Abstract
Honey is a powerful antimicrobial agent with a wide range of effects. Various components contribute to the antibacterial efficacy of honey: the sugar content; polyphenol compounds; hydrogen peroxide; 1,2-dicarbonyl compounds; and bee defensin-1. All of these elements are present at different concentrations depending on the source of nectar, bee type, and storage. These components work synergistically, allowing honey to be potent against a variety of microorganisms including multidrug resistant bacteria and modulate their resistance to antimicrobial agents. The effectiveness and potency of honey against microorganisms depends on the type of honey produced, which is contingent on its botanical origin, the health of the bee, its origin, and processing method. The application of antibiotics with honey yielded better antimicrobial potential and synergistic effects were noted against biofilms. In medicine, honey has been used in the treatment of surface wounds, burns, and inflammation, and has a synergistic effect when applied with antibiotics. Tissue repair is enhanced by the low pH of honey (3.5–4): causing a reduction in protease activity on the wound site, elevating oxygen release from hemoglobin and stimulating fibroblast and macrophage activity. Furthermore, H2O2 has antiseptic effects, and it disinfects the wound site and stimulates production of vascular endothelial growth factor. The use of honey will clean wounds or burn areas from free radicals and reduces scarring and contractures. The anti-inflammatory and antibacterial potential of honey will keep the injured area moist and as such prevents it from deterioration and fibrosis. Honey can promote fast healing and reduce scarring and is very convenient for plastic surgery. Skin maceration is protected by honey due to its high osmolarity and because it keeps the injury moist. In non-infected areas, honey still reduced pain and inflammation. In general, the use of honey in medical settings has reduced economic loss and provided proven economic benefits by lowering direct costs in comparison to conventional treatments and by using less antibiotics, faster healing and less hospitalization stay. This review is intended to provide an overview of the antibacterial activities of honey and its applications.
Keywords: Honey, Antibacterial, Antibiotics, Synergy, Wounds and burns
Abbreviations: Def-1, defensin-1; H2O2, hydrogen peroxide; MDR, multidrug resistant/resistance; MRSA, methicillin-resistant Staphylococcus aureus; TP, total phenolic
1. Introduction
Honeybees make honey after feeding on flower nectar, blossoms, or by sucking on the flower’s secretions. The collected substances are mixed together with other specific compounds from the honeybees and are then deposited by the honeybees in the wax honeycomb and allowed to mature over time. The composition of honey depends on the source of the plants that the bees feed on (Eteraf-Oskouei and Najafi, 2013, Schneider et al., 2013). However, honey of all origins is composed mainly of the sugars glucose sucrose, and fructose, which constitute ∼80% of its weight, with water composing the remaining 20%. In addition, vitamins, flavonoids, amino acids, enzymes, minerals, and phenolic acids are also present in honey. Honey has anti-inflammatory (Tonks et al. 2003), healing (Bergman et al. 1983), antioxidant (Jaganathan et al. 2010), and antineoplastic effects (Swellam et al. 2003). As a traditional remedy since ancient times, honey has been used in the treatment of bacterial infections, colds, and cough, and various infectious diseases. The production of hydrogen peroxide (H2O2), bee defensin-1, high osmolarity, and low pH seem to be important for honey’s antibacterial efficacy. It has been reported that other phytochemicals, especially phenolic compounds, are essential elements that provide honey with its antibacterial potency. Both Gram-positive and Gram-negative pathogenic bacteria are susceptible to honey, including methicillin-resistant Staphylococcus aureus (MRSA) (Jenkins et al. 2014), Shigella sonnei (Lusby et al. 2005), Helicobacter pylori (Manyi-Loh et al. 2010), and yeasts like Candida albicans (Irish et al. 2006).
The effectiveness and potency of honey against microorganisms depends on the type of the honey produced, which is contingent on its botanical origin, the health of the bee, its origin, and processing method. The antioxidant activity of honey is very much dependent on the botanical origin where the bee was reared (Chauhan et al. 2010). For example, Manuka honey has had major importance due to its broad antibacterial efficacy. Manuka honey is derived from Leptosperm sp. (Cokcetin et al. 2016), originating in New Zealand. This unifloral honey has been used in the pharmaceutical industry for the treatment of a variety of diseases and has been reverted to medical-grade honey. The antibacterial activity of honey is attributed to its osmolarity, H2O2 content, low pH, phenolic acids levels, and flavonoids. Phytochemical factors, such as tetracycline, fatty acids, peroxides, ascorbic acid, amylase, terpenes, phenols, benzoic acid and benzyl alcohols, are factors that make honey active against pathogenic bacteria and produce either bacteriostatic or bactericidal efficacy (Shears 2000). In general, no organisms have thus far developed resistance to honey. The purpose of this review is to provide an overview of the antibacterial activity of honey, the underlying factors that influence its antibacterial efficacy, and its applications.
2. Parameters related to the antimicrobial activity of honey
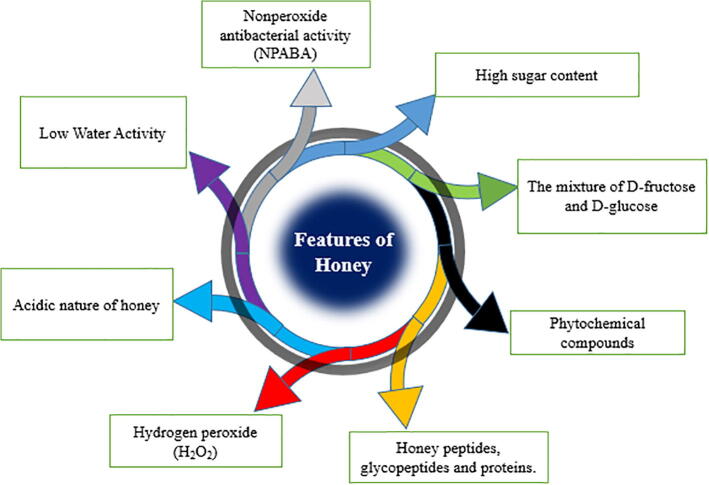
Various parameters affect the antibacterial potential of honey including its low water content (low water activity), high viscosity, acidity, and H2O2 content. In addition, various compounds are associated with honey and provide its antibacterial potential, including phytochemicals, peptides, nonperoxidase glycopeptides, and proteins that are notable features of honey and are associated with its antimicrobial effects (Fig. 1) (Molan 1992).
Fig. 1.
Schematic diagram showing the parameters that contribute to the antimicrobial potential of honey.
2.1. Low water activity
Water is a major constituent of living creatures and is found in various foods in the form of free or bound molecules. In honey, water activity measures unbound water molecules and ranges from 0.562 to 0.62, a concentration low enough to allow the growth of bacteria or other microorganisms (Molan 1992).
2.2. High sugar content
Osmosis is induced as a result of the high sugar concentration in honey. Pure, undiluted honey therefore inhibits the growth of bacteria due to its sugar content, which exerts osmotic pressure on bacterial cells, causing water to flow out of the bacterial cells via osmosis. As a result, the cells shrink due to dehydration and are unable to survive in the hypertonic sugar solution. Such antibacterial potential will be lower when honey is mixed with bodily fluids at infection sites (Molan 1992).
2.3. Acidity
The optimal growth of most microorganisms occurs at neutral pH, ranging from 6.5 to 7.5. The acidity of honey, between pH 3.2 and pH 4.5, is a very marked characteristic of its antibacterial efficacy. This acidity is caused by the presence of certain important organic acids, especially gluconic acid, found at a concentration of at ∼0.5% (w/v). Glycogenic acid is generated from glucose oxidation by an endogenous glucose oxidase enzyme and is an extremely potent antibacterial agent. In undiluted pure honey, the low pH may contribute to its antibacterial potency, however, pH alone is not sufficient to inhibit the growth of many types of bacteria when diluted in food or in other bodily liquids (Molan 1992).
2.4. Hydrogen peroxide
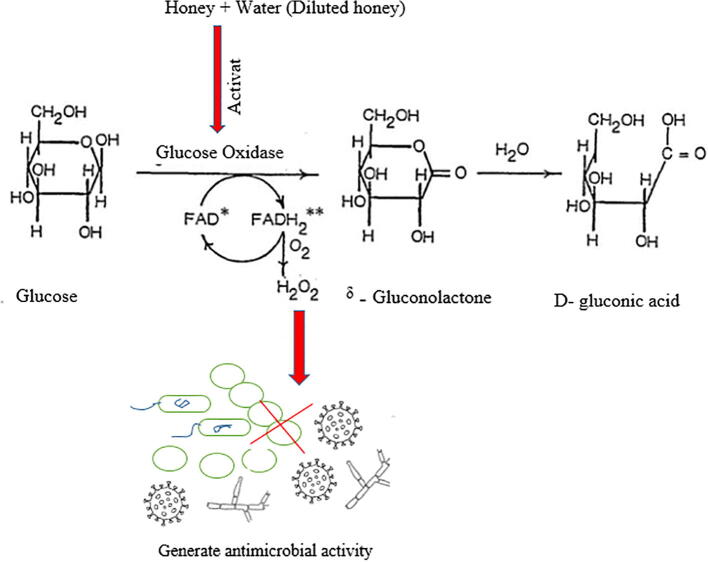
Hydrogen peroxide (H2O2) is a disinfectant and a strong oxidizing agent (Ali 2004). It provides honey with its antibacterial efficacy and is produced enzymatically (Fig. 2). The enzyme glucose oxidase is naturally present in an inactive state in honey due to the low pH conditions. When honey is diluted, glucose oxidase is activated and acts on endogenous glucose to produce H2O2. In fact, the maximum level of hydrogen peroxide can be achieved by diluting honey by 30%–50%, typically in the range of 5 to 100 μg H2O2/g honey (equivalent to ∼0.146–2.93 mM). A linear association has been reported between the H2O2 content of honey and its antibacterial potential (Brudzynski 2006).
Fig. 2.
Glucose oxidase catalyzes the generation of hydrogen peroxide (H2O2).
2.5. Proteins
To date, there have been very few published papers on the protein content of honey (Chua et al. 2013). Honey has relatively tiny amounts of proteins, ranging from ∼ 0.1%–0.5%, with molecular weights ranging from 20 to 80 kDa (Tewari and Irudayaraj 2004). Those proteins include many enzymes involved in sugar metabolism, such as alpha and beta glucosidase, glucose oxidase, and amylase (Won et al., 2008). Numerous published studies have shown that important major royal jelly proteins have antimicrobial and anticancer activities and anti-inflammatory potential (Tonks et al. 2003). The antibacterial peptide defensin-1 (Def-1) obtained from bees, is the main ingredient responsible for the antibacterial activity of honey, except for Manuka honey (Kwakman et al. 2011). Bee Def-1 works against Gram-positive bacteria. Studies with recombinant Def-1 revealed its potency against Gram-negative bacteria, including P. aeruginosa and Salmonella choleraesuis (Tseng et al. 2011).
2.6. Nonperoxide antibacterial compounds (Polyphenolic Compounds)
Polyphenols are plant secondary metabolites. They are a diverse group of chemicals, characterized by their phenolic structures and include flavonoids and phenolic acids (nonflavonoids [Table 1, Table 2]) (Cianciosi et al., 2018). These biological compounds are transmitted from nectar to honey and are important components of honey's health-promoting properties (Gunes et al. 2017). Phenolic compounds are found at high levels in honey and may contribute to its antibacterial activity. The antibacterial efficacy of both phenolic acid and flavonoids has been documented since the early 1990 s (Wahdan 1998).
Table 1.
Antimicrobial mechanism of action of common phenolic acids found in honey.
| Phenolic Acid | Structure | Mechanism | References | |
|---|---|---|---|---|
| 1 | Caffeic acid | 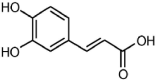 |
Oxidative stress | (Arakawa et al. 2004) |
| 2 | Chlorogenic acid | 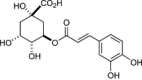 |
Cytoplasmic and nucleotide leakage as a consequence of higher membrane permeability | (Górniak et al. 2019) |
| 3 | Ferulic acid | 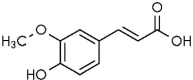 |
Malfunctioning of the cell membrane associated with morphological variations | (Verdrengh et al. 2004) |
| 4 | Gallic acid | 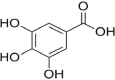 |
Intracellular leakage as a result of cell membrane disruption and increased pore formation | (Shi et al. 2016) |
| 5 | p-Coumaric acid | 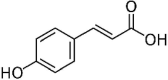 |
Disruption of cell membrane and binding to bacterial DNA | (Borges et al. 2013) |
| 6 | Syringic acid | 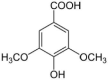 |
Cell membrane dysfunction | (Griep et al. 2007) |
Table 2.
Underlying antimicrobial mechanism of action of common flavonoids found in honey.
| Flavonoids | Structures | Mechanism | References | |
|---|---|---|---|---|
| 1 | Apigenin | 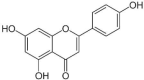 |
Inhibits DNA gyrase, chrysin, kaempferol | (Estevinho et al., 2008, Collins et al., 2019) |
| 2 | Catechin | 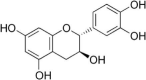 |
Hydrogen peroxide generation | (Das et al. 2015) |
| 3 | Galangin | 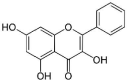 |
Inhibition of peptidoglycan and ribosome synthesis | (Lou et al. 2011) |
| 4 | Luteolin | 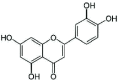 |
Inhibition of FAS-I in mycobacteria and inhibition of DNA helicases DnaB and RecBCD | (Collins et al. 2019) |
| 5 | Myricetin | 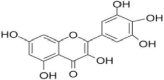 |
DNA B helicase inhibition | (Lou et al. 2012) |
| 6 | Pinocembrin | 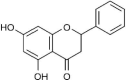 |
Induces cell lysis | (Collins et al. 2019) |
The amount of phenolic acid in honey is influenced by the geographic location and the botanical source of the nectar. In addition, it is clear that the total phenolic (TP) acid content is also significantly affected by the season. (Lachman et al. 2010) estimated the total polyphenol content of different varieties of honey harvested between May and August 2006 and found that the samples with the highest TP acid levels were collected in early June (average 170.21 mg/kg) and that TP acid levels were lower in samples collected in July (average 163.32 mg/kg). Samples collected in other months had even lower levels of TP acid (83.60 mg/kg). The type of honey also affects its phenol content. In the study by (Lachman et al. 2010), the TP acid content was very low, 82.5 to 242.5 mg/kg honey, and the main phenols were flavonoids and phenolic acids. Manuka honey has a phenolic acid content of 430 to 2,706 mg/kg compared with Kanuka honey (424 to 1,575 mg/kg) collected from the same place at the same time (Stephens et al. 2010).
Other types of honey, like viper's bugloss and heather honey, had much lower phenolic acid contents, ranging between 132.17 ± 0.05 and 727.77 ± 0.23 mg/kg (Ferreira et al. 2009). (Biesaga and Pyrzynska 2009) reported trace amounts of phenolic compounds in all types of honey investigated. However, they also reported varying concentrations of the following acids: myricetin; caffeic acid; chlorogenic acid; syringic acid; apigenin; and vanillic acid, among others.
2.6.1. Antibacterial effect of honey
The antibacterial efficacy of honey was initially recognized in 1892; however, in modern medicine, it is used only to a limited extent due to the absence of scientific support (Mohapatra et al. 2011).
Honey exhibits antibacterial activity against numerous bacteria in different environments. The natural components of honey have various activities against different microorganisms (Fig. 3). The antibacterial activity of honey is likely to depend on the pasture on which the bees were raised, climatic conditions, as well as the natural composition of the flower nectar (Abd-El Aal et al. 2007).
Fig. 3.
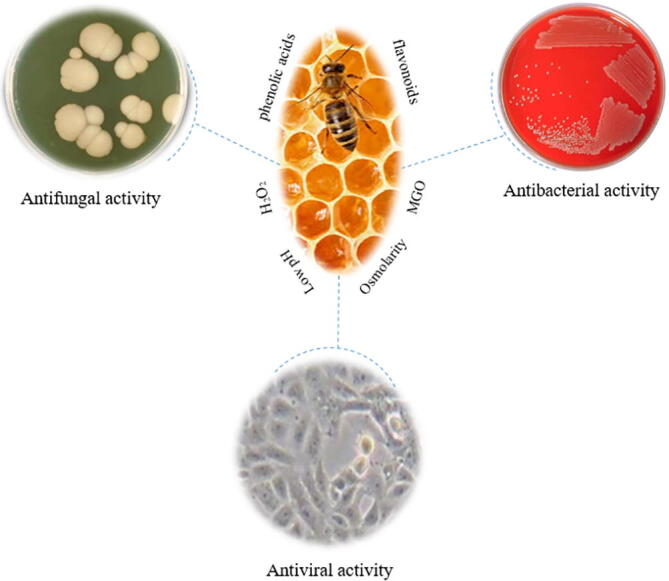
Schematic diagram showing the range of antimicrobial activities of honey.
Honey has excellent antibacterial efficacy against MRSA and a variety of Pseudomonas, which are often associated with wound and burn infections (Hazrati et al. 2010).
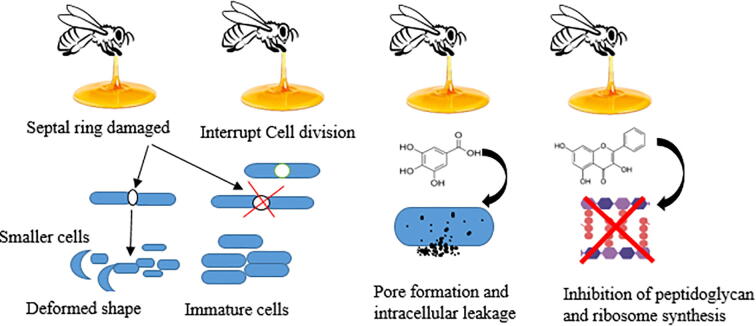
Manuka (L. scoparium) honey (Visavadia et al. 2008) showed efficacy against many pathogenic microorganisms, including: Enterobacter erogen, S. aureus, Salmonella zyphimurium, and last, but not least, Escherichia coli (E. coli) (Lusby et al. 2005). Many studies revealed that honey was effective against MRSA, haemolytic streptococci, and vancomycin-resistant enterococci (Fig. 4) (Lusby et al. 2005) . However, newly recognized local honey types may be as good as or even better than Manuka honey based on their impressive antimicrobial activity.
Fig. 4.
Schematic representation of the antimicrobial effects of honey.
Unlike Manuka honey, the activity of Almo honey is mainly due to its production of H2O2. A 25% solution (v/v) of Almo honey did not exhibit any antibacterial activity in the presence of the catalase enzyme, which scavenges H2O2 activity. This is in contrast to Manuka honey, which maintained its antibacterial potential under the same conditions (Sherlock et al. 2010). Sterilization by gamma irradiation does not interfere with Almo honey activity (Simon et al., 2009). The pH of honey is low enough to cause inhibition of certain bacterial pathogens, like S. pyogenes (4.5), E. coli (4.3), P. aeruginosa (4.4), and Salmonella spp. (4.0) and, as such therefore, acidic honey is an important antibacterial factor (Haniyeh et al. 2010).
Manuka honey can modulate bacterial size and shape, which affects the septal ring involved in cell division (Lu et al. 2013). (Henriques et al. 2010) used transmission electron microscopy to observe the effects of Manuka honey on S. aureus cultures and showed that more septal cells were found in the samples treated with Manuka honey in comparison to those treated with artificial honey made from sugars and water that mimic in its composition the natural honey. A study using phase-contrast imaging found that Bacillus subtilis and S. aureus bacteria treated with a sub-lethal dose of Manuka honey (4%, w/v) resulted in the appearance of smaller cells containing condensed chromosomes (Tonks et al. 2003). Interestingly, both (Henriques et al. 2010) and (Lu et al. 2013) showed that the stress caused by honey resulted in up-regulation of cell growth and affected bacterial cell division.
Studies by (Al-Nahari et al., 2015, Almasaudi et al., 2017) evaluated the efficacy of different types of Manuka honey (UMF 10, 16, and 20) in addition to two types of Saudi honey (Sidr and Nigella sativa) against Gram-positive methicillin resistant and sensitive S. aureus as well as Gram-negative imipenem-resistant and sensitive Pseudomonas aeruginosa. The results showed that all bacterial strains were completely inhibited at honey concentrations between 10% and 50%, depending on the strain and the efficacy of the type of honey used. Manuka UMF-20 was superior by far to all other types of honey tested and produced a bactericidal effect, whereas the Saudi honeys were bacteriostatic (Al-Nahari et al., 2015, Almasaudi et al., 2017).
The antibacterial potential of 21 types of honey collected from Mount Olympus, which is the highest mountain in Greece and known for its plant biodiversity, was tested against both S. aureus and P. aeruginosa. Antibacterial activity against S. aureus and P. aeruginosa was shown for all types of tested honey, possibly due to their H2O2 and total polyphenolic content levels. Better efficacy of free radical scavenging was seen in those honey types in comparison to Manuka honey. Moreover, antioxidant activity was noted with four types of the tested honey that were converted to powder by freeze drying. The data showed that three of the powdered honeys retained their antioxidant properties after freeze drying, which makes them suitable for further bioactive evaluation and applications (Stagos et al. 2018).
The antibacterial efficacy of honey may be attributed to its to its osmotic effect, high sugar content, and low moisture content, as well as to the presence of gluconic acid, which produces the antiseptic H2O2 (O'Grady et al. 1999). Based on an in vitro study, the antibacterial properties of honey were associated with H2O2, methylglyoxal (MGO), and the antimicrobial bee peptide defensin-1. Each of these components exhibits different mechanisms of action (Khan et al. 2007).
2.6.2. Antibiotic synergism with honey
Multidrug-resistant (MDR) bacteria are a global problem, and infections by resistant bacteria are on the rise in various animal species, including humans (Szmolka and Nagy 2013). For this reason, there is a major effort to find alternative therapies with better efficacy and to reduce antibiotic use and combat antibiotic resistance. Alternative therapies may be particularly useful in mild infections. The antibacterial properties of honey have long been known, and honey exhibits broad activity against MDR bacteria. For this reason, many studies have been carried out to evaluate the efficacy of the application of honey with antimicrobial agents, with some positive outcomes.
The application of tetracycline with Manuka honey yielded better antimicrobial potential against S. aureus and P. aeruginosa than was observed with either treatment alone. This finding suggests that such a combination is a possible treatment strategy for wound healing (Jenkins and Cooper 2012). In a different study, the combination of rifampicin with subinhibitory concentrations of Medihoney reversed rifampicin resistance in clinical isolates of S. aureus, including MRSA (Müller et al. 2013). Other data also support that the application of honey together with antibiotics can modulate antibiotic resistance. For instance, (Jenkins and Cooper 2012) reported that MRSA became susceptible to oxacillin upon the application of subinhibitory concentrations of honey.
The emergence of bacterial resistance to honey is very low because of variations in its composition due to differences in: 1) nectar source; 2) climatic conditions; 3) duration of storage; and 4) preservation conditions (Al-Waili and Boni, 2003, Sherlock et al., 2010).
Moreover, synergistic effects were noted against biofilms using combinations of honey and antibiotics. This was shown for the use of Manuka honey with vancomycin against S. aureus and for the combination of Manuka honey with gentamicin against P. aeruginosa (Campeau and Patel 2014). Additionally, a reported synergism between Portuguese honey and phage therapy showed that 25% (w/v) honey caused synergism with phage and was also efficient in the eradication of E. coli biofilms at a 50% (w/v) honey alone (Oliveira et al. 2017).
Bacterial biofilms of group A Streptococcus pyogenes, Streptococcus mutans, Proteus mirabilis, P. aeruginosa, Enterobacter cloacae, and S. aureus were removed by Manuka honey (Alandejani et al., 2008, Stephens et al., 2010, Maddocks et al., 2013, Majtan, 2014). (Merckoll et al. 2009) indicated that Norwegian honey eliminated biofilms due to its biocidal potential and was efficient in wound treatment.
These findings emphasize the potential application of honey with antibiotics to enhance their efficacy and reduce the possibility of MDR emerging. Modulation of MDR by honey is very promising and may be due to the complex composition of honey, which contains more than 180 different substances including: vitamins; minerals; amino acids; enzymes; organic acids; and other compounds that are efficacious against bacteria, either individually or in combination with each other or with other external agents in a synergistic manner to modulate MDR (Al-Nahari et al. 2015).
Glucose oxidase is an enzyme present in honey that converts glucose into gluconolactone and then to H2O2. This H2O2 is released in a slow and continuous manner to achieve a sustained antibacterial effect. This successfully eliminates microorganisms but dilutes them sufficiently so as not to damage the host tissue (Olaitan et al. 2007).
The acidity of honey (pH 3.2–4.5) inhibits the growth of many pathogenic organisms and enhances the wound healing process through epithelialization (Pieper 2009). Also, the high osmolarity of honey inhibits bacterial growth (Molan 1999).
Future clinical research is required to elucidate the efficacy of such combinations further, as well as to improve methods for application of honey to the affected areas.
2.6.3. Honey in medical settings
It has been shown that honey has very potent antibacterial activity for treatment of pathogens that infect wounds (Molan, 1999, Al-Waili, 2004). A comparative study in wound management against antibiotic-resistant plankton and biofilm-associated bacteria evaluated the antimicrobial activity of four types of honey obtained from three different floral and geographical locations: Melipona beecheii honey (Cuba) and three Apis mellifera honeys (Manuka honey [New Zealand], A. mellifera honey [Cuba], and African honey [Kenya]). The results indicated that M. beecheii honey stood out because of its acidity and produced the best antimicrobial and antibiofilm activity against Staphylococcus aureus and Pseudomonas aeruginosa cells that was associated with induced structural changes observed by transmission electron microscopy, making it a possible candidate for wound infection treatment (Morroni et al. 2018).
In medicine, honey is primarily used for the treatment of surface wounds and burns. There are two main types of medical honey with distinct active components and different mechanisms of action, Medihoney and Revamil. Medihoney is produced from Manuka honey, whereas Revamil honey is manufactured under standardized conditions in greenhouses (Molan 1992). Honey has been used to treat skin and wound lesions in both animals and humans (Carnwath et al., 2014, Di Ianni et al., 2015). Tissue repair is enhanced by the low pH of honey (3.5–4): causing a reduction in protease activity on the wound site, elevating oxygen release from hemoglobin and stimulating fibroblast and macrophage activity. Furthermore, H2O2 has antiseptic effects, and it disinfects the wound site and stimulates production of vascular endothelial growth factor (VEGF) (Molan and Rhodes, 2015, Minden-Birkenmaier and Bowlin, 2018). In addition to glucose oxidase, the enzyme invertase increases the strength of the osmotic potential of the honey and allows the sucrose to break down into fructose and glucose (Molan and Rhodes, 2015, Minden-Birkenmaier and Bowlin, 2018). Fluids are drawn out of the damaged tissues, leading to their drying and bacterial death (Molan and Rhodes 2015). Moreover, the phenolic compounds, organic acids, vitamins, and flavonoids in honey have antioxidant potential and boost its antimicrobial efficacy. Flavonoids eliminate the free radicals produced by H2O2 (Molan and Rhodes, 2015, Minden-Birkenmaier and Bowlin, 2018).
However, although there are many studies evaluating the efficacy of honey for wound healing, whether of traumatic or surgical origin, only few studies have dealt with infected wounds. Some investigations evaluated the potential of honey against certain infectious intestinal bacteria (Shin 2005) and pathogenic bacteria that frequently cause skin wound infections in both humans and animals (Basualdo et al. 2007).
Honey is widely used in the treatment of acute, chronic, traumatic, and post-surgical wounds (Ahmed et al. 2003), but also for ulcers, burns, eye diseases, skin diseases, oral mucosa problems, and necrotic areas (Molan and Rhodes 2015). Moreover, there were many instances of positive therapeutic responses to honey in patients unresponsive to traditional treatments (Schumacher 2004). In general, medical honey mainly acts as a hyperosmolar medium and presents an important physical barrier due to its viscosity.
It also exerts both bacteriostatic and/or bactericidal effects to reduce the possibility of wound infection (Ganz 2003). Moreover, it provides an osmotic gradient resulting from its high sugar content and low water activity, which causes a flow of bacteria, necrotic tissue, and debris out of the wound (Minden-Birkenmaier and Bowlin 2018). Lastly, the phenolic compounds in honey have anti-inflammatory potential and enhance the speed of wound healing and reduce scarring (Samarghandian et al. 2017). These advantages suggest that honey could result in less dependence on antibiotic use while promoting wound healing.
Furthermore, honey has immunomodulatory efficacy in wound healing and various components of honey contribute to its anti-inflammatory and antioxidant properties (Majtan 2014). Moreover, the high nutrient contents promote epithelialization and angiogenesis (Molan 2001). The carbohydrates in honey (80% of honey components), especially glucose and fructose, with maltose, sucrose, and isomaltose in smaller quantities, are considered an excellent source of nutrients for tissues (Carnwath et al., 2014, Minden-Birkenmaier and Bowlin, 2018).
Burns are associated with oxidative damage. At the site of the burn, there is an elevation in free radical activity, causing an increase in the levels of lipid peroxidation, responsible for scarring and contractures. In the treatment of burns, the use of honey will clean them from free radicals and reduces scarring and contractures (Nweze et al. 2019). As with wounds, the anti-inflammatory and antibacterial potential of honey will keep the burned area moist and such prevents it from deterioration and fibrosis. Honey can promote fast healing and reduce scarring and is very convenient for plastic surgery. Skin maceration is protected by honey due to its high osmolarity and because it keeps the wound moist. In some experimentally non-infected burns, honey still reduced pain and inflammation (Zbuchea 2014). The results of prospective randomized controlled clinical trials indicated that the use of honey for the treatment of superficial and partial-thickness burns revealed significantly faster healing than that achieved using silver sulphadiazine, amniotic membrane, polyurethane film, or potato peel (Subrahmanyam, 2007, Mashhood et al., 2017). These findings were also corroborated in a similar study, whereby honey healed partial thickness burns more rapidly than conventional treatment (Jull et al., 2015). The potentiation of the efficacy of honey when used as an adjuvant for accelerating wound healing in ulcers, infected wounds, and burns (Subrahmanyam 2007) and antioxidants (vitamins C, E, and A), has also been reported (Schencke et al. 2016). In general, the use of honey in medical settings has reduced economic loss and provided proven economic benefits by lowering direct costs in comparison to conventional treatments and by using less antibiotics, faster healing and less hospitalization stay.
2.7. A case study of two patients treated for wound healing
In the study, one patient suffered from a persistent self-inflicted wound and treated it with Manuka honey daily. The wound completely healed after six weeks, proving the potential of honey to promote re-epithelization and angiogenesis. The second patient had two large hematomas infected with P. aeruginosa and S. aureus (MRSA). After the patient failed to respond to conventional treatment, Manuka honey was applied for eight weeks, which promoted healing of the infection and the hematomas. After eight weeks, the infection was cleared (Dunford et al. 2000). In another study, the use of honey helped increase skin graft healing and reduced pain, as compared with the use of Vaseline as a control (Subrahmanyam 2015). Honey has also been effective in treatment of wounds. The use of honey and 1% silver sulfadiazine decreased the healing time by reducing burn infection and pain faster than 1% silver sulfadiazine alone (Mashhood et al. 2017). Based on these studies, the use of honey in a clinical setting should be considered in the case of burn and wound treatment in order to eliminate infections and consequently reduce the healing period and ease the patient’s pain and discomfort.
3. Conclusion
Honey has been known for its antimicrobial potential, showing a broad spectrum of potential against microorganisms including bacteria. Many important factors contribute to its antimicrobial efficacy, including osmolarity, H2O2 content, low pH, phenolic acid levels, and flavonoids. Other phytochemical factors, such as tetracycline, peroxides, amylase, fatty acids, phenols, ascorbic acid, terpenes, benzyl alcohols, and benzoic acid, make honey active against pathogenic bacteria and produce either bacteriostatic or bactericidal efficacy. All of these factors vary according to the nectar source and storage conditions. The emergence of bacterial resistance to honey is very low because of the variations in composition of various kinds of honey due to: 1) nectar source; 2) climatic conditions; 3) duration of storage; and 4) preservation conditions. Synergism was noted when using honey with antibiotics. Such findings will emphasize the potential application of honey with antibiotics and enhance their efficacy and reduce possible emerging MDR. The modulation of MDR by honey is very promising and may be due to the complex composition of honey, which contains more than 180 different substances including vitamins, minerals, amino acids, enzymes, organic acids, and other compounds that exert their antibacterial effects either individually, in combination with each other, or with other external agents in a synergistic manner to modulate MDR. A detailed analysis of the chemical composition of honey may enable us to estimate the right concentrations of these synergistic components and lead to the development of the most effective, broad-spectrum honey with activity against a variety of MDR bacteria.
Honey is widely used in the treatment of acute, chronic, traumatic, and post-surgical wounds, as well as for ulcers, burns, eye diseases, skin diseases, oral mucosa problems, and necrotic areas.
As such, honey can be a possible alternative antibacterial agent with promising therapeutic potential in the medical setting. The precise chemical composition of honey should be deciphered in order to be able to produce synthetic honey that can be used for treatment of medical conditions. The need for an improved delivery method is important to enhance its efficacy and its potential incorporation into scaffolds to provide an innovative treatment should not be overlooked.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd-El Aal A.M., El-Hadidy M.R., El-Mashad N.B., El-Sebaie A.H. Antimicrobial effect of bee honey in comparison to antibiotics on organisms isolated from infected burns. Ann Burns Fire Disasters. 2007;20(2):83–88. [PMC free article] [PubMed] [Google Scholar]
- Ahmed A.K., Hoekstra M.J., Hage J.J., Karim R.B. Honey-medicated dressing: transformation of an ancient remedy into modern therapy. Ann Plast Surg. 2003;50(2) doi: 10.1097/01.SAP.0000032306.44107.C1. 143-147; discussion 147-148. [DOI] [PubMed] [Google Scholar]
- Al-Nahari A.A.M., Almasaudi S.B., Abd El-Ghany E.S.M., Barbour E., Al Jaouni S.K., Harakeh S. Antimicrobial activities of Saudi honey against Pseudomonas aeruginosa. Saudi Journal of Biological Sciences. 2015;22(5):521–525. doi: 10.1016/j.sjbs.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Waili N.S. Investigating the Antimicrobial Activity of Natural Honey and Its Effects on the Pathogenic Bacterial Infections of Surgical Wounds and Conjunctiva. Journal of Medicinal Food. 2004;7(2):210–222. doi: 10.1089/1096620041224139. [DOI] [PubMed] [Google Scholar]
- Al-Waili N.S., Boni N.S. Natural Honey Lowers Plasma Prostaglandin Concentrations in Normal Individuals. Journal of Medicinal Food. 2003;6(2):129–133. doi: 10.1089/109662003322233530. [DOI] [PubMed] [Google Scholar]
- Alandejani T., Marsan J.G., Ferris W., Robert S., Chan F. Effectiveness of honey on S. aureus and P. aeruginosa biofilms. Otolaryngology-Head and Neck Surgery. 2008;139(2_supp;):107. doi: 10.1016/j.otohns.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Ali M. Hydrogen peroxide therapies: recent insights into oxystatic and antimicrobial actions. Townsend Letter for Doctors and Patients. 2004;255:140–144. [Google Scholar]
- Almasaudi S.B., Al-Nahari A.A.M., Abd El-Ghany E.S.M., Barbour E., Al Muhayawi S.M., Al-Jaouni S., Azhar E., Qari M., Qari Y.A., Harakeh S. Antimicrobial effect of different types of honey on. Saudi J Biol Sci. 2017;24(6):1255–1261. doi: 10.1016/j.sjbs.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H., Maeda M., Okubo S., Shimamura T. Role of Hydrogen Peroxide in Bactericidal Action of Catechin. Biol. Pharm. Bull. 2004;27(3):277–281. doi: 10.1248/bpb.27.277. [DOI] [PubMed] [Google Scholar]
- Basualdo C., Sgroy V., Finola M.S., Marioli J.M. Comparison of the antibacterial activity of honey from different provenance against bacteria usually isolated from skin wounds. Vet Microbiol. 2007;124(3–4):375–381. doi: 10.1016/j.vetmic.2007.04.039. [DOI] [PubMed] [Google Scholar]
- Bergman A., Yanai J., Weiss J., Bell D., David M.P. Acceleration of wound healing by topical application of honey. The American Journal of Surgery. 1983;145(3):374–376. doi: 10.1016/0002-9610(83)90204-0. [DOI] [PubMed] [Google Scholar]
- Biesaga M., Pyrzynska K. Liquid chromatography/tandem mass spectrometry studies of the phenolic compounds in honey. Journal of Chromatography A. 2009;1216(38):6620–6626. doi: 10.1016/j.chroma.2009.07.066. [DOI] [PubMed] [Google Scholar]
- Borges A., Ferreira C., Saavedra M.J., Simões M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids Against Pathogenic Bacteria. Microbial Drug Resistance. 2013;19(4):256–265. doi: 10.1089/mdr.2012.0244. [DOI] [PubMed] [Google Scholar]
- Brudzynski K. Effect of hydrogen peroxide on antibacterial activities of Canadian honeys. Can. J. Microbiol. 2006;52(12):1228–1237. doi: 10.1139/w06-086. [DOI] [PubMed] [Google Scholar]
- Campeau M.E., Patel R. Antibiofilm Activity of Manuka Honey in Combination with Antibiotics. Int J Bacteriol. 2014;2014 doi: 10.1155/2014/795281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnwath R., Graham E.M., Reynolds K., Pollock P.J. The antimicrobial activity of honey against common equine wound bacterial isolates. The Veterinary Journal. 2014;199(1):110–114. doi: 10.1016/j.tvjl.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Chauhan A., Pandey V., Chacko K.M., Khandal R.K. Antibacterial activity of raw and processed honey. Electronic Journal of Biology. 2010;5(3):58–66. [Google Scholar]
- Chua L.S., Lee J.Y., Chan G.F. Honey protein extraction and determination by mass spectrometry. Anal Bioanal Chem. 2013;405(10):3063–3074. doi: 10.1007/s00216-012-6630-2. [DOI] [PubMed] [Google Scholar]
- Cianciosi D., Forbes-Hernández T., Afrin S., Gasparrini M., Reboredo-Rodriguez P., Manna P., Zhang J., Bravo Lamas L., Martínez Flórez S., Agudo Toyos P., Quiles J., Giampieri F., Battino M. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules. 2018;23(9):2322. doi: 10.3390/molecules23092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cokcetin N.N., Pappalardo M., Campbell L.T., Brooks P., Carter D.A., Blair S.E., Harry E.J. The Antibacterial Activity of Australian Leptospermum Honey Correlates with Methylglyoxal Levels. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0167780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins W., Lowen N., Blake D.J. Caffeic Acid Esters Are Effective Bactericidal Compounds Against. Biomolecules. 2019;9(8) doi: 10.3390/biom9080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Datta S., Mukherjee S., Bose S., Ghosh S., Dhar P. Evaluation of antioxidative, antibacterial and probiotic growth stimulatory activities of Sesamum indicum honey containing phenolic compounds and lignan. LWT-Food Science and Technology. 2015;61(1):244–250. [Google Scholar]
- Di Ianni F., Merli E., Burtini F., Conti V., Pelizzone I., Di Lecce R., Parmigiani E., Squassino G.P., Del Bue M., Lucarelli E., Ramoni R., Grolli S. Preparation and application of an innovative thrombocyte/leukocyte-enriched plasma to promote tissue repair in chelonians. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0122595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunford, C., R. Cooper, P. Molan and R. White (2000). “The use of honey in wound treatment.” Nursing Standard (through 2013) 15(11): 63. [DOI] [PubMed]
- Estevinho L., Pereira A.P., Moreira L., Dias L.G., Pereira E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food and Chemical Toxicology. 2008;46(12):3774–3779. doi: 10.1016/j.fct.2008.09.062. [DOI] [PubMed] [Google Scholar]
- Eteraf-Oskouei T., Najafi M. Traditional and modern uses of natural honey in human diseases: a review. Iran J Basic Med Sci. 2013;16(6):731–742. [PMC free article] [PubMed] [Google Scholar]
- Ferreira I.C.F.R., Aires E., Barreira J.C.M., Estevinho L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chemistry. 2009;114(4):1438–1443. [Google Scholar]
- Ganz T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- Górniak I., Bartoszewski R., Króliczewski J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem Rev. 2019;18(1):241–272. [Google Scholar]
- Griep M.A., Blood S., Larson M.A., Koepsell S.A., Hinrichs S.H. Myricetin inhibits Escherichia coli DnaB helicase but not primase. Bioorganic Med. Chem. 2007;15:7203–7208. doi: 10.1016/j.bmc.2007.07.057. [DOI] [PubMed] [Google Scholar]
- Gunes N., Aydın L., Belenli D., Hranitz J.M., Mengilig S., Selova S. Stress responses of honey bees to organic acid and essential oil treatments against varroa mites. Journal of Apicultural Research. 2017;56(2):175–181. [Google Scholar]
- Koochak H., Seyyednejad S.M., Motamedi H. Preliminary study on the antibacterial activity of some medicinal plants of Khuzestan (Iran) Asian Pacific Journal of Tropical Medicine. 2010;3(3):180–184. [Google Scholar]
- Hazrati M., Mehrabani D., Japoni A., Montasery H., Azarpira N., Hamidian-shirazi A.R., Tanideh N. Effect of Honey on Healing of Pseudomonas aeruginosa Infected Burn Wounds in Rat. Journal of Applied Animal Research. 2010;37(2):161–165. [Google Scholar]
- Henriques A.F., Jenkins R.E., Burton N.F., Cooper R.A. The intracellular effects of manuka honey on Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 2010;29(1):45–50. doi: 10.1007/s10096-009-0817-2. [DOI] [PubMed] [Google Scholar]
- Irish J., Carter D.A., Shokohi T., Blair S.E. Honey has an antifungal effect against Candida species. Med Mycol. 2006;44(3):289–291. doi: 10.1080/13693780500417037. [DOI] [PubMed] [Google Scholar]
- Jaganathan S.K., Mandal S.M., Jana S.K., Das S., Mandal M. Studies on the phenolic profiling, anti-oxidant and cytotoxic activity of Indian honey: in vitro evaluation. Natural Product Research. 2010;24(14):1295–1306. doi: 10.1080/14786410903184408. [DOI] [PubMed] [Google Scholar]
- Jenkins R., Burton N., Cooper R. Proteomic and genomic analysis of methicillin-resistant Staphylococcus aureus (MRSA) exposed to manuka honey in vitro demonstrated down-regulation of virulence markers. Journal of Antimicrobial Chemotherapy. 2014;69(3):603–615. doi: 10.1093/jac/dkt430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R.E., Cooper R. Synergy between oxacillin and manuka honey sensitizes methicillin-resistant Staphylococcus aureus to oxacillin. Journal of Antimicrobial Chemotherapy. 2012;67(6):1405–1407. doi: 10.1093/jac/dks071. [DOI] [PubMed] [Google Scholar]
- Jull A.B., Cullum N., Dumville J.C., Westby M.J., Deshpande S., Walker N. Honey as a topical treatment for wounds. Cochrane Database of Systematic Reviews. 2015;3 doi: 10.1002/14651858.CD005083.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, F. R., U. I. Abadin and N. Rauf. (2007). “Honey; Nutritional and medical Value.” Medscape Today, from http://www.medscape.com/viewartide/565913.
- Kwakman P.H., Te Velde A.A., de Boer L., Vandenbroucke-Grauls C.M., Zaat S.A. Two major medicinal honeys have different mechanisms of bactericidal activity. PLoS One. 2011;6(3) doi: 10.1371/journal.pone.0017709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman J., Orsák M., Hejtmánková A., Kovářová E. Evaluation of antioxidant activity and total phenolics of selected Czech honeys. LWT - Food Science and Technology. 2010;43(1):52–58. [Google Scholar]
- Lou Z., Wang H., Rao S., Sun J., Ma C., Li J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control. 2012;25(2):550–554. [Google Scholar]
- Lou Z., Wang H., Zhu S., Ma C., Wang Z. Antibacterial activity and mechanism of action of chlorogenic acid. J Food Sci. 2011;76(6):M398–M403. doi: 10.1111/j.1750-3841.2011.02213.x. [DOI] [PubMed] [Google Scholar]
- Lu J., Carter D.A., Turnbull L., Rosendale D., Hedderley D., Stephens J., Gannabathula S., Steinhorn G., Schlothauer R.C., Whitchurch C.B., Harry E.J. The effect of New Zealand kanuka, manuka and clover honeys on bacterial growth dynamics and cellular morphology varies according to the species. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0055898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusby P.E., Coombes A.L., Wilkinson J.M. Bactericidal Activity of Different Honeys against Pathogenic Bacteria. Archives of Medical Research. 2005;36(5):464–467. doi: 10.1016/j.arcmed.2005.03.038. [DOI] [PubMed] [Google Scholar]
- Maddocks S.E., Jenkins R.E., Rowlands R.S., Purdy K.J., Cooper R.A. Manuka honey inhibits adhesion and invasion of medically important wound bacteria in vitro. Future Microbiology. 2013;8(12):1523–1536. doi: 10.2217/fmb.13.126. [DOI] [PubMed] [Google Scholar]
- Majtan J. Honey: An immunomodulator in wound healing: Honey: an immunomodulator. Wound Repair Regen. 2014;22(2):187–192. doi: 10.1111/wrr.12117. [DOI] [PubMed] [Google Scholar]
- Manyi-Loh C.E., Clarke A.M., Munzhelele T., Green E., Mkwetshana N.F., Ndip R.N. Selected South African Honeys and Their Extracts Possess In Vitro Anti-Helicobacter pylori Activity. Archives of Medical Research. 2010;41(5):324–331. doi: 10.1016/j.arcmed.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Mashhood A.A., Khan T.A., Sami A.N. Honey compared with 1% silver sulfadiazine cream in the treatment of superficial and partial thickness burns. Journal of Pakistan Association of Dermatology. 2017;16(1):14–19. [Google Scholar]
- Merckoll P., Jonassen T.Ø., Vad M.E., Jeansson S.L., Melby K.K. Bacteria, biofilm and honey: A study of the effects of honey on ‘planktonic’ and biofilm-embedded chronic wound bacteria. Scandinavian Journal of Infectious Diseases. 2009;41(5):341–347. doi: 10.1080/00365540902849383. [DOI] [PubMed] [Google Scholar]
- Minden-Birkenmaier B., Bowlin G. Honey-Based Templates in Wound Healing and Tissue Engineering. Bioengineering. 2018;5(2):46. doi: 10.3390/bioengineering5020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra D.P., Thakur V., Brar S.K. Antibacterial efficacy of raw and processed honey. Biotechnol Res Int. 2011;2011 doi: 10.4061/2011/917505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molan P., Rhodes T. Honey: A Biologic Wound Dressing. Wounds. 2015;27(6):141–151. [PubMed] [Google Scholar]
- Molan P.C. The Antibacterial Activity of Honey: 1. The nature of the antibacterial activity. Bee World. 1992;73(1):5–28. [Google Scholar]
- Molan P.C. The role of honey in the management of wounds. Journal of Wound Care. 1999;8(8):415–418. doi: 10.12968/jowc.1999.8.8.25904. [DOI] [PubMed] [Google Scholar]
- Molan P.C. Potential of Honey in the Treatment of Wounds and Burns. American Journal of Clinical Dermatology. 2001;2(1):13–19. doi: 10.2165/00128071-200102010-00003. [DOI] [PubMed] [Google Scholar]
- Morroni G., Alvarez-Suarez J.M., Brenciani A., Simoni S., Fioriti S., Pugnaloni A., Giampieri F., Mazzoni L., Gasparrini M., Marini E., Mingoia M., Battino M., Giovanetti E. Comparison of the Antimicrobial Activities of Four Honeys From Three Countries (New Zealand, Cuba, and Kenya) Front Microbiol. 2018;9:1378. doi: 10.3389/fmicb.2018.01378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P., Alber D.G., Turnbull L., Schlothauer R.C., Carter D.A., Whitchurch C.B., Harry E.J. Synergism between Medihoney and rifampicin against methicillin-resistant Staphylococcus aureus (MRSA) PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0057679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nweze, J. A., C. V. Olovo, I. E. Nweze, O. O. John and C. Paul (2019). Therapeutic Properties of Honey. Honey Analysis, IntechOpen.
- O'Grady N.P., Tropea M., Preas H.L., 2nd, Reda D., Vandivier R.W., Banks S.M., Suffredini A.F. Detection of macrophage inflammatory protein (MIP)-1alpha and MIP-1beta during experimental endotoxemia and human sepsis. J Infect Dis. 1999;179(1):136–141. doi: 10.1086/314559. [DOI] [PubMed] [Google Scholar]
- Olaitan P.B., Adeleke O.E., Ola I.O. Honey: a reservoir for microorganisms and an inhibitory agent for microbes. Afr Health Sci. 2007;7(3):159–165. doi: 10.5555/afhs.2007.7.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira A., Ribeiro H.G., Silva A.C., Silva M.D., Sousa J.C., Rodrigues C.F., Melo L.D.R., Henriques A.F., Sillankorva S. Synergistic Antimicrobial Interaction between Honey and Phage against. Front Microbiol. 2017;8:2407. doi: 10.3389/fmicb.2017.02407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper B. Honey-based dressings and wound care: an option for care in the United States. J Wound Ostomy Continence Nurs. 2009;36(1) doi: 10.1097/01.WON.0000345177.58740.7d. 60-66 quiz 67-68. [DOI] [PubMed] [Google Scholar]
- Samarghandian S., Farkhondeh T., Samini F. Honey and Health: A Review of Recent Clinical Research. Pharmacognosy Res. 2017;9(2):121–127. doi: 10.4103/0974-8490.204647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schencke C., Vasconcellos A., Sandoval C., Torres P., Acevedo F., Del Sol M. Morphometric evaluation of wound healing in burns treated with Ulmo (Eucryphia cordifolia) honey alone and supplemented with ascorbic acid in guinea pig (Cavia porcellus) Burns & trauma. 2016;4:1–9. doi: 10.1186/s41038-016-0050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M., Coyle S., Warnock M., Gow I., Fyfe L. Anti-Microbial Activity and Composition of Manuka and Portobello Honey: anti-microbial activity and composition of Manuka and Portobello honey. Phytother. Res. 2013;27(8):1162–1168. doi: 10.1002/ptr.4844. [DOI] [PubMed] [Google Scholar]
- Schumacher H.H.A. Use of medical honey in patients with chronic venous leg ulcers after split-skin grafting. Journal of Wound Care. 2004;13(10):451–452. doi: 10.12968/jowc.2004.13.10.26693. [DOI] [PubMed] [Google Scholar]
- Shears P. Antimicrobial Resistance in the Tropics. Trop Doct. 2000;30(2):114–116. doi: 10.1177/004947550003000225. [DOI] [PubMed] [Google Scholar]
- Sherlock O., Dolan A., Athman R., Power A., Gethin G., Cowman S., Humphreys H. Comparison of the antimicrobial activity of Ulmo honey from Chile and Manuka honey against methicillin-resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. BMC Complement Altern Med. 2010;10:47. doi: 10.1186/1472-6882-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., Zhang X., Sun Y.i., Yang M., Song K., Zheng Z., Chen Y., Liu X., Jia Z., Dong R., Cui L.u., Xia X. Antimicrobial Activity of Ferulic Acid Against Cronobacter sakazakii and Possible Mechanism of Action. Foodborne Pathogens and Disease. 2016;13(4):196–204. doi: 10.1089/fpd.2015.1992. [DOI] [PubMed] [Google Scholar]
- Shin H.S.U.Z. Carbohydrate composition of honey from different floral sources and their influence on growth of selected intestinal bacteria. Food Research international. 2005;38(6):721–728. [Google Scholar]
- Simon Arne, Traynor Kirsten, Santos Kai, Blaser Gisela, Bode Udo, Molan Peter. Medical Honey for Wound Care—Still the ‘Latest Resort’? Evidence-Based Complementary and Alternative Medicine. 2009;6(2):165–173. doi: 10.1093/ecam/nem175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagos D., Soulitsiotis N., Tsadila C., Papaeconomou S., Arvanitis C., Ntontos A., Karkanta F., Adamou-Androulaki S., Petrotos K., Spandidos D.A., Kouretas D., Mossialos D. Antibacterial and antioxidant activity of different types of honey derived from Mount Olympus in Greece. Int J Mol Med. 2018;42(2):726–734. doi: 10.3892/ijmm.2018.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens Jonathan M., Schlothauer Ralf C., Morris Bruce D., Yang Derek, Fearnley Liam, Greenwood David R., Loomes Kerry M. Phenolic compounds and methylglyoxal in some New Zealand manuka and kanuka honeys. Food Chemistry. 2010;120(1):78–86. [Google Scholar]
- Subrahmanyam M. Topical application of honey for burn wound treatment-an overview. Annals of burns and fire disasters. 2007;20(3):137. [PMC free article] [PubMed] [Google Scholar]
- Subrahmanyam M. Honey Dressing Accelerates Split-Thickness Skin Graft Donor Site Healing. Indian J Surg. 2015;77(Suppl 2):261–263. doi: 10.1007/s12262-012-0789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWELLAM TAREK, MIYANAGA NAOTO, ONOZAWA MIZUKI, HATTORI KAZUNORI, KAWAI KOJI, SHIMAZUI TORU, AKAZA HIDEYUKI. Antineoplastic activity of honey in an experimental bladder cancer implantation model: In vivo and in vitro studies. Int J Urol. 2003;10(4):213–219. doi: 10.1046/j.0919-8172.2003.00602.x. [DOI] [PubMed] [Google Scholar]
- Szmolka A., Nagy B. Multidrug resistant commensal Escherichia coli in animals and its impact for public health. Front Microbiol. 2013;4:258. doi: 10.3389/fmicb.2013.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari Jagdish, Irudayaraj Joseph. Quantification of Saccharides in Multiple Floral Honeys Using Fourier Transform Infrared Microattenuated Total Reflectance Spectroscopy. J. Agric. Food Chem. 2004;52(11):3237–3243. doi: 10.1021/jf035176+. [DOI] [PubMed] [Google Scholar]
- Tonks A.J., Cooper R.A., Jones K.P., Blair S., Parton J., Tonks A. Honey stimulates inflammatory cytokine production from monocytes. Cytokine. 2003;21(5):242–247. doi: 10.1016/s1043-4666(03)00092-9. [DOI] [PubMed] [Google Scholar]
- Tseng Jun-Ming, Huang Jun-Ru, Huang Hsiou-Chen, Tzen Jason T.C., Chou Wing-Ming, Peng Chi-Chung. Facilitative production of an antimicrobial peptide royalisin and its antibody via an artificial oil-body system. Biotechnol Progress. 2011;27(1):153–161. doi: 10.1002/btpr.528. [DOI] [PubMed] [Google Scholar]
- Verdrengh Margareta, Collins L.Vincent, Bergin Philip, Tarkowski Andrej. Phytoestrogen genistein as an anti-staphylococcal agent. Microbes and Infection. 2004;6(1):86–92. doi: 10.1016/j.micinf.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Visavadia Bhavin G, Honeysett Jan, Danford Martin. Manuka honey dressing: An effective treatment for chronic wound infections. British Journal of Oral and Maxillofacial Surgery. 2008;46(8):696–697. doi: 10.1016/j.bjoms.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Wahdan H.A.L. Causes of the antimicrobial activity of honey. Infection. 1998;26(1):26–31. doi: 10.1007/BF02768748. [DOI] [PubMed] [Google Scholar]
- Zbuchea A. Up-to-date use of honey for burns treatment. Annals of burns and fire disasters. 2014;27(1):22. [PMC free article] [PubMed] [Google Scholar]