Abstract
The intestinal epithelial layer serves as a physical and functional barrier between the microbiota in the lumen and immunologically active submucosa. Th17 T-cell function protects the gut epithelium from aggression from microbes and their by-products. Loss of barrier function has been associated with enhanced translocation of microbial products which act as endotoxins, leading to local and systemic immune activation. Whereas the inflammatory role of LPS produced by Gram-negative bacteria has been extensively studied, the role of fungal products such as β-D-glucan remains only partially understood. As HIV infection is characterized by impaired gut Th17 function and increased gut permeability, we critically review mechanisms of immune activation related to fungal translocation in this viral infection. Additionally, we discuss markers of fungal translocation for diagnosis and monitoring of experimental treatment responses. Targeting gut barrier dysfunction and reducing fungal translocation are emerging strategies for the prevention and treatment of HIV-associated inflammation and may prove useful in other inflammatory chronic diseases.
Keywords: fungi, inflammation, HIV, beta-D-glucan [BDG], immune activation
Introduction
Gut damage and increased gut permeability constitute hallmarks of both acute and chronic phases of HIV infection (1, 2). CD4+ T cells loss in the gut mucosa, including interleukin (IL)-17-producing T-helper cells (Th17), disturbs mucosal homeostasis and contributes to epithelial gut damage (3). HIV-associated loss of epithelial integrity induces the non-physiological passage of microbial by-products from the gut lumen into the systemic circulation, referred to as microbial translocation. Brenchley et al. first reported in 2006 that increased plasma levels of the Gram-negative bacterial cell wall antigen lipopolysaccharide (LPS) triggers systemic immune activation in both people living with HIV (PLWH) and SIV-infected rhesus macaques (2), and eventually contributes to disease progression in PLWH (4–8). Moreover, in macaque models, gut epithelium damage precedes immune activation (9). Although antiretroviral therapy (ART) successfully controls HIV replication and prevents AIDS, the gut epithelium is not fully repaired in long-term ART-treated PLWH (4, 10, 11). As such, microbial translocation persists along with systemic immune activation in ART-treated PLWH (4, 12–15). This chronic inflammation in ART-treated PLWH likewise increases the risks of non-AIDS comorbidities such as cardiovascular and metabolic diseases, neurocognitive dysfunction and cancer (16). Therefore, understanding the link between epithelial gut damage and systemic immune activation in PLWH is crucial in both ART-naïve and ART-treated PLWH.
On the luminal side of the gut epithelium lives a complex microbiota. Different in almost every individual, the gut microbiota composition is well-controlled by both the microbiota itself and the host. Composed of bacteria, fungi, archaea, protozoa and viruses, the microbiota plays key physiological and immune roles through the metabolism of different nutrients, regulation of the immune system and control of pathogen invasion. Yet, microbiota composition studies predominantly focus on bacteria. As such, microbial translocation of bacterial products such as LPS is primarily studied alongside the subsequent immune response, quantified by host factor soluble CD14 (sCD14) produced by macrophages/monocytes in response to LPS stimulation, and LPS-binding protein (LBP) mostly produced by the liver in the presence of LPS.
Fungal mass constitutes the second player after bacterial mass in the composition of gut microbiota. Fungi are thus found in the gut of all healthy individuals and PLWH (17, 18), with Saccharomyces cerevisiae, Malassezia restricta and Candida albicans being the most often found in stools. As such, one can hypothesize that fungal product would also translocate into the circulation in the presence of a leaky gut. (1→3)-β- D-Glucan BDG is a major cell wall component of most fungi and is used as a clinical biomarker for diagnosing and managing invasive fungal infection (IFI). Although other cell wall molecules such as mannans and galactomannans are also common across fungi species colonizing humans, BDG is the only marker associated with fungal translocation in PLWH. Morris et al. first showed elevated plasma levels of BDG in PLWH in 2012 (19). Since then, several other groups including ours reported an association between BDG and epithelial gut damage, immune activation, inflammation, and risk of developing non-AIDS comorbidities (4, 15, 20–24). These findings suggest a significant role for BDG in chronic immune activation and the development of non-AIDS comorbidities in PLWH, although the mechanisms involved remain poorly understood.
The development of non-AIDS comorbidities despite long-term ART represents the main concern in care for PLWH (16, 25–27). As fungal translocation appears to play a key role in immune activation, understanding mechanisms behind this phenomenon could help in designing novel therapies aiming at improving the quality of life of ART-treated individuals. Herein, we delve into the literature regarding the contribution and mechanism by which fungal translocation induces systemic immune activation and non-AIDS comorbidities in PLWH.
Evidence of Gut Leakage of Fungal Products in Animal Models
Fungi are peaceful colonizers of the skin but also lungs and genital tract of most mammals including humans. They’re also naturally present in the gut microbiota in absence of invasive fungal infection (IFI) (28). However, fungal products that are found in the blood usually result either from IFI or from translocation of fungal products predominantly from the gut (17, 18, 29–31).
The gastrointestinal tract (GI) encompasses multifaceted physical and immunological barriers preventing translocation of microbes and their by-products, while allowing for the absorption of nutrients. The gut mucosa is protected by both physical and immune components: the mucus and epithelial tight junctions on the apical pole of intestinal cells form a physical barrier; patrolling leukocytes in the lamina propria constitute an immune barrier ensuring that any translocated pathogens are phagocytosed, cleared, and/or transferred to mesenteric lymph nodes.
Fungi in the gut microbiota are abundant in mammals and play key roles in the balance between bacteria and other communities, as well as immune development in mice (32, 33). Animal models of gut damage that are frequently used include oral treatment with Dextran sulfate sodium (DSS), which impairs the gut epithelium and creates an experimental colitis (34). Upon DSS treatment, fungal products were found in the systemic circulation in different mice models (35, 36). Moreover, translocated fungal products, including BDG, were shown to participate in inflammation (37).
These mouse models suggest that upon gut damage, microbial translocation of fungal products occurs and participates in inflammation induction. As fungi are also present in the gut microbiota of non-human primates, studies could be performed to confirm the origin of translocated fungal products in different pathologies (31, 38).
Evidence of Gut Leakage of BDG in People Living With HIV
Increased gut permeability is a hallmark of HIV infection and has been shown to increase microbial translocation and inflammation (2). Markers of gut damage, Zonulin and intestinal fatty acid binding protein (I-FABP), as well as the marker of gut permeability regenerating islet-derived protein 3-α (REG3α) were found at higher levels in PLWH (10, 39). Beside translocation of bacterial products, higher circulating levels of fungal products were also found in PLWH, suggesting microbial translocation of fungal products (reviewed in Table 1 ).
Table 1.
Main studies assessing the influence of fungal translocation in people living with HIV.
| Country | Sample size | Population | Study design | Main findings | Reference | |
|---|---|---|---|---|---|---|
| 2012 | USA | 132 | CHI, mostly ART+ | Cross sectional | Higher BDG values associated with inflammation, CD8 T-cell activation, and pulmonary abnormalities. | (19) |
| 2015 | USA | 41 | CHI ART+ | Cross sectional | Blood BDG levels correlated with neopterin levels and tended to correlate with TNF-α levels. | (15) |
| 2016 | USA | 11 | Early infection, before and after ART | Cross sectional | Blood BDG and sCD14 levels were associated with lower colonization of Lactobacilli in stools. | (23) |
| 2016 | USA | 21 | CHI ART+ | Cross sectional | Higher blood BDG levels were associated with neurocognitive dysfunction. | (22) |
| 2018 | USA | 451 | Before and after ART | Cross sectional | suPAR and BDG plasma levels after ART initiation were associated with increased risk of non-AIDS comorbidities. | (40) |
| 2019 | Canada | 146 | Early and chronic, ART naïve or ART+ | Longitudinal Cross sectional | Plasma BDG levels were higher in chronically infected people than early infection, and were associated with inflammation and immune activation. | (4) |
| 2019 | USA | 231 | ART naïve before and after ART, comparison of TDF/FTC, ATV +DRV, or RAL | Longitudinal | BDG increased after ART initiation, in association with increase in body fat. | (20) |
| 2019 | USA | 61 | CHI ART+ | Cross sectional | BDG levels in plasma were associated with neurocognitive function. | (21) |
| 2019 | USA | 176 | CHI ART+ and uninfected controls | Cross sectional | Lower levels of BDG in HIV+ participants compared to uninfected controls. BDG levels correlated with levels of inflammation markers in HIV+ participants. No difference in levels of anti-fungal antibodies were found. | (24) |
| 2020 | USA | 14 | CHI ART+, compared to people with liver cirrhosis and healthy controls | Longitudinal and cross sectional | Oral challenge with BDG rich food did not increase blood levels of BDG. | (41) |
| 2020 | Uganda | 171 | Children (2-10 years old) HIV+ ART+, and uninfected, HIV exposed or not | Cross sectional | Blood BDG levels were higher in HIV infected children. In children with a history of breastfeeding, BDG levels correlated with soluble TNF receptor levels. | (42) |
| 2020 | Uganda | 101 | Children (10-18 years old) HIV+ ART+, and uninfected, HIV exposed or not | Cross sectional | Blood BDG levels were higher in HIV infected children. BDG levels were associated with immune activation in monocytes and T-cells. | (43) |
| 2020 | Canada | 11 | CHI ART+ | Longitudinal | 24 hours follow-up of participant showed no significant variations of BDG levels in blood. | (44) |
| 2021 | The Netherlands | 40 | CHI ART+ and uninfected controls | Cross-sectional | A higher proportion of ART-treated PLWH had detectable BDG levels in blood, and those levels were associated with inflammatory markers. | (14) |
| 2021 | Canada | 145 | CHI ART+ and uninfected control | Cross-sectional | BDG levels were associated with subclinical coronary atherosclerosis plaque in PLWH but not uninfected controls. | (45) |
CHI, chronic HIV infection; ART, antiretroviral therapy; TDF, tenofovir disoproxil fumarate; FTC, emtricitabine; ATV, atazanavir; DRV, darunavir; RAL, raltegravir.
Morris et al. were the first to report elevated levels of fungal product BDG in the blood of PLWH, grouping together ART-treated and viremic untreated individuals (19). Clinically, higher circulating BDG levels were associated with absence of ART, higher viral load and lower CD4 T-cell count (19).
Weiner et al. found lower levels of BDG in PLWH compared to uninfected controls, although high levels of BDG were also found in the control group (24). Interestingly, this study also showed no difference in levels of anti-Saccharomyces antibodies (ASCA) between both groups (IgG and IgA).
We previously compared plasma levels of distinct gut damage and microbial translocation markers in different groups of PLWH without IFI and showed that plasma levels of BDG were higher in PLWH compared to uninfected controls, while galactomannan levels were low and similar between both groups. We also found higher levels of BDG in chronically infected PLWH compared to those in the early phase of the infection (4). Surprisingly, BDG levels were not statistically lower in ART-treated PLWH compared to their ART-naïve counterpart. Moreover, those levels correlated with markers of gut damage I-FABP and gut permeability REG3α in PLWH and uninfected controls, in accordance with the hypothesis that fungal translocation originates from gut microbiota (4, 10). Also, BDG and LPS levels correlated, and both were hypothesized to originate from the gut. Moreover, after a 2-year follow-up, PLWH not taking ART had increased levels of blood BDG levels, while those treated during the early phase of the infection had stable BDG levels (4). Early ART initiation was also associated with lower BDG levels, suggesting that early ART decreases the magnitude of gut damage and prevents further BDG translocation. All in all, these results demonstrated that fungal BDG translocation occurs in PLWH and suggest that these molecules originate from the gut.
Validating BDG as a Marker of Microbial Translocation in PLWH
Recent findings tend to validate BDG as a marker of microbial translocation. Indeed, BDG can be found in several types of food including oatmeal, mushrooms, and seaweed. One would expect that increased intake of food rich in BDG might lead to its increased absorption. Therefore, Hoenigl et al. designed a clinical trial where people were fed with high-BDG food in a controlled environment (41). This study included participants with advanced HCV-associated liver cirrhosis as positive controls, as those patients have elevated microbial translocation levels (46–49). Other included participants constituted of PLWH with detectable viral loads, ART-suppressed PLWH, and HCV negative/HIV negative controls. Although BDG testing of the BDG-rich food confirmed an elevated concentration, no significant variation of plasma BDG levels were detected in any participants up to 8 hours after food intake. This study strengthened the hypothesis that translocated BDG is originating from fungal communities in the GI tract rather than from food intake.
In addition, we also demonstrated that BDG levels were stable throughout 24 hours in ART-treated PLWH, as opposed to LPS levels (44). Interestingly, LPS levels increased after lunch and dinner, and decreased during the night, while BDG levels were stable over 24 hours. Although we were not able to exclude a circadian regulation mechanism, we hypothesized that detoxification of LPS might explain its variation. Indeed, BDG levels were stable upon ART initiation in PLWH, when gut damage marker levels decreased, suggesting that translocated BDG is not detoxified as efficiently as LPS (11, 50).
Consequences of BDG Translocation in PLWH
Inflammation
Translocated products are recognized by the immune system as pathogen-associated molecular patterns (PAMPs) and induce inflammation. As such, several studies found associations between BDG and inflammation or immune-activation markers in PLWH.
Morris et al. found that participants with higher BDG levels had increased circulating levels of inflammatory cytokines IL-8 and tumor necrosis factor α (TNF-α), as well as higher levels of activated CD8 T-cells in ART-naïve and ART-treated PLWH (19).
Interestingly, in PLWH in the primary phase of the infection starting ART, circulating BDG, but not LPS, levels were inversely associated with gut colonization of Lactobacilli, which are associated with reduced colon inflammation (23). This association was demonstrated 12 weeks after ART initiation and tended to persist 12 weeks later.
In ART-treated PLWH, Hoenigl et al. found that BDG levels, although in the normal range (below 60 pg/mL), were associated with plasma levels of Neopterin, a marker of inflammation, and tended to correlate with plasma levels of pro-inflammatory cytokines IL-6 and IL-8 (15). However, no association between BDG levels and the marker of bacterial-related inflammation sCD14 could be observed.
Higher BDG levels have been associated with markers of disease progression: in ART-naïve PLWH, we found an association between viral load and BDG, but not LPS levels (4). Furthermore, in both ART-naïve and ART-treated PLWH, BDG levels were associated with lower CD4 count and lower CD4/CD8 ratio, indicating a link between BDG translocation and markers of disease progression.
BDG levels were also associated with pro-inflammatory cytokines IL-6, IL-8 and CXCL13 in blood, as well as the frequency of activated blood CD4 and CD8 cells (4, 51) ( Table 1 ). Moreover, Weiner et al. showed that levels of BDG, but not ASCA, in PLWH correlated with inflammation markers such as IP-10, IL-6, markers of monocyte/macrophage activation sCD14 and sCD163 and percentage of activated CD4 and CD8 T-cells (24).
In Ugandan ART-treated children, BDG levels were also elevated compared to HIV-exposed or unexposed children (42, 43). Also, BDG levels were associated with levels of the soluble TNF-receptor, another marker of inflammation (42).
Van der Heijden reported that PLWH with higher levels of BDG exhibited higher plasma levels of the inflammatory marker IL-1β, as well as higher response of monocytes to imiquimod or Mycobacterium tuberculosis stimulations (14).
Altogether, these findings indicate that fungal translocation of BDG is associated with inflammation, in both ART-naïve and ART-treated individuals, possibly participating in disease progression.
Non-AIDS Comorbidities
Persisting inflammation, even in ART-treated PLWH, is associated with increased risk of developing non-AIDS comorbidities including cardiovascular and metabolic diseases, and neurocognitive dysfunction. As translocation of fungal products has been associated with inflammation, the link between BDG and those comorbidities was investigated in several studies.
In 2018, Hoenigl et al. performed a cross sectional analysis of 451 PLWH, followed up to 11 years after ART initiation, and looked at the frequency of non-AIDS comorbidities, including myocardial infarction or stroke, non-AIDS malignancy or serious bacterial infection, or death from a non-AIDS related event. Among other markers of inflammations, only blood levels of soluble urokinase plasminogen activator receptor (suPAR), a marker of T-cell and monocyte activation, as well as BDG, were associated with non-AIDS comorbidity occurrence (40). Interestingly, only post-ART and pre-comorbidity BDG levels were associated with development of those comorbidities, independently of CD4 count but not smoking status pre-event.
Morris et al. found that PLWH with higher BDG levels had higher frequency of cardiopulmonary abnormalities including reduced diffusing capacity for carbon monoxide, higher pulmonary artery systolic pressure and increased tricuspid regurgitant jet velocity (19).
We also showed an association between plasma BDG levels and subclinical coronary atherosclerosis plaque in ART-treated PLWH but not uninfected controls, independently of age sex and other typical factors. Interestingly, we found that BDG levels were more strongly associated with plaque prevalence than age, smoking habits, hypertension, statin use or obesity (45).
Moreover, a study assessing metabolic and weight changes showed that after ART-initiation, blood BDG levels increased two years after ART initiation, and were associated with larger trunk and total body fat accumulation (20).
Several studies have shown a link between BDG levels and cognitive functions in PLWH. Plasma BDG levels were associated with Global Deficit Score in ART-treated PLWH (22). Interestingly, this study showed that the 2 participants (out of 21) who had the worst deficit were also the only ones with elevated BDG levels in cerebrospinal fluid. Also, although IL-8 levels in plasma were associated with the deficit score, no correlation between BDG levels and IL-8 levels was observed in this study (22). The same team expanded such findings in 61 ART-treated PLWH and found that suPAR and BDG plasmatic levels were associated with the Global Deficit Score, independently of CD4 T-cell count (21) ( Table 1 ).
Although BDG appears as a new marker of non-AIDS comorbidities, current observations rely on associations only. More studies are thus needed to puzzle out the mechanism linking fungal translocation and comorbidities.
Detection of Fungal Products in PLWH—Insights on Mechanisms
Fungal PAMPs induce inflammation following their detection by pattern recognition receptor (PRRs) expressed on different cell types. Fungal PRRs include C-type lectin receptors such as Dectin-1, Toll-like receptor 2, integrins, scavenger receptors, and hyaluronic acid receptors (52). The receptor ephrin type-A receptor 2 (EphA2) has also been shown to mediate detection of fungal BDG in the mouth and upper GI, inducing protective innate immunity (53). EphA2 is also expressed at lower levels throughout the gut. Whether this receptor is implicated in fungal product induction of inflammation in PLWH has not been elucidated yet.
Effect on Antigen Presenting Cells and Neutrophils
Antigen presenting cells (APCs) are specialized in the detection of pathogens through conserved PAMPs, allowing the development of appropriate immune responses. APC include dendritic cells, macrophages/monocytes, and B cells, and are highly abundant in tissue, notably in the gut.
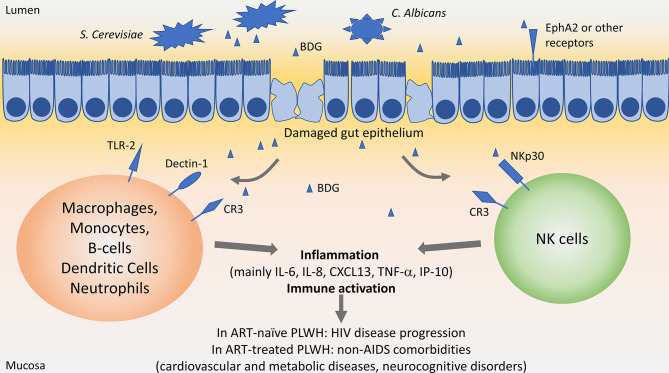
APCs can sense fungi through different receptors including Dectin-1, Toll-like receptor 2 (TLR2) and Complement receptor 3 (CR3) ( Figure 1 ).
Figure 1.
Influence of β-D-Glucan in people living with HIV. In the gut lumen, Saccharomyces Cerevisiae and Candida albicans are largely present in the microbiota. Upon HIV-associated epithelial gut damage, fungal products such as β-D-Glucan (BDG) translocate in the mucosa. BDG is recognized by immune cells through Toll-like receptor 2 (TLR-2), Dectin-1, complement receptor 3 (CR3) or NKp30, activating immune cells and inducing inflammation. Persisting inflammation has been associated with disease progression in people living with HIV (PLWH) not taking antiretroviral therapy (ART), and with increased risk of non-AIDS comorbidities in ART-treated PLWH.
Dectin-1 is the main receptor interacting with BDG on macrophages, monocytes, dendritic cells, B-cells, and neutrophils (54–57). Expressed at the cell surface, Dectin-1 recognizes circulating or membrane-bound BDG, activating the NF-κB pathway through activation of the CARD9/BCL10/MALT1 complex.
TLR2 is expressed on Dendritic cells, macrophages, and monocytes, and also activates the NF-κB pathway through activation of MyD88 upon recognition of soluble or particulate BDG.
Both stimulation of Dectin-1 and TLR2 on macrophages and monocyte induce the secretion of pro-inflammatory cytokines such as IL-6, IL-8, TNF-α, as well as anti-inflammatory mediator IL-10 (55) ( Figure 1 ). It is worth noting that stimulation of monocytes with BDG induced internalization of Dectin-1 and decreased its surface expression as soon as 30 min after stimulation (54, 55). Size of BDG molecules play a key role in the induction of inflammatory responses, with larger-sized BDG inducing higher IL-1β, IL-6 and IL-23 secretion compared to smaller-sized BDG. However, secretion of chemokines involved in recruitment and maturation of T-cells was not affected by BDG size (58).
CR3 can also trigger BDG recognition on macrophages, monocytes, and neutrophils. However, neutrophils recognize BDG through CR3 only after opsonization with complement (59).
In vitro or animal models indicated that APC and neutrophils secrete inflammatory cytokines when stimulated by BDG, however the indication of such direct effect in PLWH is still lacking. As an initial foray, we found that circulating levels of BDG, but not LPS, inversely correlated with Dectin-1 expression on monocytes in PLWH (4), suggesting a direct interaction between BDG and its receptor Dectin-1 on monocytes. To validate this mechanism, we stimulated PBMC in vitro with Saccharomyces Cerevisiae-extracted BDG and found decreased Dectin-1 expression on monocytes at 24 and 48 hours. LPS did not induce such variation [personal communication (60)]. Moreover, stimulation of monocytes and macrophages with BDG was shown to primarily induce IL-1β and IL-8, which correlated with BDG levels in plasma samples in PLWH.
Detection by NK Cells
NK cells are a key player of innate immunity responsible for eliminating infected cells, cancer cells, as well as fungi. The main fungal receptor on NK cells is NKp30, also called Natural cytotoxicity triggering receptor 3 (NCR3). Recent work has shown that NKp30 recognizes membrane bound BDG, allowing elimination of fungal cells. NKp30 is required for elimination of Cryptococcus in a mouse model (61, 62). As such, NKp30 is the PRR responsible for direct recognition of fungus and BDG by NK cells. Unexpectedly, soluble BDG also binds to NKp30, activating NK cells and allowing the secretion of cytotoxic molecules Perforins and Granzymes (61, 62). Addition of BDG to NK increased Candida-killing activity. Earlier, this group showed that NKp30 surface expression is reduced on NK cells from ART-treated PLWH (62). We later confirmed those results in both ART-naïve and ART-treated chronically infected PLWH and also found that surface NKp30 expression was negatively correlated with circulating BDG but not LPS levels (4). In vitro, stimulation with S. Cerevisiae-extracted BDG but not with LPS decreased NKp30 expression at 24 and 48h (60), confirming the direct role of BDG in reducing NKp30 expression. Reduced NKp30 expression was associated with lower cytotoxic function against fungi and cancer cells (61–63).
Altogether, these findings indicate that BDG has a direct stimulating role of NK cells, including in PLWH on ART. This could lead to inflammation and decreased efficiency in infection or cancer suppression, leading to increased non-AIDS comorbidities.
BDG and Trained Immunity in HIV
Recent findings have put fungal products under the spotlight as they robustly induce trained immunity. This type of innate immune memory has been shown to be induced by β-glucans (including BDG) and BCG vaccines (64, 65). Trained immunity is defined as the process by which a stimulation programs a cell to respond with greater efficiency to a second stimulation after returning to steady state following the first stimulation. Trained immunity is functionally different from priming and differentiation and opposed to tolerance (64). In animal and human models, BDG has been shown to activate immune cells, especially monocytes, and induce epigenetic changes allowing those cells to respond with greater intensity to a second stimulation. Trained immunity is not antigen restricted as it potentiates the response to subsequent stimuli differently from the first antigen encounter and has been shown to act throughout the body via modulation of hematopoiesis and cell trafficking.
Whether translocated BDG plays a role in inducing trained immunity in PLWH is still unknown. In 2020, Van Der Heijden identified a link between circulating BDG levels and a trained immunity phenotype in ART-treated PLWH (14). Whether this phenotype is induced by trained immunity or priming of monocytes will have to be elucidated in further studies.
However, several indications lead to the hypothesis that this trained immunity is unlikely in PLWH. The first clue concerns the dynamics: most models of trained immunity require the removal of the initial stimulus to potentiate a second response, while BDG persists chronically even at low levels in PLWH. The second clue relies on the complexity of microbial translocation in PLWH: BDG translocation is accompanied by other microbial products such as LPS, inducing various inflammatory signals, while trained immunity has been shown to mostly rely on single instances of antigenic stimulation with β-glucans or BCG. The last hint is clinically relevant: glucan-induced trained immunity has been shown to increase protective responses to diverse infections and cancer, while PLWH have increased risks of both infection and cancer. However, trained immunity could also participate in sustained chronic inflammation such as in atherosclerosis, notably through the recognition of oxidized low-density lipoprotein particles (66). Indeed, BDG levels have been linked with cardiovascular disease in PLWH (19, 40, 45).
Hence, and due to the difficulty in deciphering priming from trained immunity, the influence of microbial translocation of BDG on trained immunity in PLWH should be assessed in future studies.
Targeting Fungal Translocation in PLWH
We and others have shown that starting ART as early as possible appears to stabilize BDG levels, in accordance with current guidelines recommending ART initiation as soon as the diagnostic is confirmed (4, 20). Therefore, as fungal translocation has been associated with inflammation and non-AIDS comorbidities in ART-treated PLWH, strategies targeting fungal translocation are needed.
Treatment with the antifungal agent fluconazole in ART-treated PLWH with neurocognitive disorders barely changed levels of markers of inflammation IL-1α, IL-6, IL-8 and IP-10 (67). However, levels of fungal products translocation have not been assessed in this study, rendering it difficult to draw conclusions on the effect of anti-fungal treatment on fungal microbial translocation.
Specific strategies have not been developed to prevent microbial translocation of fungal products in ART-treated PLWH. Fecal microbiota transplantation (FMT) could influence the mass of the mycobiome and allow improvement of gut epithelium integrity, reducing fungal translocation (68). Although several pilot trials of FMT have been initiated in PLWH, few have studied fungal translocation before and after treatment. In 2020, a study by Utay et al. consisting in six weekly FMT rounds in six ART-treated PLWH reported neither significant variations of circulating BDG levels, nor changes in inflammation and gut permeability markers I-FABP (69).
However, BDG levels have been used as markers of translocation in several other clinical trials:
In one study, metformin was expected to decrease inflammation in ART-treated PLWH (70, 71). Pilot results showed that 3 months of metformin treatment in addition to ART slightly decreased the marker of inflammation sCD14, but did not decrease LPS nor BDG translocation (72).
In a randomized placebo-controlled double-blind study, dipyridamole treatment was shown to increase extracellular adenosine levels and decrease CD8 T-cell activation in ART-treated PLWH (73). However, this treatment did not modify BDG levels in either group (74).
Conclusion
Translocation of fungal products, mainly inferred from BDG levels in the blood, has been shown to be associated with inflammation and comorbidities in PLWH. Whether BDG contributes directly to inflammation remains unknown, although assessment of BDG-receptors in PLWH pledges in this favor. Further studies are required to examine the role of fungal translocation in PLWH, especially those receiving ART. Overall, BDG appears as a robust biomarker of microbial translocation linked with inflammation and non-AIDS comorbidities in PLWH. Targeted strategies are critically needed to reduce the contribution of fungal translation to inflammation in PLWH, and eventually improve the quality of life of this population.
Author Contributions
SI wrote the first draft, constructed the figure and table, and made revisions to the final draft of the manuscript. JL, SB, BF, and LR participated in the discussion and critically read and edited the manuscript. J-PR designed the review and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Canadian Institutes of Health Research (CIHR; grants MOP 103230 and PTJ 166049), the Vaccines & Immunotherapy Core of the CIHR Canadian HIV Trials Network (CTN, grant CTN 257, CTN PT032, and CTNPT038), the CIHR-funded Canadian HIV Cure Enterprise (CanCURE) Team Grant HB2-164064 and réseau Fonds de la recherche-Santé (FRQ-S) SIDA Maladies infectieuses and thérapies cellulaires. SI is a post-doctoral fellow supported by the FRQ-S and CIHR-CTN. BF is supported by a William Turner award from the McGill University Health Centre. LR is a post-doctoral fellow supported by the “Fonds de perfectionnement” of the Geneva University Hospitals, Switzerland. J-PR is the holder of the Louis Lowenstein Chair in Hematology and Oncology, McGill University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are highly grateful to Angie Massicotte, Josée Girouard, and Cezar Iovi for coordination and assistance. The authors would like to thank study participants for their time and contribution.
References
- 1. Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annu Rev Immunol (2012) 30:149–73. 10.1146/annurev-immunol-020711-075001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med (2006) 12:1365–71. 10.1038/nm1511 [DOI] [PubMed] [Google Scholar]
- 3. Ghosn J, Taiwo B, Seedat S, Autran B, Katlama C. Hiv. Lancet (2018) 392:685–97. 10.1016/s0140-6736(18)31311-4 [DOI] [PubMed] [Google Scholar]
- 4. Mehraj V, Ramendra R, Isnard S, Dupuy FP, Ponte R, Chen J, et al. Circulating (1–>3)-beta-D-Glucan is associated with immune activation during HIV infection. Clin Infect Dis (2019) 70:232–41. 10.1093/cid/ciz212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tudesq JJ, Dunyach-Remy C, Combescure C, Doncesco R, Laureillard D, Lavigne JP, et al. Microbial translocation is correlated with HIV evolution in HIV-HCV co-infected patients. PloS One (2017) 12:e0183372. 10.1371/journal.pone.0183372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Epeldegui M, Magpantay L, Guo Y, Halec G, Cumberland WG, Yen PK, et al. A prospective study of serum microbial translocation biomarkers and risk of AIDS-related non-Hodgkin lymphoma. AIDS (2018) 32:945–54. 10.1097/QAD.0000000000001771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marchetti G, Cozzi-Lepri A, Merlini E, Bellistri GM, Castagna A, Galli M, et al. Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4+ cell count. AIDS (2011) 25:1385–94. 10.1097/QAD.0b013e3283471d10 [DOI] [PubMed] [Google Scholar]
- 8. Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev (2013) 26:2–18. 10.1128/CMR.00050-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hensley-McBain T, Berard AR, Manuzak JA, Miller CJ, Zevin AS, Polacino P, et al. Intestinal damage precedes mucosal immune dysfunction in SIV infection. Mucosal Immunol (2018) 11:1429–40. 10.1038/s41385-018-0032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Isnard S, Ramendra R, Dupuy FP, Lin J, Fombuena B, Kokinov N, et al. Plasma levels of C-type lectin REG3alpha and gut damage in people with HIV. J Infect Dis (2019) 221:110–21. 10.1093/infdis/jiz423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramendra R, Isnard S, Mehraj V, Chen J, Zhang Y, Finkelman M, et al. Circulating LPS and (1–>3)-beta-D-Glucan: A Folie a Deux Contributing to HIV-Associated Immune Activation. Front Immunol (2019) 10:465. 10.3389/fimmu.2019.00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramendra R, Isnard S, Lin J, Fombuena B, Ouyang J, Merhaj V, et al. CMV seropositivity is associated with increased microbial translocation in people living with HIV and uninfected controls. Clin Infect Dis (2019) 71:1438–46. 10.1093/cid/ciz1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cassol E, Misra V, Holman A, Kamat A, Morgello S, Gabuzda D. Plasma metabolomics identifies lipid abnormalities linked to markers of inflammation, microbial translocation, and hepatic function in HIV patients receiving protease inhibitors. BMC Infect Dis (2013) 13:203. 10.1186/1471-2334-13-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Heijden WA, van de Wijer L, Keramati F, Trypsteen W, Rutsaert S, Ter Horst R, et al. Chronic HIV infection induces transcriptional and functional reprogramming of innate immune cells. JCI Insight (2021). 10.1172/jci.insight.145928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoenigl M, de Oliveira MF, Perez-Santiago J, Zhang Y, Woods SP, Finkelman M, et al. Correlation of (1–>3)-beta-D-glucan with other inflammation markers in chronically HIV infected persons on suppressive antiretroviral therapy. GMS Infect Dis (2015) 3. 10.3205/id000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsu DC, Sereti I. Serious Non-AIDS Events: Therapeutic Targets of Immune Activation and Chronic Inflammation in HIV Infection. Drugs (2016) 76:533–49. 10.1007/s40265-016-0546-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hager CL, Ghannoum MA. The mycobiome in HIV. Curr Opin HIV AIDS (2018) 13:69–72. 10.1097/COH.0000000000000432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nash AK, Auchtung TA, Wong MC, Smith DP, Gesell JR, Ross MC, et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome (2017) 5:153. 10.1186/s40168-017-0373-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morris A, Hillenbrand M, Finkelman M, George MP, Singh V, Kessinger C, et al. Serum (1–>3)-beta-D-glucan levels in HIV-infected individuals are associated with immunosuppression, inflammation, and cardiopulmonary function. J Acquir Immune Defic Syndr (2012) 61:462–8. 10.1097/QAI.0b013e318271799b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dirajlal-Fargo S, Moser C, Rodriguez K, El-Kamari V, Funderburg NT, Bowman E, et al. Changes in the Fungal Marker β-D-Glucan After Antiretroviral Therapy and Association With Adiposity. Open Forum Infect Dis (2019) 6. 10.1093/ofid/ofz434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gianella S, Letendre SL, Iudicello J, Franklin D, Gaufin T, Zhang Y, et al. Plasma (1 –> 3)-beta-D-glucan and suPAR levels correlate with neurocognitive performance in people living with HIV on antiretroviral therapy: a CHARTER analysis. J Neurovirol (2019) 25:837–43. 10.1007/s13365-019-00775-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoenigl M, de Oliveira MF, Perez-Santiago J, Zhang Y, Morris S, McCutchan AJ, et al. (1–>3)-beta-D-Glucan Levels Correlate With Neurocognitive Functioning in HIV-Infected Persons on Suppressive Antiretroviral Therapy: A Cohort Study. Med (Baltimore) (2016) 95:e3162. 10.1097/MD.0000000000003162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoenigl M, Perez-Santiago J, Nakazawa M, de Oliveira MF, Zhang Y, Finkelman MA, et al. (1–>3)-beta-d-Glucan: A Biomarker for Microbial Translocation in Individuals with Acute or Early HIV Infection? Front Immunol (2016) 7:404. 10.3389/fimmu.2016.00404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weiner L, Retuerto M, Hager C, El Kamari V, Shan L, Sattar A, et al. Fungal Translocation is Associated with Immune Activation and Systemic Inflammation in Treated HIV. AIDS Res Hum Retroviruses (2019) 25:461–72. 10.1089/AID.2018.0252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alzahrani J, Hussain T, Simar D, Palchaudhuri R, Abdel-Mohsen M, Crowe SM, et al. Inflammatory and immunometabolic consequences of gut dysfunction in HIV: Parallels with IBD and implications for reservoir persistence and non-AIDS comorbidities. EBioMedicine (2019) 46:522–31. 10.1016/j.ebiom.2019.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bonnet F, Le Marec F, Leleux O, Gerard Y, Neau D, Lazaro E, et al. Evolution of comorbidities in people living with HIV between 2004 and 2014: cross-sectional analyses from ANRS CO3 Aquitaine cohort. BMC Infect Dis (2020) 20:850. 10.1186/s12879-020-05593-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nanditha NGA, Paiero A, Tafessu HM, St-Jean M, McLinden T, Justice AC, et al. Excess burden of age-associated comorbidities among people living with HIV in British Columbia, Canada: a population-based cohort study. BMJ Open (2021) 11:e041734. 10.1136/bmjopen-2020-041734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hallen-Adams HE, Suhr MJ. Fungi in the healthy human gastrointestinal tract. Virulence (2017) 8:352–8. 10.1080/21505594.2016.1247140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Findley K, Oh J, Yang J, Conlan S, Deming C, Meyer JA, et al. Topographic diversity of fungal and bacterial communities in human skin. Nature (2013) 498:367–70. 10.1038/nature12171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hamm PS, Taylor JW, Cook JA, Natvig DO. Decades-old studies of fungi associated with mammalian lungs and modern DNA sequencing approaches help define the nature of the lung mycobiome. PloS Pathog (2020) 16:e1008684. 10.1371/journal.ppat.1008684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lai GC, Tan TG, Pavelka N. The mammalian mycobiome: A complex system in a dynamic relationship with the host. Wiley Interdiscip Rev Syst Biol Med (2019) 11:e1438. 10.1002/wsbm.1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Tilburg Bernardes E, Pettersen VK, Gutierrez MW, Laforest-Lapointe I, Jendzjowsky NG, Cavin JB, et al. Intestinal fungi are causally implicated in microbiome assembly and immune development in mice. Nat Commun (2020) 11:2577. 10.1038/s41467-020-16431-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scupham AJ, Presley LL, Wei B, Bent E, Griffith N, McPherson M, et al. Abundant and Diverse Fungal Microbiota in the Murine Intestine. Appl Environ Microbiol (2006) 72:793–801. 10.1128/aem.72.1.793-801.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang T, Fan C, Yao A, Xu X, Zheng G, You Y, et al. The Adaptor Protein CARD9 Protects against Colon Cancer by Restricting Mycobiota-Mediated Expansion of Myeloid-Derived Suppressor Cells. Immunity (2018) 49:504–514 e4. 10.1016/j.immuni.2018.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Issara-Amphorn J, Surawut S, Worasilchai N, Thim-Uam A, Finkelman M, Chindamporn A, et al. The Synergy of Endotoxin and (1–>3)-beta-D-Glucan, from Gut Translocation, Worsens Sepsis Severity in a Lupus Model of Fc Gamma Receptor IIb-Deficient Mice. J Innate Immun (2018) 10:189–201. 10.1159/000486321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leelahavanichkul A, Worasilchai N, Wannalerdsakun S, Jutivorakool K, Somparn P, Issara-Amphorn J, et al. Gastrointestinal Leakage Detected by Serum (1–>3)-beta-D-Glucan in Mouse Models and a Pilot Study in Patients with Sepsis. Shock (2016) 46:506–18. 10.1097/SHK.0000000000000645 [DOI] [PubMed] [Google Scholar]
- 37. Panpetch W, Somboonna N, Bulan DE, Issara-Amphorn J, Finkelman M, Worasilchai N, et al. Oral administration of live- or heat-killed Candida albicans worsened cecal ligation and puncture sepsis in a murine model possibly due to an increased serum (1–>3)-beta-D-glucan. PloS One (2017) 12:e0181439. 10.1371/journal.pone.0181439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mann AE, Mazel F, Lemay MA, Morien E, Billy V, Kowalewski M, et al. Biodiversity of protists and nematodes in the wild nonhuman primate gut. ISME J (2020) 14:609–22. 10.1038/s41396-019-0551-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheru LT, Park EA, Saylor CF, Burdo TH, Fitch KV, Looby S, et al. I-FABP Is Higher in People With Chronic HIV Than Elite Controllers, Related to Sugar and Fatty Acid Intake and Inversely Related to Body Fat in People With HIV. Open Forum Infect Dis (2018) 5:ofy288. 10.1093/ofid/ofy288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hoenigl M, Moser C, Funderburg N, Bosch R, Kantor A, Zhang Y, et al. Soluble Urokinase Plasminogen Activator Receptor (suPAR) is predictive of Non-AIDS Events during Antiretroviral Therapy-mediated Viral Suppression. Clin Infect Dis (2018) 69:676–86. 10.1093/cid/ciy966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoenigl M, Lin J, Finkelman M, Zhang Y, Karris MY, Letendre S, et al. Glucan rich nutrition does not increase gut translocation of Beta glucan. Mycoses (2020) 64:24–9. 10.1111/myc.13161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dirajlal-Fargo S, El-Kamari V, Weiner L, Shan L, Sattar A, Kulkarni M, et al. Altered Intestinal Permeability and Fungal Translocation in Ugandan Children With Human Immunodeficiency Virus. Clin Infect Dis (2020) 70:2413–22. 10.1093/cid/ciz561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dirajlal-Fargo S, Albar Z, Bowman E, Labbato D, Sattar A, Karungi C, et al. Increased monocyte and T-cell activation in treated HIV+ Ugandan children: associations with gut alteration and HIV factors. AIDS (2020) 34:1009–18. 10.1097/QAD.0000000000002505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ouyang J, Isnard S, Lin J, Fombuena B, Chatterjee D, Wiche Salinas TR, et al. Daily variations of gut microbial translocation markers in ART-treated HIV-infected people. AIDS Res Ther (2020) 17:15. 10.1186/s12981-020-00273-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Isnard S, Fombuena B, Sadouni M, Lin J, Richard C, Routy B, et al. Circulating β-D-Glucan as a marker of subclinical coronary plaque in ART-treated people living with HIV. Open Forum Infect Dis (2021). 10.1093/ofid/ofab109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moon MS, Quinn G, Townsend EC, Ali RO, Zhang GY, Bradshaw A, et al. Bacterial Translocation and Host Immune Activation in Chronic Hepatitis C Infection. Open Forum Infect Dis (2019) 6. 10.1093/ofid/ofz255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Balagopal A, Philp FH, Astemborski J, Block TM, Mehta A, Long R, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology (2008) 135:226–33. 10.1053/j.gastro.2008.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marchetti G, Nasta P, Bai F, Gatti F, Bellistri GM, Tincati C, et al. Circulating sCD14 is associated with virological response to pegylated-interferon-alpha/ribavirin treatment in HIV/HCV co-infected patients. PloS One (2012) 7:e32028. 10.1371/journal.pone.0032028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peters L, Neuhaus J, Duprez D, Neaton JD, Tracy R, Klein MB, et al. Biomarkers of inflammation, coagulation and microbial translocation in HIV/HCV co-infected patients in the SMART study. J Clin Virol (2014) 60:295–300. 10.1016/j.jcv.2014.03.017 [DOI] [PubMed] [Google Scholar]
- 50. Finkelman MA. Specificity Influences in (1–>3)-beta-d-Glucan-Supported Diagnosis of Invasive Fungal Disease. J Fungi (Basel) (2020) 7. 10.3390/jof7010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mehraj V, Ramendra R, Isnard S, Dupuy FP, Lebouche B, Costiniuk C, et al. CXCL13 as a Biomarker of Immune Activation During Early and Chronic HIV Infection. Front Immunol (2019) 10:289. 10.3389/fimmu.2019.00289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Patin EC, Thompson A, Orr SJ. Pattern recognition receptors in fungal immunity. Semin Cell Dev Biol (2019) 89:24–33. 10.1016/j.semcdb.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Swidergall M, Solis NV, Lionakis MS, Filler SG. EphA2 is an epithelial cell pattern recognition receptor for fungal beta-glucans. Nat Microbiol (2018) 3:53–61. 10.1038/s41564-017-0059-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, et al. Dectin-1 Is A Major β-Glucan Receptor On Macrophages. J Exp Med (2002) 196:407–12. 10.1084/jem.20020470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bonfim CV, Mamoni RL, Blotta MH. TLR-2, TLR-4 and dectin-1 expression in human monocytes and neutrophils stimulated by Paracoccidioides brasiliensis. Med Mycol (2009) 47:722–33. 10.3109/13693780802641425 [DOI] [PubMed] [Google Scholar]
- 56. Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, Gordon S, et al. The -Glucan Receptor, Dectin-1, Is Predominantly Expressed on the Surface of Cells of the Monocyte/Macrophage and Neutrophil Lineages. J Immunol (2002) 169:3876–82. 10.4049/jimmunol.169.7.3876 [DOI] [PubMed] [Google Scholar]
- 57. Ali MF, Driscoll CB, Walters PR, Limper AH, Carmona EM. beta-Glucan-Activated Human B Lymphocytes Participate in Innate Immune Responses by Releasing Proinflammatory Cytokines and Stimulating Neutrophil Chemotaxis. J Immunol (2015) 195:5318–26. 10.4049/jimmunol.1500559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Elder MJ, Webster SJ, Chee R, Williams DL, Hill Gaston JS, Goodall JC. beta-Glucan Size Controls Dectin-1-Mediated Immune Responses in Human Dendritic Cells by Regulating IL-1beta Production. Front Immunol (2017) 8:791. 10.3389/fimmu.2017.00791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McDonald JU, Rosas M, Brown GD, Jones SA, Taylor PR. Differential dependencies of monocytes and neutrophils on dectin-1, dectin-2 and complement for the recognition of fungal particles in inflammation. PloS One (2012) 7:e45781. 10.1371/journal.pone.0045781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Isnard S, Ramendra R, Dupuy FP, Mehraj V, Ponte R, Chen J, et al. Circulating beta-D-Glucan and Induction of Immune activation. In: CROI 2019 Poster 2109. Conference on Retroviruses and Opportunistic infections 2019; (2019). [Google Scholar]
- 61. Li SS, Ogbomo H, Mansour MK, Xiang RF, Szabo L, Munro F, et al. Identification of the fungal ligand triggering cytotoxic PRR-mediated NK cell killing of Cryptococcus and Candida. Nat Commun (2018) 9:751. 10.1038/s41467-018-03014-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li SS, Kyei SK, Timm-McCann M, Ogbomo H, Jones GJ, Shi M, et al. The NK receptor NKp30 mediates direct fungal recognition and killing and is diminished in NK cells from HIV-infected patients. Cell Host Microbe (2013) 14:387–97. 10.1016/j.chom.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 63. Han B, Mao FY, Zhao YL, Lv YP, Teng YS, Duan M, et al. Altered NKp30, NKp46, NKG2D, and DNAM-1 Expression on Circulating NK Cells Is Associated with Tumor Progression in Human Gastric Cancer. J Immunol Res (2018) 2018:6248590. 10.1155/2018/6248590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Divangahi M, Aaby P, Khader SA, Barreiro LB, Bekkering S, Chavakis T, et al. Trained immunity, tolerance, priming and differentiation: distinct immunological processes. Nat Immunol (2020) 22:2–6. 10.1038/s41590-020-00845-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Netea MG, Dominguez-Andres J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol (2020) 20:375–88. 10.1038/s41577-020-0285-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bekkering S, Quintin J, Joosten LA, van der Meer JW, Netea MG, Riksen NP. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler Thromb Vasc Biol (2014) 34:1731–8. 10.1161/ATVBAHA.114.303887 [DOI] [PubMed] [Google Scholar]
- 67. Sacktor N, Skolasky RL, Moxley R, Wang S, Mielke MM, Munro C, et al. Paroxetine and fluconazole therapy for HIV-associated neurocognitive impairment: results from a double-blind, placebo-controlled trial. J Neurovirol (2018) 24:16–27. 10.1007/s13365-017-0587-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ouyang J, Isnard S, Lin J, Fombuena B, Peng X, Nair Parvathy S, et al. Treating from the inside out: relevance of fecal microbiota transplantation to counteract gut damage in GVHD and HIV infection. Front Med (2020) 7:421. 10.3389/fmed.2020.00421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Utay NS, Monczor AN, Somasunderam A, Lupo S, Jiang ZD, Alexander AS, et al. Evaluation of Six Weekly Oral Fecal Microbiota Transplants in People with HIV. Pathog Immun (2020) 5:364–81. 10.20411/pai.v5i1.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Routy JP, Isnard S, Mehraj V, Ostrowski M, Chomont N, Ancuta P, et al. Effect of metformin on the size of the HIV reservoir in non-diabetic ART-treated individuals: single-arm non-randomised Lilac pilot study protocol. BMJ Open (2019) 9:e028444. 10.1136/bmjopen-2018-028444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Planas D, Pagliuzza A, Ponte R, Fert A, Marchand LR, Massanella M, et al. LILAC pilot study: Effects of metformin on mTOR activation and HIV reservoir persistence during antiretroviral therapy. EBioMedicine (2021) 65:103270. 10.1016/j.ebiom.2021.103270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Isnard S, Lin J, Fombuena B, Ouyang J, Varin TV, Richard C, et al. Repurposing metformin in non-diabetic people living with HIV: Influence on weight and gut microbiota. Open Forum Infect Dis (2020). 10.1093/ofid/ofaa338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Macatangay BJC, Jackson EK, Abebe KZ, Comer D, Cyktor J, Klamar-Blain C, et al. Placebo-Controlled, Pilot Clinical Trial of Dipyridamole to Decrease Human Immunodeficiency Virus-Associated Chronic Inflammation. J Infect Dis 221 (2020) 221:1598–606. 10.1093/infdis/jiz344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mallarino-Haeger C, Abebe KZ, Jackson EK, Zyhowski A, Klamar-Blain C, Cyktor JC, et al. Brief Report: Dipyridamole Decreases Gut Mucosal Regulatory T-Cell Frequencies Among People With HIV on Antiretroviral Therapy. J Acquir Immune Defic Syndr (2020) 85:665–9. 10.1097/QAI.0000000000002488 [DOI] [PMC free article] [PubMed] [Google Scholar]