Abstract
Cognitive deficits associated with Alzheimer's disease (AD) severely impact daily life for the millions of affected individuals. Progressive memory impairment in AD patients is associated with degeneration of the hippocampus. The dentate gyrus of the hippocampus, a region critical for learning and memory functions, is a site of adult neurogenesis in mammals. Recent evidence in humans indicates that hippocampal neurogenesis likely persists throughout life, but declines with age and is strikingly impaired in AD. Our understanding of how neurogenesis supports learning and memory in healthy adults is only beginning to emerge. The extent to which decreased neurogenesis contributes to cognitive decline in aging and AD remains poorly understood. However, studies in rodent models of AD and other neurodegenerative diseases raise the possibility that targeting neurogenesis may ameliorate cognitive dysfunction in AD. Here, we review recent progress in understanding how adult neurogenesis is impacted in the context of aging and AD.
Keywords: adult neurogenesis, aging, Alzheimer's disease, cognitive decline, neural stem cell
Main text
Introduction
Alzheimer's disease (AD) is a debilitating, relentlessly progressive neurodegenerative disease affecting millions of people worldwide. Individuals suffering from AD develop memory impairments that come to severely impact daily life. The entorhinal cortex and the hippocampus play a key role in AD etiology (Braak and Braak, 1991; Thompson et al., 2003). Histological and imaging studies indicate that the entorhinal cortex is affected early in AD (Gómez-Isla et al., 1996), followed by spread to the hippocampus and cerebral cortex (Braak et al., 2006). In addition to degeneration, AD is characterized by the buildup of pathological structures, including extracellular plaques composed of amyloid beta (Aβ), a cleavage product of the amyloid precursor protein (APP), and intracellular tangles comprising hyperphosphorylated tau. The presence of plaques and tangles in the hippocampus is strongly correlated with cognitive decline (Näslund et al., 2000). However, it is likely that additional features of the hippocampus will be important for understanding cognitive decline in AD and for developing novel treatments. Unlike most regions of the adult mammalian brain, the hippocampus harbors neural stem cells (NSCs) that have the capacity to generate new neurons, a process termed neurogenesis. Yet, our understanding of how hippocampal neurogenesis is impaired in AD or even in healthy aging is only beginning to emerge. Here, we review the recent progress in understanding how adult hippocampal neurogenesis is impacted in the context of aging and AD.
Adult Hippocampal Neurogenesis
Adult neurogenesis in rodents and non-human primates has been investigated in depth for decades (Altman and Das, 1965; Gould et al., 1999), with a number studies establishing the extent to which lifelong neurogenesis occurs in humans (Boldrini et al., 2018; Eriksson et al., 1998; Ernst et al., 2014; Johansson et al., 1999a, 1999b; Knoth et al., 2010; Moreno-Jimenez et al., 2019; Spalding et al., 2013; Tobin et al., 2019). Hippocampal NSCs in the subgranular zone (SGZ) primarily generate dentate granule neurons, which are excitatory neurons that make up the bulk of the dentate gyrus (DG) (Figure 1). Functionally, the DG receives input from the entorhinal cortex, sending information through the trisynaptic circuit to CA3 and CA1, thus playing a critical role in learning and memory. Here, we discuss the dynamics of adult neurogenesis in the rodent DG, and evidence for neurogenesis in this area in humans.
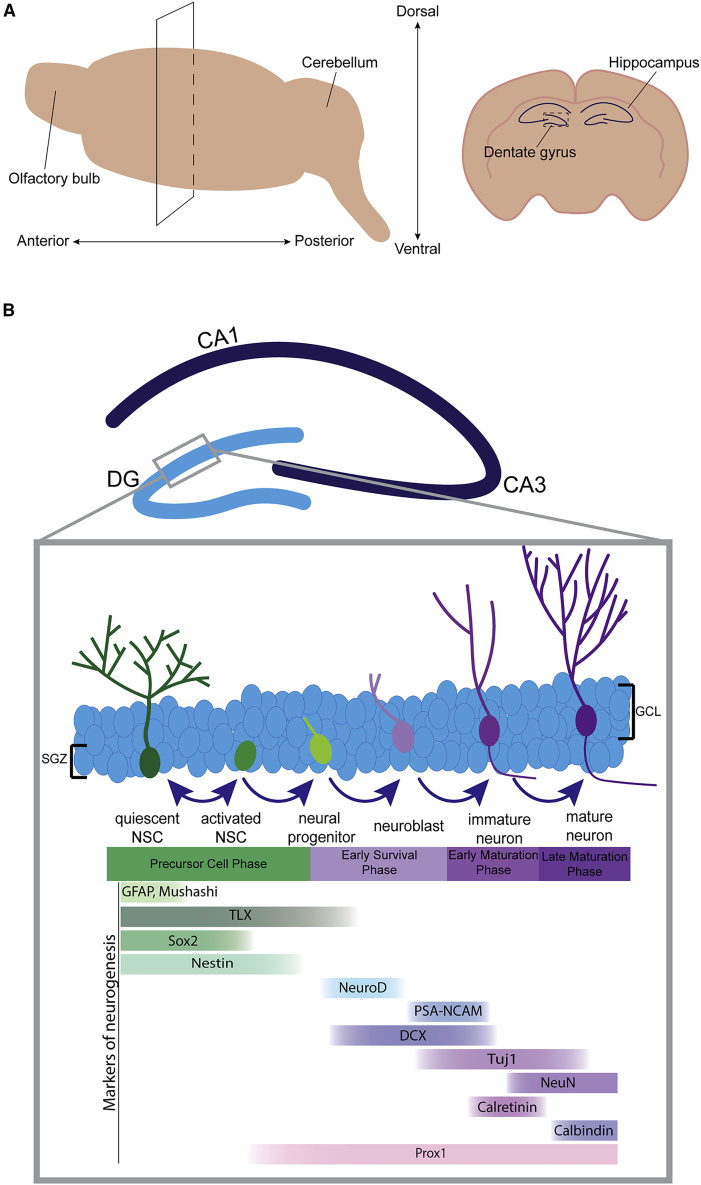
Figure 1.
Adult Hippocampal Neurogenesis in Mice
(A) Schematic representation of the mouse hippocampus. The panel on the left illustrates a coronal cut through the dorsal hippocampus of a mouse brain. The panel on the right is a coronal section through the dorsal hippocampus.
(B) Schematic representation of adult hippocampal neurogenesis in the mouse. Quiescent NSCs become activated to generate daughter cells that either return to quiescence for self-renewal of the NSC pool or differentiate into neurons. Neural progenitors that survive beyond the early survival stage mature into granule neurons and integrate into hippocampal circuitry. Markers expressed throughout the phases of neurogenesis are shown.
Adult Neurogenesis in Rodents
Most of our knowledge of adult hippocampal neurogenesis comes from work in rodents. Early experiments in rats using thymidine-H3 identified the formation of new granule neurons in the adult DG (Altman and Das, 1965; Cameron et al., 1993). Later studies combined thymidine analog incorporation with a marker of mature neurons (NeuN), further confirming that NSCs divide and can differentiate into mature granule neurons in the SGZ of the DG (Kuhn et al., 1996). Using similar methodologies, others found evidence of lifelong neurogenesis in the subventricular zone (SVZ) of the lateral ventricles (Luskin, 1993). Blocking continuous new neuron formation disrupts cognitive performance, indicating that adult neurogenesis is a critical component of the hippocampal circuitry (Imayoshi et al., 2008). A number of studies further support this notion and show that adult hippocampal neurogenesis generates neurons that are important in learning and memory, as well as in emotional regulation (Sahay et al., 2011; Shors et al., 2001). Notably, adult neurogenesis is regulated by environmental and behavioral cues. Exercise and environmental enrichment (EE) are well known to increase neurogenesis and cognitive performance in healthy rodents. In addition, training on hippocampal-dependent learning and memory tasks yields a marked increase in the survival of new granule cells in the DG (Aimone et al., 2006; van Praag et al., 2005). In contrast, stress, aging, and neurodegeneration are potent negative regulators of adult neurogenesis (Gould and Tanapat, 1999; Kuhn et al., 1996).
Most NSCs in the adult brain are in a quiescent state, and are not actively proliferating. Quiescent NSCs can become activated and divide to generate daughter cells that either return to quiescence (self-renewal) or differentiate into neurons or glia (Codega et al., 2014). In mice, the process of neurogenesis occurs over approximately 7 weeks and can be broken down into four phases: (1) precursor cell activation, (2) early survival, (3) early postmitotic maturation, and (4) late maturation (Ambrogini et al., 2004; Kempermann et al., 2015) (Figure 1). During the precursor phase, astrocyte-like quiescent NSCs activate and either divide symmetrically to generate new NSCs or asymmetrically to generate a progenitor and an NSC (Bond et al., 2015). NSCs are able to self-renew, while progenitor cells have limited proliferative capacity and can differentiate into neurons or glia. Newly generated neuroblasts, progenitors with neuronal fate, receive GABAergic input and develop into immature neurons (Tozuka et al., 2005). During the early survival phase, up to 50% of cells are eliminated through apoptosis, reducing the number of newly generated neurons (Dayer et al., 2003). New neurons that survive beyond 2 weeks migrate to the granule cell layer (GCL), begin to develop axons and dendrites, and eventually integrate into the hippocampal network (Kempermann et al., 2003). The early postmitotic maturation phase includes axon elongation, dendritic spine formation, and synapse formation (Sun et al., 2013), which is largely governed by network activity, such as GABAergic inputs (Piatti et al., 2011). Finally, the late maturation phase is characterized by a switch from calretinin expression to calbindin expression, and the development of electrophysiological signatures of older granule cells (Ambrogini et al., 2004; Brandt et al., 2003). During this time, new neurons form glutamatergic synapses and enter a critical period with a reduced threshold for long-term potentiation compared with newborn neurons. These changes are important for cellular survival, mediating synaptic plasticity and memory encoding (Aimone et al., 2006; Schmidt-Hieber et al., 2004).
The process of neurogenesis takes place in a specialized niche that includes characteristic extracellular matrix components, distinct vasculature structures, and a host of secreted factors. While a full description of the niche architecture and its influence on neurogenesis is beyond the scope of this review (see Bond et al., 2015), the niche is critical for supporting healthy neurogenesis. Disruptions to the neurogenic niche can lead to learning and memory impairments, whereas rejuvenation of the aged niche can rescue cognition (Navarro Negredo et al., 2020).
Neurogenesis in the Adult Human Brain
Three independent methods have been used to assess proliferation and neurogenesis in the adult human brain: bromodeoxyuridine (BrdU) incorporation, immunohistochemistry with markers for immature neurons, and radioactive carbon-14 DNA measurements (Boldrini et al., 2018; Eriksson et al., 1998; Ernst et al., 2014; Knoth et al., 2010; Moreno-Jimenez et al., 2019; Sorrells et al., 2018; Spalding et al., 2013). The first evidence for adult neurogenesis in humans came from a study by Eriksson et al. (1998) in which incorporation of the thymidine analog BrdU was used to identify proliferating cells in the brain. BrdU-positive cells co-expressing neuronal markers were observed in both the GCL of the DG and in the SVZ. Since that initial report, several studies have reported adult neurogenesis in humans (Boldrini et al., 2018; Ernst et al., 2014; Johansson et al., 1999a, 1999b; Knoth et al., 2010; Moreno-Jimenez et al., 2019; Spalding et al., 2013). Historically, the absolute level of new neuron formation in humans has been difficult to quantify, and there has been some controversy over the magnitude of neurogenesis postnatally. One estimate from carbon-14 incorporation data indicates continuous adult hippocampal neurogenesis occurs with an estimated annual turnover rate of 1.75% in this region (Spalding et al., 2013).
However, it should be noted that some studies have failed to detect significant generation of new neurons post-developmentally in humans (Cipriani et al., 2018; Sanai et al., 2011; Sorrells et al., 2018). Although additional factors may play a role, the inability to detect new neurons in the adult brain may be due to the different methods of tissue fixation and inconsistencies in long-term storage conditions of the autopsy samples. A recent study addressed these underlying technical issues, which may be particularly important for detection of the immature neuron marker DCX (Flor-Garcia et al., 2020). This work, along with recent advances in the development of innovative technologies, such as single-cell genomics, paves the way for future studies to precisely determine the extent to which adult neurogenesis occurs in healthy and diseased individuals.
Neurogenesis in Healthy Aging
It is well documented that adult neurogenesis declines in physiological aging in mammals. In rodents, neurogenesis significantly decreases with age in both the SVZ and the DG niches, with a severe loss of proliferation by 20–24 months (Enwere et al., 2004; Kempermann et al., 1998). Early studies using BrdU incorporation in rats reported significantly decreased progenitor proliferation in the aged hippocampus, as low as 10% of adult levels, resulting in reduced generation of new granule cells. It has also been observed that the relative proportion of cycling to quiescent NSCs shifts throughout aging, with fewer NSCs in the actively dividing state (Díaz-Moreno et al., 2018; Kalamakis et al., 2019). Moreover, the overall pool of NSCs is likely to be depleted and the ratio of symmetric to asymmetric cell divisions becomes skewed (Encinas et al., 2011; Moore et al., 2015). Further studies confirmed that there is a decrease in neurogenesis by age 6 months in the mouse, which correlates with a decline in cognitive and sensory functions (Imayoshi et al., 2008; Kempermann et al., 1997).
Both intrinsic and environmental factors influence mammalian neurogenesis, including in aged animals. For example, voluntary exercise can rescue age-related neurogenesis defects (van Praag et al., 2005). Parabiosis studies revealed that the presence of systemic inflammation in the aged environment impedes neurogenesis. Conversely, when old animals are exposed to the systematic environment of younger animals through parabiosis, neurogenesis and cognitive performance are increased (Villeda et al., 2014). In addition, a highly integrated network of signals is required to maintain healthy lifelong neurogenesis and alterations to these signaling pathways during aging have been linked to the decline in neurogenesis (e.g., BMP, Notch, Wnt, EGF, and IGF). A number of age-associated intrinsic cellular changes impact neurogenesis in aged animals, including transcriptional, metabolic, proteostatic, and epigenetic changes (for an in depth review see Audesse and Webb, 2020). For example, a major age-associated feature of NSCs is the deterioration of protein quality control, including autophagy-lysosome function, chaperone activity, and overall aggregate processing. All of these processes are altered as NSCs age (Audesse et al., 2019; Leeman et al., 2018; Moore et al., 2015; Vonk et al., 2020).
Aging and Human Neurogenesis
Similar to rodents, there is evidence indicating a decline in hippocampal neurogenesis during aging in humans. Moreno-Jimenez et al. (2019) quantified DCX-positive immature neurons in 13 healthy patients with no observable neurological disease, between 43 and 87 years of age. DCX-positive cells were identified in each of the 13 patients, with the percentage of DCX-positive cells in the DG decreasing with age. Additional markers of progressive stages of neuronal differentiation (e.g., PROX1, PSA-NCAM, NEUN, and βIII-tubulin) demonstrated defective maturation of DCX-positive cells in AD patients. These markers, as well as known markers of maturation, such as calcium binding proteins calretinin (early immature neurons) and calbindin (late-stage maturation), will be useful in future studies to fully dissect the dynamics of defective neurogenesis in aged individuals (Moreno-Jimenez et al. 2019). A second study analyzing hippocampal brain tissue from 28 human subjects between 14 and 79 years of age concluded that neurogenesis persists in the aged brain, but that the number of neural progenitors and immature neurons in the DG of the young and old patients remained similar. In aged tissue, however, there was less angiogenesis, neuroplasticity, and a smaller quiescent pool in the anterior DG compared with young tissue (Boldrini et al., 2018). Thus, in these studies, adult neurogenesis was reported to persist throughout adulthood in humans and decrease with age. The extent to which the mechanisms responsible for this decline are evolutionarily conserved remains unclear. While the study of adult neurogenesis in humans remains challenging due to limited access to human adult NSCs, recent technologies, such as single-cell genomics and cellular reprogramming, will help fill this important gap in our understanding of adult neurogenesis.
AD and the Neurogenic Niche
Symptoms of AD include progressive memory loss and severe cognitive deficits. Patients with AD undergo a significant loss of neurons and synaptic connections, including in the entorhinal cortex and hippocampus (Braak et al., 2006). Notably, the entorhinal cortex provides the major input to dentate granule cells, including those born in adulthood. The presence of extracellular plaques and intracellular neurofibrillary tangles are considered key hallmarks of AD in patients.
There are two main classes of AD, the rare familial AD (FAD) and highly prevalent sporadic AD (SAD). FAD is associated with mutations in APP, presenilin-1 (PS-1), and PS-2 genes, and pathology that typically develops between the ages of 50 and 60 years (Scheuner et al., 1996). SAD is typically later onset than FAD, and is often associated with a specific allele of the APOE gene (Corder et al., 1993). Importantly, in both classes of AD, patients exhibit plaques, tangles, and debilitating cognitive dysfunction.
The plaques and tangles present in AD comprise extracellular Aβ aggregates and neurofibrillary, misfolded tau protein, respectively. Aβ plaques generally form early in disease progression, before cognitive symptoms are noticeable (Glenner and Wong, 1984). This finding triggered the “amyloid hypothesis,” which is the idea that APP cleavage products, such as Aβ42, are the fundamental cause of AD, eventually leading to synaptic dysfunction, gliosis, neuronal loss, and tau pathology (Hardy, 2009). APP is cleaved by the beta-secretase enzyme at the N terminus and by gamma-secretase at the C-terminal end. Cleavage by gamma-secretase produces Aβ proteins of different lengths, Aβ40 and Aβ42 (Eckman and Eckman, 2007). Aβ42 levels are increased in AD and Aβ42 is more likely than Aβ40 to precipitate and form aggregates (Vassar and Citron, 2000). Many mutations associated with early onset FAD impact the cleavage of APP so that there is more Aβ42 than Aβ40 generated and present in AD brains (Scheuner et al., 1996). Evidence suggests that Aβ deposition plays a role in AD pathology (Hardy and Selkoe, 2002); however, therapeutic approaches targeting amyloid deposition and cleavage processes have failed to alleviate symptoms of the disease in clinical trials (Panza et al., 2019). Therefore, whether Aβ deposition is causal remains in question.
More recently, alternative hypotheses to an amyloid-based mechanism for AD causality have been proposed. According to the “tau hypothesis,” hyperphosphorylation of the microtubule-associated protein tau (MAPT) causes AD through formation of neurofibrillary tangles. Buildup of tau tangles is thought to disrupt critical cellular processes, such as protein transport, ultimately leading to neuronal dysfunction and death (Ebneth et al., 1998; Šimić et al., 2016). Although it is apparent that both Aβ plaques and tau tangles are present in AD brains, how these two hallmarks interact and contribute to AD pathogenesis remains unclear. Moreover, recent studies in the field implicate sustained inflammation, oxidative stress, and microglial activity as contributors to the disease (Huang et al., 2016; Sudduth et al., 2013). Other contributing mechanisms continue to emerge, including degeneration caused by interactions between amyloid peptides and macromolecules in the hippocampal niche (e.g., palmitic acid, metals) that may cause retrograde degeneration in the entorhinal cortex (Young, 2020). Future studies are necessary to fully explore these hypotheses and their long-term impact on disease progression.
Impact of AD on Adult Neurogenesis in Humans
Recent work suggests that the decline in neurogenesis that occurs in physiological aging is exacerbated in neurodegenerative diseases, including AD. However, a small number of early studies yielded contradictory and confusing results. Experiments using isolated NSCs from healthy and AD postmortem tissue showed decreased viability and precocious senescence in AD cells compared with controls (Lovell et al., 2006). In agreement with these findings, reduced numbers of progenitor cells were observed in the SVZ of AD patients using Mushashi as a marker, although the number of Nestin-positive stem cells was actually increased in that study (Ziabreva et al., 2006). Another early study reported increased neurogenesis in the hippocampus of AD patients based on expression of DCX, PSA-NCAM, and TUC4 (a neurogenic differentiation factor). It was suggested that neurogenesis is upregulated in AD brains as a compensatory mechanism to replenish cells that are lost through degeneration (Jin et al., 2004b). While these initial studies suggested changes to the NSC niches in AD, it is also worth noting that sample size was low in both cases (7–14 affected individuals).
In more recent work, an examination of 45 AD patients between the ages of 52 and 97 years at a range of Braak stages showed decreased numbers of immature neurons at all stages compared with any healthy aged patient. Immunohistochemistry of hippocampal tissue showed reduced density of DCX, and DCX-positive cells co-labeled with PROX1, NEUN, βIII-tubulin, and calbindin during disease progression, indicating deterioration across the lineage (Moreno-Jimenez et al., 2019). Intriguingly, the decline in neurogenesis was observed even in individuals at early Braak stages with low levels of tau tangles or Aβ plaques. In a separate study, Tobin et al. (2019) utilized cognitive performance as a measure of disease progression in AD patients and individuals with mild cognitive impairment. Stem cells and DCX-positive cells in the DG were observed in patients as old as 90 years, although overall numbers were reduced relative to healthy individuals. Importantly, this study demonstrates a correlation between cognitive function and neurogenesis in the disease setting. The extent to which the mechanisms responsible for reduced neurogenesis in AD patients are shared with individuals experiencing healthy aging remains to be determined.
Utilizing Mouse Models to Understand the Impact of AD on Adult Neurogenesis
Animal models are an essential resource to understand the mechanisms responsible for neurodegeneration. In the AD field, a number of models have been generated based on human mutations and phenotypes in an attempt to recapitulate the disease, mostly in mice. Although over 100 mouse models of AD exist, the field has yet to find one that fully recapitulates AD progression (LaFerla and Green, 2012). Nevertheless, these strains have been useful for modeling AD pathology and cognitive decline to some extent. Popular models harbor combinations of mutations in APP, PS-1, and MAPT. Here, we discuss recent studies using these model systems to investigate how neurogenesis is impacted in AD (Figure 2). A summary of neurogenesis studies performed in a range of AD models is presented in Table 1.
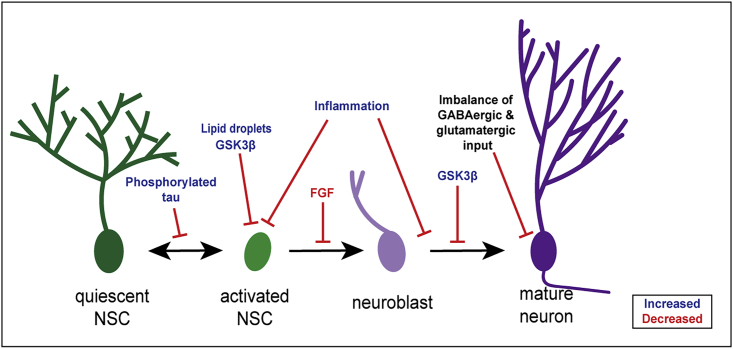
Figure 2.
The Impact of AD on Adult Hippocampal Neurogenesis
Several mechanisms contribute to the AD-associated decline in neurogenesis. Blue text indicates an increase associated with AD and red text indicates a decrease associated with AD. Phosphorylated tau buildup in interneurons contributes to reduced activation of NSCs and the presence of lipid droplets inhibits proliferation of activated NSCs. An increase in inflammation inhibits proliferation and maturation, while reduced FGF inhibits differentiation. Increased GSK3β, the kinase responsible for phosphorylating tau, inhibits both proliferation and maturation. Finally, an imbalance of GABAergic and glutamatergic input inhibits granule cell integration and disrupts granule cell morphology.
Table 1.
Adult Neurogenesis in Mouse Models of AD
| Mouse model | Mutation(s) | Plaque formation | Tangle formation | Neurogenesis | Neurogenic region | Age | Markers | Reference |
|---|---|---|---|---|---|---|---|---|
| 3xTg | APPswe KM670/671NL, PS-1 M146V, MAPT P301L | 6 months | 6 months | DG | By 4 months | HH3 | (Rodriguez et al., 2008) | |
| DG | At 11 & 18 months | BrdU, Ki67, DCX | (Hamilton et al., 2010) | |||||
| DG, SVZ | 2 months | Ki67, DCX, Ki67/DCX, Ki67/GFAP | (Hamilton et al., 2015) | |||||
| DG | 6 months | BrdU/NeuN, DCX | (Valero et al., 2014) | |||||
| 5xFAD | APPswe KM670/671NL, APPfl I716V, APPlon V717L, PS-1 M146L, PS-1 L286V | 2–3 months | N/A | DG | By 2 months | DCX | (Moon et al., 2014) | |
| DG | 2 months |
DCX No Ki67 |
(Zaletel et al., 2018) | |||||
| Tg2576, APPswe | APPswe KM670/671NL | 9–13 months | N/A | SVZ | 11–12 months | BrdU, GFAP, NeuN | (Haughey et al., 2002) | |
| SVZ | 1.5 months |
BrdU, GFAP, Sox2, BrdU/calretinin, BrdU/NeuN DCX |
(Scopa et al., 2019) | |||||
| PDAPP, APPind | APPind V717F | 6 months | N/A | DG (SGZ) | 12 months | BrdU, DCX | (Donovan et al., 2006) | |
| DG (GCL) | 12 months | BrdU, BrdU/DCX | ||||||
| J20, PDGF APPswe/ind | APPswe KM670/671NL, APPind V717F | 5–7 months | N/A | DG, SVZ | SVZ 3 months DG 1 year |
BrdU, DCX | (Jin et al., 2004a) | |
| DG | 3 months | BrdU, Ki67, NeuN, PSA-NCAM | (Lopez-Toledano and Shelanski, 2007) | |||||
| DG | 5 months | BrdU | ||||||
| DG | 2-3 months | GFP reporter, morphology | (Sun et al., 2009) | |||||
| Ps-1 knockin | PS-1 M146V | No data | N/A | DG | 3 months | BrdU, BrdU/NeuN | (Wang et al., 2004) | |
| PS-1 | PS-1 P117L | NA | N/A | DG | By 3 months | BrdU, βIII-tubulin, calbindin | (Wen et al., 2004) | |
| PS-1 | PS-1 A246E | NA | N/A | DG | 12 weeks |
BrdU No BrdU/NeuN |
(Chevallier et al., 2005) | |
| APP/PS-1 | APPswe KM670/671NL, PS-1-dE9 | 6 months | N/A | DG, SVZ | 6 months |
BrdU, BrdU/Neun No Ki67 |
(Verret et al., 2007) | |
| DG, SVZ | 2 months | BrdU, BrdU/DCX | (Demars et al., 2010) | |||||
| APP/PS-1, Nestin-GFP | APPswe KM670/671NL, PS-1-dE9 | 6 months | N/A | DG | by 3 months | BrdU, DCX, GFAP, Nestin | (Zeng et al., 2016) |
One of the most widely used mouse models of AD is the “3xTg” mouse. These mice are a transgenic model overexpressing three genes containing AD-associated mutations, one each for APPsw, PS-1, and MAPT (Oddo et al., 2003). 3xTg mice develop both tau tangles and Aβ plaques and exhibit neuroinflammation, lipid accumulation, and cognitive decline (Belfiore et al., 2019; Hamilton et al., 2015; Oddo et al., 2003). Hippocampal plaques, phosphorylated tau, neuroinflammation, and cognitive deficits occur by the age of 6 months and progress as the mice age through 20 months (Belfiore et al., 2019). 3xTg mice display clear impairments in neurogenesis at the SGZ and SVZ, but the precise timing of the defect varies from study to study (Hamilton et al., 2010, 2015; Rodriguez et al., 2008; Rodríguez et al., 2009). Despite some differences between studies, neurogenesis is clearly impacted in this model with up to a 63% decrease in hippocampal NSC proliferation as early as age 4 months in females, and little capacity to form new neurons by 12 months.
The 5xFAD mouse model has also been used to investigate the effect of AD pathology on neurogenesis. These mice harbor five separate FAD alleles: three APP mutations and two PS-1 mutations. Starting at 2 months, 5xFAD mice have reduced hippocampal neurogenesis (DCX expression), and new neuron formation is nearly undetectable by 7 months (Moon et al., 2014). These data, in conjunction with a recent report showing that NSC proliferation is not impacted in 5xFAD mice, suggest that the neurogenesis defect occurs during differentiation (Moon et al., 2014; Zaletel et al., 2018). Regardless of the mechanism responsible, altering adult hippocampal neurogenesis in the 5xFAD mice impacts cognitive function. For example, increasing neurogenesis through genetic or pharmacologic methods alone results in slight cognitive enhancement, and increasing neurogenesis while also increasing brain-derived neurotrophic factor levels, significantly rescues cognitive dysfunction (Choi et al., 2018).
A clear understanding of how AD impacts neurogenesis in mouse models of AD has been hindered by contradictory conclusions from different models (Table 1). For example, while the 3xTg, 5xFAD, and several APP overexpression models have clear neurogenesis deficits, increased neurogenesis was observed in an APPsw transgenic model (Jin et al., 2004a). PS-1 overexpression models and APP/PS-1 double transgenic mice revealed both increases and decreases in neurogenesis depending on the age and stage of disease progression (Chevallier et al., 2005; Demars et al., 2010; Sotthibundhu et al., 2009; Zeng et al., 2016). Moreover, the stage at which neurogenesis is affected differs from study to study, with some models displaying defects only at the maturation stage of neurogenesis (Hollands et al., 2017). These differences highlight the challenges involved in using mouse models to understand how AD impacts the brain, and the need for better model systems to study the disease.
Studies of neurogenesis in mouse models of tauopathy provide additional insight into how AD pathology impacts neurogenesis. Although MAPT mutations are not specifically associated with AD, tau tangles are a key hallmark of the disease, and in humans the number of neurofibrillary tangles correlates with cognitive decline and neurodegeneration better than Aβ plaques (Giannakopoulos et al., 2003). Tau plays a key role in healthy adult hippocampal neurogenesis. As newly born neurons in the SGZ mature, tau proteins facilitate the microtubule dynamics required for axonal outgrowth (Fuster-Matanzo et al., 2012). Ablation of tau in the adult hippocampus causes significant impairment in motor coordination and spatial memory (Velazquez et al., 2018). In contrast, hyperphosphorylation of tau is associated with cognitive deficits and a decline in adult neurogenesis (Boekhoorn et al., 2006). Interestingly, overexpression of human tau in DG interneurons impaired adult hippocampal neurogenesis. The number of NSCs remained constant, but tau overexpression impacted the morphology and transcriptional profiles of NSCs and caused disinhibition of GABAergic inhibitory interneurons. Importantly, this work suggests that GABA agonists could be potential therapeutics to address adult hippocampal neurogenesis defects in AD (Zheng et al., 2020). Several studies specifically examining neurogenesis in the context of tauopathies or altered tau phosphorylation revealed defects in both NSC proliferation and neuronal maturation (Komuro et al., 2015; Llorens-Martin et al., 2011, 2013). Together, these studies indicate that hyperphosphorylated tau can impact adult hippocampal neurogenesis independent of Aβ pathology.
Enhancing Neurogenesis through Environmental Changes in Healthy and AD Mice
The cognitive benefits associated with EE and voluntary exercise have been known for decades (Jones and Smith, 1980; van Praag et al., 2005). EE studies typically compare an animal housed in an enriched environment (e.g., various objects, and often with a running wheel) to animals in standard housing (Slater and Cao, 2015). A number of studies using rodent models of AD show that EE and exercise lead to increased neurogenesis and improved performance in spatial memory tasks (Kim et al., 2019; Sun et al., 2018; Valero et al., 2011). In some studies, reduction of amyloid and/or tau pathology was observed (Hu et al., 2010). Evidence suggests that EE results in not only increased progenitor proliferation but also increased survival and differentiation, as well as changes in dendritic arborization (Mirochnic et al., 2009; Valero et al., 2011). Notably, some mouse studies show limited or no effect following EE (Cotel et al., 2012). Although these reports are relatively few, they underscore the complexity of gene-environment interactions that may be unique to each model and EE method (Kempermann et al., 2018). For example, the duration of EE intervention is variable and the length of intervention appears to affect the extent to which the effect is sustained (Rodriguez and Verkhratsky, 2011; Valero et al., 2011).
The Impact of Inflammation on Neurogenesis in AD
Immune cells in the brain (e.g., microglia) contribute to the overall pathology of AD, and inflammation is a major feature of the disease (Itagaki et al., 1989). In the healthy brain, microglia remain in a “resting,” stationary state, but become activated in response to injury or disease. In AD, microglia are recruited to the site of amyloid plaques, including in the hippocampus. The overall role of inflammation in AD is beyond the scope of this discussion and has been recently reviewed elsewhere (Kinney et al., 2018). In the context of neurogenesis, studies indicate that inflammation can have different effects depending on the duration and intensity of microglial activation (Russo et al., 2011). Studies in rodents show that increased microglia activity and inflammation, as occurs in AD, correlates with a decline in NSC proliferation and neuronal maturation (Appel et al., 2018; Monje et al., 2003). Furthermore, acute overexpression of the anti-inflammatory cytokine interleukin-10 in APP/PS-1 mice significantly improves cognitive function, and rescues proliferation and neurogenesis defects (Kiyota et al., 2012). Although more research is needed, effective treatments for AD may combine approaches targeting Aβ and tau pathology, as well as inflammation and microglia activity (Kinney et al., 2018).
Future Work
At present, there is an urgent need for a better understanding of how neurogenesis is impacted in the context of aging and neurodegeneration. The field has been hindered by technological and methodological limitations, as well as by the shortcomings of existing animal models. Conceptually, progress has been slowed by a limited understanding of the dynamics of hippocampal neurogenesis in humans and the pathology and progression of AD. Future work should take into consideration recent advances in methods for handling, and analysis of, human tissue, new mouse models (e.g., APP-NLF knockin mice) (Saito et al., 2014), and genetically diverse mouse strains (Neuner et al., 2019; Onos et al., 2019). New approaches, in combination with continued advances in our understanding of adult neurogenesis in humans, are necessary to elucidate the mechanisms responsible for defective neurogenesis in the aged and diseased brain. In the long term, this knowledge may be critical to tackle devastating diseases, such as AD and other dementias.
Author contributions
K.R.B. researched and drafted the original manuscript together with A.E.W. J.S.P. contributed the section on enhancing neurogenesis in AD mice through environmental changes. J.R.F. and A.E.W. revised the manuscript for intellectual content.
Conflicts of interest
J.R.F., A.E.W., and J.S.P. are inventors on a patent owned by Brown University and co-founders of Bolden Therapeutics, a company formed to develop this work into treatments to replenish neurons in the adult brain.
Acknowledgments
We thank Lexi-Amber Hassell for critically reading and providing feedback on the manuscript. This work was supported by NIH/NIA R01 AG053268 (A.E.W.), NIH R21 NS112743 (J.R.F.) and the Edward & Della L. Thome Memorial Foundation (J.R.F. and A.E.W.). K.R.B is supported by NIH T32GM128596.
References
- Aimone J.B., Wiles J., Gage F.H. Potential role for adult neurogenesis in the encoding of time in new memories. Nat. Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Altman J., Das G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Ambrogini P., Lattanzi D., Ciuffoli S., Agostini D., Bertini L., Stocchi V., Santi S., Cuppini R. Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res. 2004;1017:21–31. doi: 10.1016/j.brainres.2004.05.039. [DOI] [PubMed] [Google Scholar]
- Appel J.R., Ye S., Tang F., Sun D., Zhang H., Mei L., Xiong W.C. Increased microglial activity, impaired adult hippocampal neurogenesis, and depressive-like behavior in microglial VPS35-depleted mice. J. Neurosci. 2018;38:5949–5968. doi: 10.1523/JNEUROSCI.3621-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audesse A.J., Dhakal S., Hassell L.A., Gardell Z., Nemtsova Y., Webb A.E. FOXO3 directly regulates an autophagy network to functionally regulate proteostasis in adult neural stem cells. PLoS Genet. 2019;15:e1008097. doi: 10.1371/journal.pgen.1008097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audesse A.J., Webb A.E. Mechanisms of enhanced quiescence in neural stem cell aging. Mech. Ageing Dev. 2020;191:111323. doi: 10.1016/j.mad.2020.111323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfiore R., Rodin A., Ferreira E., Velazquez R., Branca C., Caccamo A., Oddo S. Temporal and regional progression of Alzheimer's disease-like pathology in 3xTg-AD mice. Aging Cell. 2019;18:e12873. doi: 10.1111/acel.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekhoorn K., Terwel D., Biemans B., Borghgraef P., Wiegert O., Ramakers G.J.A., de Vos K., Krugers H., Tomiyama T., Mori H. Improved long-term potentiation and memory in young tau-P301L transgenic mice before onset of hyperphosphorylation and tauopathy. J. Neurosci. 2006;26:3514–3523. doi: 10.1523/JNEUROSCI.5425-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M., Fulmore C.A., Tartt A.N., Simeon L.R., Pavlova I., Poposka V., Rosoklija G.B., Stankov A., Arango V., Dwork A.J. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22:589–599.e5. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond A.M., Ming G.L., Song H. Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell. 2015;17:385–395. doi: 10.1016/j.stem.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Alafuzoff I., Arzberger T., Kretzschmar H., Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brandt M.D., Jessberger S., Steiner B., Kronenberg G., Reuter K., Bick-Sander A., von der Behrens W., Kempermann G. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol. Cell Neurosci. 2003;24:603–613. doi: 10.1016/s1044-7431(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Cameron H.A., Woolley C.S., McEwen B.S., Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Chevallier N.L., Soriano S., Kang D.E., Masliah E., Hu G., Koo E.H. Perturbed neurogenesis in the adult hippocampus associated with presenilin-1 A246E mutation. Am. J. Pathol. 2005;167:151–159. doi: 10.1016/S0002-9440(10)62962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.H., Bylykbashi E., Chatila Z.K., Lee S.W., Pulli B., Clemenson G.D., Kim E., Rompala A., Oram M.K., Asselin C. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer's mouse model. Science. 2018;361:eaan8821. doi: 10.1126/science.aan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani S., Ferrer I., Aronica E., Kovacs G.G., Verney C., Nardelli J., Khung S., Delezoide A.L., Milenkovic I., Rasika S. Hippocampal radial glial subtypes and their neurogenic potential in human fetuses and healthy and Alzheimer's disease adults. Cereb. Cortex. 2018;28:2458–2478. doi: 10.1093/cercor/bhy096. [DOI] [PubMed] [Google Scholar]
- Codega P., Silva-Vargas V., Paul A., Maldonado-Soto A.R., Deleo A.M., Pastrana E., Doetsch F. Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron. 2014;82:545–559. doi: 10.1016/j.neuron.2014.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder E.H., Saunders A.M., Strittmatter W.J., Schmechel D.E., Gaskell P.C., Small G.W., Roses A.D., Haines J.L., Pericak-Vance M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cotel M.C., Jawhar S., Christensen D.Z., Bayer T.A., Wirths O. Environmental enrichment fails to rescue working memory deficits, neuron loss, and neurogenesis in APP/PS1KI mice. Neurobiol. Aging. 2012;33:96–107. doi: 10.1016/j.neurobiolaging.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Dayer A.G., Ford A.A., Cleaver K.M., Yassaee M., Cameron H.A. Short-term and long-term survival of new neurons in the rat dentate gyrus. J. Comp. Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Demars M., Hu Y.-S., Gadadhar A., Lazarov O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer's disease in transgenic mice. J. Neurosci. Res. 2010;88:2103–2117. doi: 10.1002/jnr.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Moreno M., Armenteros T., Gradari S., Hortigüela R., García-Corzo L., Fontán-Lozano Á., Trejo J.L., Mira H. Noggin rescues age-related stem cell loss in the brain of senescent mice with neurodegenerative pathology. Proc. Natl. Acad. Sci. U S A. 2018;115:11625. doi: 10.1073/pnas.1813205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan M.H., Yazdani U., Norris R.D., Games D., German D.C., Eisch A.J. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer's disease. J. Comp. Neurol. 2006;495:70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- Ebneth A., Godemann R., Stamer K., Illenberger S., Trinczek B., Mandelkow E.-M., Mandelkow E. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer's disease. J. Cell Biol. 1998;143:777–794. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman C.B., Eckman E.A. An update on the amyloid hypothesis. Neurol. Clin. 2007;25:669–682, vi. doi: 10.1016/j.ncl.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas J.M., Michurina T.V., Peunova N., Park J.H., Tordo J., Peterson D.A., Fishell G., Koulakov A., Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwere E., Shingo T., Gregg C., Fujikawa H., Ohta S., Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P.S., Perfilieva E., Björk-Eriksson T., Alborn A.-M., Nordborg C., Peterson D.A., Gage F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ernst A., Alkass K., Bernard S., Salehpour M., Perl S., Tisdale J., Possnert G., Druid H., Frisen J. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Flor-Garcia M., Terreros-Roncal J., Moreno-Jimenez E.P., Avila J., Rabano A., Llorens-Martin M. Unraveling human adult hippocampal neurogenesis. Nat. Protoc. 2020;15:668–693. doi: 10.1038/s41596-019-0267-y. [DOI] [PubMed] [Google Scholar]
- Fuster-Matanzo A., Llorens-Martín M., Jurado-Arjona J., Avila J., Hernández F. Tau protein and adult hippocampal neurogenesis. Front. Neurosci. 2012;6:104. doi: 10.3389/fnins.2012.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulos P., Herrmann F.R., Bussière T., Bouras C., Kövari E., Perl D.P., Morrison J.H., Gold G., Hof P.R. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- Glenner G.G., Wong C.W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Gómez-Isla T., Price J.L., McKeel D.W., Jr., Morris J.C., Growdon J.H., Hyman B.T. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J. Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E., Reeves A.J., Graziano M.S.A., Gross C.G. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- Gould E., Tanapat P. Stress and hippocampal neurogenesis. Biol. Psychiatr. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Hamilton L.K., Aumont A., Julien C., Vadnais A., Calon F., Fernandes K.J. Widespread deficits in adult neurogenesis precede plaque and tangle formation in the 3xTg mouse model of Alzheimer's disease. Eur. J. Neurosci. 2010;32:905–920. doi: 10.1111/j.1460-9568.2010.07379.x. [DOI] [PubMed] [Google Scholar]
- Hamilton L.K., Dufresne M., Joppe S.E., Petryszyn S., Aumont A., Calon F., Barnabe-Heider F., Furtos A., Parent M., Chaurand P. Aberrant lipid metabolism in the forebrain niche suppresses adult neural stem cell proliferation in an animal model of Alzheimer's disease. Cell Stem Cell. 2015;17:397–411. doi: 10.1016/j.stem.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Hardy J. The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. J. Neurochem. 2009;110:1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Haughey N.J., Liu D., Nath A., Borchard A.C., Mattson M.P. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta-peptide: implications for the pathogenesis of Alzheimer's disease. Neuromolecular Med. 2002;1:125–135. doi: 10.1385/NMM:1:2:125. [DOI] [PubMed] [Google Scholar]
- Hollands C., Tobin M.K., Hsu M., Musaraca K., Yu T.-S., Mishra R., Kernie S.G., Lazarov O. Depletion of adult neurogenesis exacerbates cognitive deficits in Alzheimer’s disease by compromising hippocampal inhibition. Mol. Neurodegener. 2017;12:64. doi: 10.1186/s13024-017-0207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.-S., Xu P., Pigino G., Brady S.T., Larson J., Lazarov O. Complex environment experience rescues impaired neurogenesis, enhances synaptic plasticity, and attenuates neuropathology in familial Alzheimer's disease-linked APPswe/PS1DeltaE9 mice. FASEB J. 2010;24:1667–1681. doi: 10.1096/fj.09-136945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W.-J., Zhang X., Chen W.-W. Role of oxidative stress in Alzheimer's disease. Biomed. Rep. 2016;4:519–522. doi: 10.3892/br.2016.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I., Sakamoto M., Ohtsuka T., Takao K., Miyakawa T., Yamaguchi M., Mori K., Ikeda T., Itohara S., Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Itagaki S., McGeer P.L., Akiyama H., Zhu S., Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J. Neuroimmunol. 1989;24:173–182. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- Jin K., Galvan V., Xie L., Mao X.O., Gorostiza O.F., Bredesen D.E., Greenberg D.A. Enhanced neurogenesis in Alzheimer's disease transgenic (PDGF-APPSw,Ind) mice. Proc. Natl. Acad. Sci. U S A. 2004;101:13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K., Peel A.L., Mao X.O., Xie L., Cottrell B.A., Henshall D.C., Greenberg D.A. Increased hippocampal neurogenesis in Alzheimer's disease. Proc. Natl. Acad. Sci. U S A. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C.B., Momma S., Clarke D.L., Risling M., Lendahl U., Frisén J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Johansson C.B., Svensson M., Wallstedt L., Janson A.M., Frisén J. Neural stem cells in the adult human brain. Exp. Cell Res. 1999;253:733–736. doi: 10.1006/excr.1999.4678. [DOI] [PubMed] [Google Scholar]
- Jones D.G., Smith B.J. The hippocampus and its response to differential environments. Prog. Neurobiol. 1980;15:19–69. doi: 10.1016/0301-0082(80)90015-5. [DOI] [PubMed] [Google Scholar]
- Kalamakis G., Brune D., Ravichandran S., Bolz J., Fan W., Ziebell F., Stiehl T., Catala-Martinez F., Kupke J., Zhao S. Quiescence modulates stem cell maintenance and regenerative capacity in the aging brain. Cell. 2019;176:1407–1419 e1414. doi: 10.1016/j.cell.2019.01.040. [DOI] [PubMed] [Google Scholar]
- Kempermann G., Gage F.H., Aigner L., Song H., Curtis M.A., Thuret S., Kuhn H.G., Jessberger S., Frankland P.W., Cameron H.A. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell. 2018;23:25–30. doi: 10.1016/j.stem.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G., Gast D., Kronenberg G., Yamaguchi M., Gage F.H. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kempermann G., Kuhn H.G., Gage F.H. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G., Kuhn H.G., Gage F.H. Experience-Induced neurogenesis in the senescent dentate gyrus. J. Neurosci. 1998;18:3206. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G., Song H., Gage F.H. Neurogenesis in the adult hippocampus. Cold Spring Harb. Perspect. Biol. 2015;7:a018812. doi: 10.1101/cshperspect.a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Cho J., Kang H. Protective effect of exercise training against the progression of Alzheimer's disease in 3xTg-AD mice. Behav. Brain Res. 2019;374:112105. doi: 10.1016/j.bbr.2019.112105. [DOI] [PubMed] [Google Scholar]
- Kinney J.W., Bemiller S.M., Murtishaw A.S., Leisgang A.M., Salazar A.M., Lamb B.T. Inflammation as a central mechanism in Alzheimer's disease. Alzheimers Dement (N Y) 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyota T., Ingraham K.L., Swan R.J., Jacobsen M.T., Andrews S.J., Ikezu T. AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice. Gene Ther. 2012;19:724–733. doi: 10.1038/gt.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth R., Singec I., Ditter M., Pantazis G., Capetian P., Meyer R.P., Horvat V., Volk B., Kempermann G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One. 2010;5:e8809. doi: 10.1371/journal.pone.0008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro Y., Xu G., Bhaskar K., Lamb B.T. Human tau expression reduces adult neurogenesis in a mouse model of tauopathy. Neurobiol. Aging. 2015;36:2034–2042. doi: 10.1016/j.neurobiolaging.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H.G., Dickinson-Anson H., Gage F.H. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla F.M., Green K.N. Animal models of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2 doi: 10.1101/cshperspect.a006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman D.S., Hebestreit K., Ruetz T., Webb A.E., McKay A., Pollina E.A., Dulken B.W., Zhao X., Yeo R.W., Ho T.T. Lysosome activation clears aggregates and enhances quiescent neural stem cell activation during aging. Science. 2018;359:1277. doi: 10.1126/science.aag3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens-Martin M., Fuster-Matanzo A., Teixeira C.M., Jurado-Arjona J., Ulloa F., Defelipe J., Rabano A., Hernandez F., Soriano E., Avila J. GSK-3beta overexpression causes reversible alterations on postsynaptic densities and dendritic morphology of hippocampal granule neurons in vivo. Mol. Psychiatr. 2013;18:451–460. doi: 10.1038/mp.2013.4. [DOI] [PubMed] [Google Scholar]
- Llorens-Martin M., Hernandez F., Avila J. Expression of frontotemporal dementia with parkinsonism associated to chromosome 17 tau induces specific degeneration of the ventral dentate gyrus and depressive-like behavior in mice. Neuroscience. 2011;196:215–227. doi: 10.1016/j.neuroscience.2011.08.057. [DOI] [PubMed] [Google Scholar]
- Lopez-Toledano M.A., Shelanski M.L. Increased neurogenesis in young transgenic mice overexpressing human APP(Sw, Ind) J. Alzheimers Dis. 2007;12:229–240. doi: 10.3233/jad-2007-12304. [DOI] [PubMed] [Google Scholar]
- Lovell M.A., Geiger H., Van Zant G.E., Lynn B.C., Markesbery W.R. Isolation of neural precursor cells from Alzheimer's disease and aged control postmortem brain. Neurobiol. Aging. 2006;27:909–917. doi: 10.1016/j.neurobiolaging.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Luskin M.B. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Mirochnic S., Wolf S., Staufenbiel M., Kempermann G. Age effects on the regulation of adult hippocampal neurogenesis by physical activity and environmental enrichment in the APP23 mouse model of Alzheimer disease. Hippocampus. 2009;19:1008–1018. doi: 10.1002/hipo.20560. [DOI] [PubMed] [Google Scholar]
- Monje M.L., Toda H., Palmer T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Moon M., Cha M.Y., Mook-Jung I. Impaired hippocampal neurogenesis and its enhancement with ghrelin in 5XFAD mice. J. Alzheimers Dis. 2014;41:233–241. doi: 10.3233/JAD-132417. [DOI] [PubMed] [Google Scholar]
- Moore D.L., Pilz G.A., Araúzo-Bravo M.J., Barral Y., Jessberger S. A mechanism for the segregation of age in mammalian neural stem cells. Science. 2015;349:1334–1338. doi: 10.1126/science.aac9868. [DOI] [PubMed] [Google Scholar]
- Moreno-Jimenez E.P., Flor-Garcia M., Terreros-Roncal J., Rabano A., Cafini F., Pallas-Bazarra N., Avila J., Llorens-Martin M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nat. Med. 2019;25:554–560. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- Näslund J., Haroutunian V., Mohs R., Davis K.L., Davies P., Greengard P., Buxbaum J.D. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- Navarro Negredo P., Yeo R.W., Brunet A. Aging and rejuvenation of neural stem cells and their niches. Cell Stem Cell. 2020;27:202–223. doi: 10.1016/j.stem.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuner S.M., Heuer S.E., Huentelman M.J., O'Connell K.M.S., Kaczorowski C.C. Harnessing genetic complexity to enhance translatability of Alzheimer's disease mouse models: a path toward precision medicine. Neuron. 2019;101:399–411 e395. doi: 10.1016/j.neuron.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S., Caccamo A., Shepherd J.D., Murphy M.P., Golde T.E., Kayed R., Metherate R., Mattson M.P., Akbari Y., LaFerla F.M. Triple-transgenic model of Alzheimer's disease with plaques and tangles. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Onos K.D., Uyar A., Keezer K.J., Jackson H.M., Preuss C., Acklin C.J., O’Rourke R., Buchanan R., Cossette T.L., Sukoff Rizzo S.J. Enhancing face validity of mouse models of Alzheimer’s disease with natural genetic variation. PLoS Genet. 2019;15:e1008155. doi: 10.1371/journal.pgen.1008155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza F., Lozupone M., Logroscino G., Imbimbo B.P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2019;15:73–88. doi: 10.1038/s41582-018-0116-6. [DOI] [PubMed] [Google Scholar]
- Piatti V.C., Davies-Sala M.G., Esposito M.S., Mongiat L.A., Trinchero M.F., Schinder A.F. The timing for neuronal maturation in the adult hippocampus is modulated by local network activity. J. Neurosci. 2011;31:7715–7728. doi: 10.1523/JNEUROSCI.1380-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J.J., Jones V.C., Tabuchi M., Allan S.M., Knight E.M., LaFerla F.M., Oddo S., Verkhratsky A. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer's disease. PLoS One. 2008;3:e2935. doi: 10.1371/journal.pone.0002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez J.J., Jones V.C., Verkhratsky A. Impaired cell proliferation in the subventricular zone in an Alzheimer's disease model. Neuroreport. 2009;20:907–912. doi: 10.1097/WNR.0b013e32832be77d. [DOI] [PubMed] [Google Scholar]
- Rodriguez J.J., Verkhratsky A. Neurogenesis in Alzheimer's disease. J. Anat. 2011;219:78–89. doi: 10.1111/j.1469-7580.2011.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo I., Barlati S., Bosetti F. Effects of neuroinflammation on the regenerative capacity of brain stem cells. J. Neurochem. 2011;116:947–956. doi: 10.1111/j.1471-4159.2010.07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A., Scobie K.N., Hill A.S., O'Carroll C.M., Kheirbek M.A., Burghardt N.S., Fenton A.A., Dranovsky A., Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Matsuba Y., Mihira N., Takano J., Nilsson P., Itohara S., Iwata N., Saido T.C. Single App knock-in mouse models of Alzheimer's disease. Nat. Neurosci. 2014;17:661–663. doi: 10.1038/nn.3697. [DOI] [PubMed] [Google Scholar]
- Sanai N., Nguyen T., Ihrie R.A., Mirzadeh Z., Tsai H.H., Wong M., Gupta N., Berger M.S., Huang E., Garcia-Verdugo J.M. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D., Eckman C., Jensen M., Song X., Citron M., Suzuki N., Bird T.D., Hardy J., Hutton M., Kukull W. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat. Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C., Jonas P., Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Scopa C., Marrocco F., Latina V., Ruggeri F., Corvaglia V., La Regina F., Ammassari-Teule M., Middei S., Amadoro G., Meli G. Impaired adult neurogenesis is an early event in Alzheimer's disease neurodegeneration, mediated by intracellular Abeta oligomers. Cell Death Differ. 2019;27:934–948. doi: 10.1038/s41418-019-0409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors T.J., Miesegaes G., Beylin A., Zhao M., Rydel T., Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Šimić G., Babić Leko M., Wray S., Harrington C., Delalle I., Jovanov-Milošević N., Bažadona D., Buée L., De Silva R., Di Giovanni G. Tau protein hyperphosphorylation and aggregation in Alzheimer’s disease and other tauopathies, and possible neuroprotective strategies. Biomolecules. 2016;6:6. doi: 10.3390/biom6010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater A.M., Cao L. A protocol for housing mice in an enriched environment. J. Vis. Exp. 2015:e52874. doi: 10.3791/52874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells S.F., Paredes M.F., Cebrian-Silla A., Sandoval K., Qi D., Kelley K.W., James D., Mayer S., Chang J., Auguste K.I. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555:377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotthibundhu A., Li Q.X., Thangnipon W., Coulson E.J. Abeta(1-42) stimulates adult SVZ neurogenesis through the p75 neurotrophin receptor. Neurobiol. Aging. 2009;30:1975–1985. doi: 10.1016/j.neurobiolaging.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Spalding K.L., Bergmann O., Alkass K., Bernard S., Salehpour M., Huttner H.B., Bostrom E., Westerlund I., Vial C., Buchholz B.A. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudduth T.L., Schmitt F.A., Nelson P.T., Wilcock D.M. Neuroinflammatory phenotype in early Alzheimer's disease. Neurobiol. Aging. 2013;34:1051–1059. doi: 10.1016/j.neurobiolaging.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Halabisky B., Zhou Y., Palop J.J., Yu G., Mucke L., Gan L. Imbalance between GABAergic and glutamatergic Transmission impairs adult neurogenesis in an animal model of Alzheimer's disease. Cell Stem Cell. 2009;5:624–633. doi: 10.1016/j.stem.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G.J., Sailor K.A., Mahmood Q.A., Chavali N., Christian K.M., Song H., Ming G.L. Seamless reconstruction of intact adult-born neurons by serial end-block imaging reveals complex axonal guidance and development in the adult hippocampus. J. Neurosci. 2013;33:11400–11411. doi: 10.1523/JNEUROSCI.1374-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.N., Qi J.S., Gao R. Physical exercise reserved amyloid-beta induced brain dysfunctions by regulating hippocampal neurogenesis and inflammatory response via MAPK signaling. Brain Res. 2018;1697:1–9. doi: 10.1016/j.brainres.2018.04.040. [DOI] [PubMed] [Google Scholar]
- Thompson P.M., Hayashi K.M., de Zubicaray G., Janke A.L., Rose S.E., Semple J., Herman D., Hong M.S., Dittmer S.S., Doddrell D.M. Dynamics of gray matter loss in Alzheimer's disease. J. Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin M.K., Musaraca K., Disouky A., Shetti A., Bheri A., Honer W.G., Kim N., Dawe R.J., Bennett D.A., Arfanakis K. Human hippocampal neurogenesis persists in aged adults and Alzheimer’s disease patients. Cell Stem Cell. 2019;24:974–982.e3. doi: 10.1016/j.stem.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y., Fukuda S., Namba T., Seki T., Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Valero J., España J., Parra-Damas A., Martín E., Rodríguez-Álvarez J., Saura C.A. Short-term environmental enrichment rescues adult neurogenesis and memory deficits in APP(Sw,Ind) transgenic mice. PLoS One. 2011;6:e16832. doi: 10.1371/journal.pone.0016832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero J., Mastrella G., Neiva I., Sánchez S., Malva J.O. Long-term effects of an acute and systemic administration of LPS on adult neurogenesis and spatial memory. Front. Neurosci. 2014;8:83. doi: 10.3389/fnins.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H., Shubert T., Zhao C., Gage F.H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R., Citron M. Abeta-generating enzymes: recent advances in beta- and gamma-secretase research. Neuron. 2000;27:419–422. doi: 10.1016/s0896-6273(00)00051-9. [DOI] [PubMed] [Google Scholar]
- Velazquez R., Ferreira E., Tran A., Turner E.C., Belfiore R., Branca C., Oddo S. Acute tau knockdown in the hippocampus of adult mice causes learning and memory deficits. Aging Cell. 2018;17:e12775. doi: 10.1111/acel.12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verret L., Jankowsky J.L., Xu G.M., Borchelt D.R., Rampon C. Alzheimer's-type amyloidosis in transgenic mice impairs survival of newborn neurons derived from adult hippocampal neurogenesis. J. Neurosci. 2007;27:6771–6780. doi: 10.1523/JNEUROSCI.5564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda S.A., Plambeck K.E., Middeldorp J., Castellano J.M., Mosher K.I., Luo J., Smith L.K., Bieri G., Lin K., Berdnik D. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 2014;20:659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonk W.I.M., Rainbolt T.K., Dolan P.T., Webb A.E., Brunet A., Frydman J. Differentiation drives widespread rewiring of the neural stem cell chaperone network. Mol. Cell. 2020;78:329–345.e9. doi: 10.1016/j.molcel.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Dineley K.T., Sweatt J.D., Zheng H. Presenilin 1 familial Alzheimer's disease mutation leads to defective associative learning and impaired adult neurogenesis. Neuroscience. 2004;126:305–312. doi: 10.1016/j.neuroscience.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Wen P.H., Hof P.R., Chen X., Gluck K., Austin G., Younkin S.G., Younkin L.H., DeGasperi R., Gama Sosa M.A., Robakis N.K. The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Exp. Neurol. 2004;188:224–237. doi: 10.1016/j.expneurol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Young J.K. Neurogenesis makes a crucial contribution to the neuropathology of Alzheimer's disease. J. Alzheimers Dis. Rep. 2020;4:365–371. doi: 10.3233/ADR-200218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaletel I., Schwirtlich M., Perovic M., Jovanovic M., Stevanovic M., Kanazir S., Puskas N. Early impairments of hippocampal neurogenesis in 5xFAD mouse model of Alzheimer's disease are associated with altered expression of SOXB transcription factors. J. Alzheimers Dis. 2018;65:963–976. doi: 10.3233/JAD-180277. [DOI] [PubMed] [Google Scholar]
- Zeng Q., Zheng M., Zhang T., He G. Hippocampal neurogenesis in the APP/PS1/nestin-GFP triple transgenic mouse model of Alzheimer's disease. Neuroscience. 2016;314:64–74. doi: 10.1016/j.neuroscience.2015.11.054. [DOI] [PubMed] [Google Scholar]
- Zheng J., Li H.-L., Tian N., Liu F., Wang L., Yin Y., Yue L., Ma L., Wan Y., Wang J.-Z. Interneuron accumulation of phosphorylated tau impairs adult hippocampal neurogenesis by suppressing GABAergic transmission. Cell Stem Cell. 2020;26:331–345.e6. doi: 10.1016/j.stem.2019.12.015. [DOI] [PubMed] [Google Scholar]
- Ziabreva I., Perry E., Perry R., Minger S.L., Ekonomou A., Przyborski S., Ballard C. Altered neurogenesis in Alzheimer's disease. J. Psychosom Res. 2006;61:311–316. doi: 10.1016/j.jpsychores.2006.07.017. [DOI] [PubMed] [Google Scholar]