Abstract
Translocator Protein (18 kDa) (TSPO) is a mitochondrial transmembrane protein commonly used as a biomarker for neuroinflammation and is also a potential therapeutic target in neurodegenerative diseases. Despite intensive research efforts, the function of TSPO is still largely enigmatic. Deciphering TSPO structure in the native lipid environment is essential to gain insight into its cellular activities and to design improved diagnostic and therapeutic ligands. Here, we discuss the influence of lipid composition on the structure of mammalian TSPO embedded into lipid bilayers on the basis of solid-state NMR experiments. We further highlight that cholesterol can influence both the tertiary and quaternary TSPO structure and also influence TSPO localization in mitochondria-associated endoplasmic reticulum membranes.
Keywords: Cholesterol, Dynamics, Solid-state NMR, Structure, Lipid, Membrane protein, Neurodegeneration, TSPO
Introduction
Translocator Protein (18 kDa) (TSPO), initially identified and named as a peripheral benzodiazepine receptor, is a transmembrane protein mainly located in the outer mitochondrial membrane (Anholt et al. 1986; McEnery et al. 1992). The protein has been renamed as 18-kDa translocator protein (TSPO) to better reflect its cellular activities and tissue distribution (Papadopoulos 2006). TSPO is present in most peripheral organs (e.g., adrenal glands, lungs and heart) and is also expressed in microglial cells in the healthy brain, but is especially abundant in steroidogenic tissues (Banati 2002). In several neurological diseases, TSPO is markedly upregulated in microglia and astrocytes (Gui et al. 2020; Martín 2010; Wilms 2003). As a result, TSPO became one of the most used biomarkers for imaging of neuroinflammation using positron emission tomography (PET) (Wilms 2003; Schain and Kreisl 2017).
Over the years, a large number of TSPO PET radioligands have been synthesized to improve our knowledge regarding the role of neuroinflammation in central nervous system diseases and to assess the efficacy of novel anti-inflammatory therapeutic strategies. A common drawback of all TSPO radioligands is their sensitivity to a single-nucleotide polymorphism (rs6971) that results in an amino-acid substitution on the target protein: A147T-TSPO (Guo 2013; Owen 2012; Kreisl 2013). This polymorphism generates three genotypes: homozygous high-affinity binders (HABs), heterozygous mixed-affinity binders (MABs), and homozygous low-affinity binders (LABs) (Owen 2010,2011). LABs are rare and make up 9% of Caucasians, 6% of African Americans and 0.001% Han Chinese and Japanese9. Excluding LABs is important in clinical studies because brain uptake of the radioligands is too low to be quantified. Recently, two third-generation TSPO radioligands: [11C]-ER176 and [18F]-GE180 showed improved detection of TSPO signals in LABs. [11C]-ER176, a quinazoline analog of PK11195, was identified to bind TSPO across all rs6971 genotypes in membranes prepared from human brain tissue (Fujita 2017; Ikawa 2017; Boutin 2015). This radioligand has an improved lipophilicity (LogD decreased from 3.97 to 3.55) and increased the accuracy of quantification (Fujita 2017). [18F]-GE180, a tricyclic indole compound, has exhibited higher signal-to-noise ratios than [11C]PK11195 in preclinical models of stroke and Alzheimer’s diseases (Boutin 2015; Chaney 2019; Sridharan 2017; Liu 2015; López-Picón 2018). In healthy human controls, [18F]GE-180 has low brain penetration (Sridharan 2019; Zanotti-Fregonara 2018; Feeney 2016; Fan 2016); whereas, a markedly increased uptake of [18F]GE-180 was observed in lesions of patients, which have HAB, MAB and LAB genotype, with multiple sclerosis (Unterrainer 2018a; Vomacka 2017) or glioma (Albert 2017; Unterrainer 2018b, 2019). The validity of [18F]GE-180 for TSPO imaging is debated, as the origin of this increased signal is uncertain (Albert 2019; Zanotti-Fregonara 2019).
Despite its extensive use as a target in imaging studies, TSPO function is not well understood. TSPO was long considered to play an important role in the translocation of cholesterol through the mitochondrial membrane and thus execute a critical step in steroidogenesis (Costa et al. 2018; Fan et al. 2015). However, the direct role of TSPO in neurosteroidogenesis has been challenged by the development of TSPO knockout mouse models (Costa et al. 2018; Papadopoulos et al. 2018; Banati 2014). At the same time, TSPO genetic deletion and PET imaging studies in various disease models highlighted that TSPO is involved in cellular bioenergetics, associated mitochondrial functions such as redox homeostasis and apoptosis, and also plays a role in innate immune processes of microglia (Repalli 2015; Mukhin et al. 1989; Betlazar et al. 2020; Gut et al. 2015).
TSPO ligands have also been shown to have neuroprotective properties in various animal models of neurodegeneration (Jung 2020; Arbo et al. 2015). Ro5-4864 was found to attenuate the accumulation of amyloid-beta plaques and decrease microglial activation in a mouse model for Alzheimer’s disease. This was accompanied by improved behavior and cognition (Barron 2013). Administration of Emapunil (XBD173), on the other hand, ameliorated microgliosis and neuroinflammation in the MPTP mouse model for Parkinson’s disease, and protected against neuronal loss in the substantia nigra, dopamine depletion, and motor deficits (Gong 2019). Treatment with XBD173 (or Etifoxine) also improved clinical and neuropathological features in rodent models of multiple sclerosis through inhibition of inflammation, elevation of neurosteroids and prevention of demyelination (Bader 2019; Daugherty 2013; Leva 2017), and prevented microglial reactivity after injury in a mouse model of retinal degeneration (Scholz 2015; Akhtar-Schäfer et al. 2018). The preclinical studies indicate that TSPO is a potential target to delay neurodegenerative disease by the regulation of neuroinflammation, apoptosis, and steroidogenesis (Repalli 2015; Arbo et al. 2015; Bader 2019).
Decrypting the structure of TSPO in lipid bilayers is essential to understand its biological role and to design ligands for diagnostic and therapeutic applications. So far, the structure of TSPO from mouse (mTSPO) and from bacterial homologues—all solubilized in detergent—has been resolved by NMR spectroscopy and X-Ray crystallography (Jaremko et al. 2014,2015a,b; Guo 2015; Li et al. 2015; Xia 2019). Here, we describe solid-state NMR studies of mTSPO embedded into lipid bilayers, to gain insights into the lipid- and ligand-dependent structure of mammalian TSPO in a near-native environment.
Materials and methods
NMR sample preparation
Expression and purification of 13C/15N-labeled mTSPO were performed as described previously (Jaremko et al. 2014; Lacapère 2001). To obtain liposomes with homogeneous lipid composition and distribution, we dissolved the lipids in a glass vial in chloroform/methanol = 1/1 (v/v). The organic solvent was removed by a constant nitrogen stream followed by lyophilization. The resulting lipid film was dissolved in TSPO buffer by repeated sonication in a water bath. The resulting liposomes (prepared with either 1,2-dinervonoyl-sn-glycero-3-phosphocholine; 24:1 (cis) PC 850,399 Avanti) or sarcolemma lipid mix (34.7% 18:1 PC, 16.6% 18:1 PE, 2.3% 18:0 PI, 2.3% 18:1 PS, 1% 18:1 PIP2, 9.1% 18:0 SM, 31.9% Chol), percentage in molar ratio) were incubated with the foscholine-12-solubilized protein at a protein/lipid molar ratio of 1:20 for two hours at room temperature. After removal of the detergent with biobeads (BioRad), liposomes were pelleted by centrifugation at 125.000×g. The liposome pellet was washed with 10 mM sodium phosphate pH 6.0, pelleted and transferred into an NMR rotor.
Solid-state NMR spectroscopy
NMR samples contained ~ 12–15 mg of protein packed in a 3.2 mm rotor with DSS for temperature calibration. Solid-state NMR experiments were recorded either on a 850 MHz wide-bore spectrometer equipped with 3.2 mm triple-resonance probe (Bruker Biospin) or on a 950 MHz Bruker Avance III HD standard-bore spectrometer equipped with a 3.2 mm (1H, 13C, 15 N) Efree triple-resonance probe (Bruker Biospin). The (2D) 13C–1H dipolar-assisted rotational resonance experiments (Takegoshi et al. 2001,2003) were recorded at 5 °C and 35 °C with a mixing time of 20 ms. The following 90° pulse widths were used: 2.5 μs for 1H, and 4.5 μs for 13C. 1H decoupling strengths were 80–100 kHz. Spectra were processed in Topspin (Bruker) and analyzed with CcpNmr-Analysis (Stevens 2011).
Results and discussion
Interaction of cholesterol with mTSPO
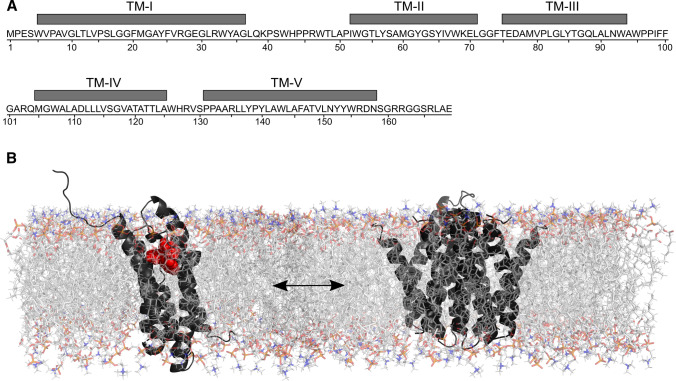
The structure, dynamics and function of membrane proteins are influenced by their lipid environment (Palsdottir and Hunte 2004). Hence, to gain insight into the activity of TSPO and its interaction with ligands it is crucial to study its conformation in the near-native environment of lipid bilayers. Solid-state NMR in combination with computational analysis suggested that mouse TSPO (mTSPO) reconstituted into liposomes and in dodecyl-phosphocholine detergent micelles fold into a bundle of five transmembrane helices (Jaremko et al. 2015a) (Fig. 1a). In these NMR studies, mTSPO is structurally stabilized through binding of high-affinity radioligands: PK11195 (Jaremko et al. 2015a,2016; Murail, et al. 1778) or DA1106 (Jaipuria 2017). While mTSPO is monomeric in detergent systems (Jaremko et al. 2015a,2016), a fraction of mTSPO associates into oligomeric species in lipids (Jaipuria 2017; Teboul 2012). High-resolution solid-state NMR further revealed that mTSPO bound to DA1106 exists in a dynamic monomer/dimer equilibrium in DMPC liposomes (Fig. 1b). The monomer/dimer equilibrium is concentration dependent, is mediated by the 83GxxxG87 motif in the transmembrane helix TM-III, and inhibited in the G87V mutant of mTSPO (Jaipuria 2017). Cholesterol, known to bind mammalian TSPO with nanomolar affinity (Lacapère 2001), influences mTSPO oligomerization by shifting the equilibrium towards the monomeric form (Jaipuria 2017). In addition, binding of cholesterol to the CRAC (cholesterol recognition amino acid consensus) motif of mTSPO (Jamin 2005) induces distal structural changes on helix TM-III, that might be a consequence of the transition from the dimeric to the monomeric form (Jaipuria 2017; Jaipuria et al. 2018a). Although details of these structural changes are unknown, they might involve changes in TM helix orientation, especially helices TM-II and TM-V (Jaipuria 2017). Interestingly, cholesterol binding is not restricted to the CRAC motif, as demonstrated using paramagnetic cholesterol analogs (Jaipuria et al. 2018a): paramagnetic broadening in the spectra of wild type and G87V-mTSPO suggested that cholesterol binds to an additional site in monomeric mTSPO (Jaipuria et al. 2018a). This site is located on helix TM-III and, thus, not available in dimeric mTSPO, where TM-III is buried in the dimer interface. Thereby, depending on the local protein and cholesterol concentration, the GxxxG motif of mammalian TSPO will be available to interact with cholesterol or other outer mitochondrial membrane proteins and, thus, can influence TSPO activity.
Fig. 1.
The structure of membrane-embedded TSPO. mTSPO amino acid sequence highlighting the location of its five transmembrane helices (a). Structural model of the mTSPO monomer–dimer equilibrium in DMPC bilayer membrane (b). DMPC lipids represented in stick, PK11195 in red spheres and mTSPO displayed in black cartoon representation
Solid-state NMR spectroscopy of mTSPO in different lipid environments
mTSPO reconstituted in either detergent or 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) liposomes displays broad NMR lines when no high-affinity ligand is bound (Xia 2019; Murail et al. 1778; Jaipuria 2017; Jaremko et al. 2015c). The broad NMR lines suggest the presence of internal dynamics and/or exchange between multiple conformations that is intermediate on the NMR time scale. The structural flexibility might arise from a hydrophobic mismatch between mTSPO and the DMPC lipid bilayer. To minimize hydrophobic mismatch, adaptations in the membrane protein can occur including tilting of TM helices or/and rotation of side chains at the ends of TM helices (Lee 2003).
To increase the membrane thickness and, thus, potentially stabilize TSPO’s TM helices, we reconstituted mTSPO into 1,2-dinervonoyl-sn-glycero-3-phosphocholine (24:1 PC). With a chain length of 24 carbons, 24:1 PC achieves a hydrocarbon region thickness of 37.5 ± 1 Å and a molecular surface area of 67.7 ± 1.9 Å2 (Lewis and Engelman 1983). We also reconstituted mTSPO in a sarcolemma lipid mixture (34.7% 18:1 PC, 16.6% 18:1 PE, 2.3% 18:0 PI, 2.3% 18:1 PS, 1% 18:1 PIP2, 9.1% 18:0 SM, 31.9% Chol), to investigate the influence of anionic lipids on the mTSPO structure. 13C–13C correlation spectra of mTSPO were recorded at 35 °C and 5 °C and compared to previous results (Jaipuria 2017), in which mTSPO had been reconstituted into DMPC:cholesterol (20:10 molar ratio).
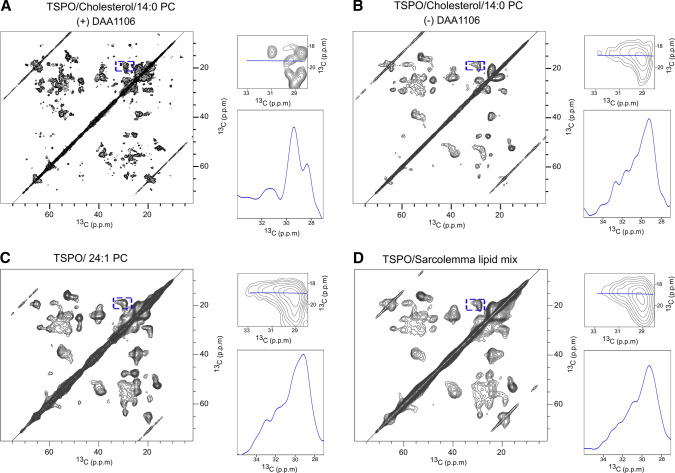
13C–13C correlation spectra recorded at 5 °C for mTSPO bound to DA1106 displayed a large number of defined NMR signals (Fig. 2a). In contrast, the same spectra recorded for mTSPO reconstituted into 24:1 PC (Fig. 2c), or sarcolemma lipid mixture (Fig. 2d), displayed mostly unresolved cross peaks, suggesting the presence of multiple mTSPO conformations. Further comparison with the spectra of mTSPO in DMPC:cholesterol (Fig. 2b), showed that the use of 24:1 PC (Fig. 2c) slightly narrowed the NMR lines, pointing to a partial stabilization of the mTSPO structure in 24:1 PC.
Fig. 2.
13C–13C correlation spectra of membrane-embedded mTSPO at 5 °C. a, b 13C–13C PDSD spectrum of mTSPO reconstituted into DMPC-cholesterol liposomes in the presence (a) or absence (b) of N‐ (2,5‐dimethoxybenzyl)‐N‐ (5‐fluoro‐2‐phenoxyphenyl)acetamide (DAA1106); protein:DMPC:cholesterol molar ratios of 1:20:10. Spectra were recorded with a spinning speed of 11 kHz (Jaipuria 2017). c, d 13C–13C DARR spectra of mTSPO reconstituted into 24:1 PC (c), and into a sarcolemma lipid mixture (d). To visualize the NMR linewidth, peaks framed in blue were enlarged and a 1D horizontal projection of these is displayed
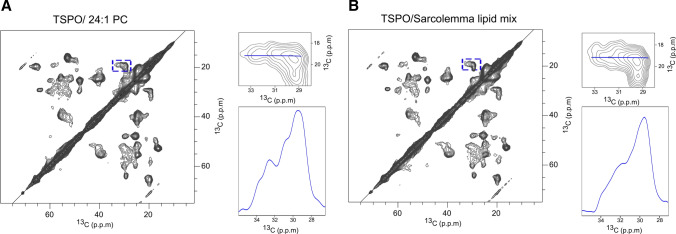
Next, we recorded the 13C–13C correlation spectra at 35 °C (Fig. 3), i.e., above the phase transition of 24:1 PC, which occurs at ~ 24 °C (Lewis and Engelman 1983). While the NMR cross peaks are still broad when compared to those of mTSPO in complex with DAA1106 (Fig. 2a), the resolution slightly improved when compared to the spectra at 5 °C (Fig. 3a when compared to Fig. 2c). Similar to the spectra recorded at 5 °C, the 13C–13C correlation spectrum of mTSPO in the sarcolemma lipid mixture (Fig. 3b) was of lower quality when compared to mTSPO in 24:1: PC (Fig. 3a). One possible reason for the lower spectral quality in the sarcolemma lipid mixture is that the anionic lipids modulate the conformational exchange in mTSPO.
Fig. 3.
13C–13C DARR correlation spectra of mTSPO reconstituted into 24:1 PC (a), and into a sarcolemma lipid mixture (b), at 35 °C. To visualize the NMR linewidth, peaks framed in blue were enlarged and a 1D horizontal projection of these is displayed
Taken together, the analysis reinforces our previous observations that mTSPO in complex with a high affinity ligand is most suitable for high-resolution NMR studies. In addition, the variation in the quality of the NMR spectra of mTSPO in different lipid bilayers stresses the potential influence of the lipid environment on the structure and, thus, activity of mammalian TSPO.
Lipid composition influences mTSPO structure and dynamics
The current results together with previous data show that the lipid composition influences the structure and dynamics of membrane-embedded mTSPO. mTSPO reconstituted into DMPC liposomes exchanges between a monomeric and dimeric structure. This equilibrium depends on both the protein and cholesterol concentration (Jaipuria 2017). Cholesterol binds to mTSPO, promotes dissociation of the mTSPO dimer and causes structural changes (Jaipuria et al. 2018a). Notably, cholesterol regulates the formation of mitochondria-associated endoplasmic reticulum membranes (MAMs), which are sites of close proximity between the endoplasmic reticulum and mitochondria (Fujimoto et al. 2012; Aufschnaiter 2017; Area-Gomez and Schon 2016). Bilayers deficient in these raft-like microdomains are linked to neurodegenerative diseases by contributing to mitochondrial dysfunction and subsequent neuronal decay (Aufschnaiter 2017; Area-Gomez and Schon 2016; Szymański 2017). MAMs incorporate a distinct set of proteins including among others VDAC, inositol 1,4,5-trisphosphate receptor, grp75, Mfn2 (Aufschnaiter 2017; Stoica 2014) and TSPO (D’Eletto 2018). VDAC and TSPO have been shown to interact directly with each other to regulate both mitochondrial structure and function (Gatliff 2015; Shoshan-Barmatz et al. 2019).
The conformational heterogeneity of mTSPO is also present when the protein is reconstituted—in the absence of a high-affinity ligand—into 24:1 PC or into a sarcolemma lipid mixture (Figs. 2, 3). Increasing the membrane thickness using 24:1 PC lipids slightly decreased the conformational heterogeneity when compared to DMPC or the sarcolemma lipid mixture (Fig. 3). The lipid composition of sarcolemma shows features in common with MAMs, such as high content in PC, cholesterol and sphingolipid. Nevertheless, the lower quality of the spectra of mTSPO in the sarcolemma lipid mixture when compared to mTSPO reconstituted into DMPC/cholesterol liposomes suggests that the mTSPO structure is less stable in the sarcolemma lipid mixture. This surprising result might be explained by the presence of anionic lipids such as PE, PI, PS, PIP2. Further studies of mTSPO embedded into MAM lipids will be needed to understand both direct and indirect (e.g., changes in membrane fluidity and thickness) effects of lipids on the structure and dynamics of mammalian TSPO.
Conclusions
Cholesterol is an important regulator of TSPO structure as well as the TSPO and VDAC co-localization in mitochondria-associated membranes. Solid-state NMR provided unique insights into the tertiary and quaternary structure of membrane-embedded mTSPO and its interaction with cholesterol (Jaipuria 2017; Jaipuria et al. 2018a,b). Future studies are required in order to fully characterize the interaction of cholesterol with mammalian TSPO and its impact on the orientation and dynamics of TSPO’s transmembrane helices. In addition, atomic-scale insight into the VDAC/TSPO complex embedded into MAM lipids is urgently required. We believe that solid-state NMR will play a critical role in these studies, because of its unique ability to investigate protein structures in native-like membranes and to probe both rigid and flexible protein structures. Moreover, the recent advancements in solid-state NMR instrumentation and radiofrequency pulse sequences have significantly enhanced the sensitivity and resolution of solid-state NMR experiments (Mandala et al. 2018), empowering the study of large membrane-embedded protein complexes.
Acknowledgements
This work was supported by the Max Planck society, and by the DFG through Collaborative Research Center 803 (project A11 to M.Z) and the Research Unit 2858 (project B1 to M.Z.; ZW 71/10-1).
Abbreviations
- NMR
Nuclear magnetic resonance
- TSPO
Translocator protein—18 kDa
- mTSPO
Mouse TSPO
- CRAC
Cholesterol recognition amino acid consensus
- DAA1106
N‐ (2,5‐Dimethoxybenzyl)‐N‐ (5‐fluoro‐2‐phenoxyphenyl)acetamide
- PET
Positron emission tomography
- HAB
High-affinity binders
- MAB
Heterozygous mixed-affinity binders
- LAB
Low-affinity binders
- DMPC
1,2-Dimyristoyl-sn-glycero-3-phosphocholine
- TM
Transmembrane
- PC
Phosphatidyl-choline
- PE
Phosphatidyl-ethanolamine
- PI
Phosphatidyl-inositol
- PS
Phosphatidyl-serine
- PIP2
Phosphatidylinositol 4,5-bisphosphate
- SM
Sphingomyelin
- VDAC
Voltage-dependent anion channel
- MAM
Mitochondria-associated endoplasmic reticulum membranes
Funding
Open Access funding enabled and organized by Projekt DEAL.
Footnotes
Special Issue: Multicomponent lipid membranes.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akhtar-Schäfer I, Wang L, Krohne TU, Xu H, Langmann T. Modulation of three key innate immune pathways for the most common retinal degenerative diseases. EMBO Mol Med. 2018;10:e8259. doi: 10.15252/emmm.201708259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert NL, et al. TSPO PET for glioma imaging using the novel ligand 18F-GE-180: first results in patients with glioblastoma. Eur J Nucl Med Mol Imaging. 2017;44:2230–2238. doi: 10.1007/s00259-017-3799-9. [DOI] [PubMed] [Google Scholar]
- Albert NL, et al. In response to: the validity of 18F-GE180 as a TSPO imaging agent. Eur J Nucl Med Mol Imaging. 2019;46:1208–1211. doi: 10.1007/s00259-019-04294-8. [DOI] [PubMed] [Google Scholar]
- Anholt RR, Pedersen PL, Souza EBD, Snyder SH. The peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membrane. J Biol Chem. 1986;261:576–583. [PubMed] [Google Scholar]
- Arbo BD, Benetti F, Garcia-Segura LM, Ribeiro MF. Therapeutic actions of translocator protein (18 kDa) ligands in experimental models of psychiatric disorders and neurodegenerative diseases. J Steroid Biochem Mol Biol. 2015;154:68–74. doi: 10.1016/j.jsbmb.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Area-Gomez E, Schon EA. Mitochondria-associated ER membranes and Alzheimer disease. Curr Opin Genet Dev. 2016;38:90. doi: 10.1016/j.gde.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufschnaiter A, et al. Mitochondrial lipids in neurodegeneration. Cell Tissue Res. 2017;367:125–140. doi: 10.1007/s00441-016-2463-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader S, et al. Differential effects of TSPO ligands on mitochondrial function in mouse microglia cells. Psychoneuroendocrinology. 2019;106:65–76. doi: 10.1016/j.psyneuen.2019.03.029. [DOI] [PubMed] [Google Scholar]
- Banati RB. Visualising microglial activation in vivo. Glia. 2002;40:206–217. doi: 10.1002/glia.10144. [DOI] [PubMed] [Google Scholar]
- Banati RB, et al. Positron emission tomography and functional characterization of a complete PBR/TSPO knockout. Nat Commun. 2014;5:5452. doi: 10.1038/ncomms6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron AM, et al. Ligand for translocator protein reverses pathology in a mouse model of Alzheimer’s disease. J Neurosci Off J Soc Neurosci. 2013;33:8891–8897. doi: 10.1523/JNEUROSCI.1350-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betlazar C, Middleton RJ, Banati R, Liu G-J. The translocator protein (TSPO) in mitochondrial bioenergetics and immune processes. Cells. 2020;9:512. doi: 10.3390/cells9020512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin H, et al. 18F-GE-180: a novel TSPO radiotracer compared to 11C-R-PK11195 in a preclinical model of stroke. Eur J Nucl Med Mol Imaging. 2015;42:503–511. doi: 10.1007/s00259-014-2939-8. [DOI] [PubMed] [Google Scholar]
- Chaney A, et al. 11 C-DPA-713 versus 18 F-GE-180: a preclinical comparison of translocator protein 18 kDa PET tracers to visualize acute and chronic neuroinflammation in a mouse model of ischemic stroke. J Nucl Med. 2019;60:122–128. doi: 10.2967/jnumed.118.209155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B, Da Pozzo E, Martini C. Translocator protein and steroidogenesis. Biochem J. 2018;475:901–904. doi: 10.1042/BCJ20170766. [DOI] [PubMed] [Google Scholar]
- D’Eletto M, et al. Transglutaminase type 2 regulates ER-mitochondria contact sites by interacting with GRP75. Cell Rep. 2018;25:3573–3581.e4. doi: 10.1016/j.celrep.2018.11.094. [DOI] [PubMed] [Google Scholar]
- Daugherty DJ, et al. A TSPO ligand is protective in a mouse model of multiple sclerosis. EMBO Mol Med. 2013;5:891–903. doi: 10.1002/emmm.201202124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z, et al. Flutriciclamide (18F-GE180) PET: First-in-human PET study of novel third-generation in vivo marker of human translocator protein. J Nucl Med. 2016;57:1753–1759. doi: 10.2967/jnumed.115.169078. [DOI] [PubMed] [Google Scholar]
- Fan J, Campioli E, Midzak A, Culty M, Papadopoulos V. Conditional steroidogenic cell-targeted deletion of TSPO unveils a crucial role in viability and hormone-dependent steroid formation. Proc Natl Acad Sci USA. 2015;112:7261–7266. doi: 10.1073/pnas.1502670112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney C, et al. Kinetic analysis of the translocator protein positron emission tomography ligand [18F]GE-180 in the human brain. Eur J Nucl Med Mol Imaging. 2016;43:2201–2210. doi: 10.1007/s00259-016-3444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Hayashi T, Su T-P. The role of cholesterol in the association of endoplasmic reticulum membranes with mitochondria. Biochem Biophys Res Commun. 2012;417:635–639. doi: 10.1016/j.bbrc.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, et al. Comparison of four 11C-labeled PET ligands to quantify translocator protein 18 kDa (TSPO) in human brain: (R)-PK11195, PBR28, DPA-713, and ER176-based on recent publications that measured specific-to-non-displaceable ratios. EJNMMI Res. 2017;7:84. doi: 10.1186/s13550-017-0334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatliff J, et al. TSPO interacts with VDAC1 and triggers a ROS-mediated inhibition of mitochondrial quality control. Autophagy. 2015;10:2279–2296. doi: 10.4161/15548627.2014.991665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, et al. Translocator protein ligand protects against neurodegeneration in the MPTP mouse model of Parkinsonism. J Neurosci. 2019;39:3752–3769. doi: 10.1523/JNEUROSCI.2070-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui Y, Marks JD, Das S, Hyman BT, Serrano-Pozo A. Characterization of the 18 kDa translocator protein (TSPO) expression in post-mortem normal and Alzheimer’s disease brains. Brain Pathol Zurich Switz. 2020;30:151–164. doi: 10.1111/bpa.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, et al. Quantification of the specific translocator protein signal of 18F-PBR111 in healthy humans: a genetic polymorphism effect on in vivo binding. J Nucl Med. 2013;54:1915–1923. doi: 10.2967/jnumed.113.121020. [DOI] [PubMed] [Google Scholar]
- Guo Y, et al. Structure and activity of tryptophan-rich TSPO translocator proteins. Science. 2015;347:551–555. doi: 10.1126/science.aaa1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gut P, Zweckstetter M, Banati RB. Lost in translocation: the functions of the 18-kD translocator protein. Trends Endocrinol Metab TEM. 2015;26:349–356. doi: 10.1016/j.tem.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa M, et al. 11C-ER176, a Radioligand for 18-kDa translocator protein, has adequate sensitivity to robustly image all three affinity genotypes in human brain. J Nucl Med. 2017;58:320–325. doi: 10.2967/jnumed.116.178996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaipuria G, et al. Cholesterol-mediated allosteric regulation of the mitochondrial translocator protein structure. Nat Commun. 2017;8:14893. doi: 10.1038/ncomms14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaipuria G, Giller K, Leonov A, Becker S, Zweckstetter M. Insights into cholesterol/membrane protein interactions using paramagnetic solid-state NMR. Chem Weinh Bergstr Ger. 2018;24:17606–17611. doi: 10.1002/chem.201804550. [DOI] [PubMed] [Google Scholar]
- Jaipuria G, Ukmar-Godec T, Zweckstetter M. Challenges and approaches to understand cholesterol-binding impact on membrane protein function: an NMR view. Cell Mol Life Sci. 2018;75:2137–2151. doi: 10.1007/s00018-018-2789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamin N, et al. Characterization of the cholesterol recognition amino acid consensus sequence of the peripheral-type benzodiazepine receptor. Mol Endocrinol Baltim Md. 2005;19:588–594. doi: 10.1210/me.2004-0308. [DOI] [PubMed] [Google Scholar]
- Jaremko L, Jaremko M, Giller K, Becker S, Zweckstetter M. Structure of the mitochondrial translocator protein in complex with a diagnostic ligand. Science. 2014;343:1363–1366. doi: 10.1126/science.1248725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaremko M, Jaremko Ł, Jaipuria G, Becker S, Zweckstetter M. Structure of the mammalian TSPO/PBR protein. Biochem Soc Trans. 2015;43:566–571. doi: 10.1042/BST20150029. [DOI] [PubMed] [Google Scholar]
- Jaremko M, Jaremko Ł, Giller K, Becker S, Zweckstetter M. Structural integrity of the A147T polymorph of mammalian TSPO. Chembiochem Eur J Chem Biol. 2015;16:1483–1489. doi: 10.1002/cbic.201500217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaremko Ł, Jaremko M, Giller K, Becker S, Zweckstetter M. Conformational flexibility in the transmembrane protein TSPO. Chem Eur J. 2015;21:16555–16563. doi: 10.1002/chem.201502314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaremko M, Jaremko Ł, Giller K, Becker S, Zweckstetter M. Backbone and side-chain resonance assignment of the A147T polymorph of mouse TSPO in complex with a high-affinity radioligand. Biomol NMR Assign. 2016;10:79–83. doi: 10.1007/s12104-015-9642-y. [DOI] [PubMed] [Google Scholar]
- Jung ME. A protective role of translocator protein in Alzheimer’s disease brain. Curr Alzheimer Res. 2020;17:3–15. doi: 10.2174/1567205017666200217105950. [DOI] [PubMed] [Google Scholar]
- Kreisl WC, et al. A genetic polymorphism for translocator protein 18 Kda affects both in Vitro and in Vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab. 2013;33:53–58. doi: 10.1038/jcbfm.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacapère JJ, et al. Structural and functional study of reconstituted peripheral benzodiazepine receptor. Biochem Biophys Res Commun. 2001;284:536–541. doi: 10.1006/bbrc.2001.4975. [DOI] [PubMed] [Google Scholar]
- Lee AG. Lipid–protein interactions in biological membranes: a structural perspective. Biochim Biophys Acta BBA Biomembr. 2003;1612:1–40. doi: 10.1016/s0005-2736(03)00056-7. [DOI] [PubMed] [Google Scholar]
- Leva G, et al. The translocator protein ligand XBD173 improves clinical symptoms and neuropathological markers in the SJL/J mouse model of multiple sclerosis. Biochim Biophys Acta Mol Basis Dis. 2017;1863:3016–3027. doi: 10.1016/j.bbadis.2017.09.007. [DOI] [PubMed] [Google Scholar]
- Lewis BA, Engelman DM. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J Mol Biol. 1983;166:211–217. doi: 10.1016/s0022-2836(83)80007-2. [DOI] [PubMed] [Google Scholar]
- Liu B, et al. In Vivo detection of age- and disease-related increases in neuroinflammation by 18F-GE180 TSPO MicroPET imaging in wild-type and Alzheimer’s Transgenic Mice. J Neurosci Off J Soc Neurosci. 2015;35:15716–15730. doi: 10.1523/JNEUROSCI.0996-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Liu J, Zheng Y, Garavito RM, Ferguson-Miller S. Crystal structures of translocator protein (TSPO) and mutant mimic of a human polymorphism. Science. 2015;347:555–558. doi: 10.1126/science.1260590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Picón FR, et al. Neuroinflammation appears early on PET imaging and then plateaus in a mouse model of Alzheimer Disease. J Nucl Med Off Publ Soc Nucl Med. 2018;59:509–515. doi: 10.2967/jnumed.117.197608. [DOI] [PubMed] [Google Scholar]
- Mandala VS, Williams JK, Hong M. Structure and dynamics of membrane proteins from solid-state NMR. Annu Rev Biophys. 2018;47:201–222. doi: 10.1146/annurev-biophys-070816-033712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín A, et al. Evaluation of the PBR/TSPO Radioligand [18 F]DPA-714 in a rat model of focal cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:230–241. doi: 10.1038/jcbfm.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEnery MW, Snowman AM, Trifiletti RR, Snyder SH. Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc Natl Acad Sci USA. 1992;89:3170–3174. doi: 10.1073/pnas.89.8.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhin AG, Papadopoulos V, Costa E, Krueger KE. Mitochondrial benzodiazepine receptors regulate steroid biosynthesis. Proc Natl Acad Sci USA. 1989;86:9813–9816. doi: 10.1073/pnas.86.24.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murail S, et al. Secondary and tertiary structures of the transmembrane domains of the translocator protein TSPO determined by NMR. Stabilization of the TSPO tertiary fold upon ligand binding. Biochim Biophys Acta BBA Biomembr. 2008;1778:1375–1381. doi: 10.1016/j.bbamem.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Owen DR, et al. Two binding sites for [3H]PBR28 in human brain: implications for TSPO PET imaging of neuroinflammation. J Cereb Blood Flow Metab. 2010;30:1608–1618. doi: 10.1038/jcbfm.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DRJ, et al. Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J Nucl Med. 2011;52:24–32. doi: 10.2967/jnumed.110.079459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2012;32:1–5. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsdottir H, Hunte C. Lipids in membrane protein structures. Biochim Biophys Acta BBA Biomembr. 2004;1666:2–18. doi: 10.1016/j.bbamem.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, et al. Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Fan J, Zirkin B. Translocator protein (18 kDa): an update on its function in steroidogenesis. J. Neuroendocrinol. 2018;30:e2500. doi: 10.1111/jne.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repalli J. Translocator protein (TSPO) role in aging and Alzheimer’s disease. Curr Aging Sci. 2015;7:168–175. doi: 10.2174/1874609808666141210103146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schain M, Kreisl WC. Neuroinflammation in neurodegenerative disorders—a review. Curr Neurol Neurosci Rep. 2017;17:25. doi: 10.1007/s11910-017-0733-2. [DOI] [PubMed] [Google Scholar]
- Scholz R, et al. Targeting translocator protein (18 kDa) (TSPO) dampens pro-inflammatory microglia reactivity in the retina and protects from degeneration. J Neuroinflammation. 2015;12:201. doi: 10.1186/s12974-015-0422-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Pittala S, Mizrachi D. VDAC1 and the TSPO: expression, interactions, and associated functions in health and disease states. Int J Mol Sci. 2019;20:3348. doi: 10.3390/ijms20133348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan S, et al. Comparative evaluation of three TSPO PET radiotracers in a LPS-induced model of mild neuroinflammation in Rats. Mol Imaging Biol. 2017;19:77–89. doi: 10.1007/s11307-016-0984-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan S, et al. Confirmation of specific binding of the 18-kDa translocator protein (TSPO) Radioligand [18F]GE-180: a blocking study using XBD173 in multiple sclerosis normal appearing white and grey matter. Mol Imaging Biol. 2019;21:935–944. doi: 10.1007/s11307-019-01323-8. [DOI] [PubMed] [Google Scholar]
- Stevens TJ, et al. A software framework for analysing solid-state MAS NMR data. J Biomol NMR. 2011;51:437–447. doi: 10.1007/s10858-011-9569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica R, et al. ER-mitochondria associations are regulated by the VAPB-PTPIP51 interaction and are disrupted by ALS/FTD-associated TDP-43. Nat Commun. 2014;5:3996. doi: 10.1038/ncomms4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymański J, et al. Interaction of mitochondria with the endoplasmic reticulum and plasma membrane in calcium homeostasis, lipid trafficking and mitochondrial structure. Int J Mol Sci. 2017;18:1576. doi: 10.3390/ijms18071576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takegoshi K, Nakamura S, Terao T. 13C–1H dipolar-assisted rotational resonance in magic-angle spinning NMR. Chem Phys Lett. 2001;344:631–637. [Google Scholar]
- Takegoshi K, Nakamura S, Terao T. 13C–1H dipolar-driven 13C–13C recoupling without 13C rf irradiation in nuclear magnetic resonance of rotating solids. J Chem Phys. 2003;118:2325–2341. [Google Scholar]
- Teboul D, et al. Mouse TSPO in a lipid environment interacting with a functionalized monolayer. Biochim Biophys Acta BBA Biomembr. 2012;1818:2791–2800. doi: 10.1016/j.bbamem.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Unterrainer M, et al. TSPO PET with [18F]GE-180 sensitively detects focal neuroinflammation in patients with relapsing–remitting multiple sclerosis. Eur J Nucl Med Mol Imaging. 2018;45:1423–1431. doi: 10.1007/s00259-018-3974-7. [DOI] [PubMed] [Google Scholar]
- Unterrainer M, et al. Detection of cerebrospinal fluid dissemination of recurrent glioblastoma using TSPO-PET With 18F-GE-180. Clin Nucl Med. 2018;43:518–519. doi: 10.1097/RLU.0000000000002113. [DOI] [PubMed] [Google Scholar]
- Unterrainer M, et al. Comparison of 18F-GE-180 and dynamic 18F-FET PET in high grade glioma: a double-tracer pilot study. Eur J Nucl Med Mol Imaging. 2019;46:580–590. doi: 10.1007/s00259-018-4166-1. [DOI] [PubMed] [Google Scholar]
- Vomacka L, et al. TSPO imaging using the novel PET ligand [18F]GE-180: quantification approaches in patients with multiple sclerosis. EJNMMI Res. 2017;7:1–9. doi: 10.1186/s13550-017-0340-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilms H, et al. Involvement of benzodiazepine receptors in neuroinflammatory and neurodegenerative diseases: evidence from activated microglial cells in vitro. Neurobiol Dis. 2003;14:417–424. doi: 10.1016/j.nbd.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Xia Y, et al. A unified structural model of the mammalian translocator protein (TSPO) J Biomol NMR. 2019;73:347–364. doi: 10.1007/s10858-019-00257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanotti-Fregonara P, et al. Head-to-head comparison of 11C-PBR28 and 18 F-GE180 for quantification of the translocator protein in the human brain. J Nucl Med. 2018;59:1260–1266. doi: 10.2967/jnumed.117.203109. [DOI] [PubMed] [Google Scholar]
- Zanotti-Fregonara P, et al. The validity of 18F-GE180 as a TSPO imaging agent. Eur J Nucl Med Mol Imaging. 2019;46:1205–1207. doi: 10.1007/s00259-019-4268-4. [DOI] [PubMed] [Google Scholar]