Abstract
Human mesenchymal stem/stromal cell (hMSC)-based cell therapies are promising for treating a variety of diseases. The unique immunomodulatory properties of hMSCs have extended their therapeutic potential beyond tissue regeneration. However, extensive pre-clinical culture expansion inevitably drives cells toward replicative “aging” and a consequent decline in quality. These “in vitro-aged” hMSCs resemble biologically aged cells, which have been reported to show senescence signatures, diminished immunosuppressive capacity, and weakened regenerative potential as well as pro-inflammatory features. In this review, we have surveyed the literature to explore the intimate relationship between the inflammatory status of hMSCs and their in vitro aging process. We posit that a shift from an anti-inflammatory to a pro-inflammatory phenotype of culture-expanded hMSCs contributes to a deterioration in their therapeutic efficacy. Potential molecular and cellular mechanisms underpinning this phenomenon have been discussed. We have also highlighted studies that leverage these mechanisms to make culture-expanded hMSCs more amenable for clinical use.
Keywords: rejuvenating mesenchymal stem cells, allogeneic stem cell therapy, aging, inflammation, immunomodulation, SASP, heparan sulfate, extracellular matrix, glycosaminoglycan, secretome
Graphical abstract

Highlights
-
•
Aged MSCs have reduced immunosuppressive potential
-
•
Chronic inflammatory microenvironments can exacerbate MSC senescence and aging
-
•
The immunomodulatory potential of MSCs should be assessed prior to clinical use
-
•
MSC immunomodulatory properties may be modified in vitro by bioengineering means
In this review, Cool and colleagues discuss the interplay between the inflammatory status, the in vitro aging process, and the immunomodulatory potential of mesenchymal stem cells (MSCs). They posit that a shift from an anti-inflammatory to a pro-inflammatory phenotype is a detrimental feature of culture-expanded MSCs and, therefore, highlight strategies to improve MSC properties prior to use in cell therapy.
Main text
Introduction
The aging of the world's population is a severe health and socioeconomic problem for most developed countries. The United Nations projects that by 2050, one in six (16%) people worldwide will be age 65 years or over, compared with one in eleven (9%) in 2019 (Department of Economic and Social Affairs, 2019). A gradual decline in mobility, physical strength, and cognitive ability, combined with increased risk of cardiovascular disease, osteoarthritis, and metabolic disorders, adversely affects the health and well-being of the elderly (Jaul and Barron, 2017). Notably, such age-associated diseases place a substantial monetary burden on society. In the United States alone, approximately 9 million people suffer age-associated fractures each year, with a health-care cost exceeding US$20 billion (Burge et al., 2007). Thus, effective therapeutic measures to repair and rejuvenate aged tissues are in great demand. In this regard, the therapeutic potential of human mesenchymal stem/stromal cells (hMSCs) in regenerative medicine has long been explored.
Owing to their multipotency, hMSCs are promising cell therapy candidates for tissue regeneration. These cells can be readily expanded and differentiated into adipocytes, chondrocytes, or osteoblasts under appropriate conditions in vitro (Kolf et al., 2007). When administered in vivo, by virtue of their trophic, growth factor-rich secretome, hMSCs attract other cells important for tissue repair and help create a regenerative environment (Lin et al., 2019). Further interest in the therapeutic application of these cells has been driven by their immunomodulatory potential. In particular, the immunosuppressive properties of hMSCs have shown promise in treating diseases characterized by highly inflammatory microenvironments, such as graft-versus-host disease (GvHD) (Le Blanc et al., 2008) and autoimmune disorders (Duijvestein et al., 2010).
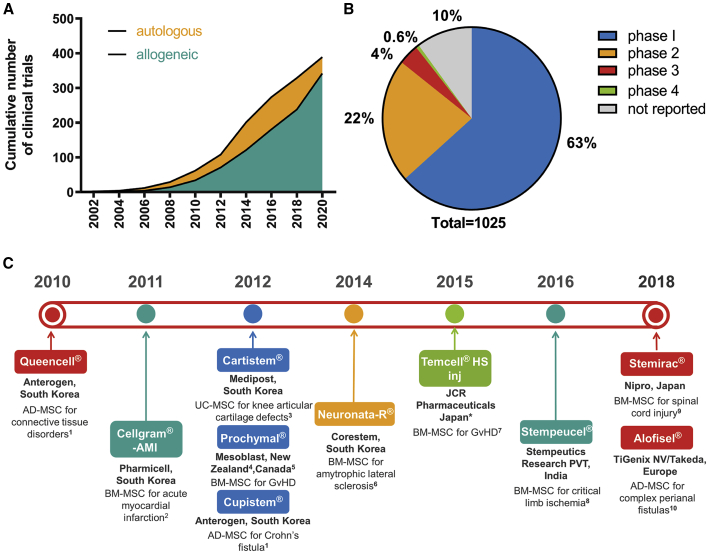
Since the first clinical trial of an hMSC-based therapy in 1995 (Lazarus et al., 1995), hMSCs have become the most clinically studied cell therapy candidates. Autologous applications are currently more widely studied than allogeneic applications, and the number of clinical trials has grown rapidly (Figure 1A). As of November 2020, there were 1,025 clinical trials of hMSC-based therapies registered worldwide (clinicaltrials.gov). The majority of these trials were at early stages (phase 1 or 2), while only 4.6% were in phase 3 or 4 (Figure 1B). To date, only 10 MSC-based products have cleared regulatory approval (Levy et al., 2020). Such approvals date back to 2010 and involve agencies from Europe, Japan, India, South Korea, New Zealand, and Canada (Figure 1C).
Figure 1.
Clinical Trials on MSC-Based Cell Therapies Listed in clinicaltrials.gov (a Total of 1,025 as of November 2020)
(A) Cumulative number of allogeneic (342) and autologous (389) trials. A further 294 trials failed to report the source of cells (mesenchymal stem cell and allogeneic or autologous were used as search strings).
(B) Percentage of trials at different phases of clinical research (mesenchymal stem cell and clinical research phase number were used as search strings).
(C) A timeline showing MSC products that have cleared regulatory approval in the stated countries. AD-MSC, adipose-derived MSC; BM-MSC, bone marrow-derived MSC; UC-MSC, umbilical cord-derived MSC; GvHD, graft-versus-host disease. ∗A licensee of Mesoblast. Sources of information are as follows: 1Korea Ministry of Food and Drug Safety website, https://www.mfds.go.kr/eng/brd/m_30/view.do?seq=71337; 2Korea Ministry of Food and Drug Safety website, https://www.mfds.go.kr/eng/brd/m_30/view.do?seq=70957; 3Korea Ministry of Food and Drug Safety website, https://www.mfds.go.kr/eng/brd/m_30/view.do?seq=69798; 4conditional approval obtained in New Zealand in 2012, approval lapsed in 2016, New Zealand Medicines and Medical Devices Safety Authority website, https://medsafe.govt.nz/regulatory/ProductDetail.asp?ID=15063; 5conditional approval obtained in Canada in 2012, approval granted in 2014, Canada Drug Product Database, https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=87195; 6Korea Ministry of Food and Drug Safety website, https://www.mfds.go.kr/eng/brd/m_30/view.do?seq=70956; 7Pharmaceuticals and Medical Devices Agency (Japan) website, https://www.pmda.go.jp/files/000215658.pdf; 8Nature India website, https://www.natureasia.com/en/nindia/article/10.1038/nindia.2016.61; 9Pharmaceuticals and Medical Devices Agency (Japan) website, https://www.pmda.go.jp/files/000231946.pdf; 10European Medical Agency website, https://www.ema.europa.eu/en/medicines/human/EPAR/alofisel.
Despite the many advantages of using hMSCs for cell therapy, the limited lifespan of these cells poses a significant challenge for culture expansion processes prior to clinical application. Among many others, a study conducted in 2008 showed that within 43–77 days of expansion, human bone marrow-derived MSCs (BM-MSCs) from eight donors underwent replicative senescence (Wagner et al., 2008). These “in vitro-aged” cells exhibit morphological abnormalities, skewed differentiation potential, attenuated surface marker expression, and, finally, proliferative arrest (Lunyak et al., 2017; Yang et al., 2018). Interestingly, parallels can be drawn between these in vitro aging phenotypes and those observed in biologically aged (or “in vivo-aged”) hMSCs from elderly donors (Alt et al., 2012; Baker et al., 2015). For example, it has been shown that at late passages, hMSCs acquire significant changes at both genetic and epigenetic levels, much like their in vivo-aged counterparts harvested from older individuals (Cakouros and Gronthos, 2019; Peffers et al., 2016). This suggests that during in vitro and in vivo aging, similar molecular mechanisms may be responsible for mediating a drastic decline in key functional attributes, such as immunomodulation and anti-inflammatory properties (Neri and Borzi, 2020).
Importantly, the significance of altered inflammatory features that in vitro-aged hMSCs tend to develop, and their impact on the quality and clinical utility of these cells, is yet to be uncovered fully. In this review, we focus on discussing the relationship between the inflammatory status of hMSCs and their in vitro-aging process, and have also compiled evidence from relevant in vivo studies that offer significant insights into the overall MSC aging process. For clarity, we designated specific terms for in vitro- and in vivo-aged cells (Figure 2).
Figure 2.
Terms that Describe In Vitro- and In Vivo-Aged Cells
(A) Early- and late-passage cells describes the in vitro age of cells according to the number of serial passages they have undergone (early passage ≤5; late passage ≥10).
(B) Young and old cells describes the in vivo age of cells depending on donor age (young donor ≤30; old donor ≥60).
(C) Youthful cells is a general term that collectively refers to early-passage (in vitro age) and young (in vivo age) cells.
(D) Aged cells is a general term that collectively refers to late-passage (in vitro age) and old (in vivo age) cells.
The Immunosuppressive and Anti-Inflammatory Effects of hMSCs
The discovery of the immunosuppressive functions of hMSCs broadened the scope of their clinical application beyond tissue regeneration. MSCs are able to suppress the activation of multiple immune cells from the innate and adaptive immune systems. Young, healthy hMSCs inhibit both the proliferation (Bartholomew et al., 2002; Meisel et al., 2004) and differentiation of T cells into Th1 and Th17 helper cells and simultaneously promote the production of regulatory T cells (Luz-Crawford et al., 2013). MSCs also attenuate the activity of natural killer cells by inhibiting their proliferation, cytotoxicity, and cytokine production (Spaggiari et al., 2006, 2008). In addition, hMSCs can induce a switch of type 1 pro-inflammatory macrophages (M1) to the type 2 anti-inflammatory phenotype (M2), suppressing inflammation as a result (Bernardo and Fibbe, 2013; Zhang et al., 2010). One way MSCs exert such immunomodulatory effects is through the production of extracellular vesicles (EVs) that carry a wide array of cargo, such as mRNAs, microRNAs (miRNAs), and proteins (Martin-Rufino et al., 2019; Witwer et al., 2019).
Priming of MSCs by a pro-inflammatory signal has emerged as a crucial requirement to activate their immunosuppressive function (Ren et al., 2008). This underpins the observations that MSCs are highly effective for treating acute and severe inflammatory disease conditions. For example, in a mouse model of GvHD, MSCs were found to be more effective when administered after the onset of inflammation (Ren et al., 2008) (that is, 1 day after bone marrow transfusion) rather than on the same day as the procedure (Sudres et al., 2006). Typically, when stimulated with the correct combination of inflammatory cytokines, hMSCs excessively express strong immunosuppressors such as indoleamine-2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), transforming growth factor β (TGF-β), tumor necrosis factor-inducible gene 6 protein, and interleukin (IL) 10 (Betancourt, 2013). As a result, primed hMSCs are able to dampen immune cell activity and counteract the effects of pro-inflammatory molecules that triggered their initial activation. Pre-conditioning hMSCs with tumor necrosis factor α (TNF-α) and interferon γ (IFN-γ) has been shown to promote the formation of anti-inflammatory M2 macrophages and inhibit mononuclear cell proliferation (Francois et al., 2012). Priming with IL-17 was also shown to enhance MSC-mediated immunosuppression by increasing the secretion of IL-10 and TGF-β, thereby prolonging the survival of allogeneic skin grafts in mice (Ma et al., 2018). Furthermore, IL-17 addition at a concentration of 0.5 ng/mL can potentiate the priming effect of TNF-α and IFN-γ in murine MSCs, in an iNOS (inducible nitric oxide synthase)-dependent manner (Han et al., 2014). However, the priming effect of IL-17 on hMSCs is yet to be validated in hMSCs, especially since hMSCs do not express iNOS. The improved immunomodulatory features of primed MSCs have also been associated with changes in the compositional and functional features of the EVs they produce. Priming MSCs with pro-inflammatory factors has been reported to stimulate the production of EVs with strong anti-inflammatory properties and increased therapeutic benefits (Ti et al., 2015; Yao et al., 2020).
Compromised Immunomodulatory Capacity of Aged hMSCs and Underpinning Mechanisms
As hMSCs age, their immunomodulatory potential is severely diminished. Youthful hMSCs have strong anti-inflammatory properties that are widely utilized in tissue regeneration strategies. By contrast, studies have shown that aged hMSCs tend to exhibit pro-inflammatory signatures with compromised immunosuppressive capacity (Betancourt, 2013; Ebert et al., 2015; Turinetto et al., 2016). Such an age-associated decline in the therapeutic features of hMSCs was evident when late-passage hMSCs were less effective than early-passage MSCs in controlling acute GvHD (von Bahr et al., 2012). In addition, a study of hMSCs from 13 different donors showed that even the least immunosuppressive early-passage hMSC lines were more effective than the most immunosuppressive late-passage cell lines (Klinker et al., 2017).
The acquisition of a pro-inflammatory signature in hMSCs has also been observed to correlate with increasing in vivo age. In a study that analyzed changes correlated with aging in BM-MSCs from donors of various ages (13–80 years), cells from older donors were reported to produce higher levels of IL-6 (Siegel et al., 2013). Similarly, adipose-derived MSCs (AD-MSCs) isolated from elderly patients (older than 65 years) with atherosclerosis displayed a pro-inflammatory secretome with increased levels of IL-6, IL-8, and monocyte chemoattractant protein 1 (MCP1), and a diminished capacity to suppress T cell proliferation and activation (Kizilay Mancini et al., 2017). Neutralizing such pro-inflammatory cytokines with monoclonal antibodies enhanced the immunosuppressive function of these hMSCs (Kizilay Mancini et al., 2017). This evidence clearly demonstrates that the immunomodulatory function of hMSCs changes markedly with aging. However, the underlying cellular and molecular mechanisms for these changes remain to be fully elucidated. In the following sections, we highlight other age-related changes in MSCs and their extracellular microenvironment, which may contribute to alterations in their functional properties.
Senescence and Senescence-Associated Secretory Phenotype
Late-passage MSCs comprise a heterogeneous population, with a high proportion of senescent cells that are under irreversible proliferative arrest and display altered differentiation potential. Unlike apoptotic cells, which are quickly cleared, senescent cells are metabolically active for a prolonged period of time, accumulate continuously, and are able to exert their effects in the surrounding environment. This is exemplified by a study showing that conditioned media from senescent hMSCs (passage 10) directly induced a senescent phenotype in early-passage cells (passage 1) (Severino et al., 2013). To study the effects of senescence on the immunomodulatory capacity of MSCs, Sepulveda and colleagues used radiation to induce cellular senescence in human BM-MSCs. These cells showed increased IL-6 expression and diminished immunosuppressive potential. A follow-up study from the same group confirmed this observation in vivo, where radiation-treated murine MSCs also lost their protective immunosuppressive function under septic conditions (Sepulveda et al., 2014).
Senescent cells are able to modulate their niche as a result of a unique secretome profile they acquire, termed the “senescence-associated secretory phenotype” or SASP (Coppe et al., 2008; Tominaga, 2015). In the case of aged hMSCs, the SASP plays an essential role in mediating their pro-inflammatory status. The potential effects of the SASP on hMSCs and their microenvironment are illustrated in Figure 3. The SASP encompasses chemotactic molecules, such as MCP2 (also known as C-C motif chemokine ligand 8, CCL8), macrophage inflammatory protein 1α (MIP1α; also known as CCL3), and the leukocyte chemotaxis molecules C-X-C motif chemokine ligands 9 and 10 (CXCL9 and CXCL10), that induce immune cell recruitment into the stem cell niche (Lasry and Ben-Neriah, 2015; Lunyak et al., 2017). In addition, pro-inflammatory cytokines in the SASP not only activate neutrophils, dendritic cells, and leukocytes recruited by chemotactic cytokines, but also modulate the proliferation and differentiation potential of hMSCs (Klinker et al., 2017). Another important class of SASP constituents, the matrix metalloproteinases (MMPs), affect the integrity of the stem cell niche by degrading protein components of the extracellular matrix (ECM). As a result, both hMSC growth and immune cell migration are affected (Lasry and Ben-Neriah, 2015; Lunyak et al., 2017).
Figure 3.
Effects of the SASP on the hMSC Microenvironment
The SASP contains a wide range of soluble factors, such as reactive oxygen species (ROS), monocyte and leukocyte chemotactic proteins (MIP1a, MCP2, CXCL9, and CXCL10), pro-inflammatory cytokines (IL-1, IL-6, and IL-8), and proteases (MMP1 and MMP3). These molecules act on both hMSCs and immune cells, as well as the extracellular matrix, to create an inflammatory microenvironment. Abbreviations: MIP, macrophage inflammatory protein; MCP, monocyte chemoattractant protein; CXCL, C-X-C motif chemokine ligand; IL, interleukin; MCP, monocyte chemotactic protein; MMP, matrix metalloproteinase.
Senescent MSCs also release a large quantity of reactive oxygen species (ROS), which induces damaging oxidative stress in the microenvironment (Lasry and Ben-Neriah, 2015). In turn, growth arrest is reinforced in senescent cells, and neighboring cells are driven into a senescent state. Senescence-related changes also influence MSC EVs, particularly their size and cargo (Boulestreau et al., 2020; Lei et al., 2017). Notably, EV preparations from young and old human donors had varying immunomodulatory capabilities, whereby MSC EVs from an old donor (72 years of age) showed reduced immunosuppression in comparison to MSC EVs from a younger donor (25 years of age) (Huang et al., 2019). Such differences were attributed to modified levels of MSC-EV miRNAs, such as miR-223-5p, miR-127-3p, and miR-125b-5p. These findings corroborate earlier data from rodent studies that also highlighted age-related changes in miRNA levels within MSC EVs and associated alterations in immunomodulatory properties (Davis et al., 2017; Fafián-Labora et al., 2017; Wang et al., 2015). Collectively, such observations indicate that age-dependent alterations in the compositional features of MSC EVs may represent a non-canonical arm of the SASP, which contributes to a decline in the immunomodulatory capacity of MSCs. The age-related changes in the effects of MSC-EV miRNAs on immune cells in the surrounding microenvironment, as well as on the parental MSCs themselves, are yet to be fully discerned. Future efforts to include MSC-EV miRNAs as a critical quality attribute of clinical-grade MSCs could be considered in the development of MSC-EV preparations with optimal immunomodulatory properties.
Extracellular Matrix Remodeling
Through the action of the SASP milieu, the ECM may be remodeled in a variety of ways. In addition to protein degradation by MMPs, components of the ECM are vulnerable targets for oxidation. For example, oxidation of carbohydrates, such as glycosaminoglycans (GAGs), by free radicals exacerbates ECM restructuring and contributes to altered cell behavior (Rees et al., 2008). GAGs, particularly heparan sulfate (HS), have an important role in mediating stem cell function (Ravikumar et al., 2020). HS can bind and sequester chemokines, cytokines, and growth factors to the ECM; regulate their bioavailability; and potentiate their binding with cognate cell-surface receptors for signaling (Xu and Esko, 2014). In addition, HS-immobilized cytokine and chemokine reserves may be liberated in biologically active forms into the extracellular space upon HS fragmentation or shedding by heparanases or MMPs, respectively. This process is crucial for increasing vascular permeability during inflammation and aiding leukocyte extravasation into the stroma (Lever et al., 2014; Parish, 2006).
Importantly, the impact of in vitro or in vivo aging and associated inflammatory events on the hMSC glycome, or vice versa, remains unexplored. We hypothesize that replicative age-related changes in HS composition may accompany the acquisition of a senescent phenotype in hMSCs. Such an in vitro-aged HS signature may preferentially bind SASP constituents, increase their local concentration in the microenvironment, and augment their pro-inflammatory functions. To address this hypothesis, HS disaccharide profiles of early- versus late-passage hMSCs may be characterized to investigate potential structural changes that correlate with in vitro aging. In addition, binding and kinetics studies employing techniques such as surface plasmon resonance may be undertaken to examine HS chain interactions with SASP molecules at different stages of culture expansion.
Alterations in Systemic Factors
Alterations in the systemic levels of certain hormones have been reported to correspond to modified MSC function. An age-associated decline in the production of estrogen has been found to correlate with a skewed differentiation potential of MSCs and an overall loss of skeletal tissue integrity (Mistry et al., 2018). In addition, testosterone has been observed to support the proliferation and stemness of MSCs (Corotchi et al., 2016), suggesting that changes in testosterone production with age may also contribute to altered MSC properties. The dysregulated lineage commitment of MSCs in an aging bone marrow niche has also been attributed in part to attenuated levels of circulating growth hormone (GH) and insulin-like growth factor I (IGF-1) during aging (Bolamperti et al., 2018; Crane et al., 2013). Interestingly, decreased IGF-1 production has been reported to correlate with increased serum IL-6 levels and associated pro-inflammatory microenvironments in aged individuals (Cappola et al., 2003). Further investigations to examine how GH and IGF-1 affect MSC function would provide valuable insights into the link between inflammation and MSC aging.
Niche Interactions
MSC function is known to depend heavily on cell-cell interactions and cross talk with other cells within the niche. In the bone marrow, MSCs lie in close apposition to a variety of cell types, especially hematopoietic stem cells (HSCs). It is highly likely that as HSCs age, the drastic changes they undergo, such as increased proliferation and lineage skewing, have a knock-on effect on the biochemical properties of neighboring MSCs and the surrounding microenvironment. In the elderly, HSCs are in a constant state of low-grade activation due to elevated levels of systemic inflammatory cytokines. This low-grade activation is associated with gradual aging and eventual senescence of the HSCs in a process termed “inflammaging,” which could propagate to MSCs via SASPs (Franceschi et al., 2000). Moreover, HSCs are known to secrete BMP that induces the differentiation of MSCs and reduces the multipotent cell reservoir. Adipocytes often also reside in close proximity to MSCs; an increased accumulation of adipocytes and fat tissue has been observed in the bone marrow of old people (Justesen et al., 2001). Leptin, a hormone released by adipocytes, was shown to induce the senescence of chondrogenic progenitor cells by activating p53/p21 (Zhao et al., 2016). This suggests a possibility that leptin may also contribute to MSC senescence during in vivo aging, which remains to be experimentally elucidated.
BM-MSCs that reside close to HSCs seem to be more vulnerable to an inflammaging effect compared with MSCs in apposition with adipocytes within adipose tissue. This hypothesis is supported by a study that assessed donor-matched BM-MSCs and AD-MSCs (Mohamed-Ahmed et al., 2018). For cells from young donors (8–14 years of age), BM-MSCs and AD-MSCs did not show any significant differences in their proliferation potential. In contrast, BM-MSCs from old donors (55–85 years of age) showed a reduced proliferative potential compared with AD-MSCs from the same donor: the average time for cells at passage 1 to reach 80% confluence was observed to be 24 ± 6 days for BM-MSCs and 13 ± 4 days for AD-MSCs. In addition to exhibiting a greater proliferative potential, AD-MSCs from old donors produced higher levels of the anti-inflammatory cytokines TGF-β1 and leukemia inhibition factor than BM-MSCs (Adolfsson et al., 2020). Similar observations were made when donor-matched BM-MSCs and AD-MSCs were expanded in vitro (Burrow et al., 2017; Wu et al., 2018). BM-MSCs showed reduced proliferative potential and longer population doubling times at late passages compared with AD-MSCs. Moreover, senescence-associated markers that are characteristic of in vitro aging, such as shortened telomeres and increased p16INK4A expression, were more apparent in BM-MSCs in comparison to AD-MSCs.
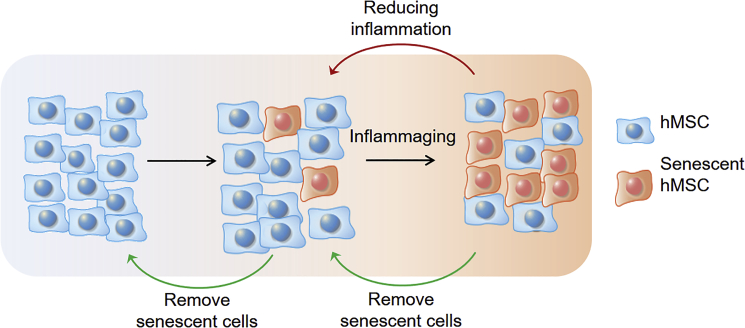
Inflammaging of hMSCs
The aging of MSCs is evidently closely related to their surrounding niche as well as age-associated systemic features. While aged hMSCs exert pro-inflammatory effects, systemic age-related chronic inflammation could, in turn, drive cellular aging of hMSCs. This leads to the formation of a self-perpetuating, degradative positive feedback loop that is detrimental to cell function and underpins the inflammaging phenomenon (Franceschi et al., 2000). Chronic undissolved inflammation is often associated with elevated ROS accumulation (Lasry and Ben-Neriah, 2015), which can cause the chemical transformation of proteins, lipids, DNA, and even carbohydrates, as discussed earlier. These irreversible changes induce genome instability and inevitably lead to cellular senescence.
Direct evidence highlighting the effects of inflammation in driving MSC aging is limited. In this regard, studies comparing MSCs from healthy individuals and patients suffering from chronic inflammation provide important insights. For example, a study from 2008 compared BM-MSCs isolated from 26 patients with rheumatoid arthritis (RA) with those isolated from 21 age-matched and sex-matched healthy control individuals (Kastrinaki et al., 2008). Cells from patients with RA, which were under constant inflammatory stress, showed significant indicators of premature senescence, with impaired clonogenicity, reduced proliferation, and telomere shortening. However, there were no significant differences between the two groups in the frequency, differentiation potential, and surface-marker profiles of the BM-MSCs. In another study, hMSCs isolated from patients with severe osteoarthritis showed significant reductions in proliferative and differentiation potential toward chondrocytes and adipocytes (Murphy et al., 2002). It was also found that repeated stimulation of dental-pulp-derived hMSCs induced an inflammatory response and triggered early onset of cellular senescence (Feng et al., 2018). These findings collectively indicate that an inflammatory microenvironment is deleterious for the lifespan of hMSCs and may induce premature cellular aging.
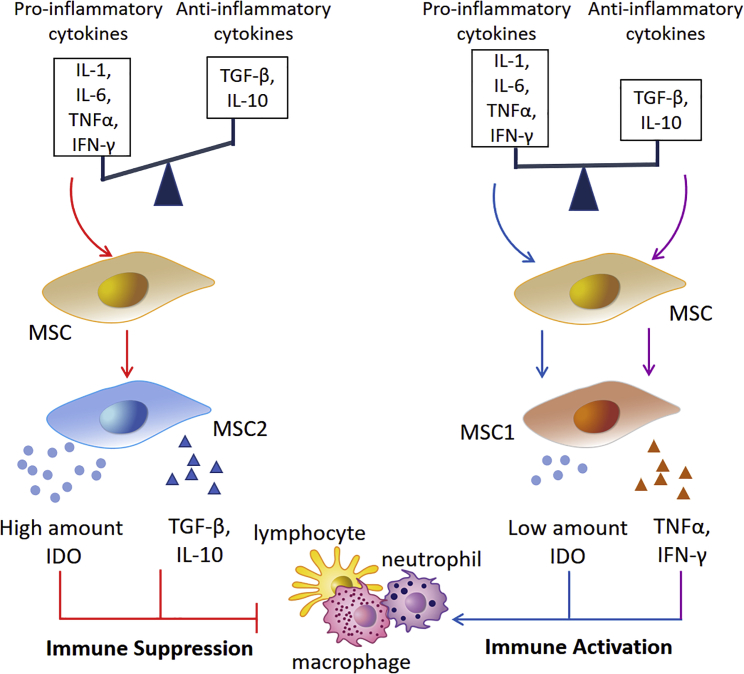
The idea that an inflammatory microenvironment can trigger hMSC senescence and diminish the therapeutic potential of these cells might seem contradictory to the clinically beneficial immunosuppressive function of hMSCs primed with pro-inflammatory signals. Notably, the opposing effects of inflammation on hMSC immunomodulatory responses are partly dictated by the level and status of inflammation. As shown in Figure 4, high levels of acute pro-inflammatory signals trigger MSCs to acquire an immunosuppressive phenotype, which has been assigned an “MSC2” classification following the nomenclature of macrophages (Waterman et al., 2010). These MSC2 cells are characterized by an ability to dampen immune cell activity via high IDO and anti-inflammatory cytokine production. IDO is an enzyme that catalyzes the breakdown of tryptophan to kynurenine. Increase in IDO expression leads to the accumulation of cytotoxic kynurenine, which inactivates immune cells and leads to a suppression in immune responses. By contrast, MSCs tend to develop immune-activating features under chronic inflammatory conditions, characteristic of an “MSC1” classification (Waterman et al., 2010). Unlike acute inflammation, chronic inflammation harbors persistently low and comparable levels of both pro- and anti-inflammatory cytokines, which may each go on to influence MSC behavior (Figure 4). Whereas a basal level of pro-inflammatory cytokines in the microenvironment triggers IDO production by MSC1 cells, albeit at low amounts, anti-inflammatory cytokines induce moderate pro-inflammatory cytokine secretion via the NF-κB signaling cascade and also function to suppress IDO production (Xu et al., 2014). The pro-inflammatory cytokines produced by the MSCs may act in an autocrine manner to further reinforce restricted IDO secretion. Overall, a secretory combination comprising low levels of IDO and a barrage of pro-inflammatory cytokines is inadequate to mount an immunosuppressive response from MSC1 cells. Unrestricted immune cell activation ensues instead, thereby exacerbating the inflammatory microenvironment. These mechanisms were confirmed by titration studies of IFN-γ and TNF-α on the immunosuppressive function of MSCs from mice, rats, and humans (Li et al., 2012; Renner et al., 2009).
Figure 4.
The Opposing Effects of Inflammation on the Immunomodulatory Function of hMSCs
MSCs can be induced to acquire distinct phenotypes (MSC2 and MSC1) depending on stimuli presented in an inflammatory microenvironment. In acute inflammation, high concentrations of pro-inflammatory cytokines (IL-1, IL-6, TNF-α, IFN-γ) and low levels of anti-inflammatory cytokines (TGF-β, IL-10) trigger hMSCs to acquire immune-suppressive features (MSC2). MSC2 cells secrete high amounts of IDO and anti-inflammatory cytokines (TGF-β, IL-10) and inhibit the activity of macrophages, neutrophils, and lymphocytes. By contrast, hMSCs acquire an immune-activation phenotype (MSC1) under chronic inflammatory conditions. Persistently low and comparable levels of pro-inflammatory and anti-inflammatory cytokines in the microenvironment lead to the production of low levels of IDO and pro-inflammatory cytokines (TNF-α, IFN-γ), respectively. However, the amount of IDO secreted by MSC1 cells is significantly lower in comparison to that produced by MSC2 cells during acute inflammation. At these reduced levels, IDO is inadequate in suppressing the activity of immune cells, and an overall pro-inflammatory state persists in the microenvironment.
Apart from pro- or anti-inflammatory stimuli, a transition of MSCs to MSC2 or MSC1 types may be induced by exogenous factors, regardless of which Toll-like receptor (TLR) agonist engagement has emerged to be essential. Short-term exposure to low levels of lipopolysaccharide (LPS), a TLR4 ligand, is capable of polarizing BM-MSCs toward the MSC1 phenotype. LPS priming has been observed to trigger secretion of primarily pro-inflammatory cytokines, such as IL-1β, IL-6, IL-8, IFN-γ, and TNF-α, by hMSCs (Tomchuck et al., 2008) and permit T cell activation (Raicevic et al., 2010). In another study, TLR stimulation in murine MSCs was found to result in the production of similar pro-inflammatory cytokines, and injecting these cells into mice led to the formation of an inflammatory site (Romieu-Mourez et al., 2009). By contrast, exposure to polyinosinic:polycytidylic acid, a TLR3 ligand, primed the cells toward the MSC2 phenotype (Waterman et al., 2010). These hMSCs primarily produced anti-inflammatory mediators, such as C-C motif chemokine ligands 10 and 5 (CCL10 and CCL5, respectively), and readily suppressed T cell activation in vitro.
Implications for Cell Therapy
The therapeutic application of hMSCs involves lengthy ex vivo expansion so as to obtain sufficient cell numbers prior to clinical administration. However, this process has been observed to induce the acquisition of premature senescence, which adversely affects the quality of hMSCs and diminishes their efficacy. To address this problem, clinical studies limit the passaging number of hMSCs to not more than four passages (Dominici et al., 2006). Although this limit reduces the risk of cell alterations and maintains efficacy for in vivo applications, it requires large quantities of highly purified hMSCs from the patient at the outset, which is a significant challenge in current cell therapy regimens. Furthermore, functional heterogeneity invariably arises during hMSC culture expansion and presents a major bottleneck in the cell therapy workflow (Phinney, 2012; Whitfield et al., 2013). Therefore, comprehensive and reliable cell characterization methods are required to determine putative in vitro aging-associated changes in the inflammatory profiles and immunomodulatory potential of hMSCs.
Although secretome profiling may be used to investigate the presence of pro- and anti-inflammatory cytokines and evaluate the inflammatory status of MSCs, there is still no straightforward way to predict the immunomodulatory capacity of MSCs based on these observations. A more direct approach to address this would be to study the ability of hMSCs to inhibit T cell proliferation in co-culture experiments. Alternatively, some companies that manufacture MSCs for research application have adopted IDO expression as a quality control indicator of the immunomodulatory capacity of the cells. Nonetheless, more robust and rigorous methods to predict the immunomodulatory behavior of hMSCs upon in vivo transplantation are in demand. Recently, Klinker and colleagues developed an in vitro assay that uses principal component analysis to incorporate multiple flow cytometry data into a single quantitative value that can be utilized to assess the overall immunosuppressive capacity of hMSCs (Klinker et al., 2017). The principal component was constructed using 16 activation variables and negative controls, and the analysis was enhanced by incorporating quantitative assessment of morphological changes. The model successfully predicted dose-dependent T cell suppression by IFN-γ from 12 different donor-derived hMSC lines for both early and late passages (Klinker et al., 2017). This study represents a step toward establishing technology suitable for the standard assessment of the immune-regulatory capacity of hMSCs.
Apart from profiling and characterization approaches, efforts need to be invested in understanding and modulating the cross talk between hMSC aging mechanisms and inflammatory mediators in the microenvironment. Because aged MSCs are associated with poor therapeutic outcomes, methods of delaying hMSC aging, or modulating their capacity to regulate inflammation, are of interest. Some of the current strategies for addressing these problems are discussed below (Figure 5).
Figure 5.
Strategies to Target the Interplay between hMSC Aging and Environmental Inflammation for Improved Therapeutic Potential
hMSC senescence can occur as a result of natural aging or be induced by external stressors, such as ROS and cytotoxic chemicals. Moreover, SASP from senescent cells causes chronic, low-level inflammatory stress. This process drives the self-perpetuating cycle of inflammaging, where inflammation induces cellular senescence and senescent cells in turn exacerbate pro-inflammatory features of the microenvironment. Strategies to dampen inflammatory signals could potentially slow down the inflammaging process. In addition, targeted removal of senescent cells from an hMSC population could be an approach to maintaining cells in a rejuvenated state.
Targeting Senescent Cells
Senescent hMSCs from elderly donors not only lose their potential for self-renewal and differentiation, but also secrete paracrine signals that suppress any “youthful” cells in the heterogeneous population. Isolating youthful cells from elderly donors (ages 60–99 years) and expanding them in media containing supernatant from hMSCs of young donors (<23 years) improved their capacity for self-renewal and differentiation (Block et al., 2017). In contrast, non-isolated parent cells showed little or no stem cell properties.
As discussed earlier, senescent cells can spread the senescence phenotype to neighboring cells via their SASP and accelerate the aging process at the tissue, and even systemic, level. Therefore, the selective elimination of senescent cells could potentially rejuvenate tissue stem cells. One group has identified a small chemical inhibitor (ABT263) that specifically inhibits the activity of B cell lymphoma (Bcl)-2 and Bcl-xL, and selectively kills senescent cells in culture. In mice, this drug successfully depleted senescent stem cells and rejuvenated stem cells with radiation-induced premature aging (Chang et al., 2016). Another emerging senolytic strategy utilizes the Forkhead box protein O4 (FOXO4), an important molecule in senescent cell viability. Senescence-specific cell death was induced using a peptide that blocks the binding between FOXO4 and p53. The peptide was also shown to restore youthful phenotypes in aged mice (Baar et al., 2017).
Targeting Pro-Inflammatory NF-kB Pathways
Many of the SASP factors from aged hMSCs, including MCP1, MCP4, MIP3a, IL-6, and IL-8, are upregulated at the transcription level by NF-κB, with detectable mRNA abundance (Coppe et al., 2008). The TLR-mediated pro-inflammatory response also functions via the NF-κB pathway. Treatment of hMSCs with microparticles loaded with TPCA-1, a small-molecule inhibitor of NF-κB, significantly reduced pro-inflammatory cytokine secretion by these cells. Conditioned medium derived from hMSCs treated with TPCA-1 showed enhanced immunosuppression on monocytes (Ranganath et al., 2016). Inhibition of the inhibitor of NF-κB kinase (IKK) has been shown to restore osteogenic differentiation in mouse MSCs treated with TNF-α and IL-17 in vitro. IKK inhibition also improved bone regeneration and repair in a rat model of chronic inflammatory disease (Chang et al., 2013). These results suggest that inhibiting NF-κB signaling reduces the inflammatory response and can restore the therapeutic potential of hMSCs.
Three-Dimensional Culture of MSCs
Aggregation of MSCs into compact three-dimensional (3D) spheroids is widely established to enhance the functionality of the cells (Jaukovic et al., 2020). Reduced oxygen pressure inside the core of the spheroid increases the expression of hypoxia-induced factor, which promotes cell survival by upregulating FGF, VEGF, and HGF expression levels (Bhang et al., 2011). Spheroid formation also enhances the secretion of anti-oxidative, anti-apoptotic, and anti-inflammatory factors (Bartosh et al., 2010; Xu et al., 2016), indicating that 3D culture systems can potentiate MSC proliferation and immunomodulatory properties. In another study, MSCs were initially expanded in monolayers (Bartosh and Ylostalo, 2019). At both early and late passages, these cells were either re-seeded in spheroid conditions or maintained in monolayer conditions. MSCs re-seeded in spheroid cultures showed better immunomodulatory potential than those maintained in monolayer for all passages, characterized by an increased production of PGE2 and TSG-6 and decreased secretion of TNF-α. Notably, such a difference in cytokine production between MSCs re-seeded in spheroid cultures and their monolayer counterparts was greater at late passages. This indicates that re-seeding MSCs into spheroids can potentially rescue their immunomodulatory properties, which may otherwise be compromised by serial passaging in monolayer.
Three-Dimensional Culture of MSCs with Biomaterials
Biomaterials can stabilize and protect biological substances such as growth factors, shield them from enzymatic degradation and immune recognition, and orchestrate cell-cell interactions by binding and releasing signaling proteins. Therefore, the clinical use of biomaterials to provide extracellular support for cell culture is becoming increasingly common. Biomaterials could potentially protect hMSCs from senescence and enhance their stemness by shielding them from inflammatory cell attack, reducing matrix stiffness, and decreasing oxidative and inflammatory stress by scavenging free radicals (Lin et al., 2019). Studies leveraging these potential advantages are discussed below.
Hydrogels made from polyacrylamide, alginate, polyethylene glycol (PEG), or collagen are some of the most popular biomaterials used in hMSC studies. Their reduced stiffness compared with plastic culture dishes helps to reduce the cytoskeletal tension within cells (Hiew et al., 2018; Mooney and Vandenburgh, 2008). In addition, the physical properties and microarchitecture of hydrogels are important for maintaining desirable cellular characteristics such as proliferation and differentiation potential of dental-derived hMSCs (Hiew et al., 2018). The porosity and elasticity of hydrogels have been found to regulate the in vivo permeation of pro-inflammatory cytokines, affecting the viability and osteogenic potential of encapsulated hMSCs (Ansari et al., 2017; Moshaverinia et al., 2015).
Biomaterials can be tailored to have moderate cell repellency, to reduce cell-matrix binding, and to free surface integrins and receptors to engage in cell-cell interactions and promote stemness. For example, Arg-Gly-Asp (RGD) repeats are commonly used in tissue regeneration to enhance cell attachment to the substrate. Reducing the RGD density of a poly(carboxybetaine) hydrogel reduced the number of cells bound to the hydrogel, increased cell-cell interactions, and enhanced the stemness of liver stem cells (Cozzolino et al., 2016). In addition, changing the molar ratio or chain length of PEG alters the cell-binding properties of PEG-poly(ε-caprolactone) block copolymers. As a result, this biomaterial can promote the aggregation of cells with enhanced stemness and reduced oxidative stress, as shown using both commercial and patient-derived hMSCs (Balikov et al., 2017). Biomaterials can also be coated onto tissue culture plates. For instance, coating a culture plate surface with poly-L-lysine improved proliferation and significantly reduced replicative senescence of hMSCs (Heo et al., 2016). Moreover, chitosan, a widely used polysaccharide, has also been observed to enhance cellular interactions among AD-hMSCs, improving their in vitro expansion, colony formation, and multipotency (Taguchi et al., 2018).
Conclusion
Research has clearly shown that hMSCs undergo a multitude of changes during in vitro expansion, including an alteration from anti-inflammatory to pro-inflammatory status. We have discussed possible mechanisms governing this shift during in vitro aging and their implications on the quality of culture-expanded hMSCs intended for clinical use. This highlights the importance of conducting more rigorous molecular characterization of hMSCs that includes profiling their inflammatory status during culture expansion. Further studies are also required to better understand molecular networks and feedback loops mediating the interplay between hMSC aging, their immunomodulatory properties, and their inflammatory signatures in vitro. As our knowledge in this area grows, we can design novel technologies and strategies to optimize hMSC-based therapies by exploring methods to modulate their inflammatory status.
Author contributions
Y.Z. and M.R. drafted the review; Y.Z. generated the graphs; S.M.C. guided the construction of the manuscript; S.M.C., Y.Z., and M.R. edited the review; L.L. and V.C. provided input on the scope and content of the review.
Acknowledgments
This work was supported by Industry Alignment Fund Pre-Positioning (IAF-PP) funding (H18/01/a0/021 and H18/AH/a0/001) from the Agency for Science, Technology and Research (A∗STAR), Singapore. We also thank A∗STAR for providing M.R. with support for her graduate studies through a Singapore International Graduate Award (SINGA). The authors further acknowledge support from the Institute of Medical Biology (IMB), Institute of Molecular and Cell Biology (IMCB), and Biomedical Research Council (BMRC), A∗STAR.
Declaration of interests
The authors declare no competing interests.
References
- Adolfsson E., Helenius G., Friberg O., Samano N., Frobert O., Johansson K. Bone marrow- and adipose tissue-derived mesenchymal stem cells from donors with coronary artery disease; growth, yield, gene expression and the effect of oxygen concentration. Scand. J. Clin. Lab. Invest. 2020;80:318–326. doi: 10.1080/00365513.2020.1741023. [DOI] [PubMed] [Google Scholar]
- Alt E.U., Senst C., Murthy S.N., Slakey D.P., Dupin C.L., Chaffin A.E., Kadowitz P.J., Izadpanah R. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res. 2012;8:215–225. doi: 10.1016/j.scr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Ansari S., Chen C., Hasani-Sadrabadi M.M., Yu B., Zadeh H.H., Wu B.M., Moshaverinia A. Hydrogel elasticity and microarchitecture regulate dental-derived mesenchymal stem cell-host immune system cross-talk. Acta Biomater. 2017;60:181–189. doi: 10.1016/j.actbio.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar M.P., Brandt R.M.C., Putavet D.A., Klein J.D.D., Derks K.W.J., Bourgeois B.R.M., Stryeck S., Rijksen Y., van Willigenburg H., Feijtel D.A. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169:132–147 e116. doi: 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N., Boyette L.B., Tuan R.S. Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone. 2015;70:37–47. doi: 10.1016/j.bone.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Balikov D.A., Crowder S.W., Boire T.C., Lee J.B., Gupta M.K., Fenix A.M., Lewis H.N., Ambrose C.M., Short P.A., Kim C.S. Tunable surface repellency maintains stemness and redox capacity of human mesenchymal stem cells. ACS Appl. Mater. Interfaces. 2017;9:22994–23006. doi: 10.1021/acsami.7b06103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew A., Sturgeon C., Siatskas M., Ferrer K., McIntosh K., Patil S., Hardy W., Devine S., Ucker D., Deans R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- Bartosh T.J., Ylostalo J.H. Efficacy of 3D culture priming is maintained in human mesenchymal stem cells after extensive expansion of the cells. Cells. 2019;8:1031. doi: 10.3390/cells8091031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosh T.J., Ylostalo J.H., Mohammadipoor A., Bazhanov N., Coble K., Claypool K., Lee R.H., Choi H., Prockop D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. U S A. 2010;107:13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo M.E., Fibbe W.E. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Betancourt A.M. New cell-based therapy paradigm: induction of bone marrow-derived multipotent mesenchymal stromal cells into pro-inflammatory MSC1 and anti-inflammatory MSC2 phenotypes. Adv. Biochem. Eng. Biotechnol. 2013;130:163–197. doi: 10.1007/10_2012_141. [DOI] [PubMed] [Google Scholar]
- Bhang S.H., Cho S.W., La W.G., Lee T.J., Yang H.S., Sun A.Y., Baek S.H., Rhie J.W., Kim B.S. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 2011;32:2734–2747. doi: 10.1016/j.biomaterials.2010.12.035. [DOI] [PubMed] [Google Scholar]
- Block T.J., Marinkovic M., Tran O.N., Gonzalez A.O., Marshall A., Dean D.D., Chen X.D. Restoring the quantity and quality of elderly human mesenchymal stem cells for autologous cell-based therapies. Stem Cell Res. Ther. 2017;8:239. doi: 10.1186/s13287-017-0688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolamperti S., Signo M., Spinello A., Moro G., Fraschini G., Guidobono F., Rubinacci A., Villa I. GH prevents adipogenic differentiation of mesenchymal stromal stem cells derived from human trabecular bone via canonical Wnt signaling. Bone. 2018;112:136–144. doi: 10.1016/j.bone.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Boulestreau J., Maumus M., Rozier P., Jorgensen C., Noël D. Mesenchymal stem cell derived extracellular vesicles in aging. Front. Cell Developmental Biol. 2020;8:107. doi: 10.3389/fcell.2020.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge R., Dawson-Hughes B., Solomon D.H., Wong J.B., King A., Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J. Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- Burrow K.L., Hoyland J.A., Richardson S.M. Human adipose-derived stem cells exhibit enhanced proliferative capacity and retain multipotency longer than donor-matched bone marrow mesenchymal stem cells during expansion in vitro. Stem Cells Int. 2017;2017:2541275. doi: 10.1155/2017/2541275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakouros D., Gronthos S. Epigenetic regulation of bone marrow stem cell aging: Revealing epigenetic signatures associated with hematopoietic and mesenchymal stem cell aging. Aging Dis. 2019;10:174–189. doi: 10.14336/AD.2017.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappola A.R., Xue Q.L., Ferrucci L., Guralnik J.M., Volpato S., Fried L.P. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J. Clin. Endocrinol. Metab. 2003;88:2019–2025. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- Chang J., Liu F., Lee M., Wu B., Ting K., Zara J.N., Soo C., Al Hezaimi K., Zou W., Chen X. NF-kappaB inhibits osteogenic differentiation of mesenchymal stem cells by promoting beta-catenin degradation. Proc. Natl. Acad. Sci. U S A. 2013;110:9469–9474. doi: 10.1073/pnas.1300532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Wang Y., Shao L., Laberge R.M., Demaria M., Campisi J., Janakiraman K., Sharpless N.E., Ding S., Feng W. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe J.P., Patil C.K., Rodier F., Sun Y., Munoz D.P., Goldstein J., Nelson P.S., Desprez P.Y., Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corotchi M.C., Popa M.A., Simionescu M. Testosterone stimulates proliferation and preserves stemness of human adult mesenchymal stem cells and endothelial progenitor cells. Rom. J. Morphol. Embryol. 2016;57:75–80. [PubMed] [Google Scholar]
- Cozzolino A.M., Noce V., Battistelli C., Marchetti A., Grassi G., Cicchini C., Tripodi M., Amicone L. Modulating the substrate stiffness to manipulate differentiation of resident liver stem cells and to improve the differentiation state of hepatocytes. Stem Cells Int. 2016;2016:5481493. doi: 10.1155/2016/5481493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane J.L., Zhao L., Frye J.S., Xian L., Qiu T., Cao X. IGF-1 signaling is essential for differentiation of mesenchymal stem cells for peak bone mass. Bone Res. 2013;1:186–194. doi: 10.4248/BR201302007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C., Dukes A., Drewry M., Helwa I., Johnson M.H., Isales C.M., Hill W.D., Liu Y., Shi X., Fulzele S. MicroRNA-183-5p increases with age in bone-derived extracellular vesicles, suppresses bone marrow stromal (stem) cell proliferation, and induces stem cell senescence. Tissue Eng. A. 2017;23:1231–1240. doi: 10.1089/ten.tea.2016.0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Economic and Social Affairs, United Nations, Population Division . United Nations; 2019. World Population Prospects 2019: Ten Key Findings. [Google Scholar]
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Duijvestein M., Vos A.C., Roelofs H., Wildenberg M.E., Wendrich B.B., Verspaget H.W., Kooy-Winkelaar E.M., Koning F., Zwaginga J.J., Fidder H.H. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn's disease: results of a phase I study. Gut. 2010;59:1662–1669. doi: 10.1136/gut.2010.215152. [DOI] [PubMed] [Google Scholar]
- Ebert R., Benisch P., Krug M., Zeck S., Meissner-Weigl J., Steinert A., Rauner M., Hofbauer L., Jakob F. Acute phase serum amyloid A induces proinflammatory cytokines and mineralization via toll-like receptor 4 in mesenchymal stem cells. Stem Cell Res. 2015;15:231–239. doi: 10.1016/j.scr.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Fafián-Labora J., Lesende-Rodriguez I., Fernández-Pernas P., Sangiao-Alvarellos S., Monserrat L., Arntz O.J., van de Loo F.J., Mateos J., Arufe M.C. Effect of age on pro-inflammatory miRNAs contained in mesenchymal stem cell-derived extracellular vesicles. Sci. Rep. 2017;7:43923. doi: 10.1038/srep43923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G., Zheng K., Cao T., Zhang J., Lian M., Huang D., Wei C., Gu Z., Feng X. Repeated stimulation by LPS promotes the senescence of DPSCs via TLR4/MyD88-NF-kappaB-p53/p21 signaling. Cytotechnology. 2018;70:1023–1035. doi: 10.1007/s10616-017-0180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C., Bonafe M., Valensin S., Olivieri F., De Luca M., Ottaviani E., De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N Y Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Francois M., Romieu-Mourez R., Li M., Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- Han X., Yang Q., Lin L., Xu C., Zheng C., Chen X., Han Y., Li M., Cao W., Cao K. Interleukin-17 enhances immunosuppression by mesenchymal stem cells. Cell Death Differ. 2014;21:1758–1768. doi: 10.1038/cdd.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J.S., Kim H.O., Song S.Y., Lew D.H., Choi Y., Kim S. Poly-L-lysine prevents senescence and augments growth in culturing mesenchymal stem cells ex vivo. Biomed. Res. Int. 2016;2016:8196078. doi: 10.1155/2016/8196078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiew V.V., Simat S.F.B., Teoh P.L. The advancement of biomaterials in regulating stem cell fate. Stem Cell Rev. 2018;14:43–57. doi: 10.1007/s12015-017-9764-y. [DOI] [PubMed] [Google Scholar]
- Huang R., Qin C., Wang J., Hu Y., Zheng G., Qiu G., Ge M., Tao H., Shu Q., Xu J. Differential effects of extracellular vesicles from aging and young mesenchymal stem cells in acute lung injury. Aging (Albany NY) 2019;11:7996–8014. doi: 10.18632/aging.102314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaukovic A., Abadjieva D., Trivanovic D., Stoyanova E., Kostadinova M., Pashova S., Kestendjieva S., Kukolj T., Jeseta M., Kistanova E. Specificity of 3D MSC spheroids microenvironment: impact on MSC behavior and properties. Stem Cell Rev. Rep. 2020;16:853–875. doi: 10.1007/s12015-020-10006-9. [DOI] [PubMed] [Google Scholar]
- Jaul E., Barron J. Age-related diseases and clinical and public health implications for the 85 Years old and over population. Front. Public Health. 2017;5:335. doi: 10.3389/fpubh.2017.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justesen J., Stenderup K., Ebbesen E.N., Mosekilde L., Steiniche T., Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- Kastrinaki M.C., Sidiropoulos P., Roche S., Ringe J., Lehmann S., Kritikos H., Vlahava V.M., Delorme B., Eliopoulos G.D., Jorgensen C. Functional, molecular and proteomic characterisation of bone marrow mesenchymal stem cells in rheumatoid arthritis. Ann. Rheum. Dis. 2008;67:741–749. doi: 10.1136/ard.2007.076174. [DOI] [PubMed] [Google Scholar]
- Kizilay Mancini O., Lora M., Shum-Tim D., Nadeau S., Rodier F., Colmegna I. A proinflammatory secretome mediates the impaired Immunopotency of human mesenchymal stromal cells in elderly patients with atherosclerosis. Stem Cells Transl. Med. 2017;6:1132–1140. doi: 10.1002/sctm.16-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinker M.W., Marklein R.A., Lo Surdo J.L., Wei C.H., Bauer S.R. Morphological features of IFN-gamma-stimulated mesenchymal stromal cells predict overall immunosuppressive capacity. Proc. Natl. Acad. Sci. U S A. 2017;114:E2598–E2607. doi: 10.1073/pnas.1617933114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolf C.M., Cho E., Tuan R.S. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res. Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasry A., Ben-Neriah Y. Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol. 2015;36:217–228. doi: 10.1016/j.it.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Lazarus H.M., Haynesworth S.E., Gerson S.L., Rosenthal N.S., Caplan A.I. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557–564. [PubMed] [Google Scholar]
- Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., Lanino E., Sundberg B., Bernardo M.E., Remberger M. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Lei Q., Liu T., Gao F., Xie H., Sun L., Zhao A., Ren W., Guo H., Zhang L., Wang H. Microvesicles as potential Biomarkers for the identification of senescence in human mesenchymal stem cells. Theranostics. 2017;7:2673–2689. doi: 10.7150/thno.18915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever R., Rose M.J., McKenzie E.A., Page C.P. Heparanase induces inflammatory cell recruitment in vivo by promoting adhesion to vascular endothelium. Am. J. Physiol. Cell Physiol. 2014;306:C1184–C1190. doi: 10.1152/ajpcell.00269.2013. [DOI] [PubMed] [Google Scholar]
- Levy O., Kuai R., Siren E.M.J., Bhere D., Milton Y., Nissar N., De Biasio M., Heinelt M., Reeve B., Abdi R. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 2020;6:eaba6884. doi: 10.1126/sciadv.aba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Ren G., Huang Y., Su J., Han Y., Li J., Chen X., Cao K., Chen Q., Shou P. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ. 2012;19:1505–1513. doi: 10.1038/cdd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Sohn J., Shen H., Langhans M.T., Tuan R.S. Bone marrow mesenchymal stem cells: aging and tissue engineering applications to enhance bone healing. Biomaterials. 2019;203:96–110. doi: 10.1016/j.biomaterials.2018.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunyak V.V., Amaro-Ortiz A., Gaur M. Mesenchymal stem cells secretory responses: senescence messaging secretome and immunomodulation perspective. Front Genet. 2017;8:220. doi: 10.3389/fgene.2017.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz-Crawford P., Kurte M., Bravo-Alegria J., Contreras R., Nova-Lamperti E., Tejedor G., Noel D., Jorgensen C., Figueroa F., Djouad F. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res. Ther. 2013;4:65. doi: 10.1186/scrt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T., Wang X., Jiao Y., Wang H., Qi Y., Gong H., Zhang L., Jiang D. Interleukin 17 (IL-17)-Induced mesenchymal stem cells prolong the survival of allogeneic skin grafts. Ann. Transplant. 2018;23:615–621. doi: 10.12659/AOT.909381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Rufino J.D., Espinosa-Lara N., Osugui L., Sanchez-Guijo F. Targeting the immune system with mesenchymal stromal cell-derived extracellular vesicles: what is the Cargo's mechanism of action? Front. Bioeng. Biotechnol. 2019;7:308. doi: 10.3389/fbioe.2019.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel R., Zibert A., Laryea M., Gobel U., Daubener W., Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- Mistry S.D., Woods G.N., Sigurdsson S., Ewing S.K., Hue T.F., Eiriksdottir G., Xu K., Hilton J.F., Kado D.M., Gudnason V. Sex hormones are negatively associated with vertebral bone marrow fat. Bone. 2018;108:20–24. doi: 10.1016/j.bone.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed-Ahmed S., Fristad I., Lie S.A., Suliman S., Mustafa K., Vindenes H., Idris S.B. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res. Ther. 2018;9:168. doi: 10.1186/s13287-018-0914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney D.J., Vandenburgh H. Cell delivery mechanisms for tissue repair. Cell Stem Cell. 2008;2:205–213. doi: 10.1016/j.stem.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Moshaverinia A., Chen C., Xu X., Ansari S., Zadeh H.H., Schricker S.R., Paine M.L., Moradian-Oldak J., Khademhosseini A., Snead M.L. Regulation of the stem cell-host immune system interplay using hydrogel Coencapsulation system with an anti-inflammatory drug. Adv. Funct. Mater. 2015;25:2296–2307. doi: 10.1002/adfm.201500055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J.M., Dixon K., Beck S., Fabian D., Feldman A., Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46:704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- Neri S., Borzi R.M. Molecular mechanisms contributing to mesenchymal stromal cell aging. Biomolecules. 2020;10:340. doi: 10.3390/biom10020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish C.R. The role of heparan sulphate in inflammation. Nat. Rev. Immunol. 2006;6:633–643. doi: 10.1038/nri1918. [DOI] [PubMed] [Google Scholar]
- Peffers M.J., Collins J., Fang Y., Goljanek-Whysall K., Rushton M., Loughlin J., Proctor C., Clegg P.D. Age-related changes in mesenchymal stem cells identified using a multi-omics approach. Eur. Cell Mater. 2016;31:136–159. doi: 10.22203/ecm.v031a10. [DOI] [PubMed] [Google Scholar]
- Phinney D.G. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J. Cell Biochem. 2012;113:2806–2812. doi: 10.1002/jcb.24166. [DOI] [PubMed] [Google Scholar]
- Raicevic G., Rouas R., Najar M., Stordeur P., Boufker H.I., Bron D., Martiat P., Goldman M., Nevessignsky M.T., Lagneaux L. Inflammation modifies the pattern and the function of Toll-like receptors expressed by human mesenchymal stromal cells. Hum. Immunol. 2010;71:235–244. doi: 10.1016/j.humimm.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Ranganath S.H., Tong Z., Levy O., Martyn K., Karp J.M., Inamdar M.S. Controlled inhibition of the mesenchymal stromal cell pro-inflammatory secretome via microparticle engineering. Stem Cell Reports. 2016;6:926–939. doi: 10.1016/j.stemcr.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar M., Smith R.A.A., Nurcombe V., Cool S.M. Heparan sulfate proteoglycans: key mediators of stem cell function. Front. Cell Dev. Biol. 2020;8:581213. doi: 10.3389/fcell.2020.581213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees M.D., Kennett E.C., Whitelock J.M., Davies M.J. Oxidative damage to extracellular matrix and its role in human pathologies. Free Radic. Biol. Med. 2008;44:1973–2001. doi: 10.1016/j.freeradbiomed.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Ren G., Zhang L., Zhao X., Xu G., Zhang Y., Roberts A.I., Zhao R.C., Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Renner P., Eggenhofer E., Rosenauer A., Popp F.C., Steinmann J.F., Slowik P., Geissler E.K., Piso P., Schlitt H.J., Dahlke M.H. Mesenchymal stem cells require a sufficient, ongoing immune response to exert their immunosuppressive function. Transplant Proc. 2009;41:2607–2611. doi: 10.1016/j.transproceed.2009.06.119. [DOI] [PubMed] [Google Scholar]
- Romieu-Mourez R., Francois M., Boivin M.N., Bouchentouf M., Spaner D.E., Galipeau J. Cytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotype. J. Immunol. 2009;182:7963–7973. doi: 10.4049/jimmunol.0803864. [DOI] [PubMed] [Google Scholar]
- Sepulveda J.C., Tome M., Fernandez M.E., Delgado M., Campisi J., Bernad A., Gonzalez M.A. Cell senescence abrogates the therapeutic potential of human mesenchymal stem cells in the lethal endotoxemia model. Stem Cells. 2014;32:1865–1877. doi: 10.1002/stem.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severino V., Alessio N., Farina A., Sandomenico A., Cipollaro M., Peluso G., Galderisi U., Chambery A. Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell Death Dis. 2013;4:e911. doi: 10.1038/cddis.2013.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel G., Kluba T., Hermanutz-Klein U., Bieback K., Northoff H., Schafer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013;11:146. doi: 10.1186/1741-7015-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaggiari G.M., Capobianco A., Abdelrazik H., Becchetti F., Mingari M.C., Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- Spaggiari G.M., Capobianco A., Becchetti S., Mingari M.C., Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- Sudres M., Norol F., Trenado A., Gregoire S., Charlotte F., Levacher B., Lataillade J.J., Bourin P., Holy X., Vernant J.P. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J. Immunol. 2006;176:7761–7767. doi: 10.4049/jimmunol.176.12.7761. [DOI] [PubMed] [Google Scholar]
- Taguchi T., Cho J.Y., Hao J., Nout-Lomas Y.S., Kang K.S., Griffon D.J. Influence of hypoxia on the stemness of umbilical cord matrix-derived mesenchymal stem cells cultured on chitosan films. J. Biomed. Mater. Res. B Appl. Biomater. 2018;106:501–511. doi: 10.1002/jbm.b.33864. [DOI] [PubMed] [Google Scholar]
- Ti D., Hao H., Tong C., Liu J., Dong L., Zheng J., Zhao Y., Liu H., Fu X., Han W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015;13:308. doi: 10.1186/s12967-015-0642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchuck S.L., Zwezdaryk K.J., Coffelt S.B., Waterman R.S., Danka E.S., Scandurro A.B. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells. 2008;26:99–107. doi: 10.1634/stemcells.2007-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga K. The emerging role of senescent cells in tissue homeostasis and pathophysiology. Pathobiol. Aging Age Relat. Dis. 2015;5:27743. doi: 10.3402/pba.v5.27743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turinetto V., Vitale E., Giachino C. Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. Int. J. Mol. Sci. 2016;17:1164. doi: 10.3390/ijms17071164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bahr L., Sundberg B., Lonnies L., Sander B., Karbach H., Hagglund H., Ljungman P., Gustafsson B., Karlsson H., Le Blanc K. Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol. Blood Marrow Transplant. 2012;18:557–564. doi: 10.1016/j.bbmt.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Wagner W., Horn P., Castoldi M., Diehlmann A., Bork S., Saffrich R., Benes V., Blake J., Pfister S., Eckstein V. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;3:e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Fu B., Sun X., Li D., Huang Q., Zhao W., Chen X. Differentially expressed microRNAs in bone marrow mesenchymal stem cell-derived microvesicles in young and older rats and their effect on tumor growth factor-β1-mediated epithelial-mesenchymal transition in HK2 cells. Stem Cell Res. Ther. 2015;6:185. doi: 10.1186/s13287-015-0179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman R.S., Tomchuck S.L., Henkle S.L., Betancourt A.M. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield M.J., Lee W.C., Van Vliet K.J. Onset of heterogeneity in culture-expanded bone marrow stromal cells. Stem Cell Res. 2013;11:1365–1377. doi: 10.1016/j.scr.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Witwer K.W., Van Balkom B.W.M., Bruno S., Choo A., Dominici M., Gimona M., Hill A.F., De Kleijn D., Koh M., Lai R.C. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell Vesicles. 2019;8:1609206. doi: 10.1080/20013078.2019.1609206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Li J.Z., Xie B.D., Tian H., Fang S.H., Jiang S.L., Kang K. Lower senescence of adipose-derived stem cells than donor-matched bone marrow stem cells for surgical ventricular restoration. Stem Cells Dev. 2018;27:612–623. doi: 10.1089/scd.2017.0271. [DOI] [PubMed] [Google Scholar]
- Xu C., Yu P., Han X., Du L., Gan J., Wang Y., Shi Y. TGF-beta promotes immune responses in the presence of mesenchymal stem cells. J. Immunol. 2014;192:103–109. doi: 10.4049/jimmunol.1302164. [DOI] [PubMed] [Google Scholar]
- Xu D., Esko J.D. Demystifying heparan sulfate-protein interactions. Annu. Rev. Biochem. 2014;83:129–157. doi: 10.1146/annurev-biochem-060713-035314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Shi T., Xu A., Zhang L. 3D spheroid culture enhances survival and therapeutic capacities of MSCs injected into ischemic kidney. J. Cell Mol. Med. 2016;20:1203–1213. doi: 10.1111/jcmm.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.K., Ogando C.R., Wang See C., Chang T.Y., Barabino G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018;9:131. doi: 10.1186/s13287-018-0876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M., Cui B., Zhang W., Ma W., Zhao G., Xing L. Exosomal miR-21 secreted by IL-1β-primed-mesenchymal stem cells induces macrophage M2 polarization and ameliorates sepsis. Life Sci. 2020;264:118658. doi: 10.1016/j.lfs.2020.118658. [DOI] [PubMed] [Google Scholar]
- Zhang Q.Z., Su W.R., Shi S.H., Wilder-Smith P., Xiang A.P., Wong A., Nguyen A.L., Kwon C.W., Le A.D. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Dong Y., Zhang J., Li D., Hu G., Yao J., Li Y., Huang P., Zhang M., Zhang J. Leptin changes differentiation fate and induces senescence in chondrogenic progenitor cells. Cell Death Dis. 2016;7:e2188. doi: 10.1038/cddis.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]