Abstract
Background and aims:
The application of prone positioning with acute hypoxemic respiratory failure (AHRF) or acute respiratory distress syndrome (ARDS) in non-intubation patients is increasing gradually, applying prone positioning for more high-flow nasal oxygen therapy (HFNC) and non-invasive ventilation (NIV) patients. This meta-analysis evaluates the efficacy and tolerance of prone positioning combined with non-invasive respiratory support in patients with AHRF or ARDS.
Methods:
We searched randomized controlled trials (RCTs) (prospective or retrospective cohort studies, RCTs and case series) published in PubMed, EMBASE and the Cochrane Central Register of Controlled Trials from 1 January 2000 to 1 July 2020. We included studies that compared prone and supine positioning with non-invasive respiratory support in awake patients with AHRF or ARDS. The meta-analyses used random effects models. The methodological quality of the RCTs was evaluated using the Newcastle–Ottawa quality assessment scale.
Results:
A total of 16 studies fulfilled selection criteria and included 243 patients. The aggregated intubation rate and mortality rate were 33% [95% confidence interval (CI): 0.26–0.42, I2 = 25%], 4% (95% CI: 0.01–0.07, I2 = 0%), respectively, and the intolerance rate was 7% (95% CI: 0.01–0.12, I2 = 5%). Prone positioning increased PaO2/FiO2 [mean difference (MD) = 47.89, 95% CI: 28.12–67.66; p < 0.00001, I2 = 67%] and SpO2 (MD = 4.58, 95% CI: 1.35–7.80, p = 0.005, I2 = 97%), whereas it reduced respiratory rate (MD = −5.01, 95% CI: −8.49 to −1.52, p = 0.005, I2 = 85%). Subgroup analyses demonstrated that the intubation rate of shorter duration prone (⩽5 h/day) and longer duration prone (>5 h/day) were 34% and 21%, respectively; and the mortality rate of shorter duration prone (⩽5 h/day) and longer duration prone (>5 h/day) were 6% and 0%, respectively. PaO2/FiO2 and SpO2 were significantly improved in COVID-19 patients and non-COVID-19 patients.
Conclusion:
Prone positioning could improve the oxygenation and reduce respiratory rate in both COVID-19 patients and non-COVID-19 patients with non-intubated AHRF or ARDS.
The reviews of this paper are available via the supplemental material section.
Keywords: acute hypoxemic respiratory failure, acute respiratory distress syndrome, meta-analysis, prone positioning
Introduction
Acute hypoxemic respiratory failure (AHRF) develops from numerous pulmonary and extrapulmonary diseases or disease processes and it is a common reason for admission to the intensive care unit (ICU). A large proportion of patients with AHRF meet the criteria for acute respiratory distress syndrome (ARDS).1–3 Non-invasive respiratory support delivered via face mask, high-flow nasal oxygen therapy (HFNC) and non-invasive ventilation (NIV) is usually the treatment to correct hypoxia in AHRF and ARDS.3 However, certain patients exhibit further progress and require invasive mechanical ventilation. Previous studies have indicated that the intubation rate of patients with AHRF is 42–58%.4,5 Invasive mechanical ventilation may lead to increased risk of ventilator associated pneumonia and utilize medical resources for a long time period, especially during the outbreak of COVID-19. The aggregated mortality rate of patients with AHRF and ARDS may reach 27–45%.6–11 The need for invasive mechanical ventilation may be associated with high mortality.12 Many factors are associated with high mortality, such as pneumothorax, as it is difficult to maintain the plateau pressure <30 cmH2O and the driving pressure <15 cmH2O. Therefore, it is necessary to actively seek other intervention measures to reduce the intubation rate and improve the prognosis of patients.
The prone positioning leads to alterations in the distribution of alveolar ventilation, improved matching of local ventilation and perfusion and reduction in regions of low ventilation/perfusion ratios through gravitational effects and reduction of ventilator-induced lung injury.13–16 Several studies have shown that the prone positioning can improve the gas exchange and progression in patients with AHRF or ARDS.17–20 All these studies included patients with AHRF or ARDS undergoing intubation for mechanical ventilation and most of them were under sedation. Valter et al.21 reported that four awake patients with hypoxemia were in the prone positioning without sedation and without intubation. All the patients were well tolerated with rapid improvement of PaO2. Previous studies demonstrated that the number of patients in the prone positioning combined with non-invasive respiratory support has gradually increased in recent years.22–36 Notably during the outbreak of COVID-19,22–33 it was reported that the prone positioning could improve oxygenation and reduce intubation rate in patients with AHRF or ARDS as well as delay or reduce the need to admit to the ICU.23 These studies suggested that the prone positioning may be used to avoid invasive mechanical ventilation and may be beneficial in several awake patients with AHRF or ARDS.
At present, the studies that have examined the prone positioning combined with non-invasive respiratory support in awake patients with AHRF or ARDS are mainly observational studies, cohort studies and case reports. Large sample-size randomized controlled trial (RCT) studies are deficient and the results are not completely consistent. The purpose of this meta-analysis was to analyze the efficacy and tolerance of prone positioning combined with non-invasive respiratory support in patients with AHRF or ARDS.
Methods
Search strategy
We searched prospective or retrospective cohort studies, RCTs and case series published in PubMed, EMBASE and the Cochrane Central Register of Controlled Trials from 1 January 2000 to 1 July 2020. The search strategy used was as follows: (respiratory distress syndrome OR ARDS) AND (Respiratory Insufficiency OR AHRF) AND (prone positioning OR prone positioning) AND (Non-invasive Ventilation OR NIV) AND (high-flow nasal cannula OR HFNC) AND Oxygen therapy. The language of publication was restricted to English. In addition, the reference lists of all primary studies and review articles were evaluated for additional relevant studies.
Study selection
The inclusion criteria were as follows: (i) cohort studies or case series; (ii) adult (⩾18 years old) patients with AHRF or ARDS and in waking state; (iii) prone positioning combined with non-invasive respiratory support (non-invasive mechanical ventilation, high flow nasal canula, venturi mask, conventional oxygen therapy); (iv) outcomes including at least one of the following measures: aggregated mortality rate, intubation rate, tolerability, prior to and following difference of arterial oxygen tension/fraction of inspired oxygen (PaO2/FiO2) ratios, peripheral oxygen saturation (SpO2) and respiratory rate.
The exclusion criteria were as follows: (i) patients who did not meet the screening criteria; (ii) studies that were not in English or commentaries, reviews, duplicate publications from the same study; (iii) data that could not be extracted by the statistical methods or non-targeted outcomes.
Data extraction and study quality
The Newcastle–Ottawa quality assessment scale (NOS) checklist (Supplementary material Figure S1 online) was used to assess the quality of eligible studies. By using this scale, each study was assessed on seven items and categorized into three groups as follows: selection, study design and outcomes. Stars were awarded for each quality item and the highest quality studies were awarded seven stars. A study was considered to be of good, normal and poor quality when it achieved 6–7, 3–5 and 0–2 stars, respectively.
Statistical analysis
The meta-analysis was performed using available data from the primary studies with the R software (R version 4.0.2; Comprehensive R Archive Network, CRAN 2020) and the Review Manager software (RevMan version 5.3; Nordic Cochrane Centre, Cochrane Collaboration, 2014). The mean difference (MD) was used to describe continuous data, while the risk ratios used were for dichotomous data. These data were assessed in the median-interquartile range and were transformed into standard mean difference format for further comparison.37 The inverse-variance method was used to pool the mean differences to yield an overall effect size with 95% confidence intervals (CIs).
The results were analyzed by the random-effects model and were presented in a forest plot. The I2 statistical index (ranges from 0% to 100%) was used to measure the heterogeneity among the studies in each analysis, with values of 25%, 50% and 75% corresponding to degrees of low, moderate and high heterogeneity, respectively. Publication bias was assessed with the Egger’s test and funnel plot. In addition, subgroup analysis was also performed to investigate different effects of prone positioning duration and etiology on treatment outcomes. A p-value of less than 0.05 (p < 0.05) was considered for significant differences.
Two investigators (Xu and Tan) independently extracted data and assessed the validity of the included studies. The definitive inclusion of the trials was made following review of the full text of the articles, including publication date, study type and design, oxygen therapy method, prone positioning duration and outcomes. Discrepancies were resolved by a consensus.
Results
Search results
A total of 275 relevant publications were obtained from the databases and a total of 16 articles21–36 which were published from 2003 to 2020 were included following further screening, with four of those being cohort studies and the other 12 case series. The main characteristics of all included articles are presented in Table 1. A total of 243 patients were included in the present meta-analysis and all of them were awake adult patients (57 ± 11 years old) with AHRF or ARDS, of which 195 patients (80%) were infected by COVID-19. The patients were admitted to the hospital and received different methods of non-invasive respiratory support, of which 32 patients (13.1%) were on NIV and 39 (16%) on HFNC (HFNC/venturi mask), 86 patients (35.4%) used nasal cannulas/facial masks, and the oxygen therapy methods for the remaining 86 patients (35.4%) were not clear. The process of searching and screening is described in the flow chart (Figure 1).
Table 1.
Characteristics of included studies.
| Reference | Study | Patients | Therapy | Outcomes | Modified NOS quality score | ||||
|---|---|---|---|---|---|---|---|---|---|
| Type | Cause | Age (mean ± SD) | No. of patients | PP duration (mean) | Oxygen therapy method | Intubation | Mortality | ||
| Bellone and Basile36 | Cohort | Non-COVID-19 | N/A | 3 | 6 h/day | HFNC | 0 | 0 | 3/7 |
| Caputo et al.32 | Case series | COVID-19 | 59 ± 5 | 50 | <1 h | Nasal cannulas or face masks | 18 | N/A | 5/7 |
| Coppo et al.25 | Cohort | COVID-19 | 57 ± 7 | 42 of 56 | 3 h/day | NIV, HFNC or face masks | 13 | 5 | 6/7 |
| Damarla et al.30 | Case series | COVID-19 | 56 ± 12 | 10 | 16 h/day | HFNC or nasal cannulas | 2 | 0 | 5/7 |
| Ding et al.23 | Cohort | Non-COVID-19 | 50 ± 10 | 20 | 4 h/day | HFNC or NIV | 9 | 1 | 6/7 |
| Elharrar et al.27 | Cohort | COVID-19 | 66 ± 10 | 24 | >3 h/day | HFNC or nasal cannulas | 5 | 0 | 5/7 |
| Huang et al.33 | Cohort | COVID-19 | 59 ± 3 | 3 | 8 h/day | HFNC and NIV | 1 | N/A | 3/7 |
| Ng et al.22 | Case series | COVID-19 | 66 | 1 of 10 | >5 h/day | Nasal cannulas or HFNC | 1 | 1 | 5/7 |
| Pérez-Nieto et al.35 | Case series | Non-COVID-19 | 36 ± 14 | 6 | 5 h/day | HFNC or NIV | 2 | 1 | 5/7 |
| Sartini et al.28 | Case series | COVID-19 | 59 ± 6 | 15 | 3 h/day | NIV | 1 | 1 | 4/7 |
| Sztajnbok et al.29 | Case series | COVID-19 | 40 ± 4 | 2 | 8 h/day | Face masks | 0 | 0 | 3/7 |
| Thompson et al.24 | Cohort | COVID-19 | N/A | 29 | 5 h/day | Nasal cannulas or face masks | 12 | 3 | 5/7 |
| Tu et al.26 | Case series | COVID-19 | 51 ± 11 | 9 | 4 h | HFNC | 2 | 0 | 6/7 |
| Valter et al.21 | Case series | Non-COVID-19 | 55 ± 27 | 4 | 3 h | Three NIV and one unknown | 0 | 1 | 4/7 |
| Scaravilli et al.34 | Case series | Non-COVID-19 | 58 ± 23 | 15 | 2 h | Nasal cannulas, HFNC or NIV | 2 | 3 | 5/7 |
| Xu et al.31 | Case series | COVID-19 | 50 ± 9 | 10 | >16 h/day | HFNC | 0 | 0 | 5/7 |
Mortality was defined as early all-cause mortality used for all studies where available. HFNC includes high flow nasal cannula and venturi mask. Non-COVID-19: acute hypoxemic respiratory failure or acute respiratory distress syndrome caused by drowning, sepsis, trauma and other diseases.
COVID-19, coronavirus disease 2019; HFNC, high flow nasal cannula; N/A, not applicable; NIV, non-invasive ventilation; NOS, Newcastle–Ottawa quality assessment scale; PP, prone positioning; SD, standard deviation.
Figure 1.
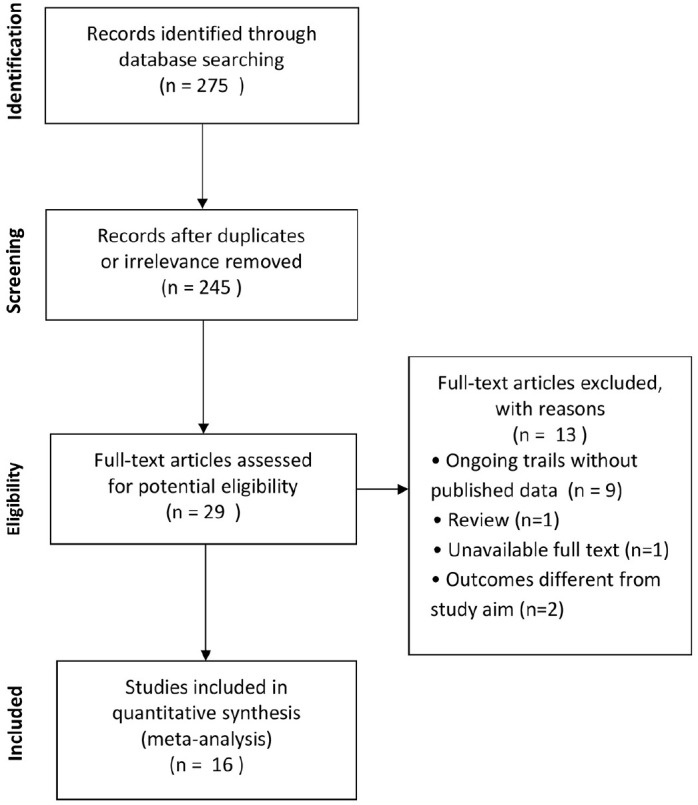
Selection of studies for the meta-analysis (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).
Literature quality and bias assessment
The quality of the included literature was assessed by a modified NOS checklist and the results are shown in Table 1. All articles were of medium quality (⩾3 stars) or above and three articles were considered as high-quality studies (⩾6 stars). The Egger’s test results (p < 0.001) and asymmetric funnel plot (Egger’s test funnel plots) suggested the presence of publication bias (Figure 2).
Figure 2.
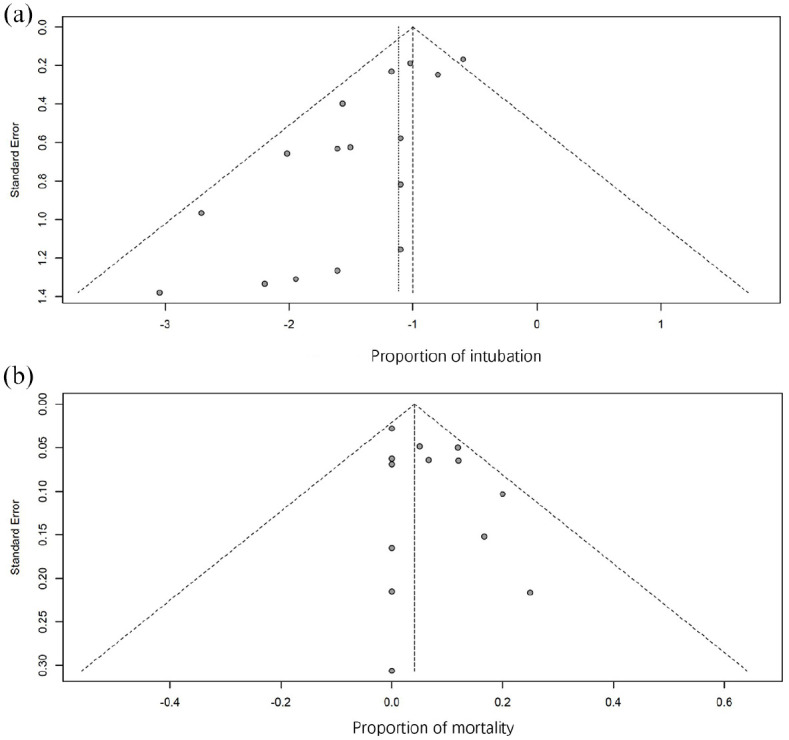
Funnel plots of the proportion versus the standard error of intubation proportion (a) and mortality proportion (b). Studies included in the meta-analysis are represented by the circles.
Intubation rate
A total of 71 out of the 243 patients (29.2%) from 16 studies21–36 were ultimately intubated and received invasive mechanical ventilation with an aggregated intubation rate of 0.33 [95% confidence interval (CI): 0.26–0.42, I2 = 25%]. Among these patients, 195 patients from 11 studies had COVID-19-related AHRF or ARDS, with an aggregated intubation rate of 0.32 (95% CI: 0.23–0.43, I2 = 36%) compared with 0.33 (95% CI: 0.20–0.54, I2 = 9%) noted in 48 non-COVID-19 patients from five studies. In 10 studies, a total of 214 patients performed prone positioning ⩽5 h/day (shorter duration prone), and their intubation rate was 0.34 (95% CI: 0.25–0.45, I2 = 44%), whereas 29 patients from six other studies who performed prone positioning >5 h/day (longer duration prone) had an intubation rate of 0.21 (95% CI: 0.10–0.45, I2 = 0%) (Table 2).
Table 2.
Comparison of different subgroups.
| COVID-19 | Non-COVID-19 | PP ⩽5 h/day | PP >5 h/day | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | No. of patients | Mean ± SD or (95% CI) | No. of studies | No. of patients | Mean ± SD or (95% CI) | No. of studies | No. of patients | Mean ± SD or (95% CI) | No. of studies | No. of patients | Mean ± SD or (95% CI) | |
| Age | 10 | 166 | 58 ± 9a | 4 | 45 | 51 ± 18 | 9 | 182 | 57 ± 12 | 5 | 29 | 54 ± 11 |
| Basic PaO2/FiO2 | 5 | 71 | 146 ± 57a | 4 | 40 | 107 ± 41 | 6 | 97 | 129 ± 55 | 3 | 14 | 148 ± 49 |
| Intubation rate | 11 | 195 | 0.32 (0.23; 0.43) | 5 | 48 | 0.33 (0.20; 0.54) | 10 | 214 | 0.34 (0.25; 0.45) | 6 | 29 | 0.21 (0.10; 0.45) |
| Mortality rate | 9 | 138 | 0.03 (0.00; 0.07) | 5 | 48 | 0.08 (0.01; 0.16) | 9 | 160 | 0.06 (0.02; 0.11) | 5 | 26 | 0.00 (0.00; 0.08) |
| Improvement in the PaO2/FiO2 | 4 | 61 | 52.06 (5.36; 98.76) | 4 | 40 | 47.11 (21.16; 73.06) | 6 | 97 | 49.16 (27.86; 70.47) | 2 | 4 | 37.00 (−29.83; 103.83) |
| Improvement in SpO2 | 5 | 112 | 5.23 (1.25; 9.22) | 1 | 43 | 2.00 (0.92; 3.08) | 4 | 144 | 4.73 (0.19; 9.28) | 2 | 11 | 4.00 (3.12; 4.88) |
| Change in respiratory rate | 4 | 68 | −5.00 (−5.58; −4.33) | 2 | 19 | −5.03 (−10.33; 0.27) | 4 | 76 | −4.70b (−5.38; −4.01) | 2 | 11 | −10.00 (−12.77; −7.23) |
| Intolerance rate | 6 | 81 | 0.06 (0.00; 0.13) | 2 | 35 | 0.11 (0.01; 0.22) | 5 | 103 | 0.09 (0.02; 0.16) | 3 | 13 | 0.00 (0.00; 0.12) |
Basic PaO2/FiO2, PaO2/FiO2 measured before prone positioning. Non-COVID-19, acute hypoxemic respiratory failure or acute respiratory distress syndrome caused by drowning, sepsis, trauma and other diseases.
Significant difference was found between COVID-19 patients and non-COVID-19 patients (p < 0.05).
Significant difference was found between PP ⩽5 h/day and PP >5 h/day (p < 0.05).
CI, confidence interval; COVID-19, coronavirus disease 2019; PP, prone positioning; SD, standard deviation.
Mortality rate
A total of 15 out of the 186 patients (8.1%) from 14 studies21–31,34–36 were ultimately deceased with an aggregated mortality rate of 0.04 (95% CI: 0.01–0.07, I2 = 0%). Among these patients, 138 patients from nine studies had COVID-19-related AHRF or ARDS with an aggregated mortality rate of 0.03 (95% CI: 0.00–0.07, I2 = 0%) compared with 0.08 (95% CI: 0.01–0.16, I2 = 0%) in 48 non-COVID-19 patients from five studies. A total of 160 patients in nine studies performed prone positioning ⩽r h/day and their aggregated mortality rate was 0.06 (95% CI: 0.02–0.11, I2 = 23%), whereas 26 patients from five other studies who performed prone positioning >5 h/day exhibited an aggregated mortality rate of 0.00 (95% CI: 0.00–0.08, I2 = 0%) (Table 2).
Improvement in the PaO2 /FiO2 ratios
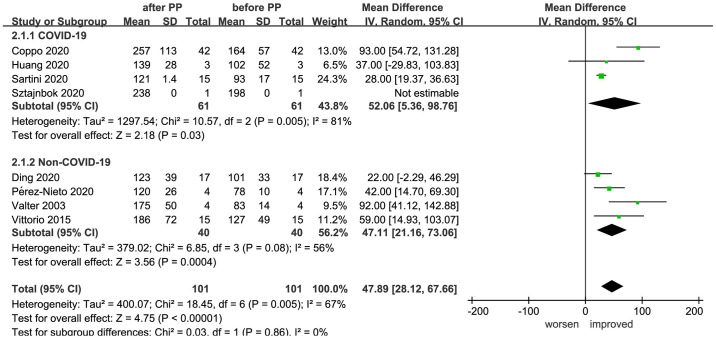
The PaO2/FiO2 ratios of a total of 101 patients were recorded prior to and following prone positioning in eight studies.21,23,25,28,29,33–35 Meta-analysis indicated that the PaO2/FiO2 ratios of the patients following prone positioning were significantly higher than prior to prone positioning (MD = 47.89, 95% CI: 28.12–67.66, p < 0.00001, I2 = 67%). Further subgroup analysis according to the causes of disease formation indicated significant improvement in PaO2/FiO2 ratios both of COVID-19 (MD = 52.06, 95% CI: 5.36–98.76, p = 0.03, I2 = 81%) and non-COVID-19 patients (MD = 47.11, 95% CI: 21.16–73.06, p = 0.0004, I2 = 56%) (Figure 3).
Figure 3.
Effect of prone positioning on PaO2/FiO2 ratios among patients with non-intubated AHRF or ARDS caused by COVID-19 and non-COVID-19 reasons (AHRF or ARDS caused by drowning, sepsis, trauma, and other diseases).
AHRF, acute hypoxemic respiratory failure; ARDS, acute respiratory distress syndrome; CI, confidence interval; COVID-19, coronavirus disease 2019; IV, inverse variance; PP, prone positioning; SD, standard deviation.
Improvement in SpO2
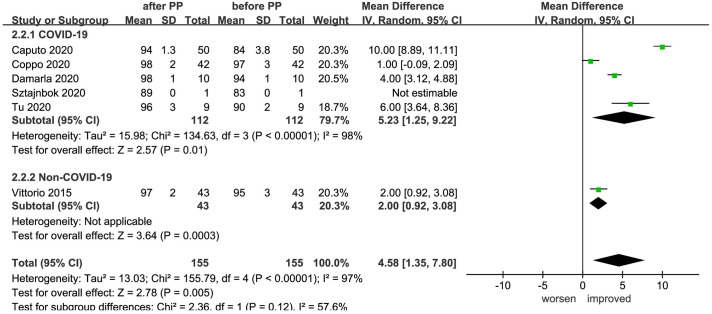
A total of six studies25,26,29,30,32,34 recorded SpO2 changes of 155 patients prior to and following prone positioning and a significant improvement was noted (MD = 4.58, 95% CI: 1.35–7.80, p = 0.005, I2 = 97%). Further subgroup analysis according to the causes of disease formation indicated significant improvement in SpO2 both for COVID-19 patients (MD = 5.23, 95% CI: 1.25–9.22, p = 0.01, I2 = 98%) and non-COVID-19 patients (MD = 2.00, 95% CI: 0.92–3.08, p = 0.0003, I2 incalculable) (Figure 4).
Figure 4.
Effect of prone positioning on SpO2 among patients with non-intubated AHRF or ARDS caused by COVID-19 and non-COVID-19 reasons (AHRF or ARDS caused by drowning, sepsis, trauma, and other diseases).
AHRF, acute hypoxemic respiratory failure; ARDS, acute respiratory distress syndrome; CI, confidence interval; COVID-19, coronavirus disease 2019; IV, inverse variance; PP, prone positioning; SD, standard deviation.
Change in the respiratory rate
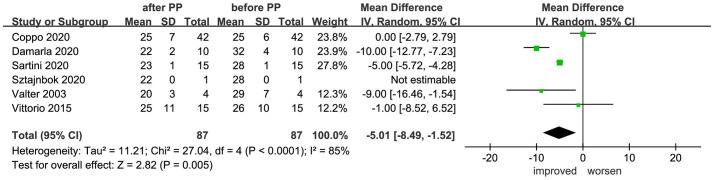
The changes in the respiratory rate of 87 patients prior to and following prone positioning were recorded in six studies.21,25,28–30,34 Meta-analysis indicated that prone positioning significantly reduced patient respiratory rate (MD = −5.01, 95% CI −8.49 to −1.52, p = 0.005, I2 = 85%) (Figure 5).
Figure 5.
Effect of prone positioning on respiratory rate among patients with non-intubated AHRF or ARDS.
AHRF, acute hypoxemic respiratory failure; ARDS, acute respiratory distress syndrome; CI, confidence interval; PP, prone positioning; SD, standard deviation.
Intolerance rate
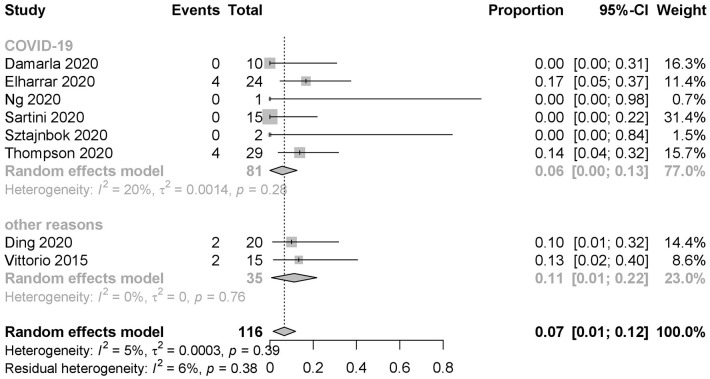
A total of 12 out of the 116 patients (10.3%) from eight studies22–24,27–30,34 could not tolerate the prone positioning treatment with an aggregated intolerance rate of 0.07 (95% CI: 0.01–0.12, I2 = 5%) (Figure 6). Among these patients, 81 patients from six studies exhibited COVID-19 related AHRF or ARDS with an aggregated intolerance rate of 0.06 (95% CI: 0.00–0.13, I2 = 20%) compared with 0.11 (95% CI: 0.01–0.22, I2 = 0%) in 35 non-COVID19 patients from two studies. A total of 103 patients in five studies performed prone positioning ⩽5 h/day and their aggregated intolerance rate was 0.09 (95% CI: 0.02–0.16, I2 = 0%), whereas 13 patients from three other studies who performed prone positioning >5 h/day exhibited an aggregated intolerance rate of 0.00 (95% CI: 0.00–0.12, I2 = 0%) (Table 2).
Figure 6.
Intolerance rate of prone positioning among patients with non-intubated AHRF or ARDS caused by COVID-19 and non-COVID-19 reasons (AHRF or ARDS caused by drowning, sepsis, trauma, and other diseases). Events: patients ceased prone positioning in advance for the unbearable discomfort brought by prone positioning.
AHRF, acute hypoxemic respiratory failure; ARDS, acute respiratory distress syndrome; CI, confidence interval; COVID-19, coronavirus disease 2019.
Discussion
A total of 243 awake patients with non-intubated AHRF or ARDS for prone positioning, whose data were derived from 16 studies, were included in our meta-analysis. The aggregated intubation rate was 33%, the aggregated mortality rate was 4% and the aggregated incidence of intolerance was 7%. The prone positioning could significantly improve the oxygenation in awake patients with AHRF or ARDS and reduce their respiratory rate. The subgroup analysis indicated that the PaO2/FiO2 ratios and SpO2 were significantly improved both in COVID-19 patients and non-COVID-19 patients. The intubation rate of shorter duration prone (⩽5 h/day) and longer duration prone (>5 h/day) was 34% and 21%, respectively; the aggregated mortality rate was 6% and 0%, respectively.
The PROSEVA trial in 2013 demonstrated that the prone positioning could significantly reduce the mortality of patients with ARDS38 and two subsequent meta-analyses indicated that the prone positioning combined with invasive mechanical ventilation could reduce the mortality of patients with severe ARDS compared with that of patients in the supine positioning (40% versus 45%, 41% versus 47%, respectively).17,19 However, it is not clear whether the prone positioning combined with non-invasive respiratory support improves the prognosis of awake patients with AHRF or ARDS. A previous study suggested that prone positioning combined with non-invasive respiratory support could delay intubation and reduce the intubation rate,23 while another study demonstrated that it did not improve patient outcome.34 The studies selected for our meta-analysis were mainly observational studies, cohort studies and case reports, including 243 awake and non-intubated patients with AHRF or ARDS. The aggregated intubation rate was 33% and the aggregated mortality rate was 4%. A previous multicenter and open-label study by Frat et al.4 included 330 patients with AHRF who received non-invasive respiratory support and demonstrated that the intubation rate was 42% and the mortality rate 17%. However, due to lack of RCTs, it is unclear whether the prone positioning reduces the intubation rate and mortality in awake patients with AHRF or ARDS.
Previous studies have shown that prolonged duration of prone positioning correlated with improved prognosis of patients with ARDS undergoing invasive mechanical ventilation.17,20 Two recent meta-analyses indicated that the duration of prone positioning higher than 12 h or 16 h could reduce the mortality of patients.18–20 The current guidelines recommend that the duration of prone positioning should last at least 12 h or 16 h.39–41 All patients included in the studies were awake and probably could not tolerate prolonged prone positioning. The duration of prone positioning was different in each study and varied from less than 1 h to more than 16 h, and the number of patients with the duration of prone positioning more than 12 h was very small. We tried to find the time point that balances effectiveness and tolerance like invasive mechanical ventilation. We divided the patients into a shorter duration prone group (⩽5 h/day) and longer duration prone group (>5 h/day) according to the general distribution of the duration of prone positioning in the studies and our experience in patient tolerance time. The aggregated intubation rate of the shorter duration prone group and longer duration prone group was 34% and 21%, respectively; the aggregated mortality rate was 6% and 0%, respectively. This may be related to the mild condition of patients who can tolerate >5 h/day of prone positioning. However, we could not make a statistical comparison between the two groups because of different interventions in the studies. Different implementation schemes, such as duration and interval, and different clinical experience of each center may lead to different results. The current data is limited and RCT studies are needed to assess these outcomes in future.
The results indicated that prone positioning combined with non-invasive respiratory support could improve oxygenation in patients with AHRF or ARDS, which was similar to the results obtained by prone positioning in patients with invasive mechanical ventilation.42–48 Recent studies demonstrated that prone positioning may provide benefits to patients with early ARDS,18,38 severe ARDS18 and with localized infiltrates49 among patients with invasive mechanical ventilation. The response of different causes of ARDS to the prone positioning was also different.50–55 The number of patients included in the present study was small and most of them were COVID-19 patients. A limited number of patients with other causes of pneumonia, trauma and drowning exhibited significantly improved PaO2/FiO2 ratios and SpO2 for the two groups. However, the awake patients with AHRF or ARDS selected in these studies demonstrated incomplete data regarding the parameters starting time of the prone positioning (early versus late), severity of hypoxemia, different causes (pulmonary versus extrapulmonary), the type of infiltrates (focal versus diffuse) and other relevant data. In addition, a lack of RCTs was also noted and patients who benefited more from prone positioning required further studies in the future.
Several observational studies demonstrated that the oxygenation could be continuously improved when patients returned from the prone to the supine positioning with limited duration of oxygenation.45,56,57 The three studies included in our meta-analysis demonstrated that the PaO2/FiO2 ratios of patients were decreased following returning from the prone to the supine positioning (1 h, 6–8 h and 6–12 h, respectively).25,27,34 Because the patient’s condition was mild and the duration of prone position was relatively short, the duration of oxygenation improvement after returning to the supine positioning was limited. Previous studies have found that the prolonged protocol of prone positioning was often adopted in patients with invasive mechanical ventilation and most of the patients were under sedation, which maintains the patient in the prone position for a prolonged time period while minimizing the number of rotations per day, thus minimizing the riskiest process.58 However, the abbreviated protocol may be another option for awake patients with AHRF or ARDS which allows the flexibility of keeping the patient prone for several hours and supine for a certain time, and then repeats the above steps. The abbreviated protocol of prone positioning may facilitate the maintenance of oxygenation because each duration of supine positioning is short, minimizes pressure-related skin ulceration and intolerance, and does not increase operational risk as the patients are awake, but may increase oxygen consumption for multiple daily repositioning maneuvers. Further clinical studies are required to determine whether the abbreviated protocol of prone positioning is more suitable for awake patients with AHRF or ARDS.
The tolerance of 116 patients to the prone positioning was reported in eight studies included in our meta-analysis. The results indicated that intolerance occurred in 12 patients with prone positioning, with an aggregated intolerance rate of 7%. The following intolerances were mainly noted: discomfort feeling, non-cooperation and aggravated cough; there was an absence of serious adverse complications. Previous studies demonstrated that the prone positioning combined with invasive mechanical ventilation could increase the risk of pressure sores,59 endotracheal tube displacement,18,20 obstruction of the endotracheal tube, dislodgement of the thoracostomy tube17 and venous access loss.19 Patients included in the present study were awake and the main complication was subjective intolerance. Overall, the patients exhibited optimal tolerance and the method used was feasible and safe. No risk of endotracheal tube displacement and low incidence of pressure scores was noted in patients with the prone positioning combined with non-invasive respiratory support compared with those with invasive mechanical ventilation, which may be associated with the patient’s waking state, the absence of sedation and the short duration of the prone positioning. Further studies are required in order to assess the potential of reducing patient intolerance and to improve patient comfort through psychological intervention, anti-anxiety or sedative drugs and palliative therapy for awake patients in the prone positioning.
The present study exhibits several limitations. First, the patients included were mainly patients with COVID-19, which may affect the results obtained. Second, our results were based on relatively small trials and the quality of the evidence in the studies was low. A lack of RCTs was noted, which may reduce accuracy and heterogeneity. Finally, the data in most of the studies were incomplete and our preliminary analysis lacked the starting time of the prone positioning (early versus late), severity of hypoxemia and the different causes responsible for the type of infiltrates (focal versus diffuse). Oxygen device differs from one study to another as well as FiO2. The time of prone positioning was very different in different studies and the number of patients with longer duration prone was also small. Whether the prone positioning could reduce the intubation rate and mortality of patients remained unclear.
Conclusions
In summary, our meta-analysis demonstrated that the aggregated intubation rate and the mortality rate of patients with non-intubation AHRF or ARDS for prone positioning was 33% and 4%, respectively, whereas the intolerance rate was 7%. The prone positioning could improve the oxygenation both in COVID-19 patients and non-COVID-19 patients and reduce the respiratory frequency of awake patients with non-intubation AHRF or ARDS.
Supplemental Material
Supplemental material, sj-jpg-2-tar-10.1177_17534666211009407 for The efficacy and tolerance of prone positioning in non-intubation patients with acute hypoxemic respiratory failure and ARDS: a meta-analysis by Wei Tan, Dong-yang Xu, Meng-jiao Xu, Zan-feng Wang, Bing Dai, Li-li Li, Hong-wen Zhao, Wei Wang and Jian Kang in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-1-tar-10.1177_17534666211009407 for The efficacy and tolerance of prone positioning in non-intubation patients with acute hypoxemic respiratory failure and ARDS: a meta-analysis by Wei Tan, Dong-yang Xu, Meng-jiao Xu, Zan-feng Wang, Bing Dai, Li-li Li, Hong-wen Zhao, Wei Wang and Jian Kang in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_17534666211009407 for The efficacy and tolerance of prone positioning in non-intubation patients with acute hypoxemic respiratory failure and ARDS: a meta-analysis by Wei Tan, Dong-yang Xu, Meng-jiao Xu, Zan-feng Wang, Bing Dai, Li-li Li, Hong-wen Zhao, Wei Wang and Jian Kang in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_17534666211009407 for The efficacy and tolerance of prone positioning in non-intubation patients with acute hypoxemic respiratory failure and ARDS: a meta-analysis by Wei Tan, Dong-yang Xu, Meng-jiao Xu, Zan-feng Wang, Bing Dai, Li-li Li, Hong-wen Zhao, Wei Wang and Jian Kang in Therapeutic Advances in Respiratory Disease
Footnotes
Author contributions: Wei Tan, Dong-yang Xu, Meng-jiao Xu, Zan-feng Wang, Li-li Li: Formal analysis; Resources; Writing-original draft.
Bing Dai, Li-li Li, Hong-wen Zhao, Wei Wang, Jian Kang: Methodology; Supervision; Writing review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Wei Tan  https://orcid.org/0000-0003-1149-4168
https://orcid.org/0000-0003-1149-4168
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Wei Tan, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of China Medical University, Shenyang, China.
Dong-yang Xu, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of China Medical University, Shenyang, China.
Meng-jiao Xu, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of China Medical University, Shenyang, China.
Zan-feng Wang, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of China Medical University, Shenyang, China.
Bing Dai, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of China Medical University, No. 155, Nanjing North Street, Heping District, Shenyang, Liaoning 110001, China.
Li-li Li, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of China Medical University, No. 155, Nanjing North Street, Heping District, Shenyang, Liaoning 110001, China.
Hong-wen Zhao, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of China Medical University, Shenyang, China.
Wei Wang, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of China Medical University, Shenyang, China.
Jian Kang, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of China Medical University, Shenyang, China.
References
- 1. Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315: 788–800. [DOI] [PubMed] [Google Scholar]
- 2. Grieco DL, Menga LS, Eleuteri D, et al. Patient self-inflicted lung injury: implications for acute hypoxemic respiratory failure and ARDS patients on noninvasive support. Minerva Anestesiol 2019; 85: 1014–1023. [DOI] [PubMed] [Google Scholar]
- 3. Frat JP, Coudroy R, Marjanovic N, et al. High-flow nasal oxygen therapy and noninvasive respiratory support in the management of acute hypoxemic respiratory failure. Ann Transl Med 2017; 5: 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015; 372: 2185–2196. [DOI] [PubMed] [Google Scholar]
- 5. Bajaj A, Kumar S, Inamdar AH, et al. Noninvasive respiratory support in acute hypoxic respiratory failure in medical intensive care unit: a study in rural medical college. Int J Crit Illn Inj Sci 2019; 9: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Villar J, Sulemanji D, Kacmarek RM. The acute respiratory distress syndrome: incidence and mortality, has it changed? Curr Opin Crit Care 2014; 2: 3–9. [DOI] [PubMed] [Google Scholar]
- 7. Milberg JA, Davis DR, Steinberg KP, et al. Improved survival of patients with acute respiratory distress syndrome (ARDS): 1983–1993. JAMA 1995; 273: 306–309. [PubMed] [Google Scholar]
- 8. Phua J, Badia JR, Adhikari NK, et al. Has mortality from acute respiratory distress syndrome decreased over time?: a systematic review. Am J Respir Crit Care Med 2009; 179: 220–227. [DOI] [PubMed] [Google Scholar]
- 9. Stapleton RD, Wang BM, Hudson LD, et al. Causes and timing of death in patients with ARDS. Chest 2005; 128: 525–532. [DOI] [PubMed] [Google Scholar]
- 10. ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012; 307: 2526–2533. [DOI] [PubMed] [Google Scholar]
- 11. Walkey AJ, Summer R, Ho V, et al. Acute respiratory distress syndrome: epidemiology and management approaches. Clin Epidemiol 2012; 4: 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Esteban A, Frutos-Vivar F, Muriel A, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med 2013; 188: 220–230. [DOI] [PubMed] [Google Scholar]
- 13. Lamm WJ, Graham MM, Albert RK. Mechanism by which the prone positioning improves oxygenation in acute lung injury. Am J Respir Crit Care Med 1994; 150: 184–193. [DOI] [PubMed] [Google Scholar]
- 14. Scholten EL, Beitler JR, Prisk GK, et al. Treatment of ARDS with prone positioning. Chest 2017; 151: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richter T, Bellani G, Harris RS, et al. Effect of prone positioning on regional shunt, aeration, and perfusion in experimental acute lung injury. Am J Respir Crit Care Med 2005; 172: 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gattinoni L, Carlesso E, Taccone P, et al. Prone positioning improves survival in severe ARDS: a pathophysiologic review and individual patient meta-analysis. Minerva Anestesiol 2010; 76: 448–454. [PubMed] [Google Scholar]
- 17. Sud S, Friedrich JO, Adhikari NK, et al. Effect of prone positioning during mechanical ventilation on mortality among patients with acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ 2014; 186: E381–E390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bloomfield R, Noble DW, Sudlow A. Prone positioning for acute respiratory failure in adults. Cochrane Database Syst Rev 2015; 2015: CD008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park SY, Kim HJ, Yoo KH, et al. The efficacy and safety of prone positioning in adults patients with acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. J Thorac Dis 2015; 7: 356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone positioning for acute respiratory distress syndrome: a systematic review and meta-analysis. Ann Am Thorac Soc 2017; 14: S280–S288. [DOI] [PubMed] [Google Scholar]
- 21. Valter C, Christensen AM, Tollund C, et al. Response to the prone positioning in spontaneously breathing patients with hypoxemic respiratory failure. Acta Anaesthesiol Scand 2003; 47: 416–418. [DOI] [PubMed] [Google Scholar]
- 22. Ng Z, Tay WC, Ho CHB. Awake prone positioning for non-intubated oxygen dependent COVID-19 pneumonia patients. Eur Respir J 2020; 56: 2001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ding L, Wang L, Ma W, et al. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care 2020; 24: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thompson AE, Ranard BL, Wei Y, et al. Prone positioning in awake, nonintubated patients with COVID-19 hypoxemic respiratory failure. JAMA Intern Med 2020; 180: 1537–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med 2020; 8: 765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tu GW, Liao YX, Li QY, et al. Prone positioning in high-flow nasal cannula for COVID-19 patients with severe hypoxemia: a pilot study. Ann Transl Med 2020; 8: 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elharrar X, Trigui Y, Dols AM, et al. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA 2020; 323: 2336–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sartini C, Tresoldi M, Scarpellini P, et al. Respiratory parameters in patients with COVID-19 after using noninvasive respiratory support in the prone positioning outside the intensive care unit. JAMA 2020; 323: 2338–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sztajnbok J, Maselli-Schoueri JH, de Resende Brasil LMC, et al. Prone positioning to improve oxygenation and relieve respiratory symptoms in awake, spontaneously breathing non-intubated patients with COVID-19 pneumonia. Respir Med Case Rep 2020; 30: 101096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Damarla M, Zaeh S, Niedermeyer S, et al. Prone positioning of nonintubated patients with COVID-19. Am J Respir Crit Care Med 2020; 202: 604–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu Q, Wang T, Qin X, et al. Early awake prone positioning combined with high-flow nasal oxygen therapy in severe COVID-19: a case series. Crit Care 2020; 24: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med 2020; 27: 375–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang CF, Tay CK, Zhuang YF, et al. Rationale and significance of patient selection in awake prone positioning for COVID-19 pneumonia. Eur Respir J 2020; 56: 2002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care 2015; 30: 1390–1394. [DOI] [PubMed] [Google Scholar]
- 35. Pérez-Nieto OR, Guerrero-Gutiérrez MA, Deloya-Tomas E, et al. Prone positioning combined with high-flow nasal cannula in severe noninfectious ARDS. Crit Care 2020; 24: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bellone A, Basile A. Prone positioning in severe acute hypoxemic respiratory failure in the emergency ward. Emerg Care J 2018; 14: 7524. [Google Scholar]
- 37. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368: 2159–2168. [DOI] [PubMed] [Google Scholar]
- 39. Griffiths MJD, McAuley DF, Perkins GD, et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir Res 2019; 6: e000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fan E, Del Sorbo L, Goligher EC, et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2017; 195: 1253–1263. [DOI] [PubMed] [Google Scholar]
- 41. Papazian L, Aubron C, Brochard L, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care 2019; 9: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roche-Campo F, Aguirre-Bermeo H, Mancebo J. Prone positioning in acute respiratory distress syndrome (ARDS): when and how? Presse Med 2011; 40: e585–e594. [DOI] [PubMed] [Google Scholar]
- 43. Johnson NJ, Luks AM, Glenny RW. Gas exchange in the prone posture. Respir Care 2017; 62: 1097–1110. [DOI] [PubMed] [Google Scholar]
- 44. Mitchell DA, Seckel MA. Acute respiratory distress syndrome and prone positioning. AACN Adv Crit Care 2018; 29: 415–425. [DOI] [PubMed] [Google Scholar]
- 45. Kallet RH. A comprehensive review of prone position in ARDS. Respir Care 2015; 60: 1660–1687. [DOI] [PubMed] [Google Scholar]
- 46. Taccone P, Presenti A, Latini R;, et al. Prone-Supine II Study Group. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 2009; 302: 1977–1984. [DOI] [PubMed] [Google Scholar]
- 47. Gattinoni L, Tognoni G, Pesenti A, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001; 345: 568–573. [DOI] [PubMed] [Google Scholar]
- 48. Guerin C, Gaillard S, Lemasson S, et al. Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA 2004; 292: 2379–2387. [DOI] [PubMed] [Google Scholar]
- 49. Gainnier M, Michelet P, Thirion X, et al. Prone positioning and positive end-expiratory pressure in acute respiratory distress syndrome. Crit Care Med 2003; 31: 2719–2726. [DOI] [PubMed] [Google Scholar]
- 50. Lim CM, Kim EK, Lee JS, et al. Comparison of the response to the prone positioning between pulmonary and extrapulmonary acute respiratory distress syndrome. Intensive Care Med 2001; 27: 477–485. [DOI] [PubMed] [Google Scholar]
- 51. Rialp G, Betbesé AJ, Pérez-Márquez M, et al. Short-term effects of inhaled nitric oxide and prone positioning in pulmonary and extrapulmonary acute respiratory distress syndrome. Am J Respir Crit Care Med 2001; 164: 243–249. [DOI] [PubMed] [Google Scholar]
- 52. Collins SR, Blank RS. Approaches to refractory hypoxemia in acute respiratory distress syndrome: current understanding, evidence, and debate. Respir Care 2011; 56: 1573–1582. [DOI] [PubMed] [Google Scholar]
- 53. Fessler HE, Talmor DS. Should prone positioning be routinely used for lung protection during mechanical ventilation? Respir Care 2010; 55: 88–99. [PubMed] [Google Scholar]
- 54. Reutershan J, Schmitt A, Dietz K, et al. Alveolar recruitment during prone position: time matters. Clin Sci (Lond) 2006; 110: 655–663. [DOI] [PubMed] [Google Scholar]
- 55. Venet C, Guyomarc’h S, Migeot C, et al. The oxygenation variations related to prone positioning during mechanical ventilation: a clinical comparison between ARDS and non-ARDS hypoxemic patients. Intensive Care Med 2001; 27: 1352–1359. [DOI] [PubMed] [Google Scholar]
- 56. Hale DF, Cannon JW, Batchinsky AI, et al. Prone positioning improves oxygenation in adult burn patients with severe acute respiratory distress syndrome. J Trauma Acute Care Surg 2012; 72: 1634–1639. [DOI] [PubMed] [Google Scholar]
- 57. Romero CM, Cornejo RA, Gálvez LR, et al. Extended prone position ventilation in severe acute respiratory distress syndrome: a pilot feasibility study. J Crit Care 2009; 24: 81–88. [DOI] [PubMed] [Google Scholar]
- 58. Athota KP, Millar D, Branson RD, et al. A practical approach to the use of prone therapy in acute respiratory distress syndrome. Expert Rev Respir Med 2014; 8: 453–463. [DOI] [PubMed] [Google Scholar]
- 59. Lucchini A, Bambi S, Mattiussi E, et al. Prone positioning in acute respiratory distress syndrome patients: a retrospective analysis of complications. Dimens Crit Care Nurs 2020; 39: 39–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-2-tar-10.1177_17534666211009407 for The efficacy and tolerance of prone positioning in non-intubation patients with acute hypoxemic respiratory failure and ARDS: a meta-analysis by Wei Tan, Dong-yang Xu, Meng-jiao Xu, Zan-feng Wang, Bing Dai, Li-li Li, Hong-wen Zhao, Wei Wang and Jian Kang in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-1-tar-10.1177_17534666211009407 for The efficacy and tolerance of prone positioning in non-intubation patients with acute hypoxemic respiratory failure and ARDS: a meta-analysis by Wei Tan, Dong-yang Xu, Meng-jiao Xu, Zan-feng Wang, Bing Dai, Li-li Li, Hong-wen Zhao, Wei Wang and Jian Kang in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_17534666211009407 for The efficacy and tolerance of prone positioning in non-intubation patients with acute hypoxemic respiratory failure and ARDS: a meta-analysis by Wei Tan, Dong-yang Xu, Meng-jiao Xu, Zan-feng Wang, Bing Dai, Li-li Li, Hong-wen Zhao, Wei Wang and Jian Kang in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_17534666211009407 for The efficacy and tolerance of prone positioning in non-intubation patients with acute hypoxemic respiratory failure and ARDS: a meta-analysis by Wei Tan, Dong-yang Xu, Meng-jiao Xu, Zan-feng Wang, Bing Dai, Li-li Li, Hong-wen Zhao, Wei Wang and Jian Kang in Therapeutic Advances in Respiratory Disease