Abstract
In the era of climate change, decreased precipitation and increased evapo-transpiration hampers the yield of several cereal crops along with the soil salinity and poor ground water resource. Wheat being the moderately tolerant crop face many challenges in the arid and semi-arid regions under irrigated agriculture. In view of this, the study was planned to explore the potential of durum wheat genotypes under salinity on the basis of physiological traits. Experiment was designed as RBD in three replications to evaluate 15 wheat genotypes with moderate saline irrigation (ECiw – 6 dS m−1) and extreme saline irrigation (ECiw – 10 dS m−1) along with one set of control (Best available water). Different physiological traits such as water potential (ψp), osmotic potential (ψs), relative water content (RWC), Na+ and K+ content were recorded in roots as well as shoots at the reproductive stage whereas photosynthetic rate and chlorophyll content were measured in the flag leaves. A significant variability (p < 0.001) was noted among the genotypes under different stress environments and it was observed that durum genotype HI 8728 and HI 8737 showed less reduction in plant water traits (RWC, ψp and ψs) than the salinity tolerant checks of bread wheat KRL 99 and KRL 3–4. HD 4728 and HI 8708 maintained higher photosynthetic rate as well as higher chlorophyll content under the extreme salinity level of ECiw – 10 dSm−1. No significant differences were found in root Na+ in genotypes KRL 99 (3.17g), KRL 3–4 (3.34g) and HI 8737 (3.41g) while in shoots, lowest accumulation was seen in KRL 99, MACS 3949 and KRL 3–4 at ECiw – 10 dSm−1. The mean range of K+ content was 7.60–9.74% in roots and 4.21–6.61% in shoots under control environment which decreased to 50.77% in roots and 46.05% in shoots under extreme salinity condition of ECiw – 10 dSm−1. At ECiw – 10 dSm−1, KRL 99 maintained highest K+/Na+ in both root and shoot followed by KRL 3–4, HI 8737, MACS 3949, HD 4728 in roots and MACS 3949, KRL 3–4, MACS 4020, HD 4758, MACS 3972 and HI 8713 in shoots. The differential response of durum wheat genotypes under salinity particularly for physiological traits, confer their adaptability towards stress environments and exhibit their potential as genetic sources in breeding programs for improving salt stress tolerance.
Keywords: Wheat, Salinity, Plant water relations, Ionic relations
Abbreviations: ψp, Water potential; ψs, Osmotic potential; RWC, Relative water content; Pn, photosynthetic rate; ECiw, Electrical conductivity of irrigation water; MPa, Mega Pascal; FW, Fresh weight; DW, Dry weight
1. Introduction
Wheat is one of the most important food crops cultivated in India to meet the food demand of burgeoning population of the country. Apart from the regular seasonal environmental constraints, in many parts of the country, wheat crop is often subjected to periods of soil and atmospheric water deficits as well as high soil salinity. These limitations are likely to increase in future as climatic change is expected to decrease precipitation and increase evapo-transpiration (World Bank, 2007, Lobell et al., 2008). Among various factors, salinity of soil and ground water resource is a major problem for irrigated agriculture. In India, salt affected soils are predicted to increase from the current 6.73 M ha to 16.2 M ha by 2050 due to planned expansion in irrigation network, use of poor quality water in irrigation and climate change impacts (Sharma and Singh, 2015, Singh et al., 2018). Globally, wheat ranks second after maize in cereal crops (Datta et al., 2009) which is consumed approximately by 36% of the population. Salinity stress negatively affects the wheat productivity and yield starts declining when ECe value exceeds 6 dS m−1 in the soil solution (Chinnusamy et al., 2005, Shahzad et al., 2016). Increasing attention to the mechanisms of salinity stress response is being emphasized now due to threats of climate change and loss of arable land during urbanization and environmental degradation.
Salt injury involves both osmotic effects and specific ion effects. In most crop plants, the main toxic components of salinity are Na+ and Cl−. These toxic ions interfere with the normal physiological processes causing membrane damage, nutrient imbalance, altered levels of growth regulators, enzymatic inhibition and metabolic dysfunction, including photosynthesis which ultimately leads to plant death (Mahajan and Tuteja, 2005, Kumar et al., 2018a, Kumar et al., 2019). Thus the plant growth is impaired by osmotic stress in a first phase and ionic stress in second phase (accumulation of high concentrations of salts (toxic ions) within the plant which damages cell functions and structure) and finally suppress the yield (Kumar et al., 2018b, Mann et al., 2019a). These negative effects include interference of root function in absorbing water, as well as the prevention of physiological and biochemical processes such as uptake of nutrient like Ca++, Mg++ and K+ and their assimilation (Carillo et al., 2011). The roots are the first plant organs that control the uptake and translocation of nutrients and salts throughout the life cycle of plant (Lata et al., 2019). In spite of the direct exposure of roots to saline environment, their growth is less vulnerable to salt than that of the shoots (Munns, 2002). Wheat is one of the world’s major crops and has been subjected to intensive breeding and selection for about a century. The bulk of the selection effort to date has been directed to improving grain yield, end use quality, and disease resistance. Bread wheat (Triticum aestivum) is always considered as a more salt-tolerant species than durum wheat (Triticum turgidum) (Munns and James, 2003, Munns and Tester, 2008); this difference has been largely attributed to its superior ability to maintain lower Na+ accumulation in the leaf/shoot (Colmer et al., 2006, Cuin et al., 2010, James et al., 2011, Munns et al., 2012). With increasing soil salinization and shortage of water supply, a major shift is now underway to improve its level of abiotic tolerance. So, to study the differential physiological and biochemical responses of roots and shoots along with mechanism of ion transport among different genotypes could serve as a selection criteria or the possible strategy to identify tolerant genotype.
2. Material and methods
2.1. Plant material, growth conditions and experimental details
The experiment was designed as Randomised Block Design (RBD) in three replications in net house of Division of Crop Improvement, ICAR-Central Soil Salinity Research Institute, Karnal, Haryana, India. Fifteen wheat genotypes were evaluated under three different environments i.e. control (best available water), moderate saline irrigation (ECiw – 6 dS m−1) and extreme saline irrigation (ECiw – 10 dS m−1). Natural saline water collected from Nain experimental farm, Panipat (Table 1) was diluted to the desired saline levels and stress treatment was imposed prior to the seed sowing. Twelve durum wheat genotypes (HD 4728, HD 4730, HI- 8708, HI-8713, HI-8758, HI-8759, HD-4758, MACS-3949, MACS-4020, MACS- 3972, MACS 4028 and HI- 8737) were collected from wheat repository, ICAR-Central Soil Salinity Research Institute, Karnal along with salt tolerant checks (KRL-99 and KRL 3–4) and salt sensitive, HD 2009. Seeds of each genotype were surface sterilized with 0.1% Bavistin and sown in 20 kg capacity clay/porcelain pots filled with sand. Nutrients were supplied with Hoagland nutrient solution. The region witnesses subtropical and sub-humid climate with hot summers. The net house was covered with a high quality transparent polythene sheet to avoid the rain water and maintain the salinity treatments.
Table 1.
Effect of saline water irrigation on water potential (ψp) of roots and shoots in wheat genotypes.
| Source Variation | Water potential root (−MPa) |
Water potential shoot (−Mpa) |
||||
|---|---|---|---|---|---|---|
| df | Mean of Square | F-ratio | df | Mean of Square | F-ratio | |
| Rep | 2 | 0.0048 | 1.94 | 2 | 0.0014 | 0.7704 |
| Genotype | 14 | 0.8865** | 361.05 | 14 | 0.582** | 318.36 |
| Trt | 2 | 27.4349** | 11763.74 | 2 | 14.95** | 9803.16 |
| Genotype × trt | 28 | 0.2212 | 94.84 | 28 | 0.147** | 96.52 |
| Treatments/Genotypes | Control | ECiw – 6 dS m−1 | ECiw – 10 dS m−1 | Control | ECiw – 6 dS m−1 | ECiw −10 dS m−1 |
| MACS 3949 | 1.08bcde | 1.97bc | 2.61e | 0.87D | 1.06F | 2.25C |
| MACS 4020 | 0.96defgh | 1.73de | 2.93c | 0.93CD | 0.95F | 1.54HI |
| HI 8737 | 0.9gh | 1.12h | 1.94g | 1.11AB | 1.43BC | 1.86E |
| KRL – 99 | 0.99cdefg | 1.17h | 1.67h | 1.06AB | 1.24DE | 1.65GH |
| HD 4728 | 1.17ab | 1.62ef | 2.74de | 1.02BC | 1.2E | 1.51I |
| MACS 4028 | 1.09bcd | 1.86cd | 3.14ab | 0.75EF | 1.02F | 2.05D |
| KRL 3–4 | 1.04bcdef | 1.17h | 1.71h | 0.62G | 0.75G | 1.56HI |
| HD 4730 | 1.27a | 2.04b | 3.12b | 0.59G | 1.71A | 2.51B |
| HI 8759 | 0.94fgh | 1.22h | 2.95c | 0.64FG | 1.26DE | 2.67A |
| HI 8758 | 0.85h | 1.18h | 1.87g | 0.85DE | 1.42C | 1.73FG |
| HD 2009 | 1.12bc | 2.35a | 3.27a | 0.83DE | 1.35CD | 2.24C |
| HD 4758 | 0.94efgh | 1.41g | 2.75d | 1.04ABC | 1.26DE | 1.84EF |
| MACS 3972 | 0.86gh | 1.21h | 2.47f | 1.07AB | 1.67A | 2.08D |
| HI 8708 | 1.05bcdef | 1.73de | 2.71de | 1.13AB | 1.71A | 2.72A |
| HI 8713 | 0.98defgh | 1.59f | 2.45f | 1.14A | 1.54B | 2.47B |
2.2. Observations and data analysis
The observations on different physiological parameters such as water potential (ψp), osmotic potential (ψs), relative water content (RWC), Na+ and K+ content were recorded in roots as well as shoots at the reproductive stage. Photosynthetic rate and chlorophyll content were also measured in the flag leaves. Water potential (ψp) was measured by chilled-mirror dew point technique using WP4C Dewpoint Potentia Meter (METER Group, Inc. USA) and expressed in -MPa. Osmotic potential was measured using Vapour Pressure Osmometer (Model 5600, ELITech Group, Belgium) and quantified as mmol kg−1. Relative water content in roots and shoots was measured by the method of Weatherley (1950). Net photosynthesis (Pn) was measured between 10:00 AM and 12.00 PM in the flag leaves using the portable photosynthetic system (Li 6800, Li-Cor Biosciences, USA). Chlorophyll content was estimated according to the method of Hiscox & Israelstam (1979) using dimethyl sulfoxide (DMSO). The leaves used for estimation of chlorophyll were tagged for measuring photosynthesis also. Chlorophyll content was expressed in mg g−1 FW according to (Welburn, 1994) formula. For ionic analysis, the plants were uprooted and washed with distilled water to remove dust and salt particles. Oven dried and finely ground root and shoot (100 mg each) were digested separately with 10 ml of HNO3:HClO4 (3:1) di-acid mixture and measurements were taken on flame photometer (Systronics Flame Photometer 128) using standard NaCl and KCl. Data was analysed using factorial RBD for two factors. For critical difference (CD), treatments and genotypes were compared at 5% level of significance using SAS (Version 9.3, SAS Institute Inc., Cary, NC, USA).
3. Results and discussion
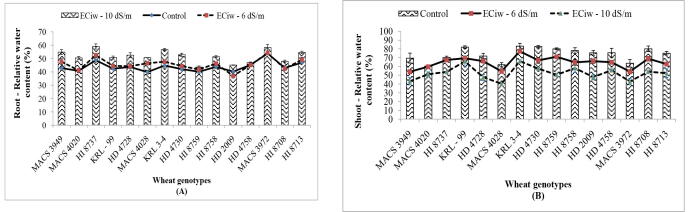
Five plants were randomly selected and tagged for recording observations for all the growth and development studies during the crop duration. Flag leaves were used for taking different observations at reproductive stage after seeing the visible effect of stresses (tip burning/yellowing of leaves). Relative water content (RWC) depicts the level of cellular and tissue hydration, important for the physiological plant metabolism and explains how plants control or maintain the hydration of cells up to an optimal level under stress conditions (Pooja et al., 2019, Sheoran et al., 2021). In the present study, root RWC was not much affected at moderate stress conditions of ECiw – 6 dS m−1 but further increase in stress level caused increase in water content and genotypes MACS 3949, MACS 4028, KRL 3–4 and HD 4730 showed more than 25 per cent increase in root RWC (Fig. 1A). It has been reported that roots showed higher RWC by sequestering toxic ions to mitigate the negative effect of stress and to maintain water balance in the cell, crucial for plant survival under osmotic stress conditions generated by high salinity (Annunziata et al., 2017). Durum wheat roots maintain plant water homeostasis under osmotic stress. On the other hand, relative water content in shoot decreased with increase of stress level and higher reduction was observed at salinity of ECiw – 10 dS m−1 (Fig. 1B). The maximum reduction in shoot RWC was recorded in genotype MACS 3949 (37.87%) followed by HI 8759 (37.12%) and HD 2009 (36.7%) and minimum reduction in MACS 4020 (15.6%), KRL 99 (19.83%) and KRL 3–4 (20.57%). Salt stress induced a reduction in the relative water content of the leaves, which indicates a loss of turgor that resulted in limited water availability for cell extension process and thus dehydration at cellular level (Kumar et al., 2018b, Makarana et al., 2019, Yadav et al., 2020). Genotype-dependent reduction trends in leaf RWC have been reported in Australian durum wheat under water deficiency and heat during reproductive stage (Liu et al., 2019). Leaf traits associated with better physiological performance are preferably used in phenotyping for breeding experiments.
Fig. 1.
Effect of saline water irrigation on relative water content (%) of roots (A) and shoots (B) in wheat genotypes.
Plant water relations, chlorophyll content, photosynthetic rate and ion content are important parameters to determine plant growth/physiological efficiency of crop plants under stress conditions. Water potential (ψp) and Osmotic potential (ψs) are the physiological parameters used for quantifying the stress level in plants (Kumar et al., 2012, Pooja et al., 2019, Lata et al., 2019). The water potential (ψp) of roots and shoots decreased significantly in all the wheat genotypes. Significant variations were observed among the genotypes over the treatments for roots and shoots, respectively. Higher reductions were noted in the values of ψp under stress condition of ECiw – 10 dSm−1 (Table 1). Maximum reduction in root ψp were observed in the genotype MACS 4028 (−3.14 MPa) and HI 4730 (−3.12 MPa) at ECiw – 10 dSm−1 than the respective control i.e. −1.09 MPa and −1.27 MPa. The genotypes, KRL 99, KRL 3–4, HI 8758 and HI 8737 showed the lowest values of root ψp under highest stress level of ECiw – 10 dSm−1. It has been reported earlier that at low ψw, root elongation takes place, although at a reduced rate, whereas shoot elongation was completely inhibited (Sharp et al., 1988, Wu and Cosgrove, 2000). Decrease in the root ψw was caused by specific toxic effects due to the accumulation of Na+ and Cl– ions in root tissues, or by the imbalance in the acquisition of other nutrients under high salinity conditions (Aroca et al., 2012). Similar results of decreasing ψp in wheat genotypes with increase of stress level were also recorded for shoot ψp but the decrease in value of ψp is less in comparison to roots (Table 1). In case of shoots, maximum decrease were observed in genotype HI 8708 (−2.72 MPa) followed by HI 8759 (−2.67 MPa) and HI 4730 (−2.51 MPa) at ECiw – 10 dSm−1 whereas genotypes showing lower values were HI 4728 (−1.51 MPa), MACS 4020 (−1.54 MPa), KRL 3–4 (−1.56 MPa) and KRL 99 (−1.65 MPa). Increase of salt in the root medium can lead to a decrease in water potential due to reduction in root hydraulic conductivity resulting in decreased water flow from roots to shoot and this decrease in water flow due to stress may cause a lowering in leaf water content (Neto et al., 2004, Nandwal et al., 2007, Pooja et al., 2019).
Osmotic potential (ψs) is another important physiological parameter used for recording the extent of stress level in plants. Generally in saline soils, plants cannot take up enough water to meet their evaporative demands and thus, low turgor creates a decrease in osmotic potential. From the results obtained, it was observed that roots showed higher values of osmotic potential (ψs) than the shoots (Table 2). Higher values of ψs (mmol kg−1) depicted lower osmotic potential in -Mpa.
Table 2.
Effect of saline water irrigation on osmotic potential (mmol kg−1) of roots and shoots in wheat genotypes.
| Source variation | Osmotic potential root (mmol kg−1) |
Osmotic potential shoot (mmol kg−1) |
||||
|---|---|---|---|---|---|---|
| df | Mean of Square | F-ratio | df | Mean of Square | F-ratio | |
| Rep | 2 | 13.655 | 1.467 | 2 | 14.73 | 2.297 |
| Genotype | 14 | 4841.821** | 520.298 | 14 | 1273.55** | 198.552 |
| Trt | 2 | 106147.05** | 8413.968 | 2 | 18991.82** | 4229.826 |
| Genotype × Trt | 28 | 815.317** | 64.627 | 28 | 290.8** | 64.758 |
| Treatments/Genotypes | Control | ECiw – 6 dS m−1 | ECiw – 10 dS m−1 | Control | ECiw – 6 dS m−1 | ECiw – 10 dS m−1 |
| MACS 3949 | 75.0de | 138.8c | 174e | 71.5a | 83cd | 119c |
| MACS 4020 | 51.0h | 98f | 162g | 54ef | 62f | 127b |
| HI 8737 | 71.0defg | 103f | 164fg | 74a | 84cd | 104.5de |
| KRL – 99 | 91.0c | 104f | 149h | 59cde | 67ef | 78gh |
| HD 4728 | 102ab | 169a | 283a | 61cd | 63.5f | 81gh |
| MACS 4028 | 107a | 152b | 185cd | 74a | 86bc | 107de |
| KRL 3–4 | 73.5defg | 101f | 122j | 53ef | 62f | 76h |
| HD 4730 | 79d | 116e | 189bcd | 70ab | 91ab | 128b |
| HI 8759 | 65fg | 86g | 137j | 65bc | 93a | 101e |
| HI 8758 | 97bc | 127d | 187bcd | 65bc | 78d | 109d |
| HD 2009 | 105ab | 136cd | 195b | 76a | 95a | 144a |
| HD 4758 | 69efg | 101f | 181de | 64bc | 86bc | 102e |
| MACS 3972 | 74def | 97f | 163g | 57.5de | 71e | 83g |
| HI 8708 | 92c | 129cd | 193bc | 61cd | 65ef | 107de |
| HI 8713 | 50f | 62f | 92f | 50f | 62f | 92f |
Significant variations were noted among the genotypes (p < 0.001) and it was found that root ψs ranged between (50 mmol kg−1 to 107 mmol kg−1) in control conditions. With the increase in salinity level the root ψs values were increased and maximum increase was observed in genotype HD 4728 (283 mmol kg−1) followed by HD 2009 (195 mmol kg−1) and HI 8708 (193 mmol kg−1) but the per cent increase was maximum in MACS 4020 (217.64%) at ECiw – 10 dSm−1 (Table 2). This showed that with the increase in intensity and duration of stress, the genotypes earmarked higher decrease in root ψs. This decrease of osmotic potential is considered to be an osmotic adaptation and is one of the defense strategies against salt stress (Nandwal et al., 2007, Hajlaoui et al., 2010). Mild and moderate salinity levels induced a significant reduction in leaf osmotic potential but the reductions were lower than the roots. Some genotype showed lesser increase in ψs at ECiw – 10 dSm−1 viz. KRL 99 (32.2%), HI 4728 (32.78%), HI 8737 (41.21%) and KRL 3–4 (43.39%) whereas MACS 4020 showed maximum increase in shoot ψs i.e. 135.18% followed by HD 2009 (89.47%) in comparison to their respective controls. The possible reason for decreasing Ψs is that plants adjust to physiological drought conditions caused by salinity to maintain pressure potential (Wright et al., 1997, Kumar et al., 2008, Kumar et al., 2012). It was also reported in literature that degree of decline in ψw and ψs depends upon the tolerance ability of genotypes which were able to absorb water from the rhizosphere (Singh, 2010) and these parameters (ψw and ψs) could be useful in determining and developing appropriate irrigation management to improve crop production in saline areas.
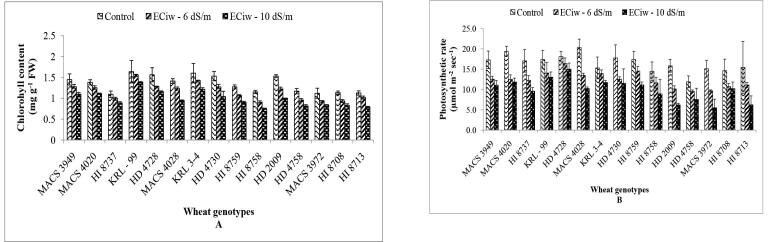
Photosynthesis, as one of the most important physiological processes, provides 90% of the plant dry matter (Steduto et al., 2000, Sheoran et al., 2021). Photosynthetic pigments particularly chlorophyll allows plants to absorb energy from light and acts as a key factor or an indicator of the photosynthetic capacity of plants (Taiz and Zeiger, 2006, Kumar et al., 2016a). Fig. 2A showed that moderate to extreme salinity stress levels, lead to decrease in chlorophyll content in all the genotypes. More than 30 per cent reduction in the chlorophyll content was observed in genotypes HI 8758, HD 2009, MACS 4028 and HD 4730 at ECiw – 10 dSm−1 whereas KRL 99 showed minimum reduction of 15.39 per cent followed by HI 8737 (18.99%) and MACS 4020 (19.53%) under highest salinity of ECiw – 10 dSm−1 (Fig. 2A). This decrease in chlorophyll content might be due to reduced activity of ALA synthase enzyme responsible for chlorophyll synthesis or due to increased activity of chlorophyll degrading enzyme, chlorophyllase (Garg and Singla, 2004, Singh et al., 2016; Mann et al., 2018) or the excessive accumulation of Na+ in the leaf tissues resulted in an alteration of the chlorophyll pigments, and/or a restriction of its biosynthesis which translates into a chlorosis of leaves (Yadav et al., 2020).
Fig. 2.
Effect of saline water irrigation on chlorophyll content (A) and photosynthetic rate (B) in wheat genotypes.
Significant variability was observed among different wheat genotypes for chlorophyll content and photosynthetic rate under the salt stress conditions. Gas exchange particularly CO2 exchange was regarded as an important indicator of the growth of plants, because of its direct link to net productivity (Ashraf, 2004). The decrease in chlorophyll content is directly correlated with a decrease in the photosynthetic activity of plants (Su et al., 2016). Under moderate stress of ECiw – 6 dSm−1, net reduction in photosynthesis was in order of MACS 3972 (36.01%) followed by HD 2009 (35.77%), MACS 4020 (35.47%) and MACS 4028 (33.63%) (Fig. 2B). Further elevation in the stress condition leads to much higher reduction i.e. 63.6% in MACS 3972, 60.71% in HD 2009 and 59.44% in HI 8713. These reductions in the net photosynthesis (Pn) might be attributed to reduced efficiency of ribulose-1, 5-bisphosphate (RuBP) carboxylase, to a reduction in RuBP regeneration capacity, or to the sensitivity of PSII to excessive accumulation of Na+ in the leaf tissues (Kumar et al., 2016b). Such inhibition in the photosynthetic rate might coincide with a strong decrease in ψp and ψs contributing to a positive water balance. While some genotype showed little resistance in terms of per cent reduction towards the effect of salinity stress on photosynthetic rate (Fig. 2B) i.e. genotypes HD 4728, KRL 99 and KRL 3–4 showed reduction of less than 25 per cent under the extreme salinity level of ECiw – 10 dSm−1. Perturbation in gas exchange attribute could be associated with decreased utilization efficiency of light, stomatal closure and the resulting CO2 deficit in the chloroplasts, which was the main cause of decreased photosynthesis under mild and moderate stresses (Kumar et al., 2016a).
Significant variation (p < 0.001) was observed for Na+ under different saline treatments among the wheat genotypes. Higher Na+ accumulation was recorded in roots as compared to shoots. Under control conditions, root Na+ content ranged from 1.06 to 1.79% among the genotypes which increased to 2.02–4.69% under moderate salinity and 3.17–6.64% under extreme salinity (Table 3). Maximum accumulation was observed in genotypes MACS 4028 (6.64%) followed by HD 4730 (6.56%), HD 2009 (6.5%) and MACS 4020 (6.31%) under highest stress of ECiw – 10 dSm−1 (Table 3) whereas non significant differences were noted for genotypes KRL 99 (3.17g), KRL 3–4 (3.34g) and HI 8737 (3.41g) which had accumulated lower Na+ in their roots at ECiw – 10 dSm−1. Ionic toxicity is one of the main components of salt stress that commences with the accumulation of injurious concentrations of ions such as Na+ and Cl– in the plant cells. The cytosol of plant cells normally contains 100–200 mM K+ and 1–10 mM Na+; this Na+/K+ ratio is optimal for many metabolic cell functions (Kader et al., 2006). Both Na+ and K+ ions compete for entry into plant root cells and the replacement of K+ by Na+ often leads to nutritional imbalances (Singh et al., 2018). Maintenance of low leaf Na+ concentration and lower Na+/K+ ratio is an important aspect of stress tolerance (Kumar et al., 2016b). The over-accumulation of Na+ may create detrimental effects on the availability of water in a root medium that can lead to cell dehydration and reduced turgor (Flowers et al., 1991).
Table 3.
Effect of saline water irrigation on Na+ content of roots and shoots in wheat genotypes.
| Source variation |
Na+ content root (% DW) |
Na+ content shoot (% DW) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| df | Mean of Square | F-ratio | df | Mean of Square | F-ratio | |||||
| Rep | 2 | 0.0004 | 0.0493 | 2 | 0.0002 | 0.057 | ||||
| Genotype | 14 | 3.856 | 493.4013 | 14 | 1.0980 | 311.809 | ||||
| Trt | 2 | 140.94 | 16221.1702 | 2 | 30.74 | 10340.4682 | ||||
| Genotype × Trt | 28 | 1.138 | 131.0597 | 28 | 0.3374 | 113.4965 | ||||
| Treatments/Genotypes | Control | ECiw – 6 dS m−1 | ECiw – 10 dS m−1 | Control | ECiw – 6 dS m−1 | ECiw – 10 dS m−1 | ||||
| Absolute value | % change | Absolute value | % change | Absolute value | % change | Absolute value | % change | |||
| MACS 3949 | 1.42bc | 3.13c | 120.42 | 4.75d | 234.50 | 0.69ef | 1.34hi | 94.20 | 1.86h | 169.56 |
| MACS 4020 | 1.08ef | 2.46d | 127.7 | 6.31b | 484.25 | 1.54a | 2.05abc | 33.11 | 2.67g | 73.37 |
| HI 8737 | 1.06f | 2.01e | 89.62 | 3.41g | 221.69 | 1.22b | 2.01abcd | 64.75 | 3.51b | 187.70 |
| KRL – 99 | 1.16def | 2.06e | 77.58 | 3.17g | 173.27 | 1de | 1.23i | 23.0 | 1.3i | 30.0 |
| HD 4728 | 1.55ab | 2.97c | 91.61 | 5.41c | 249.03 | 1.14bcd | 1.91bcde | 67.54 | 2.62g | 129.82 |
| MACS 4028 | 1.44bc | 3.21c | 122.9 | 6.64a | 361.11 | 1.18bc | 1.85def | 56.77 | 3.15cd | 166.94 |
| KRL 3–4 | 1.34bcde | 2.51d | 87.31 | 3.34g | 149.25 | 1de | 1.21i | 21.0 | 1.77h | 77.0 |
| HD 4730 | 1.79a | 4.69a | 162.01 | 6.56ab | 266.48 | 1.26b | 2.07ab | 64.28 | 2.86f | 126.98 |
| HI 8759 | 1.29bcdef | 3.11c | 141.08 | 5.54c | 329.45 | 1.28b | 1.67g | 30.46 | 2.65g | 107.03 |
| HI 8758 | 1.22cdef | 2.55d | 109.01 | 4.08f | 234.42 | 1.29b | 2.04abc | 58.13 | 3.83a | 196.89 |
| HD 2009 | 1.45bc | 4.14b | 185.51 | 6.5ab | 348.27 | 0.76f | 1.9cde | 150.0 | 3.1d | 307.89 |
| HD 4758 | 1.25cdef | 2.35d | 88.0 | 4.29ef | 243.20 | 1.03cde | 1.78efg | 72.81 | 2.92ef | 183.49 |
| MACS 3972 | 1.29bcdef | 2.52d | 95.34 | 4.13f | 220.15 | 1de | 1.73fg | 73.0 | 3.04de | 204.0 |
| HI 8708 | 1.76a | 3.22c | 82.95 | 4.86d | 176.13 | 1.3b | 2.16a | 66.15 | 3.26c | 150.76 |
| HI 8713 | 1.36bcd | 3.11c | 128.67 | 4.48e | 229.41 | 0.97e | 1.4h | 44.32 | 2.92ef | 201.03 |
Higher Na+ accumulated in shoots with the increase in salinity but to a lesser extent than roots. In shoots (particularly flag leaves), Na+ content ranged from 0.77% to 1.55% under control conditions which increased to 1.38–3.84% at ECiw – 10 dSm−1 (Table 3). Under moderate saline conditions, only 2–3 genotypes showed higher Na+ accumulation i.e. (<75% increase over control). But under extreme salinity of ECiw – 10 dSm−1 only KRL 99, MACS 3949 and KRL 3–4 showed lowest accumulation i.e. 30.0%, 73.37% and 77.0% respectively (Table 3). High Na+ reduces the amounts of available K+, Mg++ and Ca++ for plants resulting in Na+ toxicity on one hand and deficiencies of essential cations on the other hand (Kumar et al., 2018a, Lata et al., 2019).
Potassium (K+) is an essential macronutrient in plants comprising generally 4–6% of its dry matter and is recognized as a rate-limiting factor that plays important functions related to enzyme activation, osmotic adjustment and turgor generation, regulation of membrane potential, and cytoplasmatic pH homeostasis (PPI, 1998, Barragan et al., 2012, Zorb et al., 2014). Results showed that K+ content either in roots or shoots decreased with increasing salinity stress (Table 4) and significant variations were noted among the genotypes for K+ accumulation. In case of roots, K+ content ranged from 7.60 to 9.74% under the control conditions which decreased to 5.18–8.13% at ECiw – 6 dSm−1 and 2.49–6.22% at ECiw – 10 dSm−1 (Table 4) and the genotypes, KRL 99, KRL 3–4 and HD 4728 displayed the lowest decrease and the observed performance might be due to a stronger osmotic adjustment, which is considered to be important for plant adaptation to salt stress since it contributes to the maintenance of turgor and cell volume (Lata et al., 2017; Mann et al., 2019b). While in comparison to roots, shoots (flag leaves) had lower K+ accumulation. The range of K+ is 4.21–6.61% under control environment with a mean value of 5.21% among the genotypes which decreased by 28.8 per cent at ECiw – 6 dSm−1 and 46.05 per cent at ECiw −10 dSm−1 (Table 4). The lowest decrease was exhibited by KRL 99 (26.53%) followed by KRL 3–4 (29.75%), HI 8713 (29.76%) and HD 4758 (30.33%) among all the genotypes. Similar results of decrease in K+ content in both roots and shoots were also noted by Chen et al. (2005) in seven barley cultivars. Reducing salt induced K+ efflux would allow its contribution towards osmoregulation, negating the need for a high investment into the production of organic solutes and allowing the critical maintenance of optimal cytosolic K+/Na+ ratio (Cuin et al., 2010). Shabala, 2000, Shabala et al., 2003 also suggested that a cell's ability to retain K+ is at least as important for plant salt tolerance as its ability to exclude or compartmentalize toxic Na+.
Table 4.
Effect of saline water irrigation on K+ content of roots and shoots in wheat genotypes.
| Source variation |
K+ content root (% DW) |
K+ content shoot (% DW) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| df | Mean of Square | F-ratio | df | Mean of Square | F-ratio | |||||
| Rep | 2 | 0.0231 | 1.0321 | 2 | 0.0048 | 0.439 | ||||
| Genotype | 14 | 5.344** | 238.5963 | 14 | 1.6276** | 150.2015 | ||||
| Trt | 2 | 214.069** | 5408.9679 | 2 | 66.2004** | 4705.3809 | ||||
| Genotype × Trt | 28 | 1.398** | 35.319 | 28 | 0.6713** | 47.7136 | ||||
| Treatments/Genotypes | Control | ECiw – 6 dS m−1 | ECiw – 10 dS m−1 | Control | ECiw – 6 dS m−1 | ECiw – 10 dS m−1 | ||||
| Absolute value | % change | Absolute value | % change | Absolute value | % change | Absolute value | % change | |||
| MACS 3949 | 9.04b | 6.9de | −23.67 | 5.36b | −40.70 | 6.35a | 5.13a | −19.21 | 4.06a | −36.06 |
| MACS 4020 | 8.52bcde | 7.79ab | −8.56 | 3.19ef | −62.55 | 4.75fg | 3.44ef | −27.57 | 2.26h | −52.42 |
| HI 8737 | 9.71a | 6.57efg | −32.33 | 5.08b | −47.68 | 5.68b | 3.69cde | −35.03 | 2.63efg | −53.69 |
| KRL – 99 | 8.62bcde | 7.47bc | −13.34 | 6.07a | −29.58 | 4.56g | 3.92bc | −14.03 | 3.35bc | −26.53 |
| HD 4728 | 8.96b | 7.83ab | −12.61 | 5.97a | −33.37 | 5.21cde | 3.31fg | −36.46 | 2.46fgh | −52.78 |
| MACS 4028 | 8.15ef | 6.83def | −16.19 | 4.51c | −44.66 | 5.29cde | 3.31fg | −37.42 | 2.31gh | −56.33 |
| KRL 3–4 | 8.73bcd | 8.03a | −8.01 | 6.22a | −28.75 | 5.21cde | 4.21b | −19.19 | 3.66b | −29.75 |
| HD 4730 | 9.74a | 6.61efg | −32.13 | 3.62de | −62.83 | 5.05ef | 3.88c | −23.16 | 2.77def | −45.14 |
| HI 8759 | 8.31de | 6.32fgh | −23.94 | 2.73fg | −67.14 | 5.52bc | 3.77cd | −31.70 | 2.39gh | −56.70 |
| HI 8758 | 7.63fg | 5.94hi | −22.14 | 2.48g | −67.49 | 4.85fg | 3.54def | −27.01 | 2.28h | −52.98 |
| HD 2009 | 8.41cde | 7.18cd | −14.62 | 3.46e | −58.85 | 5.45bcd | 3.25fg | −40.36 | 2.42gh | −55.59 |
| HD 4758 | 7.6g | 5.59ij | −26.44 | 4.07cd | −46.44 | 4.22h | 3.53def | −16.35 | 2.94de | −30.33 |
| MACS 3972 | 8.93bc | 6.63efg | −25.75 | 3.99cd | −55.31 | 5.18de | 3.73cde | −27.99 | 3.08cd | −40.54 |
| HI 8708 | 8.22de | 6.24g | −24.08 | 3.47e | −57.78 | 6.61a | 3.1g | −53.10 | 2.57fgh | −61.11 |
| HI 8713 | 7.68fg | 5.17j | −32.68 | 2.9fg | −62.23 | 4.2h | 3.8cd | −9.52 | 2.95de | −29.76 |
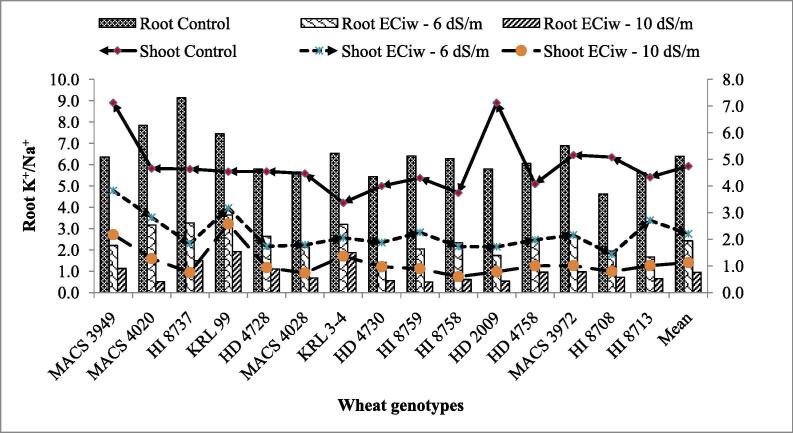
One of the key features of plant salt tolerance is the ability of plant cells to maintain optimal K+/Na+ ratio in the cytosol (Maathuis and Amtmann, 1999, Tester and Davenport, 2003, Kumar et al., 2016b) and different genotypes showed variability‘s (p < 0.001). It was observed from the results that all the genotypes exhibited higher K+/Na+ under control conditions in roots as well as shoot tissues which declined under mild and extreme stress condition (Fig. 3). Excessive Na+ accumulation in the cytosol and higher K+ leakage from the cell might be responsible for such drop down in K+/Na+ (Mann et al., 2015, Kumar et al., 2016b). Highest K+/Na+ (>1) was shown by genotypes KRL 99 followed by KRL 3–4, HI 8737, MACS 3949, HD 4728 in roots and KRL 99 followed by MACS 3949, KRL 3–4, MACS 4020, HD 4758, MACS 3972 and HI 8713 in shoots in comparison with their respective mean at ECiw – 10 dSm−1. It is reported in literature that the optimal value of K+/Na+ is about one which could be used as determinative trait in salt tolerance (Maathuis and Amtmann, 1999; Kumar et al., 2018c). Thus, it seems that any genotypes to be defined as salt tolerant might have the ability to retain K+ efficiently with the ability to prevent excessive accumulation of Na+ (Chen et al., 2007, Mann et al., 2019c).
Fig. 3.
Effect of saline water irrigation on K+/Na+ of roots and shoots in wheat genotypes.
4. Conclusion
Plant adaptive responses to salinity stress require orchestrated regulation of a plethora of physiological mechanisms and thus imply efficient root to shoot communication. Bread wheat is generally considered as a more salt-tolerant species than durum wheat but some genotypes have the potential (traits specific) to survive under saline conditions such as genotypes MACS 3949, HI 8737, MACS 4020, HD 4758 and HI 8713 maintained their K+/Na+ higher under extreme salinity of ECiw – 10 dSm−1 than the optimal value reported in literature. HD 4728 and HI 8708 showed better performance in term of photosynthesis by maintaining higher chlorophyll content. HI 8728 and HI 8737 showed less reduction in plant water traits (RWC, ψp and ψs). So based on these results, it was concluded that durum wheat also have some potential in terms of physiological traits governing the crop growth and yield under saline conditions and could be useful in crop improvement programme. The positive correlations between yield parameters and physiological traits under salt stress can pinpoint the tolerance mechanism and lead to defined strategies for breeding programmes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors are thankful to Head, Division of Crop Improvement and Director, CSSRI, Karnal for providing necessary facilities for the research work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Annunziata M.G., Ciarmiello L.F., Woodrow P., Maximova E., Fuggi A., Carillo P. Durum wheat roots adapt to salinity remodeling the cellular content of nitrogen metabolites and sucrose. Front. Plant Sci. 2017;7:1–16. doi: 10.3389/fpls.2016.02035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroca R., Porcel R., Ruiz-Lozano J.M. Regulation of root water uptake under abiotic stress conditions. J. Exp. Bot. 2012;63(1):43–57. doi: 10.1093/jxb/err266. [DOI] [PubMed] [Google Scholar]
- Ashraf M. Some important physiological selection criteria for salt tolerance in plants. Flora - Morphology Distribution. Funct. Ecol. Plants. 2004;199:361–376. doi: 10.1078/0367-2530-00165. [DOI] [Google Scholar]
- Barragan V., Leidi E., Andres Z., Rubio L., De Luca A., Fernandez J., Cubero B., Pardo J.M. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell. 2012;24:1127–1142. doi: 10.1105/tpc.111.095273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carillo P., Parisi D., Woodrow P., Pontecorvo G., Massaro G., Annunziata M.G., Fuggi A., Sulpice R. Salt-induced accumulation of glycine betaine is inhibited by high light in durum wheat. Funct. Plant Biol. 2011;38(2):139–150. doi: 10.1071/FP10177. [DOI] [PubMed] [Google Scholar]
- Chen Z., Newman I., Zhou M., Mendham N., Zhang G., Shabala S. Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant, Cell Environ. 2005;28(10):1230–1246. [Google Scholar]
- Chen Z.H., Pottosin I.I., Cuin T.A., Fuglsang A.T., Tester M., Jha D., Zepeda-Jazo I., Zhou M., Palmgren M.G., Newman I.A., Shabala S. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiol. 2007;145(4):1714–1725. doi: 10.1104/pp.107.110262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V., Jagendorf A., Zhu J.K. Understanding and improving salt tolerance in plants. Crop Sci. 2005;45(2):437–448. doi: 10.2135/cropsci2005.0437. [DOI] [Google Scholar]
- Colmer T.D., Flowers T.J., Munns R. Use of wild relatives to improve salt tolerance in wheat. J. Exp. Bot. 2006;57(5):1059–1078. doi: 10.1093/jxb/erj124. [DOI] [PubMed] [Google Scholar]
- Cuin T.A., Parsons D., Shabala S. Wheat cultivars can be screened for NaCl salinity tolerance by measuring leaf chlorophyll content and shoot sap potassium. Funct. Plant Biol. 2010;37(7):656–664. doi: 10.1071/FP09229. [DOI] [Google Scholar]
- Datta J.K., Nag S., Banerjee A., Mondal N.K. Impact of salt stress on five varieties of wheat (Triticum aestivum L.) cultivars under laboratory condition. J. Appl. Sci. Environ. Manage. 2009;13(3):93–97. [Google Scholar]
- Flowers T.J., Hajibagheri M.A., Yeo A.R. Ion accumulation in the cell walls of rice plants growing under saline conditions – evidence for the Öertli hypothesis. Plant Cell Environ. 1991;14(3):319–325. doi: 10.1111/j.1365-3040.1991.tb01507.x. [DOI] [Google Scholar]
- Garg N., Singla R. Growth, photosynthesis, nodule nitrogen and carbon fixation in the chickpea cultivars under salt stress. Braz. J. Plant Physiol. 2004;16(3):137–146. doi: 10.1590/S1677-04202004000300003. [DOI] [Google Scholar]
- Hajlaoui H., El Ayeb N., Garrec J.P., Denden M. Differential effects of salt stress on osmotic adjustment and solutes allocation on the basis of root and leaf tissue senescence of two silage maize (Zea mays L.) varieties. Ind. Crops Prod. 2010;31(1):122–130. doi: 10.1016/j.indcrop.2009.09.007. [DOI] [Google Scholar]
- Hiscox J.D., Israelstam G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979;57(12):332–334. doi: 10.1139/b79-163. [DOI] [Google Scholar]
- James R.A., Blake C., Byrt C.S., Munns R. Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1;4 and HKT1;5), decrease Na+ accumulation in bread wheat leaves under saline and waterlogged conditions. J. Exp. Bot. 2011;62(8):2939–2947. doi: 10.1093/jxb/err003. [DOI] [PubMed] [Google Scholar]
- Kader M.A., Seidel T., Golldack D., Lindberg S. Expressions of OsHKT1, OsHKT2, and OsVHA are differentially regulated under NaCl stress in salt-sensitive and salt-tolerant rice (Oryza sativa L.) cultivars. J. Exp. Bot. 2006;57(15):4257–4268. doi: 10.1093/jxb/erl199. [DOI] [PubMed] [Google Scholar]
- Kumar A., Krishnamurthy S.L., Lata C., Kumar P., Devi R., Kulshrestha N., Yadav R.K., Sharma S.K. Effect of dual stress (salinity and drought) on gas exchange attributes and chlorophyll fluorescence characteristics in rice. Ind. J. Agr. Sci. 2016;86(6):19–27. [Google Scholar]
- Kumar A., Kumar A., Kumar P., Lata C., Kumar S. Effect of individual and interactive alkalinity and salinity on physiological, biochemical and nutritional traits of Marvel grass. Ind. J. Expt. Biol. 2018;56(8):573–581. http://nopr.niscair.res.in/handle/123456789/44838 [Google Scholar]
- Kumar A., Kumar A., Lata C., Kumar S. Eco-physiological responses of Aeluropus lagopoides (grass halophyte) and Suaeda nudiflora (non-grass halophyte) under individual and interactive sodic and salt stress. South Afr. J. Bot. 2016;105:36–44. doi: 10.1016/j.sajb.2015.12.006. [DOI] [Google Scholar]
- Kumar A., Mann A., Kumar A., Devi S., Sharma P.C. Potential and role of halophyte crops in saline environments. In: Gupta S.K., Goyal M.R., Singh A., editors. Engineering Practices for Management of Soil Salinity. Apple Academic Press Inc.; Canada: 2018. pp. 329–365. [Google Scholar]
- Kumar A., Mann A., Lata C., Kumar N., Sharma P.C. Salinity induced physiological and molecular responses of halophytes. In: Dagar J.C., editor. Research developments in saline agriculture. Springer Nature Singapore Pte Ltd.; 2019. pp. 331–356. [Google Scholar]
- Kumar A., Sharma S.K., Lata C., Devi R., Kulshrestha N., Krishnamurthy S.L., Singh K., Yadav R.K. Impact of water deficit (salt and drought) stress on physiological, biochemical and yield attributes on wheat (Triticum aestivum) varieties. Ind. J. Agr. Sci. 2018;88(10):1624–1632. [Google Scholar]
- Kumar N., Nandwal A.S., Waldia R.S., Singh S., Devi S., Sharma K.D., Kumar A. Drought tolerance in chickpea as evaluated by root characteristics, plant water status, membrane integrity and chlorophyll fluorescence techniques. Exp. Agr. 2012;48(3):378–387. doi: 10.1017/S0014479712000063. [DOI] [Google Scholar]
- Kumar N., Singh S., Nandwal A.S., Waldia R.S., Sharma M.K. Genotypic differences in water status, membrane integrity, ionic content, N-fixing efficiency and dry matter of mungbean nodules under saline irrigation. Physiol. Mol. Biol. Plants. 2008;14(4):1–6. doi: 10.1007/s12298-008-0035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lata, C., Soni, S., Kumar, N., Kumar, A., Pooja, Mann, A., Rani, S., 2019. Adaptive mechanism of stress tolerance in Urochondra (grass halophyte) using roots study. Ind. J. Agr. Sci. 89(6), 1050–1052.
- Lata Charu, Kumar Ashwani, Sharma S.K., Singh Jogendra, Sheokand Shital, Pooja, Mann Anita, Rani Babita. Tolerance to combined boron and salt stress in wheat varieties: Biochemical and molecular analyses. Ind. J. Exp. Biol. 2017:321–328. [Google Scholar]
- Liu H., Able A.J., Able J.A. Genotypic performance of Australian durum under single and combined water-deficit and heat stress during reproduction. Sci. Rep. 2019;9(1):1–17. doi: 10.1038/s41598-019-49871-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobell D.B., Burke M.B., Tebaldi C., Mastrandrea M.D., Falcon W.P., Naylor R.L. Prioritizing climate change adaptation needs for food security in 2030. Nature. 2008;319(5863):607–610. doi: 10.1126/science.1152339. [DOI] [PubMed] [Google Scholar]
- Maathuis F.J.M., Amtmann A. K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Ann. Bot. 1999;84(2):123–133. doi: 10.1006/anbo.1999.0912. [DOI] [Google Scholar]
- Mahajan S., Tuteja N. Cold, salinity and drought stresses: an overview. Arch. Biochem. Biophys. 2005;444(2):139–158. doi: 10.1016/j.abb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Makarana G., Kumar A., Yadav R.K., Kumar R., Soni P.G., Lata C., Sheoran P. Effect of saline water irrigations on physiological, biochemical and yield attributes of dual purpose pearl millet (Pennisetum glaucum) varieties. Ind. J. Agr. Sci. 2019;89(4):624–633. [Google Scholar]
- Mann A., Bishi S.K., Mahatma M.K., Kumar A. Managing Salt Tolerance in Plants: Molecular and Genomic Perspectives. Taylor and Francis Group LLC.; 2015. Metabolomics and salt stress tolerance in plants; pp. 251–266. [Google Scholar]
- Mann A., Kaur G., Kumar A., Sanwal S.K., Singh J., Sharma P.C. Physiological response of chickpea (Cicer arietinum L.) at early seedling stage under salt stress conditions. Leg. Res. 2019;42:65–632. doi: 10.18805/LR-4059. [DOI] [Google Scholar]
- Mann A., Kumar A., Saha M., Lata C., Kumar A. Stress induced changes in osmoprotectants, ionic relations, antioxidants activities and protein profiling characterize Sporobolus marginatus Hochst. Ex A. Rich. Salt tolerance mechanism. Ind. J. Expt. Biol. 2019;57:672–679. http://nopr.niscair.res.in/handle/123456789/50452 [Google Scholar]
- Mann A., Kumar N., Lata C., Kumar A., Kumar A., Meena B.L. Functional annotation of differentially expressed genes under salt stress in Dichanthium annulatum. Plant Physiol. Rep. 2019;24(1):104. doi: 10.1007/s40502-019-0434-8. [DOI] [Google Scholar]
- Mann A., Singh S., Gurpreet, Kumar A., Kumar S., Kumar B. Metabolic Adaptations in Plants During Abiotic Stress. CRC Press; 2018. Plant Ionomics: An Important Component of Functional Biology. [Google Scholar]
- Munns R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002;25(2):239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- Munns R., James R.A. Screening methods for salt tolerance: a case study with tetraploid wheat. Plant Soil. 2003;253(1):239–250. doi: 10.1023/A:1024553303144. [DOI] [Google Scholar]
- Munns R., James R.A., Xu B., Athman A., Conn S.J., Jordans C., Byrt C.S., Hare R.A., Tyerman S.D., Tester M., Plett D., Gilliham M. Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nature Biotech. 2012;30(4):360–364. doi: 10.1038/nbt.2120. [DOI] [PubMed] [Google Scholar]
- Munns R., Tester M. Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Nandwal A.S., Kukreja S., Kumar N., Sharma P.K., Jain M., Mann A., Singh S. Plant water status, ethylene evolution, N2-fixing efficiency, antioxidant activity and lipid peroxidation in Cicer arietinum L. nodules as affected by short-term salinization and desalinization. J. Plant Physiol. 2007;164(9):1161–1169. doi: 10.1016/j.jplph.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Neto, M.T.C., Reinhardt, D.H., Ledo C.A. da S., 2004. Determination of water potential on mango trees by pressure chamber. Acta Hort. 645, 425–427. 10.17660/ActaHortic.2004.645.53. [DOI]
- Pooja, Nandwal, A.S., Chand, M., Singh, K., Mishra, A.K., Kumar, A., Kumari, A., Rani, B., 2019. Varietal variation in physiological and biochemical attributes of sugarcane varieties under different soil moisture regimes. Ind. J. Exp. Biol. 57(10), 721–732. http://nopr.niscair.res.in/handle/123456789/50566.
- PPI - Potash and Phosphate Institute, 1998. Better Crops with Plant Food. 3. Vol. 32. Potash and Phosphate Institute, Atlanta: 1998. Potassium for Agriculture.
- Shabala S. Ionic and osmotic components of salt stress specifically modulate net ion fluxes from bean leaf mesophyll. Plant Cell Environ. 2000;23(8):825–837. doi: 10.1046/j.1365-3040.2000.00606.x. [DOI] [Google Scholar]
- Shabala S., Shabala L., Van Volkenburgh E. Effect of calcium on root development and root ion fluxes in salinised barley seedlings. Funct. Plant Biol. 2003;30(5):507–514. doi: 10.1071/FP03016. [DOI] [PubMed] [Google Scholar]
- Shahzad M., Saqib Z.A., Hafeez F., Bilal M., Khan S.K., Asad S.A., Akhtar J. Growth-related changes in wheat (Triticum aestivum L.) genotypes grown under salinity stress. J. Plant Nutr. 2016;39(9):1257–1265. doi: 10.1080/01904167.2015.1089902. [DOI] [Google Scholar]
- Sharma D.K., Singh A. Salinity research in India-achievements, challenges and future prospects. Water Energy Internat. 2015:35–45. [Google Scholar]
- Sharp R.E., Silk W.K., Hsiao T.C. Growth of the maize primary root at low water potentials. I. Spatial distribution of expansive growth. Plant Physiol. 1988;87(1):50–57. doi: 10.1104/pp.87.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheoran P., Basak N., Kumar A., Yadav R.K., Singh R., Sharma R., Kumar S., Singh R.K., Sharma P.C. Ameliorants and salt tolerant varieties improve rice-wheat production in soils undergoing sodification with alkali water irrigation in Indo-Gangetic Plains of India. Agr. Water Manage. 2021;243:106492. doi: 10.1016/j.agwat.2020.106492. [DOI] [Google Scholar]
- Singh A., Kumar A., Datta A., Yadav R.K. Evaluation of guava (Psidium guajava) and bael (Aegle marmelos) under shallow saline watertable conditions. Ind. J. Agr. Sci. 2018;88(5):720–725. [Google Scholar]
- Singh A., Sharma P.C., Meena M.D., Kumar A., Mishra A.K., Kumar P., Chaudhari S.K., Sharma D.K. Effect of salinity on gas exchange parameters and ionic relations in bael (Aegle marmelos Correa) Ind. J. Hort. 2016;73(1):48–53. doi: 10.5958/0974-0112.2016.00017.7. [DOI] [Google Scholar]
- Singh, S., 2010. Effect of saline irrigation on anatomical, reproductive and physiological aspects of chickpea (Cicer Arietinum L.) Genotypes. Ph.D. Thesis. CCSHAU, Hisar.
- Steduto P., Albrizio R., Giorio P., Sorrentino G. Gas-exchange response and stomatal and non-stomatal limitations to carbon assimilation of sunflower under salinity. Environ. Exp. Bot. 2000;44(3):243–255. doi: 10.1016/S0098-8472(00)00071-X. [DOI] [PubMed] [Google Scholar]
- Su W.C., Ge Y.H., Wu R.H., Xu H.L., Xue F., Lu C.T. Effects of bensulfuron-methyl residue on photosynthesis traits and chlorophyll fluorescence of corn seedlings. J. Maize Sci. 2016;24:67–74. [Google Scholar]
- Taiz L., Zeiger E. Sinauer Associates Inc.; Massachusetts, USA: 2006. Plant Physiology. [Google Scholar]
- Tester M., Davenport R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003;91(5):503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherley P.E. Studies in the water relation of cotton plants. The field measurement of water deficit in leaves. New Phytol. 1950;49:81–87. https://www.jstor.org/stable/2428690 [Google Scholar]
- World Bank, 2007. Agriculture for development. Washington DC. http://siteresources.worldbank.org/INTWDR2008/Resources/WDR00 book.
- Welburn R.W. The spectral determination of chlorophyll a and b as well as total carotenoids using various solvents with spectrophotometer of different resolution. J. Plant physiol. 1994;144:307–313. [Google Scholar]
- Wright P.R., Morgan J.M., Jessop R.S. Turgor maintenance by osmo regulation in Brassica napus and B. juncea under field condition. Ann. Bot. 1997;80(3):313–319. doi: 10.1006/anbo.1997.0444. [DOI] [Google Scholar]
- Wu Y., Cosgrove D.J. Adaptation of roots to low water potentials by changes in cell wall extensibility and cell wall proteins. J. Exp. Bot. 2000;51(350):1543–1553. doi: 10.1093/jexbot/51.350.1543. [DOI] [PubMed] [Google Scholar]
- Yadav, T., Kumar, A., Yadav, R.K., Yadav, G., Kumar, R., Kushwaha, M., 2020. Salicylic acid and thiourea mitigate the salinity and drought stress on physiological traits governing yield in pearl millet–wheat. Saudi J. Biol. Sci. 27(8), 2010–2017. 10.1016/j.sjbs.2020.06.030. [DOI] [PMC free article] [PubMed]
- Zorb C., Senbayram M., Peiter E. Potassium in agriculture–status and perspectives. J. Plant Physiol. 2014;171(9):656–669. doi: 10.1016/j.jplph.2013.08.008. [DOI] [PubMed] [Google Scholar]