Abstract
The aim of this study was to assess Annona muricata L. fruit extracts as an alternative to synthetic fungicide against Alternaria alternata (Fries) Keissler, the causative agent of black spots of tomato fruit. Antifungal activities of A. muricata pulp and seed extracts were tested both in vitro and in vivo. The seed extracts were more potent at inhibiting A. alternata than the pulp extracts. The in vitro assay showed maximum inhibition of radial mycelial growth of A. alternata (90%) by methanol seed extracts, at the highest concentration of 6%. Similarly, the in vivo assay showed marked reduction in lesion diameter (2.1 mm) and consequent disease inhibition (84%) on the tomato fruit treated with methanol seed extracts. Scanning electron microscopy showed that A. muricata extracts significantly damaged the morphology of hyphae and conidial structures. The FT-IR spectrum obtained from methanol extracts showed bands representing important bioactive compounds that possess antifungal activity. Based on our findings, Annona muricata fruit extracts can be further explored as a potential, excellent alternative approach to control the postharvest Alternaria spots of tomato fruit.
Keywords: Annona muricata fruit, Alternaria black spot, Postharvest control, SEM
1. Introduction
Tomato (Solanum lycopersicum L.) is an important commercial crop cultivated and consumed worldwide, including in Saudi Arabia (Al-Harbi et al., 2017). It is estimated that, in 2018, Saudi Arabia (2018) produced about 312,343 tonnes of tomatoes on an area of 13,428 ha (FAOSTAT, 2020). Tomatoes are loaded with phytonutrients and are mostly consumed in their fresh (raw), processed or cooked form (Reimers and Keast, 2016). However, they are susceptible to attack by an array of fungal pathogens, Alternaria alternata being the most common. Alternaria alternata (Fries) Keissler is a necrotrophic latent fungus that causes black spots on the surface of ripening tomatoes, causing fruit deterioration (Encinas-Bausurto et al., 2017). Alternaria alternata enters the host tissue (tomato fruit) through wounds or natural openings (Pearson and Hall, 1975) during the harvest or preharvest period, remains quiescent for several days and then shows up as black spots causing Alternaria rot (Troncoso-Rojas and Tiznado-Hernández, 2014). These spots appear as sunken lesions and are mostly found near the blossom end or peduncle of the fruit, lead to fruit spoilage and limit marketability, besides causing huge postharvest losses (Prusky et al., 2013). Furthermore, Alternaria alternata produces mycotoxins, which have various detrimental effects on human and animal health and are also involved in the occurrence of mutations, chromosomal aberrations and DNA damage (Escrivá et al., 2017).
Alternaria black rot/spots of fruit and vegetables are primarily controlled by the extensive application of synthetic fungicides. The application of fungicides before and after harvest has proven to be very effective in controlling the growth and development of A. alternata (Troncoso-Rojas and Tiznado-Hernández, 2014). However, excessive use of fungicides not only pollutes the environment, but also results in fungicide-resistant pathogens and poses a serious threat to human and animal health (Baibakova et al., 2019).
Against this background, alternative strategies to curb the postharvest losses of fruit and vegetables by fungal pathogens are being extensively explored worldwide. Plant-derived natural products including extracts have been shown to be less toxic, economical and highly efficient for such strategies (Chen et al., 2019). Annona muricata L. (Am) is an exotic fruit, also called soursop or graviola. The bark, stem, leaves, fruits, peel and seeds of Am have substantial medicinal uses and have been used to treat various ailments in traditional medicine (Anaya Esparza and Montalvo-González, 2020).
Hence, the present research aimed to study the potential of Am fruit extracts in controlling the postharvest black rot on tomatoes, caused by A. alternata (in vivo and in vitro). To our knowledge, this is first report showing the in vivo control of Alternaria spots on tomatoes.
2. Material and methods
2.1. Plant material
The Annona muricata and tomatoes used in this study were purchased from a local supermarket in Riyadh, Kingdom of Saudi Arabia. Tomato fruit cv. Red Gold growing in Riyadh was used for all of the experiments.
2.2. Phytopathogen
A. alternata, the rot or blackspot fungi of tomato, was provided by the National Centre for Research, Agriculture and Livestock, Riyadh, Saudi Arabia. Active culture was obtained by aseptically transferring the fungi onto freshly prepared potato dextrose agar (PDA) (Sigma-Aldrich, St. Louis, MO, USA) and incubated at 25 °C for 7 days prior to the experiments. A pathogenicity test was also conducted to further characterise the disease and pathogen.
2.3. Conidial suspension of A. Alternata
Briefly, a 14-day-old A. alternata culture plate (PDA) was flooded with 15 ml of sterile distilled water (SDW). The surface of the colony was gently scraped, after which the mycelial debris was removed and filtered through three layers of sterile muslin cloth. The concentration of the spore suspension (1 × 106 spores/ ml) was adjusted using a haemocytometer (Hosseini et al., 2020).
2.4. Pathogenicity test
Green tomato fruit without any disease symptoms was washed with tap water and surface-sterilised for 2 min with 1% sodium hypochlorite solution (2 min), followed by rinsing three times with SDW. A sterilised needle was used to make a 2-mm-deep wound on the fruit surface. Each wound was inoculated with 10 μl of conidial suspension (106 conidia/ml). The inoculated tomato fruit was then placed in a plastic tray and incubated at 25 °C and 95% relative humidity for 7 days. A small portion of the lesions was aseptically transferred on PDA and incubated at 25 °C for 7 days. Colony characteristics and microscopic structures were observed to confirm and fulfil the Koch postulates. Control fruit was inoculated with SDW (10 μl). The tests were carried in triplicate (Zivkovic et al., 2010).
2.5. Preparation of plant extracts
Healthy Am fruit was purchased from a local market and washed under running tap water. The peel of the fruit was removed and the seeds were separated from the pulp. The fruit pulp and seeds were subjected to extraction. The seeds were cleaned and dried in the shade, while the pulp was lyophilised. Both were then ground into a fine powder separately with a mixer grinder and subjected to extraction with solvents. Methanol and aqueous extracts of the pulp and seeds were prepared as described by Rizwana et al. (2019). Briefly, 20 g of powdered pulp and seeds were soaked separately in methanol and distilled water and placed in a rotary shaker (Max Q-2000; Thermo Fisher Scientific) for 24 h. Then, the extracts were filtered and dried on a rotary evaporator and reconstituted with the extracting solvents to the desired concentrations (0.5%, 1%, 2%, 4% and 6% w/v). After reconstitution, the aqueous extracts were filtered with a 0.45 μm bacterial membrane Millipore filter (MF-MilliporeTM; Millipore, MA, USA).
2.6. Antifungal activity of Annona muricata extracts against Alternaria alternata in vitro
The inhibitory activity of Am (pulp and seed) extracts on the radial growth of A. alternata was evaluated by a poisoned food technique (Dwivedi and Sangeeta, 2015). A 500 μl extract was added to a sterile Petri dish, followed by sterilised molten agar (PDA; Sigma-Aldrich, St. Louis, MO, USA). The extract and molten agar were thoroughly mixed by gentle swirling of the plate and allowed to solidify. After that, a 6 mm agar plug removed from an A. alternata culture plate actively growing for 7 days was placed in the centre of the poisoned plate. The plate was incubated at 25 °C for 7 days. The diameter of the colony (radial growth) was measured after 7 days and percentage mycelial growth inhibition was calculated following the method of Thomidis and Filotheou (2016). Various concentrations of methanol and aqueous extracts (0.5%, 1%, 2%, 4% and 6% w/v) were screened against A. alternata in a similar manner. As a positive control, the extracting solvent was used, while the negative control involved the fungicides mancozeb and carbendazim (0.2% M + C). The assay was repeated twice and each treatment had three replicates.
Here, RC and RT denote the diameter of the mycelial growth (colony) on the control and treated Petri plates, respectively.
2.7. Efficacy of Am extracts at controlling the blackspots of A. Alternata on tomato fruit: In vivo assay
Briefly, fresh green tomato fruit, uniform in colour and size, was washed and surface-sterilised with 1% sodium hypochlorite solution (2 min), rinsed three times with SDW and left to air-dry (28 °C). An in vivo assay was performed via a slightly modified version of a previously described method (Rizwana, 2018). The disinfected tomatoes were wounded (wounds 2 mm wide and 2 mm deep) at three places with a sterile needle. Each wound was inoculated with a conidial suspension (106 conidia/ml), which was left in place for 30 min, and then inoculated with 20 μl of Am seed and pulp extracts individually. The inoculated fruit was left for
1 h and then placed in sterile trays, which were covered with sterile plastic wrap. The trays were incubated at 25 °C (95% relative humidity) for 7 days. The diameter of black spots/lesions was measured and percentage disease (lesion) inhibition was calculated using the formula presented below. Control fruit was inoculated with the conidial suspension of A. alternata without extracts. The assay was repeated three times, with each treatment including six tomatoes.
Here, D is the percentage disease inhibition, while L1 and L2 are the lesion diameters on control (with pathogen alone) and treated tomatoes (seed and pulp extracts and pathogen).
2.8. Scanning electron microscopy of in vitro and in vivo samples
The effect of Am extracts on the morphology of hyphal and conidial structures was observed under a scanning electron microscope (model-JSM-6060LV; JEOL, Tokyo, Japan) at the Central Laboratory, King Saud University (female section). The culture plates of A. alternata (treated with extracts) and control (not treated with extracts) were selected for observation under a scanning electron microscope. Briefly, a 6 mm disc was removed under aseptic conditions from both treated and control culture plates. The specimens were then fixed in 2.5% buffered glutaraldehyde for 24 h at 4 °C, washed three times with 0.1 M phosphate-buffered saline and then refixed for 24 h in buffered 1% osmium tetroxide. The specimens were then subjected to dehydration with various dilutions of ethanol ranging from 60% to 100%. After dehydration, the specimens were air-dried, mounted on stubs and coated with a thin layer (20–30 mm) of gold using a sputter coater. The specimens were then observed individually by SEM and microphotographs of them were taken.
Similarly, small portions (3 mm) from the lesions or black spots on tomato fruit were taken and subjected to SEM as described above. Briefly, specimens were removed from the lesions of both the control (untreated) and the treated tomato fruit. Pulp and seed extracts of methanol were chosen as specimens for SEM as water extracts did not show any significant inhibition in the in vivo assay.
2.9. FT-IR analysis of methanol extract (seed and pulp)
The methanol extracts of Am were subjected to Fourier transform infrared spectroscopic analysis (FT-IR) using a device from Thermo Scientific, USA (Nicolet-6700). The scan range was 400–4000 cm−1.
2.10. Statistical analysis
The results of this study were analysed by XLSTAT software version 2020.1.1. The data shown in results are means of triplicate values and expressed as ± SD (standard deviation) for colony diameter. All the values (colony growth, lesion diameter, percentage inhibition of mycelial growth and disease inhibition) were determined for significant differences (P ≤ 0.05) by analysis of variance (ANOVA) and Tukey’s HSD test.
3. Results
3.1. Pathogenicity test
The tomato fruit artificially inoculated with a conidial suspension of A. alternata exhibited typical Alternaria black rot symptoms at the end of 7 days of incubation. After 3 days of inoculation, sunken areas were observed around the inoculation sites. On the 7th day, the sunken areas had enlarged into black lesions, with prominent mycelial structures visible on the infected spots. However, control fruit (inoculated with distilled water) did not exhibit any lesions. The fungus was reisolated from the lesions of the inoculated tomato fruit, thus fulfilling the Koch postulates. The appearance of the lesion, in terms of the morphology of colony, hyphae and conidia, was identical to that of the inoculated isolates and as described earlier (Simmons, 1992). Thus, the pathogen was confirmed to be A. alternata and the disease as Alternaria rot or black spots of Alternaria.
3.2. In vitro growth inhibition of A. Alternata with Am extracts
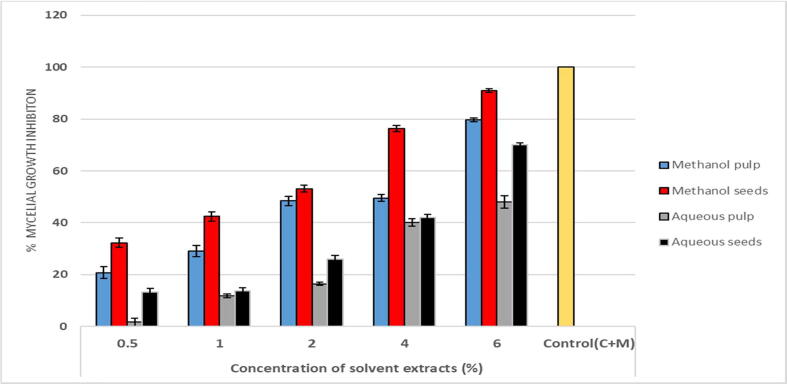
In the present study, both methanol and aqueous extracts of Am showed significant radial mycelial growth inhibition (P < 0.05) in a dose-dependent manner (Table 1). As depicted in Fig. 1, methanol seed and pulp extracts at 6% concentration showed maximum mycelial growth inhibition (90.93% and 79.56%), compared with aqueous extracts (70% and 47.9%; Fig. 1). It was observed that the methanol seed extracts caused marked reduction in colony diameter at 4% concentration and the growth was minimal at 6% concentration (Table 1). Similarly, mycelia treated with aqueous extracts (pulp and seeds) exhibited clear changes in hyphal growth; the colony had a woolly appearance compared with the control. Thus, the findings in this study showed that, upon comparing the extracts, methanol extracts were more effective at inhibiting A. alternata than aqueous extracts. Likewise, seed extracts were more potent in their antifungal activity than pulp extracts.
Table 1.
The Effect of different concentrations of Annona muricata extracts on the radial mycelial growth of Alternaria alternata. The value represents the mean of the 3 replicates ± the standard deviation. Significant difference in means (P ≤ 0.05) were determined by analysis of variance-ANOVA and Turkey HSD test.
| Solvent extracts of Annona muricata | ||||
|---|---|---|---|---|
|
Radial mycelial growth (cm) |
||||
| Concentrations (%) | Me-Pulp | Me-Seeds | Aq-Pulp | Aq-Seeds |
| 0.5 | 6.7 ± 0.2 | 5.73 ± 0.15 | 8.33 ± 0.15 | 7.33 ± 0.11 |
| 1 | 6 ± 0.17 | 4.86 ± 0.15 | 7.46 ± 0.05 | 7.3 ± 0.10 |
| 2 | 4.36 ± 0.15 | 3.96 ± 0.11 | 7.06 ± 0.05 | 6.26 ± 0.11 |
| 4 | 4.33 ± 0.11 | 2 ± 0.1 | 5.06 ± 0.11 | 4.9 ± 0.1 |
| 6 | 1.73 ± 0.05 | 0.766 ± 0.05 | 4.4 ± 0.2 | 2.53 ± 0.05 |
| Control (WE) | 8.47 ± 0.05 | 8.47 ± 0.05 | 8.47 ± 0.05 | 8.47 ± 0.05 |
| Control (C + M) | 0 | 0 | 0 | 0 |
Me-methanol extracts, Aq-aqueous extracts, WE- without extracts, C + M- carbendazim and mancozeb.
Fig. 1.
Inhibitory activity of various Annona muricata extracts at different concentrations on the percentage mycelial growth inhibition of Alternaria alternata (F) Keissler (in vitro).C + M = carbendazim and mancozeb. The value represents the mean of the 3 replicates ± the standard deviation. Significant difference in means (P ≤ 0.05) were determined by analysis of variance-ANOVA and Turkey HSD test.
3.3. Efficacy of Am extracts in controlling the lesion diameter and disease severity caused by A. Alternata (in vivo assay)
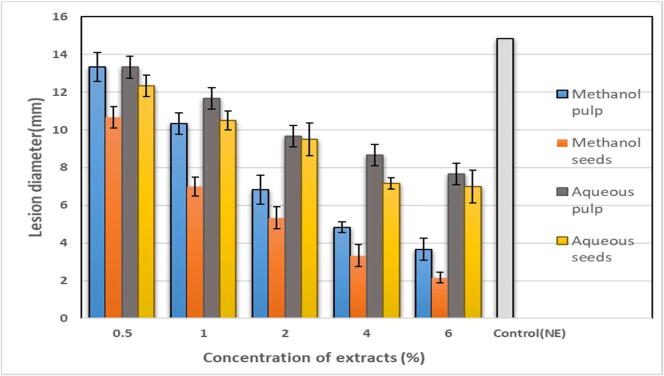
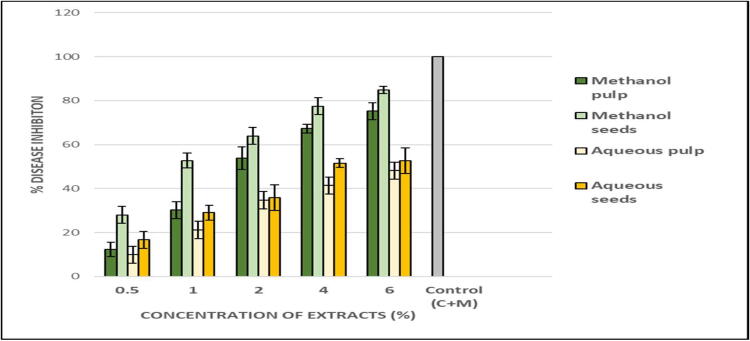
The potential of Am extracts to control the black spots on tomato fruit in vivo was assayed by assessing the reduction in decay area represented by lesion diameter in comparison to the control. Consistent with our in vitro findings, methanol seed extracts at a concentration of 6% demonstrated potent inhibitory activity against Alternaria black spots, as there was a marked reduction in the lesion diameter (2.1 mm) relative to that in the control (14.83 mm), constituting disease inhibition of 84% (Fig. 2, Fig. 3, Fig. 4). Similarly, methanol pulp extracts at 6% caused 75% disease inhibition. Conversely, aqueous extracts of both pulp and seed achieved moderate antifungal activity in the in vivo assay, with 52% and 48% disease inhibition, respectively (Fig. 2, Fig. 3, Fig. 4).
Fig. 2.
The effect of various concentrations of Annona muricata extracts on the lesion diameter on tomato fruit inoculated with Alternaria alternata. All values presented are means of three independent experimental replicates. Significant difference in means (P ≤ 0.05) were determined by analysis of variance ANOVA and Turkey HSD test.
Fig. 3.
The effect of various extracts of Annona muricata extracts on disease inhibition (black spots) on tomato fruit (in vivo assay). All values presented in standard error bars are means of three experimental replicates. Significant difference in means (P ≤ 0.05) were determined by analysis of variance ANOVA and Turkey HSD test.
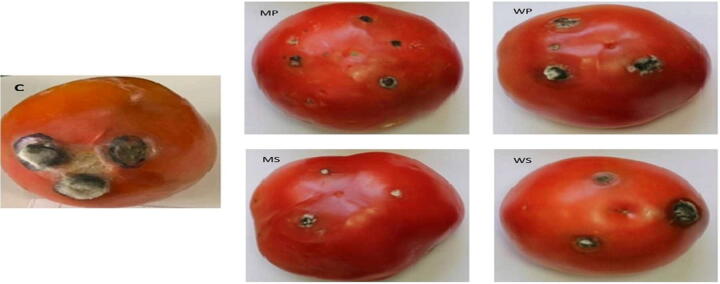
Fig. 4.
In vivo assay: tomato fruit treated with methanol and aqueous extracts of A.muricata fruit extracts (6%). C: control. Tomatoes treated with extracts (MP, MS, WP, WS): MP: methanol pulp extracts, MS: methanol seed extracts, WP: aqueous pulp extracts, WS: aqueous seed extracts.
3.4. Effect of Am extracts on the morphology of hyphae and conidia (in vivo and in vitro)
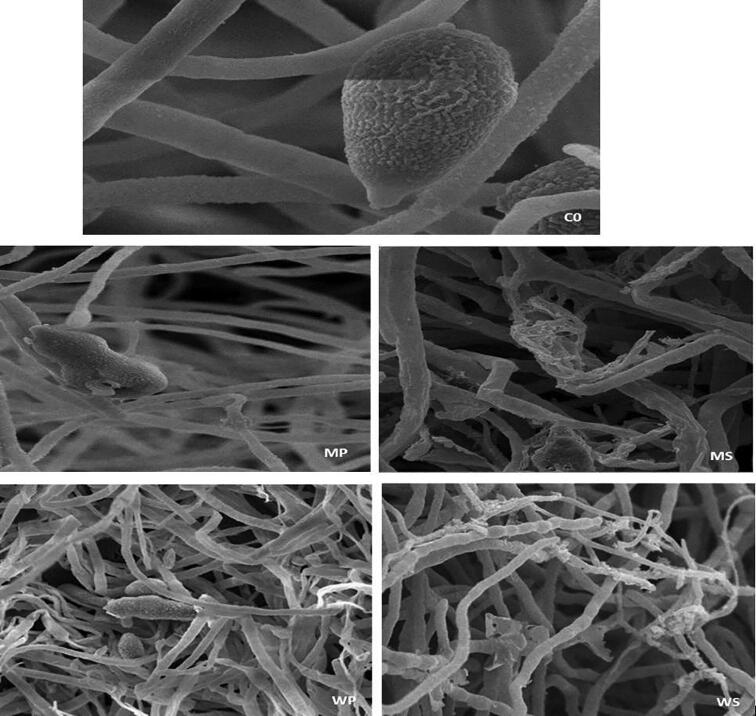
From the in vitro antifungal assay, A. alternata samples at maximum inhibitory concentrations were selected for scanning electron microscopy. The extracts had various damaging effects on the morphology of the mycelial and conidial structures, compared with the findings in the control (Fig. 5). The sample from the control plate showed intact hyphae with smooth margins. The tubular physical appearance of the hyphae was clearly demarcated, conidia were muriform in shape with a small beak and the verrucose surface of conidia was very clear (Fig. 5–Co). In contrast, the samples treated with methanol pulp extracts exhibited shrivelled and slender hyphae, while hyphal peeling was also seen. Conidia were severely damaged; ruptured conidia with leaked contents were captured in microphotographs (Fig. 5-MP). Similarly, the methanol seed extracts of Am were quite potent at inducing severe morphological alterations in hyphae. The hyphae were damaged, peeled, rough, and undulated. Some collapsed hyphae were also observed. An absence of conidia was quite evident, indicating that the seed extracts completely inhibited the formation of conidia (Fig. 5-MS). The SEM findings corresponded to the in vitro studies.
Fig. 5.
Scanning electron micrographs of A. alternata treated with methanol and aqueous extracts of A. muricata at 6% concentration (in vitro). MP and MS: methanol pulp and seed extract, the microphotograph shows ruptured conidia, shrivelled and damaged mycelium. WP and WS: aqueous pulp and seed extracts, deformed conidia and mycelium, absence of conidia in WP. Co-untreated (control): Well defined morphology of mycelium and conidia.
Similarly, the aqueous extracts of pulp and seeds had destructive effects on the fungal morphology. The hyphae exhibited peeling and some slender, distorted and shrivelled hyphae were also observed (Fig. 5). Distorted, malformed and undeveloped conidia were observed in samples treated with aqueous pulp extracts (Fig. 5-WP), while the aqueous seed extracts did not show the presence of conidia, which is evident in the microphotographs (Fig. 5- WS).
Methanol extracts exhibited potent in vivo inhibitory activity, as there was a marked reduction in lesion diameter of black spots in comparison to the findings in the control. Hence, wounded portions of tomato fruit treated with methanol extracts were chosen for SEM analysis. Samples from wounded lesions treated with methanol extracts and left untreated as a control were observed by SEM. The microphotographs of lesions from control samples showed well-defined conidial and hyphal morphology. The hyphae were smooth and tubular, while the conidia had a distinct and intact shape with a verrucose surface without any perforations (Fig. 6- Co). Meanwhile, the samples treated with methanol pulp extracts showed broken, clustered and collapsed hyphae. Very few conidia were observed, while those present had lost their shape (Fig. 6-MP). In accordance with the in vivo findings, the microphotographs of lesion sections treated with methanol seed extracts clearly showed the absence of conidia. Mycelia were shrivelled, wrinkled and sparsely distributed (Fig. 6-MS).
Fig. 6.
Scanning electron micrographs of tissue from tomato wounds inoculated with methanol pulp and seed extracts- (MP, MS) of A. muricata at 6% on the 7th day (in vivo assay). Lesion samples treated with methanol pulp (a, b) and seed extracts (c, d). Co: untreated (control). Microphotographs (MP, MS, Co) shows broken, collapsed mycelium and deformed conidia (MP), shrivelled mycelium and absence of conidia (MS), well defined morphology of mycelium and conidia (Co) .
3.5. FT-IR of methanol extract (seed and pulp)
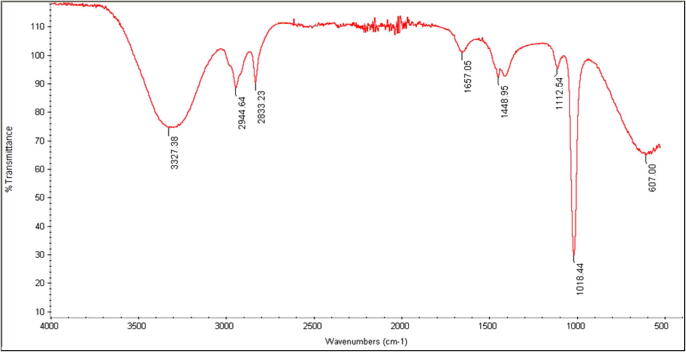
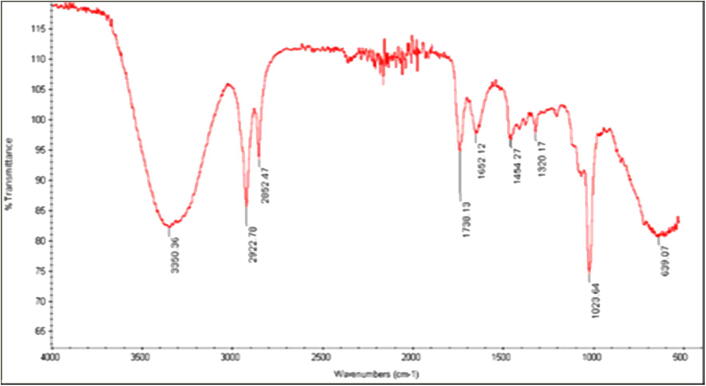
The IR spectrum of methanol extracts (seed and pulp) indicates some prominent peaks of important functional groups that possess antifungal properties (Fig. 7, Fig. 8). Both the seed and the pulp extracts showed broad peaks at 3327 cm −1 and 3350 cm −1, denoting the presence of alcohols such as phenols and amines. Similarly, the peaks at 2944 cm−1, 2922 cm−1, 2833 cm−1 and 2852 cm−1 are due to stretching bands of C–H, which arise from carbohydrates, lipids, amides and proteins. The peaks at 1657 cm−1 and 1652 cm−1 found in pulp and seed extract arose from C = C vibrational stretching of aromatic compounds or terpenes. Peaks at 1448 cm−1 and 1454 cm−1 denote the presence of alkanes or carbonyl in acids. A peak at 1112 and sharp peaks at 1018 cm−1 and 1023 cm−1 are due to the C–O stretching region and stretching vibrations of C–O–C arising from aldehydes and ketones. Seed extracts showed two peaks in the IR spectrum, which were not present in the pulp extracts (1320 and 1738 cm−1). The peak at 1320 cm−1 was related to aromatic compounds, while the sharp peak at 1738 cm−1 corresponded to carbonyl and phenols groups (Table 2).
Fig. 7.
IR spectrum of methanol pulp extract of Annona muricata.
Fig. 8.
IR spectrum of methanol seed extract of Annona muricata.
Table 2.
FT-IR spectrum of the methanol extracts of Annona muricata pulp and seed extracts.
| S.no |
Peak values (cm−1) |
Functional groups | |
|---|---|---|---|
| Pulp extracts | Seed extracts | ||
| 1 | 3327 | 3350 | Strong and broad band due to OH-stretching vibrations of hydrogen-bonded hydroxyl groups or bonding in N–H (s) |
| 2 | 2944 | 2922 | The two peaks correspond to symmetric and asymmetric stretching vibrations due to C–H (CH3 and CH2) |
| 3 | 2833 | 2852 | |
| 4 | – | 1738 | C = O stretch |
| 5 | 1657 | 1652 | C = C aromatic skeletal stretching |
| 6 | 1448 | 1454 | C–H bending (alkanes) |
| 7 | – | 1320 | CH bending |
| 8 | 1112 | – | C–O–H vibrational stretch of aliphatic compounds |
| 9 | 1018 | 1023 | Bands due to C = O stretch |
| 10 | 607 | 639 | CH bending vibrations |
3.6. Discussion
Black spots of tomato fruit are caused by the dematiaceous fungus A. alternata. Alternaria alternata is an important pathogen that causes substantial postharvest losses of tomato fruit. The objective of this study was to determine the in vitro and in vivo antifungal activities of Am extracts against A. alternata. Morphological changes induced by Am extracts on A. alternata were observed using scanning electron microscopy. Functional groups of phytocomponents present in the extracts were identified using FT-IR analysis. In the present study seed extract exhibited strong inhibition of A. alternata. In agreement with our findings, Am leaf extracts were previously reported to cause significant inhibition of Aspergillus niger, A. fumigatus, Mucor sp. and Candida albicans (Muthu and Durairaj, 2015). León-Fernández et al. (2019) reported significant antimicrobial activity of pulp extracts against an array of microorganisms, they reported 38% and 59% growth inhibition of Rhizopus stolonifer and Colletotrichum gloeosporioides, respectively.
Plants are an abundant source of various bioactive compounds, many of which are secondary metabolites that serve as chemical signals and confer resistance to numerous fungal phytopathogens (Ramírez-Gómez et al., 2019). The variable antifungal activity shown by methanol and aqueous extracts in the present study could be attributed to the solubility properties of certain solvents, as some solvents are more efficient in extracting important bioactive compounds than others, methanol being one of them (Bakari et al., 2017). Methanol extracts have the tendency to extract several polar compounds, including flavonoids, tannins, alkaloids, phenols and terpenoids (Kalidindi et al., 2015). Similar findings were reported earlier, showing low activity of aqueous extracts in comparison to methanol extracts (Solomon-Wisdom et al., 2014).
Little information is available on the in vitro antifungal activity of Am extracts against A. alternata. Moreover, to our knowledge, this the first report showing the in vivo control of Alternaria black rot by Am extracts. Consistent with our findings, in a study, bark, leaves, stalks and flowers of different plant species from Brazil were tested (in vitro and in vivo) against A. alternata (Fr) Keissl f. sp. Citri. Among numerous screened plants, five were tested in vivo on Murcott tangor fruit. Anadenanthera colubrina bark extract was the most effective and the disease inhibition (represented by decay diameter) was similar to that of the screened commercial fungicides (Carvalho et al., 2011). In addition, a recent study reported significant inhibition of A. alternata lesions on cherry tomatoes when treated with chitosan alone or in combination with methyl jasmonate (Chen et al., 2014).
Tomato fruit is prone to spoilage by fungi due to its high water content and soft endocarp (Encinas-Basurto et al., 2017). The characteristic black lesions or spots of A. alternata develop during cold storage or transport, make the fruit unfit for consumption and affect its marketability (Asai and Shirasu, 2015). Huge postharvest losses of fruit and other food commodities due to fungal pathogens have prompted researchers to explore various natural products as substitutes for synthetic fungicides. The application of natural products (plant extracts), salts and essential oils as edible coatings on fruit surfaces has shown promising results in combating postharvest fungal attack and also in managing disease (Qadri et al., 2020). Fungicides, such as carbendazim, benomyl, mancozeb and thiabendazole, are used alone or in combination to control postharvest pathogens. Generally, this involves dipping the fruit in or spraying the fruit with fungicide prior to packaging (Fu et al., 2017). As mentioned above, although fungicides are highly efficacious in controlling some postharvest pathogens, their frequent use has resulted in the emergence of fungicide-resistant strains. Moreover, the negative impact of fungicide residues on food commodities and the environment has raised serious concerns (El Ghaouth et al., 2003). Hence, A. muricata extracts could be an excellent alternative approach to control the postharvest Alternaria spots of tomato.
A recent finding shows degenerating effects on fungal morphology of various fungi when treated with extracts. Wrinkled, shrivelled, deformed and ruptured hyphae and conidia were observed when phytopathogenic fungi like A. alternata, Macrophoma phaseoloni and Fusarium species were treated with plant extracts (Otibi and Rizwana, 2019). According to previous reports, bioactive compounds such as flavonoids, phenols, polyphenols, ketones and aromatic compounds are lipophilic in nature and hence can easily diffuse through the cell membrane, disturbing cellular stability (Yusoff et al., 2020). These compounds interfere with the ABC transport system; inhibit the synthesis of ergosterol, ATP and aminoacyl tRNA synthetase leading to cellular dysfunction and impair sterol metabolism, which eventually causes leakage from fungal cells, plasmolysis and cell lysis (Rosen and Stein Gold, 2016, Yusoff et al., 2020). Hence, the morphological aberrations of hyphae and conidia, as well as the marked reduction in spore formation observed in our study, could be due to the abundance of several bioactive compounds in Am extracts, leading to the anomalies as observed by SEM.
The peaks and vibrational bands shown in the IR spectra of extracts in this study have also been reported earlier and are associated with phenols, polyphenols, aromatic compounds like flavonoids and terpenoids, ketones, carboxylic acids and aldehydes (Massoud and Nafiseh, 2018). Similar peaks from Am extracts have been reported earlier (Folorunso et al., 2019). Extensive phytochemical analysis of different parts of Am including roots, stem, leaves and fruit have shown the presence of several bioactive compounds such as alkaloids, phenols, terpenoids, flavonoids and tannins. (Solomon Wisdom et al., 2014; Anaya Esparza and Montalvo-González, 2020)
Yet, in another recent study, phytochemical analysis of various solvent extracts of A. muricata fruit showed the presence of some important phytochemicals. Seed extract followed by leaf extract showed considerably higher amounts of phenols, flavonoids and, phytosterols in comparison to pulp and peel extracts. Besides, the seed extracts exhibited the highest CUPRAC, FRAP, TEAC, and DPPH scavenging activity, followed by leaf and peel extracts. Additionally, methanol seed extracts possessed the highest total phenolic content compared to all the other solvents extracts used in the study (Orak et al, 2019). Similarly, in the present study, the IR spectrum of seed extract showed two additional peaks (1320 cm−1 and 1738 cm−1) which were not found in the IR spectrum of pulp extract. These peaks indicate the presence of phenols, terpenoids, and aromatic compounds. Hence, the potent antifungal activity (in vitro and in vivo) demonstrated by seed extracts could be due to higher amounts of phenols, terpenoids, and flavonoids when compared to pulp extracts.
Therefore, the significant fungistatic activity shown by Am extracts in our study could be attributed to the presence of several bioactive compounds, such as phenols, polyphenols, flavonoids, terpenoids, alkaloids and saponins. All of these compounds have shown wide-spectrum antifungal activity in previous studies (Bautista-Baños et al., 2002). The FT-IR analysis of Am extracts in this study also identified many of the above-mentioned compounds. Based on our in vitro and in vivo findings, Am extracts could serve as an excellent alternative for controlling blackspot of tomato. To the best of our knowledge, no reports showing the in vivo control of A. alternata on tomato fruit have previously been published.
4. Conclusions
Am extracts in the present study efficiently reduced the mycelial growth of blackspot fungi, A. alternata, in vitro. Moreover, the extracts, especially seed extracts, significantly reduced the disease incidence in an in vivo assay. Thus, our results indicate that Am extracts have the potential to be used in postharvest control of tomato rot. However, further research is needed to understand the mode of action, toxicity, and any adverse effects on fruit quality.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
This research project was supported by a grant from the Research Support Project (number RSP-2020/229), King Saud University, Riyadh, Saudi Arabia.
Acknowledgement
The authors would like to extend their sincere appreciation to the Research Support Project (number: RSP-2020/229), King Saud University, Riyadh, Saudi Arabia.
Author contributions
Conceptualisation and design of the study: HR. Experimental work: SAs, AS, RZ, and HD. Analysis of the results: SS and NB. Manuscript preparation: HR, SAs, and NB. Data collection, compilation: SAs, AS, RZ, and HD. Statistical analysis: HR. Writing of the manuscript: HR and SAs. Manuscript preparation, editing, and reviewing of the intellectual content: SAs, NB, and SS. Corresponding author and guarantor: HR.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Harbi A., Hejazi A., Al-Omran A. Responses of grafted tomato (Solanum lycopersiocon L.) to abiotic stresses in Saudi Arabia. Saudi J. Biol. Sci. 2017;24:1274–1280. doi: 10.1016/j.sjbs.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaya Esparza L.M., Montalvo-González E. Bioactive compounds of soursop (Annona muricata L.) fruit. In: Murthy H., Bapat V., editors. Bioactive Compounds in Underutilized Fruits and Nuts. Reference Series in Phytochemistry. Springer; Cham: 2020. [DOI] [Google Scholar]
- Asai S., Shirasu K. Plant cells under siege: Plant immune system versus pathogen effectors. Curr. Opin. Plant Biol. 2015;28:1–8. doi: 10.1016/j.pbi.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Baibakova E.V., Nefedjeva E.E., Suska-Malawska M., Wilk M., Sevriukova G.A., Zheltobriukhov V.F. Modern fungicides: Mechanisms of action, fungal resistance and phytotoxic effects. Annu. Res. Rev. Biol. 2019;32:1–16. doi: 10.9734/ARRB/2019/v32i330083. [DOI] [Google Scholar]
- Bakari S., Daoud A., Felhi S., Smaoui S., Gharsallah N., Kadri A. Proximate analysis, mineral composition, phytochemical contents, antioxidant and antimicrobial activities and GC-MS investigation of various solvent extracts of cactus cladode. Food Sci. Technol. 2017;37:286–293. [Google Scholar]
- Bautista-Baños S., Barrera-Necha L.L., Bravo-Luna L., Bermúdez-Torres K. Antifungal activity of leaf and stem extracts from various plant species on the incidence of Colletotrichum gloeosporioides of papaya and mango fruit after storage. Rev. Mex. Fitopatol. 2002;20:8–12. [Google Scholar]
- Carvalho D.D., Alves E., Barbosa Camargos R., Ferreira Oliveira D., Soares Scolforo J.R., de Carvalho D.A., Sâmia Batista T.R. Oct–Dec. Plant extracts to control Alternaria alternata in Murcott tangor fruits. Rev. Iberoam. Micol. 2011;28:173–178. doi: 10.1016/j.riam.2011.05.001. Epub 2011 May 13 PMID: 21635961. [DOI] [PubMed] [Google Scholar]
- Chen J., Shen Y., Chen C., Wan C. Inhibition of key citrus postharvest fungal strains by plant extracts in vitro and in vivo: A review. Plants. 2019;8:26. doi: 10.3390/plants8020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Zou X., Liu Q., Wang F., Feng W., Wan N. Combination effect of chitosan and methyl jasmonate on controlling Alternaria alternata and enhancing of cherry tomato fruits defense mechanisms. Crop Prot. 2014;56:31–36. doi: 10.1016/j.cropro.2013.10.007. [DOI] [Google Scholar]
- Dwivedi S.K., Sangeeta Efficacy of some medicinal plant extract against Fusarium oxysporum ssp. cicero causing chickpea wilt. Asian J. Crop Sci. 2015;7:138–146. [Google Scholar]
- Encinas-Basurto, D., Valenzuela-Quintanar, M.I., Sánchez-Estrada, A., Tiznado-Hernández, M.E., Rodríguez-Félix, M.E., A., Troncoso-Rojas, R. 2017. Alterations in volatile metabolites profile of fresh tomatoes in response to Alternaria alternata (Fr.) Keissl. 1912 infection. Chil. J. Agric. Res. 77:194–201. 10.4067/S0718-58392017000300194.
- El Ghaouth A., Wilson C.L., Wisniewski M. Control of postharvest decay of apple fruit with Candida saitoana and induction of defense responses. Phytopathology. 2003;93:344–348. doi: 10.1094/PHYTO.2003.93.3.344. [DOI] [PubMed] [Google Scholar]
- Escrivá, L., Oueslati, S., Font, G., Manyes, L. 2017. “Alternaria mycotoxins in food and feed: An overview.” J. Food Qual. 2017, 20 pages:article ID 1569748. https://doi.org/10.1155/2017/1569748.
- FAOSTAT 2020. Food and Agriculture Organization of the United Nations. Database. Available at http://www.fao.org/faostat/en/#data/QC/.
- Fu W., Tian G., Pei Q., Ge X., Tian P. Evaluation of berberine as a natural compound to inhibit peach brown rot pathogen Monilinia fructicola. Crop Prot. 2017;91:20–26. [Google Scholar]
- Folorunso A., Akintelu S., Oyebamiji A.K., Ajayi S., Abiola B., Abdusalam I., Morakinyo A. Biosynthesis, characterization and antimicrobial activity of gold nanoparticles from leaf extracts of Annona muricata. J. Nanostruct. Chem. 2019;9:111–117. [Google Scholar]
- Hosseini S., Amini J., Saba M.K., Karimi K., Pertot I. Preharvest and postharvest application of garlic and rosemary essential oils for controlling anthracnose and quality assessment of strawberry fruit during cold storage. Front. Microbiol. 2020;11:1855. doi: 10.3389/fmicb.2020.01855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalidindi N., Thimmaiah N.V., Jagadeesh N.V., Nandeep R., Swetha S., Kalidindi B. Antifungal and antioxidant activities of organic and aqueous extracts of Annona squamosa Linn. Leaves. J. Food Drug Anal. 2015;23:795–802. doi: 10.1016/j.jfda.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León-Fernández A.E., Martínez-Cárdenas L., Zepeda-Vallejo L.G., Arteaga-Garibay R.I., Gutiérrez-Martínez P., Montalvo-González E. Antibacterial, antifungal, antioxidant, and toxic effect of fractioned extracts from soursop pulp. Rev. Bio Cienc. 2019;6:1–17. [Google Scholar]
- Massoud K., Nafiseh J. Biosynthesis of gold nanoparticles using aqueous extract of stem of Periploca aphylla. Plant Nanostruct. 2018;8:152–158. [Google Scholar]
- Muthu S., Durairaj B. Evaluation of antimicrobial and antifungal properties of Annona muricata Leaf extracts. Br. J. Med. J. Health Res. 2015;2:1–8. [Google Scholar]
- Orak H.H., Bahrisefit I.S., Sabudak T. Antioxidant Activity of Extracts of Soursop (Annona muricata L.) Leaves, Fruit Pulps, Peels, and Seeds. Pol. J. Food Nutr. Sci. 2019;69:359–366. [Google Scholar]
- Otibi F.A., Rizwana H. Chemical composition, FTIR studies, morphological alterations, and antifungal activity of leaf extracts of Artemisia sieberi from Saudi Arabia. Int. J. Agric. Biol. 2019;21:1241–1248. [Google Scholar]
- Pearson R.C., Hall D.H. Factors affecting the occurrence and severity of black mold of ripe tomato fruit caused by Alternaria alternata. Phytopathology. 1975;57:1352–1359. [Google Scholar]
- Prusky D., Alkan N., Mengiste T., Fluhr R. Quiescent and necrotrophic lifestyle choice during postharvest disease development. Phytopathology. 2013;51:155–176. [Google Scholar]
- Qadri, R., Khan, I., Yang, Y., Ejaz, S., Akram, M.T., Khan, M.A. 2020. Conventional and modern technologies for the management of post-harvest diseases. In: Ul Haq, I., Ijaz, S. (Eds.) Plant Disease Management Strategies for Sustainable Agriculture Through Traditional and Modern Approaches. Sustainability in Plant and Crop Protection, vol 13. Springer, Cham. https://doi.org/10.1007/978-3-030-35955-3_7.
- Ramírez-Gómez, X.S., Jiménez-García, S.N. Vicente Beltrán Campos, ma. Lourdes García Campos, 2019. Chapter: Plant Metabolites in Plant Defense Against Pathogens in Plant Diseases-Current Threats and Management Trends pp 1-19.
- Reimers K.J., Keast D.R. Tomato consumption in the United States and its relationship to the US Department of Agriculture food pattern. Nutr. Today. 2016;51:198–205. [Google Scholar]
- Rizwana H. Postharvest control of anthracnose lesions and its causative agent, Colletotrichum musae by some oils. Cell. Mol. Biol. Noisy-Le-Grand. 2018;64:52–58. doi: 10.14715/cmb/2018.64.4.9. [DOI] [PubMed] [Google Scholar]
- Rizwana H., Al Otibi F., Al-Malki N. Chemical composition, FTIR Studies and antibacterial activity of Passiflora edulis f. Edulis (Fruit) J. Pure Appl. Microbiol. 2019;13:2489–2498. doi: 10.22207/JPAM.13.4.64. [DOI] [Google Scholar]
- Rosen T., Stein Gold L.F. Antifungal drugs for onychomycosis: Efficacy, safety, and mechanisms of action. Semin. Cutan. Med. Surg. 2016;35(Supplement 3):S51–S55. doi: 10.12788/j.sder.2016.009. [DOI] [PubMed] [Google Scholar]
- Simmons, E.G. 1992. Alternaria taxonomy: Current status, viewpoint, challenge. In: Chelkowski J., Visconti, A. (Eds.): Alternaria - Biology, Plant Diseases and Metabolites. Topics in Secondary Metabolism Vol 3. Elsevier Science Publishers, Amsterdam, The Netherlands.
- Solomon-Wisdom G.O., Ugoh S.C., Mohammed B. Phytochemical screening and antimicrobial activities of Annona muricata (L.) leaf extract. Am. J. Biol Chem. Pharma Sci. 2014;2:1–7. [Google Scholar]
- Thomidis T., Filotheou A. Evaluation of five essential oils as biofungicideson the control of Pilidiella granati rot in pomegranate. Crops Prod. 2016;89:66–71. [Google Scholar]
- Troncoso-Rojas R., Tiznado-Hernández M.E. Alternaria alternata (black rot, black spot) In: Bautista-Baños S., editor. Postharvest Decay of Fruits and Vegetables: Control Strategies. Elsevier; Atlanta, GA, USA: 2014. pp. 147–188. [Google Scholar]
- Yusoff S.F., Haron F.F., Tengku Muda Mohamed M., Asib N., Sakimin S.Z., Abu Kassim F., Ismail S.I. Antifungal Activity and Phytochemical Screening of Vernonia amygdalina Extract against Botrytis cinerea Causing Gray Mold Disease on Tomato Fruits. Biology. 2020;9:286. doi: 10.3390/biology9090286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivkovic S., Stojanovic S., Ivanovic Z., Trkulja N., Dolovac N., Aleksic G., Balaz J. Pestic. Phytomed. (Belgr.) 2010;25:231–239. [Google Scholar]