Abstract
To investigate the correlation between serum renin-angiotensin system (RAS) level and Symptoms of anxiety and depression in Parkinson disease patients (PD). A number of 90 PD patients (47 males and 43 females) were collected on an empty stomach 12 h after stopping taking anti-PD medicines. ELISA has been found in Serum RAS ((Ang) I, Ang II, Ang (1–7), Angiotensin converting enzyme (ACE), ACE2). Depression scale (HAMD) and Anxiety scale (HAMA) in Hamilton are used for the assessment of signs of depression and anxiety. The 90 patients were diagnosed with moderate depression (HAMD score 8 ~ 19); in 32 of those (35.56 percent), and 12 (13.33%) were diagnosed as moderate and severe depression (HAMD score ≥ 20). 20 cases (22.22%) were diagnosed as possible anxiety disorder (HAMA score 7 ~ 13) and 16 cases (17.78%) as definite anxiety disorder (HAMA score ≥ 14). The association of serum Ang I, Ang II and Ang (1–7) with HAMD (r= − 0.820, P < 0.001; r = −0.846, P < 0.001) showed negative linkage with HAMD (r = −0.887, P < 0.003; P < 0.001; Negative correlation of the settings with HAMA (r = −0.850, P < 0.001; r = −0.887, P < 0.001; r = 0.003; r = 0.001, P < 0.001, Fig. 2, Fig. 3); The HAMD score and the HAMA score (all P > 0.05) were not associated to the serum ACE and ACE2. The serum Ang I, Ang II, and Ang (1–7) were found to be adversely associated with HAMD score (r = 0.826, P < 0,001; r = −0.818, p> >0,001; r = −0.876, P < 0,001; P = 0,001) P < 0,001; And have been negatively correlated (r = 0.870, Fig. 1, Fig. 2, Fig. 3) with AMA-scores (r = −0.876, P < 0.001, Table 1, Fig. 3), R = −0.862, P > 0.001; The HAMD score and the HAMA score (all P > 0.05) were not correlated to the serum ACE and ACE2. Finally, in PD patients, non-engine signs, including depression and anxiety, are normal. Thus, Serum levels Ang I, Ang II and Ang (1–7) were substantially decreased in female and male patients and associated with symptoms of depression and anxiety, ACE and ACE2 levels have not been attributed to signs of depression and anxiety. Serum Ang I, Ang II, and Ang (1–7) are important markers of depression and anxiety prevention and diagnosis in patients with DP.
Keywords: Parkinson's disease, AngⅠ, Ang Ⅱ, Ang (1–7), Ace, ACE2, Depression, Anxiety
1. Introduction
Parkinson's disease (PD), also known as Paralysis agitans, is a common neurodegenerative disease in the middle-aged and elderly, it is estimated to occur in 1% and 4% of individuals ages over 60 and 80 years, respectively (Huang et al., 2019, Nguyen et al., 2019). PD is a clinically and biochemically heterogeneous neurodegenerative disease, and its diagnosis, prognosis, and evaluation of possible progress basically still have major challenges. PD mainly manifests as motor symptoms, such as slow motion, resting tremor, muscle stiffness, and postural instability. Parkinson’s disease also appears to be associated with non-motor symptoms, including olfactory dysfunction, sleep problems, constipation, depression, and autonomic dysfunction, due to neurons in several other brain regions that occur before or after the loss of dopaminergic neurons Lost (Fleminger, 1991). However, non-motor symptoms such as depression and anxiety will seriously affect the quality of life of PD patients, and their treatment is an important part of the comprehensive treatment of PD (Goodarzi et al., 2016). Therefore, non-motor symptoms have attracted increasing attention. Some studies have found (Maillet et al., 2016) that the pathological changes of PD are not limited to substantia nigra, and similar pathological changes exist in autonomic nerve, olfactory bulb, cortex, etc., and are closely related to non-motor symptoms of PD. At present, the cause of PD neuronal degeneration is still unclear. Oxidative stress, mitochondrial disorder and inflammatory reaction may all play an important role (Gupta et al., 2011, Aridon et al., 2011). Several clinical or biochemical markers have been identified in PD patients. In the cerebrospinal fluid, potential biomarkers include alpha synuclein (α-syn), total tau (t-tau), phosphorylated tau 181 (p-tau), and beta-amyloid 1–42 (Aβ42). In the blood, insulinlike growth factor 1 (IGF-1) and epidermal growth factor (EGF) are biomarkers.
Serum renin-angiotensin system (RAS) is one of the important systems that regulate blood pressure and water-salt balance in the body (Kobori et al., 2007). Renin released from renal near glomerular cells can act on inactive precursor angiotensinogen to form decapeptide Ang I; Ang I was hydrolyzed by angiotensin converting enzyme (ACE) to form active octapeptide Ang II; In addition to the main components of RAS mentioned above, other components are also found to participate in RAS functional mechanism (Chen et al., 2013, Bürgelová et al., 2005). If ACE2, similar to ACE, participates in RAS bypass metabolism, Its product Ang (1–7) acts through a new G protein-coupled receptor Mas, which antagonizes or cooperates with the activation of AT receptor by Ang II (van den Eijnden et al., 2002, Liu et al., 2018). In recent years, studies have found (Wan et al., 1996) that serum RAS has the effects of promoting oxidative stress and inflammatory response, which can lead to the initiation or intensification of dopaminergic cell death and play an important role in the occurrence and development of PD. Relevant experimental data have confirmed that RAS participates in the occurrence of PD, but it is mostly limited to the research of animal models (Fang et al., 2014, Yang et al., 1996). However, there are few reports on the correlation between peripheral blood RAS level and depression and anxiety symptoms in PD patients.
The purpose of this study is to discuss the correlation between serum RAS level and depression and anxiety in patients with PD and its clinical significance.
2. Data and methods
2.1. Research objects
A total of 90 PD patients, 47 males and 43 females, were selected from January 2017 to January 2020. The age ranged from 45 to 74 years, with an average of (65.32 ± 5.18) years. Body Mass Index (BMI) ranged from 21 to 32 kg/m2, with an average of (26.84 ± 1.52) kg/m2; The education level ranged from 1 to 17 years, with an average of (10.27 ± 3.14) years. The course of the disease ranged from 0.5 to 12 years, with an average course of (5.78 ± 1.04) years. The score of UPDRS Ⅰ was 0 ~ 11, the average score was (4.63 ± 1.28); The third part (UPDRS III) score of UPDRS III was 3 ~ 40, the average score was (14.77 ± 2.64); Hoehn-Yahr staging was 1 ~ 3, with an average of (1.86 ± 0.52). The written informed consents were signed by all patients or their families, and ethical approval was acquired from the Ethics Committee of Lanzhou University Second Hospital (approval no. 2020–0132).
Inclusion criteria: The diagnosis of PD conforms to the clinical diagnostic criteria of PD in the brain bank of the Parkinson's Disease Society (UK). All female patients are postmenopausal women. Exclusion criteria are Organic lesions of hypothalamus–pituitaryadrenal (HPA) axis; Is receiving angiotensin II replacement therapy; Is receiving antidepressant and anti-anxiety treatment; Unable to cooperate with the inspection.
2.2. Determination of serum RAS index
The patients collected 5 ml of elbow venous blood on an empty stomach 12 h after stopping using anti-PD medicines, injected it into a non-anticoagulant tube, put it into an icebox, let it stand for 1 h, then centrifuged to separate the serum. Serum levels of ACE, ACE2, angiotensin (Ang) I, Ang II and Ang (1–7) were detected by ELISA, and the operation was carried out strictly according to the instructions of ELISA kit.
2.3. Neuropsychological test
All patients were evaluated with HAMD and HAMA 12 h after stopping taking anti-PD medicines.
The HAMD scale includes depression, Guilt, suicidal tendencies, Difficulty in falling asleep, shallow sleep, early awakening, lack of work and interest, slow thinking and speech, agitation, mental anxiety, somatic anxiety, gastrointestinal symptoms, systemic symptoms, sexual symptoms, hypochondria, weight loss, insight, diurnal changes of symptoms, depersonalization or disintegration of reality, paranoid ideation, obsessive–compulsive symptoms, sense of impairment, sense of despair, inferiority complex, Each item has a score of 0 ~ 4 or 0 ~ 2, and the total score is 78. A score of<8 indicates normal. 8 ~ 19 points indicate mild depression; 20 ~ 35 points indicate moderate depression; > 35 indicates severe depression.
HAMA scale includes 14 items of anxiety, tension, fear, insomnia, cognitive dysfunction, depressive mood, muscle system symptoms, sensory system symptoms, cardiovascular system symptoms, respiratory system symptoms, gastrointestinal tract symptoms, genitourinary system symptoms, autonomic nervous system symptoms, and behavioral performance during talks. Each item has a score of 0 ~ 4, with a total score of 56 points. Score < 7 indicates no anxiety. A score of 7 ~ 13 indicates possible anxiety. 14 ~ 20 points indicate positive anxiety; A score of 21 ~ 29 indicates that there is obvious anxiety. > 29 indicates possible severe anxiety.
2.4. Statistical analysis method
SPSS 25.0 statistical software was used to process and analyze the data. The count data is expressed in n (%). The measurement data are expressed as mean ± standard deviation, and independent-sample t test is adopted. Correlation analysis between serum angiotensin Ⅱ level and depression and anxiety symptoms in patients with PD Pearson correlation analysis was used. The difference was statistically significant with P < 0.05.
3. Results
3.1. Results of patients' diagnosis of depression and anxiety
Of the 90 patients, 32 (35.56%) were diagnosed as mild depression (HAMD score 8 ~ 19), and 12 (13.33%) were diagnosed as moderate and severe depression (HAMD score ≥ 20). 20 cases (22.22%) were diagnosed as a possible anxiety disorder (HAMA score 7 ~ 13) and 16 cases (17.78%) as a definite anxiety disorder (HAMA score ≥ 14).
3.2. Comparison of serum ACE, ACE2, Ang I, Ang II, Ang (1–7) levels and HAMD and HAMA scores between male and female patients with PD
There was no significant difference in serum ACE, ACE2, Ang I, Ang II, Ang (1–7), HAMD and HAMA scores between male and female patients with PD according to sex (all P > 0.05, Table 1).
Table 1.
Comparison of serum ACE, ACE2, Ang I, Ang II, Ang (1–7) levels and HAMD and HAMA scores between male and female patients with PD.
| Item | Male (n = 47) |
Female (n = 43) |
P value |
|---|---|---|---|
| ACE(pg/mL) | 128.56 ± 12.07 | 127.45 ± 11.89 | 0.612 |
| ACE2(pg/mL) | 14.71 ± 3.93 | 14.47 ± 3.61 | 0.735 |
| Ang I(pg/mL) | 1270.18 ± 183.96 | 1261.00 ± 153.88 | 0.604 |
| Ang II(pg/mL) | 285.48 ± 16.68 | 284.50 ± 15.42 | 0.429 |
| Ang(1–7)(pg/mL) | 299.59 ± 18.79 | 299.98 ± 18.94 | 0.868 |
| HAMD(score) | 15.96 ± 11.57 | 16.06 ± 11.35 | 0.747 |
| HAMA(score) | 13.37 ± 8.98 | 13.53 ± 8.84 | 0.725 |
3.3. Correlation analysis of serum ACE, ACE2, Ang I, Ang II, Ang (1–7) levels and depression and anxiety symptoms in female PD patients
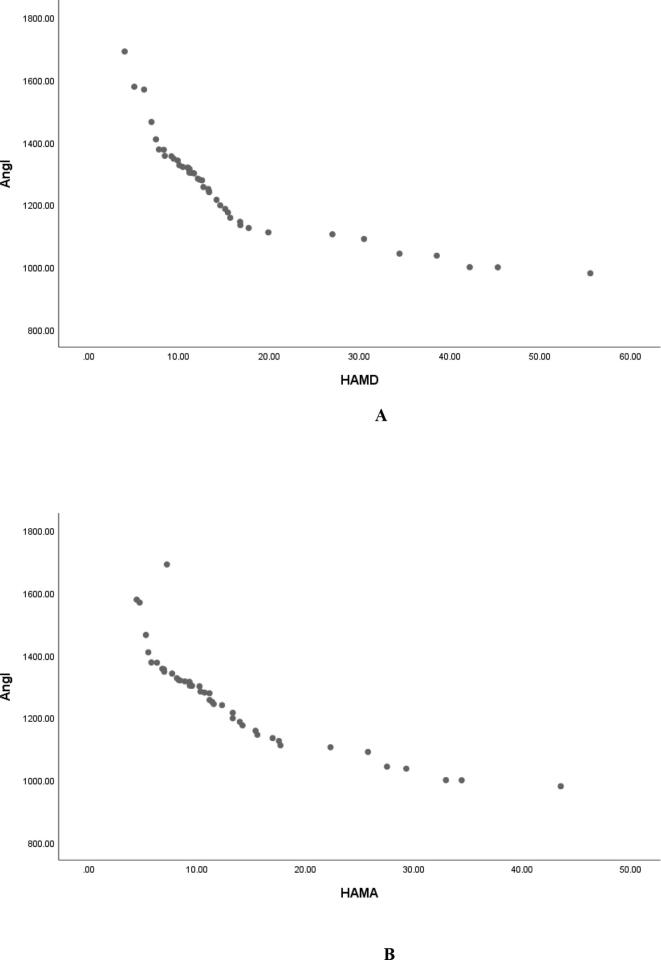
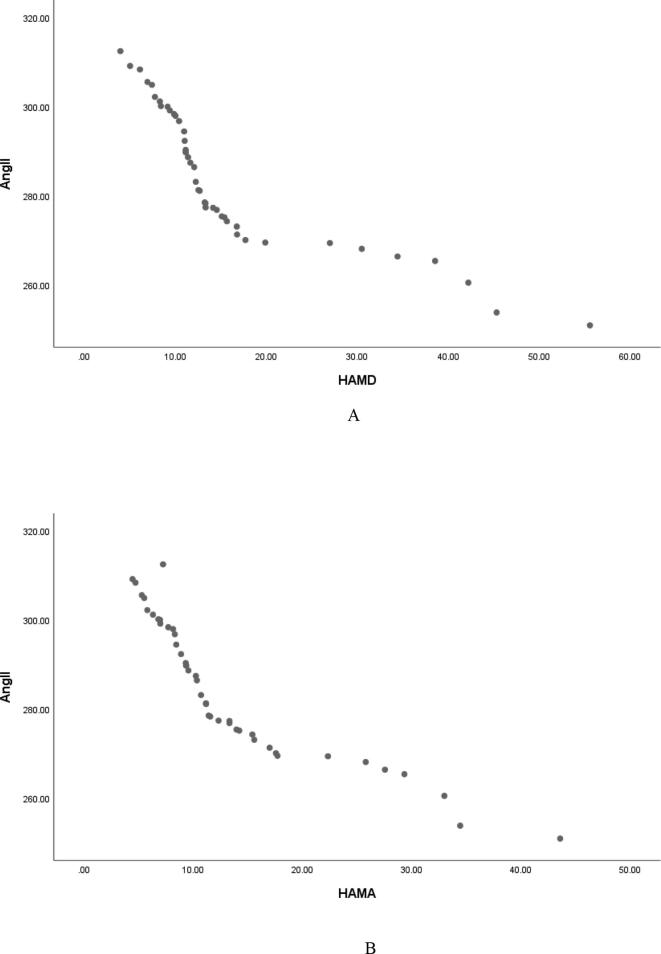
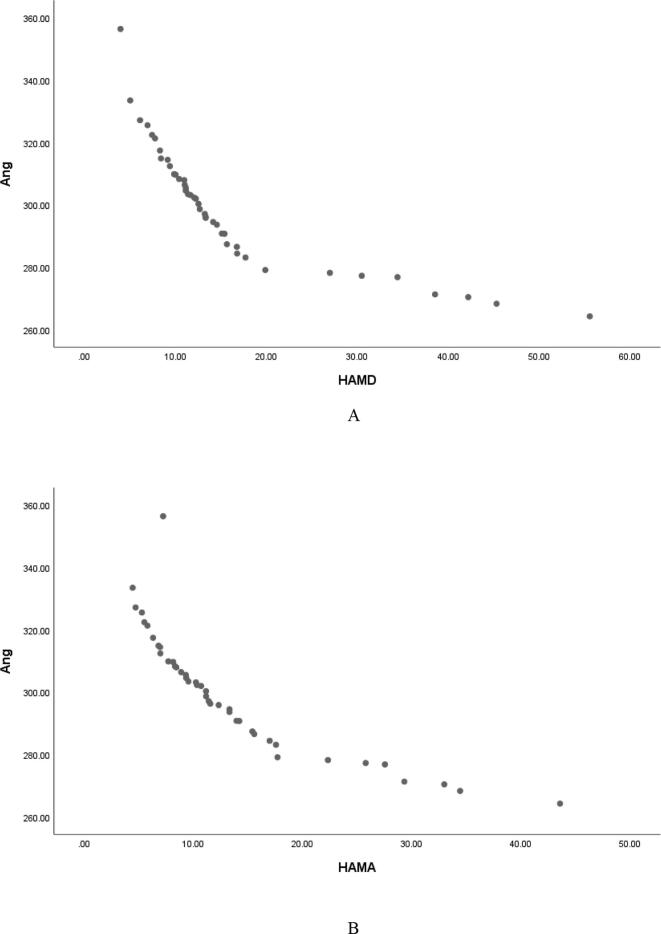
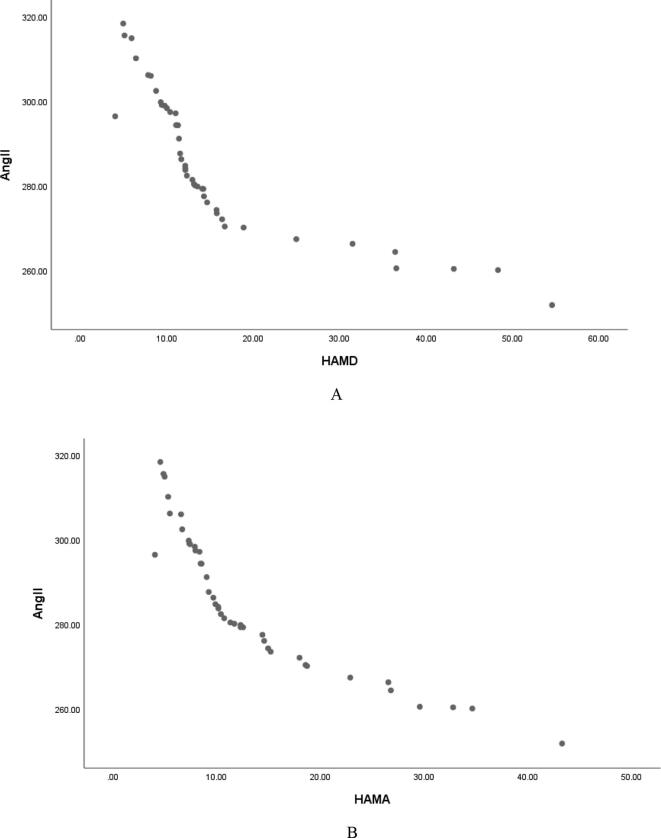
Pearson correlation analysis showed that serum Ang Ⅰ, Ang Ⅱ and Ang (1–7) were negatively correlated with HAMD score (r = −0.829, P < 0.001; r = −0.846, P < 0.001; r = −0. 887, P < 0.001, and were negatively correlated with HAMA score (r = −0.850, P < 0.001; r = −0.887, P < 0.001; r = −0. 851, P < 0.001, Table 2, Fig. 1, Fig. 2, Fig. 3); Serum ACE and ACE2 were not correlated with HAMD score and HAMA score (all P > 0.05, Table 2); It shows that the lower the levels of serum Ang Ⅰ, Ang Ⅱ and Ang (1–7) in female PD patients, the more serious the depressive symptoms and anxiety symptoms are. Fig. 4Fig. 5.Fig. 6.
Table 2.
Correlation analysis of serum ACE, ACE2, Ang I, Ang II, Ang (1–7) levels and depression and anxiety symptoms in female PD patients.
| Item | HAMD | HAMA | ||
|---|---|---|---|---|
| r value | P value | r value | P value | |
| ACE | 0.060 | 0.704 | 0.073 | 0.643 |
| ACE2 | −0.145 | 0.353 | −0.125 | 0.426 |
| Ang I | ﹣0.829 | <0.001 | ﹣0.850 | <0.001 |
| Ang II | ﹣0.846 | <0.001 | ﹣0.887 | <0.001 |
| Ang(1–7) | ﹣0.829 | <0.001 | −0.851 | <0.001 |
Fig. 1.
Serum Ang Ⅰ is negatively correlated with HAMD (A) and HAMA (B) scores in female patients with PD.
Fig. 2.
Serum Ang II was negatively correlated with HAMD (A) and HAMA (B) scores in female patients with PD.
Fig. 3.
Serum Ang (1–7) was negatively correlated with HAMD (A) and HAMA (B) scores in female patients with PD.
Fig. 4.
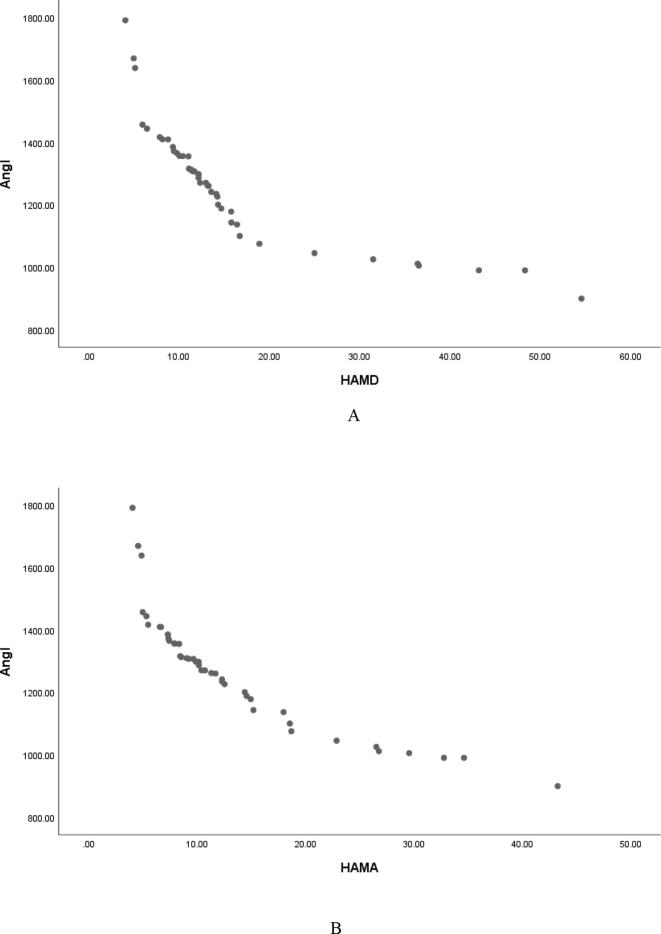
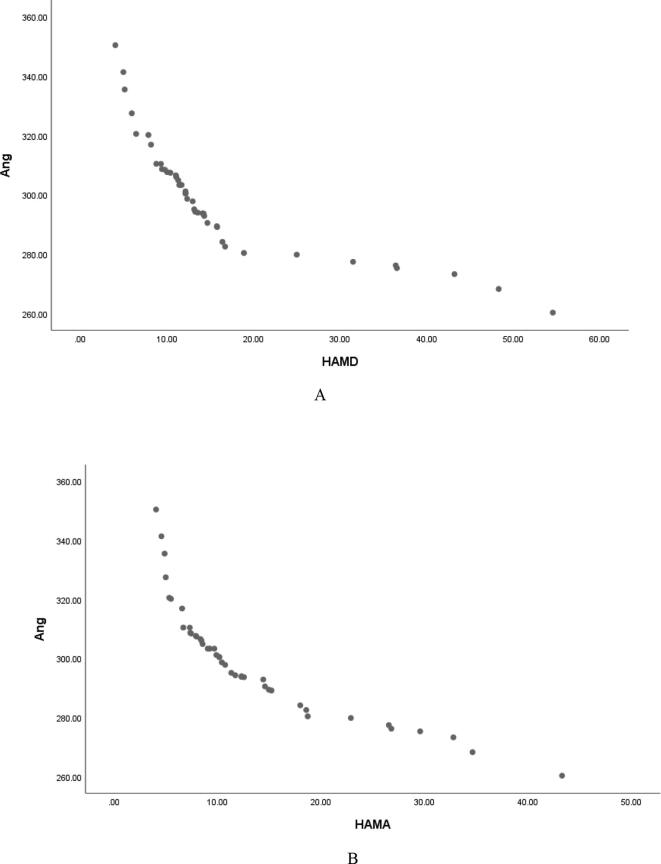
Serum Ang Ⅰ was negatively correlated with HAMD (A) and HAMA (B) scores in male patients with PD.
Fig. 5.
Serum Ang II was negatively correlated with HAMD (A) and HAMA (B) scores in male patients with PD.
Fig. 6.
Serum Ang (1–7) was negatively correlated with HAMD (A) and HAMA (B) scores in male patients with PD.
3.4. Correlation analysis of serum ACE, ACE2, Ang I, Ang II, Ang (1–7) levels and depression and anxiety symptoms in male PD patients
Pearson correlation analysis showed that serum Ang Ⅰ, Ang Ⅱ and Ang (1–7) were negatively correlated with HAMD score (r = −0.826, P < 0.001; r = −0. 818, P < 0.001; r = −0. 876, P < 0.001, and were negatively correlated with HAMA score (r = −0.870, P < 0.001; r = -0.876, P < 0.001; r = −0.862, P < 0.001, Table 3, Fig. 1, Fig. 2, Fig. 3); Serum ACE and ACE2 were not correlated with HAMD score and HAMA score (all P > 0.05, Table 3); It shows that the lower the levels of serum Ang Ⅰ, Ang Ⅱ and Ang (1–7) in male PD patients, the more serious the depressive symptoms and anxiety symptoms are.
Table 3.
Correlation analysis of serum ACE, ACE2, Ang I, Ang II, Ang (1–7) levels and depression and anxiety symptoms in male PD patients.
| Item | HAMD | HAMA | ||
|---|---|---|---|---|
| r value | P value | r value | P value | |
| ACE | 0.019 | 0.902 | 0.052 | 0.743 |
| ACE2 | −0.130 | 0.405 | −0.083 | 0.599 |
| Ang I | ﹣0.826 | <0.001 | ﹣0.870 | <0.001 |
| Ang II | ﹣0.818 | <0.001 | ﹣0.876 | <0.001 |
| Ang(1–7) | ﹣0.817 | <0.001 | −0.862 | <0.001 |
4. Discussion
Central RAS widely exists in the whole HPA axis, especially in the key areas of the stress response, such as the paraventricular nucleus, raphe nucleus, pituitary gland and adrenal medulla globose zone. During stress, the level of Ang 1I in the center and circulation increases, which can stimulate the synthesis and release of adrenocorticotropin, glucocorticoid and catecholamine, while angiotensin receptor blocker can reduce stress response, so Ang II can be considered as a stress hormone (Kim and Han, 2006, Dhanachandra Singh et al., 2014). HPA axis dysfunction exists in patients with depression. Depression can be regarded as a chronic stress state. Animal models of depression are often established by chronic restraint stress, chronic mild unpredictable stress and other methods (O'Neill et al., 2007). Therefore, central RAS may play the same important role as HPA axis in the pathological process of depression. Some studies have also shown that RAS gene polymorphism is not only associated with the onset of depression but also affects the clinical efficacy of antidepressants [19].
Animal models in vivo and in vitro show that dopamine release from striatum can be regulated by RAS and is mainly realized through AT1 receptor [20]. AT1 receptor antagonist has a protective effect on PD model. The local RAS in the basal ganglia includes high concentrations of ACE, ACE2, Ang Ⅰ and Ang Ⅱ. In the brain, Ang 1–7 is primarily formed via the degradation of Ang II by ACE-2 to activate the MasR that is widely distributed in the cerebrum. ACE inhibitor can increase striatal dopamine level and reduce neuronal necrosis, confirming the interaction between RAS system and the dopamine system. In this study, the correlation between serum RAS level and depression and anxiety symptoms in male and female PD patients was analyzed. It was found that HAMD and HAMA scores were negatively correlated with Ang I, Ang II and Ang (1–7) levels. On the one hand, patients with depression and anxiety may stimulate sympathetic nerve excitation and activate RAS due to chronic stress, thus leading to abnormal secretion of Ang I, Ang II and Ang (1–7). On the other hand, the decrease of serum ACE level in patients with depression and anxiety leads to the increase of some pre-inflammatory and oxidative stress factors, which together with RAS causes the occurrence of depressive symptoms.
Although we demonstrated that several serum parameters could be developed as potential indicators for assessment of depression and anxiety in PD patients, no significant differences of serum RAS levels between male and female PD patients were identified. The major limitation of the current study was sample size. In this study, only 90 PD patients were included, the small sample size limited conclusion of how serum RAS levels affecting PD patients, especially indicating the differences between male and female PD patients. Our ongoing study is enrolling more individuals to further expand our findings in this study.
5. Conclusion
Overall, non-motor symptoms such as depression and anxiety are more common in PD patients. Serum AngⅠ, AngⅡ, and Ang(1–7) levels of male and female patients were significantly reduced, and they were related to depression and anxiety symptoms, while ACE and ACE2 levels were not related to depression and anxiety symptoms. Serum AngⅠ, AngⅡ and Ang(1–7) can be important indicators for predicting and diagnosing depression and anxiety in PD patients.
Funding
Gansu Natural Science Foundation (20JR5RA330), and Lanzhou university second hospital (CYXZ2019-02).
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Huang T., Gao C.Y., Wu L., Gong P.Y., Wang J.Z., Tian Y.Y., Zhang Y.D. Han Chinese family with early-onset Parkinson's disease carries novel compound heterozygous mutations in the PARK2 gene. Brain Behav. 2019;9(9) doi: 10.1002/brb3.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A., Roth N., Ghassemi N.H., Hannink J., Seel T., Klucken J., Gassner H., Eskofier B.M. Development and clinical validation of inertial sensor-based gait-clustering methods in Parkinson's disease. J. Neuroeng Rehabil. 2019;16(1):77. doi: 10.1186/s12984-019-0548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleminger S. Left-sided Parkinson's disease is associated with greater anxiety and depression. Psychol Med. 1991;21(3):629–638. doi: 10.1017/s0033291700022261. [DOI] [PubMed] [Google Scholar]
- Goodarzi Z, Mele B, Guo S, Hanson H Jette N, Patten S, Pringsheim T, Holroyd-Leduc J.Guidelines for dementia or Parkinson's disease with depression or anxiety: a systematic review.BMC Neurol. 2016 ;16(1):244. [DOI] [PMC free article] [PubMed]
- Maillet A, Krack P, Lhommée E, Météreau E Klinger H, Favre E, Le Bars D, Schmitt E, Bichon A, Pelissier P, Fraix V, Castrioto A, Sgambato-Faure V, Broussolle E, Tremblay L, Thobois S.The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson's disease.Brain. 2016 ;139:2486-502. [DOI] [PubMed]
- Gupta A., Kumar A., Kulkarni S.K. Targeting oxidative stress, mitochondrial dysfunction and neuroinflammatory signaling by selective cyclooxygenase (COX)-2 inhibitors mitigates MPTP-induced neurotoxicity in mice.Prog Neuropsychopharmacol. Biol Psychiatry. 2011;35(4):974–981. doi: 10.1016/j.pnpbp.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Aridon P., Geraci F., Turturici G., D'Amelio M., Savettieri G., Sconzo G. Protective role of heat shock proteins in Parkinson's disease. Neurodegener Dis. 2011;8(4):155–168. doi: 10.1159/000321548. [DOI] [PubMed] [Google Scholar]
- Kobori H., Nangaku M., Navar L.G., Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59(3):251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- Chen L.N., Yang X.H., Nissen D.H., Chen Y.Y., Wang L.J., Wang J.H., Gao J.L., Zhang L.Y. Dysregulated renin-angiotensin system contributes to acute lung injury caused by hind-limb ischemia-reperfusion in mice. Shock. 2013;40(5):420–429. doi: 10.1097/SHK.0b013e3182a6953e. [DOI] [PubMed] [Google Scholar]
- Bürgelová M., Kramer H.J., Teplan V., Thumová M., Cervenka L. Effects of angiotensin-(1–7) blockade on renal function in rats with enhanced intrarenal Ang II activity. Kidney Int. 2005;67(4):1453–1461. doi: 10.1111/j.1523-1755.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- van den Eijnden M.M., de Bruin R.J., de Wit E., Sluiter W., Deinum J., Reudelhuber T.L., Danser A.H. Transendothelial transport of renin-angiotensin system components. J Hypertens. 2002;20(10):2029–2037. doi: 10.1097/00004872-200210000-00023. [DOI] [PubMed] [Google Scholar]
- Liu B., Zhang R., Wei S., Yuan Q., Xue M., Hao P., Xu F., Wang J. Chen Y.ALDH2 protects against alcoholic cardiomyopathy through a mechanism involving the p38 MAPK/CREB pathway and local renin-angiotensin system inhibition in cardiomyocytes. Int. J. Cardiol. 2018;257:150–159. doi: 10.1016/j.ijcard.2017.11.094. [DOI] [PubMed] [Google Scholar]
- Wan Y., Yang G., Wan S.X., Yang B., Ying Y.R., Xi Z.X. Renin-angiotension system–stress hormone response system. Sheng Li Xue Bao. 1996;48(6):521–528. [PubMed] [Google Scholar]
- Fang Y., Li S., Zhou H., Tian X., Lv S., Chen Q. Opiorphin increases blood pressure of conscious rats through renin-angiotensin system (RAS) Peptides. 2014;55:47–51. doi: 10.1016/j.peptides.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Yang G., Wan Y., Zhu Y. Angiotensin II–an important stress hormone.Biol. Signals. 1996;5(1):1–8. doi: 10.1159/000109168. [DOI] [PubMed] [Google Scholar]
- Kim K.S., Han P.L. Optimization of chronic stress paradigms using anxiety- and depression-like behavioral parameters.J. Neurosci Res. 2006;83(3):497–507. doi: 10.1002/jnr.20754. [DOI] [PubMed] [Google Scholar]
- Dhanachandra Singh Kh., Jajodia A., Kaur H., Kukreti R., Karthikeyan M. Gender specific association of RAS gene polymorphism with essential hypertension: a case-control study. Biomed. Res. Int. 2014;538053 doi: 10.1155/2014/538053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill C., Nolan B.J., Macari A., O'Boyle K.M., O'Connor J.J. Adenosine A1 receptor-mediated inhibition of dopamine release from rat striatal slices is modulated by D1 dopamine receptors. Eur. J. Neurosci. 2007;26(12):3421–3428. doi: 10.1111/j.1460-9568.2007.05953.x. [DOI] [PubMed] [Google Scholar]