Summary
ESC- and iPSC-derived retinal transplantation is a promising therapeutic approach for disease with end-stage retinal degeneration, such as retinitis pigmentosa and age-related macular degeneration. We previously showed medium- to long-term survival, maturation, and light response of transplanted human ESC- and iPSC-retina in mouse, rat, and monkey models of end-stage retinal degeneration. Because the use of patient hiPSC-derived retina with a disease-causing gene mutation is not appropriate for therapeutic use, allogeneic transplantation using retinal tissue/cells differentiated from a stocked hESC and iPSC line would be most practical. Here, we characterize the immunological properties of hESC- and iPSC-retina and present their three major advantages: (1) hESC- and iPSC-retina expressed low levels of human leukocyte antigen (HLA) class I and little HLA class II in vitro, (2) hESC- and iPSC-retina greatly suppressed immune activation of lymphocytes in co-culture, and (3) hESC- and iPSC-retina suppressed activated immune cells partially via transforming growth factor β signaling. These results support the use of allogeneic hESC- and iPSC-retina in future clinical application.
Keywords: ESCs, iPSCs, retina, suppression, inflammation
Graphical abstract

Highlights
-
•
hESC- and iPSC-retinas poorly express HLA molecules
-
•
hESC- and iPSC-retinas have immunosuppressive properties
-
•
hESC- and iPSC-retinas can suppress allogeneic lymphocytes
-
•
hESC- and iPSC-retinas suppress immune cells via TGF-β signaling
In this article, Yamasaki, Sugita, and colleagues show that the immunogenicity and immunosuppressive properties of hESC- and iPSC-retinas was differentiated in vitro outside the immune-privileged site. TGF-β secreted by these retinas played a critical role of immunosuppressive properties against activated lymphocytes. These features suggested a low risk of immune rejection after transplantation of stem cell-retina in clinical applications.
Introduction
Human embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs) have the potential to serve as an unlimited source of a variety of cell types and tissues, including retinal cells and retinal pigment epithelium (RPE) cells. Recently, several clinical studies involving the use of RPE cells derived from hESCs and hiPSCs have been conducted for age-related macular degeneration (AMD) and Stargardt disease (Da Cruz et al., 2018; Kashani et al., 2018; Mandai et al., 2017a; Mehat et al., 2018; Schwartz et al., 2015; Song et al., 2015; Sugita et al., 2020). These studies showed the overall safety of the pluripotent stem cell-based therapeutic strategy. For retinal degenerative diseases, protocols for self-organizing, three-dimensional retinal differentiation from mouse ESCs (Eiraku et al., 2011) and hESCs (Nakano et al., 2012) have emerged as a major breakthrough that allows the practical preparation of retinal tissue/cells for grafting that are almost comparable with fetal-derived retina in quality. Several groups, including ours, have further improved and optimized the differentiation protocols to now stably supply hESC- and hiPSC-derived retinal tissue/cells for clinical use (Reichman et al., 2014; Zhong et al., 2014; Capowski et al., 2019; Kuwahara et al., 2015, 2019).
Retinitis pigmentosa is a group of inherited disorders that involves the loss of photoreceptors, with more than 70 causal genes reported (Maeda et al., 2019). Several therapeutic approaches are currently being tested, including gene therapies, optogenetic therapies, replacement therapies using artificial prostheses, protective treatment using nutritional proteins, and photoreceptor transplantation. In 1990–2005, several clinical trials were conducted for retinitis pigmentosa and AMD using fetal retinal cells with or without RPE (Das et al., 1999; Humayun et al., 2000; Radtke et al., 2008). These studies showed the safety and feasibility of the use of transplantation of retinal tissue/cells for retinal degeneration. Although some patients showed possible visual improvement, the efficacy of the treatment needs to be further confirmed.
In 2006 and later, transplanted post-mitotic rod photoreceptor precursors were shown to integrate into the host retina with functional recovery in retinal degeneration mouse models, which turned out to be due to “material transfer” from the donor cells to host photoreceptors (Pearson et al., 2016; Santos-Ferreira et al., 2016; Singh et al., 2016). Using end-stage retinal degeneration models with few remaining photoreceptors, we and others reported functional recovery after neonatal or ESC- and iPSC-derived photoreceptor transplantation either in the form of a cell suspension or a retinal tissue (Barnea-Cramer et al., 2016; Iraha et al., 2018; Lin et al., 2018; Mandai et al., 2017b; Singh et al., 2013; Tu et al., 2019). The advantages of cell suspension transplantation include widespread cell delivery with minimal surgical invasion and efficient contact between partially purified graft photoreceptors and host bipolar cells (Gagliardi et al., 2018; Kruczek et al., 2017). However, isolated photoreceptor cell suspension generally do not survive for a long time after transplantation and they cannot develop an outer nuclear layer structure (Gasparini et al., 2019; Ghosh et al, 1999, 2007; West et al., 2010). On the other hand, transplanted retinal tissue tends to survive for a long period in end-stage retinal degeneration models and develop an outer nuclear layer consisting of mature photoreceptors with inner/outer segment-like structures, although these often form rosette structures (Akiba et al., 2019; Assawachananont et al., 2014; Iraha et al., 2018; Mandai et al., 2017b; Shirai et al., 2015). We previously observed the survival of abundant grafted hiPSC-derived photoreceptors in a monkey retinal degeneration model over 2 years (Tu et al., 2019).
The eye is a unique immune-privileged site where excessive inflammation is suppressed to protect the visual system (Mochizuki et al., 2013; Streilein, 2003). Several mechanisms have been proposed to explain the ocular immune-privileged status: (1) the presence of two blood-retinal barriers (BRB), namely the outer BRB as an RPE layer that separates the retina from choroidal tissue, and the inner BRB as a microvascular epithelium that separates the retina from the bloodstream (Cunha-Vaz, 2004). The composite cells of these barriers form a tight junction to restrict transport of cytokines and migration of immune cells (Streilein, 2003; Sugita, 2009). (2) RPE cells and iris/ciliary body epithelial cells have immunosuppressive properties due to secretion of soluble factors, such as transforming growth factor β (TGF-β), that inhibit activation of immune cells (Sugita, 2009; Sugita et al., 2006). Recently, we showed that retinal ganglion cells can suppress bystander T cells in vitro and produce TGF-β like RPE cells (Edo et al., 2020).
However, RPE cells, including iPSC-derived cells, also have immunogenic properties, and immune rejection after RPE cell transplantation was reported (Fujii et al., 2020; Sugita et al., 2020). Although evidence for rejection was not reported in previous clinical studies using fetus retina for transplantations (Das et al., 1999; Humayun et al., 2000; Radtke et al., 2008), whether this was due to the unique characteristics of the immune-privileged ocular environment or to immunological properties of native retinal tissues is not known. Thus, in this study, we investigated the immunologic features of hESC- and iPSC-retina that were differentiated in vitro, outside the immune-privileged environment, to assess their native immuno-competency and their clinical usefulness in future allogeneic transplantation therapy.
Results
Consistent Differentiation of hESC(KhES-1) and iPSC(TLHD2)-Retina
The Crx::Venus hESC line (KhES-1) that expresses Venus under the photoreceptor marker promotor and hiPSC line (TLHD2) established from a healthy donor were used in this study. These lines consistently expressed the pluripotent stem cell markers Oct3/4, Nanog, and SSEA-4 in feeder-free maintenance culture (Figures S1A–S1F), and were differentiated into retina using a modified serum-free floating culture of embryoid body-like aggregates with the quick aggregation (SFEBq) method (Figure 1A). Self-organized retina was consistently differentiated from these hESC and iPSC lines with a continuous neural epithelium layer (Figures 1B and 1C) expressing Venus in the hESC Crx::Venus reporter line (Figure 1B′ and 1B″) and the Crx in both hESC and iPSC lines on differentiation day 80 (DD80) (Figures 1D–1F and D′–F′). The majority of cells on DD80 in hESC- and iPSC-retina expressed the neural retinal progenitor marker Chx10 and Pax6 in the intermediate layer (Figures 1D and D′). Most of the Crx+ photoreceptor precursors were located in the apical-most layer and were positive for the cone photoreceptor marker Rxrγ (Figures 1E and 1E′). During the early differentiation period, inner retinal cells expressed Islet-1, Prox1, and Brn3, indicating the presence of retinal ganglion cells, amacrine cells, and horizontal cells (Figures 1F and 1F′). All of these indicated that these hESC- and iPSC-retina resembled those used in our previous studies that matured and elicited light responses after transplantation, and comply with our graft quality for transplantation studies (Iraha et al., 2018; Tu et al., 2019).
Figure 1.
Differentiation of hESC- and iPSC-Derived Retina and Expression of Molecules Related to Immunogenicity
(A) Schematic illustration of retinal differentiation from hESCs and iPSCs. The differentiation was pre-conditioned with SB431542 and SAG, followed by the addition of Y-27632, SAG, and BMP4, and then further differentiated by adding CHIR-99021 and SU-5402.
(B–C) Bright-field image of differentiated hESC- and iPSC-retina showing the formation of a continuous epithelial structure at DD80. (B′) hESC-retina expressed the Crx::Venus photoreceptor precursor marker. (B″) Flow cytometry analysis of Crx::Venus+ cell population on DD80 hESC-retina. Fluorescence-activated cell sorting (FACS) results are representative of three individual experiments (n = 3).
(D–F) hESC-retina (D–F) and hiPSC-retina (D′–F′) at DD80 were each stained with retinal cell markers with nuclear staining DAPI. Chx10/Pax6 (retinal progenitors)/Crx (photoreceptor precursors) (DD′); Brn3 (retinal ganglion cells)/Crx/RxRγ (cone precursors) (EE′); Islet-1/Prox1 (retinal inner cells)/Brn3 (FF′).
(G and H) Flow cytometry analysis of HLA class I and II on hESC- and iPSC-retina and hiPSC-RPE cultured for 2 days in the presence or absence of rIFN-γ (100 ng/mL). hESC- and iPSC-retina and hiPSC-RPE were stained with anti-HLA class I or anti-HLA class II antibodies. Isotype control is shown in blue. HLA class I expression in hiPSC-retina was lower compared with hiPSC-RPE (G). Crx::Venus+ population (hESC-retina Crx::Venus+; photoreceptor precursors) shows very slight expression of HLA class I (H) (independent assay, n = 3).
(I–M) Expression of HLA class I in hESC-retina (I and J). HLA class I was expressed in the whole retina with Crx::Venus+ photoreceptors after exposed to rIFN-γ (K–M). HLA class I immunostaining is shown in a single channel (I′–M′). Scale bars, 500 μm (B and C), 200 μm (I and K), 50 μm (D–F, J, and L), and 10 μm (M). RPE, retinal pigment epithelium.
Minimal and Elevated Expression of HLA Class I in hESC- and iPSC-Retina with or without Recombinant Interferon-γ
We first examined the expression of molecules related to immunogenicity in hESC- and iPSC-retina, including HLA class I, II, and E; a component of major histocompatibility complex/HLA class I molecules, β2-microglobulin (β2-MG); and the T cell co-stimulatory molecules, CD40, CD80 (B7-1), CD86 (B7-2), CD274 (PD-L1), and CD273 (PD-L2) with flow cytometry (Figures 1G–H and S1G–S1H). hiPSC-retina expressed HLA class I and β2-MG at a lower level compared with hiPSC-RPE, but did not express HLA class II. The proinflammatory cytokine recombinant interferon-γ (rIFN-γ) increased the expression of HLA class I and β2-MG, and slightly increased that of HLA class II and HLA-E, but did not lead to the expression of other immune molecules, such as CD40, CD80, CD86, or PD-L2 in hESC- and iPSC-retina (Figure S1H). rIFN-γ increased HLA class I expression in hiPSC-retina but in a broader level compared with that in hiPSC-RPE (Figure 1G). Among hESC-retina, the expression level of HLA class I and β2-MG was lower in Crx::Venus+ photoreceptor precursors than in other populations, indicating the low immunogenicity of hESC-photoreceptor precursors (Figure 1H). Also, compared with hiPSC-RPE exposed to rIFN-γ (Figure 1G), there was almost no expression of HLA class II on the Crx::Venus+ population exposed to rIFN-γ (Figure 1H). Immunohistochemical analysis confirmed the upregulation of HLA class I in hESC-retina after rIFN-γ treatment but HLA class I was barely detectable without rIFN-γ (Figures 1I–1M). In addition, hESC- and iPSC-retina expressed the suggested “don't eat me” immune ligand CD47 that can interact with several cell surface receptors to avoid phagocytosis (Jaiswal et al., 2009) regardless of rIFN-γ treatment (Figure S1I).
Since transplanted hESC- and iPSC-retina eventually matures after transplantation, which may cause different immune responses in the host retina, we also studied if HLA expression changes along with maturation of hESC-retina. hESC-retina on DD30 consisted mainly of Chx10+/Ki67+ neural retinal progenitors, with the first-born cell lineage being Brn3+/Pax6+ ganglion cells (Figures S2A and S2B). Rxrγ+ cone photoreceptors expressed the bona fide photoreceptor marker Recoverin on DD90 (Figures S2B and S2C). Around DD150, Crx::Venus+ photoreceptors in the apical-most layer further increased in number, but Rxrγ+ cone photoreceptors did not (Figure S2C). Protein kinase C α+ rod bipolar cells, glutamine synthetase (GS)+ cells, and glial fibrillary acidic protein (GFAP)− Müller glial cells emerged at this stage and were continuously present on DD200–DD240 (Figure S2D). Immunohistochemical analysis showed that the HLA class I and II expression patterns were similar at all differentiation stages (DD27, DD91, DD149, and DD239) of hESC-retina (Figures S3A and S3B). These findings imply that hESC- and iPSC-retina may consistently show low expression of HLA class I unless activated, and no detectable HLA class II at the time of transplantation, and even thereafter, toward the end of its maturation.
Different Responses of Allogeneic Peripheral Blood Mononuclear Cells against Single Cells, Semi-dissociated Cells, and Whole Retina from hESC- and iPSC-Retina
Because retinal tissues tend to survive longer compared with a cell suspension after transplantation, we then tested whether the different forms of retinal tissues/cells preparations, including (1) single cells, (2) semi-dissociated cells, and (3) whole retina, showed different immunogenicity. For this purpose, we conducted a lymphocyte graft immune reaction (LGIR) assay, in which we co-cultured allogeneic (HLA mismatched) peripheral blood mononuclear cells (PBMCs) from healthy donors with each cell form of DD78–DD100 hESC- and iPSC-retina, the developmental stage that we consider useful for transplantation in clinical application (Figure 2A). With graft retina-PBMCs co-cultured for 4–5 days, dissociated single cells from both the hESC-retina (Figure 2B) and iPSC-retina (Figure 2C) weakly enhanced the proliferation of CD4+ cells (helper T cells), CD8+ cells (cytotoxic T cells), and CD11b+ cells (macrophages/monocytes), but not natural killer group 2A (NKG2A)+ cells (natural killer cells) compared with control PBMCs without co-culturing. In contrast, semi-dissociated or whole retina failed to induce proliferation of any of these immune PBMC components, with drastic suppression by the whole retina (Figures 2B and 2C). In addition, whole tissue strongly suppressed IFN-γ secretion by PBMCs. To assess the difference in immunogenicity, we examined HLA class I expression in whole retina and single cells, but HLA class I expression was not increased in the single cells of hESC-retina (Figure 2D). Then we studied if the increase in immunogenicity results from a single-cell culture that may cause some cell death. On day 1 after dissociation of hESC-retina, approximately 70% of the cells were positive for either or both of Annexin V and SYTOX in a single-cell condition, which was also the case for less than 15% of the cells freshly prepared from the whole retina. On days 3–5, surviving cells seemed to proliferate and form small aggregates, thereby presenting an apparently improved cell viability, although this was with the absence of PBMCs, providing a less severe condition than co-culture assay (Figure S4). This implied that dying cells, although mostly apoptotic, after a single-cell dissociation may moderately enhance an immune response in co-culture assays. Another possibility for the low immunogenicity of hESC- and iPSC-retinas may be derived from the externally located Crx+ photoreceptor precursors that express lower levels of HLA class I and β2-MG compared with that in the other cell composites in hESC-retinas (Figure 1H), because these external cells are considered to be the major population to be exposed to PBMCs in co-culture assay. So, we sorted Crx::Venus+ cells in hESC-retina by fluorescence-activated cell sorting to co-culture with PBMCs. There was no significant difference between the Crx::Venus+ and Crx::Venus− cells, and the mixture of the 2, and unsorted single cells, suggesting that the low immunogenicity of the hESC- and iPSC-retinas is not due to the intrinsic property of the externally located Crx::Venus+ cells (Figure S5). These results indicated that hESC- and iPSC-retina as a whole may have an advantage in low immunogenicity, with some suppressive properties on allogeneic lymphocytes partly because of their better survival when compared with single-cell dissociation.
Figure 2.
hESC- and iPSC-Retina Elicited an Immunological Reaction in Co-culture
(A) Three types of hESC-retinal conditions, single cells, semi-dissociated cells, and whole retina of the lymphocyte graft immune rejection (LGIR) assay at DD78.
(B and C) Flow cytometry analysis of PBMCs after co-culture with hESC-retina (B) and iPSC-retina (C) using anti-CD4, anti-CD8, anti-CD11b, anti-NKG2A, anti-Ki-67 antibodies, and each isotype control antibody. IFN-γ concentration in the supernatant of LGIR culture medium was examined with enzyme-linked immunosorbent assay (ELISA) (n = 6 independent experiments).
(D) HLA class I expression level of whole retina and single-retinal cells with or without rIFN-γ. PBMCs, peripheral blood mononuclear cells.
Immunogenicity of Single Cells from hiPSC-Retina on Allogeneic PBMCs
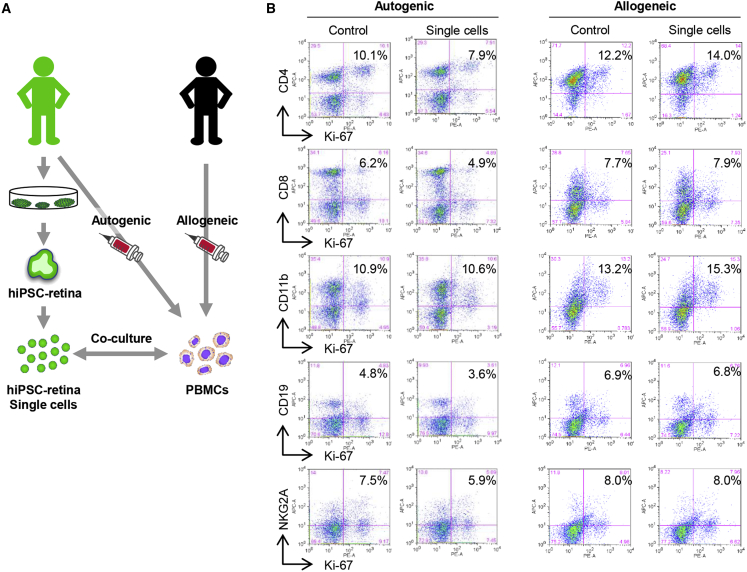
Because single cells from hESC- and iPSC-retina activated allogeneic T lymphocytes, we confirmed if this activation was due to immune reaction by directly comparing the proliferation of autogenic and HLA-mismatched allogeneic PBMCs (TLHD2 donor) against a dissociated form of iPSC-retina (Figure 3A). In the HLA-mismatched LGIR assay, proliferation of CD4+ cells and CD11b+ cells were increased, whereas the proliferation of immune cells was decreased by co-culture with autogenic iPSC-retina (Figure 3B), suggesting that single cells from iPSC-retina are immunogenic to elicit sufficient allogenic immune response.
Figure 3.
Lymphocyte Reactions of Autogenic or Allogeneic iPSC-Retina
(A) Schematic of autogenic and allogeneic LGIR assay. hiPSC-retina (TLHD2) in the single-cell condition was co-cultured with PBMCs from a TLHD2 donor or another healthy donor. The healthy donor was completely HLA mismatched.
(B) PBMCs were analyzed with flow cytometry using immune cell marker antibody FACS results are representative of independent assay (n = 2).
Active Immunosuppressive Property of hESC- and iPSC-Retina on PBMCs
Several reports have demonstrated that RPE cells and hESC- and iPSC-RPE have active immunosuppressive properties on activated immune cells (Sugita et al., 2006, 2016a, 2016b). However, immunomodulatory features of the retina have not been well studied. Because hESC- and iPSC-whole retina suppressed allogeneic PBMC proliferation, we postulated that hESC- and iPSC-retina may have some active immunosuppressive properties in addition to simply having low immunogenicity. Thus, we further tested if hESC- and iPSC-retina actively suppressed activated PBMCs, including activated T cells. PBMCs were activated by anti-human CD3/CD28 agonistic antibodies. hiPSC-retina significantly suppressed the proliferation of all immune cells, including CD4+, CD8+, CD11b+, CD19+, and NKG2A+ cells (Figure 4A). The extent of the suppression by iPSC-retina was similar to that by RPE cells established from iPSCs (Figure 4A). We also examined whether hESC-retina and hESCs as a control suppressed activated inflammatory cells in vitro. Control hESCs failed to suppress PBMCs that were activated by anti-human CD3/CD28 antibodies, whereas hESC-retina suppressed activated CD4+ cells and CD8+ cells in PBMCs (Figure 4B). In microscopy observation after the co-culture, control PBMCs without whole retina proliferated and formed large aggregates, whereas PBMCs that were co-cultured with hESC-retina formed smaller aggregates (Figure 4C). The suppressive effect was evident close to the hESC-retina in a co-culture dish, and was tissue number-dependent (5, 10, 15, and 20 retinal tissues), indicating the direct relevance of the presence of hESC-retina with the suppressive effect (Figures 4C and 4D).
Figure 4.
hESC- and iPSC-Retina Have Immunomodulatory Function against Activated Allogeneic PBMCs in the Same Manner as RPE
(A) Flow cytometry analysis of activated PBMCs using anti-CD3/CD28 agonistic antibody after co-culture with hESC-retina using anti-CD4, anti-CD8, anti-CD11b, anti-CD19, anti-NKG2A, anti-Ki-67 antibodies, and each isotype control antibody. FACS results are representative of independent experiments (n = 2).
(B) Percentage of Ki-67+ proliferating CD4+, CD8+, CD11b+, CD19+, and NKG2A+ cells compared with control were determined with flow cytometry analysis (n = 3). ∗∗p > 0.05, compared with PBMCs + hESCs. Student's t test. Data are the means ± SEM of three independent experiments.
(C) Bright-field image of aggregate formation of PBMCs. Control PBMCs proliferated and formed large aggregates. In the center of the co-culture dish with hESC-retina, PBMCs formed small aggregates. In particular, PBMCs were almost single cells near the hESC-retina. (C) PBMCs in the presence of anti-CD3/CD28 antibodies were co-cultured with iPSC-retina. These immune cells were immune suppressed when co-cultured iPSC-retina or iPSC-RPE.
(D) CD4+ and CD8+ cells were immune suppressed according to the number of whole retina cells (5, 10, 15, and 20 retinal tissues) (n = 2 independent experiments).
Immunosuppressive Property of Native Primate Retina
To confirm if this is a consistent characteristic of in vivo native retinal tissues that are freshly harvested, we isolated retinal tissue from a postmortem adult monkey (8 years old) to test the immunomodulatory properties. We co-cultured the adult monkey retinal tissue with activated allogeneic monkey PBMCs by anti-human CD3 agonistic antibodies. Compared with the control PBMCs without co-culturing, adult monkey retinal tissue dramatically suppressed the proliferation of all CD4+, CD8+, CD11b+, CD19+, and NKG2A+ cells in conditions that triggered PBMC proliferation (Figure 5A). Four adult monkey retinal tissue consistently suppressed the proliferation of T, CD4+, and CD8+ cells (Figure 5B). In co-culture, PBMCs formed aggregates without monkey adult retinal tissue, but did not do so with adult monkey retinal tissue (Figures 5C and 5D). In addition, PBMCs cultured in the fresh posterior eye cup that contained retina and RPE also suppressed allogeneic PBMCs in vitro (Figure 5A). Taken together, these data suggested that, not only the posterior eye environment, but retinal tissue alone and retinal tissue differentiated from hESCs and iPSCs in vitro have strong immunosuppressive properties.
Figure 5.
Immunosuppressive Property of Native Primate Retina
(A) Allogeneic monkey PBMCs were co-cultured in the presence of anti-CD3 antibody with a freshly isolated retina from a postmortem adult monkey, or incubated in a freshly prepared posterior eyecup that included retina, choroid, and sclera. Compared with the control without retina, monkey adult retina dramatically suppressed the proliferation of immune cells. Data are representative of n = 2 independent assays.
(B) Four adult monkey retinas consistently suppressed the proliferation of CD4+ cells and CD8+ T cells stimulated by anti-CD3 antibody.
(C and D) Control PBMCs formed aggregates in the presence of anti-CD3 antibody but no significant aggregates were observed when co-cultured with adult monkey retina (donor, n = 4). Scale bars, 500 μm (C and D).
TGF-β Is the Major Immunomodulatory Molecule Secreted by hESC- and iPSC-Retina
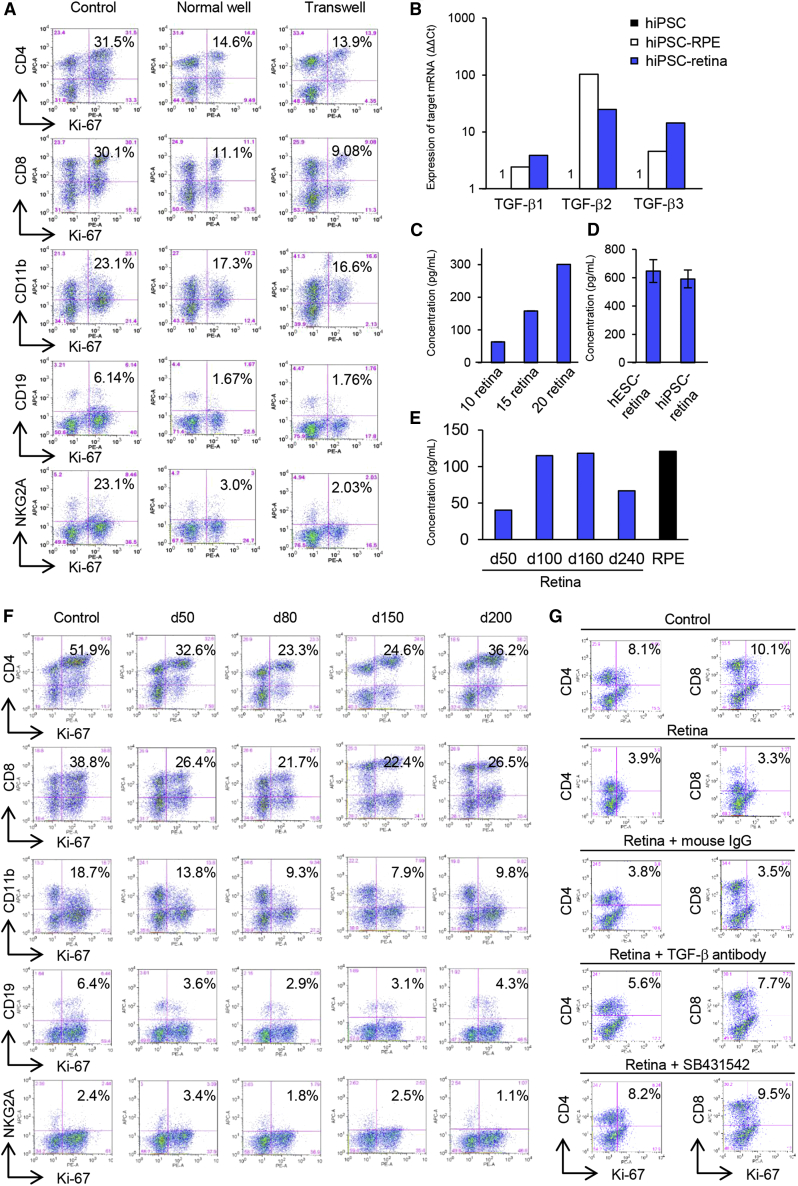
To investigate the mechanism of the immunosuppressive properties of hESC- and iPSC-retina, we first tested if direct contact between hESC-retina and PBMCs was required for immune suppression. The immunosuppressive pattern of hESC-retina did not change in a separate co-culture using a Transwell (Figure 6A). hESC-retina greatly suppressed activated immune cells in the Transwell condition. These results indicate that the immune suppression was due to humoral molecules/factors secreted by hESC-retina.
Figure 6.
TGF-β2 Expression in hESC- and iPSC-Retina and the Effect of TGF-β on Immune Suppression
(A) Flow cytometry analysis of the LGIR assay using a Transwell culture insert for separately culturing hESC-retina and PBMCs. Immunosuppressive patterns in tissues grown in a normal well and Transwell were similar (n = 2).
(B) Expression of TGF-β1, TGF-β2, and TGF-β3 by iPSC-retina was determined with quantitative real-time PCR compared with iPSCs (ΔΔCt: control iPSC = 1.0) (n = 2).
(C) Concentration of TGF-β2 was increased according to the number of retina tissues in the supernatant of hESC-retina as seen with ELISA.
(D) hESC- and iPSC-retina (10 retinas each) secreted the similar level of TGF-β2 as determined by ELISA. Data are the means ± SEM.
(E) Secretion level of TGF-β2 at d50, d100, d160, and d240 of retina and RPE. ELISA results are representative of assay (n = 3).
(F) hESC-retina showed immune modulatory properties regardless of the differentiation day. Immune suppression ability was most prominent with d80–d150 retina.
(G) LGIR neutralizing co-culture assay using TGF-β antibody or the TGF-β receptor inhibitor, SB431542. hESC-retina was co-cultured with PBMCs stimulated with anti-CD3/CD28 antibodies in the presence of the 1 μg/mL TGF-β antibody or 1 μM SB431542. The TGF-β antibody and TGF-β receptor inhibitor decreased immune suppression of hESC-retina. FACS data are representative of two independent experiments.
To identify the secreted molecule(s), we screened for the expression of immunosuppressive molecules with quantitative real-time PCR (Figure S6A). Compared with iPSCs (as a control), iPSC-retina highly expressed TGF-β1, TGF-β2, and TGF-β3, and these molecules were also expressed by immunosuppressive iPSC-RPE cells (Figure 6B). Levels of TGF-β2 in the supernatant collected from hESC-retina were tissue number-dependent as seen with an enzyme-linked immunosorbent assay (ELISA) (Figure 6C), and TGF-β2 was also secreted from iPSC-retina (32.4 ± 2.0 pg/mL per whole retina) at the same levels as hESC-retina (29.6 ± 2.8 pg/mL per whole retina) (Figure 6D).
Since transplanted hESC- and iPSC-retina mature after transplantation, we then examined whether these immunosuppressive properties and TGF-β2 secretion level changes during the maturation process of hESC-retina. TGF-β2 was consistently secreted at different developmental stages of hESC-retina between DD50 and DD240, with the highest values at DD100 and DD160. The peak secretion level of TGF-β2 by hESC-retina was similar to that by iPSC-RPE cells (Figure 6E). hESC-retina of DD50–DD200 consistently suppressed activated immune cells, and the suppression was most evident with hESC-retina of DD80 and DD150 (Figure 6F).
To see if the immunosuppressive activity of hESC- and iPSC-retina is mediated by TGF-β, we then tested if TGF-β-neutralizing antibody or TGF-β receptor inhibitor SB431542 abolish the suppressive effect. In a mixed lymphocyte reaction assay using five samples of mixed healthy donor PBMCs, the immunosuppressive activity by hESC-retina was partially inhibited by adding anti-TGF-β-neutralizing antibody (Figure 6G). Similarly, the immunosuppressive activity was blocked by adding TGF-β receptor inhibitor SB431542 (Figure 6G). These data suggested that secreted TGF-β from hESC- and iPSC-retina played a significant role among other, as yet unidentified, factors in the immunosuppressive mechanism.
Because the preparation of single cells from hESC- and iPSC-retina involves enzymatic digestion with papain, we also tested the influence of enzymatic digestion on different forms of hESC- and iPSC-retina used in Figure 2 by preparation under four tissue conditions: (1) hESC-whole retina with no enzymatic treatment, (2) hESC-whole retina with enzymatic treatment, (3) semi-dissociated hESC-retina, and (4) hESC-retina in single cells (Figure S6B). After 2 days in culture, we measured TGF-β2 secretions with ELISA and gene expression with real-time PCR. TGF-β2 was not detected in the culture medium of any of the three forms of hESC-retina after enzymatic treatment, whereas gene expression of TGF-β2 was reduced but present, implying that the reduction in secretion would be transient (Figures S6C and S6D). We further investigated if this effect was specific to papain-based neuron dissociation solution or due to enzymatic digestion in general by using other digestive reagents. We found that, similar to a papain digestion, TrypLE Select and Accumax also reduced the secretion of TGF-β2 (Figures S6E and S6F). Consistently, in PBMC co-culture assay, the dissociated cells with any enzymatic treatment increased the immunogenicity compared with non-treated retinas, which was accompanied by the simultaneous increase in the secretion of IFN-γ (Figures S7A–S7C). Thus, the absence of TGF-β seems to be a result of enzymatic digestion, which may have enhanced the immunogenicity of dissociated single cells of hESC-retinas in the LGIR assay. It is noteworthy that the immunogenicity of enzyme-treated whole retina still retained immunosuppressive property despite the evident reduction of TGF-β2 secretion and the increase in IFN-γ secretion, again suggesting the presence of some other factors contributing to the immunosuppressive mechanism other than TGF-β2. In addition, the levels of anti-inflammatory interleukin-10 secretion were low in all samples (Figure S7B).
HLA Expression Level and Distribution of hESC-Retina after Transplantation
Finally, we investigated HLA expression by hESC-retina after transplantation in a retinal degeneration model of Rho-mutant nude rats (Seiler et al., 2014). After transplantation, HLA class I expression was not apparent on day 1 but was weakly upregulated in non-Crx::Venus+ grafted cells after 7 days (Figures 7A and 7B). After 5 months, HLA class I was expressed between photoreceptor rosettes and host RPE, which was co-labeled with human-specific GFAP, indicating the activated status of distal part of grafted Müller glial cells, probably due to surgical injury, impairment of some cells in the graft, or inflammation of background disease (Figure 7D). It is at the same time noteworthy that either GFAP or HLA class I was negative in the retinal cells integrating or proximal to host retina, which was consistent with our previous report (Tu et al., 2019) that HLA class II was not expressed in any part of the graft at any of the observed time points (Figure 7C). Although transplantation was performed in lymphocyte-depleted nude rats and we could not evaluate the host immune response in this model, we also observed that hESC-retina prestimulated by IFN-γ to induce HLA class I did not activate allogeneic lymphocytes (Figure 7E). These results suggest that hESC-retina may express HLA class I in Müller glial cells after transplantation but not at the integration site, and immunogenicity may be still low.
Figure 7.
Distribution of HLA Expression of hESC-Retina after Subretinal Transplantation in SD-Foxn1 Tg (S334ter) 3LavRrrc Nude Rats
(A) Low HLA class I expression of hESC-retina in the host subretinal space after 1 day of transplantation. Stem121+ hESC-retina was confined to the area in close proximity to the transplantation site.
(B and C) Immunostaining for rat retinal sections with Stem121 and HLA class I and II after 7 days of transplantation. Crx::Venus+ photoreceptors formed rosette structures. HLA class I expression in cells was limited, and no HLA class II+ cells were seen.
(D) Rat retinal sections were stained for Stem123, human-specific GFAP, and HLA class I. Co-expression of HLA class I and Stem123 after 5 months of transplantation. Immunohistochemical analysis are representative of six animals.
(E) hESC-retina cultured for 2 days in the presence of rIFN-γ (100 ng/mL), was used for LGIR assay. hESC-retina was still suppressive on immune cells in the prestimulated rIFN-γ (independent assay, n = 2).
Scale bars, 150 μm (A), 50 μm (B and C), and 100 and 50 μm (D).
Discussion
The eye, a part of the central nervous system, is a well-known immune-privileged tissue but the immunomodulatory characteristics of isolated retina, particularly human retina, have not been studied so far. In view of recent advances in regenerative therapy and clinical application of hESC- and iPSC-retinal tissue or cells, we closely investigated the immunological characteristics of hESC- and iPSC-retina that were differentiated in vitro outside the immune-privileged site. Here, we present three unique features of hESC- and iPSC-retina. First, hESC- and iPSC-retina expressed low HLA class I and little HLA class II. Second, hESC- and iPSC-retina had low immunogenicity, and only single cells, but not tissue or semi dissociate cells, partially activated allogeneic PBMCs. Third, adult fresh monkey retina and hESC- and iPSC-retina showed active immunosuppressive properties on activated allogeneic lymphocytes, and the secretion of TGF-β was suggested to be a key mechanism. These features suggested a low risk of immune rejection after transplantation of hESC- and iPSC-retina in clinical applications, which may reduce the need for use of systemic immunosuppressive medication.
In general, neural tissues are recognized as low antigen-presenting tissues, and several neural tissues derived from hESC- and iPSC, such as neural stem/progenitor cells, neural crest cells, and dopaminergic progenitors, also show low HLA expression (Fujii et al., 2019; Itakura et al., 2017; Morizane et al., 2017; Ozaki et al., 2017). hESC- and iPSC-retina similarly showed low immunogenicity, and this was consistent throughout the different developmental/differentiation stages. Although low immunogenicity of the retina was conceptually recognized, transplanted retinal cell suspensions reportedly survive rather poorly compared with retinal sheet transplants, particularly in inflammatory conditions (Ghosh et al, 1999, 2007; West et al., 2010). In this study, although cell suspensions dissociated from hESC- and iPSC-retina had mild immunogenicity in contrast to immunosuppressive hESC- and iPSC-whole retina, the expression level of HLA was not significantly different between these two forms.
Interestingly, hESC- and iPSC-retina showed not only low immunogenicity but actively suppressed activated inflammatory immune cells. Because the inflammatory process is involved in diseases, such as AMD and retinitis pigmentosa (Ambati et al., 2003; Edwards et al., 2005; Mandai et al., 2012; Wooff et al., 2019), as well as the transplantation procedure itself, these suppressive properties of hESC- and iPSC-retina are likely advantageous for successful cell therapy. Interestingly, activated Müller marker GFAP and HLA class I were positive only in the distal to host part of the graft and were absent at the host-graft integration site. This was consistent with our previous observation that GS+ Müller glia span the entire graft, but that activated Müller marker GFAP was only positive on the distal to host part of the rosettes and was not observed at the host-graft integration site (Tu et al., 2019). Thus, we postulate that the Müller cells, which are important to retain the photoreceptor layer structure, glutamate uptake, and visual pigment recycling, may not necessarily be harmful when considering the graft immunoreaction. At the same time, tri-laminated whole retina may not be an ideal graft form for photoreceptor replacement, and we have to consider a way to retain the advantage of hESC- and hiPSC-retinas but yet facilitate the graft integration by eliminating the unnecessary retinal layers/components. In addition, hESC- and iPSC-retinas prepared in vitro entirely lack retinal microglia and vascular cells, and this may also affect the graft immunogenicity.
TGF-β is an immune modulatory factor secreted by both hESC- and iPSC-retina and iPSC-RPE. These immunosuppressive properties of hESC- and iPSC-retina contribute not only to immune rejection but also may protect against the proinflammatory status of background diseases. In this study, TGF-β played an important role in the immune modulatory properties of hESC- and iPSC-retina, and the loss of TGF-β secretion due to enzyme digestion during cell dissociation despite the presence of TGF-β mRNA may lead to lower immunogenicity or loss of immunosuppressive properties of these cells. We previously reported that iPSC-RPE induces immune tolerance and regulatory T cells (Sugita et al., 2015), which suggests a similar role for hESC- and iPSC-retina. However, in our several experiments, hESC- and iPSC-retinas is unable to induce regulatory T cells in vitro unlike RPE cells (data not shown). These finding show that the difference of immune properties between the hESC- and iPSC-retina and RPE cells.
A previous clinical trial that assessed allogeneic RPE cell transplantation for AMD patients reported a loss of transplants in 75% of the cases over 2 years when no immune suppressants were administered (Algvere et al., 1999). Interestingly, however, co-transplantation of a combination of allogeneic RPE cells with retina showed no immune rejection, even in the absence of administration of immunosuppressants (Radtke et al., 2008). In some retinal degenerative diseases, the immune-privileged environment is impaired (Welge-Lüßen et al., 1999; Lin et al., 2018). RPE cells were previously considered to have immunosuppressive properties, and RPE cells and the microvascular epithelium maintained immune privilege. However, we directly demonstrated that hESC- and iPSC-retina also have immunosuppressive properties. These findings suggest that hESC- and iPSC-retina may also contribute to maintenance of immune privilege and that transplantation of healthy hESC- and iPSC-retina in retinal degenerative diseases may also help reconstruct the ocular immune environment.
Experimental procedures
Retinal Differentiation Derived from Human ESCs/iPSCs
We used one hESC line (KhES-1) with Venus cDNA knocked in at the locus of the early photoreceptor marker gene Crx (Chen et al., 1997; Furukawa et al., 1997; Nakano et al., 2012) and a human iPSC line (TLHD2). TLHD2 iPSCs were established from skin fibroblasts of a healthy donor after informed consent was obtained. The research followed the tenets of the Declaration of Helsinki, and the study was approved by the Institutional Ethics Committees of the Center for Developmental Biology, RIKEN. An episomal vector with several genes, including, OCT3/4, SOX2, KLF4, L-MYC, LIN28, and p53 small hairpin RNA was used as described (Sugita et al., 2015). Undifferentiated hESCs and iPSCs were used and maintained in feeder-free conditions on LM511-E8 matrix (Matrixome) in Stem Fit medium (Ajinomoto) as described previously (Nakagawa et al., 2014).
We used a retinal differentiation method with the serum-free floating culture of embryoid body-like aggregates with the quick aggregation protocol (Kuwahara et al., 2015) with a minor modification (Kuwahara et al., 2019). In brief, at DD1, hESC and iPSC in Stem Fit (Ajinomoto) medium were treated with 5 μM TGF-β receptor inhibitor (SB431542: Tocris Bioscience) and 300 nM Smoothened agonist (Enzo) for 24 h before differentiation in sub-confluent culture. Dissociated hESCs and iPSCs (1.2 × 105 cells/100 μL gfCDM with 10 μM Rock inhibitor [Y-27632; Wako Pure Chemical Industries]) were reaggregated in low cell adhesion V-bottom 96-well plates (Sumilon Prime Surface plate: Sumitomo Bakelite). On DD3, 1.5 nM recombinant human BMP4 (R&D Systems) was added to the medium. Thereafter, half the medium was refreshed every third day until day 15. Retinal tissue was then transferred to low-adhesion Petri dishes (Sumitomo Bakelite) in DMEM/F12 (Gibco) containing 1% N2 supplement (Gibco), 3 μM GSK-3 inhibitor (CHIR99021; Wako Pure Chemical Industries), and a vascular endothelial growth factor receptor/fibroblast growth factor receptor inhibitor (SU5402; Wako Pure Chemical Industries). Then the medium was changed on day 18 to long-term medium (Nukaya et al. in preparation; WO2019017492A1, WO2019054514A1). The culture medium was changed every 3–4 day.
This study followed the tenets of the Declaration of Helsinki, and was approved by the Institutional Ethics Committee of the RIKEN BDR (Approval No. Kobe1 2013-01(10), 29 March 2019). Please see supplemental materials for other methods.
Author contributions
S.S., M.M., and M.T. designed and supervised the study. S.Y. and M.H. conducted in vitro experiments and immunohistochemistry, characterized hESC- and iPSC-retina, and performed data analysis. S.S. and T.M. conducted flow cytometry and data analysis and prepared and characterized iPSC-retina. M.M. conducted transplantation surgeries. A. Kuwahara, A. Kishino, and T.K., optimized the protocol of and supervised iPSC-retina differentiation. A. Kawasaki. and T.H. supported experiments using monkey samples. S.Y., S.S., and M.M. wrote the manuscript. All authors reviewed and approved the final manuscript.
Conflicts of interest
S.Y., M.H., A. Kuwahara, A. Kishino, and T.K. are employees of Sumitomo Dainippon Pharma; this study was supported by Sumitomo Dainippon Pharma. M. Takahashi: Collaboration funding from Sumitomo Dainippon Pharma (2015/10/1-2020/3/31)—26,400,000 yen (2015), 30,788,962 yen (2016), 15,000,000 yen (2017), 15,000,000 yen (2018). The other authors declare no competing interests. We have a patent related to this work (patent number: PCT/JP2019/051468).
Acknowledgments
We thank J. Sho for help with animal experiments; K. Iseki, N. Hayashi, and S. Fujino for conducting in vitro experiments. We thank T. Matsuyama, T. Hung-Ya, R. Akiba, Y. Wu, C. Morinaga, K. Matsushita, D. Nukaya, T. Kamei, and K. Watari for technical support and members of the M.T. laboratory and RACMO for discussions. We thank Mototsugu Eiraku for contribution to the development of the hESC- and iPSC-retina differentiation protocol. This study was supported by a grant from Japan Agency for Medical Research and Development (Japan) under grant number JP20bm0204002.
Published: March 25, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2021.02.021.
Contributor Information
Sunao Sugita, Email: sunao.sugita@riken.jp.
Michiko Mandai, Email: michiko.mandai@riken.jp.
Supplemental information
References
- Akiba R., Matsuyama T., Tu H.Y., Hashiguchi T., Sho J., Yamamoto S., Takahashi M., Mandai M. Quantitative and qualitative evaluation of photoreceptor synapses in developing, degenerating and regenerating retinas. Front. Cell. Neurosci. 2019;13:1–20. doi: 10.3389/fncel.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algvere P.V., Gouras P., Kopp E.D. Long-term outcome of RPE allografts in non-immunosuppressed patients with AMD. Eur. J. Ophthalmol. 1999;9:217–230. doi: 10.1177/112067219900900310. [DOI] [PubMed] [Google Scholar]
- Ambati J., Anand A., Fernandez S., Sakurai E., Lynn B.C., Kuziel W.A., Rollins B.J., Ambati B.K. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat. Med. 2003;9:1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- Assawachananont J., Mandai M., Okamoto S., Yamada C., Eiraku M., Yonemura S., Sasai Y., Takahashi M. Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice. Stem Cell Reports. 2014;2:662–674. doi: 10.1016/j.stemcr.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Cramer A.O., Wang W., Lu S.J., Singh M.S., Luo C., Huo H., McClements M.E., Barnard A.R., MacLaren R.E., Lanza R. Function of human pluripotent stem cell-derived photoreceptor progenitors in blind mice. Sci. Rep. 2016;6:1–15. doi: 10.1038/srep29784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capowski E.E., Samimi K., Mayerl S.J., Phillips M.J., Pinilla I., Howden S.E., Saha J., Jansen A.D., Edwards K.L., Jager L.D. Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Development. 2019;146:1–13. doi: 10.1242/dev.171686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Wang Q.L., Nie Z., Sun H., Lennon G., Copeland N.G., Gilbert D.J., Jenkins N.A., Zack D.J. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron. 1997;19:1017–1030. doi: 10.1016/s0896-6273(00)80394-3. [DOI] [PubMed] [Google Scholar]
- Da Cruz L., Fynes K., Georgiadis O., Kerby J., Luo Y.H., Ahmado A., Vernon A., Daniels J.T., Nommiste B., Hasan S.M. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018;36:1–10. doi: 10.1038/nbt.4114. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz J.G. The blood-retinal barriers system. Basic concepts and clinical evaluation. Exp. Eye Res. 2004;78:715–721. doi: 10.1016/s0014-4835(03)00213-6. [DOI] [PubMed] [Google Scholar]
- Das T., Del Cerro M., Jalali S., Rao V.S., Gullapalli V.K., Little C., Loreto D.A.D., Sharma S., Sreedharan A., Del Cerro C. The transplantation of human fetal neuroretinal cells in advanced retinitis pigmentosa patients: results of a long-term safety study. Exp. Neurol. 1999;157:58–68. doi: 10.1006/exnr.1998.6992. [DOI] [PubMed] [Google Scholar]
- Edo A., Sugita S., Futatsugi Y., Sho J., Onishi A., Kiuchi Y., Takahashi M. Capacity of retinal ganglion cells derived from human induced pluripotent stem cells to suppress T-cells. Int. J. Mol. Sci. 2020;21:1–18. doi: 10.3390/ijms21217831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A.O., Ritter R., Abel K.J., Manning A., Panhuysen C., Farrer L.A. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- Eiraku M., Takata N., Ishibashi H., Kawada M., Sakakura E., Okuda S., Sekiguchi K., Adachi T., Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–58. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- Fujii S., Yoshida S., Inagaki E., Hatou S., Tsubota K., Takahashi M., Shimmura S., Sugita S. Immunological properties of neural crest cells derived from human induced pluripotent stem cells. Stem Cells Dev. 2019;28:28–43. doi: 10.1089/scd.2018.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Sugita S., Futatsugi Y., Ishida M., Edo A., Makabe K., Kamao H., Iwasaki Y., Sakaguchi H., Hirami Y. A strategy for personalized treatment of iPS-retinal immune rejections assessed in cynomolgus monkey models. Int. J. Mol. Sci. 2020;21:1–16. doi: 10.3390/ijms21093077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Morrow E.M., Cepko C.L. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91:531–541. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- Gagliardi G., Ben M’Barek K., Chaffiol A., Slembrouck-Brec A., Conart J.B., Nanteau C., Rabesandratana O., Sahel J.A., Duebel J., Orieux G. Characterization and transplantation of CD73-positive photoreceptors isolated from human iPSC-derived retinal organoids. Stem Cell Reports. 2018;11:665–680. doi: 10.1016/j.stemcr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini S.J., Llonch S., Borsch O., Ader M. Transplantation of photoreceptors into the degenerative retina: current state and future perspectives. Prog. Retin. Eye Res. 2019;69:1–37. doi: 10.1016/j.preteyeres.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Ghosh F., Johansson K., Ehinger B. Long-term full-thickness embryonic rabbit retinal transplants. Invest. Ophthalmol. Vis. Sci. 1999;40:126–132. [PubMed] [Google Scholar]
- Ghosh F., Engelsberg K., English R.V., Petters R.M. Long-term neuroretinal full-thickness transplants in a large animal model of severe retinitis pigmentosa. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007;245:835–846. doi: 10.1007/s00417-006-0437-9. [DOI] [PubMed] [Google Scholar]
- Humayun M.S., De Juan E.,J., Del Cerro M., Dagnelie G., Radner W., Sadda S.R., Del Cerro C. Human neural retinal transplantation. Invest. Ophthalmol. Vis. Sci. 2000;41:3100–3106. [PubMed] [Google Scholar]
- Iraha S., Tu H.Y., Yamasaki S., Kagawa T., Goto M., Takahashi R., Watanabe T., Sugita S., Yonemura S., Sunagawa G.A. Establishment of immunodeficient retinal degeneration model mice and functional maturation of human ESC-derived retinal sheets after transplantation. Stem Cell Reports. 2018;10:1059–1074. doi: 10.1016/j.stemcr.2018.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura G., Ozaki M., Nagoshi N., Kawabata S., Nishiyama Y., Sugai K., Iida T., Kashiwagi R., Ookubo T., Yastake K. Low immunogenicity of mouse induced pluripotent stem cell-derived neural stem/progenitor cells. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-13522-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S., Jamieson C.H.M., Pang W.W., Park C.Y., Chao M.P., Majeti R., Traver D., van Rooijen N., Weissman I.L. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani A.H., Lebkowski J.S., Rahhal F.M., Avery R.L., Salehi-Had H., Dang W., Lin C.M., Mitra D., Zhu D., Thomas B.B. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci. Transl. Med. 2018;10:1–10. doi: 10.1126/scitranslmed.aao4097. [DOI] [PubMed] [Google Scholar]
- Kruczek K., Gonzalez-Cordero A., Goh D., Naeem A., Jonikas M., Blackford S.J.I., Kloc M., Duran Y., Georgiadis A., Sampson R.D. Differentiation and transplantation of embryonic stem cell-derived cone photoreceptors into a mouse model of end-stage retinal degeneration. Stem Cell Reports. 2017;8:1659–1674. doi: 10.1016/j.stemcr.2017.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara A., Ozone C., Nakano T., Saito K., Eiraku M., Sasai Y. Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue. Nat. Commun. 2015;6:1–15. doi: 10.1038/ncomms7286. [DOI] [PubMed] [Google Scholar]
- Kuwahara A., Yamasaki S., Mandai M., Watari K., Matsushita K., Fujiwara M., Hori Y., Hiramine Y., Nukaya D., Iwata M. Preconditioning the initial state of feeder-free human pluripotent stem cells promotes self-formation of three-dimensional retinal tissue. Sci. Rep. 2019;9:1–16. doi: 10.1038/s41598-019-55130-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B., McLelland B.T., Mathur A., Aramant R.B., Seiler M.J. Sheets of human retinal progenitor transplants improve vision in rats with severe retinal degeneration. Exp. Eye Res. 2018;174:13–28. doi: 10.1016/j.exer.2018.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A., Mandai M., Takahashi M. Gene and induced pluripotent stem cell therapy for retinal diseases. Annu. Rev. Genomics Hum. Genet. 2019;20:15.1–15.16. doi: 10.1146/annurev-genom-083118-015043. [DOI] [PubMed] [Google Scholar]
- Mandai M., Homma K., Okamoto S., Yamada C., Nomori A., Takahashi M. Adequate time window and environmental factors supporting retinal graft cell survival in rd mice. Cell Med. 2012;4:45–54. doi: 10.3727/215517912X639315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai M., Watanabe A., Kurimoto Y., Hirami Y., Morinaga C., Daimon T., Fujihara M., Akimaru H., Sakai N., Shibata Y. Autologous induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med. 2017;376:1038–1046. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- Mandai M., Fujii M., Hashiguchi T., Sunagawa G.A., Ito S., Sun J., Kaneko J., Sho J., Yamada C., Takahashi M. iPSC-derived retina transplants improve vision in rd1 end-stage retinal-degeneration mice. Stem Cell Reports. 2017;8:69–83. doi: 10.1016/j.stemcr.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehat M.S., Sundaram V., Ripamonti C., Robson A.G., Smith A.J., Borooah S., Robinson M., Rosenthal A.N., Innes W., Weleber R.G. Transplantation of human embryonic stem cell-derived retinal pigment epithelial cells in macular degeneration. Ophthalmology. 2018;125:1765–1775. doi: 10.1016/j.ophtha.2018.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki M., Sugita S., Kamoi K. Immunological homeostasis of the eye. Prog. Retin. Eye Res. 2013;33:10–27. doi: 10.1016/j.preteyeres.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Morizane A., Kikuchi T., Hayashi T., Mizuma H., Takara S., Doi H., Mawatari A., Glasser M.F., Shiina T., Ishigaki H. MHC matching improves engraftment of iPSC-derived neurons in non-human primates. Nat. Commun. 2017;8:1–12. doi: 10.1038/s41467-017-00926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M., Taniguchi Y., Senda S., Takizawa N., Ichisaka T., Asano K., Morizane A., Doi D., Takahashi J., Nishizawa M. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci. Rep. 2014;4:1–7. doi: 10.1038/srep03594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Ando S., Takata N., Kawada M., Muguruma K., Sekiguchi K., Saito K., Yonemura S., Eiraku M., Sasai Y. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10:771–785. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Ozaki M., Iwanami A., Nagoshi N., Kohyama J., Itakura G., Iwai H., Nishimura S., Nishiyama Y., Kawabata S., Sugai K. Evaluation of the immunogenicity of human iPS cell-derived neural stem/progenitor cells in vitro. Stem Cell Res. 2017;19:128–138. doi: 10.1016/j.scr.2017.01.007. [DOI] [PubMed] [Google Scholar]
- Pearson R.A., Gonzalez-Cordero A., West E.L., Ribeiro J.R., Aghaizu N., Goh D., Sampson R.D., Georgiadis A., Waldron P.V., Duran Y. Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nat. Commun. 2016;7:1–15. doi: 10.1038/ncomms13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke N.D., Aramant R.B., Petry H.M., Green P.T., Pidwell D.J., Seiler M.J. Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. Am. J. Ophthalmol. 2008;146:172–182. doi: 10.1016/j.ajo.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Reichman S., Terray A., Slembrouck A., Nanteau C., Orieux G., Habeler W., Nandrot E.F., Sahel J.A., Monville C., Goureaua O. From confluent human iPS cells to self-forming neural retina and retinal pigmented epithelium. Proc. Natl. Acad. Sci. U S A. 2014;111:8518–8523. doi: 10.1073/pnas.1324212111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Ferreira T., Llonch S., Borsch O., Postel K., Haas J., Ader M. Retinal transplantation of photoreceptors results in donor-host cytoplasmic exchange. Nat. Commun. 2016;7:1–7. doi: 10.1038/ncomms13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S.D., Regillo C.D., Lam B.L., Eliott D., Rosenfeld P.J., Gregori N.Z., Hubschman J.P., Davis J.L., Heilwell G., Spirn M. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- Seiler M.J., Aramant R.B., Jones M.K., Ferguson D.L., Bryda E.C., Keirstead H.S. A new immunodeficient pigmented retinal degenerate rat strain to study transplantation of human cells without immunosuppression. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014;252:1079–1092. doi: 10.1007/s00417-014-2638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai H., Mandai M., Matsushita K., Kuwahara A., Yonemura S., Nakano T., Assawachananont J., Kimura T., Saito K., Terasaki H. Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc. Natl. Acad. Sci. U S A. 2015;113:E81–E90. doi: 10.1073/pnas.1512590113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M.S., Issa P.C., Butler R., Martin C., Lipinski D.M., Sekaran S., Barnard A.R., MacLaren R.E. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc. Natl. Acad. Sci. U S A. 2013;110:1101–1106. doi: 10.1073/pnas.1119416110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M.S., Balmer J., Barnard A.R., Aslam S.A., Moralli D., Green C.M., Barnea-Cramer A., Duncan I., MacLaren R.E. Transplanted photoreceptor precursors transfer proteins to host photoreceptors by a mechanism of cytoplasmic fusion. Nat. Commun. 2016;7:1–5. doi: 10.1038/ncomms13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W.K., Park K.M., Kim H.J., Lee J.H., Choi J., Chong S.Y., Shim S.H., Del Priore L.V., Lanza R. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Reports. 2015;4:860–872. doi: 10.1016/j.stemcr.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein J.W. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol. 2003;3:879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- Sugita S. Role of ocular pigment epithelial cells in immune privilege. Arch. Immunol. Ther. Exp. 2009;57:263–268. doi: 10.1007/s00005-009-0030-0. [DOI] [PubMed] [Google Scholar]
- Sugita S., Futagami Y., Smith S.B., Naggar H., Mochizuki M. Retinal and ciliary body pigment epithelium suppress activation of T lymphocytes via transforming growth factor beta. Exp. Eye Res. 2006;83:1459–1471. doi: 10.1016/j.exer.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Sugita S., Kamao H., Iwasaki Y., Okamoto S., Hashiguchi T., Iseki K., Hayashi N., Mandai M., Takahashi M. Inhibition of T-cell activation by retinal pigment epithelial cells derived from induced pluripotent stem cells. Invest. Ophthalmol. Vis. Sci. 2015;56:1051–1062. doi: 10.1167/iovs.14-15619. [DOI] [PubMed] [Google Scholar]
- Sugita S., Iwasaki Y., Makabe K., Kamao H., Mandai M., Shiina T., Ogasawara K., Hirami Y., Kurimoto Y., Takahashi M. Successful transplantation of retinal pigment epithelial cells from MHC homozygote iPSCs in MHC-matched models. Stem Cell Reports. 2016;7:635–648. doi: 10.1016/j.stemcr.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S., Iwasaki Y., Makabe K., Kimura T., Futagami T., Suegami S., Takahashi M. Lack of T cell response to iPSC-derived retinal pigment epithelial cells from HLA homozygous donors. Stem Cell Reports. 2016;7:619–634. doi: 10.1016/j.stemcr.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S., Mandai M., Hirami Y., Takagi S., Maeda T., Fujihara M., Matsuzaki M., Yamamoto M., Iseki K., Hayashi N. HLA-matched allogeneic iPS cells-derived RPE transplantation for macular degeneration. J. Clin. Med. 2020;9:22171–221718. doi: 10.3390/jcm9072217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H.Y., Watanabe T., Shirai H., Yamasaki S., Kinoshita M., Matsushita K., Hashiguchi T., Onoe H., Matsuyama T., Kuwahara A. Medium- to long-term survival and functional examination of human iPSC-derived retinas in rat and primate models of retinal degeneration. EBioMedicine. 2019;39:562–574. doi: 10.1016/j.ebiom.2018.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welge–Lüßen U., Wilsch C., Neuhardt T., Streilein J.W., Lütjen–Drecoll E. Loss of anterior chamber-associated immune deviation (ACAID) in aged retinal degeneration (rd) mice. Invest. Ophthalmol. Vis. Sci. 1999;40:3209–3214. [PubMed] [Google Scholar]
- West E.L., Pearson R.A., Barker S.E., Luhmann U.F.O., Maclaren R.E., Barber A.C., Duran Y., Smith A.J., Sowden J.C., Ali R.R. Long-term survival of photoreceptors transplanted into the adult murine neural retina requires immune modulation. Stem Cells. 2010;28:1997–2007. doi: 10.1002/stem.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooff Y., Man S.M., Aggio-Bruce R., Natoli R., Fernando N. IL-1 family members mediate cell death, inflammation and angiogenesis in retinal degenerative diseases. Front. Immunol. 2019;10:1–21. doi: 10.3389/fimmu.2019.01618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X., Gutierrez C., Xue T., Hampton C., Vergara M.N., Cao L.H., Peters A., Park T.S., Zambidis E.T., Meyer J.S. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat. Commun. 2014;5:1–14. doi: 10.1038/ncomms5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.