Abstract
Objective:
To investigate perceptual speech outcomes following sphincter pharyngoplasty (SP) and to identify patient characteristics associated with velopharyngeal insufficiency (VPI) resolution or improvement.
Methods:
Retrospective review of prospectively collected data was performed of consecutive patients that underwent SP for management of VPI between 1994 and 2016 at a single tertiary care pediatric hospital.
Demographic data, nasendoscopic findings and speech characteristics were recorded using a standardized protocol. Pre- and post-operative VPI was graded on a 5-point Likert scale. Frequency of post-operative VPI resolution and improvement was assessed and associations with patient characteristics were analyzed. The association between odds of VPI resolution or improvement and five patient characteristics identified a priori was performed controlling for confounding factors.
Results:
Two-hundred ninety-six subjects were included. All patients had at least minimal VPI pre-operatively; 72% were graded moderate or severe. Sixty-four percent experienced resolution and 83% improved at least one point on the VPI-severity scale. Of the five patient characteristics, only history of cleft palate repair was significantly associated with decreased odds of VPI improvement but not resolution when controlling for other variables.
Conclusions:
Sphincter pharyngoplasty resulted in resolution of VPI in 64% and improvement in 83% of subjects. Children with a history of cleft palate had significantly decreased odds of VPI improvement compared to those without a history of cleft palate. Neither syndrome diagnosis nor 22q11 deletion had a significant association with speech outcomes after sphincter pharyngoplasty.
Keywords: Sphincter pharyngoplasty, velopharyngeal insufficiency, cleft palate, speech
Introduction:
Velopharyngeal dysfunction (VPD) refers to any structural or functional difference that impedes complete velopharyngeal port closure during speech production resulting in hypernasal resonance and nasal air escape. Normal speech production requires complete closure of the velopharyngeal port for all consonants except the nasal consonants (m, n and ŋ). VPD etiology is often multifactorial and may involve anatomic abnormalities (velopharyngeal insufficiency), neuromuscular dysfunction (velopharyngeal incompetence) and compensatory/maladaptive articulation patterns (velopharyngeal mislearning).1 The impact of velopharyngeal insufficiency (VPI) on speech intelligibility depends on velopharyngeal gap-size, with greater VPI severity often associated with larger gap-sizes.2 Speech intelligibility may be further impaired if there is concomitant velopharyngeal mislearning (VPM). Multiple studies have found associations between VPI and measures of decreased quality of life, including impaired scholastic, social, behavioral, emotional and physical function.3–5
While speech therapy may be helpful for patients with VPM, treatment of VPI requires physical management provided only by intra-oral appliances or surgical intervention.6,7 Various surgical approaches can narrow the velopharynx, facilitating velopharyngeal closure and eliminating nasal air escape.8 Posterior pharyngeal flap and sphincter pharyngoplasty (SP) are the most common pharyngeal procedures aimed at managing VPI,7 the latter of which is more commonly performed at our institution. SP creates a physiologic sphincter at the level of the velum by raising lateral, superiorly-based myomucosal flaps from the posterior-lateral oropharynx and rotating them medially and superiorly to the posterior nasopharynx, ideally to the level at which the velum attempts to contact the posterior pharyngeal wall.9
The definition of success after SP varies widely and depends on the outcome measured.8,10–13 A previous small study from our institution found that 63% of patients had complete resolution of VPI after SP.11 Studies investigating characteristics associated with surgical outcomes after SP have found that greater age and VPI severity, history of cleft palate, and syndromic presence may be associated with poorer surgical outcomes, though results have not been consistent across studies.8,10,12,13 Thus, patient factors affecting outcomes of SP remain poorly understood.
The purpose of this study was to describe VPI outcomes in a large group of children after SP, and to identify patient characteristics associated with VPI resolution and improvement after surgery. We hypothesized that failure to resolve or improve would be associated with 1) a history of palatal cleft, including submucous cleft, 2) any syndromic diagnosis (versus nonsyndromic), 3) a diagnosis of 22q11 deletion (versus nonsyndromic) 4) previous palatoplasty performed specifically for VPI, and 5) large pre-operative velopharyngeal gap on endoscopy.
Methods:
This study was approved by the Seattle Children’s Hospital (SCH) Institutional Review Board (STUDY15330).
Data Collection:
Patient demographic data, perceptual VPI severity ratings and nasendoscopy findings were collected in a standardized manner by the pediatric otolaryngologist and speech-language pathologist (SLP) as part of routine clinical care at each patient’s VPI clinic visits. All clinical data was entered into a secure database. Data from patients who underwent SP for VPI between February 1994 and October 2016 were downloaded from the database and additional chart review was performed to fill in missing variables. All surgeries were performed by one of 3 surgeons. The database was cross-referenced with a database of the SCH Craniofacial Center to determine syndrome diagnosis and presence of 22q11 deletion. Syndrome diagnosis was subdivided into five categories: 22q11 deletion, neuromuscular, syndromic cleft palate, central nervous system, or other (Table 1). This variable was dichotomized in two different ways: syndrome versus non-syndromic and 22q11 deletion versus non-syndromic for analysis.
Table 1.
Categorization of syndromic diagnoses.
| Syndrome Category | Included Diagnoses |
|---|---|
| 22q11 Deletion | velocardiofacial, DiGeorge |
| Neuromuscular | myopathy, Moebius, pseudobulbar palsy |
| Syndromic Cleft Palate | Hays Wells, ectrodactyly-ectodermal dysplasia, Kabuki, Stickler, Pierre-Robin, and Van de Woude |
| Central Nervous System | history of encephalitis, brain tumor, hypotonia, cerebral palsy or traumatic brain injury |
| Other | amniotic band syndrome, arthrogryposis, CHARGE, congenital hypothyroidism, frontonasal dysplasia, Klippel Feil, macro/microcephaly, craniofacial microsomia, Down Syndrome, neurofibromatosis, Pallister-Hall, in-utero drug exposure |
For children with multiple follow-up visits, we selected the follow-up visit closest to 6 months after SP; we excluded children without a follow-up visit greater than 3 months after surgery. We also excluded children who had palatoplasty on the same day as SP, those for whom SP was a revision, and those for whom pre-operative or post-operative speech perception data was unavailable. We included children with a history of cleft of the secondary and/or primary palate or submucous cleft that had been repaired, or previous Furlow palatoplasty14 for VPI (defined as that performed over the age of 2.5 years specifically to treat VPI), as well as those with non-cleft causes of VPI.
Clinical VPI Evaluation:
Patients were evaluated according to standard clinical care at our institution, including pre- and post-operative perceptual speech evaluations aimed at differential diagnosis of VPD etiology by an SLP specializing in VPD diagnosis and management. Although VPI severity was the outcome measure of interest for this study, additional speech characteristics including degree of VPM and severity of motor planning and motor execution deficits were also evaluated. Overall VPI severity was defined as the degree of passive nasal air emission and hypernasality, in the absence of maladaptive articulation, present in a variety of speech tasks. In cases where etiology of VPD was multifactorial, presence of VPI and candidacy for surgical management was confirmed through nasendoscopy. Patients whose velopharyngeal port was consistently open for consonants requiring complete closure were considered candidates for surgical management. Passive nasal air emission not attributable to oronasal fistula(e) was considered in the VPI-severity rating. Baseline and post-operative VPI were graded by the evaluating SLP on a 5-point scale from 0-none to 1-minimal, 2-mild, 3-moderate and 4-severe using a standardized institutional rubric (Table 2).15
Table 2.
Velopharyngeal insufficiency-severity rubric.
| VPI Severity |
Nasal Airflow (Nasal Air Escape/Nasal Turbulence) |
Resonance (Hypernasality) |
|---|---|---|
| 0 – None | Absent (normal intra-oral pressure for all pressure consonants) | Absent (balanced oral-nasal resonance) |
| 1 – Minimal | Absent or on auscultation only† | Borderline/minimal (present only in nasal contexts) |
| Occasional (infrequently heard) | Absent | |
| 2 – Mild | Absent or occasional | Mild (evident on high vowels only - /i, ɪ/) |
| 3 – Moderate | Occasional or frequent (consistently heard) | Moderate (evident on high & mid-vowels - /u/) |
| 4 – Severe | Frequent | Severe (present on all vowels and on voiced consonants) |
VPI=velopharyngeal insufficiency
Heard using listening tube.
The evaluating otolaryngologist performed nasendoscopy on each patient prior to SP and graded velopharyngeal gap-size as none, small, moderate, or large as described previously.16–19 Gap-size was dichotomized as none/small versus moderate/large for analysis.
Outcome Measures:
The primary outcome measure was post-surgical VPI resolution, defined as post-operative VPI severity of “0-none” or “1-minimal” and improvement of at least one point on the 5-point perceptual severity scale. Thus, children who had a pre-operative score of 1 were required to improve to 0 to be considered resolved. VPI improvement, defined as a post-operative decrease of at least one point on the VPI-severity scale, was used as a secondary outcome measure.
Statistical Analyses:
Statistical analysis was conducted with Stata/SE 15 software (StataCorp LP, College Station, Texas). An alpha level of .05 was used for all statistical tests.
For bivariate analyses, Pearson’s Chi-squared statistic for categorical variables and the Wilcoxon Rank-Sum test for continuous variables were used to test associations between each baseline variable with the two outcome measures, VPI resolution and improvement.
Multivariable logistic regression using robust standard errors was then performed to examine associations between the five pre-selected baseline patient factors hypothesized to decrease odds of resolution and improvement, (1) history of palatal cleft (including submucous cleft); 2) any syndromic diagnosis (versus nonsyndromic); 3) diagnosis of 22q11 deletion (versus nonsyndromic); 4) previous palatoplasty performed specifically for VPI; and 5) large velopharyngeal gap on pre-operativeendoscopy, while controlling for age, sex, race, and baseline VPI severity.
Results:
Three hundred sixty-three subjects underwent SP during the study period. Twenty-six were excluded for concomitant palatoplasty and 41 were excluded for missing pre- or post-operative perceptual speech data, resulting in 296 included subjects.
Baseline Characteristics:
Baseline characteristics as well as results from bivariate analyses are provided in Table 3. Subjects ranged from 2 to 23 years old at the time of SP. Median follow-up time was 6.7 months after surgery. The majority were Caucasian and just over half were male (58%). Most had either a history of repaired cleft of the secondary and/or primary palate (41%), or submucous cleft (13%). Just over one-fourth (27%) had undergone a previous Furlow palatoplasty for VPI. The majority (64%) had no syndromic diagnosis. The most common syndrome was 22q11 deletion (15%). Half had a small velopharyngeal gap on pre-operative nasendoscopy. All patients presented with at least minimal preoperative perceptual VPI, although the majority had moderate (29%) or severe (43%) pre-operative VPI.
Table 3.
Characteristics of children overall and by velopharyngeal insufficiency outcome after sphincter pharyngoplasty.
| All Subjects* |
VPI Outcome |
||||
|---|---|---|---|---|---|
| (N=296) | Improved (n=245) | p | Resolved (n=189) | p | |
| Characteristic | Median (Interquartile Range) or n (%) | ||||
| Age at surgery (years)† | 7 (5–11) | 7 (5–11) | 0.9 | 7 (5–10) | 0.9 |
| Follow up time (months)† | 6.7 (4.4–13.1) | 6.7 (4.4–13.0) | 0.3 | 6.7 (4.5–13.3) | 0.6 |
| Male‡ | 171 (58) | 142 (58) | 0.9 | 105 (56) | 0.3 |
| Race/ethnicity‡ | 1.0 | 0.2 | |||
| White | 217 (73) | 180 (74) | 143 (76) | ||
| Non-white | 77 (26) | 64 (26) | 45 (24) | ||
| Black | 4 (1) | 2 (1) | 2 (1) | ||
| Asian/Pacific Islander | 22 (7) | 19 (8) | 11 (6) | ||
| Hispanic | 22 (7) | 17 (7) | 13 (7) | ||
| Other | 29 (10) | 26 (11) | 19 (10) | ||
| Syndrome/co-morbidity‡ | 0.8 | 0.8 | |||
| None | 189 (64) | 157 (65) | 119 (64) | ||
| Any syndrome below | 105 (35) | 86 (35) | 68 (36) | ||
| 22q11deletion | 45 (15) | 40 (16) | 31 (17) | ||
| Neuromuscular | 5 (2) | 5 (2) | 5 (3) | ||
| Syndromic cleft | 27 (9) | 19 (8) | 14 (7) | ||
| CNS | 8 (3) | 6 (2) | 5 (3) | ||
| Other | 20 (7) | 16 (7) | 13 (7) | ||
| History of palatal cleft‡ | 0.02 | 0.07 | |||
| None | 137 (46) | 121 (49) | 95 (50) | ||
| Palatal cleft | 159 (54) | 124 (51) | 94 (50) | ||
| Hard/soft palatal cleft | 121 (41) | 95 (44) | 71 (43) | ||
| Submucous only | 38 (13) | 29 (14) | 23 (14) | ||
| Previous palatoplasty for VPI‡ | 80 (27) | 65 (27) | 0.7 | 44 (23) | 0.05 |
| Gap size (endoscopy)‡ | 0.8 | 0.2 | |||
| Small | 148 (50) | 120 (51) | 102 (56) | ||
| Moderate | 106 (36) | 89 (38) | 61 (33) | ||
| Large | 33 (11) | 28 (12) | 20 (11) | ||
| Baseline VPI severity‡ | <0.001 | <0.001 | |||
| Minimal | 7 (2) | 1 (<1) | 1 (1) | ||
| Mild | 77 (26) | 63 (26) | 63 (33) | ||
| Moderate | 85 (29) | 72 (29) | 56 (30) | ||
| Severe | 127 (43) | 109 (44) | 69 (37) | ||
VPI=velopharyngeal insufficiency, CNS=central nervous system
Wilcoxon Rank-Sum test used.
Pearson’s Chi-squared statistic used.
Medians are presented for continuous variables, with interquartile ranges in parenthesis. For categorical variables, the number of patient’s are presented, with percentages in parenthesis.
p – values refer to association between the given characteristic and either VPI improvement or VPI resolution.
Column percentages may not total 100 due to missing data.
VPI Severity:
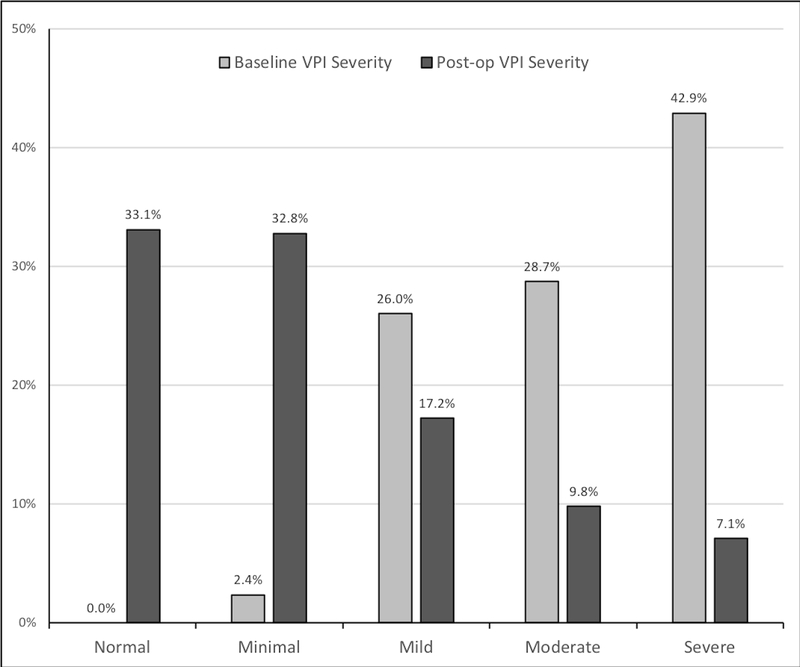
Distribution of VPI severity before and after surgery is shown in Figure 1. Nearly two-thirds (64%) of subjects presented with resolution of VPI after SP and 83% presented with VPI improvement.
Figure 1.
Perceptual VPI severity at baseline and following sphincter pharyngoplasty.
VPI=velopharyngeal insufficiency
Bivariate Analyses:
Results from bivariate analyses are provided in Table 3.
Of factors tested in bivariate analysis, only pre-operative VPI severity and history of palatoplasty for VPI, were significantly associated with VPI resolution, though there was a trend for decreased VPI resolution among those with a history of cleft palate. The proportion of those with complete resolution of VPI was highest among those with mild baseline VPI severity (82%) while those with moderate or severe baseline VPI severity demonstrated resolution 66% and 54% of the time, respectively.
Only baseline VPI severity and history of cleft palate repair were significantly associated with VPI improvement on bivariate analysis. Those with minimal pre-operative VPI severity experienced resolution at the lowest rates (14%). Notably, of the 7 patients with minimal pre-operative VPI, 5 (71%) had a history of cleft palate repair and 5 (71%) had a syndrome diagnosis; 1 (14%) had a moderate pre-operative velopharyngeal gap-size with the remaining having small gaps. Proportion of VPI improvement was similar among those with small, medium and large gap-sizes with greater than 80% of patients improving in each group (81%, 84% and 85%, respectively).
VPI resolution and improvement rates were higher among those without history of palatal cleft than those with history of cleft palate repair. Resolution was demonstrated in 69% of patients without a history of palatal cleft, versus 59% with a history of palatal cleft. Improvement was noted in 88% without history of palatal cleft versus 78% with history of cleft. We found no significant association with syndromic presence or 22q11 deletion and rates of VPI resolution or improvement.
Multivariable Analyses:
Multivariable logistic regression was performed to examine associations between baseline factors hypothesized to be associated with VPI resolution or improvement while controlling for age, sex, race and VPI severity. Results are provided in Tables 4 and 5.
Table 4.
Multivariable logistic regression of the association between baseline patient factors and velopharyngeal insufficiency resolution controlling for age, sex, race (white vs. non-white), baseline VPI severity.
| Odds Ratio (95% Confidence Interval) | P value | |
|---|---|---|
| History of cleft palate† | 0.68 (0.41 – 1.12) | 0.1 |
| Any syndrome (vs. nonsyndromic) | 1.04 (0.62 – 1.73) | 0.9 |
| 22q11 deletion (vs. nonsyndromic) | 1.18 (0.57 – 2.43) | 0.7 |
| Previous palatoplasty for VPI | 0.65 (0.37 – 1.14) | 0.1 |
| Velopharyngeal gap size‡ | 0.74 (0.43 – 1.30) | 0.3 |
VPI=velopharyngeal insufficiency
Any cleft, including submucous cleft
Moderate/large vs. small
Table 5.
Multivariable logistic regression of the association between baseline patient factors and velopharyngeal insufficiency improvement controlling for age, sex, race (white vs. non-white), baseline VPI severity.
| Odds Ratio (95% Confidence Interval) | P value | |
|---|---|---|
| History of cleft palate† | 0.46 (0.24 – 0.91) | 0.03 |
| Any syndrome (vs. nonsyndromic) | 0.92 (0.48 – 1.74) | 0.8 |
| 22q11 deletion (vs. nonsyndromic) | 1.55 (0.55 – 4.38) | 0.4 |
| Previous palatoplasty for VPI | 0.73 (0.35 – 1.53) | 0.4 |
| Velopharyngeal gap size‡ | 0.74 (0.38 – 1.44) | 0.4 |
VPI=velopharyngeal insufficiency
Any cleft, including submucous cleft
Moderate/large vs. small
No significant associations between odds of VPI resolution and baseline factors were noted on multivariable analysis.
With regards to VPI improvement, those with a history of cleft palate repair were approximately 50% less likely to demonstrate improvement in post-operative VPI severity than those without history of cleft palate, controlling for possible confounders. No other variables were significantly associated with decreased odds of VPI improvement.
Discussion:
We present a large series of patients who underwent SP for management of VPI. Preoperatively, all patients had VPI on perceptual speech assessment and transverse orientation of the levator veli palatini based on endoscopic findings. The outcome measures were based upon standardized assessments of VPI severity determined by SLPs with expertise in VPI and collected as part of routine clinical care.
Overall, 64% of children had resolution and 83% had improvement of their VPI after SP. Except for those with minimal baseline VPI, lower baseline VPI severity and lack of prior palatoplasty were associated with greater rates of VPI resolution following SP. Lower baseline VPI severity and absence of a history of cleft palate repair were associated with a greater likelihood of VPI improvement post-operatively. However, only history of palatal cleft was significantly associated with lack of VPI improvement when controlling for other patient factors.
Comparing these results to other similar studies is limited by use of different outcome measures. Losken et al. published a series of 250 patients with previously repaired cleft palate who underwent SP for VPI, using need for revision as the primary outcome. Their overall revision rate was 13%, 78% of whom underwent revision SP for persistent VPI following initial SP. They found higher rates of revision in those with larger velopharyngeal gap-size and more severe hypernasal resonance pre-operatively. Patients with 22q11 deletion syndrome and cleft palate also appeared to have higher revision rates, though these were reported as statistically nonsignificant.8 Carlisle et al. examined a cohort of 46 patients who underwent SP for VPI using rate of revision surgery as a proxy for VPI. The study found a 13% revision rate and no association with age, sex, cleft type, or syndrome. Notably, 57% of patients in their cohort underwent concomitant Furlow palatoplasty.10 The revision rate was 25% in those who underwent SP alone.10 Samoy et al. investigated speech outcomes of 62 patients who underwent VPI surgery with either SP or velopharyngoplasty. They also found no association with age, but did note poorer outcomes in patients with 22q11 deletion syndrome and those with more severe hypernasality.13
We sought to understand the outcome of SP and therefore excluded patients who had concomitant FP and SP. At our institution, combined FP and SP is typically offered to patients with hypodynamic velopharynx and evidence of sagittal orientation of the levator veli palatini. Therefore, the exclusion of these patients may have biased the outcomes reported, particularly for those patients with large gaps. Riski et al. described a series of 139 patients using nasal emissions on perceptual exam or nasal air escape on pressure-flow studies as a combined outcome measure. They found a success rate of 78%, with greater success in younger patients and those with less pre-operative hypernasality but no association with velopharyngeal gap-size, type of cleft, or sex.12 With regards to the association with age, it was hypothesized that “ingrained speech habits” (VPM) were to blame for lower success rates in older patients.12 Studies have shown that velopharyngeal closure is different when using accurate oral placement for consonants compared to maladaptive articulation (i.e. glottal stops, pharyngeal fricatives, etc.).20 Presumably, if accurate differential diagnosis of etiology of VPD is not made, VPI surgery may be tailored toward a gap size indicative of maladaptive articulation rather than gap size indicative of VPI. Accurate differential diagnosis of VPD vs. VPI vs. VPM is a primary focus of perceptual speech evaluations and care is taken to rate VPI severity based on appropriate speech characteristics. Thus our results may not be comparable to studies in which different methods are used for VPI diagnosis. The American Cleft Palate – Craniofacial Association developed the Standards for Cleft Palate and Craniofacial Teams, mandating the inclusion of SLPs among the core members of multidisciplinary craniofacial teams.21 SLPs on these teams play a vital role in the assessment and management of patients with VPI. Like the present study, more recent studies likely include greater SLP involvement, and found no association between age at surgery and VPI outcomes.8,10,13
We found that patients with previous cleft palate repair were less likely to have improvement of VPI after SP. The previously repaired cleft palate group represents a very heterogenous population and it is likely that multiple factors contributed to this result. It is also possible that patients with previous palate repair may have had more palatal scarring associated with less palatal mobility.
Evidence as to whether syndromic diagnoses are associated with poorer outcomes following SP is conflicting. While an association was suggested by Losken et al and Samoy et al, the present study, in which 39% carry a syndromic diagnosis, found no association.8,13 In this study, 22q11 deletion syndrome represented 15% of the study population. We did not find a significant association between 22q11 deletion and the odds of VPI resolution or VPI improvement when controlling for age, sex, race, and baseline VPI severity. We had postulated that patients with deletion 22q11 and large gaps may have had combined Furlow palatoplasty and sphincter pharyngoplasty and were thereby excluded from this dataset. However, upon review of our data, only one patient with 22q11 deletion syndrome was excluded for undergoing simultaneous SP and Furlow palatoplasty.
The low rates of VPI resolution and improvement among patients with pre-operative minimal VPI was remarkable. At our institution, minimal VPI is assigned to those with nasal air escape detected by auscultation only. The low rates of resolution may reflect greater degree of neuromuscular discoordination contributing to VPI, as opposed to a predominantly structural or anatomic cause. The role for surgical intervention in this group should be carefully considered based upon these findings.
Median follow-up time after SP was 6.7-months. In order to improve data uniformity, data from the follow-up visit closest to 6 months was used for patients who had multiple follow-up visits. We selected this timepoint since patients that have VPI persisting beyond 6 months will usually be considered for revision surgery. The 6 month timepoint occurs after our standard 3 month instrumental speech assessment6 but before 10 months, the reported mean time interval to revision SP.8 It is important to point out however, that changes to the surgically manipulated tissues may continue beyond this point as surgical site healing and scarring progress.
Like other studies, the present investigation found a statistically significant association between baseline VPI severity and post-operative VPI resolution, with greater odds of resolution associated with milder baseline VPI, on bivariate analysis.8,12,13 These findings are anticipated as one would expect those with less severe VPI to require less tissue volume in the transposition flaps. An association between greater velopharyngeal gap-size and decreased rate of VPI resolution would support this argument, and this was indeed demonstrated by Losken et al.8 In the present study, while a tendency for children with larger velopharyngeal gap-size to have reduced VPI resolution rates was found, it was not statistically significant. These findings may have been affected by exclusion of those patients who had concomitant FP and SP.
This study has several other limitations, primarily those related to the retrospective nature of the research design and subjective nature of perceptual speech assessments. The outcome measures in this study were limited to objective measures of (VPI resolution and improvement). While syndrome diagnosis was determined by a craniofacial pediatrician, we did not routinely test all patients for 22q11 deletion. Therefore, under-reporting of patients in this group is possible.
Conclusion:
In this study, SP is associated with VPI resolution in 64% and improvement in 83% of patients. Neither VPI resolution nor improvement were significantly associated with a history of syndrome (whether 22q11 or other syndromic diagnosis), history of previous palatoplasty for VPI, or large velopharyngeal gap-size. History of palatal cleft was significantly associated with decreased odds of VPI improvement but not resolution, independent of age, sex, race, and pre-operative VPI severity.
Acknowledgements:
Dr. Austin Lam was supported by T32DC000018 from the National Institute on Deafness and Other Communication Disorders during his work on this study.
Footnotes
Conflicts of Interest: None of the authors have any conflicts of interest to disclose.
Level of Evidence: Level 4
References:
- 1.Trost-Cardamone JE. Coming to terms with VPI: A response to Loney and Bloem. Cleft Palate J. 1989;26(1):68–70. [PubMed] [Google Scholar]
- 2.Kummer AW, Curtis C, Wiggs M, Lee L, Strife JL. Comparison of velopharyngeal gap size in patients with hypernasality, hypernasality and nasal emission, or nasal turbulence (rustle) as the primary speech characteristic. Cleft Palate-Craniofacial J Off Publ Am Cleft Palate-Craniofacial Assoc. 1992;29(2):152–156. doi: 10.1597/1545-1569_1992_029_0152_covgsi_2.3.co_2 [DOI] [PubMed] [Google Scholar]
- 3.Barr L, Thibeault SL, Muntz H, De Serres L. Quality of life in children with velopharyngeal insufficiency. Arch Otolaryngol - Head Neck Surg. 2007;133(3):224–229. doi: 10.1001/archotol.133.3.224 [DOI] [PubMed] [Google Scholar]
- 4.Hunt O, Burden D, Hepper P, Stevenson M, Johnston C. Self-reports of psychosocial functioning among children and young adults with cleft lip and palate. Cleft Palate Craniofac J. 2006;43(5):598–605. doi: 10.1597/05-080 [DOI] [PubMed] [Google Scholar]
- 5.Skirko JR, Weaver EM, Perkins JA, et al. Change in Quality of Life with Velopharyngeal Insufficiency Surgery. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2015;153(5):857–864. doi: 10.1177/0194599815591159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sie KCY, Chen EY. Management of velopharyngeal insufficiency: Development of a protocol and modifications of sphincter pharyngoplasty. Facial Plast Surg. 2007;23(2):128–139. doi: 10.1055/s-2007-979282 [DOI] [PubMed] [Google Scholar]
- 7.Crockett DJ, Goudy SL. Update on surgery for velopharyngeal dysfunction. Curr Opin Otolaryngol Head Neck Surg. 2014;22(4):267–275. doi: 10.1097/MOO.0000000000000063 [DOI] [PubMed] [Google Scholar]
- 8.Losken A, Williams JK, Burstein FD, Malick D, Riski JE. An outcome evaluation of sphincter pharyngoplasty for the management of velopharyngeal insufficiency. Plast Reconstr Surg. 2003;112(7):1755–1761. doi: 10.1097/01.PRS.0000090720.33554.8B [DOI] [PubMed] [Google Scholar]
- 9.Gart MS, Gosain AK. Surgical management of velopharyngeal insufficiency. Clin Plast Surg. 2014;41(2):253–270. doi: 10.1016/j.cps.2013.12.010 [DOI] [PubMed] [Google Scholar]
- 10.Carlisle MP, Sykes KJ, Singhal VK. Outcomes of sphincter pharyngoplasty and palatal lengthening for velopharyngeal insufficiency: a 10-year experience. Arch Otolaryngol - Head Neck Surg. 2011;137(8):763–766. doi: 10.1001/archoto.2011.114 [DOI] [PubMed] [Google Scholar]
- 11.Sie KCY, Tampakopoulou DA, de Serres LM, Gruss JS, Eblen LE, Yonick T. Sphincter pharyngoplasty: speech outcome and complications. The Laryngoscope. 1998;108(8 Pt 1):1211–1217. doi: 10.1097/00005537-199808000-00021 [DOI] [PubMed] [Google Scholar]
- 12.Riski JE, Ruff GL, Georgiade GS, Barwick WJ, Edwards PD. Evaluation of the Sphincter Pharyngoplasty. Cleft Palate Craniofac J. 1992;29(3):254–261. doi: 10.1597/1545-1569_1992_029_0254_eotsp_2.3.co_2 [DOI] [PubMed] [Google Scholar]
- 13.Samoy K, Hens G, Verdonck A, et al. Surgery for velopharyngeal insufficiency: The outcomes of the University Hospitals Leuven. Int J Pediatr Otorhinolaryngol. 2015;79(12):2213–2220. doi: 10.1016/j.ijporl.2015.10.007 [DOI] [PubMed] [Google Scholar]
- 14.Furlow LT. Cleft palate repair by double opposing Z-plasty. Plast Reconstr Surg. 1986;78(6):724–738. doi: 10.1097/00006534-198678060-00002 [DOI] [PubMed] [Google Scholar]
- 15.Sie KCY, Tampakopoulou DA, Sorom JBA, Gruss JS, Eblen LE. Results with Furlow Palatoplasty in Management of Velopharyngeal Insufficiency. Plast Reconstr Surg. 2001;108(1):17–25. doi: 10.1097/00006534-200107000-00004 [DOI] [PubMed] [Google Scholar]
- 16.Miller C, Bly R, Cofer S, et al. Multicenter Interrater Reliability in the Endoscopic Assessment of Velopharyngeal Function Using a Video Instruction Tool. Otolaryngol Neck Surg. 2019;160(4):720–728. doi: 10.1177/0194599818822989 [DOI] [PubMed] [Google Scholar]
- 17.Sie KCY, Starr JR, Bloom DC, et al. Multicenter Interrater and Intrarater Reliability in the Endoscopic Evaluation of Velopharyngeal Insufficiency. Arch Otolaryngol Neck Surg. 2008;134(7):757. doi: 10.1001/archotol.134.7.757 [DOI] [PubMed] [Google Scholar]
- 18.Tieu DD, Gerber ME, Milczuk HA, et al. Generation of Consensus in the Application of a Rating Scale to Nasendoscopic Assessment of Velopharyngeal Function. Arch Otolaryngol Neck Surg. 2012;138(10):923–928. doi: 10.1001/archotol.2013.203 [DOI] [PubMed] [Google Scholar]
- 19.Yoon PJ, Starr JR, Perkins JA, Bloom D, Sie KCY. Interrater and Intrarater Reliability in the Evaluation of Velopharyngeal Insufficiency Within a Single Institution. Arch Otolaryngol Neck Surg. 2006;132(9):947–951. doi: 10.1001/archotol.132.9.947 [DOI] [PubMed] [Google Scholar]
- 20.Henningsson GE, Isberg AM. Velopharyngeal movement patterns in patients alternating between oral and glottal articulation: a clinical and cineradiographical study. Cleft Palate J. 1986;23(1):1–9. [PubMed] [Google Scholar]
- 21.Standards for Approval of Cleft Palate and Craniofacial Teams. American Cleft Palate - Craniofacial Association. Published 2019. Accessed February 24, 2020. https://acpa-cpf.org/team-care/standardscat/standards-of-approval-for-team-care/