Abstract
The bidirectional relationship between depression and chronic pain is well-recognized, but their clinical management remains challenging. Here we characterize the shared risk factors and outcomes for their comorbidity in the Australian Genetics of Depression cohort study (N = 13,839). Participants completed online questionnaires about chronic pain, psychiatric symptoms, comorbidities, treatment response and general health. Logistic regression models were used to examine the relationship between chronic pain and clinical and demographic factors. Cumulative linked logistic regressions assessed the effect of chronic pain on treatment response for 10 different antidepressants. Chronic pain was associated with an increased risk of depression (OR = 1.86 [1.37–2.54]), recent suicide attempt (OR = 1.88 [1.14–3.09]), higher use of tobacco (OR = 1.05 [1.02–1.09]) and misuse of painkillers (e.g., opioids; OR = 1.31 [1.06–1.62]). Participants with comorbid chronic pain and depression reported fewer functional benefits from antidepressant use and lower benefits from sertraline (OR = 0.75 [0.68–0.83]), escitalopram (OR = 0.75 [0.67–0.85]) and venlafaxine (OR = 0.78 [0.68–0.88]) when compared to participants without chronic pain. Furthermore, participants taking sertraline (OR = 0.45 [0.30–0.67]), escitalopram (OR = 0.45 [0.27–0.74]) and citalopram (OR = 0.32 [0.15–0.67]) specifically for chronic pain (among other indications) reported lower benefits compared to other participants taking these same medications but not for chronic pain. These findings reveal novel insights into the complex relationship between chronic pain and depression. Treatment response analyses indicate differential effectiveness between particular antidepressants and poorer functional outcomes for these comorbid conditions. Further examination is warranted in targeted interventional clinical trials, which also include neuroimaging genetics and pharmacogenomics protocols. This work will advance the delineation of disease risk indicators and novel aetiological pathways for therapeutic intervention in comorbid pain and depression as well as other psychiatric comorbidities.
Keywords: depression, chronic pain, suicide, treatment response, comorbidity, antidepressant
Introduction
Depression is estimated to affect over 264 million people worldwide and is a leading cause of global disability (1). Its clinical manifestations and outcomes are highly heterogeneous, with multiple factors underlying susceptibility, progression and treatment response (2). One key factor that frequently complicates the diagnosis of depression is comorbid chronic pain, as patients presenting with pain are more likely to be investigated medically rather than as part of a broader biopsychosocial framework (3). Depression and chronic pain frequently coexist, with up to 60% of chronic pain patients also presenting with depression (4, 5). Furthermore, the combination of chronic pain and depression leads to poorer treatment outcomes and overall functioning than either condition alone (6). This problem is underlined by depression and chronic pain being among the top three leading causes of global disability over the past three decades (1).
Chronic pain has been defined by the International Association for the Study of Pain (IASP) as pain persisting or recurring for longer than 3 months (7, 8). In contrast to acute pain, which alerts individuals to potential or real tissue damage, chronic pain serves no apparent physiological purpose and persists beyond normal healing time (7). In Australia (2015–2016), the disease group with the highest expenditure was musculoskeletal disorders (9). In 2016, more than 1.5 million people over the age of 45 had chronic pain (10) and nearly half of adult patients referred to a pain specialist have comorbid anxiety or depression (11). In 2018 the cost to the Australian economy was around $139 billion, mostly due to lost productivity and impaired quality of life (10) with predictions it will almost triple by 2050 (12). In the United States, chronic pain is already costing well over $500 billion per year (13, 14).
The relationship between chronic pain and depression is bidirectional, as having either disorder increases the risk of developing the other condition (15–19) and pain, in particular, is strongly associated with depression onset and relapse (20–26). Furthermore, the relationship is dose-dependent with more severe pain being associated with greater severity of depression (23, 27–31). That is especially true for older age populations which report the highest prevalence (13%) (15) of comorbid chronic pain and depression out of all age groups (3, 32–34). However, the evidence for comorbid psychiatric disorders predicting pain intensity and worse outcomes is much weaker (18, 35). Nevertheless, recent large-scale human genetic studies (36–52), animal models (53–56) and neuroimaging in antidepressant treatment trials (57–59) have made essential inroads toward delineating the causal mechanisms between chronic pain and depression.
Serotonin noradrenaline reuptake inhibitors (SNRIs; e.g., duloxetine) and selective serotonin reuptake inhibitors (SSRIs; e.g., paroxetine, sertraline) are commonly used antidepressants for the treatment of comorbid chronic pain and depression (60). Other antidepressant options include tricyclic antidepressants (TCAs) such as amitriptyline (61, 62). While these medications have been found to reduce the symptoms of both depression and pain partially, no significant differences in efficacy between them have been established so far (63), thus further research is required (60, 64). For example, the efficacy of TCAs against other antidepressants for the treatment of comorbid chronic pain and depression remains unclear due to a lack of rigorous studies (35, 65, 66).
Despite the high prevalence and cost of comorbid chronic pain and depression (1, 4, 5, 15–17, 19, 67–69), research efforts have yet to deliver clinically useful findings and recommendations specifically for this comorbid indication (66, 70, 71). For example, a recent review highlighted that it was unclear which specific antidepressant should be prescribed as the first-line treatment for comorbid chronic pain and depression (60), while others have recommended non-opioid medications as first-line therapy for chronic neuropathic pain (72, 73). To address this issue, pharmacoepidemiological studies—which examine the use and effect of medications in large population cohorts—have been proposed as a cost-effective method for reviewing pharmaceutical safety and effectiveness, as well as helping to inform clinical guideline development (74).
In the current study, we examined the pharmacoepidemiology of comorbid chronic pain and depression in the Australian Genetics of Depression Study (AGDS)—one of the world's largest participant cohorts with a detailed history of depression and its comorbidities (75). Here, we sought to: (i) quantify the association between depression and chronic pain; (ii) assess the dependency between chronic pain severity, depression severity and recent suicidality; (iii) identify other psychiatric disorders and patterns of recent substance use associated with comorbid chronic pain and depression; and (iv) assess whether comorbid chronic pain and depression is associated with differential antidepressant effectiveness.
Methods
Participants
This study comprised data from two cohorts: AGDS and the Prospective Imaging Study of Aging (PISA). Participants in both groups provided informed consent before participating. These studies, including all questionnaires used, were approved by QIMR Berghofer Medical Research Institute's Human Research Ethics Committee.
AGDS Cohort
Twenty thousand six hundred eighty-nine participants from across Australia were recruited through an open media campaign and targeted mailout. The publicity campaign, from which 86% of participants were recruited, including both conventional and online social media. The campaign appealed for anyone who “had been treated by a doctor, psychiatrist or psychologist for depression” to visit this website—https://www.geneticsofdepression.org.au. For the targeted mailout, invitation letters were sent by the Australian Government Department of Human Services (DHS) to individuals who, according to their records, had received at least four prescriptions for any of the 10 most commonly used antidepressants in the last 4.5 years. DHS did not, at any time, share any personal information with the research team. Potential participants were directed to the above website, which contained information about the study, a registration and consent form, and a comprehensive online questionnaire. The essential inclusion criteria included having been prescribed and taken antidepressants and providing consent to donate a saliva sample for subsequent genotyping. No participant was excluded based on comorbid conditions. The online survey assessed mental health diagnoses, antidepressant response, suicidality, general health and substance use, among several other variables. A detailed baseline description of the cohort has been published elsewhere (75). The full list and details of instruments used for AGDS phenotyping are available at: https://bmjopen.bmj.com/content/bmjopen/10/5/e032580/DC2/embed/inline-supplementary-material-2.pdf.
PISA Cohort
The Prospective Imaging Study of Aging (PISA) is a longitudinal cohort of Australian adults (76). The population-based sample recruitment pool comprised adult twins, their spouses, and first-degree relatives of twins and spouses who over previous decades, had volunteered for studies on risk factors or biomarkers for physical or psychiatric conditions and had previously been genome-wide genotyped (77, 78). The PISA protocol consisted of online questionnaires, including a history of mental health diagnoses and the same pain questionnaire in AGDS. It was completed by N = 2,469 PISA participants. For that reason, AGDS and PISA data were used in the present study to assess the effect of depression and demographics (e.g., age, sex) on chronic pain. All other analyses described in this manuscript were performed only in the AGDS cohort.
Depression and Chronic Pain Ascertainment
AGDS participants were asked to self-report whether they had ever been diagnosed with depression by a health professional, and similarly for 19 other psychiatric conditions. Individuals were classified as depression cases if they had reported both a depression diagnosis and had been prescribed antidepressants in the past 5 years (N = 17,849). Of these, 92% fulfilled the DSM-5 diagnostic criteria for a lifetime depressive episode based on detailed descriptions of this cohort (75). Importantly, this figure is within the test-retest reliability estimates of depression ascertainment from DSM-5 based instruments (79, 80). Participants were administered a pain severity numerical rating scale (81). Briefly, patients were asked to indicate whether they experienced chronic pain in their daily life and to rank its intensity on a scale from 0 to 10. Only those reporting a pain rating >0 progressed to the remainder of the pain module, which included questions about the duration and location of their primary pain. Following the IASP guidelines, chronic pain was defined as pain persisting or recurring for at least 3 months (7, 8). Cases were classified as having comorbid chronic pain and depression if they fulfilled the criteria for both conditions (N = 6,895), and controls were classified as those who reported depression but no chronic pain (N = 4,475). We performed a complete case analysis. Thus, participants with missing data for chronic pain (i.e., those who did not complete the section; N = 6,463) were excluded from analyses that needed data for both chronic pain and depression.
Recent Suicidality and Substance Use
Suicidality was assessed using the SIDAS instrument (82). Briefly, suicidal ideation over the last month was measured on a 10-point scale: 0 indicated having no suicidal ideation in the past month (never), and 10 denoted persistent suicidal ideation. Participants with a score >0 were classified as positive cases for suicidal ideation. Suicide attempt was measured using a similar 10-point scale in regard to how close a participant had come to making an attempt. Only those with a score of 10 (labeled as “I have made an attempt”) were considered cases for a suicide attempt. Participants also reported their frequency in using a range of substances over the last 3 months. Alcohol consumption frequency was measured as the number of days the participant drank three or more standard drinks. For all other substances, the response options were: “never” (0), “once or twice” (1), “monthly” (2), “weekly” (3), or “daily” (4). These responses were modeled as continuous variables when assessing their correlation with chronic pain.
Antidepressant Use and Response
Participants were asked whether they had ever been prescribed any of the 10 most commonly used antidepressants in Australia for any indication. These are sertraline, escitalopram, venlafaxine, amitriptyline, mirtazapine, desvenlafaxine, citalopram, fluoxetine, duloxetine and paroxetine. Information regarding the reason(s) for prescription was collected using a checklist of 17 possible responses, including depression, chronic pain, and anxiety (among others). Multiple selections were possible. Participants were asked to report on the best aspects of taking antidepressants using the following item: “What were the best aspects of taking the antidepressant(s)? Include any antidepressant you have taken.” Participants were then able to select all that apply out of a list including relief of depressive symptoms, relief of other symptoms, e.g., sleep disturbance, reduction in suicidal ideation, return of normal emotion, improved relationships, returning to normal activities and restored control over mood. Moreover, participants rated the effectiveness of each antidepressant they had taken, using a scale ranging from 0 (e.g., “sertraline works not at all well for me”) to 2 (e.g., “sertraline works very well for me”). Two analyses were performed: (i) first, antidepressant effectiveness was compared between participants who reported taking an antidepressant prescribed for chronic pain against the rest of the participants (i.e., not prescribed for chronic pain); and (ii) we compared antidepressant effectiveness between participants reporting chronic pain and those reporting no chronic pain (regardless of explicit indication).
Statistical Analyses
In this study, we used complete case analysis and thus removed participants who did not have the required data from specific analyses. The relationship between chronic pain and several other variables of interest was assessed using multivariable logistic regression. This approach enabled us to quantify the associations while adjusting for age, sex and all other relevant factors (e.g., the correlation between alcohol and chronic pain while keeping usage of all other substances equal). Fully adjusted odds ratios were calculated from effect sizes on the logit scale, and p-values were estimated using Wald-tests. For all analyses, the presence of chronic pain was modeled as a binary variable, while chronic pain severity was modeled as a quantitative score from zero to 10. The relationship between chronic pain and antidepressant effectiveness was examined using cumulative link logistic regressions to accurately model treatment response, which was coded on an ordinal scale. Furthermore, to assess the effect of chronic pain across all antidepressants, a random effect was included to account for repeated responses from participants. This analysis was performed in R using the ordinal package and the clm and clmm functions, adjusting for the effects of sex and age when antidepressant treatment started. All other statistical analyses were performed and figures generated in python using these modules: statsmodels, scipy, numpy, pandas, matplotlib, and seaborn.
Results
Sample Demographics and Association Between Chronic Pain and Depression
Demographics and chronic pain prevalence for both AGDS (enriched for depression) and PISA (not enriched for depression) cohorts are shown in Table 1, Supplementary Figures 1, 2. Figure 1 shows the prevalence of chronic pain by age, stratified by cohort. A significant cohort effect is evident. Nonetheless, this cohort effect may be attributable (at least in part) to other differences such as age, sex and education rather than depression. Chronic pain is positively associated with age but despite the PISA cohort being older on average (Supplementary Figure 1), the AGDS cohort showed a higher prevalence of chronic pain. After adjusting for all the relevant factors, the cohort effect was found to be partly attributable to depression status (OR = 1.86 [1.37–2.54]) because residual cohort effects were non-significant after accounting for the effect of depression (CohortAGDS OR = 1.32 [0.97–1.79]). Furthermore, a higher age (OR = 1.02 [1.02–1.03]), lower educational attainment (OR = 0.89 [0.86–0.91]), and being female (OR = 1.16 [1.07–1.25]) were associated with chronic pain in the pooled PISA and AGDS sample (Supplementary Table 1).
Table 1.
Chronic pain prevalence in AGDS and PISA cohorts.
| Cases | Controls | |
|---|---|---|
| AGDS (depression cohort) | ||
| Sample size N (%) | 6,895 (60.6%) | 4,475 (39.4%) |
| Female N (%) | 5,215 (60%) | 3,402 (40%) |
| Age mean (sd)* | 45 (15.1) | 40 (14.3) |
| PISA | ||
| Sample size N (%) | 1,248 (50%) | 1,221 (50%) |
| Depression* N (%) | 119 (64%) | 68 (36%) |
| Female N (%) | 882 (51%) | 854 (49%) |
| Age mean (sd) | 60 (6.8) | 60 (6.9) |
Cases: participants reporting chronic pain.
Controls: participants reporting no chronic pain.
p < 0.05 two-sample t-test or χ2 test.
Figure 1.
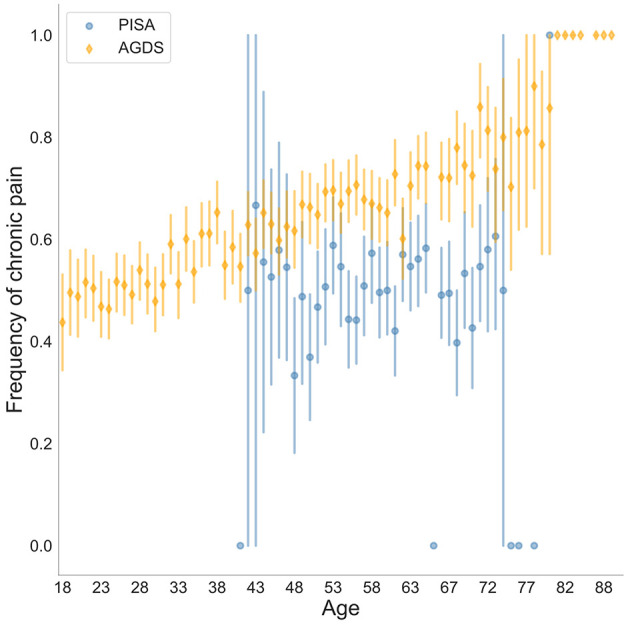
Prevalence of chronic pain stratified by age and cohort (AGDS vs. PISA). Self-reported chronic pain was significantly higher in the AGDS cohort (N = 6,895/11,370) compared with the PISA cohort (N = 1,248/2,469)—OR = 1.31 (0.96–1.77); p = 0.086. For other statistical significance results see Supplementary Table 1. Both cohorts are population-based samples with AGDS being enriched for depression.
Chronic Pain Is Associated With Severity of Depression and Recent Suicidality
Results presented here are from AGDS where all participants reported depression. Higher pain severity (intensity) was found to be associated with longer durations of pain (Supplementary Figure 3). Increased pain severity was also associated with an increased number of depressive episodes (Supplementary Figure 4). The prevalence of suicidal ideation was higher in the comorbid chronic pain group (OR = 1.49 [1.38–1.61]). Likewise, recent suicide attempt was associated with chronic pain (OR = 1.88 [1.14–3.09]). Within the chronic pain group, recent suicidal thoughts and suicide attempt scores were also positively correlated with chronic pain severity scores (Supplementary Figure 4).
Comorbid Psychiatric Diagnoses and Recent Substance Use
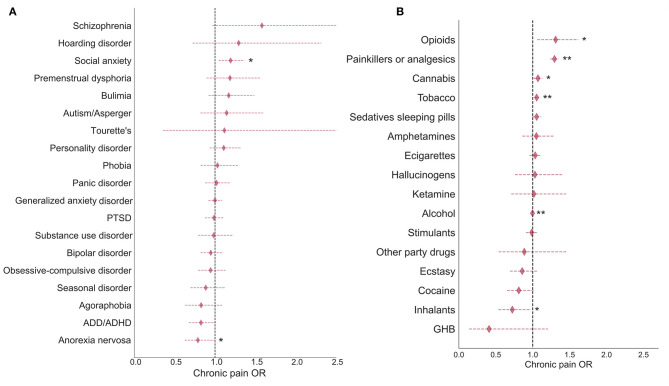
In this subsection, the results presented are from AGDS where all participants reported depression. Out of the 19 mental health conditions examined, social anxiety disorder was found to have the strongest association with chronic pain (p < 0.01), however, this association did not survive multiple-testing correction. Anorexia nervosa was found to be negatively associated with the likelihood of developing chronic pain (p < 0.05). Although both of these results were nominally significant, no association survived correction for multiple testing (Figure 2; Supplementary Table 2). Notably, chronic pain was significantly associated with decreased use of alcohol, increased use of tobacco, and painkiller misuse (e.g., opioids). Nominal associations were observed for other drugs such as cocaine (negative relationship) and opioids (Figure 2; Supplementary Table 3).
Figure 2.
Comorbidities and substance use associations with chronic pain in AGDS cohort. Forest plots depict the chronic pain odds ratios (ORs) for (A) comorbid disorders and (B) recent substance use during the past 3 months. Chronic pain was significantly associated with decreased use of alcohol, increased use of tobacco, and painkiller misuse (e.g., opioids). Diamonds represent ORs and horizontal lines depict 95% CI. ORs were estimated from a multivariate logistic regression accounting for all relevant covariates (see Methods). *p < 0.05; **p < 0.05 after Bonferroni correction for multiple testing (AGDS data only).
Chronic Pain and Antidepressant Response
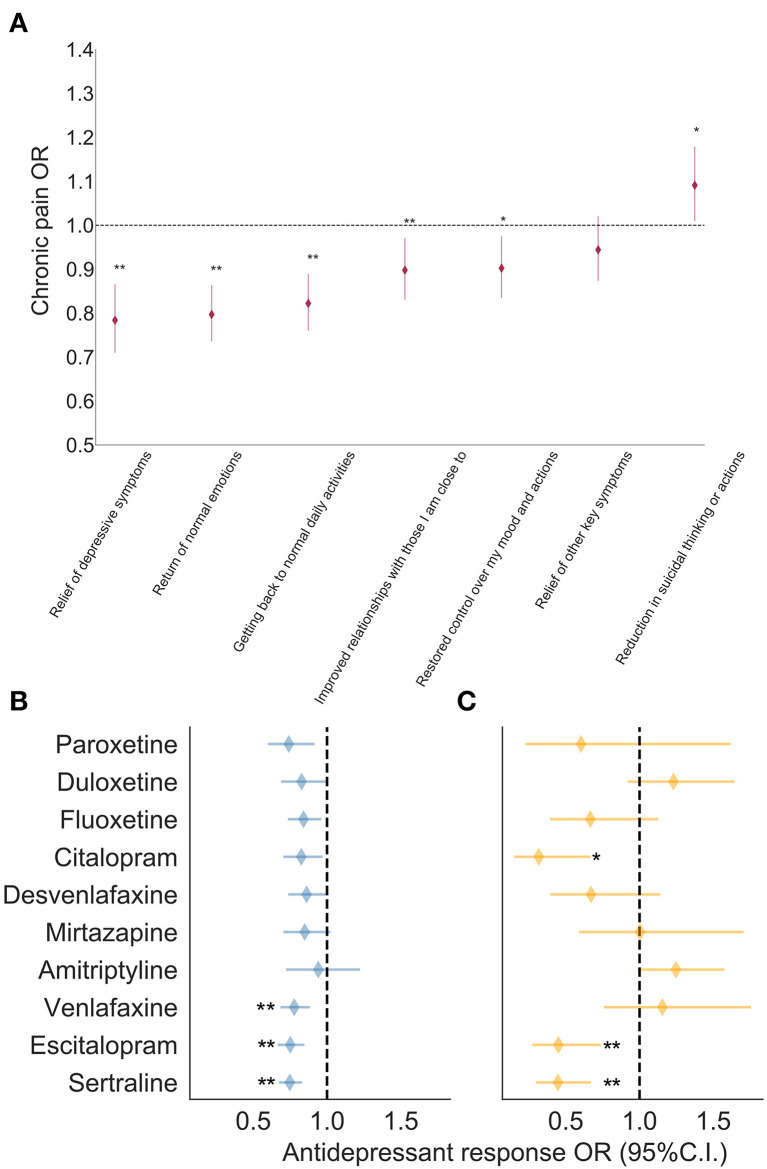
In this subsection, the results presented are from AGDS, where all participants reported depression. The three most commonly prescribed antidepressants for the indication of chronic pain—over and above depression—were amitriptyline (N = 606), duloxetine (N = 288) and sertraline (N = 160; Supplementary Figure 5). Overall, the overwhelming majority of participants with chronic pain (i.e., 75–98% across all antidepressants) reported that their antidepressant prescription did not consider chronic pain. Furthermore, participants with chronic pain predominantly reported taking antidepressants for depression (i.e., more than 90% of participants reported their prescription was for depression). The exception was amitriptyline, for which only 60% of participants with chronic pain reported the prescription was for depression (Supplementary Table 4). Compared to participants without chronic pain, those with comorbid chronic pain were less likely to report positive functional benefits from taking antidepressants such as “relief of depressive symptoms,” “return of normal emotions,” and “getting back to normal daily activities” (Figure 3A). A trend was noted—whereby participants with chronic pain were more likely to report a reduction in suicidal symptoms as a positive aspect of antidepressant treatment—but this finding did not survive correction for multiple testing (Supplementary Table 5). Furthermore, in the chronic pain group, the average self-reported benefits from taking antidepressants were significantly lower compared to the group without chronic pain (OR = 0.75 [0.71–0.80]; p < 2 × 10−16). A similar but non-significant finding was observed between the average response of participants prescribed antidepressants for chronic pain vs. those without a chronic pain indication (OR = 0.94 [0.80–1.1]; Supplementary Figure 6). Next, we examined whether these findings held true for each antidepressant under investigation. For most antidepressants, no statistically significant difference in effectiveness was found between participants with chronic pain (or an indication for chronic pain) and participants without chronic pain. Participants with chronic pain who had taken sertraline, escitalopram or venlafaxine, reported significantly lower effectiveness than participants without chronic pain (Figure 3B). At this point, it remained unclear whether the antidepressant was taken before or after the commencement of chronic pain. To address this question, we performed a secondary analysis defining cases as participants who reported taking an antidepressant where the prescription explicitly considered chronic pain. This analysis revealed lower effectiveness for sertraline, escitalopram and citalopram (Figure 3C). The only antidepressants with a positive effect (i.e., greater effectiveness) were duloxetine, venlafaxine and amitriptyline, but only when prescribed for comorbid chronic pain and depression. However, none of these positive associations reached statistical significance (Figure 3B; Supplementary Tables 6, 7).
Figure 3.
Effect of chronic pain on antidepressant benefits in AGDS cohort. Forest plots depicting the results from: (A) association of chronic pain with self-reported benefits from general antidepressant treatment; and associations between antidepressant treatment response and (B) self-reported chronic pain or (C) self-reported prescription for chronic pain, while adjusting for the effects of sex and age at commencement of taking the antidepressant (*p < 0.05; **p < 0.005 statistical significance after Bonferroni correction for multiple testing; AGDS data only). Further details are in Supplementary Figure 4 and Supplementary Tables 4, 5.
Discussion
We have reported the largest study to date on comorbid chronic pain and depression assessing the risk factors and treatment outcomes through comprehensive phenotyping of a depression-enriched sample. A key finding is that participants with comorbid chronic pain and depression reported significantly lower benefits from taking particular SSRI and SNRI antidepressants (i.e., sertraline, escitalopram, venlafaxine) compared to participants with depression but no chronic pain. Participants with comorbid chronic pain and depression also reported fewer functional benefits from taking antidepressants compared to those without chronic pain. For example, participants with comorbid chronic pain and depression treated with antidepressants were 22% less likely to report relief of depressive symptoms and 18% less likely to get back to normal daily activities compared to depression patients without comorbid chronic pain. The fewer functional benefits reported from antidepressants in those with comorbid chronic pain is consistent with prior research showing that pain is a strong predictor of non-remission with antidepressant medication treatment (83, 84).
We also found that participants prescribed particular SSRI antidepressants (i.e., sertraline, escitalopram, citalopram) for chronic pain reported significantly lower benefits (e.g., 55% lower odds of response from sertraline) compared to those taking the same medications but for a different indication. These results suggest that while SSRI and SNRI antidepressant classes may be equally effective in the treatment of comorbid chronic pain and depression (63, 85), specific antidepressants have differential effectiveness depending on certain common disease modifiers such as chronic pain. The lower effectiveness of sertraline is particularly important, as it is commonly used for the treatment of comorbid chronic pain and depression (60). Here we have shown evidence for differential effectiveness between several specific antidepressants in comorbid chronic pain and depression. We consider these findings to be robust because our methodological approach took into account the inherent clinical heterogeneity, high comorbidity and wide individual variation commonly observed in psychiatric disorders.
The current study's main findings of differential antidepressant effectiveness and fewer functional benefits from antidepressant use in comorbid chronic pain and depression are further underlined by demonstrating several results consistent with previous research. These include: (i) a strong association between depression and chronic pain (86, 87); (ii) increasing severity of chronic pain was associated with a higher number of depressive episodes experienced by participants (23); and (iii) older age, lower educational attainment and female sex were associated with higher chronic pain prevalence (3, 32–34, 88–90).
In the current study, amitriptyline was found to be the most commonly prescribed antidepressant to individuals with comorbid chronic pain. Indeed, it was prescribed over two times more than the next most commonly prescribed medication—duloxetine. Moreover, amitriptyline was the medication with, by far, the lowest indication for depression followed by duloxetine. Amitriptyline has traditionally been the first-line treatment for chronic neuropathic pain (61), however, its side-effect profile and mortality risk in overdose often limit its use (66). Our results are consistent with these medications being effective at treating chronic pain. In the current study, when the antidepressant prescription was for chronic pain—amitriptyline, duloxetine and venlafaxine showed a positive association with treatment effectiveness. This effect did not survive multiple-testing correction, which may be explained by the reduced power from further stratifying the sample. Our findings highlight the inadequate treatment recommendations for comorbid chronic pain and depression, as most participants with chronic pain did not report their antidepressant prescriptions were for chronic pain. The current study thus reaffirms the critical unmet need in this patient population.
While there have been conflicting reports regarding the link between chronic pain severity and suicidal behaviors (91–96), we provide evidence that supports an association between comorbid chronic pain and depression with both an increased risk for suicidal ideation and suicide attempt. Given suicide is a leading cause of death—particularly for young people (97)—and that depression and chronic pain are both treatable conditions, assessing their comorbidity in both at-risk youth and older adult populations may help to reduce suicide rates (98–101).
Consistent with previous observations (87), we also found comorbid chronic pain and depression was associated with recent increased use of tobacco and painkillers (e.g., opioids). However, we did not observe a significant association between comorbid chronic pain and depression with a self-reported substance use disorder. Previous reports suggest chronic pain, depression and substance use disorder are often comorbid (102). It is possible that screening and diagnosis of substance use disorders in Australia may be lacking in those with comorbid chronic pain and depression. As such, clinicians need to consider substance use disorder in patients presenting with this comorbidity, as all three conditions increase the risk for other chronic diseases such as cardiovascular disease and cancer, while also increasing the risk of premature death (70).
Strengths, Limitations, and Further Research
The current study is the largest to date examining the relationship between depression and chronic pain with a novel pharmacoepidemiological approach that has yielded new insights into the medical treatment of these highly morbid conditions. However, there are also a number of limitations to be acknowledged. Data were primarily drawn from AGDS, which is a multi-aim study investigating the risk factors for depression and treatment response to antidepressants. As the AGDS employed phenotyping across an extensive range of complex traits (75), on balance it was not feasible to also collect detailed information from participants on dosages and (polypharmacy) combinations taken of the prescribed antidepressants (103); the duration and magnitude of benefits (e.g., for cost-effectiveness analyses) (65, 104, 105); drug tolerability and adverse events (65, 106–111); adjunct psychological therapies and multidisciplinary treatment/rehabilitation programs (112); other prescribed pain pharmacotherapies and questionnaires (113–117). Further pharmacoepidemiological studies focusing on chronic pain and a large range of psychiatric comorbidities (51, 52, 118). can directly address and collect data on these specific issues. As the current data were based on self-reported responses, they may also be subject to a degree of participant recall bias (e.g., time-specific details). For example, the substance use and suicidality phenotypes may be subject to non-disclosure effects due to the potential stigma associated with these conditions and the antidepressant response data may include non-specific (placebo/nocebo) effects (59, 119). However, these effects would be present across all antidepressants and thus alone are highly unlikely to explain the observed differences between the chronic pain (cases) and control participants. Randomized interventional studies comparing treatments for participants with comorbid chronic pain and depression are required to validate our results and elucidate the causal direction of associations reported here. Genetic-based methods can also aid in further examination of our findings by performing discovery genome-wide association studies (GWAS) of comorbid chronic pain and depression to determine individuals' polygenic risk irrespective of whether they have developed chronic pain or not. Furthermore, GWAS will enable: (i) the elucidation of causality between chronic pain and treatment response by using methods such as Mendelian randomization; and (ii) replication in antidepressant treatment-resistant depression cohorts with primary care and genotype data (120).
Conclusions
In summary, we found patients with comorbid depression and chronic pain were less likely to derive functional benefits from antidepressants (especially sertraline, escitalopram, and venlafaxine) than patients with depression but no chronic pain. Compared to patients with depression but no chronic pain, those with comorbid chronic pain and depression were also more likely to have had a recent suicide attempt, use tobacco and misuse painkillers. To further assess differences in effectiveness between specific antidepressants, targeted interventional trials can directly address the other phenotypes not captured in the current study. Nevertheless, our large-scale data-driven approach—like recent human genetic (36–52) and neuroimaging (57–59) antidepressant treatment studies—have revealed novel insights into the relationship between chronic pain and depression. Along with animal model and human pharmacogenetic studies (53–56, 116, 117, 121–125), there is also independent converging evidence for the critical role of subcortical brain regions in mediating pain and mood (126). The application of rigorous statistical genetics methodologies to large-scale neuroimaging data, for example, has already produced several major discoveries, such as advancing our understanding of causal pathways (subcortical), brain networks and medication response markers in mood disorders (127–133). Our study suggests pharmacoepidemiological approaches in psychiatry and pain medicine research will be increasingly valuable as a cost-effective, first-line strategy to enhance the design, feasibility, and clinical utility of randomized controlled trials (134), particularly as they routinely exclude patients with specific comorbidities and thus are not representative of the inherent individual variation across the population (104, 107, 135). Finally, the current study also has important implications for Australia's mental health system and chronic disease policy reforms, such as addressing problems concerning medication overprescription and effectiveness, their side-effects and suicide prevention (136–138).
Data Availability Statement
The datasets generated for this article are not readily available because Access to the data in this study, even in anonymized formats is restricted due to ethical considerations. Access to the dataset can be granted only after review and approval by the QIMR Berghofer Human and Research Ethics Committee as well as the studies' private investigators. Requests to access the datasets should be directed to nick. martin@qimrberghofer.edu.au.
Ethics Statement
The studies involving human participants were reviewed and approved by QIMR Berghofer Medical Research Institute's Human Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
WR, AC, TN, and MR designed this study and wrote the first version of the manuscript. AC performed the analyses with input from MR, TN, and WR. NM, SM, NW, and IH designed and directed the AGDS data collection efforts. NM and ML led the PISA study data collection efforts. TN designed the pain module in both the AGDS & PISA online surveys and conceived the genetic & epidemiological investigation of comorbid pain & depression in these cohorts. LG-M and GC-P contributed to data analyses. All authors contributed to the interpretation of the results and provided feedback on the preliminary versions of the manuscript.
Conflict of Interest
IH has been Commissioner of Australia's National Mental Health Commission (2012–2018); Co-director of Health and Policy at the Brain and Mind Centre, University of Sydney; leading community-based and pharmaceutical industry-supported projects (Wyeth, Eli Lilly, Servier, Pfizer, AstraZeneca) focused on the identification and better management of anxiety and depression; a member of the Medical Advisory Panel for Medibank Private until October 2017; a board member of Psychosis Australia Trust; a member of the Veterans Mental Health Clinical Reference Group; and Chief Scientific Advisor to and an equity shareholder in Innowell. GC-P contributed to this study while employed at The University of Queensland. He is now an employee of 23andMe Inc and he may hold stock or stock options from the company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank our colleagues Richard Parker, Simone Crossm, and Kerrie McAloney for their valuable work coordinating all the administrative and operational aspects of the AGDS and PISA projects.
Footnotes
Funding. AC and LG-M were supported by UQ Research Training Scholarships from The University of Queensland (UQ). MR thanks the support of NHMRC and the Australian Research Council (ARC), through an NHMRC-ARC Dementia Research Development Fellowship (GNT1102821). The views expressed are those of the authors and not necessarily those of the affiliated or funding institutions. Data collection for AGDS was possible thanks to funding from the Australian National Health & Medical Research Council (NHMRC) to NM (GNT1086683). PISA was possible thanks to an NHMRC Dementia Research Team Grant administered by QIMR Berghofer Medical Research Institute (GNT1095227).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.643609/full#supplementary-material
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392:1789–858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mora C, Zonca V, Riva MA, Cattaneo A. Blood biomarkers and treatment response in major depression. Expert Rev Mol Diagn. (2018) 18:513–29. 10.1080/14737159.2018.1470927 [DOI] [PubMed] [Google Scholar]
- 3.Jaracz J, Gattner K, Jaracz K, Górna K. Unexplained painful physical symptoms in patients with major depressive disorder: prevalence, pathophysiology and management. CNS Drugs. (2016) 30:293–304. 10.1007/s40263-016-0328-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooten WM. Chronic pain and mental health disorders: shared neural mechanisms, epidemiology, and treatment. Mayo Clin Proc. (2016) 91:955–70. 10.1016/j.mayocp.2016.04.029 [DOI] [PubMed] [Google Scholar]
- 5.Armbrecht E, Shah A, Schepman P, Shah R, Pappadopulos E, Chambers R, et al. Economic and humanistic burden associated with non-communicable diseases among adults with depression and anxiety in the United States. J Med Econ. (2020) 23:1032–42. 10.1080/13696998.2020.1776297 [DOI] [PubMed] [Google Scholar]
- 6.Dhanju S, Kennedy SH, Abbey S, Katz J, Weinrib A, Clarke H, et al. The impact of comorbid pain and depression in the United States: results from a nationally representative survey. Scand J Pain. (2019) 19:319–25. 10.1515/sjpain-2018-0323 [DOI] [PubMed] [Google Scholar]
- 7.Treede R-D, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain. (2019) 160:19–27. 10.1097/j.pain.0000000000001384 [DOI] [PubMed] [Google Scholar]
- 8.Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. A classification of chronic pain for ICD-11. Pain. (2015) 156:1003–7. 10.1097/j.pain.0000000000000160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Australian Institute of Health and Welfare . Health Expenditure Australia 2018-19. (2020). Available online at: https://www.aihw.gov.au/reports/health-welfare-expenditure/health-expenditure-australia-2018-19/contents/data-visualisation (accessed December 4, 2020).
- 10.Australian Institute of Health and Welfare . Chronic Pain in Australia. Canberra, ACT: Australian Government; (2020). [Google Scholar]
- 11.Tardif H, Blanchard MB, White JM, Bryce MP. Normative Data for Adults Referred for Specialist Pain Management in Australia. (2018). Available online at: https://ro.uow.edu.au/ahsri/940 (accessed November 23, 2020).
- 12.Deloitte Access Economics . The Cost of Pain in Australia. Canberra, ACT: (2019). [Google Scholar]
- 13.Gaskin DJ, Richard P, Walburn J. The economical impact of pain. In: Saba L. ed Neuroimaging of Pain. Cham: Springer International Publishing; (2017). p. 1–17. 10.1007/978-3-319-48046-6_1 [DOI] [Google Scholar]
- 14.Lin SX, Patel K, Younge RG. Opioid medications prescribing and the pain-depression dyad in primary care: analysis of 2014–2015 National Ambulatory Medical Care Survey (NAMCS) data. J Am Board Family Med. (2019) 32:614–8. 10.3122/jabfm.2019.04.180311 [DOI] [PubMed] [Google Scholar]
- 15.Zis P, Daskalaki A, Bountouni I, Sykioti P, Varrassi G, Paladini A. Depression and chronic pain in the elderly: links and management challenges. Clin Interv Aging. (2017) 12:709–20. 10.2147/CIA.S113576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain. (1997) 13:116–37. 10.1097/00002508-199706000-00006 [DOI] [PubMed] [Google Scholar]
- 17.Viana MC, Lim CCW, Garcia Pereira F, Aguilar-Gaxiola S, Alonso J, Bruffaerts R, et al. Previous mental disorders and subsequent onset of chronic back or neck pain: findings from 19 countries. J Pain. (2018) 19:99–110. 10.1016/j.jpain.2017.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bondesson E, Larrosa Pardo F, Stigmar K, Ringqvist Å, Petersson IF, Jöud A, et al. Comorbidity between pain and mental illness - Evidence of a bidirectional relationship. Eur J Pain. (2018) 22:1304–11. 10.1002/ejp.1218 [DOI] [PubMed] [Google Scholar]
- 19.Antioch I, Ilie O-D, Ciobica A, Doroftei B, Fornaro M. Preclinical considerations about affective disorders and pain: a broadly intertwined, yet often under-explored, relationship having major clinical implications. Medicina. (2020) 56:504. 10.3390/medicina56100504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerrits MM, van Oppen P, Leone SS, van Marwijk HWJ, van der Horst HE, Penninx BW. Pain, not chronic disease, is associated with the recurrence of depressive and anxiety disorders. BMC Psychiatry. (2014) 14:187. 10.1186/1471-244X-14-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerrits MMJG, Marloes MJ, van Oppen P, van Marwijk HWJ, Brenda WJ, van der Horst HE. Pain and the onset of depressive and anxiety disorders. Pain. (2014) 155:53–9. 10.1016/j.pain.2013.09.005 [DOI] [PubMed] [Google Scholar]
- 22.Fava M, Wiltse C, Walker D, Brecht S, Chen A, Perahia D. Predictors of relapse in a study of duloxetine treatment in patients with major depressive disorder. J Affect Disord. (2009) 113:263–71. 10.1016/j.jad.2008.05.023 [DOI] [PubMed] [Google Scholar]
- 23.DeVeaugh-Geiss AM, West SL, Miller WC, Sleath B, Gaynes BN, Kroenke K. The adverse effects of comorbid pain on depression outcomes in primary care patients: results from the ARTIST trial. Pain Med. (2010) 11:732–41. 10.1111/j.1526-4637.2010.00830.x [DOI] [PubMed] [Google Scholar]
- 24.Novick D, Montgomery W, Aguado J, Kadziola Z, Peng X, Brugnoli R, et al. Which somatic symptoms are associated with an unfavorable course in Asian patients with major depressive disorder? J Affect Disord. (2013) 149:182–8. 10.1016/j.jad.2013.01.020 [DOI] [PubMed] [Google Scholar]
- 25.Means-Christensen AJ, Roy-Byrne PP, Sherbourne CD, Craske MG, Stein MB. Relationships among pain, anxiety, and depression in primary care. Depress Anxiety. (2008) 25:593–600. 10.1002/da.20342 [DOI] [PubMed] [Google Scholar]
- 26.Rhee TG, Mohamed S, Rosenheck RA. Stages of major depressive disorder and behavioral multi-morbidities: findings from nationally representative epidemiologic study. J Affect Disord. (2021) 278:443–52. 10.1016/j.jad.2020.09.081 [DOI] [PubMed] [Google Scholar]
- 27.Liu M, McCurry SM, Belza B, Dobra A, Buchanan DT, Vitiello MV, et al. Effects of osteoarthritis pain and concurrent insomnia and depression on health care use in a primary care population of older adults. Arthritis Care Res. (2019) 71:748–57. 10.1002/acr.23695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sil S, Cohen LL, Dampier C. Psychosocial and functional outcomes in youth with chronic sickle cell pain. Clin J Pain. (2016) 32:527–33. 10.1097/AJP.0000000000000289 [DOI] [PubMed] [Google Scholar]
- 29.de Heer EW, Gerrits MMJG, Beekman ATF, Dekker J, van Marwijk HWJ, de Waal MWM, et al. The association of depression and anxiety with pain: a study from NESDA. PLoS ONE. (2014) 9:e106907. 10.1371/journal.pone.0106907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angst F, Benz T, Lehmann S, Wagner S, Simmen BR, Sandòr PS, et al. Extended overview of the longitudinal pain-depression association: a comparison of six cohorts treated for specific chronic pain conditions. J Affect Disord. (2020) 273:508–16. 10.1016/j.jad.2020.05.044 [DOI] [PubMed] [Google Scholar]
- 31.Reed C, Hong J, Novick D, Lenox-Smith A, Happich M. Health care costs before and after diagnosis of depression in patients with unexplained pain: a retrospective cohort study using the United Kingdom general practice research database. Clinicoecon Outcomes Res. (2013) 5:37–47. 10.2147/CEOR.S38323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. (2016) 6:e010364. 10.1136/bmjopen-2015-010364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaakxs R, Comijs HC, van der Mast RC, Schoevers RA, Beekman ATF, Penninx BWJH. Risk factors for depression: differential across age? Am J Geriatr Psychiatry. (2017) 25:966–77. 10.1016/j.jagp.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 34.Case A, Deaton A, Stone AA. Decoding the mystery of American pain reveals a warning for the future. Proc Natl Acad Sci USA. (2020) 117:24785–9. 10.1073/pnas.2012350117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Institute for Health and Care Excellence . Guideline Chronic Pain in over 16s: Assessment and Management (Draft for Consultation). NICE; (2020). Available online at: https://www.nice.org.uk/guidance/gid-ng10069/documents/draft-guideline (accessed August 17, 2020). [Google Scholar]
- 36.Johnston KJA, Adams MJ, Nicholl BI, Ward J, Strawbridge RJ, Ferguson A, et al. Genome-wide association study of multisite chronic pain in UK Biobank. PLoS Genet. (2019) 15:e1008164. 10.1371/journal.pgen.1008164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundberg M, Campos AI, Farrell SF, Wang G, Sterling M, Renteria ME, et al. Genetic, lifestyle and environmental risk factors for chronic pain revealed through GWAS. bioRxiv [preprint]. (2020). 10.1101/2020.05.26.115568 [DOI] [Google Scholar]
- 38.Shen X, Howard DM, Adams MJ, David Hill W, Clarke T-K, Deary IJ, et al. A phenome-wide association and mendelian randomisation study of polygenic risk for depression in UK Biobank. Nat Commun. (2020) 11:1–16. 10.1038/s41467-020-16022-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun J, Yan W, Zhang X-N, Lin X, Li H, Gong Y-M, et al. Polygenic evidence and overlapped brain functional connectivities for the association between chronic pain and sleep disturbance. Transl Psychiatry. (2020) 10:252. 10.1038/s41398-020-00941-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnston KJA, Ward J, Ray PR, Adams MJ, McIntosh AM, Smith BH, et al. Sex-stratified genome-wide association study of multisite chronic pain in UK biobank. medRxiv [preprint]. (2020). 10.1101/2020.06.25.20140087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLean SA, Ressler K, Koenen KC, Neylan T, Germine L, Jovanovic T, et al. The AURORA Study: a longitudinal, multimodal library of brain biology and function after traumatic stress exposure. Mol Psychiatry. (2020) 25:283–96. 10.1038/s41380-019-0581-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levey DF, Stein MB, Wendt FR, Pathak GA, Zhou H, Aslan M, et al. GWAS of depression phenotypes in the million veteran program and meta-analysis in more than 1.2 million participants yields 178 independent risk loci. medRxiv [preprint]. (2020). 10.1101/2020.05.18.20100685 [DOI] [Google Scholar]
- 43.Bortsov AV, Parisien M, Khoury S, Zaykin DV, Martinsen AE, Lie MU, et al. Genome-wide analysis identifies significant contribution of brain-expressed genes in chronic, but not acute, back pain. medRxiv [preprint]. (2020). 10.1101/2020.09.04.20187575 [DOI] [Google Scholar]
- 44.Nilufer R, Karina B, Paraskevi C, Rebecca D. Large-scale genome-wide association meta-analysis of endometriosis reveals 13 novel loci and genetically-associated comorbidity with other pain conditions. BioRxiv [preprint]. (2018). 10.1101/406967 [DOI] [Google Scholar]
- 45.Rahman MS, Winsvold BS, Chavez SOC, Borte S, Tsepilov YA, Shapov SZ, et al. Genome-wide association study identifies RNF123 locus as associated with chronic widespread musculoskeletal pain. medRxiv [preprint]. (2020). 10.1101/2020.11.30.20241000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meng W, Adams MJ, Reel P, Rajendrakumar A, Huang Y, Deary IJ, et al. Genetic correlations between pain phenotypes and depression and neuroticism. Eur J Hum Genet. (2020) 28:358–66. 10.1038/s41431-019-0530-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meng W, Chan BW, Harris C, Freidin MB, Hebert HL, Adams MJ, et al. A genome-wide association study finds genetic variants associated with neck or shoulder pain in UK Biobank. Hum Mol Genet. (2020) 29:1396–404. 10.1093/hmg/ddaa058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosoff DB, Smith GD, Lohoff FW. Prescription opioid use and risk for major depressive disorder and anxiety and stress-related disorders: a multivariable mendelian randomization analysis. JAMA Psychiatry. (2020) 78:151–60. 10.1001/jamapsychiatry.2020.3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsepilov YA, Freidin MB, Shadrina AS, Sharapov SZ, Elgaeva EE, van Zundert J, et al. Analysis of genetically independent phenotypes identifies shared genetic factors associated with chronic musculoskeletal pain conditions. Commun Biol. (2020) 3:329. 10.1038/s42003-020-1051-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adewuyi EO, Mehta D, Sapkota Y, International Endogene Consortium, 23andMe Research Team. Auta A, Yoshihara K, et al. Genetic analysis of endometriosis and depression identifies shared loci and implicates causal links with gastric mucosa abnormality. Hum Genet. (2021) 140:529–52. 10.1007/s00439-020-02223-6 [DOI] [PubMed] [Google Scholar]
- 51.Johnson JS, Cote AC, Dobbyn A, Sloofman LG, Xu J. The phenome-wide consequences of anorexia nervosa genes. medRxiv [preprint]. (2021). 10.1101/2021.02.12.21250941 [DOI] [Google Scholar]
- 52.Smail MA, Wu X, Henkel ND, Eby HM, Herman JP, McCullumsmith RE, et al. Similarities and dissimilarities between psychiatric cluster disorders. Mol Psychiatry. (2021). 10.1038/s41380-021-01030-3. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou W, Jin Y, Meng Q, Zhu X, Bai T, Tian Y, et al. A neural circuit for comorbid depressive symptoms in chronic pain. Nat Neurosci. (2019) 22:1649–58. 10.1038/s41593-019-0468-2 [DOI] [PubMed] [Google Scholar]
- 54.Kremer M, Becker LJ, Barrot M, Yalcin I. How to study anxiety and depression in rodent models of chronic pain? Eur J Neurosci. (2020) 53:236–70. 10.1111/ejn.14686 [DOI] [PubMed] [Google Scholar]
- 55.Bravo L, Llorca-Torralba M, Suárez-Pereira I, Berrocoso E. Pain in neuropsychiatry: insights from animal models. Neurosci Biobehav Rev. (2020) 115:96–115. 10.1016/j.neubiorev.2020.04.029 [DOI] [PubMed] [Google Scholar]
- 56.Cunha AM, Pereira-Mendes J, Almeida A, Guimarães MR, Leite-Almeida H. Chronic pain impact on rodents' behavioral repertoire. Neurosci Biobehav Rev. (2020) 119:101–27. 10.1016/j.neubiorev.2020.09.022 [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Bernanke J, Peterson BS, McGrath P, Stewart J, Chen Y, et al. The association between antidepressant treatment and brain connectivity in two double-blind, placebo-controlled clinical trials: a treatment mechanism study. Lancet Psychiatry. (2019) 6:667–74. 10.1016/S2215-0366(19)30179-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheline YI, Yu M. Linking antidepressant performance with pain network connectivity. Lancet Psychiatry. (2019) 6:635–6. 10.1016/S2215-0366(19)30250-0 [DOI] [PubMed] [Google Scholar]
- 59.Brown V, Peciña M. Neuroimaging studies of antidepressant placebo effects: challenges and opportunities. Front Psychiatry. (2019) 10:669. 10.3389/fpsyt.2019.00669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.IsHak WW, Wen RY, Naghdechi L, Vanle B, Dang J, Knosp M, et al. Pain and depression: a systematic review. Harv Rev Psychiatry. (2018) 26:352–63. 10.1097/HRP.0000000000000198 [DOI] [PubMed] [Google Scholar]
- 61.Moore RA, Andrew Moore R, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain in adults. Cochrane Datab Syst Rev. (2015) 2015:CD008242. 10.1002/14651858.CD008242.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brueckle M-S, Thomas ET, Seide SE, Pilz M, Gonzalez-Gonzalez AI, Nguyen TS, et al. Adverse drug reactions associated with amitriptyline — protocol for a systematic multiple-indication review and meta-analysis. Syst Rev. (2020) 9:59. 10.1186/s13643-020-01296-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gebhardt S, Heinzel-Gutenbrunner M, König U. Pain relief in depressive disorders: a meta-analysis of the effects of antidepressants. J Clin Psychopharmacol. (2016) 36:658–68. 10.1097/JCP.0000000000000604 [DOI] [PubMed] [Google Scholar]
- 64.Skånland SS, Cieślar-Pobuda A. Off-label uses of drugs for depression. Eur J Pharmacol. (2019) 865:172732. 10.1016/j.ejphar.2019.172732 [DOI] [PubMed] [Google Scholar]
- 65.Caruso R, Ostuzzi G, Turrini G, Ballette F, Recla E, Dall'Olio R, et al. Beyond pain: can antidepressants improve depressive symptoms and quality of life in patients with neuropathic pain? A systematic review and meta-analysis. Pain. (2019) 160:2186–98. 10.1097/j.pain.0000000000001622 [DOI] [PubMed] [Google Scholar]
- 66.Urits I, Peck J, Orhurhu MS, Wolf J, Patel R, Orhurhu V, et al. Off-label antidepressant use for treatment and management of chronic pain: evolving understanding and comprehensive review. Curr Pain Headache Rep. (2019) 23:66. 10.1007/s11916-019-0803-z [DOI] [PubMed] [Google Scholar]
- 67.Stubbs B, Vancampfort D, Veronese N, Thompson T, Fornaro M, Schofield P, et al. Depression and pain: primary data and meta-analysis among 237 952 people across 47 low- and middle-income countries. Psychol Med. (2017) 47:2906–17. 10.1017/S0033291717001477 [DOI] [PubMed] [Google Scholar]
- 68.Vwaire Orhurhu MD, Mayowa Olusunmade MD, Yinka Akinola MD, Ivan Urits MD. Depression trends in patients with chronic pain: an analysis of the nationwide inpatient sample. Pain Phys. (2019) 22:E487–94. 10.36076/ppj/2019.22.E487 [DOI] [PubMed] [Google Scholar]
- 69.Gold SM, Köhler-Forsberg O, Moss-Morris R, Mehnert A, Miranda JJ, Bullinger M, et al. Comorbid depression in medical diseases. Nat Rev Dis Primers. (2020) 6:69. 10.1038/s41572-020-0200-2 [DOI] [PubMed] [Google Scholar]
- 70.Haibach JP, Beehler GP, Dollar KM, Finnell DS. Moving toward integrated behavioral intervention for treating multimorbidity among chronic pain, depression, and substance-use disorders in primary care. Med Care. (2014) 52:322–7. 10.1097/MLR.0000000000000098 [DOI] [PubMed] [Google Scholar]
- 71.Du L, Luo S, Liu G, Wang H, Zheng L, Zhang Y. The 100 top-cited studies about pain and depression. Front Psychol. (2019) 10:3072. 10.3389/fpsyg.2019.03072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bates D, Schultheis BC, Hanes MC, Jolly SM, Chakravarthy KV, Deer TR, et al. A comprehensive algorithm for management of neuropathic pain. Pain Med. (2019) 20:S2–12. 10.1093/pm/pnz075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moisset X, Bouhassira D, Avez Couturier J, Alchaar H, Conradi S, Delmotte MH, et al. Pharmacological and non-pharmacological treatments for neuropathic pain: systematic review and French recommendations. Revue Neurol. (2020) 176:325–52. 10.1016/j.neurol.2020.01.361 [DOI] [PubMed] [Google Scholar]
- 74.Davis KAS, Farooq S, Hayes JF, John A, Lee W, MacCabe JH, et al. Pharmacoepidemiology research: delivering evidence about drug safety and effectiveness in mental health. Lancet Psychiatry. (2020) 7:363–70. 10.1016/S2215-0366(19)30298-6 [DOI] [PubMed] [Google Scholar]
- 75.Byrne EM, Kirk KM, Medland SE, McGrath JJ, Colodro-Conde L, Parker R, et al. Cohort profile: the Australian genetics of depression study. BMJ Open. (2020) 10:e032580. 10.1136/bmjopen-2019-032580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lupton MK, Robinson GA, Adam RJ, Rose S, Byrne GJ, Salvado O, et al. A prospective cohort study of prodromal Alzheimer′s disease: prospective imaging study of ageing: genes, brain and behavior (PISA). medRxiv. (2020) 29:1. 10.1101/2020.05.04.20091140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Benyamin B, Ferreira MAR, Willemsen G, Gordon S, Middelberg RPS, McEvoy BP, et al. Common variants in TMPRSS6 are associated with iron status and erythrocyte volume. Nat Genet. (2009) 41:1173–5. 10.1038/ng.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heath AC, Whitfield JB, Martin NG, Pergadia ML, Goate AM, Lind PA, et al. A quantitative-trait genome-wide association study of alcoholism risk in the community: findings and implications. Biol Psychiatry. (2011) 70:513–8. 10.1016/j.biopsych.2011.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoyer J, Voss C, Strehle J, Venz J, Pieper L, Wittchen H-U, et al. Test-retest reliability of the computer-assisted DIA-X-5 interview for mental disorders. BMC Psychiatry. (2020) 20:280. 10.1186/s12888-020-02653-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shankman SA, Funkhouser CJ, Klein DN, Davila J, Lerner D, Hee D. Reliability and validity of severity dimensions of psychopathology assessed using the Structured Clinical Interview for DSM-5 (SCID). Int J Methods Psychiatr Res. (2018) 27:e1590. 10.1002/mpr.1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. (1992) 50:133–49. 10.1016/0304-3959(92)90154-4 [DOI] [PubMed] [Google Scholar]
- 82.van Spijker BAJ, Batterham PJ, Calear AL, Farrer L, Christensen H, Reynolds J, et al. The suicidal ideation attributes scale (SIDAS): community-based validation study of a new scale for the measurement of suicidal ideation. Suicide Life Threat Behav. (2014) 44:408–19. 10.1111/sltb.12084 [DOI] [PubMed] [Google Scholar]
- 83.Fishbain DA, Cole B, Lewis JE, Gao J. Does pain interfere with antidepressant depression treatment response and remission in patients with depression and pain? An evidence-based structured review. Pain Med. (2014) 15:1522–39. 10.1111/pme.12448 [DOI] [PubMed] [Google Scholar]
- 84.Bair MJ, Robinson RL, Eckert GJ, Stang PE, Croghan TW, Kroenke K. Impact of pain on depression treatment response in primary care. Psychosom Med. (2004) 66:17–22. 10.1097/01.PSY.0000106883.94059.C5 [DOI] [PubMed] [Google Scholar]
- 85.Thaler KJ, Morgan LC, Van Noord M, Gaynes BN, Hansen RA, Lux LJ, et al. Comparative effectiveness of second-generation antidepressants for accompanying anxiety, insomnia, and pain in depressed patients: a systematic review. Depress Anxiety. (2012) 29:495–505. 10.1002/da.21951 [DOI] [PubMed] [Google Scholar]
- 86.Holmes A, Christelis N, Arnold C. Depression and chronic pain. Med J Aust. (2013) 199:S17–20. 10.5694/mja12.10589 [DOI] [PubMed] [Google Scholar]
- 87.Mills SEE, Nicolson KP, Smith BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth. (2019) 123:e273–83. 10.1016/j.bja.2019.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bierman A, Lee Y. Chronic pain and psychological distress among older adults: a national longitudinal study. Res Aging. (2018) 40:432–55. 10.1177/0164027517704970 [DOI] [PubMed] [Google Scholar]
- 89.Goosby BJ. Early life course pathways of adult depression and chronic pain. J Health Soc Behav. (2013) 54:75–91. 10.1177/0022146512475089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Van Hecke O, Torrance N, Smith BH. Chronic pain epidemiology and its clinical relevance. Br J Anaesth. (2013) 111:13–8. 10.1093/bja/aet123 [DOI] [PubMed] [Google Scholar]
- 91.Racine M. Chronic pain and suicide risk: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. (2018) 87:269–80. 10.1016/j.pnpbp.2017.08.020 [DOI] [PubMed] [Google Scholar]
- 92.Bailly F, Belaid H. Suicidal ideation and suicide attempt associated with antidepressant and antiepileptic drugs: Implications for treatment of chronic pain. Joint Bone Spine. (2020) 88:105005. 10.1016/j.jbspin.2020.04.016 [DOI] [PubMed] [Google Scholar]
- 93.Calati R, Laglaoui Bakhiyi C, Artero S, Ilgen M, Courtet P. The impact of physical pain on suicidal thoughts and behaviors: Meta-analyses. J Psychiatr Res. (2015) 71:16–32. 10.1016/j.jpsychires.2015.09.004 [DOI] [PubMed] [Google Scholar]
- 94.Edwards RR, Smith MT, Kudel I, Haythornthwaite J. Pain-related catastrophizing as a risk factor for suicidal ideation in chronic pain. Pain. (2006) 126:272–9. 10.1016/j.pain.2006.07.004 [DOI] [PubMed] [Google Scholar]
- 95.Tang NKY, Beckwith P, Ashworth P. Mental Defeat is associated with suicide intent in patients with chronic pain. Clin J Pain. (2016) 32:411–9. 10.1097/AJP.0000000000000276 [DOI] [PubMed] [Google Scholar]
- 96.Breslau N, Schultz L, Lipton R, Peterson E, Welch KMA. Migraine headaches and suicide attempt. Headache. (2012) 52:723–31. 10.1111/j.1526-4610.2012.02117.x [DOI] [PubMed] [Google Scholar]
- 97.Handley T, Rich J, Davies K, Lewin T, Kelly B. The challenges of predicting suicidal thoughts and behaviours in a sample of rural Australians with depression. Int J Environ Res Public Health. (2018) 15:928. 10.3390/ijerph15050928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hinze V, Crane C, Ford T, Buivydaite R, Qiu L, Gjelsvik B. The relationship between pain and suicidal vulnerability in adolescence: a systematic review. Lancet Child Adolesc Health. (2019) 3:899–916. 10.1016/S2352-4642(19)30267-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Santos J, Martins S, Azevedo LF, Fernandes L. Pain as a risk factor for suicidal behavior in older adults: a systematic review. Arch Gerontol Geriatr. (2020) 87:104000. 10.1016/j.archger.2019.104000 [DOI] [PubMed] [Google Scholar]
- 100.Petrosky E, Harpaz R, Fowler KA, Bohm MK, Helmick CG, Yuan K, et al. Chronic pain among suicide decedents, 2003 to 2014: findings from the national violent death reporting system. Ann Intern Med. (2018) 169:448–55. 10.7326/M18-0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kirtley OJ, Rodham K, Crane C. Understanding suicidal ideation and behaviour in individuals with chronic pain: a review of the role of novel transdiagnostic psychological factors. Lancet Psychiatry. (2020) 7:282–90. 10.1016/S2215-0366(19)30288-3 [DOI] [PubMed] [Google Scholar]
- 102.Barrett K, Chang YP. Behavioral interventions targeting chronic pain, depression, and substance use disorder in primary care. J Nurs Scholarsh. (2016) 48:345–53. 10.1111/jnu.12213 [DOI] [PubMed] [Google Scholar]
- 103.Scherf-Clavel M, Breisinger S, Fischer M, Deckert J, Unterecker S, Rittner HL. Therapeutic drug monitoring of antidepressants for the treatment of chronic musculoskeletal pain with and without depression. Ther Drug Monit. (2020) 42:893–901. 10.1097/FTD.0000000000000783 [DOI] [PubMed] [Google Scholar]
- 104.Baune BT, Boyce P, Morris G, Hamilton A, Bassett D, Hopwood M, et al. Organising the front line: Is there a rationale for the first-line pharmacotherapy of major depressive disorder? Austr N Zeal J Psychiatry. (2019) 53:279–81. 10.1177/0004867418824026 [DOI] [PubMed] [Google Scholar]
- 105.Pan Y-J, Kuo K-H, Wang S-J. Pharmacological treatment of depression with and without headache disorders: an appraisal of cost effectiveness and cost utility of antidepressants. J Affect Disord. (2015) 170:255–65. 10.1016/j.jad.2014.08.034 [DOI] [PubMed] [Google Scholar]
- 106.Pradier MF, McCoy TH, Jr, Hughes M, Perlis RH, Doshi-Velez F. Predicting treatment dropout after antidepressant initiation. Transl Psychiatry. (2020) 10:60. 10.1038/s41398-020-0716-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kendrick T, Taylor D, Johnson CF. Which first-line antidepressant? Br J Gen Pract. (2019) 69:114–5. 10.3399/bjgp19X701405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tomlinson A, Efthimiou O, Boaden K, New E, Mather S, Salanti G, et al. Side effect profile and comparative tolerability of 21 antidepressants in the acute treatment of major depression in adults: protocol for a network meta-analysis. Evid Based Ment Health. (2019) 22:61–6. 10.1136/ebmental-2019-300087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kernot C, Tomlinson A, Chevance A, Cipriani A. One step closer to personalised prescribing of antidepressants: using real-world data together with patients and clinicians' preferences. Evid Based Ment Health. (2019) 22:91–2. 10.1136/ebmental-2019-300105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sinyor M, Cheung CP, Abraha HY, Lanctôt KL, Saleem M, Liu CS, et al. Antidepressant-placebo differences for specific adverse events in major depressive disorder: a systematic review. J Affect Disord. (2020) 267:185–90. 10.1016/j.jad.2020.02.013 [DOI] [PubMed] [Google Scholar]
- 111.Dragioti E, Solmi M, Favaro A, Fusar-Poli P, Dazzan P, Thompson T, et al. Association of antidepressant use with adverse health outcomes: a systematic umbrella review. JAMA Psychiatry. (2019) 76:1241–55. 10.1001/jamapsychiatry.2019.2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dragioti E, Evangelou E, Larsson B, Gerdle B. Effectiveness of multidisciplinary programmes for clinical pain conditions: an umbrella review. J Rehabil Med. (2018) 50:779–91. 10.2340/16501977-2377 [DOI] [PubMed] [Google Scholar]
- 113.Tardif H, Arnold C, Hayes C, Eagar K. Establishment of the Australasian electronic persistent pain outcomes collaboration. Pain Med. (2016) 18:1007–18. 10.1093/pm/pnw201 [DOI] [PubMed] [Google Scholar]
- 114.Lord SM, Tardif HP, Kepreotes EA, Blanchard M, Eagar K. The paediatric electronic persistent pain outcomes collaboration (PaedePPOC). Pain. (2019) 160:1572–85. 10.1097/j.pain.0000000000001548 [DOI] [PubMed] [Google Scholar]
- 115.Nicholas MK, Costa DSJ, Blanchard M, Tardif H, Asghari A, Blyth FM. Normative data for common pain measures in chronic pain clinic populations: closing a gap for clinicians and researchers. Pain. (2019) 160:1156–65. 10.1097/j.pain.0000000000001496 [DOI] [PubMed] [Google Scholar]
- 116.Yamamoto PA, Conchon Costa AC, Lauretti GR, de Moraes NV. Pharmacogenomics in chronic pain therapy: from disease to treatment and challenges for clinical practice. Pharmacogenomics. (2019) 20:971–82. 10.2217/pgs-2019-0066 [DOI] [PubMed] [Google Scholar]
- 117.Kaye AD, Garcia AJ, Hall OM, Jeha GM, Cramer KD, Granier AL, et al. Update on the pharmacogenomics of pain management. Pharmgenomics Pers Med. (2019) 12:125–43. 10.2147/PGPM.S179152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Velly AM, Mohit S. Epidemiology of pain and relation to psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. (2018) 87:159–67. 10.1016/j.pnpbp.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 119.Slomski A. Important conversations are needed to explain the nocebo effect. JAMA. (2021) 325:707–9. 10.1001/jama.2020.25840 [DOI] [PubMed] [Google Scholar]
- 120.Fabbri C, Hagenaars SP, John C, Williams AT, Shrine N, Moles L, et al. Genetic and clinical characteristics of treatment-resistant depression using primary care records in two UK cohorts. Mol Psychiatry. (2021). 10.1038/s41380-021-01062-9. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maciukiewicz M, Marshe VS, Tiwari AK, Fonseka TM, Freeman N, Kennedy JL, et al. Genome-wide association studies of placebo and duloxetine response in major depressive disorder. Pharmacogenomics J. (2018) 18:406–12. 10.1038/tpj.2017.29 [DOI] [PubMed] [Google Scholar]
- 122.Dorfman R, London Z, Metias M, Kabakchiev B, Mukerjee G, Moser A. Individualized medication management in ontario long-term care clinical impact on management of depression, pain, and dementia. J Am Med Dir Assoc. (2020) 21:823–9.e5. 10.1016/j.jamda.2020.04.009 [DOI] [PubMed] [Google Scholar]
- 123.Bousman CA, Arandjelovic K, Mancuso SG, Eyre HA, Dunlop BW. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. (2019) 20:37–47. 10.2217/pgs-2018-0142 [DOI] [PubMed] [Google Scholar]
- 124.Milosavljevic F, Bukvic N, Pavlovic Z, Miljevic C, Pešic V, Molden E, et al. Association of CYP2C19 and CYP2D6 poor and intermediate metabolizer status with antidepressant and antipsychotic exposure: a systematic review and meta-analysis. JAMA Psychiatry. (2020) 78:270–80. 10.1001/jamapsychiatry.2020.3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bousman CA, Bengesser SA, Aitchison KJ, Amare AT, Aschauer H, Baune BT, et al. Review and consensus on pharmacogenomic testing in psychiatry. Pharmacopsychiatry. (2020) 54:5–17. 10.1055/a-1288-1061 [DOI] [PubMed] [Google Scholar]
- 126.Kuner R, Kuner T. Cellular circuits in the brain and their modulation in acute and chronic pain. Physiol Rev. (2021) 101:213–58. 10.1152/physrev.00040.2019 [DOI] [PubMed] [Google Scholar]
- 127.Thompson PM, Jahanshad N, Ching CRK, Salminen LE, Thomopoulos SI, Bright J, et al. ENIGMA and global neuroscience: a decade of large-scale studies of the brain in health and disease across more than 40 countries. Transl Psychiatry. (2020) 10:100. 10.1038/s41398-020-0705-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schmaal L, Pozzi E, Ho T, van Velzen LS, Veer IM, Opel N, et al. ENIGMA MDD: seven years of global neuroimaging studies of major depression through worldwide data sharing. Transl Psychiatry. (2020) 10:172. 10.1038/s41398-020-0842-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Satizabal CL, Adams HHH, Hibar DP, White CC, Knol MJ, Stein JL, et al. Genetic architecture of subcortical brain structures in 38,851 individuals. Nat Genet. (2019) 51:1624–36. 10.1038/s41588-019-0511-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Buch AM, Liston C. Dissecting diagnostic heterogeneity in depression by integrating neuroimaging and genetics. Neuropsychopharmacology. (2020) 46:156–75. 10.1038/s41386-020-00789-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hibar DP, Stein JL, Renteria ME, Arias-Vasquez A, Desrivières S, Jahanshad N, et al. Common genetic variants influence human subcortical brain structures. Nature. (2015) 520:224–9. 10.1038/nature14101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hibar DP, Westlye LT, Doan NT, Jahanshad N, Cheung JW, Ching CRK, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA bipolar disorder working group. Mol Psychiatry. (2017) 23:932–42. 10.1038/mp.2017.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Medland SE, Grasby KL, Jahanshad N, Painter JN, Colodro-Conde L, Bralten J, et al. Ten years of enhancing neuro-imaging genetics through meta-analysis: An overview from the ENIGMA genetics working group. Hum Brain Mapp. (2020). 10.1002/hbm.25311. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. (2018) 391:1357–66. 10.1016/S0140-6736(17)32802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gutsmiedl K, Krause M, Bighelli I, Schneider-Thoma J, Leucht S. How well do elderly patients with major depressive disorder respond to antidepressants: a systematic review and single-group meta-analysis. BMC Psychiatry. (2020) 20:102. 10.1186/s12888-020-02514-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Whitely M, Raven M, Jureidini J. Antidepressant prescribing and suicide/self-harm by young australians: regulatory warnings, contradictory advice, and long-term trends. Front Psychiatry. (2020) 11:478. 10.3389/fpsyt.2020.00478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pain Australia . The National Strategic Action Plan for Pain Management. (2019). Available online at: https://www.painaustralia.org.au/media/newsletters/89/the-national-strategic-action-plan-for-pain-management (accessed December 3, 2020).
- 138.Productivity Commission . Mental Health Report no.95. (2020). Available online at: https://www.pc.gov.au/inquiries/completed/mental-health/report (accessed November 13, 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this article are not readily available because Access to the data in this study, even in anonymized formats is restricted due to ethical considerations. Access to the dataset can be granted only after review and approval by the QIMR Berghofer Human and Research Ethics Committee as well as the studies' private investigators. Requests to access the datasets should be directed to nick. martin@qimrberghofer.edu.au.