Abstract
The family Calliphoridae is a group of heterogenous calyptrate flies with a worldwide distribution including species of ecological, veterinary, medical, and forensic importance. Notorious for their parasitic habits, the larvae of many blowflies are characterised – like some other dipteran larvae – by their ability to develop in animal flesh. When parasitism affects a living host, it is termed “myiasis”. This has led the Calliphoridae to be considered as a pivotal family in its relationship with a man. Nevertheless, even after more than 50 years of research, the phylogenetic relationships among calliphorid subfamilies together with the evolutionary origin of myiasis remain unclear. In order to elucidate these problems, we constructed three phylogenetic trees by using nucleotide sequence data from cytochrome oxidase subunit one (COI), representing a mitochondrial conservative gene, and nuclear 28S subunit of ribosomal RNA gene (28S rRNA) in order to interpret the evolutionary profile of myiasis in the family Calliphoridae. The sequenced data represented species associated with ectoparasitic life-styles, either saprophagy or facultative and obligate parasitism. A total number of 50 accessions were collected for 28S rRNA, 56 for COI, and 38 for combined sequences phylogeny. Molecular Evolutionary Genetics Analysis (MEGA) software was used to align 2197 nucleotide positions of 28S rRNA and 1500 nucleotide positions of COI with a gap opening penalties and gap extension penalties equalling 20 and 0.1 respectively. The results reveal the non-monophyly of the family Calliphoridae despite the stable monophyletic status of the Chrysomyinae, Luciliinae, and Auchmeromyiinae. Also, our findings recommend ranking the Toxotarsinae as a separate family. Furthermore, comparative analysis of the phylogenetic trees shows that the habit of obligatory myiasis originated independently more than five times. This strengthens our hypothesis that the origin of eating fresh meat is a case of convergent evolution that has taken place after speciation events millions of years ago. Finally, estimating the divergence dates between lineages from molecular sequences provides a better chance of understanding their evolutionary biology.
Keywords: Blowflies, Conservative genes, Evolution, Data science, Phylogeny
1. Introduction
The true flies (Diptera) include cosmopolitan and ubiquitous flies which have had an immense impact on mankind and his activities and possessions. Calyptrate flies comprise one of the major clades of the Schizophora, the latter representing the most rampant radiation of Diptera which includes about 22,000 extant species (about 14% of all flies) and is abundantly represented in nearly all terrestrial ecosystems, from tropical forests, savannas, and deserts to the extreme High Arctic (Wiegmann, 2011, Yeates and Wiegmann, 2005). It is currently classified into the Hippoboscoidea (tsetse, louse, and bat flies), the Muscoid grade (house flies and relatives), and the Oestroidea (blowflies, bot flies, flesh flies, parasitic flies). The most diverse group within the calyptrates is the superfamily Oestroidea (Cerretti et al., 2017). It is a large and ecologically diverse clade with about 15,000 described species with a variety of larval feeding habits as saprophages, endoparasites, parasitoids, and predators (Marinho et al., 2012, El-Hawagry and El-Azab, 2019). Among the oestroid families, the Calliphoridae is a crucial one (Singh and Wells, 2011).
The family Calliphoridae (blowflies), with about 1500 species, comprises almost 8% of the calyptrate flies and occupies all continents except Antarctica (Verves, 2007, Kutty et al., 2010). Furthermore, it includes a variety of subfamilies such as the Calliphorinae, Chrysomyinae, Luciliinae, Ameniinae, Bengaliinae, Helicoboscinae, Polleniinae, Melanomyinae, Rhiniinae, Mesembrinellinae and Toxotarsinae (Rognes, 1997, Kutty et al., 2010). Blowflies are a miscellaneous group of flies with medical, veterinary, and forensic importance (Zumpt, 1965, Hall and Wall, 1995, Amendt et al., 2004). Moreover, the diversity of their breeding environments encompasses different feeding habits and distinct types of parasitism (Marinho et al., 2012).
Most calliphorid adults are oviparous but others are larviparous, either unilarviparous (Helicoboscinae and Mesembrinellinae) or multilarviparous (Onesia Robineau-Desvoidy and Bellardia Robineau-Desvoidy) (Singh and Wells, 2013). Within the Calliphoridae, there is a wide range of feeding habits. While adults are better known as nectar feeders, larvae are characterised by other feeding behaviors including saprophagy, hematophagy, coprophagy and ectoparasitism, either obligatory or facultative (Zumpt, 1965, Stevens, 2003, Mcdonagh and Stevens, 2011).
In addition to the ability of blowfly larvae to develop on the flesh of a vertebrate host (e.g. Chrysomya bezziana and Cochliomyia hominivorax), others parasitise birds (e.g. species of Protocalliphora) or terrestrial gastropods (e.g. species of Melanomya) or earthworms (e.g. species of Bellardia and Pollenia) and even nests of termites and ants (e.g. species of Bengalia and Tricyclea) (Zumpt, 1965, Marinho et al., 2012). However, a protein-rich meals are necessary for blowflies to complete their life-cycle (Zumpt, 1965, Stevens, 2003).
The invasion of living animal tissues by dipterous larvae is defined as myiasis (Zumpt, 1965). This type of parasitism can take place externally by some blowfly species and is classified as ectoparasitism or can take place internally by the bot and warble flies and is classified as endoparasitism (Stevens, 2003). Blowfly species with the parasitic lifestyle can be divided into (i) saprophagous larvae, which feed on decaying matter and can cause a secondary infestation in living tissue (e.g. Calliphora); (ii) facultative ectoparasites, which mainly live as saprophagous larvae and are able to initiate myiasis in an animal host (e.g. Lucilia); and (iii) obligatory ectoparasites like Chrysomya bezziana which only feed on the living tissue of the host (Stevens, 2003).
This proposed division, established by Zumpt (1965), has driven the evolution of the myiasis habit along two pathways, either saprophagous or hematophagous (Zumpt, 1965). But an understanding of the different forms of parasitism leads to the separation of myiasis-causing flies from the parasitic habit of myiasis itself (McDonagh and Stevens, 2011). To resolve the debate around the evolutionary scenario outlined above, a precise phylogenetic analysis will reveal the relationship of the calliphorid subfamilies, especially as there are additional complicated parasitic habits among the blowflies such as those found in the voracious blood-sucking Auchmeromyia luteola (Congo floor maggot) and Cordylobia anthropophaga (African Tumbu fly) which develops in a solitary furuncle under the skin of the host (Zumpt, 1965, Stevens, 2003).
Although the family Calliphoridae includes several economically significant myiasis-causing flies prominent in the field of livestock parasitism, the evolutionary relationships among its species are still ambiguous (Stevens and Wall, 1997). To date, several phylogenetic analyses of the family Calliphoridae have been made based on morphological characters (Rognes, 1997). However, convergent evolution among these morphological traits is misleading for an overall assessment of the phylogenetic relationships among calliphorid clades.
Historically, even the monophyly of the family Calliphoridae has been contentious as the morphological evidence supporting monophyly is fragile (Rognes, 1997, Pape and Arnaud, 2001, Kutty et al., 2010, Marinho et al., 2012). Further studies based on molecular characters have opened the door to different hypotheses and a molecular phylogeny has shown that the Calliphoridae as it was then constituted is not monophyletic (Kutty et al., 2010). In addition, the systematic relationship between subfamilies and even among specific taxa needs to be addressed (Marinho et al., 2012).
Fortunately, the development of advanced molecular techniques can help in the construction of a robust phylogeny that enables accurate reassessment to be made of the evolutionary processes within calliphorid flies. Furthermore, a revision of the status of blowfly subfamilies using modern phylogenetic analysis could serve as a cornerstone to clarify more precisely the possible scenario for the origin of myiasis, whether saprophagous or hematophagous.
In molecular phylogenetic studies, mitochondrial DNA (mtDNA) and nuclear ribosomal DNA are usually used as these different data evolved under different constraints (Shao and Barker, 2007). Otherwise, the 28S rRNA and COI subunits are characterised by a lack of recombination during cell division, ease of isolation, susceptibility to universal primers and presence of both variable and conserved regions, and this makes them suitable either for studying hierarchical relationships or for distinguishing between closely related species (Baker et al., 2001, Otranto et al., 2005, Mcdonagh and Stevens, 2011). Recently, data science and bioinformatics methodology were helping in studying evolutionary biology in some insect groups (Shobrak et al., 2015, Nasser et al., 2019). With huge accumulative nucleotide data representing members of family Calliphoridae around the globe -especially for the last two decades-, the bioinformatic data could aid in a better understanding of evolutionary pathways of the family. Moreover, such data will help in clarifying the way by which the myiasis habit originating.
Accordingly, the present study describes a phylogenetic analysis using newly available nucleotide sequence data for cytochrome oxidase I (COI), representing mitochondrial genes, and 28S large subunit ribosomal RNA (28S rRNA), representing nuclear genes, to illustrate the evolutionary profile of the origin of myiasis in the family Calliphoridae.
2. Methods
2.1. Taxa selection and DNA sequence collection
All taxa used in this study belong to the family Calliphoridae, and all subfamily groups are included in the phylogenetic analysis. Furthermore, the data input includes the different parasitic lifestyles exhibited by calliphorid larvae, such as obligatory or facultative parasitism in both vertebrates and invertebrates or saprophagy (Table 1).
Table 1.
Taxa used in the study along with the myiasis habit exhibited by their larvae on the targeted host.
| Sub-family | Species | Myiasis habit and targeted host | Reference source |
|---|---|---|---|
| Auchmeromyiinae | Auchmeromyia luteola (Fabricius, 1805) | Obligate myiasis (hematophagous) on vertebrates | (McDonagh and Stevens, 2011) |
| Cordylobia anthropophaga (Blanchard, 1872) | Obligate parasitism on vertebrates | (McDonagh and Stevens, 2011) | |
| Hemigymnochaeta unicolor (Bigot, 1888) | Obligate parasitism on termites’ and ants’ nests | (Marinho et al., 2012) | |
| Pachychoeromyia praegrandis (Austen, 1910) | Unknown | (McDonagh and Stevens, 2011 | |
| Tricyclea sp. | Obligate parasitism on termites’ and ants’ nests | (Marinho et al. 2012) | |
| Calliphorinae | Calliphora croceipalpis (Jaennicke, 1867) | Secondary facultative on carrion and vertebrates | (McDonagh and Stevens, 2011 |
| Calliphora vicina (Robineau-Desvoidy, 1830) | Secondary facultative on carrion and vertebrates | (McDonagh and Stevens, 2011) | |
| Calliphora vomitoria (Linnaeus, 1758) | Secondary facultative on carrion and vertebrates | (McDonagh and Stevens, 2011) | |
| Calliphora dubia (Macquart, 1855) | Secondary facultative on carrion. | (McDonagh and Stevens, 2011) | |
| Calliphora quadrimaculata (Swederus, 1787) | Secondary facultative on carrion and vertebrates | (McDonagh and Stevens, 2011) | |
| Cynomya mortuorum (Linnaeus, 1758.) | Secondary facultative on vertebrates | (McDonagh and Stevens, 2011) | |
| Cynomyopsis cadaverina (Robineau-Desvoidy, 1830) | Saprophagous on vertebrates | (McDonagh and Stevens, 2011) | |
| Bellardia vulgaris (Robineau Desvoidy, 1830) | Obligatory on earth-worms | (Marinho et al., 2012) | |
| Chrysomyinae | Chloroprocta idioidea (Robineau-Desvoidy, 1830) | Unknown | (McDonagh and Stevens, 2011) |
| Chrysomya albiceps (Wiedemann, 1819) | Secondary facultative on carrion and vertebrates | (McDonagh and Stevens, 2011) | |
| Chrysomya bezziana (Villeneuve, 1914) | Obligatory myiasis on vertebrates | (McDonagh and Stevens, 2011) | |
| Chrysomya chloropyga (Wiedemann, 1818) | Secondary facultative on carrion and vertebrates | (McDonagh and Stevens, 2011) | |
| Chrysomya megacephala (Fabricius, 1794) | Secondary facultative on carrion and vertebrates | (McDonagh and Stevens, 2011) | |
| Chrysomya putoria (Wiedemann, 1830) | Secondary myiasis on carrion | (McDonagh and Stevens, 2011) | |
| Chrysomya rufifacies (Macquart, 1843) | Secondary facultative on carrion and vertebrates | (McDonagh and Stevens, 2011) | |
| Cochliomyia hominivorax (Coquerel, 1858) | Obligatory myiasis on vertebrates | (McDonagh and Stevens, 2011) | |
| Cochliomyia macellaria (Fabricius, 1775) | Secondary facultative on vertebrates | (McDonagh and Stevens, 2011) | |
| Hemilucilia segmentaria (Fabricius, 1805) | Secondary facultative on carrion and vertebrates | (Sanit et al. 2018) | |
| Phormia regina(Meigen, 1826) | Facultative (unknown degree) on carrion and vertebrates | (McDonagh and Stevens, 2011) | |
| Protophormia terraenovae (Robineau-Desvoidy, 1830) | Secondary facultative on carrion and vertebrates | (McDonagh and Stevens, 2011) | |
| Compsomyiops fulvicrura (Robineau- Desvoidy, 1830) | Saprophagous on carrion | (McDonagh and Stevens, 2011) | |
| Protocalliphora sialia (Shannon & Dobroscky, 1924) | Obligatory myiasis on birds and mammals | (Marinho et al. 2012) | |
| Protocalliphora azurea (Fallén, 1817) | Obligatory myiasis on birds | (McDonagh and Stevens, 2011) | |
| Luciliinae | Lucilia eximia (Wiedemann, 1819) | Unknown | (McDonagh and Stevens, 2011) |
| Lucilia sericata (Meigen, 1826) | Primary facultative on carrion and vertebrates | (McDonagh and Stevens, 2011) | |
| Hypopygiopsis infumata (Bigot, 1877) | Human carrion | (Sanit et al., 2018) | |
| Hemipyrellia fernandica (Macquart, 1855) | Saprophagous on carrion | (McDonagh and Stevens, 2011) | |
| Lucilia ampullaceal (Villeneuve, 1922) | Secondary facultative on frogs | (McDonagh and Stevens, 2011) | |
| Lucilia caesar (Linnaeus, 1758) | Secondary facultative on carrion and vertebrates | (McDonagh and Stevens, 2011) | |
| Lucilia cluvia (Walker, 1849) | Saprophagous on carrion | (McDonagh and Stevens, 2011) | |
| Lucilia cuprina (Wiedemann, 1830) | Primary facultative on carrion and vertebrates | (McDonagh and Stevens, 2011) | |
| Lucilia illustris (Meigen, 1826) | Secondary facultative on carrion and vertebrates | (McDonagh and Stevens, 2011) | |
| Lucilia mexicana (Macquart, 1844) | Saprophagous on carrion | (McDonagh and Stevens, 2011) | |
| Lucilia richardsi (Collin, 1926) | Facultative (unknown degree) on vertebrates | (McDonagh and Stevens, 2011) | |
| Lucilia silvarum (Meigen, 1826) | Facultative (unknown degree) on toads/ frogs | (McDonagh and Stevens, 2011) | |
| Lucilia bufonivora (Moniez, 1876) | Obligate on frogs / toads | (McDonagh and Stevens, 2011) | |
| Hemipyrellia ligurriens (Wiedemann, 1830) | Human carrion | (Sukontason et al. 2010) | |
| Polleniinae | Pollenia rudis (Fabricius, 1794) | Obligatory on earth-worms | (Marinho et al. 2012) |
| Pollenia amentaria (Scopoli, 1763) | Unknown | (McDonagh and Stevens, 2011) | |
| Bengaliinae | Bengalia depressa (Walker, 1857) | Obligatory myiasis on termites and ant pupae | (McDonagh and Stevens, 2011) |
| Bengalia peuhi (Villeneuve, 1914) | Unknown | (McDonagh and Stevens, 2011) | |
| Mesembrinellinae | Eumesembrinella quadrilineata (Fabricius, 1805) | Unknown | (McDonagh and Stevens, 2011) |
| Eumesembrinella benoisti (Séguy, 1925) | Unknown | (McDonagh and Stevens, 2011) | |
| Mesembrinella bellardiana (Aldrich, 1922) | Unknown | (McDonagh and Stevens, 2011) | |
| Mesembrinella bicolor (Fabricius, 1805) | Unknown | (McDonagh and Stevens, 2011) | |
| Mesembrinella peregrina (Aldrich, 1922) | Unknown | (McDonagh and Stevens, 2011) | |
| Rhiniinae | Cosmina fuscipennis (Robineau-Desvoidy, 1830) | Unknown | (McDonagh and Stevens, 2011) |
| Rhinia sp. | Unknown | (McDonagh and Stevens, 2011) | |
| Rhyncomya soyauxi (Karsch, 1886) | Unknown | (McDonagh and Stevens, 2011) | |
| Thoracites sp. | Unknown | (McDonagh and Stevens, 2011) | |
| Isomyia gomezmenori(Peris, 1951) | Unknown | (McDonagh and Stevens, 2011) | |
| Toxotarsinae | Sarconesia chlorogaster (Wiedemann, 1830) | Necrophagous | (Flissak et al. 2018) |
| Helicoboscinae | Eurychaeta palpalis (Robineau-Desvoidy, 1830) | Saprophagous on slugs and snails (gastropods) | (McDonagh and Stevens, 2011) |
| Melanomyinae | Melinda viridicyanea Robineau (Desvoidy, 1830) | Saprophagous on slugs and snails (gastropods) | (McDonagh and Stevens, 2011) |
Sequences in the database of the National Center for Biotechnology Information (NCBI) were used to investigate the evolution of myiasis in the family Calliphoridae. Two conservative genes were applied for studying the relationships among calliphorid species. The first is nuclear 28S large subunit ribosomal RNA gene (28S rRNA) and the other is mitochondrial cytochrome oxidase subunit I (COI). Accession numbers were retrieved from NCBI (http://www.ncbi.nim.nih.gov). A total number of 50 accessions were collected for 28S rRNA, 56 for COI, and 38 for combined sequences (Table 2). Based on the availability of data, sequences of 28S rRNA and COI were extracted from Gene bank in FASTA format and were used to construct the phylogenetic analyses.
Table 2.
Species used in the phylogenetic analysis with collected accession numbers.
| Sub-family | Species |
Accession numbers |
|
|---|---|---|---|
| 28S | COI | ||
| Auchmeromyiinae | Auchmeromyia luteola (Fabricius, 1805) | AJ551431.1 | FR719153.1 |
| Cordylobia anthropophaga (Blanchard, 1872) | AJ551432.1 | FR719158.1 | |
| Hemigymnochaeta unicolor (Bigot, 1888) | JQ246628.1 | JQ246682.1 | |
| Pachychoeromyia praegrandis (Austen, 1910) | JQ246629.1 | JQ246683.1 | |
| Tricyclea sp. | JQ246630 | JQ246684 | |
| Calliphorinae | Calliphora croceipalpis (Jaennicke, 1867) | JQ246616 | JQ246671 |
| Calliphora vicina (Robineau-Desvoidy, 1830) | JQ246617 | JQ246672 | |
| Calliphora vomitoria (Linnaeus, 1758) | JQ246618 | JQ246673 | |
| Calliphora dubia (Macquart, 1855) | AJ558185.1 | KJ719470.1 | |
| Calliphora quadramaculata (Swederus, 1787) | AJ558187 | – | |
| Cynomya mortuorum (Linnaeus, 1758) | AJ300135 | KU874773.1 | |
| Cynomyopsis cadaverina (Robineau-Desvoidy, 1830) | KU873244.1 | – | |
| Bellardia vulgaris (Robineau Desvoidy, 1830) | GQ409231.1 | MG673858.1 | |
| Chrysomyinae | Chloroprocta idioidea (Robineau-Desvoidy, 1830) | JQ246603.1 | JQ246658.1 |
| Chrysomya albiceps (Wiedemann, 1819) | JQ246604.1 | JQ246659.1 | |
| Chrysomya bezziana (Villeneuve, 1914) | JQ246605.1 | JQ246660.1 | |
| Chrysomya chloropyga (Wiedemann, 1818) | JQ246606 | JQ246661 | |
| Chrysomya megacephala (Fabricius, 1794) | JQ246607 | JQ246662.1 | |
| Chrysomya putoria (Wiedemann, 1830) | JQ246608 | JQ246663 | |
| Chrysomya rufifacies (Macquart, 1843) | JQ246609 | JQ246664.1 | |
| Cochliomyia hominivorax (Coquerel, 1858) | JQ246610 | JQ246665.1 | |
| Cochliomyia macellaria (Fabricius, 1775) | JQ246611 | JQ246666.1 | |
| Hemilucilia segmentaria (Fabricius, 1805) | JQ246612 | JQ246667 | |
| Hemilucilia semidiaphana (Rondani, 1850) | JQ246613 | JQ246668 | |
| Phormia regina (Meigen, 1826) | JQ246614 | JQ246669 | |
| Protophormia terraenovae (Robineau-Desvoidy, 1830) | JQ246615 | JQ246670.1 | |
| Compsomyiops fulvicrura (Robineau- Desvoidy, 1830) | FJ025504.1 | FJ025607.2 | |
| Protocalliphora sialia (Shannon & Dobroscky, 1924) | AJ558190 | AF295559 | |
| Protocalliphora azurea (Fallén, 1817) | AJ551439.1 | HE614022.1 | |
| Luciliinae | Lucilia eximia (Wiedemann, 1819) | JQ246623 | JQ246678 |
| Lucilia sericata (Meigen, 1826) | JQ246624 | JQ246679 | |
| Hypopygiopsis infumata (Bigot, 1877) | JF439575 | JF439550 | |
| Hemipyrellia fernandica (Macquart, 1855) | – | FR719160 | |
| Lucilia ampullacea (Villeneuve, 1922) | AJ300137 | KY031826.1 | |
| Lucilia caesar (Linnaeus, 1758) | AJ300138 | JX295699.1 | |
| Lucilia cluvia (Walker, 1849) | AJ551440.1 | JN280714.1 | |
| Lucilia cuprina (Wiedemann, 1830) | AJ417709.1 | KX053871.1 | |
| Lucilia illustris (Meigen, 1826) | AJ300136 | KM571189.1 | |
| Lucilia mexicana (Macquart, 1844) | AJ551441 | JN280725.1 | |
| Lucilia richardsi (Collin, 1926) | AJ551442 | FR872384.1 | |
| Lucilia silvarum (Meigen, 1826) | AJ551443 | MG118877.1 | |
| Lucilia bufonivora (Moniez, 1876) | FR719294.1 | KF751384.1 | |
| Hemipyrellia ligurriens (Wiedemann, 1830) | JQ246621 | JQ246676 | |
| Polleniinae | Pollenia rudis (Fabricius, 1794) | AJ558192 | KT368817.1 |
| Pollenia amentaria (Scopoli, 1763) | GQ409262 | GQ409350 | |
| Bengaliinae | Bengalia depressa (Walker, 1857) | FR719270 | FR719154 |
| Bengalia peuhi (Villeneuve, 1914) | JQ246631 | JQ246685 | |
| Mesembrinellinae | Eumesembrinella quadrilineata (Fabricius, 1805) | JQ246633 | JQ246687 |
| Eumesembrinella benoisti (Séguy, 1925) | JQ246632 | JQ246686 | |
| Mesembrinella bellardiana (Aldrich, 1922) | JQ246635 | JQ246688 | |
| Mesembrinella bicolor (Fabricius, 1805) | JQ246637 | JQ246689 | |
| Mesembrinella peregrina (Aldrich, 1922) | JQ246638 | JQ246690 | |
| Rhiniinae | Cosmina fuscipennis (Robineau-Desvoidy, 1830) | JQ246639 | JQ246691 |
| Rhinia sp. | JQ246640 | JQ246692 | |
| Rhyncomya soyauxi (Karsch, 1886) | JQ246641 | JQ246693 | |
| Thoracites sp. | JQ246642 | JQ246694 | |
| Isomyia gomezmenori (Peris, 1951) | JF439579 | JF439553 | |
| Rhyncomya nigripes (Séguy, 1933) | GQ409268 | GQ409356 | |
| Metallea erinacea (Fang & Fan, 1984) | – | GQ409337 | |
| Toxotarsinae | Sarconesia chlorogaster (Wiedemann, 1830) | KJ438987 | GQ409359 |
| Helicoboscinae | Eurychaeta palpalis (Robineau-Desvoidy, 1830) | FJ025512 | FJ025612 |
| Melanomyinae | Melinda viridicyanea (Robineau-Desvoidy, 1830) | GQ409248 | GQ409335 |
2.2. Sequence alignment
Initially, pairwise alignments (PA) and multiple sequence alignments (MSA) were carried out by the Clustal W algorithm implemented into Molecular Evolutionary Genetics Analysis (MEGA) software 6. The 28S rRNA alignment included about 2197 nucleotide positions whereas, the COI alignment comprised 1500 nucleotide positions. Next, the gap opening penalties and gap extension penalties for PA and MSA were 20 and 0.1 respectively. According to the Maximum Composite Likelihood (MCL) approach, the Maximum Likelihood (ML) analysis produced the most suited phylogenetic trees for 28S rRNA and COI aligned sequences. Also, another confirmatory tree showed the combination of aligned sequences from the same conservative genes (28SrRNA and COI). Finally, MSA for 28S and COI genes were verified by checking with the translation of proteins for sequence homology.
2.3. Phylogenetic analysis
Molecular Evolutionary Genetics Analysis (MEGA) software 6 was used to construct the phylogenetic analysis among the 28S and COI sequences (Tamura et al., 2013). Nearest-Neighbor-Interchange (NNI) was used as an ML heuristic method for trees interference (Tajima and Nei, 1984). The aligned data for both 28S and COI genes were analyzed using the Maximum Likelihood model. All positions containing gaps and missing data were eliminated. Also, combined data from 28S and COI genes were used to test the phylogenetic homogeneity. Differences between 28S and COI genes against combined genes tree topologies were assessed by the Tamura-Nei model test for DNA substitution models with uniform rates among the sites (Tajima and Nei, 1984). For all phylogenetic tests, 500 bootstrap replications were used (Felsenstein, 1985). Finally, the outgroup was removed to minimize the number of inapplicable sequences data. Also, heterogeneity of Calliphoridae as a group form a limitation to use any outgroup especially with using the bioinformatic technique.
3. Results
3.1. Phylogenetic analysis
Three phylogenetic trees were obtained from aligned DNA sequences collected from NCBI. In general, the inferred trees clarified the relationships among the calliphorid subfamilies. Also, a separate phylogenetic tree was added to illustrate the relationships among obligatory myiasis causing flies belonging to family Calliphoridae.
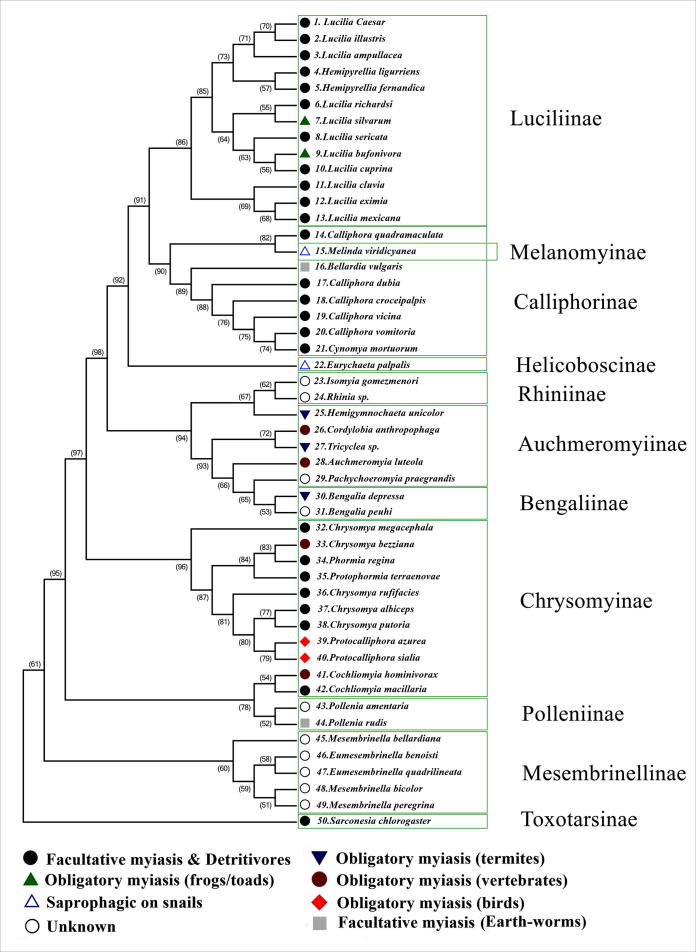
In general, the inferred trees clarified the relationships among the calliphorid subfamilies. The phylogenetic tree of 28S rRNA (Fig. 1) gave a clear resolution about sister-groups within the family Calliphoridae. As shown in Fig. 1, the subfamilies Luciliinae and Calliphorinae are sister-groups and with 91% bootstrap value, but the subfamily Helicoboscinae (Eurychaeta palpalis) is paraphyletic to them. Moreover, the Rhiniinae, Auchmeromyiinae, and Bengaliinae are monophyletic subfamilies with a bootstrap value of 94%. The topology of the tree also illustrated that the Mesembrinellinae falls outside the family Calliphoridae. The Toxotarsinae acts as an outgroup, and the phylogenetic tree suggests it to be a separate family.
Fig 1.
Phylogenetic tree constructed by Maximum Likelihood (ML) analysis of 2197 aligned 28S rRNA nucleotides. Fifty sequences representing blowfly taxa in eleven subfamilies of the Calliphoridae: Luciliinae, Melanomyinae, Calliphorinae, Helicoboscinae, Rhiniinae, Auchmeromyiinae, Bengaliinae, Chrysomyinae, Polleniinae, Mesembrinellinae and Toxotarsinae.
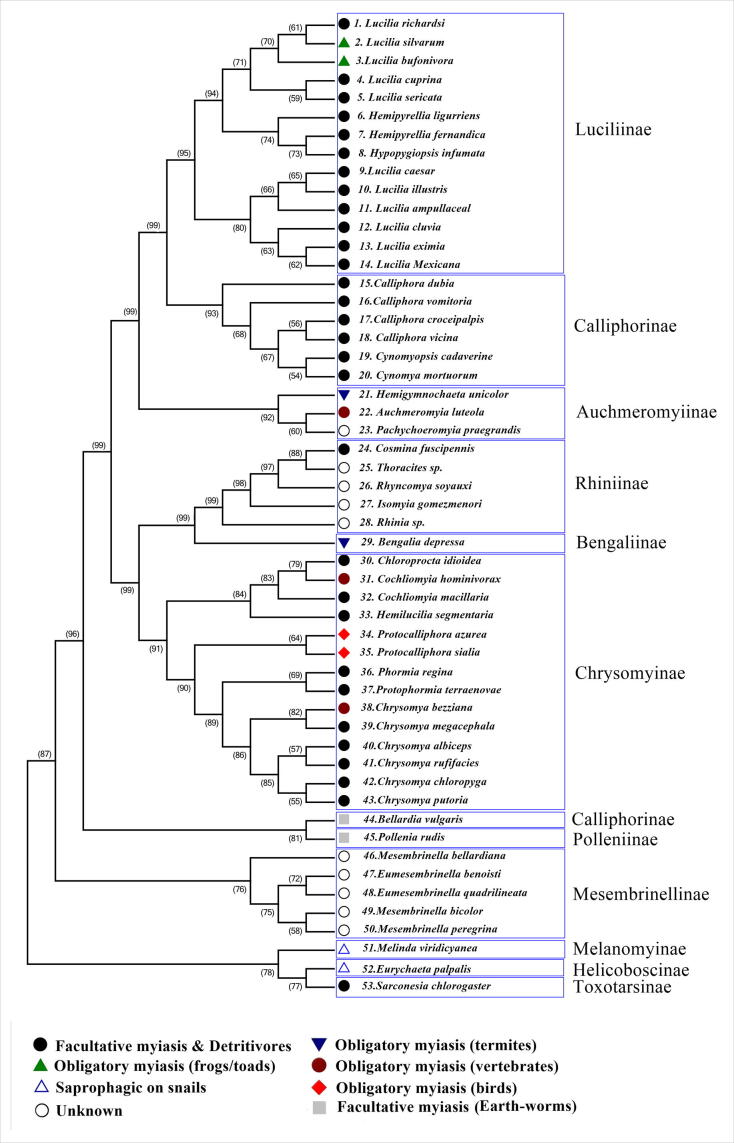
The phylogenetic tree of COI sequences (Fig. 2) revealed that the Luciliinae, Auchmeromyiinae, and Chrysomyinae are monophyletic groups. Furthermore, the Chrysomyinae and Rhiniinae are sister-groups with a bootstrap value equal to 99%, but the subfamily Bengaliinae represented by Bengalia depressa is a sister-group to Rhiniinae. Also, as demonstrated in Fig. 2, the phylogenetic analysis supports the Melanomyinae, Helicoboscinae, and Toxotarsinae as separate families with bootstrap values equal to 87%.
Fig 2.
Phylogenetic tree constructed by Maximum Likelihood (ML) analysis of 1500 aligned COI nucleotides. Fifty-six sequences representing blowfly taxa in eleven subfamilies of the Calliphoridae: Luciliinae, Calliphorinae, Auchmeromyiinae, Rhiniinae, Bengaliinae, Chrysomyinae, Polleniinae, Mesembrinellinae, Melanomyinae, Helicoboscinae, and Toxotarsinae.
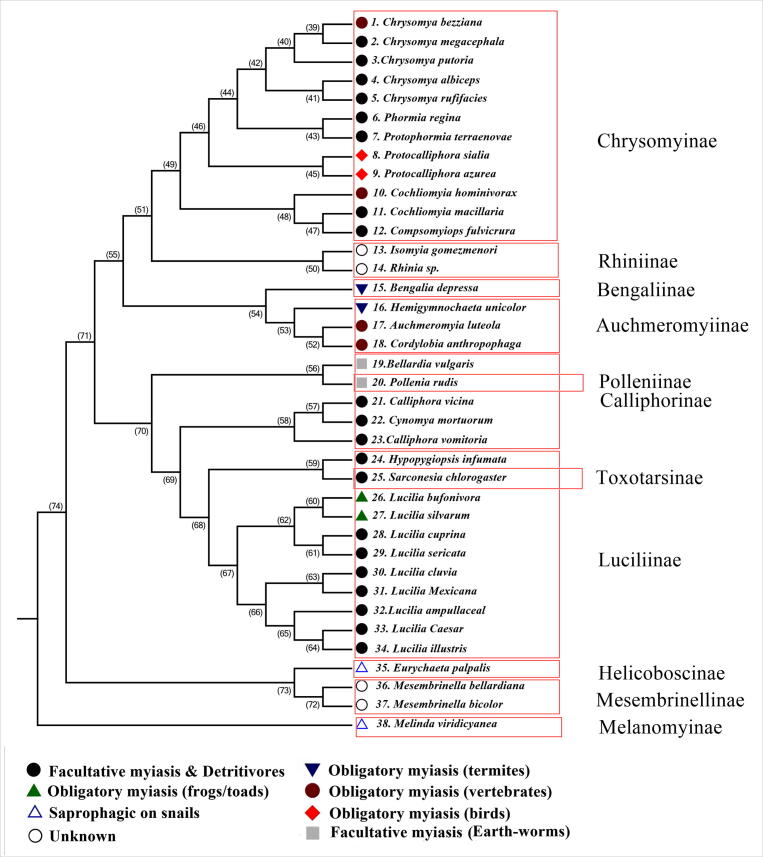
A combined phylogenetic tree with 38 taxa representing all the calliphorid subfamilies (Fig. 3) confirmed most of the previous results. The Chrysomyinae and Rhiniinae are sister-groups and the Bengaliinae is paraphyletic to the subfamily Rhiniinae. The Melanomyinae and Helicoboscinae fall in a clade outside other calliphorid subfamilies with a bootstrap value of 74%. Both need to be ranked as separate families, distinct from the Calliphoridae. There was just one remarkable change that took place in the subfamily Toxotarsinae, as represented by Sarconesia chlorogaster, which falls in the same clade as a member of subfamily Luciliinae, Hypopygiopsis infumata (Fig. 3).
Fig 3.
Combined phylogenetic tree representing thirty-eight sequences of taxa in eleven sub-families of Calliphoridae: Chrysomyinae, Rhiniinae, Bengaliinae, Auchmeromyiinae, Polleniinae, Calliphorinae, Toxotarsinae, Luciliinae, Helicoboscinae, Mesembrinellinae and Melanomyinae.
3.2. Myiasis status in blowflies
The evolution of eating fresh meat as an idea is linked to the evolution of myiasis itself. Biological or parasitological types of myiasis, either obligatory or facultative, clearly appeared on many occasions in the three phylogenetic trees (Fig 1, Fig 2, Fig 3). Within the blowflies, the obligatory myiasis of mammals was represented in different clades by Chrysomya bezziana, Cochliomyia hominivorax, and Auchmeromyia luteola. Obligatory myiasis on other hosts also emerged in different positions. Protocalliphora sialia and Protocalliphora azurea feed on birds, whilst Lucilia bufonivora and Lucilia silvarum are obligatory parasites on toads and frogs. Also, obligatory myiasis on termites as established by Bengalia depressa (Bengaliinae), Hemigymnochaeta unicolor, and Tricyclea sp. (Auchmeromyiinae) arose independently on several occasions. Obligatory myiasis on earthworms appeared in the Calliphorinae clade (Bellardia vulgaris) and the Polleniinae clade (Pollenia rudis). Moreover, secondary facultative myiasis is represented in different positions by Chrysomya albiceps, Chrysomya rufifacies, and Lucilia caesar. Finally, the saprophagous origin of myiasis is illustrated by the Melanomyinae and Helicoboscinae clades, where Melinda viridicyanea and Eurychaeta palpalis parasitise snails and gastropods (Fig 1, Fig 2, Fig 3).
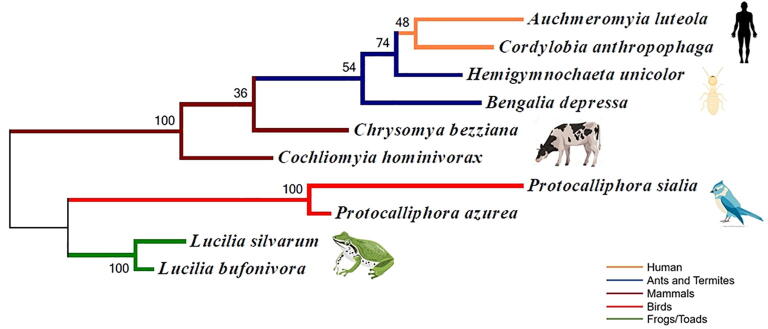
The annotated phylogenetic tree of obligatory myiasis species (Fig. 4) shows an impressive evolutionary way of myiasis. The tree represents paraphyletic relation to flies which parasitised amphibians and birds, and to flies which have a parasitic relationship with mammals. Also, it indicates that Lucilia bufonivora could be one of the oldest flies that causing myiasis in a vertebrate host. The appearance of Bengalia depressa (Bengaliinae) and Hemigymnochaeta unicolor which parasitised ants and termites in the same clade of flies causing myiasis is controversial and appeared late in the evolution of the group.
Fig 4.
The annotated tree illustrates phylogenetic relations among obligatory myiasis calliphorids.
4. Discussion
The family Calliphoridae is considered to be a key family for describing the evolution of the superfamily Oestroide (Rognes, 1997). This controversial status of the family Calliphoridae is due to the homogeneity of calliphorid species that could not be assigned to other families, the diversity of feeding habits, and the non-host specificity (Rognes, 1997, Stevens, 2003). Furthermore, there has been a shortage of hypotheses that discuss the taxonomic classification and evolutionary relationships within the family.
In this study, several taxa are used to complement the clades of three independent gene trees. The sequence data from 28S rRNA, COI gene, and the confirmatory combined sequence tree define the phylogenetic analysis of the family Calliphoridae. The resulting trees support each other.
Several previous studies have regarded the Calliphoridae as a monophyletic family (Singh and Wells, 2013). Other studies provide evidence for the non-monophyly of the Calliphoridae based on morphological and molecular clues (Rognes, 1997, Kutty et al., 2010). Our phylogenetic analysis based on 28S rRNA and COI subunits support the studies of Kutty et al. (2010) which concludes that the Calliphoridae is a non-monophyletic group (Fig 1, Fig 2, Fig 3) (Kutty et al., 2010, Singh and Wells, 2013). On the other hand, the calliphorid subfamilies selected for this study (Chrysomyinae, Calliphorinae, Luciliinae, Auchmeromyiinae, Bengaliinae, Helicoboscinae, Polleniinae, Melanomyinae, Mesembrinellinae, Rhiniinae, Toxotarsinae) are each monophyletic.
At the subfamily level, the resulting phylogenetic relationships among calliphorid subfamilies do not agree with the relationship models revealed by previous studies. Our model trees support the sister lineage of both Luciliinae-Calliphorinae, which agrees with recent molecular studies but disagree with the morphology-based study which supports a Calliphorinae-Chrysomyinae grouping (Rognes, 1997, Stevens, 2003, Wallman et al., 2005). Although the monophyly of Chrysomyinae and Luciliinae is well supported (Fig 1, Fig 2, Fig 3), the status of the Calliphorinae is less robust. This agrees with the studies of Kutty et al., 2010, Singh and Wells, 2013 in which the Melanomyinae is nested within the Calliphorinae (Fig. 1) (Kutty et al., 2010, Singh and Wells, 2013). This is a surprising and unexpected position for the Melanomyinae because its species have previously been classified predominantly as Calliphorinae (Kurahashi, 1970).
The Toxotarsinae is considered to be one of the obscure calliphorid subfamilies. Based on some morphological characters, such as subcostal sclerite setulose and upper surface of stem-vein setose, the Toxotarsinae was grouped with the Chrysomyinae (Singh and Wells, 2013). The phylogenetic analysis of 28S rRNA and COI suggests raising the Toxotarsinae to the family level (Fig 1, Fig 2). Similarly, the phylogenetic positions of the Helicoboscinae, Mesembrinellinae, and Melanomyinae are unstable and provide evidence of their paraphyletic status with the remaining calliphorid subfamilies (Fig 2, Fig 3). This has been confirmed elsewhere by raising the Mesembrinellinae to family level (Mesembrinellidae), based on phylogenetic analysis and as a sister-family to the Ulurumyiidae (McAlpine's fly) (Cerretti et al., 2017).
Previous studies separated the Bengaliidae as a sister-family to the Calliphoridae (Rognes, 2005). But a recent molecular analysis nested Bengaliinae within the Chrysomyinae (Kutty et al., 2010). Moreover, our phylogenetic analysis recommends it as a sister-group of the Chrysomyinae (Fig 1, Fig 2).
Previously, the Rhiniinae was classified inside a monophyletic Calliphoridae (Singh and Wells, 2013). But recent phylogenetic analysis has supported raising it to family level as sister-group of the Oestridae, Calliphoridae, and Tachinidae or as sister-group of the Calliphoridae and Sarcophagidae (Kutty et al., 2010, Marinho et al., 2012). Our phylogeny suggests that the Rhiniinae is nested within the family Calliphoridae (Fig 1, Fig 2, Fig 3). The position of the Rhiniinae thus remains controversial and needs further investigation.
Although most blowfly larvae are saprophagous (carrion breeders), other members of family Calliphoridae parasitise vertebrate and invertebrate hosts (Stevens, 2003). The parsimonious phylogenetic analysis provided in this study suggests that the ectoparasitic behavior in the form of myiasis has arisen on more than one occasion. It can be seen that obligate parasitism arose autonomously on more than five occasions (Fig 1, Fig 2, Fig 3). Cordylobia anthropophaga and Auchmeromyia luteola of the subfamily Auchmeromyiinae, and Chrysomya bezziana and Cochliomyia hominivorax of the Chrysomyinae, are able to parasitise warm-blooded vertebrates (Zumpt, 1965, Stevens, 2003), whilst Lucilia silvarum and Lucilia bufonivora of the Luciliinae, and Protocalliphora azurea and Protocalliphora sialia of the Chrysomyinae, are parasites of toads and birds respectively (Marinho et al., 2012, Arias-Robledo et al., 2019). This study also shows that myiasis occurs in less evolutionarily advanced hosts like earthworms and termites which are parasitised by Pollenia rudis of the Polleniinae and Bengalia depressa of the Bengaliinae, respectively (Fig 1, Fig 2, Fig 3). Moreover, members of the subfamilies Bengaliinae and Rhiniinae which parasitise ants and termites are nested in the same clade (Fig 1, Fig 2). This monophyletic conclusion in our results is incongruous with Singh and Wells (2013) and suggests that parasitism on social insects diverged only once in the family Calliphoridae (Singh and Wells, 2013).
Focusing on the above results, it could be considered that eating fresh meat of both vertebrates and invertebrates is an ancestral state of the myiasis habit (Stevens, 2003). But the presence of other primary obligatory myiasis-causing agents and species of the primarily facultative ectoparasitic Luciliinae (L. sericata and L. cuprina) with other saprophagous blowfly clades suggest multiple independent evolutions of myiasis as a parasitic habit in the family Calliphoridae. Furthermore, the hematophagous behavior of C. bezziana and Co. hominivorax could be considered as an opportunistic myiasis mode adopted by these larvae to overcome stressful conditions. This conclusion agrees with the functional development of the origins of myiasis, whether saprophagous or hematophagous (Zumpt, 1965, Hosni et al., 2019).
Through the evolutionary history, coevolution appears several times between the parasite and its host (Shobrak et al., 2015, Nasser et al., 2019, Nasser et al., 2020). But it is too difficult to describe and analyze any pattern of co-evolution among obligatory myiasis calliphorids and their host. As members of family Calliphoridae show no host specificity. In context, although the resultant annotated tree (Fig. 4) illustrated that obligatory parasitism appeared several times through the evolutionary history of myiasis, it appeared firstly on toads and frogs via Lucilia bufonivora and Lucilia silvarum that could be appeared during the Devonian period, around 370 million years ago (George and Blieck, 2011), and ended by Auchmeromyia luteola and Cordylobia anthropophaga which parasitise human around 200,000 years ago (Villa and Roebroeks, 2014). The occurrence of Bengalia depressa (Bengaliinae) and Hemigymnochaeta unicolor in the same clade of mammalian parasites could be questionable and need more investigations, especially their relationship with Auchmeromyia luteola and Cordylobia anthropophaga which could share a common ancestor (Fig. 4).
However, the similarity of myiasis behavior between the Old World screwworm (C. bezziana) and the New World screwworm (Co. hominivorax) could be considered as a type of convergent evolution that occurred ecologically due to geographic isolation resulting in a speciation event (Hosni et al., 2020). This occurred at the beginning of the Late Cretaceous period accompanied by the expansion of flowering plants (146–65 Myr ago) which allowed adult blowflies to feed on flower nectar (Amendt et al., 2004). The later diversification of the mammals (65–1.8 Myr ago), which offered a range of potential hosts, facilitated the subsequent evolutionary pathways for larvae to diverge in their feeding behavior (Stevens et al., 2006). This agrees with the available fossil evidence of a calliphorid species, Cretaformia fowleri (105–65 Myr ago), from Canada (McAlpine, 1970). Unfortunately, there is no clue as to what its feeding behavior may have been.
Finally, myiasis is a major area of uncertainty in flies' evolution and the evidence on their history has been challenging to interpret from both molecular and morphological perspectives. So, our work forms a step in a long way of understanding such a phenomenon. The study gives an important insight into some hot areas of myiasis evolution, especially parasitism on living tissue, and the generated trees came compatible with recently published work concerning myiasis (Narayanan Kutty et al., 2019). Incorporation of more species of the family to the study could improve the results, but the used taxa form the most significant members of the family and cover the area of our main question. Further studies are still needed to complete the picture of the family taxonomy and evaluation.
5. Conclusions
Our study has suggested answers to several systematic and phylogenetic problems surrounding the family Calliphoridae. 28S rRNA and COI subunits are considered to be recent and successful molecular techniques that enable the possible construction of robust phylogenies. With these, we are able to reassess the hypotheses of blowfly evolution and the divergent and convergent evolutionary pathways of the myiasis habit. Our results support the polyphyletic origin of the family Calliphoridae despite the monophyletic status of some of the subfamilies such as the Chrysomyinae and Luciliinae. Moreover, our phylogenetic analysis has endorsed the elevation of the subfamily Toxotarsinae to family level and has highlighted the unstable phylogenetic positions of the subfamilies Helicoboscinae, Mesembrinellinae, and Melanomyinae which need further systematic clarification. Furthermore, our parsimonious phylogenies demonstrate that the eating of fresh meat appeared independently on more than five occasions among different calliphorid taxa in the course of the evolutionary history of myiasis. Finally, filling the evolutionary gaps by incorporating other myiasis-causing families (Oestridae, Gastrophilidae, and Sarcophagidae) along with fundamental life-history studies that deal with biology, physiology, feeding behavior and host specificity in addition to a phylogenetic analysis could give a more precise answer about the exact origin of myiasis.
Ethical approval
This article does not contain any studies dealing directly with animals and all applicable international, national, and /or institutional ethical guidelines were taken into consideration during the preparation of this manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
This project was supported by Researchers Supporting Project number (RSP-2020/283) King Saud University, Riyadh, Saudi Arabia. Thanks, extended to Entomology Department staff, Faculty of Science, Ain Shams University for their continuous help and support during the study.
Funding
Researchers Supporting Project number (RSP-2020/283) King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Amendt J., Krettek R., Zehner R. Forensic entomology. Naturwissenschaften. 2004;91:51–65. doi: 10.1007/s00114-003-0493-50. [DOI] [PubMed] [Google Scholar]
- Arias-Robledo G., Wall R., Szpila K., Shpeley D., Withworth T., Stark T., King R.A., Stevens J.R. Ecological and geographical speciation in Lucilia bufonivora: The evolution of amphibian obligate parasitism. IJP-PAW. 2019;10:218–230. doi: 10.1016/j.ijppaw.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R.H., Wilkinson G.E.S., Desalle R. Phylogenetic utility of different types of molecular data used to infer evolutionary relationships among stalk-eyed flies (Diopsidae) Syst. Biol. 2001;50:87–105. doi: 10.1080/106351501750107512. [DOI] [PubMed] [Google Scholar]
- Cerretti P., Stireman J.O., Pape T., O’hara J.E., Marinho M.A.T., Rognes K., Grimaldi D.A. First fossil of an oestroid fly (Diptera: Calyptratae: Oestroidea) and the dating of oestroid divergences. PLoS ONE. 2017;12:1–24. doi: 10.1371/journal.pone.0182101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hawagry M.S., El-Azab S.A. Catalog of the calliphoridae, rhiniidae, and sarcophagidae of Egypt (Diptera: Oestroidea) EJBPC. 2019;29 doi: 10.1186/s41938-019-0118-8. [DOI] [Google Scholar]
- Flissak J.C., Moura M.O., Kaufman P. Intrapuparial development of Sarconesia chlorogaster (Diptera: Calliphoridae) for Postmortem Interval Estimation (PMI) J. Med. Entomol. 2018;55:277–284. doi: 10.1093/jme/tjx214. [DOI] [PubMed] [Google Scholar]
- George D., Blieck A. Rise of the earliest tetrapods: An Early Devonian origin from marine environment. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0022136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M., Wall R. Myiasis of humans and domestic animals. Adv. Parasitol. 1995;35:257–334. doi: 10.1016/S0065-308X(08)60073-1. [DOI] [PubMed] [Google Scholar]
- Hosni E.M., Nasser M.G., Al-Ashaal S.A., Magda H.R., Mohamed A.K. Modeling current and future global distribution of Chrysomya bezziana under changing climate. Sci. Rep. 2020;10:4947. doi: 10.1038/s41598-020-61962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosni E.M., Nasser M.G., Al-Ashaal S.A., Magda H.R., Mohamed A.K. A brief review of myiasis with special notes on the blow flies’ producing myiasis (F.: Calliphoridae). Egypt. Acad. J. Biol, Sci. 2019;11:25–32. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Kurahashi H. Tribe Calliphorini from Australian and Oriental Regions, I. Melinda-group (Diptera: Calliphoridae) Pacific Insects. 1970;12:519–542. [Google Scholar]
- Kutty S.N., Pape T., Wiegmann B.M., Meier R. Molecular phylogeny of the Calyptratae (Diptera: Cyclorrhapha) with an emphasis on the superfamily Oestroidea and the position of Mystacinobiidae and McAlpine’s fly. Syst. Entomol. 2010;35:614–635. doi: 10.1111/j.1365-3113.2010.00536.x. [DOI] [Google Scholar]
- Marinho M.A.T., Junqueira A.C.M., Paulo D.F., Esposito M.C., Villet M.H., Azeredo-Espin A.M.L. Molecular phylogenetics of Oestroidea (Diptera: Calyptratae) with emphasis on Calliphoridae: Insights into the inter-familial relationships and additional evidence for paraphyly among blowflies. Mol. Phylogenet. Evol. 2012;65:840–854. doi: 10.1016/j.ympev.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Mcalpine J.F. First record of calypterate flies in the mesozoic era (Diptera: Calliphoridae) Can. Entomol. 1970;102:342–346. doi: 10.4039/Ent102342-3. [DOI] [Google Scholar]
- Mcdonagh L.M., Stevens J.R. The molecular systematics of blowflies and screwworm flies (Diptera: Calliphoridae) using 28S rRNA, COX1 and EF-1: Insights into the evolution of dipteran parasitism. Parasitology. 2011;138:1760–1777. doi: 10.1017/S0031182011001089. [DOI] [PubMed] [Google Scholar]
- Kutty S.N., Meusemann K., Bayless K.M., Marinho M.A.T., Pont A.C., Zhou X., Misof B., Wiegmann B.M., Yeates D., Cerretti P., Meier R., Pape T. Phylogenomic analysis of Calyptratae: resolving the phylogenetic relationships within a major radiation of Diptera. Cladistics. 2019;35:605–622. doi: 10.1111/cla.12375. [DOI] [PubMed] [Google Scholar]
- Nasser M., Alahmed A., Ansari M., Adly E., Shobrak M. An analysis of osprey/chewing lice interaction, with a new record for Saudi Arabia. Afr. Entomol. 2019;27:178–184. [Google Scholar]
- Nasser M., Alahmed A., Shobrak M. Host habitat and position on host affecting the evolution of chewing lice (Phthiraptera): Phylogenetic analysis of Ischnocera in Saudi Arabia. J. Insect Biodivers. Syst. 2020;6:101–112. [Google Scholar]
- Otranto D., Traversa D., Milillo P., De Luca F., Stevens J. Utility of mitochondrial and ribosomal genes for differentiation and phylogenesis of species of gastrointestinal bot flies. J. Econ. Entomol. 2005;98:2235–2245. doi: 10.1093/jee/98.6.2235. [DOI] [PubMed] [Google Scholar]
- Pape T., Arnaud P.H. Bezzimyia - A genus of native new world Rhinophoridae (Insecta, Diptera) Zool. Scr. 2001;30:257–297. doi: 10.1046/j.1463-6409.2001.00064.x. [DOI] [Google Scholar]
- Rognes K. The Calliphoridae (Blowflies) (Diptera: Oestroidea) are not a monophyletic group. Cladistics. 1997;13:27–66. doi: 10.1006/clad.1997.0031. [DOI] [PubMed] [Google Scholar]
- Rognes K. Bengalomania – A review of Andy Z. Lehrer’s book on Bengalia Robineau-Desvoidy, 1830 and related works (Diptera, Calliphoridae) Studia dipterological. 2005;12:443–471. [Google Scholar]
- Sanit S., Limsopatham K., Klong-Klaew T., Samerjai C., Yasanga T., Sukontason K., Tomberlin J.K., Sukontason K.L. Morphology of immature blow fly Hypopygiopsis infumata (Bigot) (Diptera: Calliphoridae), a potential species of forensic importance. Acta Trop. 2018;188:168–179. doi: 10.1016/j.actatropica.2018.08.037. [DOI] [PubMed] [Google Scholar]
- Shao R., Barker S.C. Mitochondrial genomes of parasitic arthropods: Implications for studies of population genetics and evolution. Parasitology. 2007;134:153–167. doi: 10.1017/S0031182006001429. [DOI] [PubMed] [Google Scholar]
- Shobrak M., Alahmed A., Palma R., Almalki M., Nasser M.G. New records of species of Saemundssonia (Insecta: Phthiraptera: Philopteridae) infesting breeding terns in the Arabian Peninsula, with notes on their phylogeny and ecology. Parasito. Res. 2015;114:2587–2597. doi: 10.1007/s00436-015-4463-6. [DOI] [PubMed] [Google Scholar]
- Singh B., Wells J.D. Chrysomyinae (Diptera: Calliphoridae) is monophyletic: A molecular systematic analysis. Syst. Entomol. 2011;36:415–420. doi: 10.1111/j.1365-3113.2011.00568.x. [DOI] [Google Scholar]
- Singh B., WELLS J.D. Molecular systematics of the Calliphoridae (Diptera: Oestroidea): evidence from one mitochondrial and three nuclear genes. J. Med. Entomol. 2013;50:15–23. doi: 10.1603/me11288. [DOI] [PubMed] [Google Scholar]
- Stevens J., Wall R. The evolution of ectoparasitism in the genua Lucilia (Diptera: Calliphoridae) Int. J. Parasitol. 1997;27:51–59. doi: 10.1016/s0020-7519(96)00155-5. [DOI] [PubMed] [Google Scholar]
- Stevens J.R. The evolution of myiasis in blowflies (Calliphoridae) Int. J. Parasitol. 2003;33:1105–1113. doi: 10.1016/S0020-7519(03)00136-X. [DOI] [PubMed] [Google Scholar]
- Stevens J.R., Wallman J.F., Otranto D., Wall R., Pape T. The evolution of myiasis in humans and other animals in the old and new Worlds (part II): Biological and life-history studies. Trends Parasitol. 2006;22:181–188. doi: 10.1016/j.pt.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Sukontason K., Sribanditmongkol P., Ngoen-Klan R., Klong-Klaew T., Moophayak K., Sukontason K.L. Differentiation between Lucilia cuprina and Hemipyrellia ligurriens (Diptera: Calliphoridae) larvae for use in forensic entomology applications. Parasitol. Res. 2010;106:641–646. doi: 10.1007/s00436-009-1711-7. [DOI] [PubMed] [Google Scholar]
- Tajima F., Nei M. Estimation of evolutionary distance between nucleotide sequences. Mol. Biol. Evol. 1984;1:269–285. doi: 10.1093/oxfordjournals.molbev.a040317. [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verves Y.G. The new faunistic data on Calliphoridae and Sarcophagidae (Diptera) of the Republic of Seychelles. Phelsuma. 2007;15:71–81. [Google Scholar]
- Villa P., Roebroeks W. Neandertal demise: An archaeological analysis of the modern human superiority complex. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0096424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallman J.F., Leys R., Hogendoorn K. Molecular systematics of Australian carrion-breeding blowflies (Diptera: Calliphoridae) based on mitochondrial DNA. Invertebr. Syst. 2005;19:1–15. doi: 10.1071/IS04023. [DOI] [Google Scholar]
- Wiegmann B.M. Episodic radiations in the fly tree of life. PNAS. 2011;108:5690–5695. doi: 10.1073/pnas.1012675108//DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1012675108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates K.D., Wiegmann B.M. Columbia University Press; 2005. The Evolutionary Biology of Flies. [Google Scholar]
- Zumpt F. Butterworths; London, U.K.: 1965. Myiasis in Man and Animals in the Old World. [Google Scholar]