Graphical abstract
Keywords: Granulomatosis with polyangiitis, Acute pericarditis, Recurrent pericarditis, Anakinra, Corticosteroids, Cardiac magnetic resonance imaging
Highlights
-
•
GPA is a systemic necrotizing vasculitis of medium and small vessels.
-
•
GPA classically involves the upper and lower respiratory tracts and the kidneys.
-
•
Pericarditis is a common cardiac manifestation, but RP is rarely described.
-
•
A systematic literature search yielded 13 cases of acute pericarditis secondary to GPA, which are analyzed in this review.
-
•
The potential role of Anakinra for debilitating RP secondary to GPA is described.
Introduction
Granulomatosis with polyangiitis (GPA; formerly known as Wegener's granulomatosis) is a disease characterized by necrotizing granulomatous vasculitis involving the upper and lower respiratory tracts and the kidneys.1 Cardiac involvement is reported in 6%-44% of cases, and pericarditis is the most common cardiac manifestation.2,3 The pathophysiology is postulated to be necrotizing vasculitis secondary to granulomatous infiltrates.4,5 Prognosis is same as for other forms of pericarditis, and in cases where constrictive pericarditis develop, surgical intervention is associated with good outcome.3,6,7
Herein, we report two cases of pericarditis secondary to GPA. Furthermore, the systematic review of literature outlines our current understanding of the epidemiology, clinical presentations, diagnostic modalities, clinical course, and outcomes of pericardial diseases in GPA.1, 2, 3, 4, 5, 6, 7, 8, 9, 10
Case Presentation
Patient 1
A 44-year-old woman, presented to the Emergency Department (ED) from her dialysis center with pleuritic chest pain, shortness of breath, and fever during her dialysis session. The patient has a past medical history significant for GPA diagnosed by renal biopsy revealing crescentic glomerulonephritis and positive p-ANCA/MPO serology. Despite treatment with prednisone and rituximab, the patient progressed to end-stage renal disease. At the time of admission, the patient's physical examination revealed clear lung fields, regular heart rate and rhythm, a pericardial friction rub, flat jugular veins, and no pedal edema.
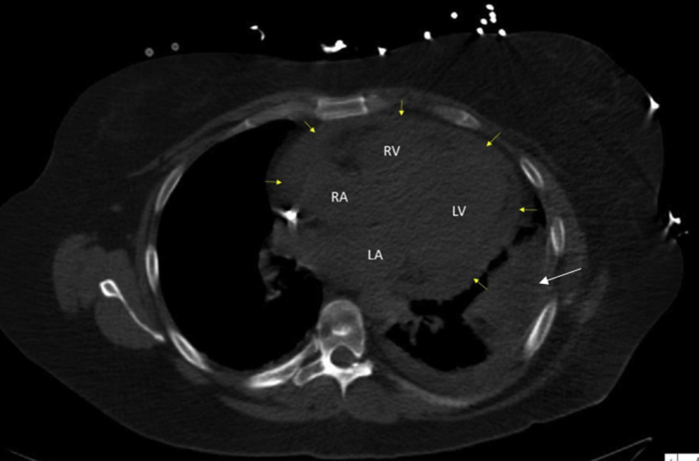
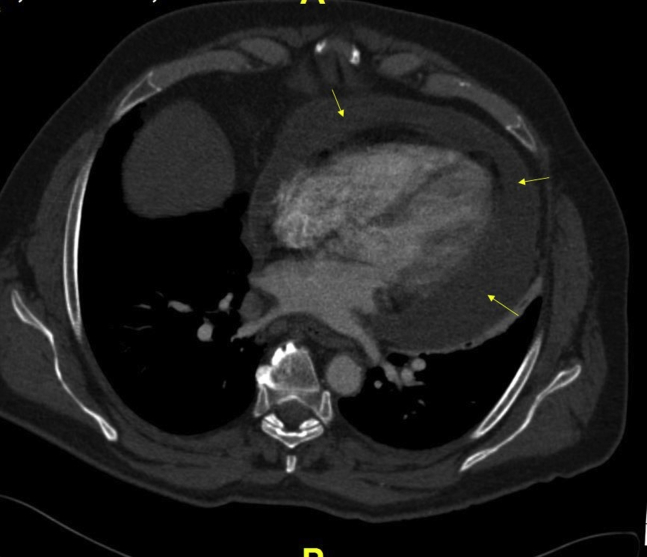
Initial laboratory studies revealed mild leukocytosis of 11.84 K/uL (normal, 3.7-11.0 K/uL), ESR 88 mm/hour, and CRP 20.3 mg/dL. Blood cultures were negative, but viral swab revealed respiratory syncytial virus. Chest computed tomography (CT) scan on presentation showed a new-onset moderate to large sized circumferential pericardial effusion (Figure 1).
Figure 1.
Chest CT scan axial view showing moderate to large circumferential pericardial effusion (yellow arrows) and left pelural effusion (white arrow). LA, Left atrium; LV left ventricle; RA, right atrium; RV, right ventricle.
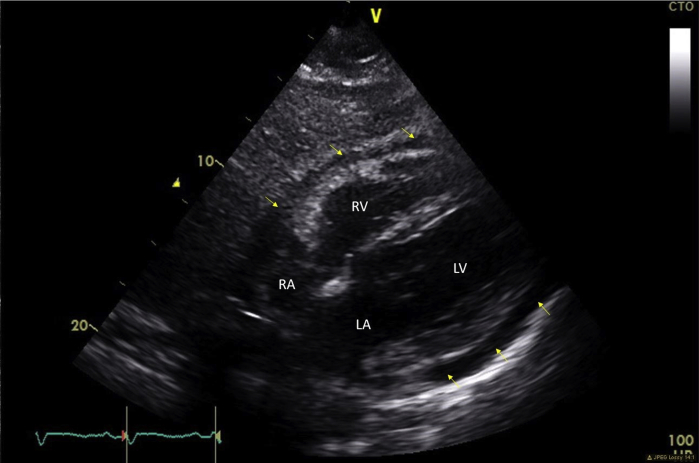
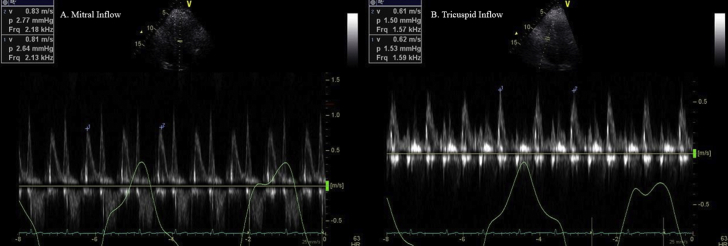
Electrocardiogram showed normal sinus rhythm. Cardiac enzymes were within normal range. Transthoracic echocardiogram (TTE) revealed a moderate sized pericardial effusion and inferior vena cava (IVC) plethora, but no chamber collapse or significant respiratory variation of Doppler inflows to suggest tamponade (Figures 2 and 3; Video 1 available at www.onlinejase.com).
Figure 2.
Transthoracic echocardiogram two-dimensional subcostal view of the heart showing moderate size circumferential pericardial effusion without tamponade (yellow arrows). LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
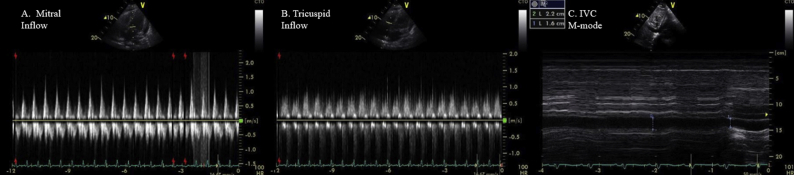
Figure 3.
Pulsed-wave-Doppler recordings of (A) mitral and (B) tricuspid inflow showing mild respiratory variation of mitral and tricuspid inflows in setting of moderate pericardial effusion. (C) M-mode echocardiogram of IVC showing dilated IVC (>21 mm diameter) with plethora.
In the setting of classic chest pain, elevated inflammatory markers, and moderate pericardial effusion, the patient was diagnosed with GPA relapse manifesting as acute pericarditis with moderate pericardial effusion with only mild respiratory variation of Doppler inflows (Figure 3A–C; Video 2 available at www.onlinejase.com).
Cardiac magnetic resonance imaging (MRI) was deferred due to renal failure. The patient's prednisone was escalated to 60 mg daily and colchicine 0.6 mg twice daily was added to treat the acute pericarditis in the setting of GPA. Avoidance of physical activity was recommended upon discharge. At 3-month follow-up, the patient reported no symptoms and a trivial pericardial effusion was found on TTE. There was no abnormal respirophasic ventricular septal shift (ventricular interdependence) observed on M-mode echocardiogram. (Video 3 available at www.onlinejase.com).
Continuation of colchicine and a prolonged prednisone taper was advised. At 6-month follow-up, the patient remained asymptomatic. The prednisone was tapered down to 12.5 mg, and colchicine dose was decreased to once daily.
The patient noted recurrence of her pleuritic chest pains as the prednisone was further tapered prompting multiple visits to the ED, subsequently her prednisone dose was increased to 20 mg, resulting in improvement of her symptoms. Inflammatory markers remained normal. Instructions were given for a very slow prednisone taper (5 mg every 2 weeks then subsequently 2.5 mg every 2 weeks), and the patient was approved for renal transplant.
The patient continued to have flares of recurrent pericarditis post–renal transplant and she presented 1.5 years later to our pericardial center with an active flare of her recurrent pericarditis. She was on immunosuppressants tacrolimus and everolimus for her renal transplant. A decision was made to initiate the patient on anakinra (interleukin blocker), in addition to her current medical therapy. Colchicine was stopped due to elevated hepatic enzymes and the patient was continued on quadruple immunosuppressive therapy with tacrolimus, everolimus, prednisone and anakinra, with symptomatic improvement.
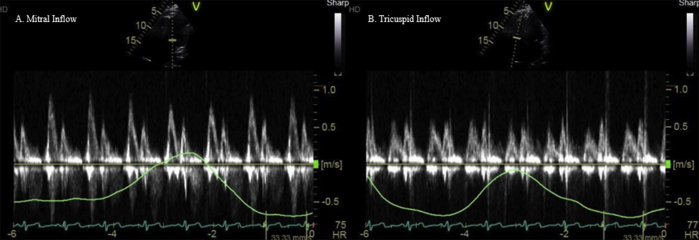
On most recent follow-up at the pericardial center, 3 years after the index hospitalization, the patient had been asymptomatic with no reported recurrences, ED visits, or hospitalizations in the last 12 months. Transthoracic echocardiogram showed no pericardial effusion or signs of constrictive physiology (Figure 4A and B; Videos 4 and 5 available at www.onlinejase.com). Prednisone were slowly tapered off, given concerns of developing undesirable side effects with chronic steroid therapy, followed by a prolonged anakinra taper with an aim to taper off all medical therapy in a span of next 1 year.
Figure 4.
Pulsed-wave-Doppler recordings of (A) mitral (B) tricuspid inflow velocities, with simultaneous respirometric recording, showing no significant respirophasic variations of mitral and tricuspid inflows.
Patient 2
A 63-year-old man was referred to our clinic for investigation of pericardial effusion. He had recurrent otitis media, resistant to steroid and antibiotic therapy, with hearing loss for last 5 years. The patient was recently at the hospital for worsening shortness of breath, weight loss, night sweats and dry cough for 5 months. An elevated ESR of 71 mm/hour and CRP of 17.5 mg/dL were significant. Chest CT scan showed a moderate-to-large pericardial effusion with flattening of interventricular septum (Figure 5) followed by a TTE showing a moderate-to-large organized circumferential pericardial effusion with no evidence of pericardial tamponade.
Figure 5.
Chest CT scan axial view showing moderate-to-large pericardial effusion (yellow arrows).
The patient underwent pericardiocentesis with 400 mL of fluid drained, analysis of which revealed an exudative effusion with acute and chronic inflammation and no malignant cells. The patient improved symptomatically postprocedure and a repeat TTE revealed a moderate organized pericardial effusion measuring 1.8 cm adjacent to the right atrium and right ventricle. The patient was diagnosed with acute pericarditis and started on colchicine 0.6 mg twice daily and ibuprofen 800 mg thrice daily.
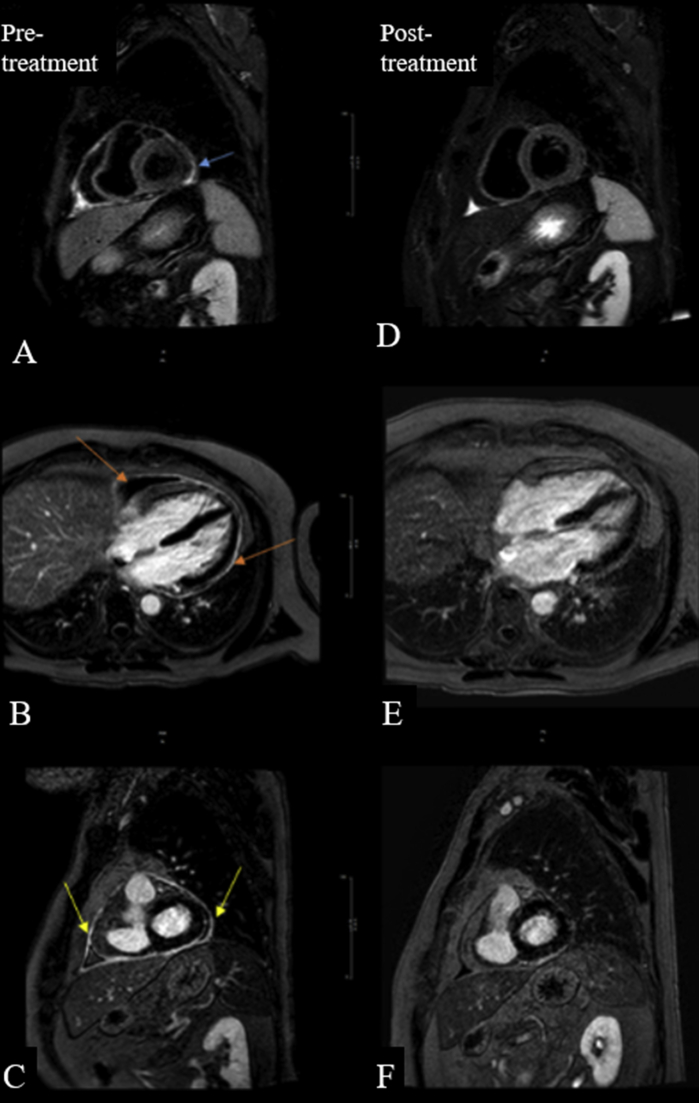
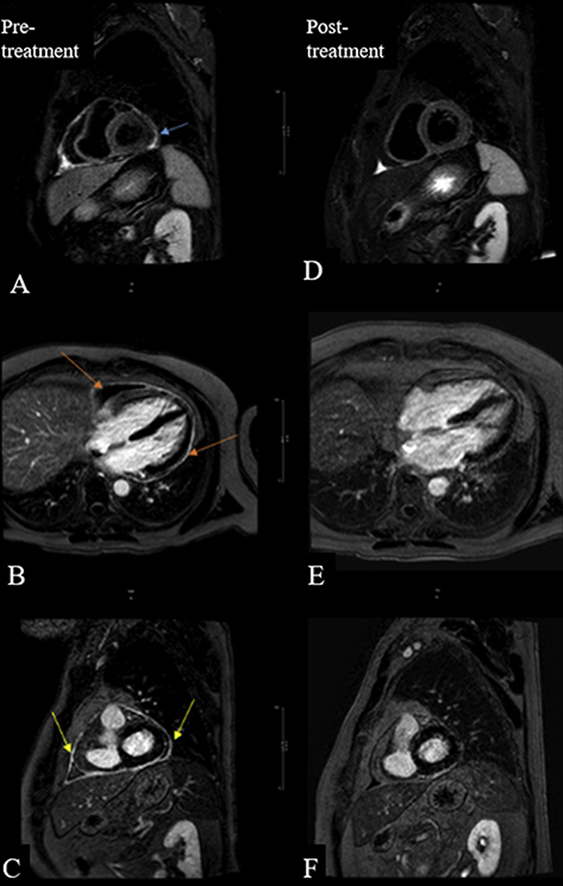
The patient continued to have night sweats and was readmitted 1 month later with fever and proximal muscle weakness. Laboratory workup revealed prominent leukocytosis of 18.68 K/uL, aspartate aminotransferase of 56 U/L (normal, 7-40 U/L), alanine aminotransferase of 71 U/L (normal, 5-50 U/L) and an elevated p-ANCA level (detected by indirect immunofluorescence) of 44 U (normal, 0-20 U). Repeat TTE shows small pericardial effusion. A diagnosis of GPA was made and the patient was started on prednisone 40 mg daily and cyclophosphamide 150 mg daily. A cardiac MRI was performed due to persistent symptoms and poor response to medical therapy. It revealed increased pericardial thickening of 2-3 mm with moderate circumferential enhancement of the pericardium on late gadolinium enhancement sequences, increased signal on T2 edema weighted imaging consistent with active pericarditis without any features of constrictive physiology (Figure 6A–C). The patient showed a dramatic improvement on combination cyclophosphamide and prednisone prolonged taper and a repeat cardiac MRI 4 weeks later showed reduction in pericardial effusion, enhancement and thickening (Figure 6D–F).
Figure 6.
Cardiac MRI with pretreatment images showing (A) increased T2 STIR signal intensity indicating acute inflammation, small pericardial effusion, and late gadolinium enhancement in (B) four-chamber and (C) short-axis views. Cardiac MRI posttreatment images showing no more increased pericardial signal T2 STIR, indicating edema has resolved (D); resolution of late gadolinium enhancement in (E) four-chamber (F) short-axis views indicating the pericardial inflammation is resolving.
The patient continued to follow at the pericardial center. He reported developing fatigue with worsening hearing loss 3 years after the index admission and was started on rituximab infusions. The patient's GPA is currently managed on low-dose prednisone and rituximab infusions with no recurrence of pericarditis (Figure 7A and B; Videos 6–8 available at www.onlinejase.com).
Figure 7.
On-treatment Doppler recording of (A) mitral and (B) tricuspid inflow with a respirometer showing no evidence of constrictive physiology.
Discussion
First described in 1931 by Klinger and further characterized by Wegener in 1936, GPA (formerly called Wegener's granulomatosis) most commonly involves the sinuses, lungs and kidneys with necrotizing granulomatous vasculitis.1,3 Cardiac complications can occur in up to 44% of patients with GPA. Pericarditis is the most frequently reported cardiac manifestation of GPA (50% of cases), but myocarditis, endocarditis and conduction system granulomata are also described.3 It has been reported that pathologic involvement of the pericardium is found in as many as 50% of patients with GPA at autopsy. Mild subclinical pericardial effusions are found in the majority of patients, and large pericardial effusions and tamponade requiring pericardiocentesis with or without pericardial window is rare.11,12 Pericardial effusions can develop in the absence of severe renal dysfunction, indicating that besides uremia, GPA vasculitis has a direct pathogenic role.13 Absence of pathological evidence of granulomata or active vasculitis in pericardial tissue suggests that other inflammatory mechanisms associated with disease exacerbations are involved.1,3,7 Constrictive pericarditis with GPA is rarely reported.3,7
The etiology of pericardial diseases is diverse and is broadly classified into infectious causes (viral, bacterial, fungal or parasitic) and noninfectious causes (autoimmune, neoplastic, metabolic, traumatic/iatrogenic, drug-related and congenital malformations). Pericarditis related to systemic vasculitis is categorized under autoimmune causes of acute pericarditis.13 Pericardial involvement in systemic vasculitides is relatively rare in large-vessel vasculitis while it is more common in medium- and small-vessel vasculitis such as Kawasaki disease, eosinophilic granulomatosis with polyangiitis and GPA (Wegener's granulomatosis).13
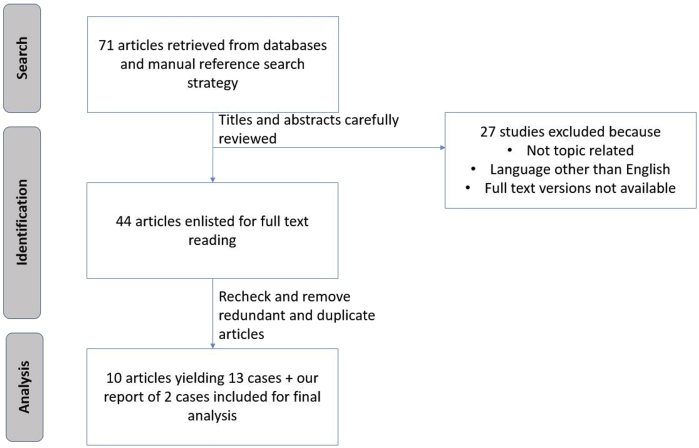
We systemically searched the published medical literature to retrieve the available case reports for pericardial involvement in GPA/Wegener's granulomatosis (Figure 8). The data of these patients including demographics, clinical features, diagnostic tools, prognosis and outcomes are summarized in Table 1.1, 2, 3, 4, 5, 6, 7, 8, 9, 10
Figure 8.
Flow diagram depicting the selection of the articles included in this review.
Table 1.
Literature review of case reports of pericardial diseases in GPA (Wegener's granulomatosis)
| Author | Publication year | Country | Age/gender | Prior organ involvement | Clinical presentation | Relevant examination findings | Diagnostic EKG and laboratory findings | Multimodality cardiac imaging findings | Pericardial fluid drain/biopsy | Treatment | Clinical course and outcomes | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schiavone et al Patient 11 | 1985 | USA | 60/M | Nose, lung, kidney | Weight gain, abdominal distension, edema | Pericardial knock | Renal failure | TTE: loculated posterior pericardial effusion, thickened pericardium, abrupt halt in diastolic filling pressure. Cardiac catheterization: equalization of diastolic pressures | Biopsy: fibrosis | Surgical pericardiectomy | Improvement on cyclophosphamide and prednisone | None |
| Schiavone et al Patient 21 | 1985 | USA | 43/M | Nose, kidney | Edema, fevers, night sweats, hemoptysis and conjunctival erythema | Pericardial friction rub | EKG: diffuse STE, accelerated junctional rhythm and AV dissociation | TTE: small pericardial effusion | Not required/performed | Prednisone, cyclophosphamide | Symptomatic improvement on medical management | None |
| Schiavone et al Patient 31 | 1985 | USA | 56/M | Lung | Productive cough, fever, night sweats | Transient pericardial friction rub, expiratory wheezing | EKG: atrial tachycardia with 2:1 conduction | TTE: small pericardial effusion | Not required/performed | Intravenous methylprednisolone and nitrogen mustard; digoxin and quinidine for heart block with conversion to sinus rhythm | Symptomatic improvement on medical management | None |
| Meryhew et al2 | 1988 | USA | 59/M | Lung, kidney | Dyspnea, fever, hemoptysis | Systolic ejection murmur | EKG normal, anemia, neutrophilia, thrombocytosis, elevated ESR, antinuclear antibody+ | Chest CT: Large pericardial effusion. TTE: Large pericardial effusion with right atrial systolic and right ventricular diastolic collapse. Cardiac catheterization: equalization of diastolic intracardiac pressures | Pericardial window. Biopsy: acute inflammation, granulation tissue | Emergency pericardiectomy, intravenous methylprednisolone and cyclophosphamide | Improved symptomatically after hospital discharge on prednisone taper and cyclophosphamide but died suddenly at home 8 months later | None |
| Grant et al Patient 23 | 1994 | UK | 45/M | Sinus | Heart failure | Engorged neck veins, peripheral edema, hepatomegaly, ascites, basal crackles in lungs | EKG: widespread nonspecific ST-T wave changes. Anemia, elevated ESR, c ANCA+ | TTE: Small left ventricular cavity, no pericardial fluid or abnormality. Cardiac catheterization: constrictive pericarditis with equalization of diastolic pressures | Biopsy: Fibrosis | Prednisone, cyclophosphamide with mesna followed by pericardiectomy | Improvement on medical management | None |
| Grant et al Patient 33 | 1994 | UK | 35/F | Sinus, kidney | Chest pain and increasing shortness of breath | Pale, puffy face and ankle swelling | Anemia, - c ANCA | TTE: moderately large pericardial effusion, abnormal right atrial movement, left ventricle small and vigorous with an estimated ejection fraction of 60% | Biopsy: Fibrinous hemorrhagic pericarditis | Pericardial fenestration followed by pericardiectomy | Did well and discharged home | None |
| Yildizer et al4 | 1996 | Turkey | 50/F | Sinus, lung, kidney | Cough, weakness, anorexia, pleuritic chest pain | Reduced breath sounds at right lung base | Anemia, elevated ESR, renal failure on dialysis | TTE: pericardial tamponade | Biopsy: necrotizing vasculitis | Prednisone, cyclophosphamide | Died during hospitalization | Not available |
| Florian et al5 | 2011 | Belgium | 38/M | Lung | Shortness of breath and position-related chest pain | No friction rub or murmur heard | EKG: diffuse T-wave flattening. Elevated ESR and CRP, c ANCA+ | Chest CT: Mild cardiomegaly, discrete posterobasal pleural effusion, thickened pericardium without calcification. TTE: Thickened pericardium with circumferential, homogenous pericardial effusion (14 mm along LV wall), mitral valve: E inspiration 75 cm/sec, E expiration 97 cm/sec, 23% variation, E wave deceleration time 166 msec, normal BiV function. Cardiac MRI: real-time cine imaging showed minor septal flattening but no septal inversion no shift (argues against pericardial constriction), morphologic analysis by T1-weighted sequences showed normal myocardium and thickened pericardium (6 mm) with hyperintense circumferential pericardial effusion (up to 7 mm along the LV lateral wall). STIR imaging showed intense circumferential edema of both pericardial layers and limited subepicardial edema in inferolateral LV wall. Late post-gadolinium administration imaging showed strong enhancement of both pericardial layers, subtle subepicardial enhancement of inferolateral LV wall. | Not required/performed | Not reported | Not reported | Not reported |
| Somaliy et al6 | 2012 | Saudi Arabia | 34/M | Nasal sinus, lung, kidney | Chest pain and productive cough for 5 days, fever and arthralgia for 1 month | High jugular venous pulse, +Kussmaul sign, distant heart sounds | Neutrophilia, leukocytosis, elevated ESR, c ANCA+ | Chest CT and TTE: Large pericardial effusion | Not required/performed | Intravenous prednisolone | Switched to oral prednisone with a prolonged taper; asymptomatic with resolution of pericardial effusion on 2-week follow-up | None |
| Horne et al7 | 2014 | UK | 42/F | Sinus, lung | Dyspnea, peripheral edema, orthopnea | Peripheral edema | EKG: normal; c ANCA+ | TTE: good left ventrucular systolic function, diastolic septal bounce, increased respirophasic variaton of atrioventricular flows. Cardiac MRI: pericardial thickening (7 mm), and inspiratory septal flattening with no evidence of infiltrative/inflammatory myocardial disease | Biopsy: collagenous fibrous tissue with no evidence of inflammation or vasculitis | Surgical pericardiectomy | Improved symptomatically | None |
| Dewan et al8 | 2015 | USA | 57/M | None | Syncopal episode, frontal headache | None | Elevated ESR, p ANCA+ | Chest CT: soft tissue attenuation around the coronary arteries, bypass grafts, pericardium. Cardiac MRI: enhancing soft tissue around the graft and coronary arteries with nodular appearance of pericardium | Biopsy: Dense scar tissue with mononuclear infiltrates: granulomatous capillaritis with leukocytoclasis and mononuclear infiltrate | Prednisone, rituximab | Improvement in soft tissue thickening around coronary arteries and pericardium at 3-year follow-up CT scan | None |
| Miyawaki et al9 | 2017 | Japan | 60/M | Lung, kidney | Fever, cough | Conjunctival hyperemia | Anemia, elevated CRP, c ANCA+ | Chest CT: Thickened pericardium | Not required/performed | Methylprednisolone and cyclophosphamide | At 2- month follow-up: marked reduction in size of multicenter nodular pulmonary lesions, concentric soft tissue cuff around aortic arch and pericardial thickening | None |
| Parmar et al10 | 2019 | USA | 49/M | Sinus, Kidney | Dyspnea, chest pain | Saddle nose deformity, distant heart sounds, elevated jugular venous pulse, AV fistula bruit | EKG: electrical alternans. Anemia, elevated BUN and Cr, elevated ESR and CRP, p ANCA+ | Chest CT: moderate pericardial effusion. TTE: pericardial effusion with tamponade | Pericardial window. Biopsy: acute inflammation, granulation tissue, fibrinopurulent exudate. | Pulse dose steroids with prolonged taper | Hospitalized within a month of discharge for arteriovenous fistula occlusion and sepsis/bacteremia; passed away secondary to cardiogenic shock and hypoxic respiratory failure during hospitalization. | Not available |
| Cleveland Clinic Patient 1 | 2020 | USA | 44/F | Sinus, lung, kidney | Positional chest pain, shortness of breath, fever | Friction rub | Leukocytosis, elevated ESR and CRP | Chest CT: Moderate-to-large sized pericardial effusion and left pleural effusion. TTE: moderate pericardial effusion without tamponade TEE: moderate pericardial effusion, no evidence of constriction |
Not required | Prednisone 60 mg daily (with prolonged taper) and colchicine 0.6 mg twice daily | Multiple recurrences over the next 3 years, persistent after kidney transplant, requiring immunomodulatory therapy with anakinra with resolution of symptoms | Multiple |
| Cleveland Clinic Patient 2 | 2020 | USA | 63M | Sinus, ears | Shortness of breath, night sweats, dry cough | None | Leukocytosis, elevated ESR and CRP, elevated p-ANCA |
Chest CT: large pericardial effusion, flattening of interventricular septum TTE: moderate organized circumferential pericardial effusion Cardiac MRI: Increased pericardial thickening of 2-3 mm with moderate circumferential enhancement of the pericardium on late gadolinium enhancement T1 sequence, increased signal on T2 edema weighted imaging |
Pericardiocentesis, 400 mL of exudative effusion revealing acute and chronic inflammation | Colchicine 0.6 mg twice daily and ibuprofen 800 mg three times daily | Admitted with acute pericarditis after 1 month and managed with prednisone and cyclophosphamide. Currently on prednisone and rituximab with no reported recurrences. | None |
EKG, Electrocardiogram; F, female; LV, left ventricular; M, male.
A comprehensive review of these cases revealed a male predominance (men, n = 10; women n = 3). The mean age of the patients was 48 years (range, 35-60 years). The common clinical presentation was cough with or without hemoptysis, fever, chest pain, and shortness of breath consistent with our experience of such patients. Physical examination findings of volume overload state were most frequently observed, whereas a pericardial friction rub and pericardial knock were less documented. Biochemical evaluation predominantly showed elevated ESR and CRP, as well as anemia, thrombocytosis, and neutrophilia associated with GPA. Patients 1 and 2 exhibited elevated inflammatory markers and leukocyte counts. Echocardiographic evidence of a new or worsening pericardial effusion was found to be present in most cases along with findings of tamponade and constrictive pathology in four and three patients, respectively. Two patients underwent advanced imaging with cardiac MRI, probably due to the recent surge in the use of this modality for the diagnosis of pericardial diseases. Patient 1 could not get an MRI in the setting of renal failure. In the case of patient 2, the diagnosis of acute pericarditis was evident on presentation; however, the cardiac MRI evaluation illustrating the findings associated with acute inflammatory pericarditis aided with the treatment response.
The 2015 European Society of Cardiology guidelines for the diagnosis and management of pericardial diseases define an episode of acute pericarditis as an inflammatory pericardial syndrome diagnosed with at least two of the following four criteria: (1) pericardial chest pain, (2) pericardial rubs, (3) new or widespread ST elevation or PR depression on the electrocardiogram, or (4) evidence of pericardial effusion (new or worsening) on imaging. The diagnosis is supported in the presence of elevated markers of inflammation (ESR, CRP, white blood cell count) and evidence of pericardial inflammation on imaging techniques (CT or MRI).14 Recurrent pericarditis (RP) is defined as recurrence of pericarditis after a documented first episode of acute pericarditis and a symptom-free interval of 4-6 weeks or longer.14 Acute pericarditis with transient constriction is stated to occur when the acute inflammation as well as constrictive features resolve with anti-inflammatory therapy.14
Transthoracic echocardiogram in suspected acute pericarditis provides confirmatory evidence of the diagnosis when a new or increasing pericardial effusion is found. As per our review, TTE was performed in all but two cases, in which the findings on a CT scan were found to be sufficient.8,9 Although not routinely used for the initial diagnosis of pericarditis, cardiac MRI is highly sensitive for the diagnosis of active pericarditis, and can be useful in cases with an otherwise uncertain diagnosis.14 Florian et al5 superbly described the cardiac MRI findings of pericardial involvement in GPA, suggesting that this imaging modality has a unique role to play, consistent with our observations with patient 2.
In this review, out of a total of 13, eight patients underwent pericardial biopsy, and evidence of acute inflammation and granuloma formation was found in five of these patients. In both patients 1 and 2, pericardial biopsy was avoided due to low diagnostic yield and patient comfort. A majority of the patients were treated with steroid therapy, often a combination of prednisone and cyclophosphamide. Surgical pericardiotomy was performed in patients with constrictive pericarditis. An interesting observation is that all patients improved clinically (except one case who died during hospitalization) with the interventions performed, with no reported recurrences during follow-up. Our experience is unique in patient 1, who presented with RP despite being on prolonged steroid therapy and subsequently responded to an immunomodulatory agent.
The pathophysiology of RP is postulated to be an amplified and self-sustained autoinflammatory and/or autoimmune response to exogenous or endogenous triggers. Anakinra, an interleukin-1 receptor antagonist, interferes with this self-sustained pathway and is among the emerging therapies for RP refractory to standard medical therapy to control symptoms and avoid the long-term effects of corticosteroids.14 Patient 1 developed steroid-refractory colchicine-resistant RP, which happened in the context of renal transplantation and did not respond to conventional anti-inflammatory therapy. Our patient showed a remarkable recovery with anakinra (Kineret, Sobi, Stockholm, Sweden). Currently approved by the U.S. Food and Drug Administration for the treatment of rheumatoid arthritis (off label for recurrent pericarditis), daily subcutaneous injections of anakinra at 1-2 mg/kg/day, up to 100 mg, for several months has been shown to lower the risk of recurrence, ED admissions, and hospitalizations and decreased use of corticosteroids in a multicenter observational cohort study.14,15 Anakinra is generally reserved for the most refractory pericarditis cases (especially if they are corticosteriod-dependent and colchicine-resistant) owing to high cost, daily subcutaneous injections and limited published data.15
Conclusion
Cardiac involvement in GPA is found in advanced disease, with pericarditis being the most common clinical manifestation. Patients with a GPA flare can present with acute/RP as a manifestation of their flare. Pericarditis secondary to GPA, in the absence of constrictive physiology, usually responds to standard medical management. Steroid refractory pericarditis from GPA has not been previously reported, likely due to a paucity of long-term follow-up data. In patients with steroid-dependent/steroid-refractory pericarditis with multiple recurrences, anakinra seems promising for symptom control and to prevent recurrences and avoid the long-term effects of glucocorticoids.
Footnotes
Conflicts of Interest: Kiniksa pharmaceuticals: research grant and scientific advisory board.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2020.11.008.
Supplementary Data
Transthoracic echocardiogram with circumferential pericardial effusion on parasternal short-axis view.
Transthoracic echocardiogram with circumferential pericardial effusion on apical four-chamber view.
Transthoracic echocardiogram with apical four-chamber view with a respirometer showing no ventricular interdependence.
On-treatment TTE videos of patient 1 showing no pericardial effusion or signs of pericardial constriction on parasternal short-axis view.
On-treatment TTE videos of patient 1 showing no pericardial effusion or signs of pericardial constriction on parasternal four-chamber view.
On-treatment TTE videos of patient 2 showing no pericardial effusion or signs of pericardial constriction on parasternal long-axis view.
On-treatment TTE videos of patient 2 showing no pericardial effusion or signs of pericardial constriction on parasternal short-axis view.
On-treatment TTE videos of patient 2 showing no pericardial effusion or signs of pericardial constriction on parasternal four-chamber view.
References
- 1.Schiavone W.A., Ahmad M., Ockner S.A. Unusual cardiac complications of Wegener’s granulomatosis. Chest. 1985;88:745–748. doi: 10.1378/chest.88.5.745. [DOI] [PubMed] [Google Scholar]
- 2.Meryhew N.L., Bache R.J., Messner R.P. Wegener’s granulomatosis with acute pericardial tamponade. Arthritis Rheum. 1988;31:300–302. doi: 10.1002/art.1780310224. [DOI] [PubMed] [Google Scholar]
- 3.Grant S.C.D., Levy R.D., Venning M.C., Ward C., Brooks N.H. Wegener’s granulomatosis and the heart. Br Heart J. 1994;71:82–86. doi: 10.1136/hrt.71.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yildizer K., Paydas S., Serin E., Sagliker Y. Wegener’s granulomatosis complicated by pericardial tamponade and renal failure. Nephron. 1996;72:339–340. doi: 10.1159/000188876. [DOI] [PubMed] [Google Scholar]
- 5.Florian A., Slavich M., Blockmans D., Dymarkowski S., Bogaert J. Cardiac involvement in granulomatosis with polyangiitis (Wegener granulomatosis) Circulation. 2011;124:e342–e344. doi: 10.1161/CIRCULATIONAHA.111.030809. [DOI] [PubMed] [Google Scholar]
- 6.Somaily M., Al Arfaj A.S. Wegner’s granulomatosis with very unusual presentation. Intl J Case Rep Images. 2012;12:47–51. [Google Scholar]
- 7.Horne A.E., Henriksen P.A., Amft E.N. Granulomatosis with polyangiitis and constrictive pericarditis—a case report. J R Coll Physicians Edinb. 2014;44:283–285. doi: 10.4997/JRCPE.2014.406. [DOI] [PubMed] [Google Scholar]
- 8.Dewan R., Bittar H.E.T., Lacomis J., Ocak I. Granulomatosis with polyangiitis presenting with coronary artery and pericardial involvement. Case Rep Radiol. 2015:516437. doi: 10.1155/2015/516437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyawaki M., Oda S., Hirata K., Yuki H., Utsunomiya D., Hayashi H. Granulomatosis with polyangiitis can cause periaotitis and pericarditis. Clinic Case Rep. 2017;5:1732–1733. doi: 10.1002/ccr3.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parmar M.K., Alikhan M., Hsu V.M., Borham A. Echocardiogram: the GPS to GPA’s heart (granulomatosis with polyangiitis) Case Rep Rheumatol. 2019:7609386. doi: 10.1155/2019/7609386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliveria G.H., Sweard J.B., Tsang T.S., Specks U. Echocardiographic findings in patients with Wegener granulomatosis. Mayo Clin Proc. 2005;80:1435–1440. doi: 10.4065/80.11.1435. [DOI] [PubMed] [Google Scholar]
- 12.Życińska K., Borowiec A., Zielonka T.M., Rusinowicz T., Hadzik-Błaszczyk M., Cieplak M. Echocardiographic assessment in patients with granulomatosis with polyangiitis. Adv Exp Med Biol. 2017;1022:27–33. doi: 10.1007/5584_2017_43. [DOI] [PubMed] [Google Scholar]
- 13.Imazo M. Pericardial involvement in systemic inflammatory disease. Heart. 2011;97:1882–1892. doi: 10.1136/heartjnl-2011-300054. [DOI] [PubMed] [Google Scholar]
- 14.Cremer P.C., Kumar A., Kontzias A., Tan C.D., Rodriguez R., Imazo M. Complicated pericarditis: understanding risk factors and pathophysiology to inform imaging and treatment. J Am Coll Cardiol. 2016;68:2311–2328. doi: 10.1016/j.jacc.2016.07.785. [DOI] [PubMed] [Google Scholar]
- 15.Imazio M., Andreis A., De Ferrari G.M.D., Cremer P.C., Mardigyan V., Maestroni S. Anakinra for corticosteroid-dependent and colchicine-resistant pericarditis: the IRAP (International Registry of Anakinra for Pericarditis) Study. Eur J Prev Cardiol. 2020;27:956–964. doi: 10.1177/2047487319879534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic echocardiogram with circumferential pericardial effusion on parasternal short-axis view.
Transthoracic echocardiogram with circumferential pericardial effusion on apical four-chamber view.
Transthoracic echocardiogram with apical four-chamber view with a respirometer showing no ventricular interdependence.
On-treatment TTE videos of patient 1 showing no pericardial effusion or signs of pericardial constriction on parasternal short-axis view.
On-treatment TTE videos of patient 1 showing no pericardial effusion or signs of pericardial constriction on parasternal four-chamber view.
On-treatment TTE videos of patient 2 showing no pericardial effusion or signs of pericardial constriction on parasternal long-axis view.
On-treatment TTE videos of patient 2 showing no pericardial effusion or signs of pericardial constriction on parasternal short-axis view.
On-treatment TTE videos of patient 2 showing no pericardial effusion or signs of pericardial constriction on parasternal four-chamber view.