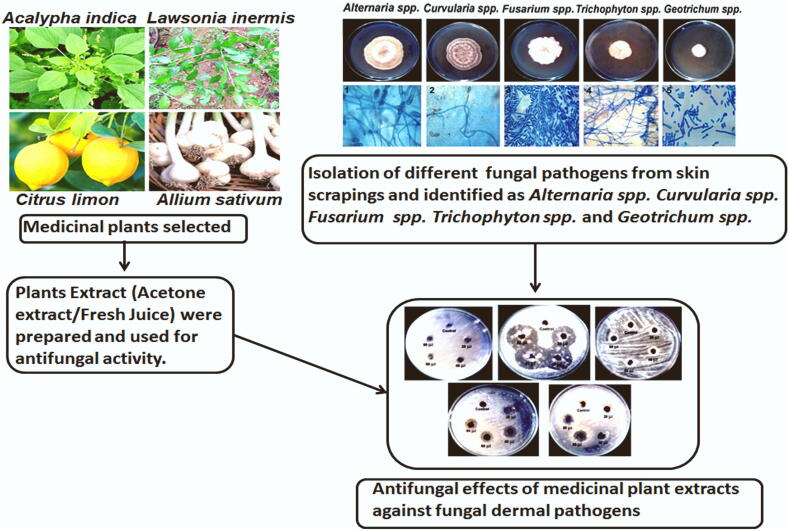
Abstract
A broad spectrum of medicinal plants was used as traditional remedies for various infectious diseases. Fungal infectious diseases have a significant impact on public health. Fungi cause more prevalent infections in immunocompromised individuals mainly patients undergoing transplantation related therapies, and malignant cancer treatments. The present study aimed to investigate the in vitro antifungal effects of the traditional medicinal plants used in India against the fungal pathogens associated with dermal infections. Indian medicinal plants (Acalypha indica, Lawsonia inermis Allium sativum and Citrus limon) extract (acetone/crude) were tested for their antifungal effects against five fungal species isolated from skin scrapings of fungal infected patients were identified as including Alternaria spp., Curvularia spp., Fusarium spp., Trichophyton spp. and Geotrichum spp. using well diffusion test and the broth micro dilution method. All plant extracts have shown to have antifungal efficacy against dermal pathogens. Particularly, Allium sativum extract revealed a strong antifungal effect against all fungal isolates with the minimum fungicidal concentration (MFC) of 50–100 μg/mL. Strong antifungal activity against Curvularia spp., Trichophyton spp., and Geotrichum spp. was also observed for the extracts of Acalypha indica, and Lawsonia inermis with MFCs of 50–800 μg/mL respectively. The extracts of Citrus limon showed an effective antifungal activity against most of the fungal strains tested with the MFCs of 50–800 μg/mL. Our research demonstrated the strong evidence of conventional plants extracts against clinical fungal pathogens with the most promising option of employing natural-drugs for the treatment of skin infections. Furthermore, in-depth analysis of identifying the compounds responsible for the antifungal activity that could offer alternatives way to develop new natural antifungal therapeutics for combating resistant recurrent infections.
Keywords: Oppourtunistic pathogens, Immunocompromised individuals, Skin infections, Fungal pathogens, Antifungal activity, Indian medicinal plants
1. Introduction
Universally, humans are co-habiting earth with plentiful ecosystems comprises of various classes of microorganisms that may be beneficial or harmful to them. However, humans are evolved in such a way that their inherent innate immune system protects the body from harmful pathogens. It is well known that our human skin is featured to have abundant diversified groups of microorganisms ranging from bacteria, fungi to parasites. Such colonies of microorganism particularly fungi are forming a commensal relationship with human skin (Hurabielle et al., 2020). Thus, fungi contribute to play a role of an essential member of the dermal microbiome; however, such relationships between epithelial-fungal partnerships (mycobiomes) in maintaining skin homeostasis are not well understood. In specific cases, during the immunocompromised state, fungi will act as opportunistic pathogen and begin to evade host immune system and cause serious and frequent infections (Köhler et al., 2015).
Opportunistic infections occur mainly in immunocompromised hosts, but primary infections may also occur in hosts with a healthy immune system. Besides that, fungal infections can be systemic or local (Janbon et al., 2019). The majority of fungal infections are common in individuals working in agriculture or forest division. These fungi are soil dwellers and gain entry to humans via penetrating the injuries or wounds and capable of causing cutaneous and subcutaneous fungal infections. The hind and forelimbs are the commonly affected areas where fungal infections often causing local or systemic infections (Seyedmousavi et al., 2018).
In recent years the occurrence of fungal infections has become more prevalent and additionally leading to the cause of mortality and morbidity in immunocompromised individuals. Out of which 74.3% contributes for dermal infections both cutaneous and subcutaneous, then 9.5% constitutes for oculomycosis, then 8.1% for invasive and non-invasive rhinosinusitis and onychomycosis respectively (Deutsch et al., 2019). A fungal infection may occur following trauma or wound contamination (Ganesan et al., 2019). Recurrent and sustained contact of immunocompromised patients to variable environmental circumstances has ensued new opportunistic fungal infections (Badiee and Hashemizadeh, 2014).
Recently, much newer opportunistic pathogens are emerging and causing life-threatening infections worldwide. This is due to the repeated usage of currently available antifungal drugs and constant use of this resulting in the fungal strain acquiring resistance to particular drugs, which are difficult to treat further. Moreover, the known antifungal drug amphotericin B is shown to cause systemic toxicity and other health-related problems (Pierce et al., 2013). Therefore, novel compounds having a different approach of action, with high fungicidal activity and minimal systemic toxicity are the current prerequisite for treating the resistant strains of clinical pathogens. Emerging reports of antimicrobial properties of our traditional medicinal plant extracts suggesting an alternative for chemically synthesized molecules. The World Health Organization also recognized the use of plant extracts or their active components in the name of folk medicine as traditional therapies in 80% of the world's population (Shaik et al.,1994). Plants are a chemical and biologically complex source used traditionally throughout human history as a common medicine. Plants remain an essential source of medicines, especially where conventional plant-based medicines still serve the needs of health (Salim et al., 2008). Medicinal plants are habitually used by ethnic people to treat various diseases comprising ringworm and other fungal dermal infections (Rajan et al., 2001). While drug-driven compounds have recently demonstrated their interest in molecular modeling, combinatory chemistry, natural derived compounds, and other synthetic approaches are still an indispensable source of medicaments for humans (Salim et al., 2008). Several methods of research support that the variety of biological and pharmacological characteristics of plant materials shown increased interest (Dhanasekaran, 2020, Ocheng et al., 2014, Wu and Dhanasekaran, 2020).
The present study was indented to validate the traditional use of selected Indian medicinal plants against opportunistic fungal pathogens including Alternaria spp., Curvularia spp., and Fusarium solani spp., Trichophyton spp. and Geotrichum spp. by evaluating their in vitro antifungal activity. The plants examined in this research are frequently used to treat skin infections and the associated symptoms in regular day today use in local areas of parts of Tamil Nadu, India.
2. Materials and methods
2.1. Isolation fungal pathogens
2.1.1. Sample collection
Samples were collected from 30 clinically suspected cases of cutaneous fungal infections admitted in the outpatient clinic of the dermatology department of Government Hospital, Tuticorin, Tamil Nadu, India. After the informed consent, the patient was registered with demographic data and clinical conditions were recorded. The infected areas were rubbed with 70% alcohol to remove the dirt and other ointments. The skin scrapings of the fungal infections were collected by using sterile blunt scalpel from the advancing borders of the fungal infections. The collected samples were aseptically transported shortly to the laboratory for further processing like cultural analysis and microscopic (Singh and Beena, 2003). The fungi were maintained and preserved on Sabouraud dextrose agar (SDA) (Hi-Media, Cat No: MHO63) slants at 4 °C and aseptically subcultured throughout the study.
2.1.2. Processing of fungal culture
Each sample was cultured on Sabouraud’s Dextrose agar supplemented with chloramphenicol. The plates were incubated at 28 °C for up to a week and everyday examined the fungal growth. After the incubation period, the mycelial morphology of various fungal isolates grown on the Sabouraud’s Dextrose agar plates was observed and recorded. Isolated fungi were further subcultured onto Sabouraud’s Dextrose agar slants.
2.1.3. Microscopic examination
Adhesive tape was cut into 1 cm squares and was placed with the help of sterile forceps on the surface of mycelia and lifted off. Few drops of lactophenol cotton blue (LPCB) were placed on another slide. Then the adhesive tape containing the fungal mycelia was placed on it, then mounted and observed under low power 10X and high power 45X objective lens of the bright field light microscope.
2.1.4. Antifungal activity of commercial drugs
Antifungal activity of commercial drugs against the isolated non-dermatophytic opportunistic cutaneous fungi were done by the Kirby Bauer method. Commercially available antifungal such as Amphotericin-B, Fluconazole, Clotrimazole, Ketokonaze from were tested against the selected fungal skin infection. (All antifungals were purchased from Hi-Media, and the Cat No: A011-1X20ML, EM072-30ST, SD115-1PL and SD274-1PL respectively).
2.2. Plant materials and extraction
2.2.1. Plant collection
The plants (Acalypa indica, Lawsonia inermis) and spices (Citrus limon and Allium sativum) were collected from in and around Srivaikundam Tuticorin, Tamil Nadu, India between January to August 2018. The collected plant material and spices were authenticated by the Department of Botany, Kamaraj College, Tuticorin, Tamil Nadu, India. The specimens of the plants were deposited in the Herbarium of the Department of Botany, Kamaraj College, Tuticorin.
2.2.2. Preparation of plant extracts
The collected plant leaves of Acalypa indica and Lawsonia inermis were air-dried and finely powdered using a blender. To prepare acetone extracts of the plant materials, 50 g of each powdered leaf material was soaked in 500 ml of acetone for 72 h with constant rocking at room temperature. The samples were then sieved over Whatman No:1 filter paper. The filtrates were vaporized to dryness using a rotary evaporator. For antifungal activity assays, a stock solution was made for each extract with 0.2 g/mL in dimethyl sulfoxide (DMSO). The extracts were stored at −20 °C until further use and the stock were stored at 4 °C until used.
2.2.3. Preparation of spices extracts
The spice Allium sativum gloves were descaled and washed in sterile distilled water. The extract was prepared as follows. Approximately 100 g of spices weighed and chopped into small pieces and let for air-dried. The dried pieces were then crushed using a blender to get the powder form of the extract. To prepare acetone extracts of Allium sativum, 50 g of powdered Allium sativum was soaked in 500 ml of acetone for 72 h with constant rocking at room temperature. The samples were then sieved over the Whatman No:1 filter paper. The filtrate was evaporated to dryness using a rotary evaporator. Similarly, fresh juice of Citrus limon (Lemon) were collected from fresh lemon (cut and squeezed, the juice in a sterile flask). The extracts were strained through a fine mesh cloth and sterilized using a membrane filter.
For antifungal activity assays, a stock solution was made for each extract with 0.2 g/mL in dimethyl sulfoxide (DMSO). The stock was stored at 4 °C, until used.
2.3. Antifungal assays
The fungicidal activity of extracts was assessed by the agar well diffusion method using Sabouraud’s Dextrose Agar. The isolated fungal skin pathogens were inoculated into 10 ml of sterile Sabouraud’s Dextrose Broth and incubated at room temperature for 5 days. Using a sterile cotton swab, fungal spores were swabbed on the sterile Sabouraud’s Dextrose agar plates. Agar wells were prepared with the help of a sterile cork borer with a 10 mm diameter. Using a micropipette, different concentrations of medicinal plant extracts were added to different wells on the plate separately. The plates were incubated in an upright position at room temperature. The diameter of inhibition zones was measured in mm and the results were recorded (Indu et al., 2006).
2.4. Determination of antifungal efficacy
The minimum inhibitory concentration (MIC) of each medicinal plant extract was determined using the broth microdilution method according to (Gerald, et al., 1996). Briefly, a fresh colony of fungal isolates were inoculated into Sabouraud’s dextrose broth (SDB) and incubated at 37 °C for 4 h equivalent to 1.5 × 108 Colony Forming Unit adjusted to 0.5 McFarland standard. Fluconazole infusion (2 mg/ml; Pfizer, (Pharmacia India Pvt Ltd) India) was used as a positive control for the antifungal assay. Cultures were incubated at 37 °C for 24–48 h, and the tubes were examined for fungal growth to determine the minimum inhibitory concentration (MIC). The antifungal activity was repeated twice with two replicates for all clinical isolates with each plant extract at all the test concentrations.
3. Results
3.1. Antifungal effects of clinical isolates
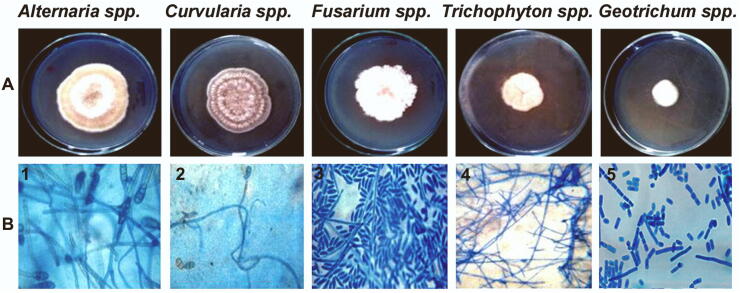
Of the 30 clinical samples processed, all samples 30 (100%) displayed fungal morphology on KOH preparation and further clinical specimens were confirmed by culture. In positive clinical cases, the ratio of men and women was 2:1. (Table 1). Fig. 1 showed the colony morphology of fungal strains isolates and the microscopic examination of isolated fungus by Lactophenol cotton blue staining from a skin infection. The fungal isolates were identified as Alternaria spp., Curvularia spp., and Fusarium spp., Trichophyton spp. and Geotrichum spp. The commercial antibiotic Amphotericin – B and Ketoconazole showed high inhibitory activity against Alterneria sp, but, Fluconazole and Clotrimazole antibiotics showed highly resistant against Alterneria spp. In Curvularia spp., antibiotics showed less inhibitory activity (slightly sensitive) against antibiotics. These antibiotics did not show any inhibitory activity (resistant) against the Fusarium spp. Trichophyton spp. and Geotrichum spp.
Table 1.
Distribution of dermatophytes in relation to sex of the patient.
| Fungus Isolated | Male | Female |
|---|---|---|
| Alternaria sp, | 3 | 2 |
| Curvularia sp, | 3 | 1 |
| Fusarium sp, | 3 | 2 |
| Trichophyton sp. | 7 | 3 |
| Geotrichum sp. | 4 | 2 |
| Total | 20 | 10 |
Fig. 1.
Colony morphology and microscopic photographs of dermal fungal strains isolated from skin scrapings. A. Macroscopic morphology of fungal isolates on Sabouraud dextrose agar (SDA) incubated at 30 °C for 5 days. B. Microscopic observation of fungal isolates by lactophenol cotton blue stain (LPCB) wet mount preparation. 1. Microscopically, the tapering conidia arise in chains and have both transverse and vertical septae giving a muriform appearance. (x40) 2. The thallus consists of dark septate hyphae with conidia arising sympodially from the conidiophore. Conidia have transverse septae with 3–5 cells. (x40) 3. Septate hyphae with short tapering conidiophore (conidiophore may be long in some species) are seen. (x40) 4. Sparse clubbed microconidia, formed along the sides of the hyphae. (x40) 5. True hyphae giving lateral branches at right angle and break into rectangular arthroconidia of variable lengths. (x40).
3.2. Antifungal effects of Acalypha indica
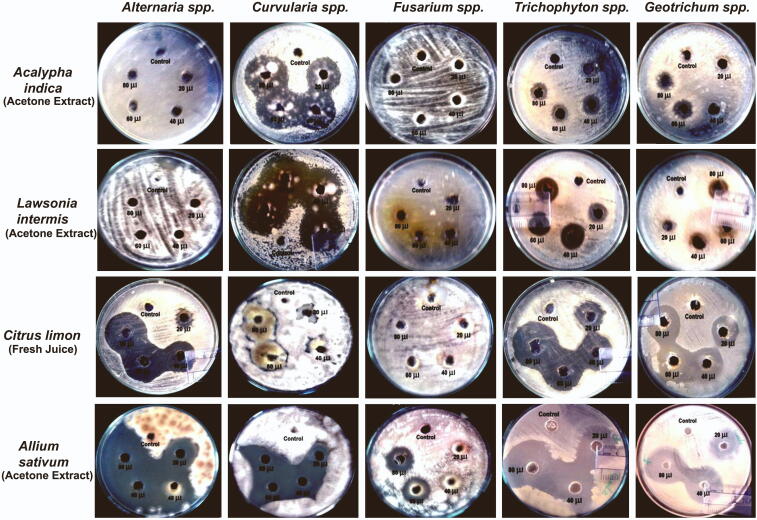
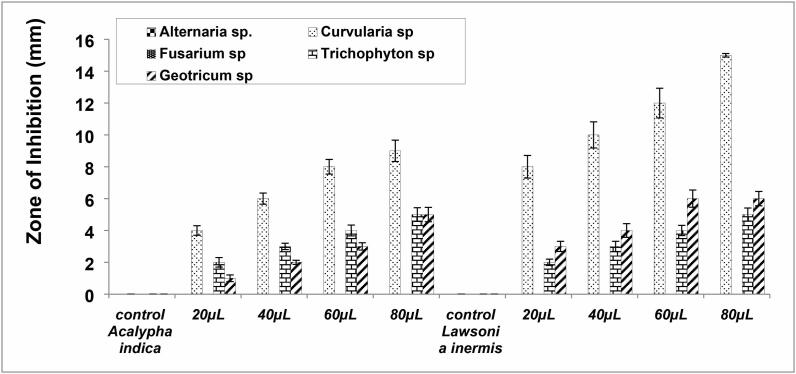
In this study, 4 plants were selected according to their traditional usage for the treatment of fungal skin infection and associated symptoms. Table 2. revealed the description of the plants examined by common and scientific names, traditional medicinal uses. Acetone leaf extract of Acalypha indica showed an effective dose-dependent antifungal effects against Curvularia spp. and showed negligible inhibitory activity against Trichophyton spp. and Geotrichum spp. whereas did not show any antifungal activity against Alternaria spp. and Fusarium spp. (Fig. 2 and Fig. 3).
Table 2.
Selected Indian medicinal plants used for treatment of skin diseases.
| Plant species (Family); | Common name | Part Used | Traditional uses | Previous reports on antimicrobial activities |
|---|---|---|---|---|
| Acalypha indica Linn. (Euphorbiaceae) | kuppaimeni or kucing galak | Leaves, root | Emetic, ophthalmic, vermifuge, asthma, stomach-ache bronchitis, intestinal worms | Ethanolic, water and chloroform extract of leaves (Somchit et al., 2010). |
| Lawsonia inermis Linn (Lythraceae) | Marudani or Henna | Leaves, bark, root, flower, seeds | skin diseases, leprosy, wounds, ulcers, herpes hair coloring, cosmetics, liver problems, nervous symptoms, toothache. | Ethanol and petroleum ether extract of leaves (Suleiman and Mohamed, 2014) |
| Citrus limon Linn (Rutaceae). | Elumicchai, or Lemon | Leaves, bud, Fruit (Juice, Pericap, powder, peel), seed, stem | Loss of weigh, diabetic, inflammation, allergic, menstrual disorders, skin disease, cough, abdominal pain, | Acetone and ethanol extract of fruit peel (Otang and Afolayan, 2016) |
| Allium sativum (Alliaceae) | Poondu or garlic | Garlic cloves | Hemorrhoids, rheumatism, dermatitis, abdominal pain, cough, loss of appetite, loss of weigh, liver disorders, bronchitis, colic, flatulence, dysentery, intestinal worms | Ethanol extract of garlic gloves (Karuppiah and Rajaram, 2012) |
Fig. 2.
Antifungal activity of acetone extract of Medicinal plants against dermal fungal isolates.
Fig. 3.
Antifungal activity of acetone extract of Acalypha indica and Lawsonia inermis against dermal fungal isolates.
3.3. Antifungal effects of Lawsonia inermis
Acetone leaf extract of Lawsonia inermis showed negligible inhibitory activity against Geotrichum spp. and Trichophyton spp. However, the absence of inhibitory activity observed against Alternaria spp. and Fusarium spp. (Fig. 2 and Fig. 3). Chloroform leaf extract of Lawsonia inermis did not show antifungal activity.
3.4. Antifungal effects of Citrus limon
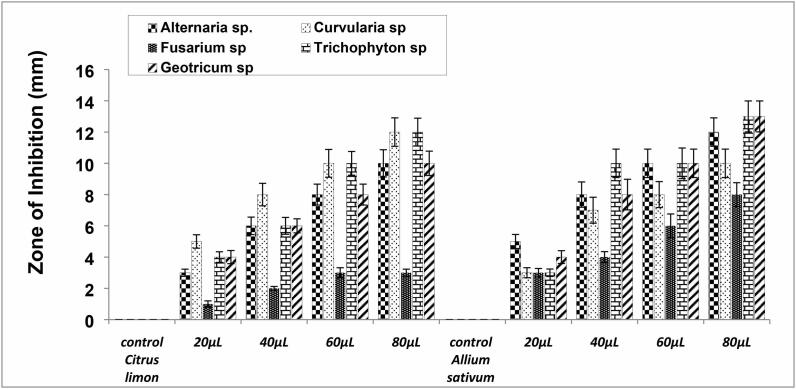
Citrus limon juice effectively inhibited the growth of Alternaria spp., Geotrichum spp. and Trichophyton sp. and mild inhibitory effects on Curvularia spp. whereas, no effects against Fusarium sp. This result revealed that Citrus limon juice showed the highest antifungal activity against Geotrichum spp. and Alternaria spp. as shown in Fig. 2 and Fig. 4.
Fig. 4.
Antifungal activity of acetone extract of citrus limon and Allium sativum against dermal fungal isolates.
3.5. Antifungal effects of Allium sativum
Acetone extract of Allium sativum gloves showed excellent inhibition against all opportunistic cutaneous fungi isolates in dose-dependent manner (Fig. 2 and Fig. 4). Allium sativum extract showed strong antifungal activity against Alternaria spp., followed by Trichophyton sp. Geotrichum spp., Curvularia spp., and Fusarium spp. Upon the use of traditional plant extract in this study, Allium sativum extract alone has a strong and effective antifungal activity against Fusarium spp. shown in Fig. 2 and Fig. 4.
3.6. Minimum fungal concentration (MFC) of plants extract
The in vitro antifungal effects of traditional plant extracts were evaluated against fungal isolates using the microdilution method to investigate its MFC values. The MFC values of the medicinal plant extracts against test fungi are listed in Table 3. Most of the plant extracts shown a strong and effective antifungal spectrum against clinical isolates. All tested plant extracts were more strong effects against Curvularia spp., Trichophyton spp., and Geotrichum spp. with less MFC concentration of the extract (50–150 μg/mL). The extracts of Allium sativum showed the MFC values in the ranges of 50–100 μg/mL, whereas, all the other extract showed the MFC values in the ranges 50–800 μg/mL. Most of the plant extract revealed the highest concentration of MFC values for Fusarium spp. On the other hand, fluconazole used as positive controls showed strong antifungal effects.
Table 3.
Minimum Fungicidal Concentration (MFC) Indian medicinal plants extracts against fungal isolates.
| Minimum Fungicidal Concentration (MFC) µg/mL |
|||||
|---|---|---|---|---|---|
| Plant species | Alternaria spp. | Curvularia spp. | Fusarium spp. | Trichophyton spp. | Geotrichum spp. |
| Acalypha indica | 600 | 50 | 800 | 100 | 100 |
| Lawsonia inermis | 400 | 50 | 800 | 50 | 100 |
| Citrus limon | 50 | 150 | 800 | 50 | 50 |
| Allium sativum | 50 | 50 | 100 | 50 | 50 |
| Fluconazole | 12.5 | 12.5 | 25 | 12.5 | 12.5 |
4. Discussion
The practice of using plant-derived bioactive products is the secondary metabolites are regularly followed in various tribal communities all over the world for more than many centuries. Similarly, our Indian traditional medicine or Ayurveda, also known to practice the use of many herbs as medicine (Supreetha et al., 2011). Therefore, the burgeoning search for identifying the traditional plants for its novel fungicidal property is now extensively accomplished and as a consequence, antifungal treatment is succeeding the better cases in health care and management of disease (Motsei et al., 2003). The global rise in the occurrence of fungal infection has been documented in the last few decades. Commonly using antifungal agents has varied toxicity, efficacy, and cost differences, and its repeated usage leading to the emergence of resistant strains that cannot be treated with the normal antifungal drugs. Here, the challenge remains to be establishing effective antifungal strategies for treating the common fungal disease such as candidiasis and other frequent pathogens. (Abad et al., 2007). The recent emergence of resistant in identified fungal pathogens and the occurrence of fungal pathogens which are inherently unaffected by the current available antibiotics, are the prevalent reasons for developing the need for identifying the novel antifungal agents (Ficker et al., 2003). Here in our current study revealed the antifungal activity of traditional medicinal plant extracts that had a negligible inhibitory effect on fungal isolates, however, it is not as good as effect of Allium sativum. Acetone extract of Allium sativum gloves had significant fungal inhibitory actions of all selected dermal fungal pathogens.
Traditionally, the decoction of leaves and spices of these medicinal plants has been used to treat indigestion, menstrual irregularities, dysentery, intestinal worms, wounds and ulcers as mentioned in Table 2. These medicinal plants extract were reported in previous studies against bacterial and candidiasis. However, detailed evidence on the antifungal properties is lacking. These plant extracts have been used for the treatment of diarrhea food poisoning and diuretic and indigestion (Somchit et al., 2010, Suleiman and Mohamed, 2014, Otang and Afolayan, 2016, Karuppiah and Rajaram, 2012). These plants have been used traditionally to treat ailments, skin problem and aid digestion in India (Chaudhari et al., 2016, Petrovska and Cekovska, 2010, Chekuri et al., 2020, Buddhadev and Buddhadev, 2016).
Medicinal plants produce various types of secondary metabolites showed strong antifungal effects against several dermal fungal pathogens. Allicin, the most potent bioactive compound reported from Allium sativum (Martins et al., 2015) possesses a wide variety of activities includes; antioxidant, fungicidal, bactericidal, virucidal, and antiparasitic effects. Allium sativum cloves extract to have noble and diverse standards of antifungal activity against Aspergillus niger, Candida albicans and Trichophyton rubrum (Ikegbunam et al., 2016). Sudanese origin garlic bulb has reported to exhibit a substantial level of fungicidal property towards Candida albicans and Aspergilllus niger, comparable with the commercial antifungal drug (Abdallah, 2017). Nikpay and Soltani (2018) reported that fungicidal activity due to the presence of flavonoids, alkaloids, tannins, citronellol, geraniol, thymoquinone, and phenolic compounds. The antifungal effects might be attributed due to the presence of tannins, alkaloids, flavonoids, steroids, glycosides, saponins and phenolic compounds in the Acalypha indica leaves extract (Mohan et al., 2012). Furthermore, the research revealed that the leaf extract of Acalypha indica consists of phenolic compounds such as anthraquinones, catechols, triterpenoids, and kaempferol derivatives (Chitravadivu et al., 2009) actively inhibits the fungal isolates. Lawsonia inermis extract consists of various bioactive phytochemicals including aromatic compounds, flavonoids (quercetin, apigenin, apigenin-7-glucoside, apigenin-4-glycoside, luteoline, luteolin-7-glucoside, luteolin-3-glucoside), naphthoquinone (lawsoniaside and lawsone), saponins, triterpenoids, dioxin derivatives, polyphenolic components (lalioside, lawsoniaside B, syringinoside, daphneside, daphnorin, isoscutellarin, gallic acid), terpenes and terpenoids, phytosterols and aliphatic compounds (lawsaritol, stigmasterol and β-sitosterol), xanthones (laxanthone I-III), benzopyrone (Singh et al., 2015). The antifungal activities of Lawsonia inermis extract against the selected dermal fungal pathogens are due to presence of the bioactive phytochemicals. In the current study, Citrus limon crude extracts have exhibited comparable antifungal effects to the presence of phenolic compounds (Marzouk, 2013). Citrus limon crude extracts consists of 68% d-limonene, a dominant antioxidant that is favourable to the skin due to its purifying and cleansing properties (Bickers and Athar, 2006). Therefore, the discoveries of these potential herbal antifungal agents are encouraging in replacing the current commericial antifungal drugs that induce many types of toxicities in patients. This study showed that, fluconazole commercial antifungal was found to exhibit 12.5–25 μg/ml whereas the extracts of plant species shows 50–600 μg/ml due to less concentration of pure bioactive components. Furthermore, future studies to identifying bioactive molecules and its molecular mechanisms responsible for promising therapeutic applications in the rescue of antifungal efficacy.
Therefore, the current study, we have demonstrated the acetone extract of Allium sativum has excellent fungicidal activity compared to other traditionally used plant extract. The prevalence of antimicrobial resistance is an ongoing issue attributable to the emergence of a strong antibiotic defence mechanism. The antifungal activity of these extract is likely to be associated with the bioactive compounds. Therefore, it is important to exploit and produce novel inhibitors against resistant microbial pathogens.
5. Conclusions
Currently, all the medicinal plants investigated in this analysis are traditionally used to combat different fungal diseases (Table. 2), and have demonstrated strong antifungal effects against the fungal isolates including Alternaria spp., Curvularia spp., Fusarium spp., Trichophyton spp. and Geotrichum spp. The antifungal effects of acetone extract of Allium sativum gloves were highly significant with the MIC values of 50 μg/mL. Furthermore, this is the first report that revealed the antifungal effects of acetone extract of medicinal plants investigated in this study (Fig. 5). Further phytochemical research is important to provide relevant information for the development of potential bioactive compounds that benefit the development of new therapeutic needs for fungal infections. Finally, the findings of this analysis clearly demonstrate the antifungal effects of medicinal plant extract produce evidence to endorse in traditional medicine.
Fig. 5.
Summary revealed the antifungal activity of acetone extract of medicinal plants.
Acknowledgments
Acknowledgments
The author is very thankful to the Deanship of Scientific Research, Prince Sattam bin Abdulaziz University for supporting this work. The author expresses hearty thanks to research team for their timely assistant and personally grateful to Mr. Muthamil Selvan for his understanding, full-hearted support and encouragement. Further, this research holds no conflict of interest.
Authors’ contributions
AS, ERB, TS, MK, SD concept and design of the study, data acquisition, and supervision of the study. AS, ERB, TS, MK, literature search, AS, ERB, SD, NAD, AHM manuscript preparation, critical and AS, SD, MK, final revision of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Financial disclosures
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Sasi Abirami, Email: abisasi@gmail.com.
Marikani Kannan, Email: kannan.m@vhnsnc.edu.in.
Dhanasekaran Sugapriya, Email: sughaphd@yahoo.com, s.narayanaswamy@psau.edu.sa.
Noura Al-Dayan, Email: n.aldayan@psau.edu.sa.
References
- Abad M., Ansuategui M., Bermejo P. Active antifungal substances from natural sources. Arkivoc. 2007;7:116–145. [Google Scholar]
- Abdallah E.M. Potential Antifungal Activity of Fresh Garlic Cloves (Allium sativum L.) from Sudan. J. Biotechnol. Res. 2017;3:106–109. [Google Scholar]
- Badiee P., Hashemizadeh Z. Opportunistic invasive fungal infections: diagnosis & clinical management. Indian J. Med. Res. 2014;139:195–204. [PMC free article] [PubMed] [Google Scholar]
- Bickers D.R., Athar M. Oxidative stress in the pathogenesis of skin disease. J. Invest. Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- Buddhadev S.G., Buddhadev S.S. Ayurvedic medicininal plant Lawsonia inermis Linn.: a complete review. Pharma Sci. Monit. 2016;7(2) [Google Scholar]
- Chaudhari S.Y., Ruknuddin G., Prajapati P. Ethno medicinal values of Citrus genus: a review. Med. J. DY. Patil. Vidyapeeth. 2016;9:560. doi: 10.4103/0975-2870.192146. [DOI] [Google Scholar]
- Chekuri S., Lingfa L., Panjala S., Bindu K.S., Anupalli R.R. Acalypha indica L.-an important medicinal plant: a brief review of its pharmacological properties and restorative potential. Eur. J. Med. Plant. 2020;1–10 doi: 10.9734/ejmp/2020/v31i1130294. [DOI] [Google Scholar]
- Chitravadivu C., Manian S., Kalaichelvi K. Qualitative analysis of selected medicinal plants, Tamilnadu, India, Middle East. J. Sci. Res. 2009;4:144–146. http://www.idosi.org/mejsr/mejsr4(3)/4.pdf [Google Scholar]
- Deutsch P.G., Whittaker J., Prasad S. Invasive and non-invasive fungal rhinosinusitis—a review and update of the evidence. Medicina. (Kaunas). 2019;55:319. doi: 10.3390/medicina55070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran S. Phytochemical characteristics of aerial part of Cissus quadrangularis (L) and its in-vitro inhibitory activity against leukemic cells and antioxidant properties. Saudi. J. Biol. Sci. 2020;27:1302–1309. doi: 10.1016/j.sjbs.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficker C.E., Smith M.L., Susiarti S., Leaman D.J., Irawati C., Arnason J.T. Inhibition of human pathogenic fungi by members of Zingiberaceae used by the Kenyah (Indonesian Borneo) J. Ethnopharmacol. 2003;85:289–293. doi: 10.1016/S0378-8741(03)00009-6. [DOI] [PubMed] [Google Scholar]
- Ganesan A., Shaikh F., Bradley W., Blyth D.M., Bennett D., Petfield J.L., Carson M.L., Wells J.M., Tribble D.R., Infectious Disease Clinical Research Program Trauma Infectious Disease Outcomes Study Group Classification of trauma-associated invasive fungal infections to support wound treatment decisions. Emerg. Infect. Dis. 2019;25:1639–1647. doi: 10.3201/eid2509.190168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerald C.J., Marmion Barrie P., Robert I., Fraser Andrew G., Anthony S. Churchill Livingstone; London: 1996. Mackie & McCartney Practical Medical Microbiology; pp. 151–178. [Google Scholar]
- Hurabielle C., Link V.M., Bouladoux N., Han S.J., Merrill E.D., Lightfoot Y.L., Seto N., Bleck C.K., Smelkinson M., Harrison O.J., Linehan J.L. Immunity to commensal skin fungi promotes psoriasiform skin inflammation. PNAS U.S.A. 2020;117:16465–16474. doi: 10.1073/pnas.2003022117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegbunam M., Ukamaka M., Emmanuel O. Evaluation of the antifungal activity of aqueous and alcoholic extracts of six spices. Am. J. Plant Sci. 2016;7:118–125. doi: 10.4236/ajps.2016.71013. [DOI] [Google Scholar]
- Indu M.N., Hatha A.A.M., Abirosh C., Harsha U., Vivekanandan G. Antimicrobial activity of some of the south-Indian spices against serotypes of Escherichia coli, Salmonella, Listeria monocytogenes and Aeromonashydrophila. Braz. J. Microbiol. 2006;37:153–158. doi: 10.1590/S1517-83822006000200011. [DOI] [Google Scholar]
- Janbon G., Quintin J., Lanternier F., d’Enfert C. Studying fungal pathogens of humans and fungal infections: fungal diversity and diversity of approaches. Genes Immun. 2019;20:403–414. doi: 10.1038/s41435-019-0071-2. [DOI] [PubMed] [Google Scholar]
- Karuppiah P., Rajaram S. Antibacterial effect of Allium sativum cloves and Zingiber officinale rhizomes against multiple-drug resistant clinical pathogens. Asian Pac. J. Trop. Biomed. 2012;2:597–601. doi: 10.1016/S2221-1691(12)60104-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler J.R., Casadevall A., Perfect J. The spectrum of fungi that infects humans. Cold. Spring. Harb. Perspect. Med. 2015;5:a019273. doi: 10.1101/cshperspect.a019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins N., Barros L., Henriques M., Silva S., Ferreira I.C. Activity of phenolic compounds from plant origin against Candida species. Ind. Crops Prod. 2015;74:648–670. doi: 10.1016/j.indcrop.2015.05.067. [DOI] [Google Scholar]
- Marzouk B. Characterization of bioactive compounds in Tunisian bitter orange (Citrus aurantium L.) peel and juice and determination of their antioxidant activities. BioMed. Res. Inter. 2013;2014 doi: 10.1155/2013/345415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan S.C., Dinakar S., Anand T., Elayaraja R., SathiyaPriya B. Phytochemical, GC-MS analysis and antibacterial activity of a medicinal plant Acalypha indica. Int. J. Pharm. Technol. Res. 2012;4(3):1050–1054. [Google Scholar]
- Motsei M.L., Lindsey K.V., Van Staden J., Jäger A.K. Screening of traditionally used South African plants for antifungal activity against Candida albicans. J. Ethnopharmacol. 2003;86:235–241. doi: 10.1016/S0378-8741(03)00082-5. [DOI] [PubMed] [Google Scholar]
- Nikpay A., Soltani M. In vitro anti-parasitic activities of Pulicariadysenterica and Lycopuseuropaeusmethanolic extracts against Trichomonasgallinae. J. Herbmed. Pharmacol. 2018;7:112–118. doi: 10.15171/jhp.2018.19. [DOI] [Google Scholar]
- Ocheng F., Bwanga F., Joloba M., Borg-Karlson A.K., Gustafsson A., Obua C. Antibacterial activity of extracts from Ugandan medicinal plants used for oral care. J. Ethnopharmacol. 2014;155:852–855. doi: 10.1016/j.jep.2014.06.027. [DOI] [PubMed] [Google Scholar]
- Otang W.M., Afolayan A.J. Antimicrobial and antioxidant efficacy of Citrus limon L. peel extracts used for skin diseases by Xhosa tribe of Amathole District, Eastern Cape. S. Afr. J. Bot. 2016;102:46–49. doi: 10.1016/j.sajb.2015.08.005. [DOI] [Google Scholar]
- Petrovska B.B., Cekovska S. Extracts from the history and medical properties of garlic. Pharmacogn. Rev. 2010;4:106. doi: 10.4103/0973-7847.65321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce C.G., Srinivasan A., Uppuluri P., Ramasubramanian A.K., López-Ribot J.L. Antifungal therapy with an emphasis on biofilms. Curr. Opin. Pharmacol. 2013;13:726–730. doi: 10.1016/j.coph.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S., Baburaj D.S., Sethuraman M., Parimala S. Stem and stem bark used medicinally by the Tribals Irulas and Paniyas of Nilgiri District, Tamilnadu. J. Nat. Remed. Ethnobot. 2001;6:19–24. doi: 10.18311/jnr/2001/62. [DOI] [Google Scholar]
- Salim A.A., Chin Y.W., Kinghorn A.D. Drug discovery from plants. In: Ramawat K.G., Merillon J.M., editors. Bioactive Molecules and Medicinal Plants. Springer; Berlin Heidelberg: 2008. pp. 1–24. [Google Scholar]
- Seyedmousavi S., Bosco S.D.M., De Hoog S., Ebel F., Elad D., Gomes R.R., Jacobsen I.D., Jensen H.E., Martel A., Mignon B., Pasmans F. Fungal infections in animals: a patchwork of different situations. Med. Mycol. 2018;56(suppl_1):S165–S187. doi: 10.1093/mmy/myx104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaik D., Malika F.A., Rafi S., Naqui B. Studies of antibacterial activity of ethanolic extract from Nericumindicum and Hibiscus rosasinensis. J. Islamic. Acad. Sci. 1994;7(167–16):8. [Google Scholar]
- Singh D.K., Luqman S., Mathur A.K. Lawsonia inermis L.-A commercially important primaeval dying and medicinal plant with diverse pharmacological activity: a review. Ind. Crops Prod. 2015;65:269–286. doi: 10.1016/j.indcrop.2014.11.025. [DOI] [Google Scholar]
- Singh S., Beena P.M. Profile of dermatophyte infections in Baroda. Indian J. Dermatol. Venereol. Leprol. 2003;69:281–283. http://www.ijdvl.com/text.asp?2003/69/4/281/4994 [PubMed] [Google Scholar]
- Somchit M.N., Rashid R.A., Abdullah A., Zuraini A., Zakaria Z.A., Sulaiman M.R., Arifah A.K., Mutalib A.R. In vitro antimicrobial activity of leaves of Acalypha indica Linn (Euphorbiaceae) Afr. J. Microbiol. Res. 2010;4:2133–2136. doi: 10.5897/AJMR.9000388. [DOI] [Google Scholar]
- Suleiman E.A., Mohamed E.A. In vitro activity of Lawsonia inermis (Henna) on some pathogenic fungi. J. Mycol. 2014;201:4. doi: 10.1155/2014/375932. Article ID 375932. [DOI] [Google Scholar]
- Supreetha S., Mannur S., Simon S.P., Jain J., Tikare S., Mahuli A. Antifungal activity of ginger extract on Candida albicans: an in-vitro study. J. Dental Sci. Res. 2011;2:18–21. https://www.ssdctumkur.org/jdsr4_05.pdf [Google Scholar]
- Wu X., Dhanasekaran S. Protective effect of leaf extract of Abutilon indicum on DNA damage and peripheral blood lymphocytes in combating the oxidative stress. Saudi. Pharm. J. 2020;28:943–950. doi: 10.1016/j.jsps.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]