Abstract
Increasing clinical evidence shows that acute kidney injury (AKI) is a common and severe complication in critically ill COVID-19 patients. The older age, the severity of COVID-19 infection, the ethnicity, and the history of smoking, diabetes, hypertension, and cardiovascular disease are the risk factor for AKI in COVID-19 patients. Of them, inflammation may be a key player in the pathogenesis of AKI in patients with COVID-19. It is highly possible that SARS-COV-2 infection may trigger the activation of multiple inflammatory pathways including angiotensin II, cytokine storm such as interleukin-6 (IL-6), C-reactive protein (CRP), TGF-β signaling, complement activation, and lung-kidney crosstalk to cause AKI. Thus, treatments by targeting these inflammatory molecules and pathways with a monoclonal antibody against IL-6 (Tocilizumab), C3 inhibitor AMY-101, anti-C5 antibody, anti-TGF-β OT-101, and the use of CRRT in critically ill patients may represent as novel and specific therapies for AKI in COVID-19 patients.
Keywords: COVID-19, AKI, cytokines, inflammation, mechanisms
Introduction
COVID-19 is a progressive viral pneumonia with a broad spectrum of clinical manifestations, ranging from asymptomatic to mild (80%), severe (10-15%) or critical and death (2-5%) 1, 2. Among critically ill COVID-19 patients, acute respiratory distress syndrome (ARDS) and multiorgan failure including acute kidney injury (AKI) are the most common co-morbidities 3-5. In this review article, we are focusing on SARS-CoV-2-associated AKI. The possible mechanisms and pathways related to SARS-CoV-2-associated AKI are discussed.
Epidemiology of AKI in COVID-19 patients
Increasing evidence shows that there is high prevalence of AKI in COVID-19 patients 6, 7. The manifestations of AKI are diverse, from proteinuria, hematuria, elevated serum creatinine (Scr) or blood urea nitrogen (BUN) levels to acute renal failure. A meta-analysis shows that more than half (57%) of COVID-19 patients develop proteinuria, accompanied by elevated serum levels of Scr (9.6%-15.5%) and BUN (13.7-14.1%) 5, 6. The CT scan also shows renal inflammation and edema 8. Pathologically, diffuse proximal tubule injury with loss of brush border and frank necrosis is found in COVID-19 patients with AKI 9, 10.
Compared to patients with Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS) in which the incidence of AKI is 6.7% and 42% respectively 11, 12. The incidence of AKI in COVID-19 patients is highly variable. In the early reports from China, COVID-19 patients with AKI was rare 13, 14, but increased to 10% in a later study 15, and became more severe with the incident rate of 25%-29% in those admitted to ICU 16, 17. The large cohort studies in the western countries revealed that the incidence of AKI was 27%-37% 18, 19 and became more severe (68%) in critically ill COVID-19 patients who were admitted to ICU in the New York city 20. Nevertheless, it is now clear that the incidence of AKI in COVID-19 patients is associated with the age, smoking, the cytokine storm, the severity of disease, the ethnicity, and the history of diabetes, hypertension, and cardiovascular disease 7. Thus, AKI is an independent risk factor for the poor long-term renal outcome and mortality in critically ill COVID-19 patients 21, 22. During the follow-up study, AKI is a major cause of in-hospital mortality. In addition, the complete kidney recovery rate of AKI in COVID-19 infection is only about 30-45% based on the recent reports 15, 20, 23. Thus, AKI is one of severe complications and mortality of in-hospital COVID-19 patients, however, mechanisms of COVID-19-associated AKI remain largely unclear and need further studies.
Inflammation may be a mechanism of AKI in COVID-19 patients
Multiple factors such as direct virus infection, cytokine storm, hypoxia, sepsis shock, hemodynamic instability and rhabdomyolysis, hypertension, and diabetes may be associated with AKI in COVID-19 patients. Of these factors, inflammation stress may be a mechanism of AKI in COVID-19 patients, which is discussed below.
Angiotensin II (Ang II) and hypertensive stress
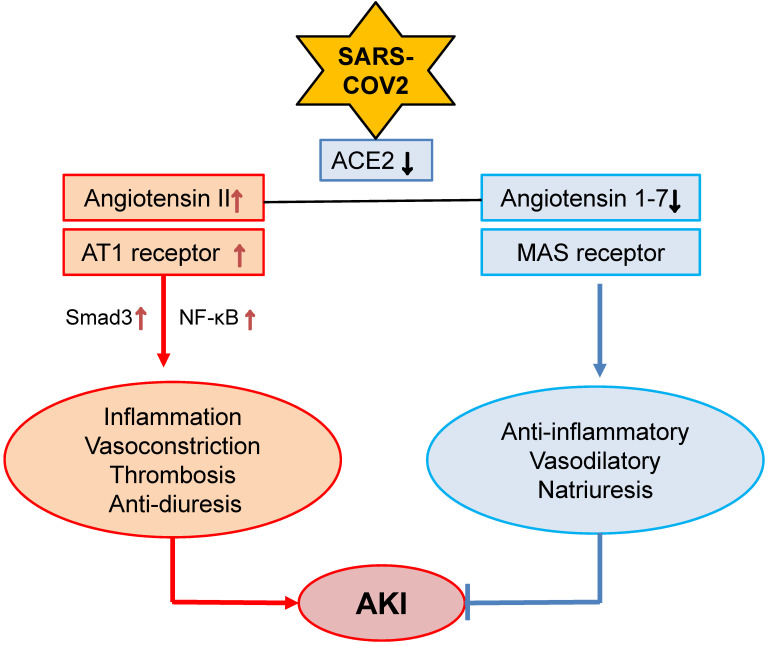
Kidney is a target organ of SARS-COV-2 virus infection due to the high expression levels of angiotensin-converting enzyme 2 (ACE2), a receptor for SARS-COV-2 virus 24, in the kidney tissues, particularly in renal tubular epithelial cells (TECs) 25-27. Thus, SARS-COV-2 may be able to directly bind to ACE2 and infect kidney cells, which is supported by high levels of SARS-COV-2 spike (S) and nucleoprotein (N protein) in COVID-19 patients with AKI 9, 10, 28. In the kidney, renin-angiotensin-aldosterone system (RAAS) maintains renal hemodynamic and regulates renal sodium transport in both normal physiological states and pathological conditions. Ang II and Ang 1-7 are the two major effectors of RAAS and are tightly controlled by two major enzymes of ACE and ACE2 29. Ang II acts via its receptor-1 (AT1) to mediate renal inflammation and fibrosis by activating NF-kB and Smad signaling crosstalk pathways, whereas Ang 1-7 binds receptor Mas to counter-regulate these pathological effects of Ang II 29. The primary function of ACE2 is to covert Ang II to Ang 1-7 to exert its anti-inflammatory, vasodilatory and natriuretic properties 30 (Figure 1). After binding to ACE2, SARS-COV-2 significantly downregulates ACE2 expression 31, 32, resulting in a inhibition or loss of Ang 1-7 while enhancing Ang II-AT1-dependent renal inflammation, vasoconstriction, thrombosis and anti-diuresis effects 33 (Figure 1). It has been well documented that Ang II is a key mediator of AKI 34-36, whereas, the ACE2-Ang-1-7-Mas axis is renoprotective 37. Thus, SARS-COV-2 viral infection to the kidney may downregulate ACE2-Ang1-7-Mas signaling while promoting the Ang II-AT1 signaling to mediate renal inflammation and AKI. A similar mechanism is also found in patients with ARDS 38. Thus, the interaction between SARS-COV-2 virus and ACE2 may eventually impair the ACE2-Ang 1-7 while enhancing Ang II signaling, resulting in hypertension and inflammatory stress both systemically and locally in the kidney. This may well explain that hypertension is an independent risk factor in COVID-19 patients 39. However, the role of Ang II signaling in COVID-19 patients with progressive renal injury remains yet to be determined.
Figure 1.
Alterations of Ang II and Ang 1-7 signaling in COVID-19 associated AKI. SARS-COV-2 binds and downregulates ACE2, which may result in downregulation of Ang 1-7 while upregulating Ang II-AT signaling to promote AKI.
Diabetes and metabolic stress
Diabetes is also a risk factor for AKI 40. Patients with diabetes are associated with the severity and death in pandemic influenza (H1N1) 41, SARS-COV 42 and MERS-COV 43. Recent studies also reported that COVID-19 patients with diabetes have higher AKI and mortality rate than those with non-diabetes 14, 44. This is also confirmed in a recent meta-analysis in 5497 COVID-19 patients 45.
It is now well accepted that metabolic stress including hyperglycemia, obesity, insulin resistance and high levels of glycosylation end products (AGEs) in patients with diabetes can trigger the production of pro-inflammatory cytokines and promotes the oxidative stress 46. Hyperglycemia is a risk factor for AKI in patients with diabetes 21. The IκB kinase-β (IKKβ)/NF-κB axis is a key inflammatory pathway in diabetes in response to hyperglycemia and insulin resistance 47. AGEs can also induce activation of NF-κB, resulting in production of pro-inflammatory cytokines 48. By comparing with non-diabetic COVID-19 patients, COVID-19 patients with diabetes have significantly higher levels of IL-6 and CRP 49. In addition, patients with diabetes also develop hypertension, which is associated with activation of Ang II-AT1 while inhibiting ACE2-Ang 1-7 signaling 50. In addition, since ACE2 is also expressed in pancreas, infection of SARS-COV-2 may also damage pancreatic islet β-cells and aggravate hyperglycemia 51. Thus, enhanced metabolic inflammation in the diabetic kidney may be another mechanism contributing to the development of AKI in COVID-19 patients. However, mechanisms responsible for the metabolic stress in AKI after COVID-19 infection remain yet to be explored.
Cytokine storm
Inflammatory cytokines has been recognized as a critical factor in the progression of COVID-19 52, 53. The inflammatory response triggered by SARS-COV, MERS-COV or SARS-COV-2 can recruit and activate monocytes, macrophage and dendritic cells to produce inflammatory cytokines 54, 55, which may be essential in controlling the viral replication and cleaning the infected cells 56. However, overactive immune responses may cause excessive and persistent cytokine production that leads to cytokine storm and results in multiple organ dysfunction as seen in patients with severe SARS 57, MERS 58 and COVID-19 infection 59. In these patients, a number of proinflammatory cytokines such as interleukin (IL)-1β, IL-6, IL-12, interferon γ (IFN-γ) and monocyte chemoattractant peptide (MCP-1) are associated with extensive lung damage in SARS patients 60. The blood levels of IL-10, IL-15 and TGF-β1 are also positively correlated with the disease severity in patients with MERS 61. In COVID-19 patients, levels of IL-1β, IL-1RA, IL-7, IL-8, IL-9, IL-10, granulocyte colony stimulating factor (G-CSF), IFN-γ, interferon γ inducible protein (IP)-10, tumor necrosis factor-alpha (TNF-α) and MCP-1 are also increased over the healthy controls and become worsen in those admitted to ICU with severe acute lung injury 62, 63.
Cytokine storm can also trigger AKI under various clinical conditions including secondary haemophagocytic lymphohistiocytosis (sHLH) 64-68. sHLH is also found in patients with SARS and COVID-19 69, 70. In addition, cytokine storm may also result in the development of antiphospholipid syndrome in AKI patients with COVID-19 71, 72. Of these inflammatory cytokines, IL-6 has been recognized as a key mediator in COVID-19 patients, which is further described below.
IL-6
Many studies have demonstrated that among the inflammatory cytokines, IL-6 is a most strong and important mediator in COVID-19 patients 73, 74. Meta-analysis involving 12681 COVID-19 patients confirms that IL-6 is significantly higher in those with severe disease conditions 75, 76. Indeed, serum levels of IL-6 positively correlate with the severity of COVID-19 75, 77, 78 and also predict the mechanical ventilation need for COVID-19 patients 79. In COVID-19 patients with older age, IL-6 is an independent risk factor for in-hospital mortality 80.
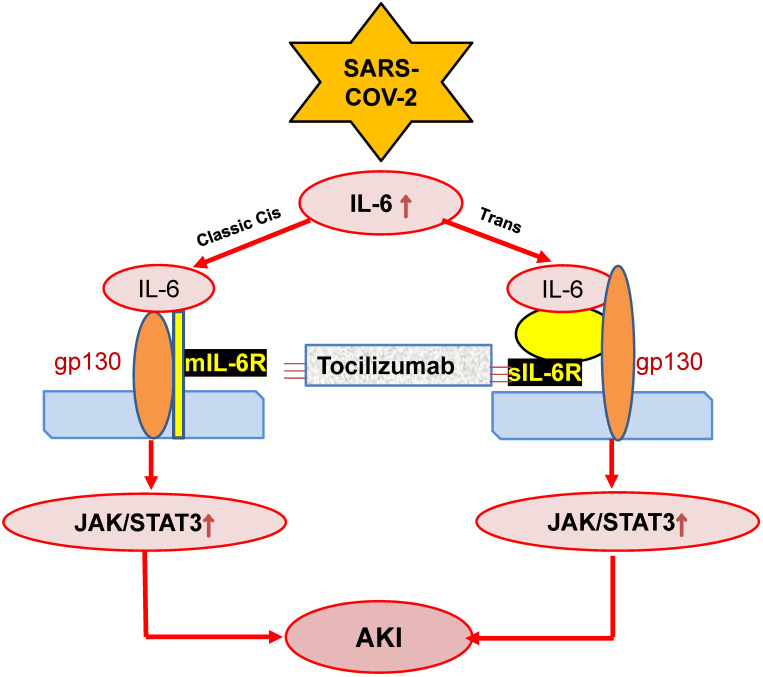
IL-6 is also a predictor for AKI in patients under various clinical conditions including cardiovascular disease, kidney diseases and liver transplantation 81-83. This is also found in ischemic AKI animal model 84. In response to injury, IL-6 is upregulated and released from renal TECs and plays an important role in the pathophysiology of AKI 85. Increasing evidence shows that IL-6 is not only a biomarker but also a mediator for AKI as mice lacking IL-6 are resistant to HgCl2-induced AKI 85-88. In patients with COVID-19, serum levels of IL-6 are elevated in those with AKI 89, and become further increased in those with critically ill 71, 90. In addition, serum levels of IL-6 can also predict the clinical outcomes of AKI as it is significantly reduced in those when AKI is recovered after effective treatment 91. Mechanistically, JAK-STAT3 is a downstream signal transduction of IL-6-membrane-bound-IL-6 receptor (mIL-6R)/soluble-bound-IL-6 receptor (sIL-6R). The IL-6-mIL-6R/sIL-6R-JAK-STAT3 signing pathways are activated during cytokine storm in severe COVID-19 patients 54, which is outlined in Figure 2. However, the functional role and molecular mechanisms of IL-6 in the pathogenesis of COVID-19 associated AKI remain largely unclear.
Figure 2.
Possible mechanisms through which SARS-COV-2 may induce AKI by stimulating IL-6 signaling. SARS-COV-2 may activate IL-6-mIL-6/sIL6-JAK-STAT3 signaling, resulting in AKI, which can be blocked by Tocilizumab antibodies.
C-reactive protein (CRP)
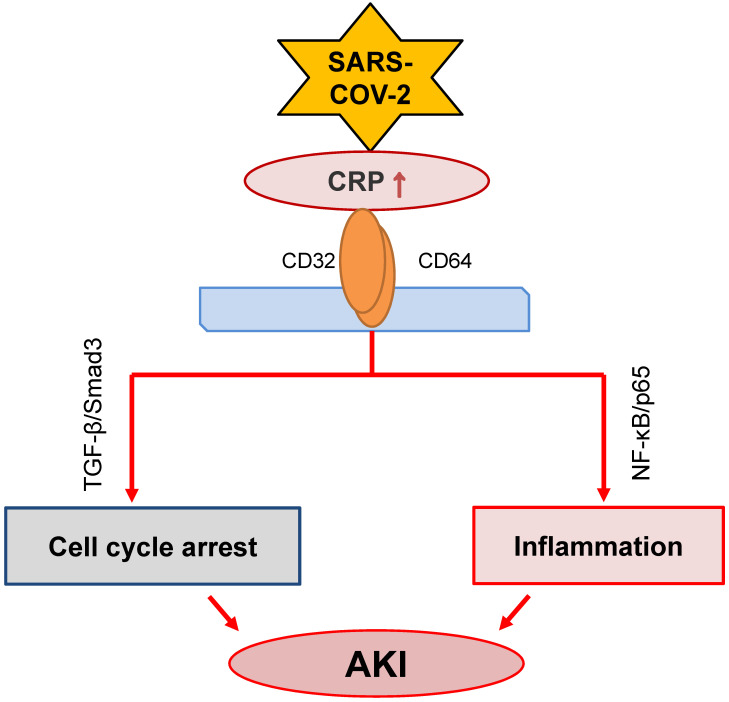
CRP, produced by liver and many inflammatory cells, is an acute phase protein. It has been widely used in clinical settings as an acute inflammation biomarker. CRP is proved as a predictor of postoperative AKI in patients undergoing Coronary Artery Bypass Graft (CABG) 92. High sensitive CRP is associated with AKI in patients with acute myocardial infarction 93, 94. Meanwhile, it is an independent predictor for AKI among ST elevation myocardial infarction patients undergoing primary percutaneous intervention 95. Increasing evidence has suggested that CRP is also a pathogenic factor contributing to the development of inflammatory diseases including atherosclerosis 96 and AKI 97-101. The mechanisms of CRP in the progression of AKI include stimulating macrophage activation 97, inducing cell death by causing G1 cell cycle arrest and autophagy 99, and promoting inflammation 101 (Figure 3). The activation of NF-κB/p65 and TGF-β/Smad3 signaling pathways are the major mechanisms through which CRP mediates AKI 98, 100, 101.
Figure 3.
Possible role of CRP signaling in COVID-19-associated AKI. SARS-COV-2 infection may activate CRP signaling to cause AKI via TGF-β/Smad3-mediated G1 cell cycle arrest and NF-κB-dependent renal inflammation.
COVID-19 patients with AKI show higher levels of serum CRP over those without AKI 102. Serum levels of CRP are also a risk factor of AKI in COVID-19 patients 103. Tan et al. reported that serum levels of CRP are significantly elevated after SARS-CoV-2 infection, which becomes further increased when the disease is progressive but declines dramatically when COVID-19 is recovered 104. Thus, levels of serum CRP may be a predictor for the clinical outcomes of COVID-19 patients. Meta-analysis confirms this notion that in contrast to mild and survival subgroup of COVID-19 patients, high levels of CRP are associated with severe and death subgroup of COVID-19 patients 75, 76, 105. In addition, CRP is also an indicator for renal replacement therapy and the need for mechanical ventilation in COVID-19 patients 106. Thus, elevated CRP is independently associated with poor clinical outcomes in COVID-19 patients 107, 108. However, the pathogenic role and mechanisms of CRP in COVID-19-associated AKI remain largely unknown.
TGF-β
TGF-β is a pleiotropic cytokine and signals through its downstream canonical and non-canonical pathways to diversely regulate renal inflammation and fibrosis 109, 110. It has been reported that SARS-COV nucleocapsid protein can interact with Smad3 to activate the canonical pathway 111. Whereas, the non-canonical TGF-β signaling pathway is also activated by the papain-like protease of SARS-COV by inducing expression of TGF-β1 112. In COVID-19 patients, plasma levels of TGF-β are significantly elevated and associated with the disease severity and poor clinical outcomes 113, 114. Elevated TGF-β2 mRNA is also found in the bronchoalveolar lavage (BAL) fluid of COVID-19 patients 115, which may contribute to lung inflammation and fibrosis because TGF-β1 is also a growth factor associated with fibrosis [109.110]. It is reported that SARS-COV-2 encoded microRNAs are able to target TGF-β signaling pathway to induce TGF-β-dominated adaptive immune response 116. Upregulation of TGF-β1 in COVID-19 patients is responsible for the recruitment of neutrophils into the site of inflammation 117. TGF-β can also induce MCP-1 to activate macrophage-dependent inflammation in the diabetic kidney via a Smad3-dependent LRNA9884 118. Induction of IL-6 production by TGF-β1 also leads to systemic inflammation and “cytokine storm” 119. Furthermore, TGF-β can induce IgA class switching, which may contribute to vasculitis in critically ill patients with COVID-19 120. Thus, TGF-β may significantly contribute to the immediate and long-term effects of COVID-19.
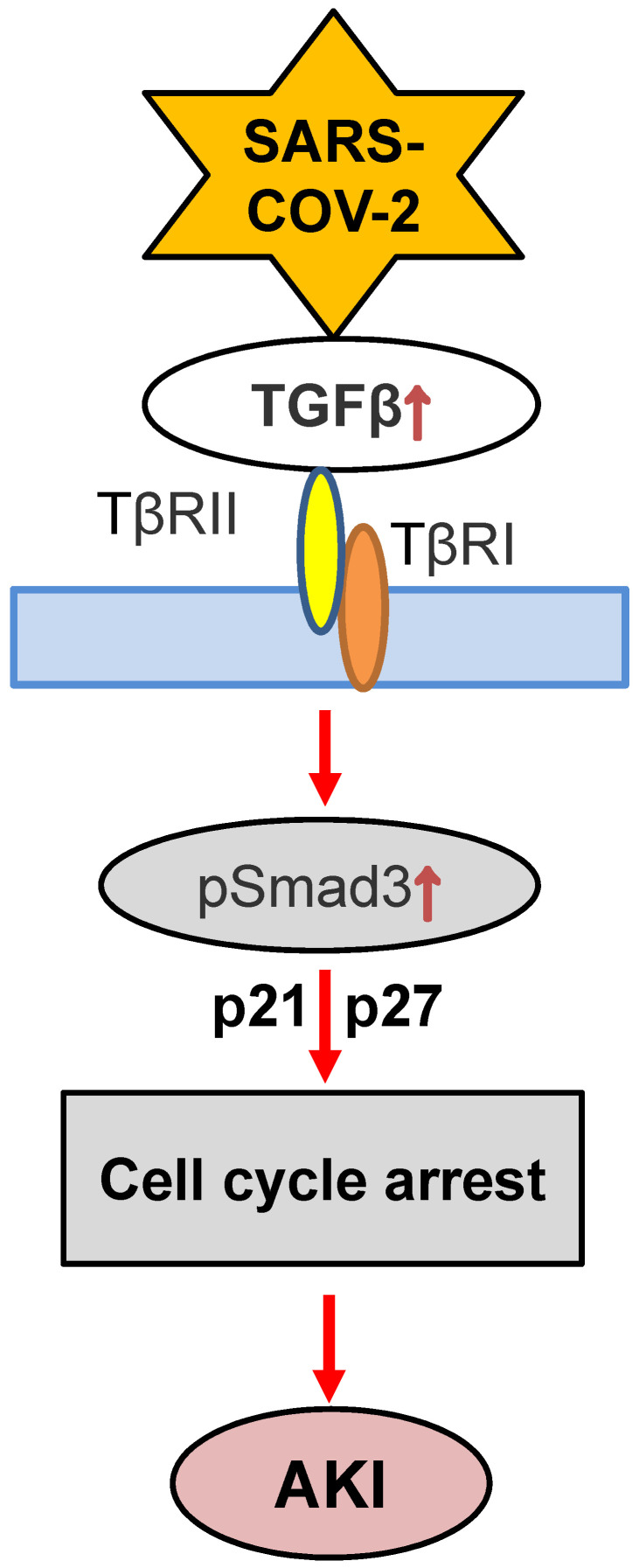
Although the role of TGF-β in COVID-19 associated AKI is still unclear, recent findings that conditional deletion of TGF-β receptor II from renal proximal tubules protects against mercuric chloride and cisplatin-induced AKI reveal a critical role of TGF-β signaling in AKI 121, 122. Furthermore, mice specifically lacking bronchial epithelial TGF-β1 (epTGFβKO) are protected against influenza-induced weight loss, airway inflammation, and viral replication 123, suggesting the impact of TGF-β in viral infection. Mechanistically, TGF-β1 may act via Smad3 to cause AKI as genetic deletion or pharmacological inhibition of Smad3 can block AKI in ischemic mice with or without high human CRP conditions 124. Indeed, Smad3 can be activated by both TGF-β-dependent and independent mechanisms including Ang II, advanced end products (AGE), and CRP under various disease conditions such as hypertension and diabetes 125-127. Thus, activation of Smad3 signaling may account for the clinical notion that patients with diabetes and hypertension are high risk for COVID-19 7. Mechanistically, Smad3 promotes AKI by directly binding to p21/p27 to suppress CDKs/cyclin E to cause the G1 cell cycle arrest 128, 129 (Figure 4). Thus, it is highly possible that TGF-β/Smad3 signaling may contribute to COVID-19-associated AKI, which is warranted for further investigation.
Figure 4.
Proposed TGF-β signaling in COVID-19-associated AKI. SARS-COV-2 may induce G1 cell cycle arrest and cell death via TGF-β/Smad3-p21/p27 mechanism.
Complement activation
The complement system is the first response of host immune system that recognizes and eliminates virus, such as SARS-COV or SARS-COV-2 7, 130. There are several pathways involving in systemic complement activation including the lectin pathway, classical pathway, and alternative pathway 130, 131. The lectin pathway is triggered by the binding of mannose-binding lectin (MBL) with SARS-COV spike (S) protein 132, which leads to the activation of mannan-binding lectin-associated serine protease 2 (MASP-2). The N protein of coronavirus is also associated with the severity of lung injury according to MASP-2-mediated complement overactivation. Thus, alteration of MASP-2-binding motif or blocking the MASP-2-N protein interaction attenuates lung injury 133. The classical pathway is activated by the binding of antibodies, which forms the immune complexes with viral antigens to complement C1 complex 130, 131. The classical, lectin and alternative pathways resulting in the formation of C3 convertase to activate the complement system, which is observed in the lung after SARS-COV infection 130, 131, 134. The role of C3 in SARS-COV-induced lung injury is confirmed in C3 deficient mice in which deletion of C3 protects against ARDS with lower levels of cytokine and inflammatory monocytes infiltration 135.
The complement system is activated during the progression of COVID-19 136. The activation of complement system is related to the disease severity and the respiratory failure in COVID-19 patients 134, 136, 137. It has been shown that the C5a-C5aR1 axis plays an important role in the development of ARDS in COVID-19 138, whereas, C4d and C5-9 are colocalized with the SARS-COV-2 S protein in the lung and skin vasculature 139.
The activation of alternative pathway of complement also participates in the pathogenesis of AKI 140, 141. It has been reported that local synthesis or deposition and activation of complement by renal epithelium is an important cause of AKI 142, 143. In addition, strong C5b-9 staining has been demonstrated on the apical brush border of TECs of the kidney with SARS-COV-2 infection 10. Activation of the classical complement pathway is also observed in the AKI kidney in critically ill children with COVID-19 90. However, the functional role and mechanisms of complement activation in COVID-19-assicated AKI remain largely unclear.
The lung-kidney crosstalk pathway
The crosstalk between the lung and kidney has been observed in patients with AKI and ARDS 144. Clinically, acute lung injury and AKI are complications often encountered in patients with critical illness 145. Mechanical ventilation can improve lung function but is a risk factor for AKI in critically ill patients 146, 147. It has been reported that positive pressure of mechanical ventilation can increase the risk of AKI by almost 8-fold 106, which is associated with systemic hemodynamic and neurohormonal changes and biotrauma 65, 148, 149. ARDS can trigger AKI via mechanisms associated with systemic hypoxia, hypercapnia, systemic inflammatory response syndrome (SIRS), and mechanical ventilation. Severe hypoxemia in ICU patients is associated with AKI required for renal replacement therapy 145. Kidney is susceptible to hypoxic injury due to the high rate of oxygen consumption 65, 150. Thus, hypoxia can induce AKI and tubular necrosis or apoptosis 149. Hypercapnia in COVID-19 patients can also affect the renal blood flow by stimulating renal vasoconstriction 151. More importantly, the lung-kidney cross-talk is also associated with the cytokine storm 152. The inflammatory reaction caused by the lung injury can damage the kidney to release abundant inflammatory cytokines, which, in turn, promotes the damage in the lung 8. Thus, the approaches to limit ventilator-induced lung injury and decrease the duration of mechanical ventilatory support to protect against AKI in critically ill patients are proposed for treatment of critically ill COVID-19 with acute lung and kidney injury.
Therapeutic potential for COVID-19-associated AKI
Continuous renal replacement therapy (CRRT)
CRRT is an advanced approach to treat patients with AKI by improving overload water status and removing inflammatory factors 153, particularly in those with septic AKI 154. CRRT has been utilized in AKI patients with severe MERS and in critically ill COVID-19 155. However, whether the early initiation or high intensity of CRRT can improve the progression of AKI COVID-19 patients remains to be determined.
Tocilizumab (TCZ)
TCZ, a recombinant humanized monoclonal antibody against the human membrane and soluble IL-6 receptors, is widely used for treatment of immunoinflammatory rheumatic diseases 156, 157. TCZ has been shown to block the IL-6/NF-κB/JNK pathway to have a protective effect against sepsis-induced acute lung injury and AKI 158. As IL-6 is important in COVID-19, TCZ has been used to treat COVID-19 patients clinically (Figure 2). The early treatment with TCZ has been shown to effectively improve the oxygen status in COVID-19 patients 156. The meta-analysis of TCZ studies in a total of 1675 and 6279 COVID-19 patients with critically ill shows that TCZ treatment can significantly reduce the in-hospital mortality rate, although patients remain the need for hemodialysis and ventilation 159. Thus, the therapeutic efficacy of TCZ on COVID-19-associated AKI needs to be further studied.
Complement inhibitor
A C3 inhibitor AMY-101
AMY-101 is a highly selective and potent C3 inhibitor and is currently tested in Phase II clinical trials in patients with sepsis, hemodialysis-induced inflammation or malarial anemia 160-163. It is reported that treatment with AMY-101 is safe and can significantly improve the clinical manifestations in severe COVID-19 patients 164. Further Phase II and III clinical trials are still going.
An anti-C5 antibody
An anti-C5 antibody has been clinically used in patients with C3 glomerulopathy and several types of AKI including atypical hemolytic uremic syndrome and paroxysmal nocturnal hemoglobinuria 165. Treatment with the anti-C5 antibody has shown to improve the kidney function and ameliorate the intra-renal complement activation and systemic inflammation in ischemia reperfusion-induced AKI mouse model 165. The first result of anti-C5 treatment has also revealed a rapid and promising effect on COVID-19 patients 166. However, more clinical trials are needed for the conclusive results of the anti-C5 antibody treatment on COVID-19 patients with AKI.
Anti-TGF-β treatment
As SARS coronavirus can upregulate TGF-β and patients with coronavirus infection have elevated levels of TGF-β 113, 114, it has been proposed that TGF-β could be a valid target for the treatment of COVID-19 167, 168. In a recent Phase II clinical trial, inhibition of TGF-β expression by OT-101, an anti-sense to TGF-β1, has been shown to suppress SARS-COV and SARS-COV-2 replication and allow patients to recover without going into respiratory crisis 169, suggesting that COVID-19 can be treated with TGF-β inhibition. However, it should be noted that TGF-β has diverse roles in renal inflammation and fibrosis 109, 110 and targeting the upstream of TGF-β may also cause adverse effects. Our recent studies showed that TGF-β can trigger AKI via the Smad3-dependent mechanism and treatment with Smad3 inhibitors such as SIS3 or a natural product of Traditional Chinese Medicine Quercetin can effectively suppress AKI even under high human CRP conditions 100, 170. These findings suggest that targeting Smad3 specifically, rather than the entire TGF-β signaling, may represent as a novel and effective therapy for AKI in COVID-19 patients clinically.
Conclusion
AKI is a common complication in critically ill COVID-19 patients. Inflammation may be a key mechanism triggering this process. Many inflammatory stress molecules and pathways including Ang II-associated hypertensive stress, diabetes-related metabolic stress, cytokine storm, high CRP, overreactive TGF-β signaling, complement activation, and lung-kidney crosstalk may promote AKI in COVID-19 patients. Thus, treatments by targeting these molecules and pathways may represent as a novel and specific therapy for AKI in COVID-19 patients.
Acknowledgments
This study was supported by Lui Che Woo Institute of Innovative Medicine (CARE program), Research Grants Council of Hong Kong (GRF 14163317, 14106518, 14111019, C7018‐16G, R4012-18), Health and Medical Research Fund (03140486, 05161326, 14152321), and the Guangdong-Hong Kong-Macao-Joint Labs Program from Guangdong Science and Technology Department (2019B121205005).
Author Contributions
J.C. and W.W. wrote the manuscript and Y.T and X.R.H collected and analyzed data. X.Y. and H.Y.L. designed and revised the manuscript.
Abbreviations
- ACE2
angiotensin-converting enzyme 2
- Ang I
Angiotensin II
- AKI
Acute kidney injury
- ARDS
acute respiratory distress syndrome
- BUN
Blood urea nitrogen
- CRP
C-reactive protein
- CABG
Coronary Artery Bypass Graft
- CRRT
Continuous renal replacement therapy
- G-CSF
granulocyte colony stimulating factor
- IFN-γ
interferon γ
- IKKβ
IκB kinase-β
- IL-6
Interleukin-6
- IP
inducible protein
- MASP-2
mannan-binding lectin-associated serine protease 2
- MCP-1
monocyte chemoattractant peptide
- mIL-6R
membrane-bound-IL-6 receptor
- RAAS
renin-angiotensin-aldosterone system
- Scr
serum creatinine
- sIL-6R
soluble-bound-IL-6 receptor
- TCZ
Tocilizumab
- TNF-α
tumor necrosis factor-alpha
References
- 1.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Gagliardi I, Patella G, Michael A. et al. COVID-19 and the Kidney: From Epidemiology to Clinical Practice. J Clin Med. 2020;9:2506. doi: 10.3390/jcm9082506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soleimani M. Acute Kidney Injury in SARS-CoV-2 Infection: Direct Effect of Virus on Kidney Proximal Tubule Cells. Int J Mol Sci. 2020;21:3257. doi: 10.3390/ijms21093275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Z, Shi L, Wang Y. et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng YC, Luo R, Wang K. et al. Kidney disease is associated with in -hospital death of patients with COVID-19. Kidney International. 2020;97:829–38. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Jin Y, Li R. et al. Prevalence and impact of acute renal impairment on COVID-19: a systematic review and meta-analysis. Crit Care. 2020;24:356. doi: 10.1186/s13054-020-03065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stasi A, Castellano G, Ranieri E. et al. SARS-CoV-2 and Viral Sepsis: Immune Dysfunction and Implications in Kidney Failure. J Clin Med. 2020;9:4057. doi: 10.3390/jcm9124057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z WM, Yao JW, Caution on Kidney Dysfunctions of COVID-19 Patients. medRxiv. 2020.
- 9.Su H, Yang M, Wan C. et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–27. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diao B WC, Wang RH, Human Kidney is a Target for Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. medRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 11.Chu KH, Tsang WK, Tang CS. et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67:698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou S, Xu J, Xue C. et al. Coronavirus-associated kidney outcomes in COVID-19, SARS, and MERS: a meta-analysis and systematic review. Ren Fail. 2020;43:1–15. doi: 10.1080/0886022X.2020.1847724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang LW, Li X, Chen H. et al. Coronavirus Disease 19 Infection Does Not Result in Acute Kidney Injury: An Analysis of 116 Hospitalized Patients from Wuhan, China. American Journal of Nephrology. 2020;51:343–8. doi: 10.1159/000507471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan WJ, Ni ZY, Hu Y. et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pei G, Zhang Z, Peng J. et al. Renal Involvement and Early Prognosis in Patients with COVID-19 Pneumonia. J Am Soc Nephrol. 2020;31:1157–65. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Yu Y, Xu J. et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu Y, Xu D, Fu S. et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care. 2020;24:219. doi: 10.1186/s13054-020-02939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch JS, Ng JH, Ross DW. et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–18. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson S, Hirsch JS, Narasimhan M. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052–9. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan L, Chaudhary K, Saha A, Acute Kidney Injury in Hospitalized Patients with COVID-19. medRxiv. 2020.
- 21.Chen J, Zeng H, Ouyang X. et al. The incidence, risk factors, and long-term outcomes of acute kidney injury in hospitalized diabetic ketoacidosis patients. BMC Nephrol. 2020;21:48. doi: 10.1186/s12882-020-1709-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Y, Chen J, Huang K. et al. The incidence, risk factors and in-hospital mortality of acute kidney injury in patients after abdominal aortic aneurysm repair surgery. BMC Nephrol. 2017;18:184. doi: 10.1186/s12882-017-0594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng JH, Hirsch JS, Hazzan A. et al. Outcomes Among Patients Hospitalized With COVID-19 and Acute Kidney Injury. Am J Kidney Dis. 2021;77:204–15. doi: 10.1053/j.ajkd.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staico MF, Zaffanello M, Di Pietro G. et al. The kidney in COVID-19: protagonist or figurant? Panminerva Med. doi: 10.23736/S0031-0808.20.03965-8. in press. [DOI] [PubMed] [Google Scholar]
- 25.Wan Y, Shang J, Graham R. et al. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94:e00127–20. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wrapp D, Wang N, Corbett KS. et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–3. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu H, Uchimura K, Donnelly EL. et al. Comparative Analysis and Refinement of Human PSC-Derived Kidney Organoid Differentiation with Single-Cell Transcriptomics. Cell Stem Cell. 2018;23:869–81. doi: 10.1016/j.stem.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braun F, Lutgehetmann M, Pfefferle S. et al. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet. 2020;396:597–8. doi: 10.1016/S0140-6736(20)31759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ni J, Yang F, Huang XR. et al. Dual deficiency of angiotensin-converting enzyme-2 and Mas receptor enhances angiotensin II-induced hypertension and hypertensive nephropathy. J Cell Mol Med. 2020;24:13093–103. doi: 10.1111/jcmm.15914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos RAS, Sampaio WO, Alzamora AC. et al. The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7) Physiol Rev. 2018;98:505–53. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malha L, Mueller FB, Pecker MS. et al. COVID-19 and the Renin-Angiotensin System. Kidney Int Rep. 2020;5:563–5. doi: 10.1016/j.ekir.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. European Journal of Internal Medicine. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizuiri S, Ohashi Y. ACE and ACE2 in kidney disease. World J Nephrol. 2015;4:74–82. doi: 10.5527/wjn.v4.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kontogiannis J, Burns KD. Role of AT1 angiotensin II receptors in renal ischemic injury. Am J Physiol. 1998;274:79–90. doi: 10.1152/ajprenal.1998.274.1.F79. [DOI] [PubMed] [Google Scholar]
- 35.Jerkic M, Miloradovic Z, Jovovic D. et al. Relative roles of endothelin-1 and angiotensin II in experimental post-ischaemic acute renal failure. Nephrol Dial Transplant. 2004;19:83–94. doi: 10.1093/ndt/gfg521. [DOI] [PubMed] [Google Scholar]
- 36.Mackie FE, Campbell DJ, Meyer TW. Intrarenal angiotensin and bradykinin peptide levels in the remnant kidney model of renal insufficiency. Kidney Int. 2001;59:1458–65. doi: 10.1046/j.1523-1755.2001.0590041458.x. [DOI] [PubMed] [Google Scholar]
- 37.da Silveira KD, Pompermayer Bosco KS, Diniz LR. et al. ACE2-angiotensin-(1-7)-Mas axis in renal ischaemia/reperfusion injury in rats. Clin Sci (Lond) 2010;119:385–94. doi: 10.1042/CS20090554. [DOI] [PubMed] [Google Scholar]
- 38.Reddy R, Asante I, Liu S. et al. Circulating angiotensin peptides levels in Acute Respiratory Distress Syndrome correlate with clinical outcomes: A pilot study. PLoS One. 2019;14:e0213096. doi: 10.1371/journal.pone.0213096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lippi G, Wong J, Henry BM. Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020;130:304–9. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 40.Muroya Y, He X, Fan L. et al. Enhanced renal ischemia-reperfusion injury in aging and diabetes. Am J Physiol Renal Physiol. 2018;315:1843–54. doi: 10.1152/ajprenal.00184.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoen K, Horvat N, Guerreiro NFC. et al. Spectrum of clinical and radiographic findings in patients with diagnosis of H1N1 and correlation with clinical severity. BMC Infect Dis. 2019;19:964. doi: 10.1186/s12879-019-4592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang JK, Feng Y, Yuan MY. et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23:623–8. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 43.Banik GR, Alqahtani AS, Booy R, Rashid H. Risk factors for severity and mortality in patients with MERS-CoV: Analysis of publicly available data from Saudi Arabia. Virol Sin. 2016;31:81–4. doi: 10.1007/s12250-015-3679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020;323:1775–6. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 45.Hansrivijit P, Qian C, Boonpheng B. et al. Incidence of acute kidney injury and its association with mortality in patients with COVID-19: a meta-analysis. J Investig Med. 2020;68:1261–70. doi: 10.1136/jim-2020-001407. [DOI] [PubMed] [Google Scholar]
- 46.Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142. doi: 10.1016/j.diabres.2020.108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shoelson SE, Lee J, Yuan M. Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance. International Journal of Obesity. 2003;27:S49–S52. doi: 10.1038/sj.ijo.0802501. [DOI] [PubMed] [Google Scholar]
- 48.Ott C, Jacobs K, Haucke E. et al. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–29. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo WN, Li MY, Dong YL. et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes-Metabolism Research and Reviews. doi: 10.1002/dmrr.3319. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tikellis C, Pickering R, Tsorotes D. et al. Interaction of diabetes and ACE2 in the pathogenesis of cardiovascular disease in experimental diabetes. Clin Sci (Lond) 2012;123:519–29. doi: 10.1042/CS20110668. [DOI] [PubMed] [Google Scholar]
- 51.Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetologica. 2010;47:193–9. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mehta P, McAuley DF, Brown M. et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–4. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stebbing J, Phelan A, Griffin I. et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–2. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–4. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 55.Ye Q, Wang BL, Mao JH. The pathogenesis and treatment of the 'Cytokine Storm' in COVID-19. Journal of Infection. 2020;80:607–13. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tufan A, Avanoglu Guler A, Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci. 2020;50:620–32. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–39. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fehr AR, Channappanavar R, Perlman S. Middle East Respiratory Syndrome: Emergence of a Pathogenic Human Coronavirus. Annu Rev Med. 2017;68:387–99. doi: 10.1146/annurev-med-051215-031152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lipworth B, Chan R, Lipworth S, RuiWen Kuo C. Weathering the Cytokine Storm in Susceptible Patients with Severe SARS-CoV-2 Infection. J Allergy Clin Immunol Pract. 2020;8:1798–801. doi: 10.1016/j.jaip.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong CK, Lam CW, Wu AK. et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Min CK, Cheon S, Ha NY. et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep. 2016;6:25359. doi: 10.1038/srep25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang C, Wang Y, Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Y SC, LI JX, Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. medRxiv. 2020.
- 64.Ahmed AR, Ebad CA, Stoneman S. et al. Kidney injury in COVID-19. World J Nephrol. 2020;9:18–32. doi: 10.5527/wjn.v9.i2.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chong WH, Saha BK. Relationship Between Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and the Etiology of Acute Kidney Injury (AKI) Am J Med Sci. doi: 10.1016/j.amjms.2020.10.025. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramos-Casals M, Brito-Zeron P, Lopez-Guillermo A. et al. Adult haemophagocytic syndrome. Lancet. 2014;383:1503–16. doi: 10.1016/S0140-6736(13)61048-X. [DOI] [PubMed] [Google Scholar]
- 67.Aulagnon F, Lapidus N, Canet E. et al. Acute Kidney Injury in Adults With Hemophagocytic Lymphohistiocytosis. American Journal of Kidney Diseases. 2015;65:851–9. doi: 10.1053/j.ajkd.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 68.Karras A. What nephrologists need to know about hemophagocytic syndrome. Nature Reviews Nephrology. 2009;5:329–36. doi: 10.1038/nrneph.2009.73. [DOI] [PubMed] [Google Scholar]
- 69.McGonagle D, Sharif K, O'Regan A, Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun Rev. 2020;19:102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dimopoulos G, de Mast Q, Markou N. et al. Favorable Anakinra Responses in Severe Covid-19 Patients with Secondary Hemophagocytic Lymphohistiocytosis. Cell Host Microbe. 2020;28:117–23. doi: 10.1016/j.chom.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xia P, Wen Y, Duan Y. et al. Clinicopathological Features and Outcomes of Acute Kidney Injury in Critically Ill COVID-19 with Prolonged Disease Course: A Retrospective Cohort. J Am Soc Nephrol. 2020;31:2205–21. doi: 10.1681/ASN.2020040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Xiao M, Zhang S. et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen XH, Zhao BH, Qu YM. et al. Detectable Serum Severe Acute Respiratory Syndrome Coronavirus 2 Viral Load (RNAemia) Is Closely Correlated With Drastically Elevated Interleukin 6 Level in Critically Ill Patients With Coronavirus Disease 2019. Clinical Infectious Diseases. 2020;71:1937–42. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fara A, Mitrev Z, Rosalia RA, Assas BM. Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol. 2020;10:200160. doi: 10.1098/rsob.200160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ji P, Zhu J, Zhong Z. et al. Association of elevated inflammatory markers and severe COVID-19: A meta-analysis. Medicine (Baltimore) 2020;99:e23315. doi: 10.1097/MD.0000000000023315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeng F, Huang Y, Guo Y. et al. Association of inflammatory markers with the severity of COVID-19: A meta-analysis. Int J Infect Dis. 2020;96:467–74. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen L, Liu HG, Liu W. et al. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:203–8. doi: 10.3760/cma.j.issn.1001-0939.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 78.Luo X, Zhou W, Yan X. et al. Prognostic Value of C-Reactive Protein in Patients With Coronavirus 2019. Clin Infect Dis. 2020;71:2174–9. doi: 10.1093/cid/ciaa641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herold T, Jurinovic V, Arnreich C. et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146:128–36. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yan Q, Zuo P, Cheng L. et al. Acute Kidney Injury Is Associated With In-hospital Mortality in Older Patients With COVID-19. J Gerontol A Biol Sci Med Sci. doi: 10.1093/gerona/glaa181. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chae MS, Kim Y, Chung HS. et al. Predictive Role of Serum Cytokine Profiles in Acute Kidney Injury after Living Donor Liver Transplantation. Mediators Inflamm. 2018;2018:8256193. doi: 10.1155/2018/8256193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang WR, Garg AX, Coca SG. et al. Plasma IL-6 and IL-10 Concentrations Predict AKI and Long-Term Mortality in Adults after Cardiac Surgery. J Am Soc Nephrol. 2015;26:3123–32. doi: 10.1681/ASN.2014080764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Greenberg JH, Whitlock R, Zhang WR. et al. Interleukin-6 and interleukin-10 as acute kidney injury biomarkers in pediatric cardiac surgery. Pediatric Nephrology. 2015;30:1519–27. doi: 10.1007/s00467-015-3088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grigoryev DN, Liu M, Hassoun HT. et al. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol. 2008;19:547–58. doi: 10.1681/ASN.2007040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nechemia-Arbely Y, Barkan D, Pizov G. et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol. 2008;19:1106–15. doi: 10.1681/ASN.2007070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kielar ML, John R, Bennett M. et al. Maladaptive role of IL-6 in ischemic acute renal failure. J Am Soc Nephrol. 2005;16:3315–25. doi: 10.1681/ASN.2003090757. [DOI] [PubMed] [Google Scholar]
- 87.Patel NS, Chatterjee PK, Di Paola R. et al. Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. J Pharmacol Exp Ther. 2005;312:1170–8. doi: 10.1124/jpet.104.078659. [DOI] [PubMed] [Google Scholar]
- 88.Lemay S, Rabb H, Postler G, Singh AK. Prominent and sustained up-regulation of gp130-signaling cytokines and the chemokine MIP-2 in murine renal ischemia-reperfusion injury. Transplantation. 2000;69:959–63. doi: 10.1097/00007890-200003150-00049. [DOI] [PubMed] [Google Scholar]
- 89.Joseph A, Zafrani L, Mabrouki A. et al. Acute kidney injury in patients with SARS-CoV-2 infection. Ann Intensive Care. 2020;10:117. doi: 10.1186/s13613-020-00734-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang XW, Chen XF, Tang F. et al. Be aware of acute kidney injury in critically ill children with COVID-19. Pediatric Nephrology. 2021;36:163–9. doi: 10.1007/s00467-020-04715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Villa G, Romagnoli S, De Rosa S. et al. Blood purification therapy with a hemodiafilter featuring enhanced adsorptive properties for cytokine removal in patients presenting COVID-19: a pilot study. Crit Care. 2020;24:605. doi: 10.1186/s13054-020-03322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Han SS, Kim DK, Kim S. et al. C-Reactive Protein Predicts Acute Kidney Injury and Death After Coronary Artery Bypass Grafting. Ann Thorac Surg. 2017;104:804–10. doi: 10.1016/j.athoracsur.2017.01.075. [DOI] [PubMed] [Google Scholar]
- 93.Cosentino N, Genovese S, Campodonico J. et al. High-Sensitivity C-Reactive Protein and Acute Kidney Injury in Patients with Acute Myocardial Infarction: A Prospective Observational Study. J Clin Med. 2019;8:2192. doi: 10.3390/jcm8122192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fu EL, Franko MA, Obergfell A. et al. High-sensitivity C-reactive protein and the risk of chronic kidney disease progression or acute kidney injury in post-myocardial infarction patients. Am Heart J. 2019;216:20–9. doi: 10.1016/j.ahj.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 95.Shacham Y, Leshem-Rubinow E, Steinvil A. et al. High sensitive C-reactive protein and the risk of acute kidney injury among ST elevation myocardial infarction patients undergoing primary percutaneous intervention. Clinical and Experimental Nephrology. 2015;19:838–43. doi: 10.1007/s10157-014-1071-1. [DOI] [PubMed] [Google Scholar]
- 96.Verma S. C-reactive protein incites atherosclerosis. Can J Cardiol. 2004;20:S29–S31. [PubMed] [Google Scholar]
- 97.Pegues MA, McCrory MA, Zarjou A, Szalai AJ. C-reactive protein exacerbates renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2013;304:1358–65. doi: 10.1152/ajprenal.00476.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tang Y, Huang XR, Lv J. et al. C-reactive protein promotes acute kidney injury by impairing G1/S-dependent tubular epithelium cell regeneration. Clin Sci (Lond) 2014;126:645–59. doi: 10.1042/CS20130471. [DOI] [PubMed] [Google Scholar]
- 99.Bian A, Shi MJ, Flores B. et al. Downregulation of autophagy is associated with severe ischemia-reperfusion-induced acute kidney injury in overexpressing C-reactive protein mice. Plos One. 2017;12:e0181848. doi: 10.1371/journal.pone.0181848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lai W, Tang Y, Huang XR. et al. C-reactive protein promotes acute kidney injury via Smad3-dependent inhibition of CDK2/cyclin E. Kidney Int. 2016;90:610–26. doi: 10.1016/j.kint.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li ZI, Chung AC, Zhou L. et al. C-reactive protein promotes acute renal inflammation and fibrosis in unilateral ureteral obstructive nephropathy in mice. Lab Invest. 2011;91:837–51. doi: 10.1038/labinvest.2011.42. [DOI] [PubMed] [Google Scholar]
- 102.Taha M, Sano D, Hanoudi S. et al. Platelets and renal failure in the SARS-CoV-2 syndrome. Platelets. 2021;32:130–7. doi: 10.1080/09537104.2020.1817361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hamer M, Kivimaki M, Gale CR, Batty GD. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: A community-based cohort study of 387,109 adults in UK. Brain Behav Immun. 2020;87:184–7. doi: 10.1016/j.bbi.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tan C, Huang Y, Shi F. et al. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J Med Virol. 2020;92:856–62. doi: 10.1002/jmv.25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smilowitz NR, Kunichoff D, Garshick M. et al. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J. doi: 10.1093/eurheartj/ehaa1103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Doher MP, Torres de Carvalho FR, Scherer PF, Acute Kidney Injury and Renal Replacement Therapy in Critically Ill COVID-19 Patients: Risk Factors and Outcomes: A Single-Center Experience in Brazil. Blood Purif. 2020. pp. 1–11. [DOI] [PMC free article] [PubMed]
- 107.Malik P, Patel U, Mehta D. et al. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid Based Med. doi: 10.1136/bmjebm-2020-111536. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vena A, Giacobbe DR, Di Biagio A. et al. Clinical characteristics, management and in-hospital mortality of patients with coronavirus disease 2019 in Genoa, Italy. Clin Microbiol Infect. 2020;26:1537–44. doi: 10.1016/j.cmi.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–38. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 110.Gu YY, Liu XS, Huang XR. et al. TGF-beta in renal fibrosis: triumphs and challenges. Future Med Chem. 2020;12:853–66. doi: 10.4155/fmc-2020-0005. [DOI] [PubMed] [Google Scholar]
- 111.Zhao X, Nicholls JM, Chen YG. Severe acute respiratory syndrome-associated coronavirus nucleocapsid protein interacts with Smad3 and modulates transforming growth factor-beta signaling. J Biol Chem. 2008;283:3272–80. doi: 10.1074/jbc.M708033200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li SW, Wang CY, Jou YJ. et al. SARS coronavirus papain-like protease induces Egr-1-dependent up-regulation of TGF-beta1 via ROS/p38 MAPK/STAT3 pathway. Sci Rep. 2016;6:25754. doi: 10.1038/srep25754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ghazavi A, Ganji A, Keshavarzian N. et al. Cytokine profile and disease severity in patients with COVID-19. Cytokine. 2021;137:155323. doi: 10.1016/j.cyto.2020.155323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Agrati C, Sacchi A, Bordoni V. et al. Expansion of myeloid-derived suppressor cells in patients with severe coronavirus disease (COVID-19) Cell Death Differ. 2020;27:3196–207. doi: 10.1038/s41418-020-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xiong Y, Liu Y, Cao L. et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerging Microbes & Infections. 2020;9:761–70. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ferreira-Gomes M. KA, Durek P. et al. In severe COVID-19, SARS-CoV-2 induces a chronic, TGF-β-dominated adaptive immune response. medRxiv. 2020.
- 117.Barnes BJ, Adrover JM, Baxter-Stoltzfus A. et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020;217:e20200652. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang YY, Tang PMK, Tang PCT. et al. LRNA9884, a Novel Smad3-Dependent Long Noncoding RNA, Promotes Diabetic Kidney Injury in db/db Mice via Enhancing MCP-1-Dependent Renal Inflammation. Diabetes. 2019;68:1485–98. doi: 10.2337/db18-1075. [DOI] [PubMed] [Google Scholar]
- 119.Turner M, Chantry D, Feldmann M. Transforming growth factor beta induces the production of interleukin 6 by human peripheral blood mononuclear cells. Cytokine. 1990;2:211–6. doi: 10.1016/1043-4666(90)90018-o. [DOI] [PubMed] [Google Scholar]
- 120.Yu HQ, Sun BQ, Fang ZF. et al. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur Respir J. 2020;56:2001526. doi: 10.1183/13993003.01526-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gewin L, Vadivelu S, Neelisetty S. et al. Deleting the TGF-beta receptor attenuates acute proximal tubule injury. J Am Soc Nephrol. 2012;23:2001–11. doi: 10.1681/ASN.2012020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang Q, Ren GL, Wei B. et al. Conditional knockout of TGF-betaRII /Smad2 signals protects against acute renal injury by alleviating cell necroptosis, apoptosis and inflammation. Theranostics. 2019;9:8277–93. doi: 10.7150/thno.35686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Denney L, Branchett W, Gregory LG. et al. Epithelial-derived TGF-beta 1 acts as a pro-viral factor in the lung during influenza A infection. Mucosal Immunology. 2018;11:523–35. doi: 10.1038/mi.2017.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nath KA, Croatt AJ, Warner GM, Grande JP. Genetic deficiency of Smad3 protects against murine ischemic acute kidney injury. Am J Physiol Renal Physiol. 2011;301:436–42. doi: 10.1152/ajprenal.00162.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang W, Huang XR, Canlas E. et al. Essential role of Smad3 in angiotensin II-induced vascular fibrosis. Circ Res. 2006;98:1032–9. doi: 10.1161/01.RES.0000218782.52610.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li JH, Huang XR, Zhu HJ. et al. Advanced glycation end products activate Smad signaling via TGF-beta-dependent and independent mechanisms: implications for diabetic renal and vascular disease. FASEB J. 2004;18:176–8. doi: 10.1096/fj.02-1117fje. [DOI] [PubMed] [Google Scholar]
- 127.You YK, Huang XR, Chen HY. et al. C-Reactive Protein Promotes Diabetic Kidney Disease in db/db Mice via the CD32b-Smad3-mTOR signaling Pathway. Sci Rep. 2016;6:26740. doi: 10.1038/srep26740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Matsuura I, Denissova NG, Wang G. et al. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature. 2004;430:226–31. doi: 10.1038/nature02650. [DOI] [PubMed] [Google Scholar]
- 129.Fu S, Tang Y, Huang XR. et al. Smad7 protects against acute kidney injury by rescuing tubular epithelial cells from the G1 cell cycle arrest. Clin Sci (Lond) 2017;131:1955–69. doi: 10.1042/CS20170127. [DOI] [PubMed] [Google Scholar]
- 130.Java A, Apicelli AJ, Liszewski MK. et al. The complement system in COVID-19: friend and foe? JCI Insight. 2020;5:e140711. doi: 10.1172/jci.insight.140711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Noris M, Benigni A, Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020;98:314–22. doi: 10.1016/j.kint.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ip WK, Chan KH, Law HK. et al. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J Infect Dis. 2005;191:1697–704. doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gao T HM, Zhang XP, Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. medRxiv. 2020.
- 134.de Nooijer AH, Grondman I, Janssen NAF, Complement activation in the disease course of COVID-19 and its effects on clinical outcomes. J Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 135.Gralinski LE, Sheahan TP, Morrison TE. et al. Complement Activation Contributes to Severe Acute Respiratory Syndrome Coronavirus Pathogenesis. mBio. 2018;9:e01753–18. doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cugno M, Meroni PL, Gualtierotti R. et al. Complement activation and endothelial perturbation parallel COVID-19 severity and activity. J Autoimmun. 2021;116:102560. doi: 10.1016/j.jaut.2020.102560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Holter JC, Pischke SE, de Boer E. et al. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proceedings of the National Academy of Sciences of the United States of America. 2020;117:25018–25. doi: 10.1073/pnas.2010540117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Carvelli J, Demaria O, Vely F. et al. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature. 2020;588:146–50. doi: 10.1038/s41586-020-2600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Magro C, Mulvey JJ, Berlin D. et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol. 2006;176:1305–10. doi: 10.4049/jimmunol.176.3.1305. [DOI] [PubMed] [Google Scholar]
- 141.David S, Biancone L, Caserta C. et al. Alternative pathway complement activation induces proinflammatory activity in human proximal tubular epithelial cells. Nephrol Dial Transplant. 1997;12:51–6. doi: 10.1093/ndt/12.1.51. [DOI] [PubMed] [Google Scholar]
- 142.Farrar CA, Zhou WD, Lin T, Sacks SH. Local extravascular pool of C3 is a determinant of postischemic acute renal failure. Faseb Journal. 2006;20:217–26. doi: 10.1096/fj.05-4747com. [DOI] [PubMed] [Google Scholar]
- 143.Thurman JM, Lucia MS, Ljubanovic D, Holers VM. Acute tubular necrosis is characterized by activation of the alternative pathway of complement. Kidney International. 2005;67:524–30. doi: 10.1111/j.1523-1755.2005.67109.x. [DOI] [PubMed] [Google Scholar]
- 144.Panitchote A, Mehkri O, Hastings A. et al. Factors associated with acute kidney injury in acute respiratory distress syndrome. Ann Intensive Care. 2019;9:74. doi: 10.1186/s13613-019-0552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Darmon M, Clec'h C, Adrie C. et al. Acute Respiratory Distress Syndrome and Risk of AKI among Critically Ill Patients. Clinical Journal of the American Society of Nephrology. 2014;9:1347–53. doi: 10.2215/CJN.08300813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Doi K, Ishizu T, Fujita T, Noiri E. Lung injury following acute kidney injury: kidney-lung crosstalk. Clin Exp Nephrol. 2011;15:464–70. doi: 10.1007/s10157-011-0459-4. [DOI] [PubMed] [Google Scholar]
- 147.Husain-Syed F, Birk HW, Seeger W, Ronco C. A Protective Kidney-Lung Approach to Improve Outcomes in Mechanically Ventilated Patients. Blood Purif. 2016;42:214–8. doi: 10.1159/000448471. [DOI] [PubMed] [Google Scholar]
- 148.Basu RK, Wheeler DS. Kidney-lung cross-talk and acute kidney injury. Pediatr Nephrol. 2013;28:2239–48. doi: 10.1007/s00467-012-2386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Domenech P, Perez T, Saldarini A. et al. Kidney-lung pathophysiological crosstalk: its characteristics and importance. Int Urol Nephrol. 2017;49:1211–5. doi: 10.1007/s11255-017-1585-z. [DOI] [PubMed] [Google Scholar]
- 150.Ricksten SE, Bragadottir G, Redfors B. Renal oxygenation in clinical acute kidney injury. Crit Care. 2013;17:221. doi: 10.1186/cc12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Husain-Syed F, Slutsky AS, Ronco C. Lung-Kidney Cross-Talk in the Critically Ill Patient. Am J Respir Crit Care Med. 2016;194:402–14. doi: 10.1164/rccm.201602-0420CP. [DOI] [PubMed] [Google Scholar]
- 152.Ahmadian E, Hosseiniyan Khatibi SM, Razi Soofiyani S, Covid-19 and kidney injury: Pathophysiology and molecular mechanisms. Rev Med Virol. 2020:e2176. [DOI] [PMC free article] [PubMed]
- 153.Silvester W. Mediator removal with CRRT: Complement and cytokines. American Journal of Kidney Diseases. 1997;30:S38–S43. doi: 10.1016/s0272-6386(97)90541-2. [DOI] [PubMed] [Google Scholar]
- 154.Tian H, Sun T, Hao D. et al. The optimal timing of continuous renal replacement therapy for patients with sepsis-induced acute kidney injury. Int Urol Nephrol. 2014;46:2009–14. doi: 10.1007/s11255-014-0747-5. [DOI] [PubMed] [Google Scholar]
- 155.Cha RH, Joh JS, Jeong I. et al. Renal Complications and Their Prognosis in Korean Patients with Middle East Respiratory Syndrome-Coronavirus from the Central MERS-CoV Designated Hospital. Journal of Korean Medical Science. 2015;30:1807–14. doi: 10.3346/jkms.2015.30.12.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Okoh AK, Bishburg E, Grinberg S, Nagarakanti S. Tocilizumab use in COVID-19-associated pneumonia. Journal of Medical Virology. 2021;93:1023–8. doi: 10.1002/jmv.26471. [DOI] [PubMed] [Google Scholar]
- 157.Choy EH, De Benedetti F, Takeuchi T. et al. Translating IL-6 biology into effective treatments. Nature Reviews Rheumatology. 2020;16:335–45. doi: 10.1038/s41584-020-0419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ibrahim YF, Moussa RA, Bayoumi AMA, Ahmed AF. Tocilizumab attenuates acute lung and kidney injuries and improves survival in a rat model of sepsis via down-regulation of NF-kappa B/JNK: a possible role of P-glycoprotein. Inflammopharmacology. 2020;28:215–30. doi: 10.1007/s10787-019-00628-y. [DOI] [PubMed] [Google Scholar]
- 159.Aziz M, Haghbin H, Abu Sitta E. et al. Efficacy of tocilizumab in COVID-19: A systematic review and meta-analysis. J Med Virol. doi: 10.1002/jmv.26509. in press. [DOI] [PubMed] [Google Scholar]
- 160.Lindorfer MA, Cook EM, Reis ES. et al. Compstatin Cp40 blocks hematin-mediated deposition of C3b fragments on erythrocytes: Implications for treatment of malarial anemia. Clin Immunol. 2016;171:32–5. doi: 10.1016/j.clim.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.van Griensven M, Ricklin D, Denk S. et al. Protective Effects of the Complement Inhibitor Compstatin CP40 in Hemorrhagic Shock. Shock. 2019;51:78–87. doi: 10.1097/SHK.0000000000001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mastellos DC, Reis ES, Biglarnia AR. et al. Taming hemodialysis-induced inflammation: Are complement C3 inhibitors a viable option? Clin Immunol. 2019;198:102–5. doi: 10.1016/j.clim.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Reis ES, Berger N, Wang X. et al. Safety profile after prolonged C3 inhibition. Clin Immunol. 2018;197:96–106. doi: 10.1016/j.clim.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mastaglio S, Ruggeri A, Risitano AM. et al. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin Immunol. 2020;215:108450. doi: 10.1016/j.clim.2020.108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zilberman-Itskovich S, Abu-Hamad R, Stark M, Efrati S. Effect of anti-C5 antibody on recuperation from ischemia/reperfusion-induced acute kidney injury. Ren Fail. 2019;41:967–75. doi: 10.1080/0886022X.2019.1677248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Laurence J, Mulvey JJ, Seshadri M. et al. Anti-complement C5 therapy with eculizumab in three cases of critical COVID-19. Clin Immunol. 2020;219:108555. doi: 10.1016/j.clim.2020.108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Al-helfawi MA. Potential Approach for Fighting Against Corona Virus Disease. ASRJETS. 2020;66:127–44. [Google Scholar]
- 168.Chen W. A potential treatment of COVID-19 with TGF-beta blockade. Int J Biol Sci. 2020;16:1954–5. doi: 10.7150/ijbs.46891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Uckun FM TV. Medical-Scientific Rationale for a Randomized, Placebo-Controlled, Phase 2 Study of Trabedersen/OT-101 in COVID-19 Patients with Hypoxemic Respiratory Failure. Anna pul and Cri Car Med. 2020;3:01–9. [Google Scholar]
- 170.Gu YY, Zhang M, Cen H. et al. Quercetin as a potential treatment for COVID-19-induced acute kidney injury: Based on network pharmacology and molecular docking study. PLoS One. 2021;16:e0245209. doi: 10.1371/journal.pone.0245209. [DOI] [PMC free article] [PubMed] [Google Scholar]