NY, USA) was used.
RESULTS
Growth hormone levels in Hanwoo beef calves
Blood GH levels in calves castrated at 4 to 5 months of age (early castrated) or 7 to 8 months of age (late castrated), and female calves were analyzed via ELISA (Table 2). Serum GH levels were the highest in the late castrated group (16.66 ± 8.26 ng/mL), and the lowest in the early castrated group (3.94 ± 1.62 ng/mL). There was no significant difference between GH levels in early castrated and female calves. Thus, to test the effects of hormonal level on the proliferation, development, and differentiation of SVCs and C2C12 cells, the groups were divided into the low group treated with 5 ng/mL GH and the high group treated with 15 ng/mL GH.
Table 2. Serum growth hormone (GH) levels in calves.
| Early castrated (n = 8) | Late castrated (n = 8) | Female (n = 4) | |
|---|---|---|---|
| GH level in blood serum (ng/mL) | 3.94 ± 1.62a | 16.66 ± 8.26b | 4.91 ± 1.15a |
Data are presented as the means ± SD.
Superscripts indicate statistically significant differences (p < 0.05).
Effect of growth hormone on stromal vascular cell and C2C12 myoblast proliferation
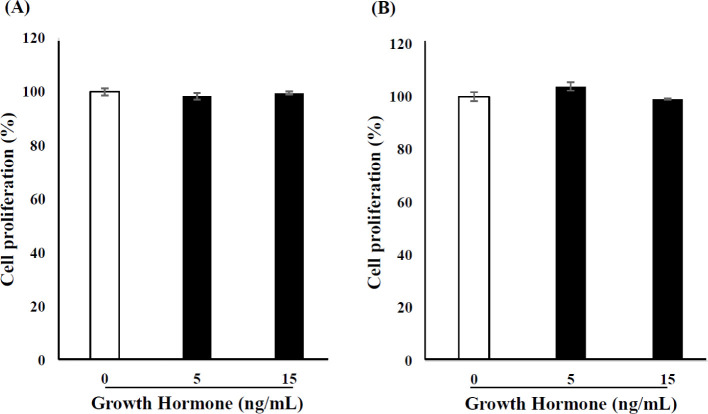
SVCs and C2C12 myoblasts were exposed to low and high doses of GH (5 or 15 ng/mL) for 24 h. GH had no significant effect on the proliferation of SVCs or C2C12 myoblasts at either dose (Fig. 1).
Fig. 1. Effect of GH on SVC and C2C12 myoblast proliferation.
(A) SVCs and (B) C2C12 myoblasts were treated with 0, 5, and 15 ng/mL GH for 24 h, and cell viability was determined using cell counting kit-8 (CCK-8) assay. Values are expressed as a percentage of that in the control. Each value represents the mean ± SD (n = 3). GH, growth hormone; SVCs, stromal vascular cells.
Effect of growth hormone on adipogenesis in stromal vascular cells
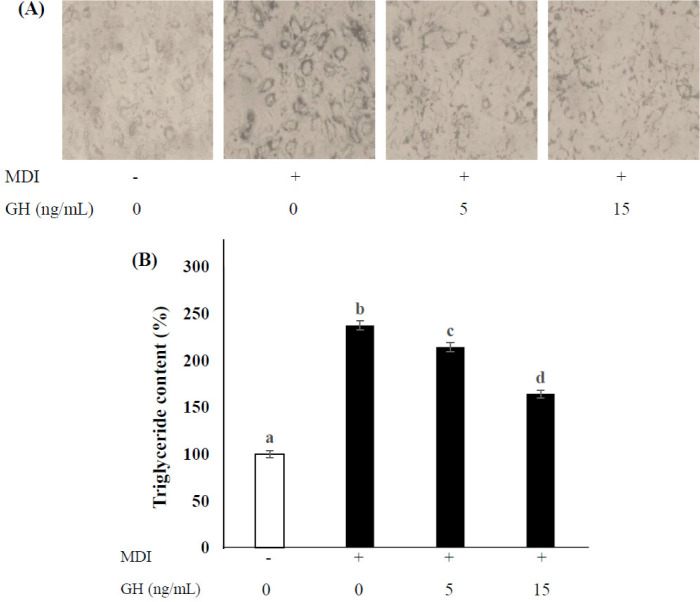
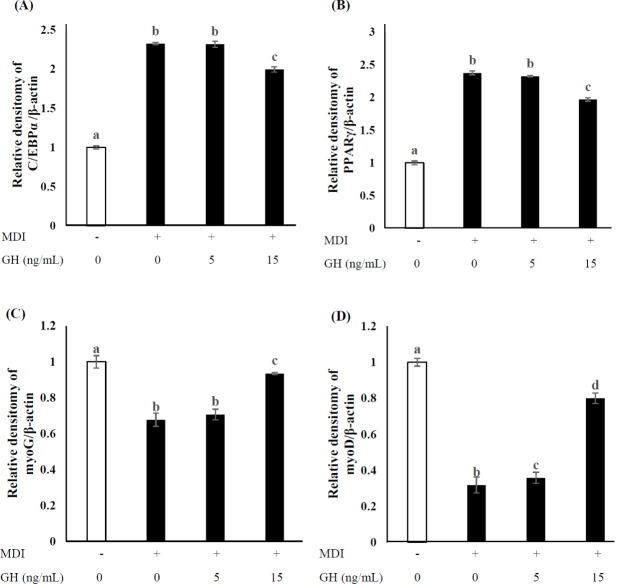
To examine the effect of hormones on adipocyte differentiation in SVCs, cells were incubated with MDI. SVCs were differentiated upon exposure to 5 or 15 ng/mL GH for 14 d. MDI treatment significantly increased TG accumulation (p <0 .05), whereas GH treatment exhibited dose-dependent inhibitory effects (Fig. 2). In addition, the expression of the adipogenic genes C/EBP α and PPAR γ was significantly decreased in the high-dose group (treated with 15 ng/mL GH) compared to that in the low-dose group (treated with 5 ng/mL GH), as shown in Fig. 3A and b (p < 0.05). The expression of the myogenic genes myoG and myoD (Fig. 3C and D) increased significantly in the high-dose group compared to that in the low-dose and untreated groups (p < 0.05). MyoG expression in the low-dose group was similar to that in the untreated group, though the myoD expression in the low-dose group was significantly higher than that in the untreated group (p < 0.05).
Fig. 2. Effect of GH on TG accumulation and adipocyte differentiation in SVCs.
Cells were incubated with GH (5 or 15 ng/mL) in differentiation medium for 2 wk. Untreated cells (0 ng/mL GH, no MDI) were used as negative controls, and MDI-only-treated cells were used as positive controls. The MDI mixture comprised 1 µM dexamethasone (DEX), 0.5 mM isobutylmethylxanthine (IBMX), and 10 µg/mL insulin (INS). (A) Differentiated cells were stained with Oil Red O and visualized under a microscope. (B) Oil Red O staining was performed and quantified to measure TG accumulation. Values are expressed as a percentage of that in the negative controls, and each value represents the mean ± SD (n = 3). Superscripts indicate statistically significant differences (p< 0.05). MDI, methylene diphenyl diisocyanate; GH, growth hormone; TG, triglyceride; SVCs, stromal vascular cells.
Fig. 3. Effect of GH on myogenic and adipogenic gene expression after 2-wk differentiation in SVCs.
mRNA expression was calculated via real-time PCR using SYBR green. Untreated cells (0 ng/mL GH, no MDI) were used as negative controls, and MDI-only-treated cells were used as positive controls. The MDI mixture comprised 1 µM DEX, 0.5 mM IBMX, and 10 µg/mL INS. (A, B) Expression of adipogenic genes (CCAAT-enhancer-binding protein [C/EBP]α and peroxisome proliferator-activated receptor [PPAR]γ) and (C, D) myogenic genes (myogenin [myo]G and myoD) relative to that of β-actin. Values are expressed relative to those in negative control considering as 1.00. Data represent the means ± SD (n = 3). Superscripts indicate statistically significant differences (p < 0.05). MDI, methylene diphenyl diisocyanate; GH, growth hormone; SVCs, stromal vascular cells; PCR, polymerase chain reaction; DEX, dexamethasone; IBMX, isobutylmethylxanthine; INS, insulin.
Effect of growth hormone on lipolysis in stromal vascular cells
As shown in Fig. 4a, when SVCs was treated with isoproterenol which is a nonselective β- adrenergic agonist, free glycerol concentration in the media was drastically increased compared to those cells not treated with isoproterenol. However, the free glycerol concentration of the SVCs treated with GH (5 or 15 ng/mL) was significantly higher compared to the cells without GH treatment (p < 0.05). As shown in Fig. 4B, HSL gene expression was significantly suppressed by the differentiation via MDI media. Meanwhile, SVCs treated with GH (0, 5, or 15 ng/mL) showed increased HSL expression in a dose-dependent manner (p < 0.05).
Fig. 4. Effect of GH on lipolysis and HSL expression in 2-wk differentiated SVCs.
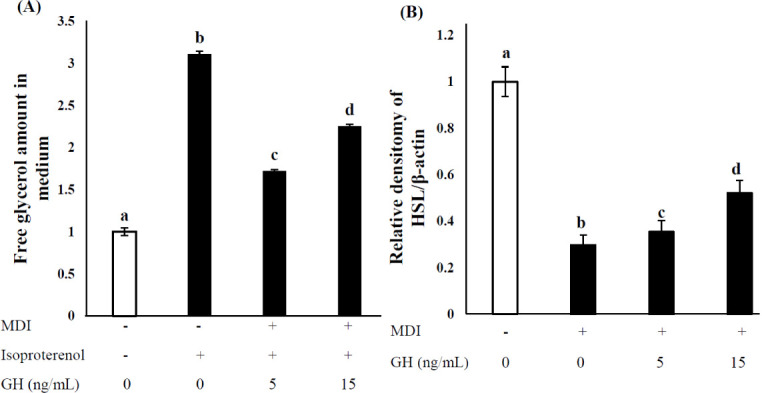
Untreated cells (0 ng/ mL GH, no MDI) were used as negative controls, and MDI- and isoproterenol-treated cells (no GH) were used as positive controls. The MDI mixture comprised 1 μM DEX, 0.5 mM IBMX, and 10 μg/mL INS. (A) The extent of lipolysis was expressed relative to the amount of free glycerol in the medium. (B) HSL mRNA expression was calculated via real-time PCR using SYBR green and expressed relative to that of β-actin. Values are expressed relative to those in negative control considering as 1.00. Values represent the means ± SD (n = 3). Superscripts indicate statistically significant differences (p < 0.05). MDI, methylene diphenyl diisocyanate; GH, growth hormone; HSL, hormone-sensitive lipase; SVCs, stromal vascular cells; DEX, dexamethasone; IBMX, isobutylmethylxanthine; INS, insulin; PCR, polymerase chain reaction.
Effect of growth hormone on transdifferentiation of C2C12 myoblasts
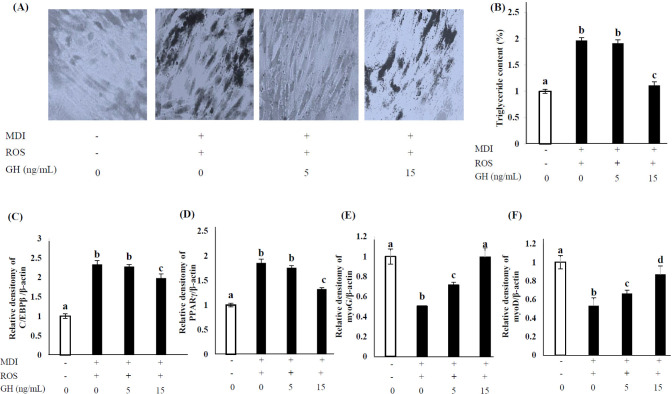
Adipogenic transdifferentiation of myoblasts was examined in C2C12 cells treated with MDI and ROS. GH treatment suppressed adipogenic differentiation and promoted myotube formation in the high-dose group (treated with 15 ng/mL GH), whereas treatment with 5 ng/mL GH (low-dose group) had no significant effect on adipogenic transformation. Additionally, C/EBP β and PPAR γ expression was significantly inhibited in the high-dose group, whereas low-dose treatment caused a slight repressive effect on C/EBP β expression (p < 0.05). Conversely, adipogenic induction considerably suppressed myoG and myoD expression, though GH treatment upregulated the expression of both in a dose-dependent manner (Fig. 5).
Fig. 5. Effect of GH on TG accumulation, morphological changes, and adipogenic and myogenic gene expression in C2C12 myoblasts after 1-wk adipogenic differentiation with ROS.
Untreated cells (0 ng/mL GH, no MDI) were used as negative controls, and MDI-only-treated cells were used as positive controls. The MDI mixture comprised 1 µM DEX, 0.5 mM IBMX, and 10 µg/mL INS. (A) Differentiated cells were stained with Oil Red O, counterstained with crystal violet, and visualized under a microscope. (B) Oil Red O stain was extracted and quantified to measure TG accumulation. (C, D) The expression of adipogenic genes (C/EBPβ and PPARγ) and (E, F) myogenic genes (myoG and myoD) was calculated via real-time PCR using SYBR green and expressed relative to that of β-actin. Values are expressed relative to those in negative control considering as 1.00. Values represent the means ± SD (n = 3). Superscripts indicate statistically significant differences (p < 0.05). MDI, methylene diphenyl diisocyanate; ROS, reactive oxygen species; GH, growth hormone; TG, triglyceride; DEX, dexamethasone; IBMX, isobutylmethylxanthine; INS, insulin; C/EBP, CCAAT-enhancer-binding protein; PPAR, peroxisome proliferator-activated receptor; myo, myogenin; PCR, polymerase chain reaction.
Effect of growth hormone on brown adipocyte-specific gene expression in C2C12 myoblasts
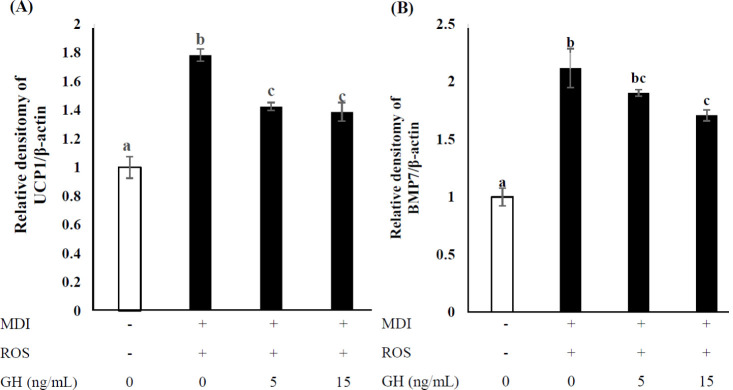
The expression of brown adipose tissue (BAT)-specific genes increased during the adipogenic differentiation of MDI- and ROS-treated cells, though this expression decreased in groups treated with GH. As shown in Fig. 6A, UCP1 expression levels in the high- and low-dose groups were similar but decreased significantly compared to those of cells treated with MDI and ROS (p < 0.05). BMP7 expression levels in the high-dose group were significantly reduced compared with those of cells treated with MDI and ROS (p < 0.05; Fig. 6B).
Fig. 6. Effect of GH on brown adipocyte-specific gene expression in C2C12 myoblasts after 1-wk adipogenic differentiation with ROS.
Untreated cells (0 ng/mL GH, no MDI) were used as negative controls and MDI-only-treated cells were used as positive controls. The MDI mixture comprised 1 µM DEX, 0.5 mM IBMX, and 10 µg/mL INS. (A) Uncoupling protein (UCP) 1 and (B) bone morphogenetic protein (BMP) 7 mRNA expression was calculated via real-time PCR using SYBR green and expressed relative to that of β-actin. Values are expressed relative to those in negative control considering as 1.00. Values represent the means ± SD (n = 3). Superscripts indicate statistically significant differences (p < 0.05). MDI, methylene diphenyl diisocyanate; ROS, reactive oxygen species; GH, growth hormone; DEX, dexamethasone; IBMX, isobutylmethylxanthine; INS, insulin; PCR, polymerase chain reaction.
Effect of castration timing on carcass characteristics
The carcass yield and quality traits of early castrated and late castrated Hanwoo beef cattle are shown in Table 3. There was no significant difference in back fat thickness, rib-eye area, carcass weight, dressing percentage, marbling score, meat color and fat color between early and late castrated steers.
Table 3. Effect of castration timing on carcass yield and quality traits of Hanwoo steers.
| Early castration (at 4–5 month of age, n=8) | Late castration (at 7–8 month of age, n=8) | |
|---|---|---|
| Carcass yield traits | ||
| Back fat thickness (cm) | 15.2 ± 3.02 | 11 ± 0.7 |
| Rib-eye area (cm2) | 98.8 ± 5.11 | 96.2 ± 8.58 |
| Carcass weight (kg) | 432.6 ± 25.2 | 450.2 ± 15.7 |
| Dressing percentage (%) | 63.94 ± 2.27 | 64.84 ± 1.86 |
| Quality traits | ||
| Marbling score1) | 7.8 ± 0.58 | 7.6 ± 0.75 |
| Meat color2) | 4.6 ± 0.24 | 4.6 ± 0.28 |
| Fat color3) | 3 ± 0.01 | 3 ± 0.01 |
Data are presented as the means ± SD.
Low fat (1 point) – high fat (9 point).
Very light cherry red (1 point) – very dark red (7).
White (1 point) – yellow (7 point).
DISCUSSION
Bovine GH plays a major role in the body growth and development of various tissues in calves by promoting gluconeogenesis in muscles and lipolysis in adipocytes, and bone growth. Moreover, blood GH levels vary depending on the age of the cattle and the time of day; however, in general, GH levels peak during puberty [9]. GH levels were almost 4-fold higher in late-castrated calves than in early-castrated calves (Table 2), suggesting that castration at a later stage of growth may improve body growth in male cattle. However, high GH concentrations may suppress INS-induced lipogenesis in adipose tissue [10]. To clarify the effect of different doses of GH on adipocyte differentiation and myoblast transdifferentiation, we treated SVCs isolated from the cervical longissimus muscle of Hanwoo beef cattle and C2C12 mouse myoblasts with low or high doses (5 or 15 ng/mL, respectively) of GH and examined the effects of GH treatment on lipolysis, adipogenesis, and adipogenic and myogenic gene expression.
In adipose tissue, there are heterogenous cell population known as SVCs, which contain muscle cells, adipocytes, blood cells, fibroblasts, and their progenitors. However, SVCs mostly contains adipogenic precursor known as adipose derived stem cells (ASCs) [11]. The SVCs used in current study were analyzed using surface markers and confirmed characterized ASCs in our previous published work [12].
GH induces bovine muscle hypertrophy and the fusion of myoblasts into myotubes but does not affect the proliferation of myoblasts or satellite cells [13]. As shown in Fig. 5, GH had no effect on the proliferation of SVCs or C2C12 myoblasts at either concentration.
Expression of the early adipogenic markers C/EBP α and PPAR γ is positively correlated with the number of adipocytes and stimulates lipid accumulation in adipocytes [14]. Conversely, the myogenic differentiation markers such as myoD and myoG are key regulators of myotube formation and myoblast fusion [15]. MyoG plays an important role in skeletal muscle development of fetus, and its deletion causes premature or non-functional muscle development [16]. Likewise, myoD is responsible for the terminal commitment of mesenchymal stem cells (MSCs) into the myogenic lineage and early satellite cell fusion into myotubes [17]. MSCs may develop either into preadipocytes or myoblasts, the main progenitor components of intramuscular fat [18]. High GH levels repressed adipocyte differentiation in SVCs by downregulation of C/EBP α and PPAR γ mRNA expression while upregulation of myoD and myoG mRNA expression (Figs. 2 and 3).
Mature adipocytes store TGs as lipid droplets and INS maintains lipid accumulation by inhibition of HSL protein expression. HSL is an intracellular lipase that hydrolyzes TG into monoglyceride and free fatty acids [17]. However, GH prevents lipid accumulation in adipocytes by encouraging the lipolytic activity of HSL, which is highly expressed in white adipose tissue (WAT) and BAT [19]. In the present study, the GH treatment decreased TG amount in SVCs dose-dependently, however, the lipolysis capacity was significantly increased by GH treatment by upregulating HSL mRNA expression (Figs. 2 and 4B). According to these results, low-dose GH treatment does not appear to direct cells into myogenic differentiation; thus, ASCs may differentiate into the adipogenic lineage. SVCs, an important source of ASCs, consist primarily of adipogenic precursors [20]. Moreover, TG accumulation is not suppressed in the low-dose treatment of GH, thus enabling mature adipocytes to retain lipid droplets.
Another method used to increase the fat content between muscle bundles is the trans-differentiation of myogenic precursor cells into adipocyte-resembling cells. PPAR γ is upregulated during the transdifferentiation of myogenic satellite cells into adipocyte-like cells [21]. Myocytes and BAT are derived from the same myogenic factor (MYF)5-expressing progenitors; therefore, undifferentiated precursor cells, such as satellite cells and myoblasts, may be manipulated to differentiate into BAT by PPAR γ activators including thiazolidinedione and ROS [22–25]. On the contrary, high myoD expression inhibited cell self-renewal and promoted terminal differentiation into myotubes; moreover, the loss of myoD in myoblasts facilitated transdifferentiation into BAT [26].
Furthermore, several studies have reported increased BAT-specific gene expression, such as that of UCP1 and BMP7, during myoblast transdifferentiation [27–31]. UCP1 is known to participate in thermogenesis in BAT and is remarkably expressed in brown as well as beige/brite adipocytes [28,29]. Similarly, BMP7 activates BAT differentiation in ASCs and is well-known as a key inducer of BAT differentiation [29–31]. High-dose GH treatment suppressed adipogenic transdifferentiation in C2C12 myoblasts by upregulating myoG and myoD expression as well as promoting myotube formation (Fig. 5). Conversely, low-dose GH treatment resulted in higher TG accumulation as well as PPAR γ and C/EBP β mRNA expression compared to that in high-dose-treated cells (Fig. 5). Moreover, the expression of the BAT-specific genes UCP1 and BMP7 was lower after high-dose GH treatment (Fig. 6). These results suggest that high-dose GH treatment inhibited the efficient transdifferentiation of myoblasts into adipocytes by favoring myogenic over adipogenic differentiation.
Thus, high-dose GH treatment suppressed adipogenic differentiation and lipid accumulation in both myogenic and adipogenic precursor cells by promoting myogenesis. Adipocyte development between muscle bundles can be attained in two ways: by promoting adipogenic commitment and differentiation from MSCs or by reprogramming myogenic progenitors into adipocytes. At low concentrations, GH favor adipogenic determination of ASCs and adipocyte differentiation from committed cells. Furthermore, low concentration of GH sustains transdifferentiation of myoblasts and lipid accumulation in mature adipocytes.
Regardless of castration timing, blood testosterone level, another important factor that influences marbling score, drops after castration. Our previous study showed that, low levels of testosterone also had increased adipocyte differentiation in SVC but exposure to high levels of testosterone may have negative effect on marbling in late castrated calves [12]. Next, in vivo experiment was conducted to investigate whether the results of the cellular study showing marked differences in the effect of GH levels were also reflected in calves with different castration timing. The results indicated that calves castrated at 4–5 or 7–8 months of age were reared for 22–25 months more and then slaughtered to observe the characteristics of meat quality and there was no significant difference between them (Table 3). Knight et al. reported that carcass weight, total meat weight, the cross-section area of the longissimus muscle, and marbling score did not show any difference with castration timing when the interval from bull castration to slaughter was longer than 160 days [32]. In addition, it was reported that castration age did not affect carcass weight and at 14 months of slaughter age, meat from castrated calves, regardless of castration timing, had less hardness values than that of Holstein bulls [33]. Our findings that castration timing did not affect carcass characteristics were similar to their results. It has been thought to be due to 22 months or longer interval from bull castration to slaughter in the present study. High levels of GH and testosterone had an adverse effect on adipocyte differentiation on myoblast and SVC but hormonal effect of different GH level by the different castration timing during growing stage on carcass characteristics were not significantly remarkable.
Taken together, it is suggested that considering the slaughter age is generally 30 months, castration timing at a little earlier age of 4–5 months or later age of 7–8 months does not seem to be an important factor in determining carcass qualities, especially carcass weight and marbling score in Hanwoo beef cattle. Additionally, further studies are needed to clarify the appropriate castration timing in a large number of calves.
Acknowledgements
Not applicable.
Competing interests
No potential conflict of interest relevant to this article was reported.
Funding sources
This work was financially supported by grants from Hoengseong Prefecture, Gangwondo, Korea.
Availability of data and material
Upon reasonable request, the datasets of this study can be available from the corresponding author.
Authors’ contributions
Conceptualization: Hwang SG.
Data curation: Hong H, Baatar D, Hwang SG.
Formal analysis: Hong H, Baatar D.
Methodology: Hong H, Baatar D, Hwang SG.
Software: Baatar D.
Validation: Hong H, Baatar D, Hwang SG.
Investigation: Baatar D.
Writing - original draft: Hong H, Baatar D.
Writing - review & editing: Hong H, Baatar D, Hwang SG.
Ethics approval and consent to participate
This study was undertaken in accordance with the Guideline for the Care and Use of Experimental Animals of Hankyong National University Animal Welfare Committee (Hankyong 2020-1).
REFERENCES
- 1.Mwangi FW, Charmley E, Gardiner CP, Malau-Aduli BS, Kinobe RT, Malau-Aduli AEO. Diet and genetics influence beef cattle performance and meat quality characteristics. Foods. 2019;8:648. doi: 10.3390/foods8120648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee B, Choi YM. Correlation of marbling characteristics with meat quality and histochemical characteristics in longssimus thoracis muscle from Hanwoo steers. Food Sci Anim Resour. 2019;39:151–61. doi: 10.5851/kosfa.2019.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vettor R, Milan G, Franzin C, Sanna M, De Coppi P, Rizzuto R, et al. The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab. 2009;297:E987–98. doi: 10.1152/ajpendo.00229.2009. [DOI] [PubMed] [Google Scholar]
- 4.Ryan KJP, Daniel ZCTR, Craggs LJL, Parr T, Brameld JM. Dose-dependent effects of vitamin D on transdifferentiation of skeletal muscle cells to adipose cells. J Endocrinol. 2013;217:45–58. doi: 10.1530/JOE-12-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bretschneider G. Effects of age and method of castration on performance and stress response of beef male cattle: a review. Livest Prod Sci. 2005;97:89–100. doi: 10.1016/j.livprodsci.2005.04.006. [DOI] [Google Scholar]
- 6.Wang J, Chen J, Zhang J, Gao B, Bai X, Lan Y, et al. Castration-induced changes in the expression profiles and promoter methylation of the GHR gene in Huainan male pigs. Anim Sci J. 2017;88:1113–9. doi: 10.1111/asj.12739. [DOI] [PubMed] [Google Scholar]
- 7.Bolamperti S, Guidobono F, Rubinacci A, Villa I. The role of growth hormone in mesenchymal stem cell commitment. Int J Mol Sci. 2019;20:5264. doi: 10.3390/ijms20215264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kopchick JJ, Berryman DE, Puri V, Lee KY, Jorgensen JOL. The effects of growth hormone on adipose tissue: old observations, new mechanisms. Nat Rev Endocrinol. 2020;16:135–46. doi: 10.1038/s41574-019-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burton JL, McBride BW, Block E, Glimm DR, Kennelly JJ. A review of bovine growth hormone. Can J Anim Sci. 1994;74:167–201. doi: 10.4141/cjas94-027. [DOI] [Google Scholar]
- 10.Škarda J. Effect of bovine growth hormone on growth, organ weights, tissue composition and adipose tissue metabolism in young castrated male goats. Livest Prod Sci. 1998;55:215–25. doi: 10.1016/S0301-6226(98)00138-9. [DOI] [Google Scholar]
- 11.Han S, Sun HM, Hwang KC, Kim SW. Adipose-derived stromal vascular fraction cells: update on clinical utility and efficacy. Crit Rev Eukaryot Gene Expr. 2015;25:145–52. doi: 10.1615/CritRevEukaryotGeneExpr.2015013057. [DOI] [PubMed] [Google Scholar]
- 12.Baatar D, Hwang SG. Effect of testosterone on the differentiation control of stromal vascular cells isolated from longissimus muscle of Hanwoo beef cattle. Meat Sci. 2020;159:1079616. doi: 10.1016/j.meatsci.2019.107916. [DOI] [PubMed] [Google Scholar]
- 13.Ge X, Yu J, Jiang H. Growth hormone stimulates protein synthesis in bovine skeletal muscle cells without altering insulin-like growth factor-I mRNA expression. J Anim Sci. 2012;90:1126–33. doi: 10.2527/jas.2011-4358. [DOI] [PubMed] [Google Scholar]
- 14.Sarjeant K, Stephens JM. Adipogenesis. Cold Spring Harb Perspect Biol. 2012;4:a008417. doi: 10.1101/cshperspect.a008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo W, Li E, Nie Q, Zang X. Myomaker, regulated by MYOD, MYOG and miR-140-3p, promotes chicken myoblast fusion. Int J Mol Sci. 2015;16:26186–201. doi: 10.3390/ijms161125946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meadows E, Cho JH, Flynn JM, Klein WH. Myogenin regulates a distinct genetic program in adult muscle stem cells. Dev Biol. 2008;322:406–14. doi: 10.1016/j.ydbio.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Tapscott SJ. The circuitry of a master switch: myod and the regulation of skeletal muscle gene transcription. Development. 2005;132:2685–95. doi: 10.1242/dev.01874. [DOI] [PubMed] [Google Scholar]
- 18.Miao ZG, Zhang LP, Fu X, Yang QY, Zhu MJ, Dodson MV, et al. Invited review: mesenchymal progenitor cells in intramuscular connective tissue development. Animals. 2016;10:75–81. doi: 10.1017/S1751731115001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi SM, Tucker DF, Gross DN, Easton RM, DiPilato LM, Dean AS, et al. Insulin regulates adipocyte lipolysis via an akt-independent signaling pathway. Mol Cell Biol. 2010;30:5009–20. doi: 10.1128/MCB.00797-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen TS, Jessen N, Jørgensen JO, Møller N, Lund S. Dissecting adipose tissue lipolysis: molecular regulation and implicatins for metabolic disease. J Mol Endocrinol. 2014;52:R199–222. doi: 10.1530/JME-13-0277. [DOI] [PubMed] [Google Scholar]
- 21.Aronowitz JA, Lockhart RA, Hakakian CS. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus. 2015;4:713. doi: 10.1186/s40064-015-1509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee EJ, Bajracharya P, Lee DM, Kang SW, Lee YS, Lee JL, et al. Gene expression profiles during differentiation and transdifferentiation of bovine myogenic satellite cells. Genes Genom. 2012;34:133–48. doi: 10.1007/s13258-011-0096-z. [DOI] [PubMed] [Google Scholar]
- 23.Shan T, Liang X, Bi P, Zhang P, Liu W, Kuang S. Distinct populations of adipogenic and myogenic Myf5-lineage progenitors in white adipose tissue. J Lipid Res. 2013;54:2214–24. doi: 10.1194/jlr.M038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh NK, Chae HS, Hwang IH, Yoo YM, Ahn CN, Lee HJ, et al. Conversion of C2C12 myoblast into adipoblast with thiazolidinediones: a possible basis for intramuscular fat generation in meat animals. Asian Australas J Anim Sci. 2007;20:432–9. doi: 10.5713/ajas.2007.432. [DOI] [Google Scholar]
- 25.Zhu Y, Yang R, McLenithan J, Yu D, Wang H, Wang Y, et al. Direct conversion of human myoblasts into brown-like adipocytes by engineered super-active PPARy. Obesity Soc. 2015;23:1014–21. doi: 10.1002/oby.21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C, Liu W, Nie Y, Qaher M, Horton HE, Yue F, et al. Loss of myoD promotes fate transdifferentiation of myoblasts into brown adipocytes. EBioMedicine. 2017;16:212–23. doi: 10.1016/j.ebiom.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boon MR, van den Berg SAA, Wang Y, van den Bossche J, Karkampouna S, Bauwens M, et al. BMP7 activates brown adipose tissue and reduces diet-induced obesity only at subthermoneutrality. PLOS ONE. 2013;8:e74083. doi: 10.1371/journal.pone.0074083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalinovich AV, de Jong JMA, Cannon B, Nedergaard J. UCP1 in adipose tissues: two steps to full browning. Biochimie. 2017;134:127–37. doi: 10.1016/j.biochi.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Porter C. Quantification of UCP1 function in human brown adipose tissue. Adipocyte. 2017;6:167–74. doi: 10.1080/21623945.2017.1319535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okla M, Ha JH, Temel RE, Chung S. BMP7 drives human adipogenic stem cells into metabolically active beige adipocytes. Lipids. 2015;50:111–20. doi: 10.1007/s11745-014-3981-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saini S, Duraisamy AJ, Bayen S, Vats P, Singh SB. Role of BMP7 in appetite regulation, adipogenesis, and energy expenditure. Endocrine. 2015;48:405–9. doi: 10.1007/s12020-014-0406-8. [DOI] [PubMed] [Google Scholar]
- 32.Knight TW, Cosgrove GP, Death AF, Anderson CB. Effect of interval from castration of bulls to slaughter on carcass characteristics and meat quality. New Zealand J Agric Res. 1999;42:269–77. doi: 10.1080/00288233.1999.9513376. [DOI] [Google Scholar]
- 33.Marti S, Realini CE, Bach A, Pérez-Juan M, Devant M. Effect of castration and slaughter age on performance, carcass, and meat quality traits of Holstein calves fed a high-concentrate diet. J Anim Sci. 2013;91:1129–40. doi: 10.2527/jas.2012-5717. [DOI] [PubMed] [Google Scholar]