Abstract
The severe cases of Coronavirus Disease 2019 (COVID-19) frequently exhibit excessive inflammatory responses, acute respiratory distress syndrome (ARDS), coagulopathy, and organ damage. The most striking immunopathology of advanced COVID-19 is cytokine release syndrome or “cytokine storm” that is attributable to the deficiencies in immune regulatory mechanisms. CD4+FoxP3+ regulatory T cells (Tregs) are central regulators of immune responses and play an indispensable role in the maintenance of immune homeostasis. Tregs are likely involved in the attenuation of antiviral defense at the early stage of infection and ameliorating inflammation-induced organ injury at the late stage of COVID-19. In this article, we review and summarize the current understanding of the change of Tregs in patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and discuss the potential role of Tregs in the immunopathology of COVID-19. The emerging concept of Treg-targeted therapies, including both adoptive Treg transfer and low dose of IL-2 treatment, is introduced. Furthermore, the potential Treg-boosting effect of therapeutic agents used in the treatment of COVID-19, including dexamethasone, vitamin D, tocilizumab and sarilumab, chloroquine, hydroxychloroquine, azithromycin, adalimumab and tetrandrine, is discussed. The problems in the current study of Treg cells in COVID-19 and future perspectives are also addressed.
Keywords: COVID-19, SARS-CoV-2, CD4+FoxP3+ regulatory T cells, immunopathology
Introduction
The infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is initiated by the binding of the virus to angiotensin-converting enzyme 2 (ACE2) and the internalization of the complex to the host cells 1, 2. Patients with Coronavirus disease 2019 (COVID-19) manifest a range of symptoms of varying severity from no symptoms (asymptomatic) to severe pneumonia, which can progress to acute respiratory distress syndrome (ARDS), metabolic acidosis, septic shock, coagulopathy, organ failure, and even to death 3, 4. Deaths of COVID-19 patients are frequently associated with a cytokine storm syndrome 5, a common feature of several infectious and non-infectious disease, including H5N1 influenza, severe acute respiratory syndrome coronavirus (SARS-CoV), Middle-East respiratory syndrome coronavirus (MERS-CoV), Epstein-Barr virus, cytomegalovirus, group A streptococcus, and graft-versus-host disease 6, 7. Marked elevation of cytokine levels such as interleukin (IL)-6, IL-7, IL-1, IL-10, IL-2, granulocyte colony-stimulating factor, C-X-C motif chemokine ligand 10, monocyte chemoattractant protein-1, macrophage inflammatory proteins-1 alpha, and tumor necrosis factor (TNF) in patients with severe COVID-19 is the characteristics of cytokine storm 8. Moreover, clinical data indicate that lymphopenia (i.e., decrease in CD4+ and CD8+ T cells, NK cells, and B cells) is common in COVID-19 patients as well, which may be associated with the negative prognosis of COVID-19 patients 4, 9, 10. Remarkably, dysregulation of the T cell populations is strongly correlated with severity in COVID-19 patients 11, 12.
CD4+ FoxP3+ Tregs are a subset of potent immunosuppressive cells that play a vital role in maintaining immune homeostasis and in the prevention of autoimmune responses 13, 14. Presumably, Tregs are required to induce immune tolerance specific to SARS-CoV-2 and suppress the excessive inflammation in patients, while they also likely attenuate the host defense capacity to eliminate infection of SARS-CoV-2. The possibly important role of Tregs in immunopathology and Tregs may represent therapeutic targets have spurred the investigators to examine the change of Tregs in COVID-19 patients, especially those with ARDS 11, 15, 16.
In this article, we review and summarize the current understanding of the change of Tregs in patients infected with SARS-CoV-2 and discuss the potential role of Tregs in the immunopathology of COVID-19. The emerging concept of Treg-targeting therapies, including both adoptive Treg transfer and a low dose of IL-2 treatment, is introduced. Furthermore, the potential Treg-boosting effect of therapeutic agents used in the treatment of COVID-19 is analyzed. We also discuss the problems in the current study of Treg cells in COVID-19 and future perspectives.
Overview of immunopathology in COVID-19 infection
Previously, there were two major coronavirus (CoVs) outbreaks, e.g., SARS-CoV and MERS-CoV, which resulted in fatal respiratory diseases with alarming morbidity and mortality 17. The current worldwide outbreak of coronavirus disease 2019 (COVID-19) is caused by a new lineage beta CoVs, namely SARS-CoV-2 3. The genome of SARS-CoV-2 contains 3′ and 5′ untranslated regions and open reading frames that code the non-structural and structural proteins essential for the virus, including spike, nucleocapsid, membrane, and envelope structural proteins 18. A genome analysis from a patient with COVID-19 showed nearly 90% nucleotide identity with SL-CoVZXC21, the bat SARS-related-CoV and 82% identity with human SARS-CoV Tor2 and SARS- CoV BJ01 2003 19. However, the external subdomain of SARS-CoV-2 Spike's receptor binding domain just shares less than half of amino acid identity with other SARS-related coronaviruses 19. The infection of SARS-CoV-2 was due to the binding of S protein to ACE2 expressed by the host cells, which caused a further release of the RNA of SARS-CoV-2 1, 20.
Innate immunity is the first line of defense against viruses. RNA of SARS-CoV-2 that works as a pathogen‐associated molecular pattern can be recognized by host-pathogen recognition receptors, including cytosolic RIG-I-like receptors and extracellular and endosomal Toll-like receptors (TLR) 21. Then specific signal adapter proteins like NF-κB and interferon-regulatory factor family protein are activated and finally trigger the production of type I interferons, which is considered the most important cytokine for antiviral defense 1, 21. In the alveoli of the lungs of COVID-19 patients, the infiltration of CD68+ macrophage is increased, accompanied by the production of cytokines (IL-6, TNFα, and IL-10) 22. More than half of ARDS in all COVID-19 patients manifested macrophage activation syndrome with high levels of TNF and IL-6 in the circulation 23. In the peripheral blood of COVID-19 patients, the proportion of CD14+CD16+ inflammatory monocytes in CD45+ leukocytes is increased, especially in the patients with severe-pulmonary-syndrome 24. These inflammatory monocytes have the capacity to produce high levels of GM-CSF and IL-6 24. As shown by a study on 452 patients, markedly elevated levels of IL-6 and slightly enhanced levels of TNF and IL-2R, while no marked change of IL-1β, were observed in COVID-19 patients 11. Notably, lymphopenia is a common clinical manifestation in patients with COVID-19 3. In addition to inflammatory macrophages, the number of neutrophils is also increased in the circulation, which can be an indicator to predict the severity of COVID-19 8. The neutrophil-to-lymphocyte ratio is a clinical inflammation biomarker. This ratio is elevated in the peripheral blood of patients with COVID-19, and the enhanced ratio is associated with the severity of infection at the early stage of disease 25. Patients treated in the ICU tend to have higher cytokines, including TNF, IL-2, IL-7, IL-10, G-CSF, and monocyte chemoattractant protein-1 in the plasma, and the elevation of these cytokines is related to the progress of disease 3. These cytokines further activate immune cells and increase the pulmonary infiltration that causes lung damage or even ARDS.
CD4 and CD8 T cells are important for the elimination of pathogens through cell-mediated immune responses. Although the absolute number of CD4 and CD8 T cells is lower in patients with COVID-19, these two subsets of T cells are hyperactivated 26. A high rate of asymptomatic infected individuals who could not be detected by serological testing may be due to the inhibition of virus replication through immune cell-mediated responses 27. A recent study shows that in mild COVID-19, SARS-CoV-2 induces strong responses of CD8 T cells, characterized by the expression of granzyme A, B, and perforin, without obvious responses of CD4 T cells 28. However, the responses of cytotoxic CD8 T cells are not detectable in COVID-19 patients over the age of 80 years, which may explain why the incidence of severe or critically severe illness was high in elderly patients 28. It was shown that SARS-CoV-2-specific CD8 T cells express IFNγ and CD107a, a degranulation marker of CD8 T cells, while SARS-CoV-2-specific CD4 T cells had the capacity to produce IFNγ, IL-2, and TNF 29. A recent study found that CD4 T cells in patients with COVID-19 express higher levels of CD38, HLA-DR, and Ki-67 30. With a memory and Th1 phenotype, CD4 T cells in COVID-19 could express IFNγ, the signature cytokine of Th1 responses 30. These CD4 T cells could respond to the second peptide pool PepMix 2 of SARS-CoV-2, which spans the entire S protein and contains putative MHC-II epitopes in SARS-CoV. However, although early T cell responses against SARS-CoV-2 are likely to be protective, SARS-CoV-2 manages to develop a mechanism to escape T cell-mediated immunity 31, 32, presumably partially through the activation or expansion of Tregs. Therefore, Treg cells may play dual and/or biphasic roles: downregulation of T cell-mediated immune responses against SARS-CoV-2 in the early stage of infection as well as dampening the excessive inflammation in severe COVID-19 patients in the late stage.
The role of Tregs in the immunopathology of COVID-19: current understanding
CD4+FoxP3+ regulatory T cells (Tregs) are a subset of CD4+ T cells that typically develop in the thymus (aka. thymus-derived Treg cells or naturally-occurring Treg cells) and can also be induced in the periphery (aka. In vitro-induced Treg cells) 33. Tregs play a crucial role in the maintenance of immune homeostasis and inhibition of autoimmune inflammatory responses by potently inhibiting the activation, proliferation, and effector functions of other immune cells 34. The identification of human Tregs based on surface markers remains challenging. Earlier studies found that human Treg cells express high levels of CD25 (IL-2 receptor alpha chain), e.g., CD25+/hi, and are negative or low expression of CD127 (IL-7 receptor alpha chain), e.g., CD127-/lo 35, 36. Consequently, the conjunction of CD25+/hi and CD127-/lo, as well as lineage marker CD4, are frequently used as surrogate surface makers of FoxP3-expressing Treg cells in human 37, 38. Our own previous study revealed that the combination of CD25 and TNFR2 is able to identify more of FoxP3-expressing Tregs in human peripheral blood 39. FoxP3 is a master nuclear transcript factor that determines the development, function, and phenotype of Treg cells. To date, FoxP3 remains to be the most reliable and specific marker of Treg lineage 40. Moreover, Tregs also express different surface and intracellular markers including co-inhibitory/co-stimulatory molecules (CTLA-4, PD-1, TIM3, LAG3, TIGIT, ICOS, and CD28), Toll-like receptors (such as TLR1, -2, -4, -5, -6, -7, -8 and -9), chemokine receptors (CCR2, -4, -5, -6, -7 and -8, CXCR3 and -4) and TNF receptor superfamily (TNFR2, OX40, 4-1BB, GITR, and FAS) 41. The mechanism of Treg-mediated immunosuppression has been extensively studied. A number of putative mechanisms have been reported, including the production of immunosuppressive cytokines (IL-10, IL-35, and TGFβ), consumption of IL-2, induction of death of effector cells via granzyme and perforin, inhibition of the activation of antigens presenting cells (APC), metabolic disruption (such as the generation of adenosine) and others 42. However, the universal molecular mechanism is still elusive, and further research is needed. We (Xin Chen and Joost J. Oppenheim) for the first time found and reported that TNF through TNFR2 signaling could enhance the expression FoxP3, promote the proliferative expansion and increase the immunosuppressive function of Treg cells 43. Interestingly, TNF preferentially upregulates the expression of TNFR2, along with other members of the TNF receptor superfamily (OX40, 4-1BB, and FAS), on the surface of Tregs 44. We also demonstrated that TNFR2 and its major signaling component IKKα are crucial for the in vivo function and phenotypical stability of Tregs 45, 46.
It was previously reported that, in respiratory virus infection, Treg cells could quench cytokine storm, ameliorate virus-induced pneumonia and acute lung injury 47-49. In human and mouse acute lung injury, accumulation of Tregs is attributable to the attenuation of immunopathology by inhibition of the innate immune responses 49. Therefore, it is likely that Tregs are protective in COVID-19 patients with excessive inflammation and cytokine storm.
Several studies indicate that the number of immunosuppressive Tregs in peripheral blood is moderately increased in patients with mild COVID-19 16, 50, 51. Elevated levels of Tregs are also observed in some patients with more severe disease 50, 51. For example, it was reported that the proportion of conventional T cells, B cells, and NK cells in COVID-19 patients were reduced, while Tregs (defined by CD4+CD25+CD127- ) were increased by 7% and 5% in the mild and severe cases, respectively 50. Since their surface expression of CD25 was up-regulated, and CD127 was down-regulated, Tregs in COVID-19 may be activated, with an enhanced suppressive activity 50. Interestingly, soluble CD25 was increased in the peripheral blood of COVID-19 patients, while its ligand IL-2 was also increased 3, 52. In mechanically ventilated COVID-19 patients with ARDS, in sharp contrast to the lymphopenia in both subsets of CD4 and CD8 T cells, the proportion of Treg cells (defined by CD4+FoxP3+) was increased in the lungs and peripheral blood mononuclear cells (PBMC) 53. The degree of Treg recruitment into the lungs of COVID-19 patients may determine the severity of the disease since patients with more Treg cells experienced milder disease 54, 55. Interestingly, it was reported that in convalescent COVID-19 patients, the expression of FoxP3 in circulating CD4 T cells was higher than that in uninfected individuals 56. Another study demonstrates that, although the total number of Tregs (CD4+ CD25+ CD127-) in an asymptomatic infected person did not change, the percentage of activated Tregs (CD45RA- FoxP3hi) was increased 4.4-fold, as compared with healthy controls 57. In the rhesus monkey model infected with SARS-CoV-2, the proportion of CD4+FoxP3+ Tregs, as well as CD4+ IFNγ+ Th1 and CD4+ IL-4+ Th2 cells, in the lungs was increased at 3 days post-infected (dpi). Furthermore, an increased proportion of Tregs in PBMCs could also be observed from 3 to 21 dpi (P<0.05, 21 dpi), while the proportions of Th1 and Th2 cells were decreased as the severity of the illness was increased in the following days 55. It was demonstrated by a previous study that, in mouse experimental acute lung injury model, increased accumulation of Treg cells in bronchoalveolar lavage fluid (BALF) mediated the resolution of lung injury by induction of neutrophil apoptosis, macrophage efferocytosis, and decrease of fibrocyte recruitment 58.
Although plenty of studies indicate the proportion or number of Tregs is increased in patients with COVID-19 (especially those with the milder disease), it was also reported that the number of Tregs is reduced in the patients. For example, it has been reported that Tregs (CD3+ CD4+ CD25hi CD127lo FoxP3+) in PBMCs were markedly decreased in severe COVID-19 patients 59-61. The result of single-cell analysis showed that FoxP3 expression was remarkedly reduced in severe patients with COVID-19, although the expression of CD25 was higher 62. High expression of CD25 on T cell correlates to furin protease that eases the entry of virus particles 62. A recent study examined Tregs (CD4+ FoxP3+ CD25+) in PBMC derived from the COVID-19 patients treated in ICU and found a marked decline in the number of Tregs with a decreased expression of FoxP3 mRNA and immunosuppressive cytokines (IL-10 and TGFβ) 63. Another study reported that the expression of CD25 and FoxP3 mRNA by Tregs was markedly decreased in COVID-19 patients, compared to healthy donors 60. Interestingly, a report shows that the frequency of Tregs (CD3+ CD4+ CD25hi CD127lo) was significantly declined in critically ill patients, while that was increased in mild and severe cases after SARS-CoV-2 infection 51. Other studies also found that the proportion of Tregs in CD4 T was reduced in adults and children with severe COVID-19 11, 64, 65. These studies conclude that a reduced number of Tregs, as well as increased Th17 responses, may be attributable to the excessive inflammation and pathogenesis of COVID-19. Moreover, a recent study reported that the proportion of Tregs (defined by CD3+ CD4+ CD25+ FoxP3+) and the levels of FoxP3 expression by Tregs were increased in COVID-19 patients, and that was correlated with poor outcome 66. In addition to expressing immunosuppressive molecules, these Tregs also expressed pro-inflammatory cytokine IL-32, suggesting that Treg cells in acute patients with severe disease may inhibit anti-viral T cell responses while promoting inflammatory responses 66.
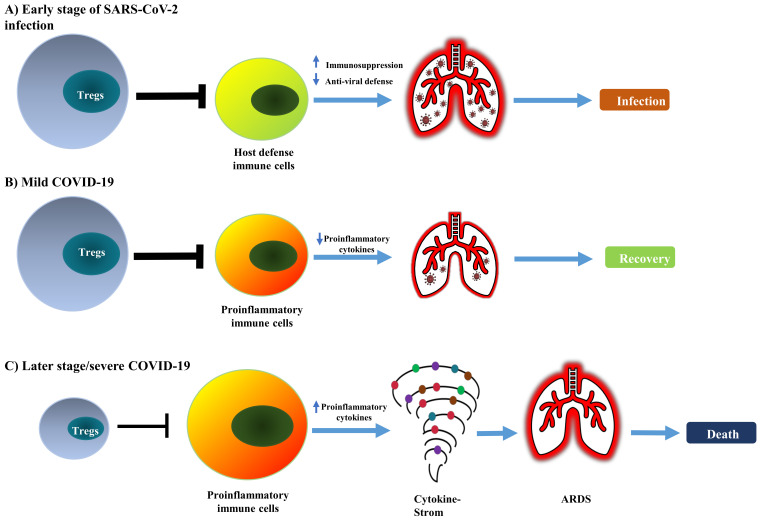
It is worth noting that some studies did not observe any change in the frequency of Tregs (CD4+ CD25+ FoxP3+) in the peripheral blood of COVID-19 patients 67, 68, including in cancer patients infected with SARS-CoV-2 69. Therefore, current reports on the change of absolute and relative number of Treg cells in COVID-19 patients remains controversial (as summarized in Table 1). This should be mainly caused by the different criteria used in the identification of Tregs and the observation was made in different stages of the disease. Nevertheless, the majority of studies indicate that Treg cells in COVID-19 patients are activated, which presumably represents a negative feedback mechanism of the immune system to avoid damage of self-tissues by activated immune cells. It is also possible that, in the early stage of infection, an increased number of activated Tregs may reduce antiviral defense by potently inhibiting the immune responses against SARS-CoV-2. In contrast, a reduction in the number of impaired functions of Tregs in severe cases or later stages of the disease may contribute to the excessive production of pro-inflammatory cytokines that lead to ARDS (schematically shown in Figure 1).
Table 1.
Summary of current studies on Treg cells in COVID-19 patients
| Number of patients or healthy donor controls | Source of Tregs | Markers of Tregs | Changes of Tregs | References |
|---|---|---|---|---|
| N=57 (7 mild, 26 severe and 24 recovered cases) | PBMCs | CD25+FoxP3+ | Increase (proportion) in severe cases | 66 |
| N=4 (mechanically ventilated cases) | BALF and PBMC | FoxP3+ | Increase (proportion, both in lungs and blood) | 53 |
| N=39 | PBMC | CD4+CD25+CD127lo/- | Increase (proportion) | 160 |
| N=169 (80 mild, 22 severe, 61 mild-recovery and 6 severe-recovery cases) | PBMC | CD4+CD25+CD127lo | Increase (number and proportion) | 50 |
| N=1/11 (1 asymptomatic case and 11 healthy controls) | PBMC | CD4+CD25+CD127- CD45RA-FoxP3hi |
Increase (proportion) | 57 |
| N=12/12 (4 mild, 5 severe, 3 critical cases and 12 heathy controls ) | PBMC | CD4+CD25+CD127- | Increase (proportion, in mild and severe cases) Decrease (proportion, in critical cases) |
51 |
| N=40 (22 Severe, 18 milder disease and 9 ICU cases) | UMAP | FoxP3 | Decrease (proportion) | 61 |
| N=30/8 (30 cases and 8 healthy controls) | PBMC | CD4+FoxP3+CD25+ | Decrease (mRNA level) | 60 |
| N=452 (166 mild or moderate and 286 severe cases) | PBMC | CD3+CD4+CD25+ CD127lo | Decrease (numbers) | 11 |
| N=40 (ICU cases) | PBMC | CD4+FoxP3+CD25+ | Decrease (proportion and mRNA level) | 63 |
| N=19 (Pericardial effusion cases) | PBMC | CD3+CD4+CD25+CD127- | Decrease (proportion) | 64 |
| N=19/18 (19 Children cases and 18 healthy controls) | PBMC | CD3+CD4+CD25+CD127- | Decrease (proportion, in acute phase) No change (proportion, in convalescent phase) |
65 |
| N=109/98 (109 convalescent cases and 98 healthy controls) | PBMC | CD25+CD127-FoxP3+ | Decrease (number) | 59 |
| N=99 (93 moderate, 1 severe and 5 critical cases) | PBMC | CD4+ CD25+FoxP3+ | Decrease (proportion) | 62 |
| N=22/10 (11 covid-19 and 11 no-covid-19 cases with cancer, and 10 healthy controls) | PBMC | CD4+CD25+CD127- | No change (proportion) | 69 |
| N=52 (17 moderate, 27 severe and 8 critical cases) | PBMC | CD4+CD25+FoxP3+ | No change (proportion and number) | 67 |
| N=2/2 (A 22-year-old immunocompetent boy, a 63-year-old female COVID cases and 2 healthy controls) | PBMC | CD4+CD25+CD127- FoxP3+ | No change (proportion) | 68 |
Figure 1.
Conceptual model of dual and biphasic roles of Tregs in SARS-CoV-2 infection and immunopathology of COVID-19. In SARS-CoV-2 infection, Tregs are likely to play detrimental and beneficial dual roles and biphasic roles in the early and late stages of the disease. (A) The augmented Treg population in the early stage of infection potently suppresses the mobilization of host defensive immune cells such as Th1 cells and CD8+ CTLs, consequently reducing anti-viral immune responses. (B) An increased number of Tregs could attenuate the inflammatory responses and quench the cytokine storm; this can promote the recovery of patients. (C) In severe COVID-19, depletion of Tregs can enhance the activation of pro-inflammatory immune cells and production of pro-inflammatory cytokines that cause cytokine storm and lead to lung injury, ARDS, and eventually the death of patients.
Treg-targeted treatment for COVID-19: promising results from case reports
The excessive inflammatory responses in COVID-19 patients with ARDS suggest that Tregs should be protective. Therefore, the immunosuppressive property of Tregs may be harnessed to curtail the cytokine storm seen in the critically ill COVID-19 patients 70, 71. In fact, Treg-targeted treatments, including the adoptive transfer of Tregs and rIL-2 administration, have been proposed and studied.
Adoptive Transfer of Tregs
Adoptive Treg transfer is a promising cellular therapy for the treatment of graft-versus-host-disease and autoimmune diseases 72, 73. Importantly, previous studies show that this treatment is effective in a variety of preclinical models of ARDS 49, 74. Moreover, animal studies indicate that the transfer of Tregs could improve the survival rate of mice infected with coronavirus-induced encephalitis and reduce virus-induced cardiac fibrosis 75, 76. Therefore, infusion of ex vivo expanded Treg cells may be able to restore Treg homeostasis in patients with insufficient Treg activity caused by SARS-CoV-2 infection and consequently ameliorate life-threatening manifestations by inhibiting excessive inflammation and quenching cytokine storm.
This idea was tested and supported by a recent case study that examined the effect of adoptive transfer of allogeneic HLA-matched umbilical cord blood-derived Tregs 77. In this report, two critically ill COVID-19 patients with ARDS were intravenously administered with allogeneic Tregs derived from cord blood (1×108 cells per dose). Prior to treatment with Tregs, both patients received tocilizumab (anti- IL-6 receptor antibody), vasopressors, and the first patient also received hydroxychloroquine and broad-spectrum antimicrobial agents. After 2 rounds (Patient 1; on day 13 and 17) to 3 rounds (Patient 2; on day 8, 11, and 15) of Treg infusion, the conditions of both patients markedly improved, accompanied by reduced levels of proinflammatory cytokines (IL-6, TNF, IFNγ, IL-8, IL-12, and MCP-4). None of them demonstrated any infusion reaction, inflammatory rebound, or other adverse reaction 77. Therefore, infusion of cord blood-derived Treg cells (designated as CK0802) may serve as an off-the-shelf cellular therapy in the treatment of COVID-19 with ARDS. The efficacy and safety are under evaluation by an ongoing clinical trial (NCT04468971). Furthermore, RAPA-501-ALLO hybrid TREG/Th2 cells with the potential to reduce inflammation and mediate a protective effect on tissues are under study in another clinical trial as an off-the-shelf therapy for patients with severe COVID-19 and ARDS (NCT04482699) 78.
Recombinant interleukin-2 (rIL-2)
It has been reported that COVID-19 patients have elevated levels of circulating soluble IL-2 receptors 11, 15, 52, 79. Soluble IL-2 receptors may restrain the expansion of Treg cells in COVID-19 patients by reducing the bioavailability of IL-2 to Treg cells 52. A recent case study reported that the treatment with rIL-2 (1 million IU per day) could markedly increase the number of lymphocytes in the peripheral blood (p<0.01) 80. Furthermore, the level of C-reactive protein was decreased after rIL-2 treatment (although no statistically significant, p>0.05) 80. Prompted by the previous studies showing that in vivo treatment with low-dose IL-2 (≤1 million IU per day) was able to specifically induce Treg expansion in patients with type 1 diabetes and graft-versus-host disease 81, 82, a clinical trial was registered to study the efficacy of low-dose IL-2 in the treatment of ARDS related to COVID-19 (NCT04357444).
Thus, two treatments aiming to boost Treg activity in COVID-19 patients have been studied and reported. Their pros and cons are listed in Table 2. Although the outcomes of such treatments are promising based on the case reports, ongoing large-scale clinic trials may provide more convincing results to verify the efficacy and safety of such therapies.
Table 2.
Comparison of Treg-targeted treatments
| Treatment | Advantages | Disadvantages | References |
|---|---|---|---|
| Adoptive Transfer of Tregs | Infusion possible to allogeneic patients, off-the-shelf “living drug” Large scale expansion in vitro; expansion rate is much higher than in vivo Phenotypical and functional features can be analyzed prior to infusion Dosage can be precisely controlled |
Requires 2-3 weeks for o control for possible phenotypical and functional change after infusion Requires specific facility |
161, 162 |
| Low-dose of rIL-2 | Effectively expand of Tregs in autoimmune patients Therapeutic agent readily available Applicable in all health care settings |
May activate CD25-expressing proinflammatory T cells | 163, 164 |
Upregulation of Treg activity by COVID-19 therapeutic agents used in clinic
Dexamethasone
Glucocorticoids have been widely used in the treatment of inflammatory and autoimmune diseases, including asthma, chronic obstructive pulmonary disease (COPD), and rheumatic diseases 83, 84. The common glucocorticoids used in the clinic including prednisone, prednisolone, budesonide, and dexamethasone 83. Previously, glucocorticoids were widely used in the treatment of SARS and MERS, which share some similarities with COVID-19 85. On 2nd September 2020, an interim guideline regarding the use of dexamethasone and other glucocorticoids for COVID-19 patients was declared by World Health Organization, which strongly recommends glucocorticoids including dexamethasone for severe and critical COVID-19 patients 86. Dexamethasone proves effective in the treatment of critically ill COVID-19 patients with hypoxic respiratory failure 87. In a controlled, open-label RECOVERY trial, dexamethasone treatment markedly reduced mortality 85. The immunosuppressive effect of dexamethasone is attributable to its efficacy in the treatment of COVID-19 88.
Our previous study showed that in vivo treatment with dexamethasone increased in the proportion of Treg cells and an elevated ratio of Tregs/Teffs 89. We also found that dexamethasone could allow Treg expansion induced by IL-2, and the combination of IL-2 and dexamethasone synergistically increased the number of Tregs and inhibited the development of experimental autoimmune encephalomyelitis (EAE) in mice 90. Dexamethasone was also reported to improve the function of Treg in human patients with Graves' disease 91. Therefore, dexamethasone may be able to boost Treg activity in COVID-19 patients.
Vitamin D
A number of recent studies suggest that vitamin D deficiency was associated with an increased risk of infection, mortality, and even requirement for intensive care among hospitalized patients of COVID-19 92, 93. For example, a study based on bioinformatics and systems biology found that vitamin D suppressed cytokine storm and enhanced antiviral response by binding with its receptor 94. It could either inhibit the expression of pro-inflammatory cytokines by blocking the TNF-induced NFκB1 signaling pathway or initiated the expression of interferon-stimulating genes for the antiviral defense program through activating the interferon-induced JAK/STAT signaling pathway 94. To date, there are 76 registered clinical trials in ClinicalTrials.gov to evaluate the effect of vitamin D on COVID-19 95, 96.
Although the exact correlation between vitamin D deficiency and susceptibility of SARS-CoV-2 infection remains elusive, the immunomodulatory effect of vitamin D in viral disease and autoimmune disease provides a strong rationale for using it in COVID-19 97, 98. There is evidence that vitamin D has the capacity to inhibit the production of pro-inflammatory cytokines (IL-6 and TNF) and increase the production of immunosuppressive cytokines, and this property of vitamin D could be harnessed to curtail excessive inflammation triggered by viral infections 99, 100. Vitamin D can directly act on cells in innate and adaptive immune systems to regulate various immune pathways 101. In fact, a wide spectrum of immune cells, including macrophages, neutrophils, dendritic cells, B and T lymphocytes, express vitamin D receptor and these cells can convert vitamin D into its active form that can modulate the adaptive and innate immunity 102, 103. It has been reported that Vitamin D induces the generation of tolerogenic dendritic cells that have the capacity to induce IL-10-producing CD4 T cells and antigen-specific Tregs 104.
Additionally, previous studies have demonstrated that dendritic cells induced by vitamin D3 treatment express high levels of transmembrane TNF (mTNF), and such dendritic cells could induce antigen-specific Tregs through the interaction of mTNF-TNFR2 105. In addition, vitamin D3 inhibited T cell-mediated inflammation and promoted the proliferation of Treg cells 106, 107. Vitamin D supplements can also upregulate the expression of FoxP3, IL-10, and TGF-β1 gene in Tregs and increase the number and boost the function of Tregs 108, 109. A recent animal study reported that the treatment with vitamin D resulted in the marked reduction of the methylation of the conserved non-coding sequence 2 region of FoxP3 109. Moreover, there is experimental and clinical evidence that high vitamin D levels are associated with an enhanced ratio of Treg/total T cells 110, 111. Oral administration of vitamin D3 can increase the absolute number of Tregs (CD4+CD25+FoxP3+CD127lo) in the peripheral blood and enhance the immunosuppressive activity of Tregs in healthy adults and patients with autoimmune inflammatory diseases 111, 112. Therefore, vitamin D may have a beneficial effect in the management of SARS-CoV-2 infection by boosting Treg activity.
Tocilizumab and Sarilumab
Tocilizumab and sarilumab are monoclonal antibodies specific to the IL-6 receptor that is used to control cytokine release syndrome, which is characterized by a marked increase in proinflammatory cytokines, including IL-6 113, 114. The effect of tocilizumab on patients with COVID-19 was investigated in China previously. This study found that the treatment was effective in the majority of patients and the responsive patients recovered within 2-weeks 115. Another study also found a similar effect 116. The therapeutic effect of sarilumab, another antibody specific for IL-6R, on COVID-19 is under study in a clinical trial in the US (NCT04315298).
There is some evidence that blockade of IL-6 receptor can promote Treg activity. For example, it was shown that in murine models of autoimmune diseases, anti-IL-6RAb induces the generation of Treg while inhibiting the differentiation of Th17 and Th1 cells 117. In patients with rheumatoid arthritis, the proportions of CD4+CD25+CD127lo Tregs and HLA-DR+ activated Tregs are markedly increased after tocilizumab therapy 118. Therefore, tocilizumab and sarilumab may also promote the generation of Tregs in COVID-19 patients, and this possibility should be further studied.
Chloroquine and Hydroxychloroquine
Chloroquine is an effective schistosome insecticide with a quinoline skeleton that can fight against malaria parasites (mainly Plasmodium falciparum) 119. Hydroxychloroquine, one of the analogs synthesized on the base of the chloroquine molecule, is also frequently used in malaria treatment 120. Chloroquine was listed in the Chinese "Guideline on diagnosis and treatment of COVID-19 (Trail sixth edition)" in Feb 2020 as a recommended therapeutic agent 121. US Food and Drug Administration also granted an emergency authorization to use chloroquine and hydroxychloroquine for the treatment of COVID-19 122, although its efficacy remains controversial 123, 124. It has been reported that chloroquine treatment promoted the expansion of Treg cells in EAE mice and consequently inhibited the development of EAE 125. In patients with systemic lupus erythematosus (SLE), in vitro experiment indicated that chloroquine could rebalance Th17 cells and Treg cells, while in vivo study showed that hydroxychloroquine treatment restored the balance of the immune system and increased the levels of FoxP3 in Treg cells 126. Therefore, it is interesting to ask if the therapeutic effect of chloroquine or hydroxychloroquine, if any, is partially based on their activities in promoting Treg activity.
Azithromycin
Azithromycin (9-deoxo-9a-aza-9a-methyl-9a-homoerythromycin) is a bacteriostatic macrolide with immunoregulatory activity and mTOR inhibitory activity. The result of a clinic trial (EU Clinical Trials Register: 2020-000890-25) in France shows that azithromycin could enhance the efficacy of hydroxychloroquine in the treatment of COVID-19 123. Previously, it was reported that azithromycin, same as rapamycin, could promote Treg phenotype, including FoxP3 expression, in bulk Tregs 127. It was also reported that in vivo treatment with azithromycin could result in the expansion of Tregs in mouse model of graft-versus-host disease 128. Thus, the combination of azithromycin and hydroxychloroquine may synergistically increase the number of Tregs in COVID-19.
Adalimumab
Adalimumab is an anti-TNF monoclonal antibody approved by the US Food and Drug Administration for the treatment of autoimmune diseases including rheumatoid arthritis, Crohn's disease and others 129. A number of case reports showed that adalimumab treatment received by patients with autoimmune diseases has a beneficial effect in the prevention of the clinical progression of severe COVID-19 3, 130-133. This provides a strong rationale for the clinical trial to examine the effect of anti-TNF therapy on COVID-19 134. Two randomized controlled trials (NCT04705844 & ChiCTR2000030089) have been registered to evaluate the efficacy of adalimumab in the treatment of COVID-19 patients with ARDS. Interestingly, it was reported that adalimumab could upregulate the expression of transmembrane TNF on monocytes, and through the TNF-TNFR2 pathway, promoted the activation and expansion of Tregs in rheumatoid arthritis patients 135-138. Therefore, the Treg-boosting effect of adalimumab may contribute to its preventive effect on severe COVID-19 progression.
Tetrandrine
Tetrandrine is an anti-inflammatory bis-benzylisoquinoline alkaloid isolated from the root of Stephania tetrandra S Moore, a traditional Chinese herb 139. This compound was approved for the treatment of silicosis in China 140. It has also been used as an analgesic and diuretic agent 140. Recently, researchers urged to repurpose tetrandrine as adjuvant therapy for the treatment of COVID-19, which might reduce pulmonary fibrosis, and a phase IV clinical trial has been registered (NCT04308317) 141. Tetrandrine is a known blocker of the two‐pore channel, and this property of tetrandrine can inhibit the host cell entrance of ebola virus, MERS-CoV, and SARS-CoV-2 spike protein pseudovirions 142-144. Recently, we reported that inhibition of the two‐pore channel in APC by tetrandrine enhanced the expression of mTNF on APC and consequently induced the proliferative expansion of Tregs via TNFR2 signaling 145, 146. Further, we also found that tetrandrine potently inhibited the differentiation of Th1, Th2 and Th17 cells, while sparing the differentiation of Treg cells 139. Thus, in addition to interfering with cellular entrance and replication of the virus, tetrandrine may attenuate the excessive inflammation in severe COVID-19 by stimulation of Treg proliferative expansion.
Taken together, therapeutic agents currently used in the clinic for the treatment of COVID-19 have the potential to promote Treg activity (summarized in Table 3). It is possible that the Treg-boosting effect of these agents may contribute to their therapeutic effect and this possibility merit future study.
Table 3.
COVID-19 therapeutic agents and their Treg-boosting effect
| Therapeutic agent | Effect on COVID-19 | Action on Tregs | References |
|---|---|---|---|
| Dexamethasone | Improves the outcomes of critically ill patients with ARDS (NCT04445506) Reduces mortality (NCT04381936) Reduces duration for mechanical ventilation (NCT04327401) |
Increases in the proportion of Tregs and the ratio of Treg/effector T cells Increases the number of with combination of IL-2 Enhances the function of Tregs in human patients |
85, 87, 89-91, 165 |
| Vitamin D | Reduces mortality Reduces the need for ICU treatment Deficiency is associated with increased COVID-19 risk |
Induces generation of tolerogenic DCs, IL-10-producing CD4 T cells and antigen-specific Tregs Increases Tregs/T cells ratio and enhances Treg function Increases Foxp3 expression in Tregs and increases Treg number |
92, 104, 108, 166-168 |
| Tocilizumab and Sarilumab | Reduces the incidence or duration of ICU and reduces length of hospital stay Rapidly inhibits excessive inflammation (NCT04331795) |
Increases the proportions of CD4+CD25+CD127lo Tregs and HLA-DR+ activated Tregs Tips the balance of Th17/Tregs toward Tregs cells |
118, 169-171 |
| Chloroquine and hydroxychloroquine | Possesses antiviral activity, inhibits SARS-CoV-2 infection (in vitro) Reduces hospitalization rate among mild outpatients |
Promotes the expansion and increases the number of Treg cells Promotes Foxp3 expression and differentiation of Treg cells Rebalances Th17/Treg cells |
125, 126, 172-174 |
| Azithromycin | Further reduces viral load used together with hydroxychloroquine | Promotes Treg phenotype including Foxp3 expression in bulk Tregs | 123, 127, 175 |
| Tetrandrine | Inhibits the entry of SARS-CoV-2 spike-protein pseudovirions (in vitro) | Increases the expression of mTNF on APC and induces expansion of Tregs through TNFR2 Inhibit differentiation of Th1, Th2 and Th17 cells, but sparing Treg differentiation |
139, 144, 145 |
| Adalimumab | Inhibits the progression of severe COVID-19 | Increases mTNF expression on monocyte and promotes expansion of Tregs through TNFR2 | 132, 134, 137 |
Conclusions and perspectives
To date, the reports regarding the change of Treg cell number in COVID-19 patients remains controversial (Table 1). It should be mainly caused by the use of different markers in the identification of Tregs since CD25+/hi, CD127-/lo, FoxP3+, and their various combinations were used. Furthermore, Tregs number is likely different in COVID-19 patients in the various stages or with various severity of illness. CD25+/hi and CD127-/lo are just surrogate surface markers of FoxP3-expressing Tregs; thus, it would be ideal only to use CD4 and FoxP3 to identify Tregs in the future study 37, 38. Nevertheless, most studies indicate that Treg cells in COVID-19 patients are activated. Unfortunately, the suppressive function of Treg cells in COVID-19 patients has not to be evaluated with a standard in vitro Treg functional assay.
Immunosuppressive Tregs cells and proinflammatory Th17 cells are two major subsets of CD4 T cells. If the balance of Th17/Treg cell shifts towards Th17 cells the release of other pro-inflammatory cytokines will be triggered and inflammatory responses in COVID-19 patients will be exaggerated 147. It was shown that the proportion of Tregs was markedly decreased, while the proportion of CCR6+ Th17 cells was increased in COVID-19 patients 148, 149. TGF-β is required for the differentiation of both Tregs and Th17 cells 150. IL-6 can promote the differentiation of Th17 cells through phosphorylating and activating STAT3, while STAT3 can downregulate TGF-β-induced Foxp3 expression and consequently inhibit Tregs cell differentiation 151. Intriguingly, the levels of IL-6 were markedly elevated in COVID-19 patients 11, and that can tip the balance of Treg/Th17 toward Th17 responses.
To harness the potential beneficial and protective effect of immune suppressors on severe cases, Treg cell-targeted treatments have studied, as shown in two case reports 77, 80. Adoptive transfer of cord blood-derived allogenic Tregs had a clear beneficial effect in critically ill patients 77. Hopefully, the efficacy and safety profile of such treatment could be verified by large-scale clinical trial (NCT04468971). The low dose of IL-2 was proposed as a treatment for COVID-19. However, the levels of IL-2 are increased in severe COVID-19 patients 3, 152. Thus, the possibility that rIL-2 may further fuel inflammatory responses should be closely monitored since the activated pro-inflammatory T cells also express CD25 (the alpha chain of IL-2 receptor). It was reported that dexamethasone could permit IL-2-induced expansion of Tregs while potently inhibiting the activation of Teff cells, and dexamethasone is used in the treatment of COVID-19 as an immunosuppressive agent 87, 90. Thus, it would be reasonable to propose the combination of dexamethasone and rIL-2 in the treatment of critically ill COVID-19 patients. Hopefully, this combination therapy can reduce the dose of dexamethasone as well as its potential side effect 90.
Providing enhanced Treg activity is beneficial in severe COVID-19; other agents with the capacity to promote the induction or expansion of Treg cells should also be considered. For example, rapamycin and all-trans retinoic acid can promote TGFβ-induced differentiation of Tregs from naïve CD4 cells 153, 154. TNFR2-agonist antibody 155, 156 and IL-2/anti-IL-2 monoclonal antibodies 157-159 can stimulate the expansion of pre-existing naturally occurring Tregs. These agents may also have beneficial effect in the treatment of COVID-19 by boosting Treg activity.
The clinical manifestations of patients infected with SARS-CoV-2 are caused by the cytopathic effects of the virus and by excessive inflammatory responses. Treg cells may be involved in the attenuation of antiviral defense at the early stage of infection and inhibition of cytokine storm in critically ill patients. The exact role of Tregs in the pathogenesis of COVID-19 should be further defined. Treg-based therapy appears to be an innovative and promising approach (schematically shown in Figure 2). Nevertheless, its efficacy and safety should be carefully investigated. Furthermore, other therapeutic agents used in COVID-19 treatment may be attributable to their Treg-boosting effect, and this possibility merits future investigation.
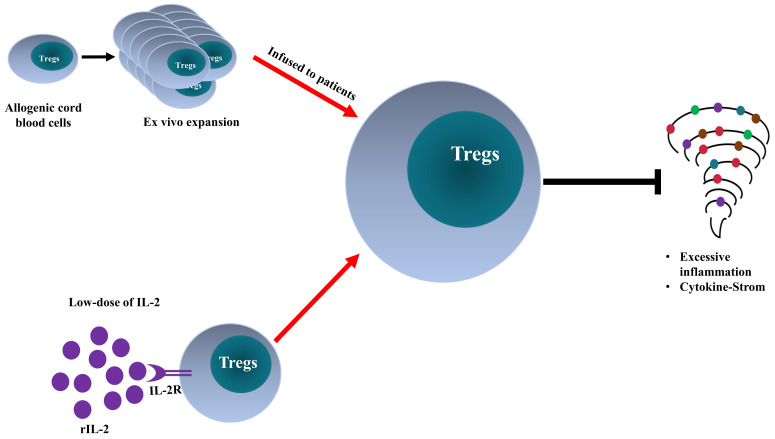
Figure 2.
Comparison of Treg-targeted treatments for COVID-19. Adoptive transfer of ex vivo activated and expanded allogeneic cord-blood derived Tregs and low-dose of recombinant interleukin-2 (rIL-2) enhance Treg activity in severe COVID-19 patients, consequently, dampen the excessive inflammation and quench cytokine storm.
Acknowledgments
This project was funded by The Science and Technology Development Fund, Macau SAR (FDCT, File No. 201/2017/A3 and 0056/2019/AFJ), University of Macau (File No. MYRG2016-00023-ICMS-QRCM, MYRG2017-00120-ICMS, MYRG2019-00169-ICMS and CPG202-00007-ICMS) and the 2020 Guangdong Provincial Science and Technology Innovation Strategy Special Fund, Guangdong-Hong Kong-Macau Joint Lab (File No. 2020B1212030006).
Abbreviations
- APC
Antigens presenting cells
- ARDS
Acute respiratory distress syndrome
- COVID-19
Coronavirus disease 2019
- EAE
Experimental autoimmune encephalomyelitis
- mTNF
Transmembrane TNF
- PD-1
Programmed cell death protein 1
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- Th1
T helper 1 cells
- Th2
T helper 2 cells
- Th17
T helper 17 cells
- TNF
Tumor necrosis factor
- TNFR2
TNF receptor type II
- Treg
CD4+Foxp3+ regulatory T cell
References
- 1.Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H. et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–74. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W-j, Ni Z-y, Hu Y, Liang W-h, Ou C-q, He J-x. et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson LA, Canna SW, Schulert GS, Volpi S, Lee PY, Kernan KF. et al. On the Alert for Cytokine Storm: Immunopathology in COVID-19. Arthritis Rheumatol. 2020;72:1059–63. doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong JP, Viswanathan S, Wang M, Sun L-Q, Clark GC, D'Elia RV. Current and future developments in the treatment of virus-induced hypercytokinemia. Future Med Chem. 2017;9:169–78. doi: 10.4155/fmc-2016-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the Eye of the Cytokine Storm. Microbiol Mol Biol Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Jiang M, Chen X, Montaner LJ. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. 2020;108:17–41. doi: 10.1002/JLB.3COVR0520-272R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19) Front Immunol. 2020. 11. [DOI] [PMC free article] [PubMed]
- 10.Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M. et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–9. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y. et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–8. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalfaoglu B, Almeida-Santos J, Tye CA, Satou Y, Ono M. T-cell dysregulation in COVID-19. Biochem Biophys Res Commun. 2021;538:204–10. doi: 10.1016/j.bbrc.2020.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of Early Protective Immunity to Viral Infection by Regulatory T Cells. Science. 2008;320:1220–4. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T Cells and Immune Tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020. 130. [DOI] [PMC free article] [PubMed]
- 16.Tan M, Liu Y, Zhou R, Deng X, Li F, Liang K. et al. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology. 2020;160:261–8. doi: 10.1111/imm.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilgenfeld R, Peiris M. From SARS to MERS: 10 years of research on highly pathogenic human coronaviruses. Antiviral Res. 2013;100:286–95. doi: 10.1016/j.antiviral.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satarker S, Nampoothiri M. Structural Proteins in Severe Acute Respiratory Syndrome Coronavirus-2. Arch Med Res. 2020;51:482–91. doi: 10.1016/j.arcmed.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan JF-W, Kok K-H, Zhu Z, Chu H, To KK-W, Yuan S. et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221–36. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318:H1084–h90. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M. et al. Immunology of COVID-19: Current State of the Science. Immunity. 2020;52:910–41. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang C, Xie J, Zhao L, Fei X, Zhang H, Tan Y. et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. EBioMedicine. 2020;57:102833. doi: 10.1016/j.ebiom.2020.102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020. 27. [DOI] [PMC free article] [PubMed]
- 24.Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y. et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev. 2020;7:998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu J, Kong J, Wang W, Wu M, Yao L, Wang Z. et al. The clinical implication of dynamic neutrophil to lymphocyte ratio and D-dimer in COVID-19: A retrospective study in Suzhou China. Thromb Res. 2020;192:3–8. doi: 10.1016/j.thromres.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C. et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lisboa Bastos M, Tavaziva G, Abidi SK, Campbell JR, Haraoui L-P, Johnston JC. et al. Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ. 2020;370:m2516. doi: 10.1136/bmj.m2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westmeier J, Paniskaki K, Karaköse Z, Werner T, Sutter K, Dolff S. et al. Impaired Cytotoxic CD8+ T Cell Response in Elderly COVID-19 Patients. mBio. 2020;11:e02243–20. doi: 10.1128/mBio.02243-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sekine T, Perez-Potti A, Rivera-Ballesteros O, Strålin K, Gorin J-B, Olsson A. et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell. 2020;183:158–68.e14. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braun J, Loyal L, Frentsch M, Wendisch D, Georg P, Kurth F. et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587:270–4. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- 31.Blanco-Melo D, Nilsson-Payant BE, Liu W-C, Uhl S, Hoagland D, Møller R. et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181:1036–45.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maggi E, Canonica GW, Moretta L. COVID-19: Unanswered questions on immune response and pathogenesis. J Allergy Clin Immunol. 2020;146:18–22. doi: 10.1016/j.jaci.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S. et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013;14:307–8. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 34.Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T Cells and Human Disease. Annu Rev Immunol. 2020;38:541–66. doi: 10.1146/annurev-immunol-042718-041717. [DOI] [PubMed] [Google Scholar]
- 35.Hartigan-O'Connor DJ, Poon C, Sinclair E, McCune JM. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods. 2007;319:41–52. doi: 10.1016/j.jim.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 37.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 38.Simonetta F, Chiali A, Cordier C, Urrutia A, Girault I, Bloquet S. et al. Increased CD127 expression on activated FOXP3+CD4+ regulatory T cells. Eur J Immunol. 2010;40:2528–38. doi: 10.1002/eji.201040531. [DOI] [PubMed] [Google Scholar]
- 39.Chen X, Subleski JJ, Hamano R, Howard OM, Wiltrout RH, Oppenheim JJ. Co-expression of TNFR2 and CD25 identifies more of the functional CD4+FOXP3+ regulatory T cells in human peripheral blood. Eur J Immunol. 2010;40:1099–106. doi: 10.1002/eji.200940022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramsdell F, Rudensky AY. Foxp3: a genetic foundation for regulatory T cell differentiation and function. Nat Immunol. 2020;21:708–9. doi: 10.1038/s41590-020-0694-5. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Oppenheim JJ. Resolving the identity myth: key markers of functional CD4+ FoxP3+ regulatory T cells. Int Immunopharmacol. 2011;11:1489–96. doi: 10.1016/j.intimp.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt A, Oberle N, Krammer P. Molecular Mechanisms of Treg-Mediated T Cell Suppression. Front Immunol. 2012. 3. [DOI] [PMC free article] [PubMed]
- 43.Chen X, Bäumel M, Männel DN, Howard OMZ, Oppenheim JJ. Interaction of TNF with TNF Receptor Type 2 Promotes Expansion and Function of Mouse CD4+CD25+ T Regulatory Cells. J Immunol. 2007;179:154–61. doi: 10.4049/jimmunol.179.1.154. [DOI] [PubMed] [Google Scholar]
- 44.Hamano R, Huang J, Yoshimura T, Oppenheim JJ, Chen X. TNF optimally activatives regulatory T cells by inducing TNF receptor superfamily members TNFR2, 4-1BB and OX40. Eur J Immunol. 2011;41:2010–20. doi: 10.1002/eji.201041205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X, Wu X, Zhou Q, Howard OM, Netea MG, Oppenheim JJ. TNFR2 is critical for the stabilization of the CD4+Foxp3+ regulatory T. cell phenotype in the inflammatory environment. J Immunol. 2013;190:1076–84. doi: 10.4049/jimmunol.1202659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X, Willette-Brown J, Wu X, Hu Y, Howard OM, Oppenheim JJ. IKKalpha is required for the homeostasis of regulatory T cells and for the expansion of both regulatory and effector CD4 T cells. Faseb J. 2015;29:443–54. doi: 10.1096/fj.14-259564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veiga-Parga T, Sehrawat S, Rouse BT. Role of regulatory T cells during virus infection. Immunol Rev. 2013;255:182–96. doi: 10.1111/imr.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin S, Wu H, Wang C, Xiao Z, Xu F. Regulatory T Cells and Acute Lung Injury: Cytokines, Uncontrolled Inflammation, and Therapeutic Implications. Front Immunol. 2018. 9. [DOI] [PMC free article] [PubMed]
- 49.D'Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF. et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898–913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X, Huang J, Huang Y, Chen J, Huang Y, Jiang X. et al. Characteristics of immune cells and cytokines in patients with coronavirus disease 2019 in Guangzhou, China. Hum Immunol. 2020;81:702–8. doi: 10.1016/j.humimm.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang W, Su B, Pang L, Qiao L, Feng Y, Ouyang Y. et al. High-dimensional immune profiling by mass cytometry revealed immunosuppression and dysfunction of immunity in COVID-19 patients. Cell Mol Immunol. 2020;17:650–2. doi: 10.1038/s41423-020-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Wang X, Li X, Xi D, Mao R, Wu X. et al. Potential contribution of increased soluble IL-2R to lymphopenia in COVID-19 patients. Cell Mol Immunol. 2020;17:878–80. doi: 10.1038/s41423-020-0484-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ronit A, Berg RM, Bay JT, Haugaard AK, Ahlström MG, Burgdorf KS. et al. Compartmental immunophenotyping in COVID-19 ARDS: A case series. J Allergy Clin Immunol. 2021;147:81–91. doi: 10.1016/j.jaci.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taefehshokr N, Taefehshokr S, Heit B. Mechanisms of Dysregulated Humoral and Cellular Immunity by SARS-CoV-2. Pathogens. 2020;9:1027. doi: 10.3390/pathogens9121027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng H, Li H, Guo L, Liang Y, Li J, Wang X. et al. Virulence and pathogenesis of SARS-CoV-2 infection in rhesus macaques: A nonhuman primate model of COVID-19 progression. PLoS Pathog. 2020;16:e1008949. doi: 10.1371/journal.ppat.1008949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J, Yang X, Wang H, Li Z, Deng H, Liu J, The analysis of the long-term impact of SARS-CoV-2 on the cellular immune system in individuals recovering from COVID-19 reveals a profound NKT cell impairment. medRxiv. 2020. 2020. 08.21.20179358.
- 57.Yang J, Zhang E, Zhong M, Yang Q, Hong K, Shu T, Impaired T cell functions along with elevated activated Tregs at the early stage of asymptomatic SARS-CoV-2 infection. medRxiv (Preprint). 2020. 2020. 05.25.20108852.
- 58.Wang L, Zhao L, Lv J, Yin Q, Liang X, Chu Y. et al. BLT1-dependent Alveolar Recruitment of CD4+CD25+ Foxp3+ Regulatory T Cells Is Important for Resolution of Acute Lung Injury. Am J Respir Crit Care Med. 2012;186:989–98. doi: 10.1164/rccm.201202-0261OC. [DOI] [PubMed] [Google Scholar]
- 59.Kratzer B, Trapin D, Ettel P, Körmöczi U, Rottal A, Tuppy F. et al. Immunological imprint of COVID-19 on human peripheral blood leukocyte populations. Allergy. 2021;76:751–65. doi: 10.1111/all.14647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohebbi SR, Baghaei K, Rostami-Nejad M, Mojarad EN, Mirjalali H, Yadegar A. et al. Significant changes of CD4, FOXP3, CD25, and IL6 expression level in Iranian COVID-19 patients. Gastroenterol Hepatol Bed Bench. 2020;13:388. [PMC free article] [PubMed] [Google Scholar]
- 61.Meckiff BJ, Ramírez-Suástegui C, Fajardo V, Chee SJ, Kusnadi A, Simon H. et al. Imbalance of Regulatory and Cytotoxic SARS-CoV-2-Reactive CD4+ T Cells in COVID-19. Cell. 2020;183:1340–53.e16. doi: 10.1016/j.cell.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalfaoglu B, Almeida-Santos J, Tye CA, Satou Y, Ono M. T-Cell Hyperactivation and Paralysis in Severe COVID-19 Infection Revealed by Single-Cell Analysis. Front Immunol. 2020. 11. [DOI] [PMC free article] [PubMed]
- 63.Sadeghi A, Tahmasebi S, Mahmood A, Kuznetsova M, Valizadeh H, Taghizadieh A. et al. Th17 and Treg cells function in SARS-CoV2 patients compared with healthy controls. J Cell Physiol. 2021;236:2829–39. doi: 10.1002/jcp.30047. [DOI] [PubMed] [Google Scholar]
- 64.Duerr GD, Heine A, Hamiko M, Zimmer S, Luetkens JA, Nattermann J. et al. Parameters predicting COVID-19-induced myocardial injury and mortality. Life Sci. 2020;260:118400. doi: 10.1016/j.lfs.2020.118400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jia R, Wang X, Liu P, Liang X, Ge Y, Tian H, Mild cytokine elevation, moderate CD4+ T cell response and abundant antibody production in children with COVID-19. Virol Sin. 2020. pp. 1–10. [DOI] [PMC free article] [PubMed]
- 66.Galván-Peña S, Leon J, Chowdhary K, Michelson DA, Vijaykumar B, Yang L, Profound Treg perturbations correlate with COVID-19 severity. bioRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 67.Rezaei M, Marjani M, Mahmoudi S, Mortaz E, Mansouri D. Dynamic Changes of Lymphocyte Subsets in the Course of COVID-19. Int Arch Allergy Immunol. 2021. pp. 1–9. [DOI] [PMC free article] [PubMed]
- 68.Gupta S, Su H, Narsai T, Agrawal S. SARS-CoV-2-Associated T-Cell Responses in the Presence of Humoral Immunodeficiency. Int Arch Allergy Immunol. 2021. pp. 1–15. [DOI] [PMC free article] [PubMed]
- 69.Thibaudin M, Fumet JD, Bon M, Hampe L, Limagne E, Ghiringhelli F. Immunological features of coronavirus disease 2019 in patients with cancer. Eur J Cancer. 2020;139:70–80. doi: 10.1016/j.ejca.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stephen-Victor E, Das M, Karnam A, Pitard B, Gautier J-F, Bayry J. Potential of regulatory T-cell-based therapies in the management of severe COVID-19. Eur Respir J. 2020;56:2002182. doi: 10.1183/13993003.02182-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y, Qi G, Bellanti JA, Moser R, Ryffel B, Zheng SG. Regulatory T cells: A potential weapon to combat COVID-19? MedComm (Beijing) 2020;1:157–64. doi: 10.1002/mco2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nguyen VH, Zeiser R, daSilva DL, Chang DS, Beilhack A, Contag CH. et al. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood. 2006;109:2649–56. doi: 10.1182/blood-2006-08-044529. [DOI] [PubMed] [Google Scholar]
- 73.Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK. et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7:315ra189–315ra189. doi: 10.1126/scitranslmed.aad4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun J, Han Z-B, Liao W, Yang SG, Yang Z, Yu J. et al. Intrapulmonary delivery of human umbilical cord mesenchymal stem cells attenuates acute lung injury by expanding CD4+ CD25+ Forkhead Boxp3 (FOXP3)+ regulatory T cells and balancing anti-and pro-inflammatory factors. Cell Physiol Biochem. 2011;27:587–96. doi: 10.1159/000329980. [DOI] [PubMed] [Google Scholar]
- 75.Anghelina D, Zhao J, Trandem K, Perlman S. Role of regulatory T cells in coronavirus-induced acute encephalitis. Virology. 2009;385:358–67. doi: 10.1016/j.virol.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cao Y, Xu W, Xiong S. Adoptive Transfer of Regulatory T Cells Protects against Coxsackievirus B3-Induced Cardiac Fibrosis. PLoS One. 2013;8:e74955. doi: 10.1371/journal.pone.0074955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gladstone DE, Kim BS, Mooney K, Karaba AH, D'Alessio FR. Regulatory T cells for treating patients with covid-19 and acute respiratory distress syndrome: two case reports. Ann Intern Med. 2020;173:852–3. doi: 10.7326/L20-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hossein-khannazer N, Shokoohian B, Shpichka A, Aghdaei HA, Timashev P, Vosough M. An update to "novel therapeutic approaches for treatment of COVID-19". Journal of Molecular Medicine-Jmm. 2021;99:303–10. doi: 10.1007/s00109-020-02027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang F, Hou H, Luo Y, Tang G, Wu S, Huang M, The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020. 5. [DOI] [PMC free article] [PubMed]
- 80.Zhu M-E, Wang Q, Zhou S, Wang B, Ke L, He P. Recombinant interleukin-2 stimulates lymphocyte recovery in patients with severe COVID-19. Exp Ther Med. 2021;21:227. doi: 10.3892/etm.2021.9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hartemann A, Bensimon G, Payan CA, Jacqueminet S, Bourron O, Nicolas N. et al. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2013;1:295–305. doi: 10.1016/S2213-8587(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 82.Kennedy-Nasser AA, Ku S, Castillo-Caro P, Hazrat Y, Wu M-F, Liu H. et al. Ultra Low-Dose IL-2 for GVHD Prophylaxis after Allogeneic Hematopoietic Stem Cell Transplantation Mediates Expansion of Regulatory T Cells without Diminishing Antiviral and Antileukemic Activity. Clin Cancer Res. 2014;20:2215–25. doi: 10.1158/1078-0432.CCR-13-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol Sci. 2013;34:518–30. doi: 10.1016/j.tips.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li P, Zheng Y, Chen X. Drugs for Autoimmune Inflammatory Diseases: From Small Molecule Compounds to Anti-TNF Biologics. Front Pharmacol. 2017. 8. [DOI] [PMC free article] [PubMed]
- 85.Group TRC. Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report. N Engl J Med. 2020.
- 86.World Health Organization.Coronavirus disease (COVID-19) Dexamethasone. https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-dexamethasone. 2021.
- 87.Selvaraj V, Dapaah-Afriyie K, Finn A, Flanigan TP. Short-Term Dexamethasone in Sars-CoV-2 Patients. R I Med J. 2020;103:39–43. [PubMed] [Google Scholar]
- 88.Cain DW, Cidlowski JA. After 62 years of regulating immunity, dexamethasone meets COVID-19. Nat Rev Rheumatol. 2020;20:587–8. doi: 10.1038/s41577-020-00421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen X, Murakami T, Oppenheim JJ, Howard OMZ. Differential response of murine CD4+CD25+ and CD4+CD25- T cells to dexamethasone-induced cell death. Eur J Immunol. 2004;34:859–69. doi: 10.1002/eji.200324506. [DOI] [PubMed] [Google Scholar]
- 90.Chen X, Oppenheim JJ, Winkler-Pickett RT, Ortaldo JR, Howard OMZ. Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3+CD4+CD25+ T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur J Immunol. 2006;36:2139–49. doi: 10.1002/eji.200635873. [DOI] [PubMed] [Google Scholar]
- 91.Hu Y, Tian W, Zhang L-L, Liu H, Yin G-P, He B-S. et al. Function of regulatory T-cells improved by dexamethasone in Graves' disease. Eur J Endocrinol. 2012;166:641. doi: 10.1530/EJE-11-0879. [DOI] [PubMed] [Google Scholar]
- 92.Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Netw Open. 2020;3:e2019722–e. doi: 10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brenner H, Holleczek B, Schöttker B. Vitamin D insufficiency and deficiency and mortality from respiratory diseases in a cohort of older adults: potential for limiting the death toll during and beyond the COVID-19 pandemic? Nutrients. 2020;12:2488. doi: 10.3390/nu12082488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ahmed F. A Network-Based Analysis Reveals the Mechanism Underlying Vitamin D in Suppressing Cytokine Storm and Virus in SARS-CoV-2 Infection. Front Immunol. 2020. 11. [DOI] [PMC free article] [PubMed]
- 95.Amin HA, Drenos F. No evidence that vitamin D is able to prevent or affect the severity of COVID-19 in individuals with European ancestry: a Mendelian randomisation study of open data. BMJ Nutrition. 2021: bmjnph-2020-000151. [DOI] [PMC free article] [PubMed]
- 96.Torrey H, Butterworth J, Mera T, Okubo Y, Wang L, Baum D, Targeting TNFR2 with antagonistic antibodies inhibits proliferation of ovarian cancer cells and tumor-associated Tregs. Sci Signal. 2017. 10. [DOI] [PubMed]
- 97.Zdrenghea MT, Makrinioti H, Bagacean C, Bush A, Johnston SL, Stanciu LA. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol. 2017. 27. [DOI] [PubMed]
- 98.Handel AE, Sandve GK, Disanto G, Berlanga-Taylor AJ, Gallone G, Hanwell H. et al. Vitamin D receptor ChIP-seq in primary CD4+ cells: relationship to serum 25-hydroxyvitamin D levels and autoimmune disease. BMC Med. 2013;11:163. doi: 10.1186/1741-7015-11-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khare D, Godbole NM, Pawar SD, Mohan V, Pandey G, Gupta S. et al. Calcitriol [1, 25[OH]2 D3] pre- and post-treatment suppresses inflammatory response to influenza A (H1N1) infection in human lung A549 epithelial cells. Eur J Nutr. 2013;52:1405–15. doi: 10.1007/s00394-012-0449-7. [DOI] [PubMed] [Google Scholar]
- 100.Veugelers PJ, Pham TM, Ekwaru JP. Optimal Vitamin D Supplementation Doses that Minimize the Risk for Both Low and High Serum 25-Hydroxyvitamin D Concentrations in the General Population. Nutrients. 2015;7:10189–208. doi: 10.3390/nu7125527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yusupov E, Li-Ng M, Pollack S, Yeh JK, Mikhail M, Aloia JF. Vitamin d and serum cytokines in a randomized clinical trial. Int J Endocrinol. 2010. 2010. [DOI] [PMC free article] [PubMed]
- 102.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol Rev. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57:1308–10. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 104.Unger WWJ, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: Differential role for PD-L1. Eur J Immunol. 2009;39:3147–59. doi: 10.1002/eji.200839103. [DOI] [PubMed] [Google Scholar]
- 105.Kleijwegt FS, Laban S, Duinkerken G, Joosten AM, Zaldumbide A, Nikolic T. et al. Critical role for TNF in the induction of human antigen-specific regulatory T cells by tolerogenic dendritic cells. J Immunol. 2010;185:1412–8. doi: 10.4049/jimmunol.1000560. [DOI] [PubMed] [Google Scholar]
- 106.Adorini L, Penna G. Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Hum Immunol. 2009;70:345–52. doi: 10.1016/j.humimm.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 107.Chun RF, Liu PT, Modlin RL, Adams JS, Hewison M. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Front Physiol. 2014;5:151. doi: 10.3389/fphys.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M. et al. 1,25-Dihydroxyvitamin D3 and IL-2 Combine to Inhibit T Cell Production of Inflammatory Cytokines and Promote Development of Regulatory T Cells Expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–67. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mona O, Sama B, Seyed Alireza M-N, Ali N-Z, Fatemeh M, Karim P, Immunomodulatory Effects of Calcitriol through DNA Methylation Alteration of FOXP3 in the CD4+ T Cells of Mice. Iran J Allergy Asthma Immunol. 2020. 19. [DOI] [PubMed]
- 110.Khoo A-L, Koenen HJPM, Chai LYA, Sweep FCGJ, Netea MG, van der Ven AJAM. et al. Seasonal Variation in Vitamin D3 Levels Is Paralleled by Changes in the Peripheral Blood Human T Cell Compartment. PLoS One. 2012;7:e29250. doi: 10.1371/journal.pone.0029250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prietl B, Treiber G, Mader JK, Hoeller E, Wolf M, Pilz S. et al. High-dose cholecalciferol supplementation significantly increases peripheral CD4+ Tregs in healthy adults without negatively affecting the frequency of other immune cells. Eur J Nutr. 2014;53:751–9. doi: 10.1007/s00394-013-0579-6. [DOI] [PubMed] [Google Scholar]
- 112.Savastio S, Cadario F, D'Alfonso S, Stracuzzi M, Pozzi E, Raviolo S. et al. Vitamin D Supplementation Modulates ICOS+ and ICOS- Regulatory T Cell in Siblings of Children With Type 1 Diabetes. J Clin Endocrinol Metab. 2020;105:e4767–e77. doi: 10.1210/clinem/dgaa588. [DOI] [PubMed] [Google Scholar]
- 113.Kim GW, Lee, N.R, Pi, R.H. et al.. IL-6 inhibitors for treatment of rheumatoid arthritis: past, present, and future. Arch Pharm Res. 2015;38:575–84. doi: 10.1007/s12272-015-0569-8. [DOI] [PubMed] [Google Scholar]
- 114.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, Cytokine release syndrome. J Immunother Cancer. 2018. 6. [DOI] [PMC free article] [PubMed]
- 115.Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020. 18. [DOI] [PMC free article] [PubMed]
- 116.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020;92:814–8. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tanaka T, Narazaki M, Kishimoto T. Therapeutic targeting of the interleukin-6 receptor. Annu Rev Pharmacol Toxicol. 2012;52:199–219. doi: 10.1146/annurev-pharmtox-010611-134715. [DOI] [PubMed] [Google Scholar]
- 118.Kikuchi J, Hashizume M, Kaneko Y, Yoshimoto K, Nishina N, Takeuchi T. Peripheral blood CD4(+)CD25(+)CD127(low) regulatory T cells are significantly increased by tocilizumab treatment in patients with rheumatoid arthritis: increase in regulatory T cells correlates with clinical response. Arthritis Res Ther. 2015;17:10. doi: 10.1186/s13075-015-0526-4. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Parhizgar AR, Tahghighi A. Introducing New Antimalarial Analogues of Chloroquine and Amodiaquine: A Narrative Review. Iran J Med Sci. 2017;42:115–28. [PMC free article] [PubMed] [Google Scholar]
- 120.Lei ZN, Wu ZX, Dong S, Yang DH, Zhang L, Ke Z. et al. Chloroquine and hydroxychloroquine in the treatment of malaria and repurposing in treating COVID-19. Pharmacol Ther. 2020;216:107672. doi: 10.1016/j.pharmthera.2020.107672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Qiu T, Liang S, Dabbous M, Wang Y, Han R, Toumi M. Chinese guidelines related to novel coronavirus pneumonia. J Mark Access Health Policy. 2020;8:1818446. doi: 10.1080/20016689.2020.1818446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.US Food and Drug Administration. request for emergency use authorization for use of chloroquine phosphate or hydroxychloroquine sulfate supplied from the strategic National Stockpile for treatment of 2019 coronavirus disease. https://www.fda.gov/media/136534/download. 2021.
- 123.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020. 56. [DOI] [PMC free article] [PubMed] [Retracted]
- 124.Geleris J, Sun YF, Platt J, Zucker J, Baldwin M, Hripcsak G. et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020;382:2411–8. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thomé R, Moraes AS, Bombeiro AL, Farias Ados S, Francelin C, da Costa TA. et al. Chloroquine treatment enhances regulatory T cells and reduces the severity of experimental autoimmune encephalomyelitis. PLoS One. 2013;8:e65913. doi: 10.1371/journal.pone.0065913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.An N, Chen Y, Wang C, Yang C, Wu Z, Xue J. et al. Chloroquine Autophagic Inhibition Rebalances Th17/Treg-Mediated Immunity and Ameliorates Systemic Lupus Erythematosus. Cell Physiol Biochem. 2017;44:412–22. doi: 10.1159/000484955. [DOI] [PubMed] [Google Scholar]
- 127.Bergström M, Müller M, Karlsson M, Scholz H, Vethe NT, Korsgren O. Comparing the Effects of the mTOR Inhibitors Azithromycin and Rapamycin on In Vitro Expanded Regulatory T Cells. Cell Transplant. 2019;28:1603–13. doi: 10.1177/0963689719872488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Radhakrishnan SV, Palaniyandi S, Mueller G, Miklos S, Hager M, Spacenko E. et al. Preventive azithromycin treatment reduces noninfectious lung injury and acute graft-versus-host disease in a murine model of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21:30–8. doi: 10.1016/j.bbmt.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 129.U.S. Food and Drug Administration. HUMIRA® (adalimumab) https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125057s415lbl.pdf. 2021.
- 130.Conti A, Lasagni C, Bigi L, Pellacani G. Evolution of COVID-19 infection in four psoriatic patients treated with biological drugs. J Eur Acad Dermatol Venereol. 2020;34:e360–e1. doi: 10.1111/jdv.16587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tursi A, Angarano G, Monno L, Saracino A, Signorile F, Ricciardi A. et al. COVID-19 infection in Crohn's disease under treatment with adalimumab. Gut. 2020;69:1364–5. doi: 10.1136/gutjnl-2020-321240. [DOI] [PubMed] [Google Scholar]
- 132.Vechi HT, Maia LR, Alves MdM, Rodrigues-Neto JF. Favorable outcome of COVID-19 in a young woman with severe Crohn's disease on regular use of adalimumab and prednisone: a case report. Rev Inst Med Trop Sao Paulo. 2020. 62. [DOI] [PMC free article] [PubMed]
- 133.Fakharian A, Barati S, Mohamadi M, Dastan F. Successful Management of COVID-19 with Adalimumab in a Post-Coronary Artery Bypass Graft Surgery Patient. J Cardiothorac Vasc Anesth. 2020: S1053-0770 (20) 31370-7. [DOI] [PMC free article] [PubMed]
- 134.Feldmann M, Maini RN, Woody JN, Holgate ST, Winter G, Rowland M. et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395:1407–9. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dao X. Nguyen B, Alice Cotton R, BSca, Laura Attipoe M, Coziana Ciurtin M, PhDa, Caroline J. Dore Bb, Michael R. Ehrenstein M, PhD. Regulatory T cells as a biomarker for response to adalimumab in rheumatoid arthritis. J Allergy Clin Immunol. 2018;142:978–80. doi: 10.1016/j.jaci.2018.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Onuora S. Adalimumab drives Treg cell expansion via membrane TNF. Nat Rev Rheumatol. 2016;12:438. doi: 10.1038/nrrheum.2016.109. [DOI] [PubMed] [Google Scholar]
- 137.Nguyen DX, Ehrenstein MR. Anti-TNF drives regulatory T cell expansion by paradoxically promoting membrane TNF-TNF-RII binding in rheumatoid arthritis. J Exp Med. 2016;213:1241–53. doi: 10.1084/jem.20151255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen X, Oppenheim JJ. Therapy: Paradoxical effects of targeting TNF signalling in the treatment of autoimmunity. Nat Rev Rheumatol. 2016;12:625–6. doi: 10.1038/nrrheum.2016.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zou H, He T, Chen X. Tetrandrine inhibits differentiation of proinflammatory subsets of T helper cells but spares de novo differentiation of iTreg cells. Int Immunopharmacol. 2019;69:307–12. doi: 10.1016/j.intimp.2019.01.040. [DOI] [PubMed] [Google Scholar]
- 140.Bhagya N, Chandrashekar KR. Tetrandrine - A molecule of wide bioactivity. Phytochemistry. 2016;125:5–13. doi: 10.1016/j.phytochem.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 141.Heister PM, Poston RN. Pharmacological hypothesis: TPC2 antagonist tetrandrine as a potential therapeutic agent for COVID-19. Pharmacol Res Perspect. 2020;8:e00653. doi: 10.1002/prp2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sakurai Y, Kolokoltsov AA, Chen C-C, Tidwell MW, Bauta WE, Klugbauer N. et al. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science. 2015;347:995–8. doi: 10.1126/science.1258758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gunaratne GS, Yang Y, Li F, Walseth TF, Marchant JS. NAADP-dependent Ca2+ signaling regulates Middle East respiratory syndrome-coronavirus pseudovirus translocation through the endolysosomal system. Cell Calcium. 2018;75:30–41. doi: 10.1016/j.ceca.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ou X, Liu Y, Lei X, Li P, Mi D, Ren L. et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.He T, Yang D, Li X-Q, Jiang M, Islam MS, Chen S. et al. Inhibition of two-pore channels in antigen-presenting cells promotes the expansion of TNFR2-expressing CD4+Foxp3+ regulatory T cells. Sci Adv. 2020;6:eaba6584. doi: 10.1126/sciadv.aba6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen X, Oppenheim JJ. Tetrandrine (TET), an immunosuppressive component of Chinese herb, induces tolerogenic dendritic cells and consequently expands regulatory T cells. J Immunol. 2016;196:70.8–8. [Google Scholar]
- 147.Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2020;53:368–70. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C. et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory medicine. 2020;8:420–2. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang F, Hou H, Luo Y, Tang G, Wu S, Huang M. et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI insight. 2020;5:e137799. doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 151.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS. et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–63. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 152.Shi H, Wang W, Yin J, Ouyang Y, Pang L, Feng Y. et al. The inhibition of IL-2/IL-2R gives rise to CD8+ T cell and lymphocyte decrease through JAK1-STAT5 in critical patients with COVID-19 pneumonia. Cell Death Dis. 2020;11:429. doi: 10.1038/s41419-020-2636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kopf H, de la Rosa GM, Howard OM, Chen X. Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int Immunopharmacol. 2007;7:1819–24. doi: 10.1016/j.intimp.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lu L, Zhou X, Wang J, Zheng SG, Horwitz DA. Characterization of Protective Human CD4+CD25+ FOXP3+ Regulatory T Cells Generated with IL-2, TGF-β and Retinoic Acid. PLoS One. 2010;5:e15150. doi: 10.1371/journal.pone.0015150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.He X, Landman S, Bauland SCG, van den Dolder J, Koenen HJPM, Joosten I. A TNFR2-Agonist Facilitates High Purity Expansion of Human Low Purity Treg Cells. PLoS One. 2016;11:e0156311. doi: 10.1371/journal.pone.0156311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chopra M, Biehl M, Steinfatt T, Brandl A, Kums J, Amich J. et al. Exogenous TNFR2 activation protects from acute GvHD via host T reg cell expansion. J Exp Med. 2016;213:1881–900. doi: 10.1084/jem.20151563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu R, Zhou Q, La Cava A, Campagnolo DI, Van Kaer L, Shi F-D. Expansion of regulatory T cells via IL-2/anti-IL-2 mAb complexes suppresses experimental myasthenia. Eur J Immunol. 2010;40:1577–89. doi: 10.1002/eji.200939792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD. et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–60. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yokoyama Y, Iwasaki T, Kitano S, Satake A, Nomura S, Furukawa T. et al. IL-2-Anti-IL-2 Monoclonal Antibody Immune Complexes Inhibit Collagen-Induced Arthritis by Augmenting Regulatory T Cell Functions. J Immunol. 2018;201:1899–906. doi: 10.4049/jimmunol.1701502. [DOI] [PubMed] [Google Scholar]
- 160.De Biasi S, Meschiari M, Gibellini L, Bellinazzi C, Borella R, Fidanza L. et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun. 2020;11:3434. doi: 10.1038/s41467-020-17292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Riley JL, June CH, Blazar BR. Human T Regulatory Cell Therapy: Take a Billion or So and Call Me in the Morning. Immunity. 2009;30:656–65. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gliwiński M, Iwaszkiewicz-Grześ D, Trzonkowski P. Cell-Based Therapies with T Regulatory Cells. BioDrugs. 2017;31:335–47. doi: 10.1007/s40259-017-0228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ye C, Brand D, Zheng SG. Targeting IL-2: an unexpected effect in treating immunological diseases. Sig Transduct Target Ther. 2018;3:2. doi: 10.1038/s41392-017-0002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.He J, Zhang R, Shao M, Zhao X, Miao M, Chen J. et al. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2020;79:141–9. doi: 10.1136/annrheumdis-2019-215396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC. et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA. 2020;324:1307–16. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Merzon E, Tworowski D, Gorohovski A, Vinker S, Golan Cohen A, Green I. et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020;287:3693–702. doi: 10.1111/febs.15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcala Diaz JF, Lopez Miranda J, Bouillon R. et al. "Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study". J Steroid Biochem Mol Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fisher SA, Rahimzadeh M, Brierley C, Gration B, Doree C, Kimber CE. et al. The role of vitamin D in increasing circulating T regulatory cell numbers and modulating T regulatory cell phenotypes in patients with inflammatory disease or in healthy volunteers: A systematic review. PLoS One. 2019;14:e0222313. doi: 10.1371/journal.pone.0222313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Samson M, Audia S, Janikashvili N, Ciudad M, Trad M, Fraszczak J. et al. Brief Report: Inhibition of interleukin-6 function corrects Th17/Treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheumatol. 2012;64:2499–503. doi: 10.1002/art.34477. [DOI] [PubMed] [Google Scholar]
- 170.Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19 - Preliminary report. medRxiv (Preprint). 2021. 2021. 01.07.21249390.
- 171.Strohbehn GW, Heiss BL, Rouhani SJ, Trujillo JA, Yu J, Kacew AJ, COVIDOSE: Low-dose tocilizumab in the treatment of Covid-19. medRxiv. 2020. 2020. 07.20.20157503. [DOI] [PMC free article] [PubMed]
- 172.Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H. et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discovery. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Park T-Y, Jang Y, Kim W, Shin J, Toh HT, Kim C-H. et al. Chloroquine modulates inflammatory autoimmune responses through Nurr1 in autoimmune diseases. Sci Rep. 2019;9:15559. doi: 10.1038/s41598-019-52085-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Piconi S, Parisotto S, Rizzardini G, Passerini S, Terzi R, Argenteri B. et al. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood. 2011;118:3263–72. doi: 10.1182/blood-2011-01-329060. [DOI] [PubMed] [Google Scholar]
- 175.Echeverría-Esnal D, Martin-Ontiyuelo C, Navarrete-Rouco ME, De-Antonio Cuscó M, Ferrández O, Horcajada JP. et al. Azithromycin in the treatment of COVID-19: a review. Expert Rev Anti Infect Ther. 2021;19:147–63. doi: 10.1080/14787210.2020.1813024. [DOI] [PubMed] [Google Scholar]