Abstract
Cheeseweed mallow (Malva parviflora L.) was used to biosynthesize silver nanoparticles. The biosynthesized silver nanoparticles were classified by UV–vis Spectroscopy and Fourier-Transform Infrared Spectroscopy (FT-IR). The shape and size distribution were visualized by Transmission Electron Microscopy (TEM), Field Emission Scanning Electron Microscopy (FE-SEM), and Zeta potential analysis. The chemical composition of M. parviflora leaf extract was identified by Gas Chromatography and Mass Spectroscopy (GC/MS). Finally, in vitro antifungal assay was done to assess the potential of biosynthesized silver nanoparticles and crude leaf extract of M. parviflora for inhibiting the mycelial growth of phytopathogenic fungi. The UV–vis analysis manifests the formation of silver nanoparticles. FTIR analysis established that chemicals of the leaf extract stabilized the biosynthesized silver nanoparticles by binding with the free silver ions. The TEM, FE-SEM and zeta potential analyzer confirmed that the biosynthesized silver nanoparticles were mostly spherical with an average diameter of 50.6 nm. The biosynthesized silver nanoparticles and leaf extract of M. parviflora effectively mitigate the mycelial growth of Helminthosporium rostratum, Fusarium solani, Fusarium oxysporum, and Alternaria alternata. The maximum reduction in mycelial growth by biosynthesized nanoparticles was observed against H. rostratum (88.6%). Whereas, the leaf extract of M. parviflora was most effective against F. solani (65.3%). Thus, the biosynthesis of nanoparticle assisted by M. parviflora is a feasible and eco-friendly method for the synthesis of silver nanoparticles. Further the silver nanoparticles and leaf extract of M. parviflora could be explored for the development of the fungicide.
Keywords: Antifungal activity, FE-SEM, FT-IR, Helminthosporium rostratum, Malva parviflora, Silver nanoparticles
Abbreviations: AgNPs, silver nanoparticles; LEMP, leaf extract of M. parviflora; FT-IR, Fourier-Transform Infrared Spectroscopy; FE-SEM, Field Emission Scanning Electron Microscopy; GC/MS, Gas Chromatography/Mass Spectrometry; DLS, Dynamic Light Scattering; PDI, Polydispersity Index; SPR, Surface Plasmon Resonance
1. Introduction
Cheeseweed mallow (Malva parviflora L.) belongs to the family Malvaceae. It is an annual plant found in several regions of Saudi Arabia. M. parviflora inhabits various regions of the world and mostly grows wild in uncultivated land, roadsides, and marshy places. The plant is rich in phenolic compounds, anthocyanin, and flavonoids. It contains many active substances such as Asparagine, Ascorbic Acid, Flavonol glycosides (including josepine-3-sulfate), Pectin, phenolic acid, Quercetin, Salicylic acid, Anthocyanin, vitamins A, B & C (Akbar et al., 2014, Singh, 2017). In ancient Rome and Greece, M. parviflora was used in traditional medicines to treat mouth and throat infection (Shale et al., 2005, Aslam and Sial, 2014). In South Africa people of Lesotho and Xhosa use parts of M. parviflora to clean wounds, sores, and to treat inflammation (Almagboul et al., 1985, Shale et al., 1999). Besides, M. parviflora has been reported for antibacterial and antifungal properties (Shale et al., 2005, Farhan et al., 2012a, Singh, 2017). There are some reports on the use of Malva spp. in the biosynthesis of silver nanoparticles (Zayed et al., 2012, Miavaghi and Pourakbar, 2016, Esfanddarani et al., 2018). Silver nanoparticles (AgNPs) bear distinctive physical and chemical properties. These AgNPs have versatile applications in pharmaceutical, cosmetics, engineering, medicine and other technologies. Generally, nanomaterials differ from their bulk form due to their considerably smaller size and large surface area. The nanoparticles are synthesized through various methods that uses diverse means such as mechanical, heat, and chemicals. For the synthesis of nanoparticles by such type of methods hazardous radiations and chemicals are used eventually damaging the environment and living things (Duan et al., 2015, Roy et al., 2019). Therefore, in this era there is a demand for another strategy for the synthesis of nanoparticles that are ecofriendly and cost effective as well. The synthesis of nanoparticles by utilizing natural sources like microorganisms and plants has been evolved as an alternate solution (Akhtar et al., 2013, Duan et al., 2015, Roy et al., 2019). In the biosynthesis of metallic nanoparticles by plants, the metabolites of plant extracts such as phenols, alkaloids, proteins, and sugar mediate the synthesis and stabilize the nanoparticles (Nguyen et al., 2020). The biosynthesis of nanoparticles primarily depends upon the type of plant extract, temperature, pH, and solvent. M. parviflora possesses several metabolites such as phenols, flavonoids, esters, and peptides that can mediate the synthesis of silver nanoparticles (Farhan et al., 2012b, Wang et al., 2001).
Plant pathogenic fungi cause various diseases to plants including economically important crops, they can cause damage to crops at pre- or post-harvest stages. The most effective way to control fungal diseases is the use of fungicides. These fungicides are mostly synthetic and have several side effects to the environment and human (Lushchak et al., 2018). In recent years, several natural and harmless substitutes of chemical fungicides have been explored. In the same context the prospect of nanoparticles as a fungicide has been evaluated by several workers (Bahrami-Teimoori et al., 2017, Roy et al., 2019, Ghojavand et al., 2020, Jebril et al., 2020). The antimicrobial values of silver have been known globally since ages. Similarly, AgNPs have been recognized for antimicrobial properties. However, there are limited records on the potential of AgNPs to control phytopathogenic fungi. Hence, the present study is targeted at biosynthesis of AgNPs using Malva parviflora and evaluate the potential of synthesized AgNPs in limiting the growth of phytopathogenic fungi.
2. Materials and methods
2.1. Biosynthesis of silver nanoparticles
For the biosynthesis of AgNPs, the leaf extract of M. parviflora was used. The fresh plant material was collected from the area of Wadi Hanifa, Riyadh Saudi Arabia. The identity of the plant as Malva parviflora L. was confirmed by the plant taxonomist Dr. Najat Bukhari, King Saud University (Radcliffe-Smith, & Collenette, 1987). The leaf extract of M. parviflora was prepared with the deionized water. Initially, the collected plants were washed with tap water till all the dirt and debris was removed from the plants, later the leaves were plucked and rinsed with the deionized water. After that, the leaves (10 g) were cut into the small pieces and boiled with 200 ml deionized water at 100 °C for 10 min and the solution was allowed to cool at room temperature and then filtered through the Whatman filter paper no. 40. The obtained filtrate leaf extract of M. parviflora (LEMP) was stored in the refrigerator at 4 °C in a dark-colored bottle until further analysis was conducted.
For the biosynthesis of silver nanoparticles, the methodology mentioned earlier was followed (Zayed et al., 2012). The reaction mixture was prepared by mixing 5 ml of LEMP with 45 ml of silver nitrate (0.01 mM) the prepared reaction mixture was left undisturbed at room temperature (23 °C) till color of reaction mixture changed to reddish-brown. The color change is the primary indication of the synthesis of the AgNPs. Later, to verify the formation, stabilization and morphology and dimensions of biosynthesized AgNPs, UV–vis Spectroscopy, Fourier-Transform Infrared Spectroscopy (FT-IR), Field Emission Scanning Electron Microscopy (FE-SEM), and Zeta potential analysis was conducted.
2.2. Characterization of biosynthesized AgNPs
UV–visible spectrophotometer (Shimadzu, Tokyo, Japan) was used to determine the formation of AgNPs. The absorbance was measured at 200–800 nm at UV-2450 double-beam.
FT-IR (Nicolet 6700 FT-IR Spectrometer, Waltham, MA, USA) analysis was performed to substantiate the capping and stabilizing of the biosynthesized AgNPs.
The FE-SEM (JEOL 7500FA JEOL, Peabody, MA, USA) analysis was used to ascertain the morphology and size of the biosynthesized AgNPs. A drop (8 μL) of AgNPs suspension was put onto grids (200 mesh) with a carbon support film (Agar Scientific, London, UK) and then the sample was dried. After that, the obtained dried sample was rinsed with EtOH and dried again, then later fixed on an appropriate SEM holder. Images were captured at an accelerating voltage of 30 kV.
2.3. Gas chromatography/mass spectrometry technique (GC/MS)
The phenolic compounds of LEMP were detected by GC/MS analysis. The thermo-gas chromatograph /mass spectrometer (model Shimadzu 2010) equipped with Rtx-5MS capillary column (30 m long, 0.25 mm in diameter, film thickness 0.25 μm) was used. The carrier gas was helium and the maximum usable temperature was 280 °C. The separated compounds were identified by computer searches in commercial libraries of NIST14 and WILEY.
2.4. Evaluation of the antifungal potential of biosynthesized AgNPs and LEMP, in vitro
2.4.1. Fungal culture
The plant pathogenic fungi Helminthosporium rostratum, Fusarium solani, Fusarium oxysporum, and Alternaria alternata, previously identified at laboratory of plant protection were obtained from the College of Food and Agriculture, King Saud University. The cultures were maintained on the slants of Potato Dextrose Agar (PDA) and were stored in a refrigerator (4 °C).
2.4.2. In vitro antifungal activity
The poisoned food technique was employed to assess the antifungal potential of biosynthesized AgNPs and LEMP (Singh et al., 2007). In brief, pre-calculated amount of the LEMP or AgNPs was added to the autoclaved molten PDA to get the 5% final concentration, the PDA receiving deionized water served as control. The PDA medium (20 ml) was then poured into Petri plates and allowed to solidify. The prepared PDA plates were inoculated with 6 days old culture of phytopathogenic fungi. A mycelial plug of 6 mm was placed (surface of mycelial growth facing the agar) at the center of the Petri plates. There were three replicates for each treatment, Petri plates were incubated at 25 ± 2 °C till the fungal growth in the control plates reached the edge of the plate (7–9 days). After incubation diameter of the mycelial growth of the fungi was recorded and the percent inhibition was calculated. The following formula was used for the calculation:
2.5. Statistical analysis
Each test was performed twice and Microsoft Excel 2013 was used for calculation of data and standard error.
3. Results
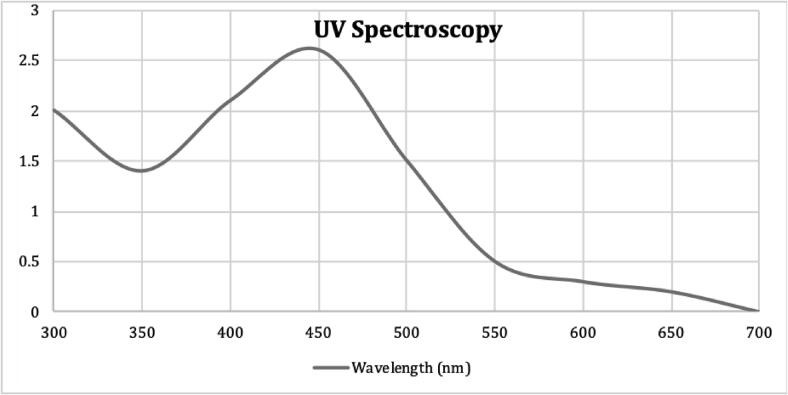
The AgNPs were synthesized by using 0.01 mM AgNO3 and LEMP, the reaction mixture prepared by mixing these two solutions was incubated at room temperature till the change in color was visible. The color change from colorless to reddish-brown was apparent after 60 min. and it was the first indication of efficient synthesis of AgNPs. Further, the biosynthesized AgNPs were analyzed by UV–visible Spectroscopy. The wavelength of the nanoparticles extract was measured using UV–visible Spectroscopy to ascertain the formation of AgNPs. The peaks in the Fig. 1 clearly shows that there was a Surface Plasmon Resonance Phenomena. Moreover, the UV–vis absorption band was observed between 430 and 455 nm.
Fig. 1.
UV–vis absorbance spectra of biosynthesized AgNPs assisted by aqueous extract of M. parviflora.
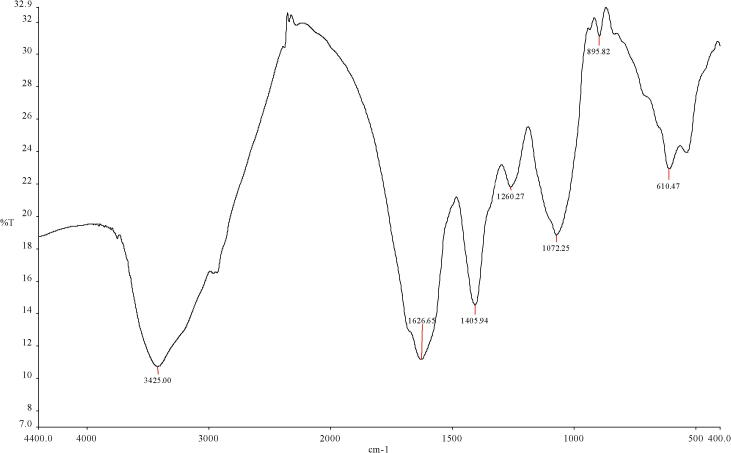
The Fourier-Transform Infrared Spectroscopy (FT-IR.) established the presence of functional groups in the biosynthesized AgNPs solution. The FT-IR spectra show the absorption bands in regions ranging from 3425.00 to 610.47 cm−1. Further, the spectrum shows prominent absorption peaks at 3425.00, 1626.65, 1405.94, 1260.27, 1072.25, 895.82, and 610.47 cm−1. These peaks reveal that the several functional groups like alcohols, carboxylic acids, amides, alkynes, alkanes, alkyl amines, halogen, and cycloalkanes groups present in the solution have acted as capping and stabilizing agents (Fig. 2).
Fig. 2.
FT-IR absorbance spectra of biosynthesized AgNPs assisted by aqueous extract of M. parviflora.
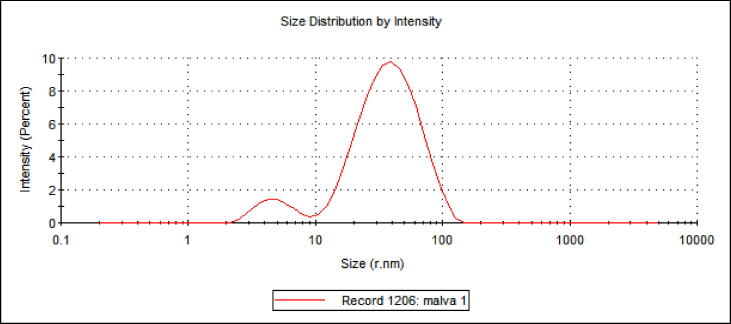
To determine the particle size and distribution, the biosynthesized AgNPs were characterized by zeta potential analyzer with the DLS technique. The DLS measurement as represented in Fig. 3 depicts that the average size of the biosynthesized AgNPs was 50.6 nm (diameter) and the polydispersity index (PdI) was 0.412 (Fig. 3).
Fig. 3.
DLS analysis to determine size of biosynthesized AgNPs assisted by aqueous extract of M. parviflora.
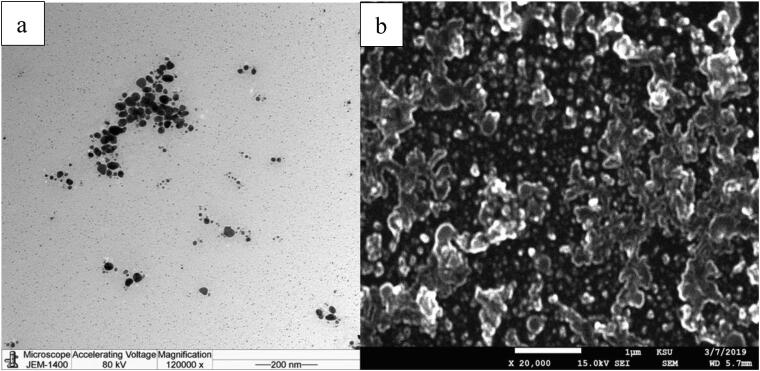
Transmission electron microscopy (TEM) and Field Emission Scanning Electron Microscopy (FE-SEM) was used to determine the morphological characteristics of the nanoparticles. Mainly the shape of the biosynthesized AgNPs were spherical, however, some irregularly shaped particles were marked. In addition, a few agglomerations were noticed (Fig. 4a and 4b).
Fig. 4.
TEM (a) and FE-SEM (b) image of biosynthesized AgNPs assisted by aqueous extract of M. parviflora.
The LEMP was analyzed by GC/MS to detect the phenolic compounds in it. The results of the analysis are presented in Table 1. Primarily, the LEMP contains Anthocyanins, Ferulic acid, Hydroxycinnamic, and Sterol.
Table 1.
Chemical constituents of LEMP analyzed by GC/MS.
| Phenolic compounds | Formula | Molecular weight | MS fragments m/z | Rel. Int. |
|---|---|---|---|---|
| Sterol | C17H28O | 248.41 | 248.00 | 33.48 |
| Hydroxycinnamic | C9H8O3 | 164.16 | 164.00 | 68.30 |
| Anthocyanins | C15H11O+ | 207.252 | 207.00 | 48.42 |
| Ferulic acid | C10H10O4 | 194.186 | 194.00 | 71.74 |
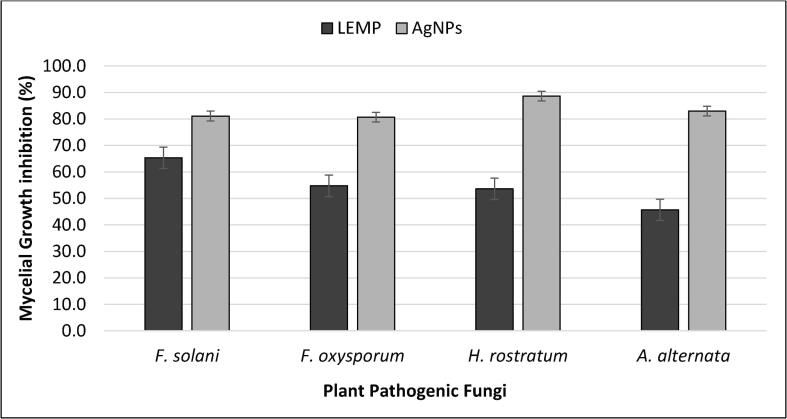
The results of in vitro antifungal activity of AgNPs and LEMP presented in Fig. 5, Fig. 6 exhibit that the biosynthesized AgNPs and LEMP have repressed the mycelial growth of all tested fungi. However, the percent inhibition of mycelial growth by the AgNPs and LEMP varies among tested species (Fig. 5, Fig. 6). Furthermore, the reduction in the mycelial growth of all tested phytopathogenic fungi was more by AgNPs than LEMP. The maximum reduction in the mycelial growth by AgNPs was against H. rostratum (88.6%), followed by A. alternata (83.0%), F. solani (81.1%), and F. oxysporum (80.7%). The Fig. 5, Fig. 6 also depicts that there was a considerable reduction in the mycelial growth of all tested fungi by LEMP as compared to control, the maximum reduction in mycelial growth by LEMP was against F. solani (65.3%) followed by F. oxysporum (54.7%), H. rostratum (53.6%), A. alternata (45.6%).
Fig. 5.
In vitro Antifungal activity of biosynthesized AgNPs assisted by aqueous extract of M. parviflora against H. rostratum, F. solani, F. oxysporum and A. alternate. The vertical bars represent ± standard error (n = 3).
Fig. 6.
Mycelial growth of F. solani (a), H. rostratum (b), F. oxysporum (c) and A. alternaria (d) treated with 5% AgNPs (2.2a-d); LEMP (2.3a-d) and untreated (2.1a-d).
4. Discussion
Plants contain many primary and secondary metabolites with distinctive properties. Nowadays the AgNPs are being investigated extensively for their application in managing the pathogenic microorganisms. For the synthesis of AgNPs a reducing agent is required, mainly they are harsh chemicals. To substitute these hazardous chemicals the plant extracts have been emerged as an alternative reducing agent. M. parviflora contains composite biochemicals and metabolites that can assist the synthesis of AgNPs. In the current study, LEMP mediated the synthesis of AgNPs and the first indication of their synthesis was the color change to reddish-brown after an hour of incubation at room temperature. The chemical composition of the LEMP acted as an electron donor and mediated the reduction of silver ions that resulted in synthesis of AgNPs, further the surface plasmon resonance (SPR) phenomena causes the change in color to reddish-brown (Shankar et al., 2003, Fan et al., 2009) The synthesis of AgNPs was affirmed by the UV–vis spectroscopy through the recognition of the SPR property by absorption spectra band (Zhang et al., 2016). The absorption peak depends on the characteristics of the biochemicals of the plant extracts and the concentration used for the AgNPs synthesis (Balavijayalakshmi and Ramalakshmi, 2017, Kumar et al., 2017, Yasir et al., 2017). Whereas, the FT-IR analysis ascertain the presence of several functional groups in the solution of the biosynthesized AgNPs. The source of these functional groups was M. parviflora extract that was used as a reducing agent for the synthesis of AgNPs. Probably, these chemicals and proteins of the LEMP have served as reducing, capping and stabilizing agent for AgNPs. The strong band at 3425.00 cm−1 was observed in the FT-IR chromatograph, the value indicate presence of –OH groups. It indicates the strong affinity of plant extract towards the surface of AgNPs thus the stabilization of biosynthesized AgNPs (Zayed et al., 2012, Ajitha et al., 2015, Balavijayalakshmi and Ramalakshmi, 2017). The image obtained through FE-SEM analysis of biosynthesized AgNPs exhibited that the prominent shape of nanoparticles was spherical with some irregular shapes. The results of present study are in agreement with previous studies that have observed the spherical AgNPs when synthesis was assisted by seed extract of dates (Ansari & Alzohairy, 2018) and leaf extract of Artemisia princeps (Gurunathan et al., 2015). Zeta size analysis also confirm the fact that the synthesized AgNPs were discrete with an average diameter of 42.3 nm. Zayed et al., (2012) reported the average size of the AgNPs synthesized using M. parviflora ranges between 19 and 25 nm and was dependent on the concentration of the plant extract. The molarity of initial substrate and concentration of the reducing agent mainly regulate the size of the nanoparticles. In addition, the size of AgNPs also rely on the types of plant species and extract. AgNPs of 40–60 nm were recorded from Pulicaria glutinosa plant extract (Khan et al., 2013), while average size was 30–70 nm of the AgNPs synthesised Berberis vulgaris leaf and root extract (Behravan et al., 2019) and 105 nm from Calligonum comosom (Mohammed et al., 2018).
In the present study, the noteworthy antifungal activity was recorded from the biosynthesized AgNPs against four important phytopathogenic fungi (H. rostratum, F. solani, F. oxysporum, and A. alternata), additionally LEMP also exhibited a considerable amount of antifungal activity. The AgNPs and LEMP both reduced the mycelial growth of the tested plant pathogenic fungi. Previously several scientists have reported the antifungal activity of AgNPs against different plant pathogenic fungi (Buzea et al., 2007, Lee et al., 2013, Roy et al., 2019, Jebril et al., 2020). However, the reports are lacking on the effect of AgNPs on H. rostratum. Bahrami-Teimoori et al., (2017) documented an appreciable decrease in mycelial growth of M. phaseolina, A. alternata, and F. oxysporum treated with AgNPs synthesized using Amaranthus retroflexus leaf extract. Recently Ghojavand et al., (2020) observed that the AgNPs synthesized using stems and flowers of Teucrium polium exhibited antifungal activity against F. oxysporum.
The current study demonstrated the positive potential of biosynthesized AgNPs in mitigating the mycelial growth of all tested phytopathogens. However, the mechanisms of control of fungi by AgNPs is still a topic of investigation. Presumably, minute size, shape and form of the AgNPs supported the entry of nanoparticles in the plasma membrane. The nanoparticles may have caused hindrance in the normal functioning of the proteins present in the cell membrane, which may have resulted in the collapse of the cells (Buzea et al., 2007). The spore germination and development of hyphae may have also been affected by the AgNPs as noticed by Bocate et al., (2019) that the Aspergillus treated with AgNPs had abnormal spore germination and distorted hyphae. The current study also demonstrated that the LEMP can render the reduction in mycelial growth of the tested fungi. The chemical analysis of the LEMP identified several phenolic compounds specifically Anthocyanins, Ferulic acid, Hydroxycinnamic. These findings are in agreement with the previous reports on the chemical components of M. parviflora (Akbar et al., 2014; A. Singh, 2017). The bioactive compounds such as flavonoid glycosides, coumarins derivatives, and anthraquinones possess antimicrobial activity. These compounds may have additive or synergistic effect on the fungi (Abad et al., 2007).
5. Conclusion
The synthesis of AgNPs by M. parviflora is easy, fast, and eco-friendly. Moreover, the plant is easy to obtain and as it is found in abundance with no commercial use these plants can be used to get extract or AgNPs. The AgNPs and leaf extract can be used for the protection of plants/crops against some important pre and post-harvest diseases caused by phytopathogenic fungi. Furthermore, to ascertain the mechanism of AgNPs to inhibit fungi more studies are needed.
Declaration of Competing Interest
The authors declare that they have no conflict of interest
Acknowledgement
“Research Center of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University for funding this work through research group No (SMRC -1904).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abad, M. J., Ansuategui, M., Bermejo, P., 2007. Active antifungal substances from natural sources. In Arkivoc (Vol. 2007, Issue 7). doi: 10.3998/ark.5550190.0008.711.
- Ajitha B., Ashok Y., Kumar, Reddy, Sreedhara P., Reddy Green synthesis and characterization of silver nanoparticles using Lantana camara leaf extract. Mater. Sci. Eng., C. 2015;49 doi: 10.1016/j.msec.2015.01.035. [DOI] [PubMed] [Google Scholar]
- Akbar S., Hanif U., Ali J., Ishtiaq S. Pharmacognostic studies of stem, roots and leaves of Malva parviflora L. Asian Pacific J. Tropical Biomed. 2014;4(5) doi: 10.12980/APJTB.4.2014C1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar M.S., Panwar J., Yun Y.S. Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sustain. Chem. Eng. 2013;1(6) doi: 10.1021/sc300118u. [DOI] [Google Scholar]
- Almagboul A.Z., Bashir A.K., Farouk A., Salih A.K.M. Antimicrobial activity of certain Sudanese plants used in folkloric medicine. Screening for antibacterial activity (IV) Fitoterapia. 1985;56(6) doi: 10.1016/s0367-326x(01)00310-0. [DOI] [PubMed] [Google Scholar]
- Ansari M.A., Alzohairy M.A. One-pot facile green synthesis of silver nanoparticles using seed extract of phoenix dactylifera and their bactericidal potential against MRSA. Evidence-Based Complement. Alternative Med. 2018;2018 doi: 10.1155/2018/1860280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M., Sial A.A. Neuroprotective Effect of Ethanol Extract of Leaves of Malva parviflora against Amyloid- β - (A β -) Mediated Alzheimer’s Disease. Int. Scholarly Res. Notices. 2014 doi: 10.1155/2014/156976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami-Teimoori B., Nikparast Y., Hojatianfar M., Akhlaghi M., Ghorbani R., Pourianfar H.R. Characterisation and antifungal activity of silver nanoparticles biologically synthesised by Amaranthus retroflexus leaf extract. J. Exp. Nanosci. 2017 doi: 10.1080/17458080.2017.1279355. [DOI] [Google Scholar]
- Balavijayalakshmi J., Ramalakshmi V. Carica papaya peel mediated synthesis of silver nanoparticles and its antibacterial activity against human pathogens. J. Appl. Res. Technol. 2017 doi: 10.1016/j.jart.2017.03.010. [DOI] [Google Scholar]
- Behravan M., Hossein Panahi A., Naghizadeh A., Ziaee M., Mahdavi R., Mirzapour A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 2019;124:148–154. doi: 10.1016/j.ijbiomac.2018.11.101. [DOI] [PubMed] [Google Scholar]
- Bocate K.P., Reis G.F., de Souza P.C., Oliveira Junior A.G., Durán N., Nakazato G., Furlaneto M.C., de Almeida R.S., Panagio L.A. Antifungal activity of silver nanoparticles and simvastatin against toxigenic species of Aspergillus. Int. J. Food Microbiol. 2019;291 doi: 10.1016/j.ijfoodmicro.2018.11.012. [DOI] [PubMed] [Google Scholar]
- Buzea C., Pacheco I.I., Robbie K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases. 2007;2(4) doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- Duan H., Wang D., Li Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015;44(16) doi: 10.1039/c4cs00363b. [DOI] [PubMed] [Google Scholar]
- Esfanddarani H.M., Kajani A.A., Bordbar A.K. Green synthesis of silver nanoparticles using flower extract of Malva sylvestris and investigation of their antibacterial activity. IET Nanobiotechnol. 2018;12(4) doi: 10.1049/iet-nbt.2017.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Liu Z., Wang L., Zhan J. Synthesis of starch-stabilized Ag nanoparticles and Hg 2+ recognition in aqueous media. Nanoscale Res. Lett. 2009 doi: 10.1007/s11671-009-9387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhan H., Rammal H., Hijazi A., Hamad H., Daher A., Reda M., Badran B. In vitro antioxidant activity of ethanolic and aqueous extracts from crude Malva parviflora L. grown in Lebanon. Asian J. Pharm. Clin. Res. 2012;5(Suppl 3) [Google Scholar]
- Farhan Hussein, Rammal H., Hijazi A., Badran B. Preliminary phytochemical screening and extraction of polyphenol from stems and leaves of a lebanese plant Malva parviflora L. Int. J. Current Pharm. Res. 2012;4(1) [Google Scholar]
- Ghojavand S., Madani M., Karimi J. Green Synthesis, Characterization and Antifungal Activity of Silver Nanoparticles Using Stems and Flowers of Felty Germander. J. Inorg. Organomet. Polym Mater. 2020;30(8):2987–2997. doi: 10.1007/s10904-020-01449-1. [DOI] [Google Scholar]
- Gurunathan S., Jeong J.K., Han J.W., Zhang X.F., Park J.H., Kim J.H. Multidimensional effects of biologically synthesized silver nanoparticles in Helicobacter pylori, Helicobacter felis, and human lung (L132) and lung carcinoma A549 cells. Nanoscale Res. Lett. 2015;10(1) doi: 10.1186/s11671-015-0747-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jebril S., Khanfir R., Ben, Jenana, Dridi C. Green synthesis of silver nanoparticles using Melia azedarach leaf extract and their antifungal activities: In vitro and in vivo. Mater. Chem. Phys. 2020;248 doi: 10.1016/j.matchemphys.2020.122898. [DOI] [Google Scholar]
- Khan M., Khan M., Adil S.F., Tahir M.N., Tremel W., Alkhathlan H.Z., Al-Warthan A., Siddiqui M.R.H. Green synthesis of silver nanoparticles mediated by Pulicaria glutinosa extract. Int. J. Nanomed. 2013;8 doi: 10.2147/IJN.S43309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Sharma P., Bamal A., Negi S., Chaudhary S. A safe, efficient and environment friendly biosynthesis of silver nanoparticles using Leucaena leucocephala seed extract and its antioxidant, antimicrobial, antifungal activities and potential in sensing. Green Process. Synth. 2017;6(5) doi: 10.1515/gps-2016-0146. [DOI] [Google Scholar]
- Lee K.J., Park S.H., Govarthanan M., Hwang P.H., Seo Y.S., Cho M., Lee W.H., Lee J.Y., Kamala-Kannan S., Oh B.T. Synthesis of silver nanoparticles using cow milk and their antifungal activity against phytopathogens. Mater. Lett. 2013;105 doi: 10.1016/j.matlet.2013.04.076. [DOI] [Google Scholar]
- Lushchak V.I., Matviishyn T.M., Husak V.V., Storey J.M., Storey K.B. Pesticide toxicity: A mechanistic approach. Excli J. 2018;17 doi: 10.17179/excli2018-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miavaghi M.B., Pourakbar L. Phytosynthesis of Silver Nanoparticles by Medicinal Plant Malva neglecta. QOM Univ. Med. Sci. J. 2016;10(3) [Google Scholar]
- Mohammed A.E., Bin Baz F.F., Albrahim J.S. Calligonum comosum and Fusarium sp. extracts as bio-mediator in silver nanoparticles formation: characterization, antioxidant and antibacterial capability. 3 Biotech. 2018;8(1) doi: 10.1007/s13205-017-1046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D.H., Lee J.S., Park K.D., Ching Y.C., Nguyen X.T., Phan V.H.G., Thi T.T.H. Green silver nanoparticles formed by Phyllanthus urinaria, Pouzolzia zeylanica, and scoparia dulcis leaf extracts and the antifungal activity. Nanomaterials. 2020;10(3) doi: 10.3390/nano10030542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe-Smith, A., Collenette, S., 1987. An Illustrated Guide to the Flowers of Saudi Arabia. Kew Bull. doi: 10.2307/4109716.
- Roy A., Bulut O., Some S., Mandal A.K., Yilmaz M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019;9(5) doi: 10.1039/c8ra08982e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shale T.L., Stirk W.A., Van Staden J. Screening of medicinal plants used in Lesotho for anti-bacterial and anti-inflammatory activity. J. Ethnopharmacol. 1999;67(3) doi: 10.1016/S0378-8741(99)00035-5. [DOI] [PubMed] [Google Scholar]
- Shale T.L., Stirk W.A., Van Staden J. Variation in antibacterial and anti-inflammatory activity of different growth forms of Malva parviflora and evidence for synergism of the anti-inflammatory compounds. J. Ethnopharmacol. 2005;96(1–2) doi: 10.1016/j.jep.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Shankar, S. S., Ahmad, A., Sastry, M., 2003. Geranium Leaf Assisted Biosynthesis of Silver Nanoparticles. Biotechnol. Prog. 19(6). doi: 10.1021/bp034070w. [DOI] [PubMed]
- Singh A. Ethnomedicinal, Antimicrobial and Pharmacological aspects of Malva parviflora Linn.: A review. J. Phytopharmacol. 2017 [Google Scholar]
- Singh G., Maurya S., deLampasona M.P., Catalan C.A.N. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem. Toxicol. 2007 doi: 10.1016/j.fct.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Wang X., Bunkers G.J., Walters M.R., Thoma R.S. Purification and characterization of three antifungal proteins from cheeseweed (Malva parviflora) Biochem. Biophys. Res. Commun. 2001;282(5) doi: 10.1006/bbrc.2001.4716. [DOI] [PubMed] [Google Scholar]
- Yasir M., Singh J., Tripathi M.K., Singh P., Shrivastava R. Green synthesis of silver nanoparticles using leaf extract of common arrowhead houseplant and its anticandidal activity. Pharmacognosy Magazine. 2017;13(52) doi: 10.4103/pm.pm_226_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayed M.F., Eisa W.H., Shabaka A.A. Malva parviflora extract assisted green synthesis of silver nanoparticles. Spectrochim. Acta - Part A: Mol. Biomol. Spectroscopy. 2012;98:423–428. doi: 10.1016/j.saa.2012.08.072. [DOI] [PubMed] [Google Scholar]
- Zhang X.F., Liu Z.G., Shen W., Gurunathan S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016;17(9) doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]