Abstract
Background:
In the 2-year CARE-MS I and II trials, alemtuzumab 12 mg administered on 5 consecutive days at core study baseline and on 3 consecutive days 12 months later significantly improved outcomes versus subcutaneous interferon beta-1a (SC IFNB-1a) in relapsing–remitting multiple sclerosis patients. Here, we present the final 6-year CARE-MS extension trial results (CAMMS03409), and compare outcomes over 6 years in patients randomized to both treatment groups at core study baseline.
Methods:
Over a 4-year extension, alemtuzumab patients (alemtuzumab-only) received as-needed additional alemtuzumab (⩾12 months apart) for disease activity after course 2. SC IFNB-1a patients who entered the extension discontinued SC IFNB-1a and received 2 alemtuzumab 12 mg courses (IFN–alemtuzumab), followed by additional, as-needed, alemtuzumab.
Results:
Through year 6, 63% of CARE-MS I and 50% of CARE-MS II alemtuzumab-only patients received neither additional alemtuzumab nor other disease-modifying therapy, with lasting suppression of disease activity, improved disability, and slowing of brain volume loss (BVL). In CARE-MS I patients (treatment-naive; less disability; shorter disease duration), disease activity and BVL were significantly reduced in IFN–alemtuzumab patients, similar to alemtuzumab-only patients at year 6. Among CARE-MS II patients (inadequate response to prior treatment; more disability; longer disease duration), alemtuzumab significantly improved clinical and magnetic resonance imaging outcomes, including BVL, in IFN–alemtuzumab patients; however, disability outcomes were less favorable versus alemtuzumab-only patients. Safety profiles, including infections and autoimmunities, following alemtuzumab were similar between treatment groups.
Conclusion:
This study demonstrates the high efficacy of alemtuzumab over 6 years, with a similar safety profile between treatment groups.
ClinicalTrials.gov identifiers:
Keywords: alemtuzumab, brain volume, CD52, disability, disease activity, efficacy, extension, MRI, multiple sclerosis, relapse, safety
Introduction
Multiple sclerosis (MS) immunotherapy is intended to prevent inflammatory demyelination and axonal loss, thereby reducing long-term disability accumulation.1 However, trials typically report only 2–3 years of therapy exposure.1 Here, we report 6-year efficacy and safety of alemtuzumab—a high-efficacy disease-modifying therapy (DMT) for MS that selectively targets CD52-expressing cells for depletion, allowing for subsequent repopulation2,3—and examine the impact of early versus delayed alemtuzumab treatment in two patient populations: treatment-naive patients and patients who were treated previously with other DMTs.
Alemtuzumab demonstrated significantly greater efficacy versus subcutaneous interferon beta-1a (SC IFNB-1a) in clinical and magnetic resonance imaging (MRI) outcomes in the two 2-year phase III clinical trials of relapsing–remitting MS (RRMS) patients [CARE-MS I (treatment-naive; NCT00530348) and II (inadequate response to prior therapy; NCT00548405)].4,5 The most frequent adverse events (AEs) with alemtuzumab in clinical trials were infusion-associated reactions (IARs); other treatment-associated AEs included autoimmune AEs, which were mostly thyroid AEs [less often immune thrombocytopenia (ITP) and nephropathies], leading to a comprehensive risk management plan.4,5 In the postmarketing setting, there have been reports of rare, but severe and potentially fatal, AEs in alemtuzumab-treated patients. These cases have included opportunistic infections, such as listeriosis, cytomegalovirus infection, pulmonary aspergilosis, and cerebral or pulmonary nocardiosis; autoimmune cytopenias; autoimmune hepatitis; autoimmune hemolytic anemia; hemophagocytic lymphohistiocytosis; acute acalculous cholecystitis; and potentially infusion-related cardiovascular and pulmonary events.4–21 Patients who completed the CARE-MS core studies could enter a 4-year extension [CAMMS03409 (NCT00930553)]. Previous interim analyses have shown 5- and 6-year efficacy and safety in alemtuzumab-treated patients, but did not provide outcomes beyond year 2 in patients who were first randomized to SC IFNB-1a.22–24
Here we report the final 6-year follow-up of alemtuzumab-randomized patients who received treatment (alemtuzumab-only group). We also report for the first time follow-up through year 6 in the SC IFNB-1a-randomized patients who switched to alemtuzumab at the start of the extension (IFN–alemtuzumab group), and provide statistical comparisons between treatment arms from core study baselines to the end of the extension. In addition, we assessed the efficacy of alemtuzumab on SC IFNB-1a-randomized patients who did or did not show disease activity during the 2-year core study before receiving alemtuzumab. Differing disability levels and disease durations between the populations at core study baseline enabled assessment of the impact of initiating alemtuzumab at different stages of MS disease.4,5
Methods
Patients and procedures for the CARE-MS core studies
The 2-year CARE-MS core study designs have been published previously.4,5 Briefly, the CARE-MS I and II core studies were randomized, rater-blinded, active-controlled, head-to-head trials of alemtuzumab (12 mg/day; core study baseline: 5 consecutive days; 12 months later: 3 consecutive days) versus SC IFNB-1a 44 µg 3 times/week.
Procedures for the extension study
The group who received alemtuzumab in the core studies (“alemtuzumab-only” group) could receive as-needed additional courses in the extension study (12 mg/day intravenous; 3 consecutive days ⩾12 months after the previous course) for disease activity, defined as ⩾1 protocol-specified relapse or ⩾2 unique lesions on brain or spinal cord MRI consisting of new/enlarging T2 hyperintense and/or gadolinium (Gd)-enhancing lesions. The group who received SC IFNB-1a in the 2-year core studies (“IFN–alemtuzumab” group) discontinued SC IFNB-1a at the start of the extension study, and received alemtuzumab 12 mg/day (extension baseline: 5 consecutive days; 12 months later: 3 consecutive days), with as-needed additional alemtuzumab courses (as described above). All patients could also receive other licensed DMT at the treating neurologist’s discretion. Although the extension study also enrolled CARE-MS II patients who received a 24 mg/day alemtuzumab dose and patients from a phase II alemtuzumab study, these patients are not included in this report.
Efficacy assessments
Relapse events were confirmed by the Relapse Adjudication Panel during the core studies and by the investigator during the extension. Confirmed relapses met protocol-specified criteria including an objective change on neurological examination lasting ⩾48 h in the absence of fever. Expanded Disability Status Scale (EDSS) evaluations were conducted quarterly and at the time of suspected relapse by raters blinded to treatment assignment. Annual MRI scans were obtained locally and scored, blinded to treatment, by imaging specialists at NeuroRx Research (Montréal, Canada; for lesion-based analyses) and at the Cleveland Clinic MS MRI Analysis Center [Cleveland, OH; for brain parenchymal fraction (BPF) analysis].
Clinical efficacy endpoints included: annualized relapse rate (ARR); proportion of relapse-free patients; mean change in EDSS score relative to core study baseline EDSS score; proportions of patients with EDSS scores that were improved (⩾1.0-point decrease), worsened (⩾1.0-point increase), or stable (⩽0.5-point change in either direction) versus core study baseline; 6-month confirmed disability worsening [CDW; ⩾1-point EDSS score increase (⩾1.5 if core study baseline EDSS = 0)], formerly termed sustained accumulation of disability;25 and 6-month confirmed disability improvement (CDI; ⩾1.0-point decrease from core study baseline EDSS score, assessed in patients with core study baseline EDSS scores ⩾2.0), formerly termed sustained reduction of disability.26 Brain volume loss (BVL), both cumulatively from core study baseline and as an annual rate, was based on median percentage changes in BPF (i.e. volume of the brain parenchyma divided by the outer contour volume),27 which were determined using proton density and T2-weighted MRI scans.
Safety monitoring
Patients were monitored for safety for ⩾48 months following their last alemtuzumab administration, according to the recommended risk minimization protocol, which included hematology (complete blood counts with differential; at least monthly), renal examinations (serum creatinine and urinalysis with microscopy; monthly), and thyroid function (at least quarterly). All AEs, serious AEs, and medical events of interest were recorded. Any AE with onset during or ⩽24 h after the end of infusion was defined as an IAR.
Statistical analysis
Analyses included all alemtuzumab-only and IFN–alemtuzumab patients, and were based on all available data (without imputation) through year 6. Efficacy endpoints were compared within the IFN–alemtuzumab group before and after alemtuzumab initiation. Endpoints included ARR using repeated negative binomial regression with robust variance estimation, and the proportions of patients free of MRI disease activity, new Gd-enhancing lesions, and new/enlarging T2 hyperintense lesions using the McNemar test.
Comparative efficacy between the alemtuzumab-only and IFN–alemtuzumab groups was also analyzed. ARR was modeled using negative binomial regression with robust variance estimation. Mean EDSS scores from core study baseline through year 6 were evaluated using a mixed model for repeated measures with treatment, visit, visit-by-treatment interaction, geographic region, and core study baseline EDSS score as fixed effects. Percentages of patients with improved, stable, or worsened EDSS scores were compared between groups using the chi-square test. Kaplan–Meier estimates were used to determine the percentages of patients free of 6-month CDW or with 6-month CDI; these percentages were compared between groups using the Cox proportional hazard model with robust variance estimation and adjustment for geographic region. Percentages of patients free of MRI lesions were compared using logistic regression adjusted for core study baseline lesion status. No evidence of disease activity (NEDA) was defined as the absence of relapse, 6-month CDW, and MRI disease activity (i.e. Gd-enhancing lesions and new/enlarging T2 hyperintense lesions). Assessments in IFN–alemtuzumab patients who did or did not achieve NEDA in the core studies for changes in EDSS score, 6-month CDI, and cumulative change in brain volume (BV), were carried out after rebaselining at extension start. Percentage change in BPF from core study baseline was evaluated at each time point using the ranked analysis of covariance model with adjustment for geographic region and core study baseline BPF. All statistical tests used a two-sided 5% significance level without adjustment for multiple comparisons.
Safety data are reported as incidences (percentage of patients with ⩾1 event) and exposure-adjusted incidence rates per 100 patient-years [100 × (number of patients with the specific event divided by total follow-up time in years among patients at risk of initial occurrence of the event during the specified time interval)].28 Incidence of IARs was analyzed independently for each alemtuzumab course.
All analyses were carried out using SAS statistical software (version 9.4, The SAS Institute, Cary, NC, USA).
Standard protocol approvals, registrations, and patient consents
CARE-MS I, CARE-MS II, and CAMMS03409 are registered with ClinicalTrials.gov (NCT00530348; NCT00548405; NCT00930553). Patients provided written informed consent, and all procedures were approved by local institutional ethics review boards of participating sites.
Results
Patients and treatment
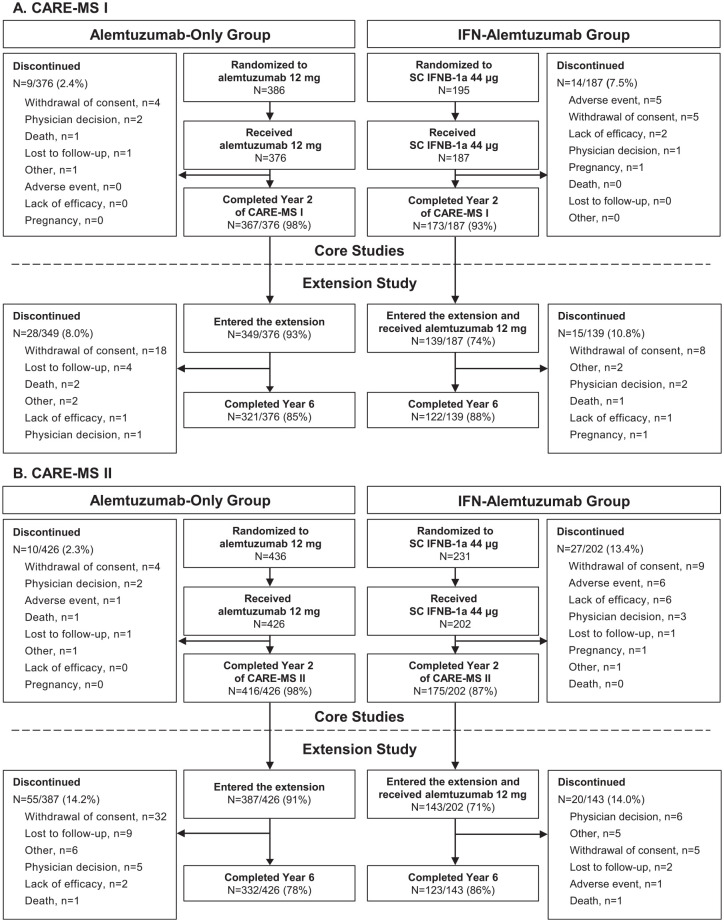
Patient characteristics at core study baseline were comparable between study arms within each study, specifically mean EDSS scores and median disease durations, and have been reported previously.4,5 Of those patients who were randomized to and received alemtuzumab 12 mg in the core studies (alemtuzumab-only group), 93% (349/376) from CARE-MS I and 91% (387/426) from CARE-MS II entered the extension. Proportions of randomized patients who received SC IFNB-1a in the core study, entered the extension, and received alemtuzumab (IFN–alemtuzumab group) were 74% (139/187) for CARE-MS I and 71% (143/202) for CARE-MS II. After initiating alemtuzumab treatment at core study baseline (alemtuzumab-only group), 85% (321/376) and 78% (332/426) of CARE-MS I and II patients, respectively, remained on study at year 6. Of those who initiated alemtuzumab at extension study baseline (IFN–alemtuzumab group), 88% (122/139) and 86% (123/143) of CARE-MS I and II patients, respectively, remained on study at year 6 (Figure 1).
Figure 1.
CARE-MS I and II patient disposition.
Schematic of the as-randomized patient population from the core CARE-MS studies through the extension study, CAMMS03409. Patients randomized to SC IFNB-1a 44 μg who received treatment in the core studies discontinued SC IFNB-1a before initiating alemtuzumab 12 mg in the extension. (A) CARE-MS I patients who were randomized to either alemtuzumab or SC IFNB-1a at core study start. (B) CARE-MS II patients who were randomized to either alemtuzumab or SC IFNB-1a at core study start.
CARE-MS, Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; IFN, interferon; SC IFNB-1a, subcutaneous interferon beta-1a.
Through year 6, 221/349 (63%) alemtuzumab-only patients and 104/139 (75%) IFN–alemtuzumab patients from CARE-MS I, and 192/387 (50%) alemtuzumab-only patients and 101/143 (71%) IFN–alemtuzumab patients from CARE-MS II received neither additional courses of alemtuzumab nor another DMT in the extension (Table 1). Over the 4-year extension study (years 3–6), 23%, 8%, 3%, and 0.3% of CARE-MS I alemtuzumab-only patients, and 29%, 13%, 2%, and 1% of CARE-MS II alemtuzumab-only patients, received a total of 3, 4, 5, and 6 courses of alemtuzumab, respectively (Supplemental material Table 1 online). In the last 2 years of the extension study (years 5 and 6), 21% and 4% of CARE-MS I IFN–alemtuzumab patients, and 20% and 5% of CARE-MS II IFN–alemtuzumab patients, received a total of 3 or 4 courses of alemtuzumab, respectively (as IFN–alemtuzumab patients did not receive their first and second courses of alemtuzumab until years 3 and 4, respectively, and additional courses could not be given until ⩾12 months after the previous one, the maximum number of courses this group could receive was four within the 6-year trials; Supplemental Table 1).
Table 1.
CARE-MS I and II patients who received no as-needed additional alemtuzumab treatment or no other DMT in the extension.
| No as-needed additional courses of alemtuzumab and no other DMT | No as-needed additional courses of alemtuzumab | No other DMT | |
|---|---|---|---|
| CARE-MS I, n (%) | |||
| Alemtuzumab-only group over years
3–6 n = 349 |
221 (63.3%) | 225 (64.5%) | 340 (97.4%) |
| IFN–alemtuzumab group over years 5 and
6 n = 139 |
104 (74.8%) | 105 (75.5%) | 137 (98.6%) |
| CARE-MS II, n (%) | |||
| Alemtuzumab-only group over years
3–6 n = 387 |
192 (49.6%) | 210 (54.3%) | 348 (89.9%) |
| IFN–alemtuzumab group over years 5 and
6 n = 143 |
101 (70.6%) | 107 (74.8%) | 134 (93.7%) |
CARE-MS, Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; DMT, disease-modifying therapy; IFN, interferon.
The mean interval (SD) and the median between courses 2 and 3 for CARE-MS I patients were 2.2 (1.2) and 2.0 years for the alemtuzumab-only group and 1.7 (0.7) and 1.5 years for the IFN–alemtuzumab group. The mean interval (SD) and the median between courses 2 and 3 for CARE-MS II patients were 2.3 (1.1) and 2.1 years for the alemtuzumab-only group and 1.8 (0.7) and 1.8 years for the IFN–alemtuzumab group (Table 2). Time between courses 3 and 4 for the alemtuzumab-only and IFN–alemtuzumab groups, and between courses 4 and 5, and 5 and 6 for the alemtuzumab-only group in the CARE-MS I and II trials is shown in Table 2.
Table 2.
Times between alemtuzumab courses in CARE-MS I and II patients.
| Time between courses, years | ||||
|---|---|---|---|---|
| Course 2 to course 3 | Course 3 to course 4 | Course 4 to course 5 | Course 5 to course 6 | |
| CARE-MS I | ||||
| Alemtuzumab-only group over years 3–6 | n = 124 | n = 42 | n = 13 | n = 1 |
| Mean (SD) | 2.2 (1.2) | 1.8 (0.9) | 1.4 (0.5) | 1.0 (NE) |
| Median | 2.0 | 1.5 | 1.2 | 1.0 |
| IFN–alemtuzumab group over years 5 and 6 | n = 34 | n = 5 | − | − |
| Mean (SD) | 1.7 (0.7) | 1.3 (0.4) | − | − |
| Median | 1.5 | 1.1 | − | − |
| CARE-MS II | ||||
| Alemtuzumab-only group over years 3–6 | n = 178 | n = 63 | n = 13 | n = 4 |
| Mean (SD) | 2.3 (1.1) | 1.7 (0.7) | 1.3 (0.3) | 1.1 (0.2) |
| Median | 2.1 | 1.6 | 1.2 | 1.0 |
| IFN–alemtuzumab group over years 5 and 6 | n = 36 | n = 7 | − | − |
| Mean (SD) | 1.8 (0.7) | 1.2 (0.1) | − | − |
| Median | 1.8 | 1.2 | − | − |
CARE-MS, Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; IFN, interferon; NE, not estimable.
Efficacy outcomes: CARE-MS I (treatment-naive patients)
Alemtuzumab-only group
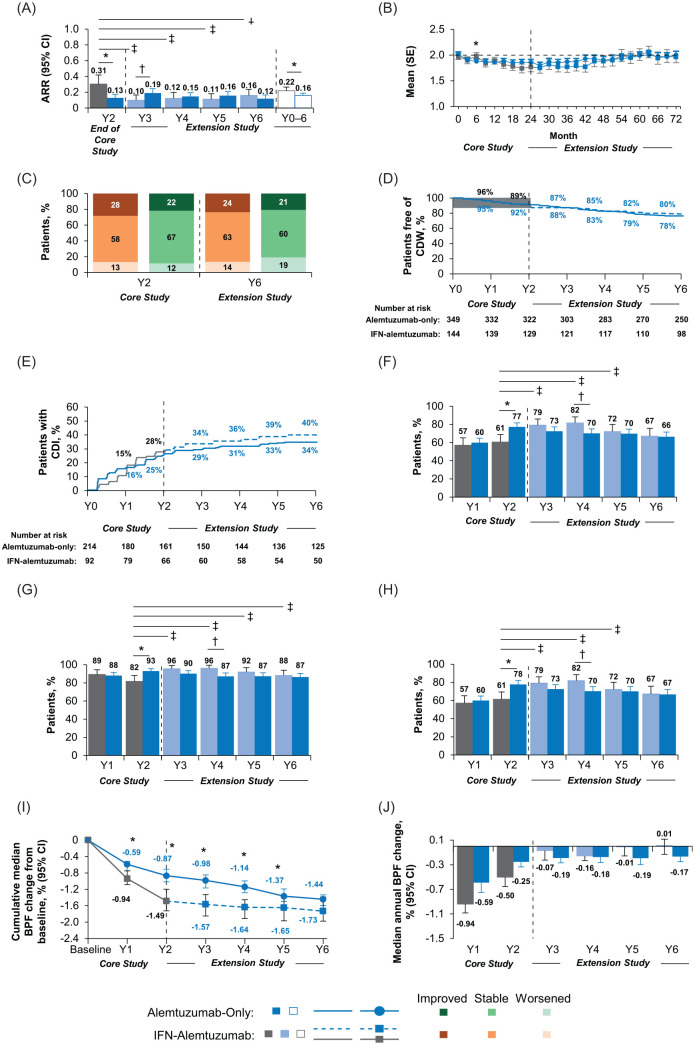
Relapse rates remained low throughout the extension. ARR at year 6 was 0.12 (Figure 2A), and cumulative ARR over years 0–6 was 0.16; 84–89% of patients were free of relapse annually over years 3−6. Mean EDSS score change over years 0–6 was +0.09 (Figure 2B). At year 6 compared with core study baseline, 21% of patients had improved and 60% had stable EDSS scores (Figure 2C), 78% were free of 6-month CDW (Figure 2D), and 34% achieved 6-month CDI (Figure 2E). At year 6, 66% of patients were free of MRI disease activity (Figure 2F–H). Over years 0–6, median cumulative change in BV was −1.44% (Figure 2I) and annual median change in BV ranged from −0.19% in year 3 to −0.17% in year 6 (Figure 2J).
Figure 2.
Efficacy outcomes in CARE-MS I patients through year 6.
Results are shown for the CARE-MS I alemtuzumab-only and IFN–alemtuzumab groups. (A) Yearly ARR from year 2 of the core study to the end of the CAMMS03409 extension study, and cumulative ARR from core study baseline through year 6. Core study ARR values are presented for year 2 only in this analysis, and were reported previously for years 0–2 cumulatively (alemtuzumab: 0.18; SC IFNB-1a: 0.39).4 (B) Change in mean (SE) EDSS score from core study baseline through year 6. (C) Percentages of patients with improved, stable, and worsened EDSS scores at year 2 and year 6 from core study baseline. Percentages may not sum to 100% due to rounding. (D) Kaplan–Meier estimates of the percentages of patients free of 6-month CDW. (E) Kaplan–Meier estimates of the percentages of patients with 6-month CDI. (F) Percentages of patients free of MRI disease activity each year from core study baseline through year 6. For IFN–alemtuzumab patients, MRI disease activity values for year 1 and year 2 are presented only for patients who entered the extension only. (G) Percentages of patients free of new Gd-enhancing lesions each year from core study baseline through year 6. Core study values for proportions of patients free of new Gd-enhancing lesions are presented separately for year 1 and year 2 in this analysis, and were reported previously for year 2 only (alemtuzumab: 93%; SC IFNB-1a: 81%).4 (H) Percentages of patients free of new/enlarging T2 hyperintense lesions each year from core study baseline through year 6. Core study values for proportions of patients free of new/enlarging T2 hyperintense lesions are presented separately for year 1 and year 2 in this analysis, and were reported previously for years 0–2 cumulatively (alemtuzumab: 52%; SC IFNB-1a: 42%).4 For IFN–alemtuzumab patients, values for proportions free of Gd-enhancing lesions and new/enlarging T2 lesions for year 1 and year 2 are presented for patients who entered the extension only, and were reported previously for all patients who received SC IFNB-1a in the core study.4 (I) Median cumulative percentage BVL from core study baseline to the end of CAMMS03409. (J) Median annual percentage BVL.
Alemtuzumab-only group versus IFN–alemtuzumab group: *p < 0.05 indicates significant between-treatment group differences in favor of the alemtuzumab-only group and †p < 0.05 indicates significant between-treatment group differences in favor of the IFN–alemtuzumab group. Year 2 of SC IFNB-1a treatment versus each year (years 3–6) after initiating alemtuzumab treatment: ‡p < 0.05.
ARR, annualized relapse rate; BPF, brain parenchymal fraction; BVL, brain volume loss; CARE-MS, Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; CDI, confirmed disability improvement; CDW, confirmed disability worsening; CI, confidence interval; EDSS, Expanded Disability Status Scale; Gd, gadolinium; IFN, interferon; MRI, magnetic resonance imaging; SC IFNB-1a, subcutaneous interferon beta 1-a; SE, standard error; Y, year.
IFN–alemtuzumab group
Compared with year 2 of SC IFNB-1a, ARR was significantly improved after initiating alemtuzumab at year 3, and this improvement was maintained through year 6 (0.16 at year 6 compared with 0.31 at year 2; p < 0.05; Figure 2A). Cumulative ARR over years 0–6 was 0.22. After receiving alemtuzumab, cumulative ARR in years 3–6 was 0.12, compared with 0.34 in years 0–2 while receiving SC IFNB-1a. Over years 3–6, 86–91% of patients were relapse-free each year, increasing from 79% at year 2 with SC IFNB-1a. The overall change in mean EDSS score from core study baseline through year 6 was +0.11 (Figure 2B). Compared with core study baseline, 24% of patients had improved and 63% had stable EDSS scores at year 6 (Figure 2C). Over 6 years, 80% were free of 6-month CDW (Figure 2D), and 40% achieved 6-month CDI (Figure 2E). The percentage of patients free of MRI disease activity increased significantly from year 2 with SC IFNB-1a treatment (61%) to year 3 with alemtuzumab (79%; p < 0.05) and was 67% at year 6 (Figure 2F–H). Over years 0–6, median cumulative change in BV was −1.73% (Figure 2I) and annual median change in BV ranged from −0.16% to +0.01% over years 3–6, improving from −0.50% at year 2 with SC IFNB-1a (Figure 2J). To determine whether NEDA during years 0–2 while receiving SC IFNB-1a affected outcomes after switching to alemtuzumab, data prior to and after switch were calculated. Mean change in EDSS score in those who did (27%) and did not (73%) achieve NEDA during years 0–2 was −0.62 and −0.06, respectively; 32% and 26% of patients achieved 6-month CDI across this interval and cumulative change in BV was −1.47% and −1.52%, respectively. After rebaselining at the start of the extension, mean change in EDSS score was +0.56 and +0.02 through year 6 with alemtuzumab in those who had and had not achieved NEDA during years 0–2, respectively; of these, 23% and 27% achieved 6-month CDI, and cumulative change in BV was −0.40% and +0.08%, respectively (Supplemental Figure 1A, C, and E).
CARE-MS I: alemtuzumab-only group versus IFN–alemtuzumab group
ARR at year 2 and cumulative ARR over years 0–6 were significantly lower, and proportions of patients free of MRI disease activity at year 2 were significantly higher in the alemtuzumab-only group than in the IFN–alemtuzumab group (all p < 0.05; Figure 2A and F–H). In the CARE-MS I core trial, there were no significant differences in clinical disability outcomes between the alemtuzumab-only group and the IFN–alemtuzumab group. Over years 3–6, differences in disability outcomes between the treatment groups were marginal (Figure 2B–E). Median annual change in BV at year 2 was less in the alemtuzumab-only group versus the IFN–alemtuzumab group, with annual changes ⩽0.19% for both groups thereafter in the extension (Figure 2J). Differences between the treatment groups in cumulative change in BV since core study baseline were statistically significant each year over years 1–5 (p < 0.05), but not at year 6 (Figure 2I). The greatest difference in cumulative BVL was at year 2 when patients were still receiving different therapies. After IFN–alemtuzumab patients began receiving alemtuzumab, the cumulative BVL trajectories became less divergent and were only non-statistically different at year 6.
Efficacy outcomes: CARE-MS II (previously treated patients)
Alemtuzumab-only group
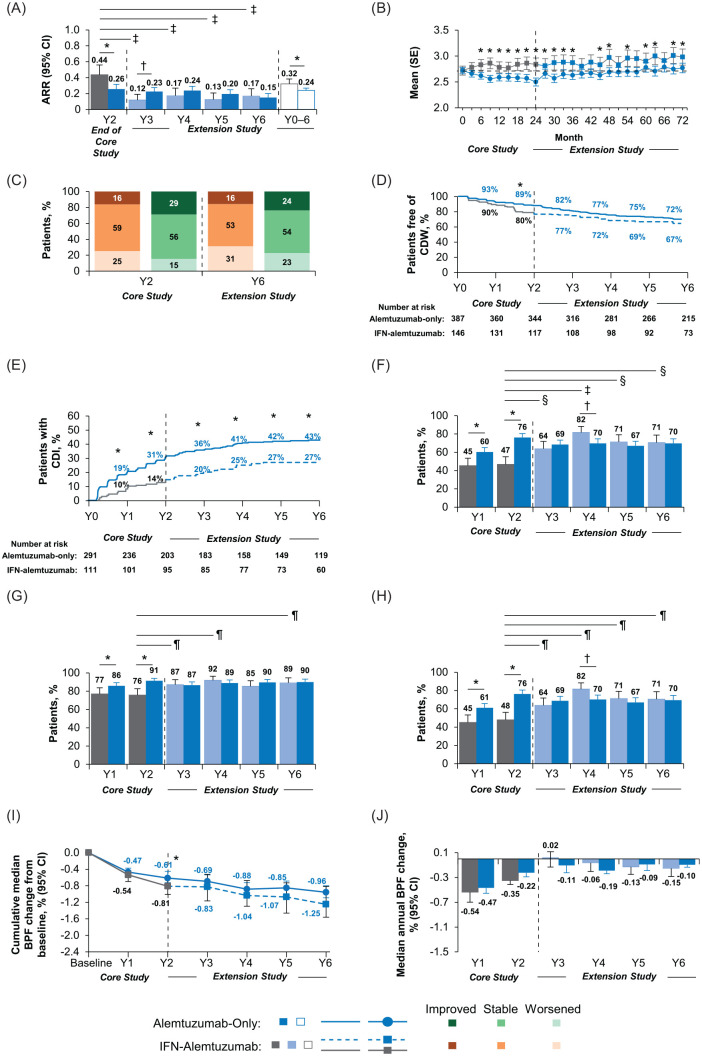
Relapse rates remained low throughout the extension, with ARR of 0.15 during year 6 and 0.24 over years 0–6 (Figure 3A); 79–87% of patients were relapse-free annually in years 3–6. At year 6 compared with core study baseline, the mean EDSS score change was +0.18 (Figure 3B) and EDSS scores were improved in 24% of patients and stable in 54% (Figure 3C). Through year 6, 72% of patients were free of 6-month CDW (Figure 3D) and 43% achieved 6-month CDI (Figure 3E). At year 6, 70% of patients were free of MRI disease activity (Figure 3F–H). Median cumulative change in BV over years 0–6 was −0.96% (Figure 3I), with annual median change in BV during the extension ranging from −0.19% to −0.09% (Figure 3J).
Figure 3.
Efficacy outcomes in CARE-MS II patients through year 6.
Results are shown for the CARE-MS II alemtuzumab-only and IFN–alemtuzumab groups. (A) Yearly ARR from year 2 of the core study to the end of the CAMMS03409 extension study, and cumulative ARR from core study baseline through year 6. Core study ARR values are presented for year 2 only in this analysis, and were reported previously for years 0–2 cumulatively (alemtuzumab: 0.26; SC IFNB-1a: 0.52).5 (B) Change in mean (SE) EDSS score from core study baseline through year 6. (C) Percentages of patients with improved, stable, and worsened EDSS scores at year 2 and year 6 from core study baseline. Percentages may not sum to 100% due to rounding. (D) Kaplan–Meier estimates of the percentages of patients free of 6-month CDW. (E) Kaplan–Meier estimates of the percentages of patients with 6-month CDI. (F) Percentages of patients free of MRI disease activity each year from core study baseline through year 6. For IFN–alemtuzumab patients, MRI disease activity values for year 1 and year 2 are presented for patients who entered the extension only. (G) Percentages of patients free of new Gd-enhancing lesions each year from core study baseline through year 6. Core study values for proportions of patients free of new Gd-enhancing lesions are presented separately for year 1 and year 2 in this analysis, and were reported previously for year 2 only (alemtuzumab: 91%; SC IFNB-1a: 77%).5 (H) Percentages of patients free of new/enlarging T2 hyperintense lesions each year from core study baseline through year 6. Core study values for proportions of patients free of new/enlarging T2 hyperintense lesions are presented separately for year 1 and year 2 in this analysis, and were reported previously for years 0–2 cumulatively (alemtuzumab: 54%; SC IFNB-1a: 32%).5 For IFN–alemtuzumab patients, values for proportions free of Gd-enhancing lesions and new/enlarging T2 lesions for year 1 and year 2 are presented for patients who entered the extension only, and were reported previously for all patients who received SC IFNB-1a in the core study.5 (I) Median cumulative percentage BVL from core study baseline to the end of CAMMS03409. (J) Median annual percentage BVL.
Alemtuzumab-only group versus IFN–alemtuzumab group: *p < 0.05 indicates significant between-treatment group differences in favor of the alemtuzumab-only group and †p < 0.05 indicates significant between-treatment group differences in favor of the IFN–alemtuzumab group. Year 2 of SC IFNB-1a treatment versus each year (years 3–6) after initiating alemtuzumab treatment: ‡p<0.0001, §p<0.001, and ¶p<0.05.
ARR, annualized relapse rate; BPF, brain parenchymal fraction; BVL, brain volume loss; CARE-MS, Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; CDI, confirmed disability improvement; CDW, confirmed disability worsening; CI, confidence interval; EDSS, Expanded Disability Status Scale; Gd, gadolinium; IFN, interferon; MRI, magnetic resonance imaging; SC IFNB-1a, subcutaneous interferon beta 1-a; SE, standard error; Y, year.
IFN–alemtuzumab group
Compared with year 2 of SC IFNB-1a, ARR was significantly improved after initiating alemtuzumab at year 3, and this improvement was maintained through year 6 [0.17 at year 6 compared with 0.44 at year 2; p < 0.0001 (Figure 3A)]. Cumulative ARR over years 0–6 was 0.32; cumulative ARR was 0.15 in years 3–6 after receiving alemtuzumab, compared with 0.55 in years 0–2 while receiving SC IFNB-1a. In years 3–6 with alemtuzumab, 85–90% of patients were relapse-free annually, increasing from 70% at year 2 with SC IFNB-1a. Change in mean EDSS score from core study baseline was +0.46 at year 6 (Figure 3B). In year 6 versus core study baseline, 16% had improved and 53% had stable EDSS scores (Figure 3C). Over years 0–6, 67% remained free of 6-month CDW (Figure 3D) and 27% achieved 6-month CDI (Figure 3E). There was a significant increase in the percentage of patients free of MRI disease activity in year 6 with alemtuzumab compared with year 2 of SC IFNB-1a treatment (71% versus 47%, p < 0.05; Figure 3F–H). Median cumulative change in BV over years 0–6 was −1.25% (Figure 3I), and annual change in BV slowed from −0.35% at year 2 with SC IFNB-1a to ⩽0.15% each year following alemtuzumab initiation (Figure 3J). To determine whether NEDA during years 0–2 while receiving SC IFNB-1a affected outcomes after switching to alemtuzumab, data prior to and after switch were calculated. Mean change in EDSS score in those who did (14%) and did not (86%) achieve NEDA during years 0–2 was −0.26 and +0.29, respectively; 23% and 13% of patients achieved 6-month CDI across this interval and cumulative change in BV was −0.89% and −0.81%, respectively. After rebaselining at the start of the extension, mean change in EDSS score was +0.43 and +0.19 through year 6 with alemtuzumab in those who had and had not achieved NEDA during years 0–2, respectively; of these, 13% and 23% achieved 6-month CDI, and cumulative change in BV was +0.27% and −0.26%, respectively (Supplemental Figure 1B, D, and F).
CARE-MS II: alemtuzumab-only group versus IFN–alemtuzumab group
Compared with the IFN–alemtuzumab group, the alemtuzumab-only group had significantly lower ARR at year 2 and cumulatively over years 0–6 (p < 0.05; Figure 3A), a smaller worsening of EDSS score at year 6 [mean EDSS score difference between groups: −0.28 (95% confidence interval: −0.54 to −0.02); p < 0.05; Figure 3B], and significantly more patients free of MRI disease activity at years 1 and 2 (p < 0.05; Figure 3F–H). The percentage of patients with 6-month CDI was significantly greater in the alemtuzumab-only group compared with the IFN–alemtuzumab group at each year from year 1 through year 6 (p < 0.05; Figure 3E); the percentage difference between both groups remained approximately the same throughout the extension as that at the end of the core study, with IFN–alemtuzumab patients maintaining the same trajectory as alemtuzumab-only patients. After 2 years, the alemtuzumab-only group accumulated less clinical disability than the IFN–alemtuzumab group. Compared with the IFN–alemtuzumab group over the subsequent 4 extension years, the alemtuzumab-only group experienced marginal increases in percentages of patients with improved or stable EDSS scores during year 6 (Figure 3C) and patients free of 6-month CDW at year 6 (Figure 3D). Slowing of median annual change in BVL was numerically greater for alemtuzumab-only patients compared with IFN–alemtuzumab patients at year 2, but was similar between groups (⩽0.19% change) after IFN–alemtuzumab patients switched to alemtuzumab (Figure 3J). Nonetheless, by year 6, the cumulative change in BVL was numerically greater in the IFN–alemtuzumab group than in the alemtuzumab-only group who received alemtuzumab 2 years sooner. However, the differential between the treatment groups was established by year 2 when the two groups were on separate treatments and cumulative BVL trajectories diverged; once alemtuzumab was initiated in the extension, the rate of cumulative BVL in IFN–alemtuzumab patients was aligned with that in the alemtuzumab-only patients (Figure 3I).
Safety
The safety profile of alemtuzumab in the pooled CARE-MS I and II alemtuzumab-only group over 6 years was consistent with the 2-year core study and interim 5-year reports.4,5,22,23 Annual incidences of any AE in the pooled CARE-MS alemtuzumab-only group decreased across each study year (Table 3), with serious AE incidence ⩽12.3% per year. Incidences of infections peaked at year 1 (59.9%) after initiating alemtuzumab, with serious infection incidences not exceeding 1.8% throughout the study. Thyroid AE incidence peaked at year 3 (16.2%) and serious thyroid AE incidence was ⩽3.5% per year. In the extension, there were a total of 14 cases of ITP; annual incidence of ITP ranged from 0.1% to 1.1%. A total of two cases of immune-mediated nephropathy were reported. As reported previously, one CARE-MS I patient initially presented with nephrotic syndrome in year 3; however, there was evidence only of mild membranous nephropathy with anti-glomerular basement membrane (anti-GBM) antibodies in the absence of typical anti-GBM disease. The patient experienced small increases in serum anti-GBM antibody titer, which subsequently prompted treatment with plasmapheresis, glucocorticosteroids, and cyclophosphamide. The patient was considered to be in remission 39 months after the last treatment for nephropathy, with no detectable anti-GBM antibodies or proteinuria, serum creatinine within normal limits, and no need for medication.22,29 One CARE-MS II patient was reported previously as having confirmed membranous glomerulonephritis in year 2 and received four treatments for nephrotic syndrome (furosemide, valsartan, metolazone, and oral potassium chloride).23,29 All ITP and nephropathy events occurred within 48 months after receiving the last dose of alemtuzumab and while undergoing routine protocol-specified safety monitoring; of 342 total thyroid events over 6 years, 11 (3.2%) occurred in year 6 beyond the 48-month monitoring period after the last dose of alemtuzumab. Incidence of malignancy was ⩽0.4% per year. Three deaths occurred during the extension phase in the alemtuzumab-only group [one death deemed unrelated to treatment: non-small cell lung cancer (year 6); two deaths deemed possibly related to treatment: sepsis (year 3, reported previously4) and pulmonary embolism (year 6)].
Table 3.
AEs by year in pooled CARE-MS I and II patients.
| Incidence, n (%) | Exposure-adjusted incidence rate per 100 patient-years, a (n) | |||||||
|---|---|---|---|---|---|---|---|---|
| Y1 | Y2 | Y3 | Y4 | Y5 | Y6 | Y0–2 | Y3–6 | |
| Alemtuzumab treatment | As-needed additional courses of alemtuzumab | |||||||
| Alemtuzumab-only group | n = 811b | n = 810 | n = 77222,23,c | n = 731 | n = 707 | n = 692 | n = 811 | n = 772 |
| Any AE | 764 (94.2) | 718 (88.6) | 616 (79.8) | 569 (77.8) | 529 (74.8) | 486 (70.2) | 771.9 (788) | 150.2 (696) |
| Any AE excluding IARsd | 667 (82.2) | 662 (81.7) | 612 (79.3) | 564 (77.2) | 527 (74.5) | 481 (69.5) | 206.2 (738) | 146.0 (695) |
| Serious AEs | 100 (12.3) | 74 (9.1) | 81 (10.5) | 86 (11.8) | 55 (7.8) | 46 (6.6) | 10.8 (154) | 8.4 (197) |
| Infections | 486 (59.9) | 446 (55.1) | 372 (48.2) | 337 (46.1) | 300 (42.4) | 275 (39.7) | 78.5 (588) | 44.6 (544) |
| Serious infections | 15 (1.8) | 9 (1.1) | 11 (1.4) | 12 (1.6) | 9 (1.3) | 8 (1.2) | 1.5 (23) | 1.2 (34) |
| Thyroid AEse | 46 (5.7) | 74 (9.1) | 125 (16.2) | 48 (6.6) | 24 (3.4) | 25 (3.6) | 7.9 (120) | 10.0 (222) |
| Serious thyroid AEse | 2 (0.2) | 5 (0.6) | 27 (3.5) | 6 (0.8) | 3 (0.4) | 1 (0.1) | 0.4 (7) | 1.4 (37) |
| ITPe | 2 (0.2) | 5 (0.6) | 2 (0.3) | 8 (1.1) | 1 (0.1) | 3 (0.4) | 0.4 (7) | 0.5 (14) |
| Nephropathiese | 0 | 1 (0.1) | 1 (0.1) | 0 | 0 | 0 | 0.1 (1) | 0 (1) |
| Malignancies | 1 (0.1) | 3 (0.4) | 3 (0.4) | 1 (0.1) | 3 (0.4) | 3 (0.4) | 0.2 (4) | 0.4 (10) |
| SC IFNB-1a treatment | Alemtuzumab treatment | As-needed additional courses of alemtuzumab | SC IFNB-1a treatment | Alemtuzumab treatment | ||||
| IFN–alemtuzumab group | n = 282 | n = 281 | n = 282 | n = 278 | n = 275 | n = 261 | n = 282 | n = 282 |
| Any AE | 249 (88.3) | 224 (79.7) | 261 (92.6) | 230 (82.7) | 203 (73.8) | 185 (70.9) | 285.0 (265) | 458.6 (273) |
| Any AE excluding IARsd | 249 (88.3) | 224 (79.7) | 217 (77.0) | 208 (74.8) | 202 (73.5) | 185 (70.9) | 285.0 (265) | 134.7 (264) |
| Serious AEs | 35 (12.4) | 33 (11.7) | 30 (10.6) | 26 (9.4) | 25 (9.1) | 27 (10.3) | 11.5 (57) | 8.6 (78) |
| Infections | 124 (44.0) | 118 (42.0) | 147 (52.1) | 138 (49.6) | 128 (46.5) | 99 (37.9) | 46.9 (161) | 49.0 (213) |
| Serious infections | 1 (0.4) | 2 (0.7) | 5 (1.8) | 11 (4.0) | 5 (1.8) | 4 (1.5) | 0.5 (3) | 2.3 (24) |
| Thyroid AEse | 7 (2.5) | 5 (1.8) | 6 (2.1) | 23 (8.3) | 33 (12.0) | 17 (6.5) | 2.2 (12) | 8.3 (79) |
| Serious thyroid AEse | 0 | 0 | 0 | 1 (0.4) | 4 (1.5) | 0 | 0 | 0.5 (5) |
| ITPe | 0 | 0 | 0 | 0 | 2 (0.7) | 0 | 0 | 0.2 (2) |
| Nephropathiese | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Malignancies | 1 (0.4) | 0 | 3 (1.1) | 4 (1.4) | 0 | 1 (0.4) | 0.2 (1) | 0.7 (7) |
(Number of patients with a specific AE divided by the total follow-up time in years among patients at risk of an initial occurrence of the event during the specified time interval) × 100.
Safety analysis included nine patients who received alemtuzumab 12 mg/day in the CARE-MS II core study, despite randomization to the alemtuzumab 24 mg/day core study arm.
A total of 772 patients were followed up for safety during year 3 in the alemtuzumab-only group, including 349 CARE-MS I and 393 as-treated CARE-MS II patients who enrolled in the extension, and 30 additional patients from the CARE-MS core studies who did not enroll in the extension but were assessed for safety in year 3.
IARs were any AE that occurred during the infusion or within 24 h after the end of the infusion.
First occurrence of AE within the time period.
AE, adverse event; CARE-MS, Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; IAR, infusion-associated reaction; IFN, interferon; ITP, immune thrombocytopenia; SC IFNB-1a, subcutaneous interferon beta-1a; Y, year.
The safety profile of alemtuzumab in the pooled CARE-MS I and II IFN–alemtuzumab group was similar to that in the pooled alemtuzumab-only group, with decreasing annual incidences of AEs following initiation of alemtuzumab in year 3 (Table 3). Most AEs in the IFN–alemtuzumab group were mild to moderate in severity, with serious AE incidences ⩽10.6% per year after switching to alemtuzumab. Incidences of infections declined each year following initiation of alemtuzumab treatment, with serious infection incidences ⩽4.0% in years 3–6. Thyroid AE incidences peaked in year 5, the third year after initiating alemtuzumab treatment (thyroid AEs: 12.0%; serious thyroid AEs: 1.5%). In the extension, two cases of ITP (both in CARE-MS II IFN–alemtuzumab patients in year 5; incidence of 0.7% in that year), no nephropathies, and eight malignancies (incidence ⩽1.4% per year) were reported. Two deaths occurred in the IFN–alemtuzumab group. The cause of one death could not be ascertained, as the patient lived alone and was found at home approximately 4–5 days after death (year 5; unable to determine relationship to alemtuzumab treatment). The other death was due to a suicide (year 6; classified as unrelated to alemtuzumab treatment).
In both treatment groups, IAR incidences peaked during alemtuzumab course 1 and decreased with each exposure to subsequent treatment courses (Table 4). Serious IAR incidence over all courses of alemtuzumab ranged from 0% to 2.0% in the alemtuzumab-only group and 0–1.4% in the IFN–alemtuzumab group.
Table 4.
IARs by course in pooled CARE-MS I and II patients.
| Incidence, n (%) | ||||||
|---|---|---|---|---|---|---|
| Course 1 | Course 2 | Course 3 | Course 4 | Course 5 | Course 6 | |
| Alemtuzumab-only group | n = 811a | n = 791 | n = 302 | n = 105 | n = 26 | n = 5 |
| Any IAR eventsb | 687 (84.7) | 544 (68.8) | 188 (62.3) | 66 (62.9) | 11 (42.3) | 2 (40.0) |
| Any serious IAR eventsb | 16 (2.0) | 8 (1.0) | 2 (0.7) | 0 | 0 | 0 |
| IFN–alemtuzumab group | n = 282 | n = 266 | n = 70 | n = 12 | – | – |
| Any IAR eventsb | 232 (82.3) | 174 (65.4) | 45 (64.3) | 5 (41.7) | − | − |
| Any serious IAR eventsb | 1 (0.4) | 2 (0.8) | 1 (1.4) | 0 | − | − |
Safety analysis included nine patients who received alemtuzumab 12 mg/day in the CARE-MS II core study, despite randomization to the alemtuzumab 24 mg/day core study arm.
IARs were any adverse event that occurred during or within 24 h after the end of the infusion.
CARE-MS, Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; IAR, infusion-associated reaction; IFN, interferon.
Discussion
We have shown that both first-line treatment with alemtuzumab and switching to alemtuzumab from SC IFNB-1a lead to sustained reduction in disease activity, along with slowing of disability accumulation and brain atrophy. However, prolonged delay in switching to alemtuzumab leads to potentially unrecoverable loss of function. Principal differences at core study baseline between the cohorts in this study were disability and disease duration, and history of prior treatment for RRMS.4,5 These findings suggest that initiating a high-efficacy therapy, as either a first-line therapy or after switching from another therapy, has greatest impact when done early in the disease course, and resonates both with the reduced conversion to secondary-progressive MS (SPMS) with higher- versus lower-efficacy therapies reported in a recent registry-based study,30 and with the SPMS conversion rates of 1.2% and 4.2% among CARE-MS I and II alemtuzumab-only patients, respectively, through year 6.31 Despite findings supporting early switch to high-efficacy therapy, no substantial differences were observed in the year 6 outcomes for CARE-MS I and II IFN–alemtuzumab patients who did or did not achieve NEDA in years 1 and 2 (Supplemental Figure 1).
When alemtuzumab was used as a first-line treatment for patients with RRMS, it offered superior efficacy on clinical and MRI disease activity outcomes compared with SC IFNB-1a over 2 years, as demonstrated in the phase III CARE-MS I trial.4 Furthermore, alemtuzumab slowed brain atrophy by 42% compared with SC IFNB-1a over 2 years, although this did not translate into significant differences in clinical disability outcomes; the lack of significance for the 30% reduction in 6-month CDW with alemtuzumab (p = 0.22) may be partly attributed to reduced power for statistical calculations due to the lower-than-expected rate of CDW among SC IFNB-1a patients.4 We now report that disease activity remained suppressed in the CARE-MS I alemtuzumab-only group over an additional 4 years, with 63% of patients requiring no further treatment. Importantly, the IFN–alemtuzumab group had improved clinical and MRI disease activity outcomes after switching to alemtuzumab, along with slowing of BVL, such that cumulative BVL at year 6 was not statistically different from the alemtuzumab-only group.
When alemtuzumab was given to patients who had ⩾1 relapse on prior therapy (i.e. the CARE-MS II population), clinical and MRI disease activity outcomes were superior over 2 years compared with SC IFNB-1a treatment.5 We now show that alemtuzumab-only patients continued to experience disease suppression over 4 more years, with 50% needing no further treatment. The IFN–alemtuzumab group benefited rapidly from alemtuzumab treatment, with improved clinical efficacy and MRI disease activity outcomes, which translated into slowed BVL and reduced disability accumulation but not to the extent of the alemtuzumab-only group. Interestingly, the IFN–alemtuzumab patients did not experience improvement in disability through year 6 as often as the alemtuzumab-only group.
Taken together, these results indicate that either first-line treatment (i.e. the CARE-MS I alemtuzumab-only group) or early elective switching to alemtuzumab (i.e. the CARE-MS I IFN–alemtuzumab group) and earlier initiation of treatment (i.e. the CARE-MS II alemtuzumab-only group) offers control of disease activity and slowing of BVL over 6 years, whereas delaying treatment with alemtuzumab (i.e. the CARE-MS II IFN–alemtuzumab group) may reduce its potential positive impact.
The 6-year safety profile of alemtuzumab was consistent between both treatment groups, regardless of whether patients had received previous treatment with SC IFNB-1a. AEs were mostly mild to moderate in severity, and incidences generally diminished with time. Autoimmune events, including thyroid AEs and serious thyroid AEs, were reported with similar frequency in both groups. No nephropathies and two cases of ITP were reported in the IFN–alemtuzumab group. One of the IFN–alemtuzumab patients with ITP was in complete remission for 15 months at the time of last follow-up; the other patient was in remission following a splenectomy.32 These findings highlight the value of continued clinical monitoring for at least 48 months after alemtuzumab treatment, which is consistent with the required risk management programs specified under product labeling.6,7 In the postmarketing setting, rare but serious AEs have been observed in alemtuzumab-treated patients, including cases of ischemic or hemorrhagic stroke or dissection of the cervicocephalic arteries soon after alemtuzumab infusion, and less common autoimmune events, such as autoimmune hepatitis and hemophagocytic lymphohistiocytosis.33,34 It is notable that, in this controlled trial with a comparatively lower number of patients compared with the postmarketing experience, these potential safety concerns recently identified through postmarketing surveillance were not seen. Owing to the postmarketing evidence, limitations have been implemented in the European Union that confine alemtuzumab treatment to patients with highly active RRMS treated previously with a full and adequate course of treatment with at least one other DMT, or those who have rapidly evolving severe disease defined by ⩾2 disabling relapses in 1 year and with ⩾1 Gd-enhancing lesions on brain MRI or a significant increase in T2 lesion load compared with recent MRI.7
Infrequent dosing, consisting of 5 consecutive days of infusions at treatment initiation followed by 3 consecutive days of infusions 12 months later, with optional additional courses per approved local labels, is a distinct advantage of alemtuzumab treatment.6,7 After the initial two courses, a majority of CARE-MS patients did not require further alemtuzumab treatment or another DMT through year 6; however, those meeting criteria for additional courses mostly needed only 3 more days of infusions (i.e. course 3). Although the therapeutic mechanism of alemtuzumab is not fully elucidated, it is partly reflected by the persistent alteration of the immune cell repertoire by T- and B-cell reconstitution after alemtuzumab-induced lymphocyte depletion.35,36 Following alemtuzumab treatment and depletion of T and B cells, rebalance of the immune system occurs within 1 year, with relative increases in immunoregulatory T-cell, B-cell, and natural killer-cell populations and a less inflammatory cytokine profile.2,37,38 Potential neuroprotective effects have also been seen in in vitro murine models, including reductions in spinal cord inflammation, demyelination, and axonal damage.36 Imaging data of alemtuzumab-treated patients in exploratory studies have demonstrated potential neuroprotective effects, with increased retinal nerve fiber layer thickness consistent with reduced neurodegeneration,39 increased myelin water fraction suggestive of remyelination,40 and stabilized magnetization transfer ratio indicative of preserved myelination.41,42 Whether apparent neuroprotective effects of alemtuzumab are due to direct effects on neural cells or to reduction of the immunological assault associated with MS has yet to be determined.
We conclude that alemtuzumab offers the greatest benefit when administered earlier in the disease course, whether as first-line therapy in treatment-naive patients or after switching sooner from other DMTs. Long-term safety and efficacy follow-up continue in the subsequent 5-year long-Term follow-up study for multiple sclerOsis Patients who have completed the AlemtuZumab extension study (TOPAZ; NCT02255656).43
Supplemental Material
Supplemental material, sj-pdf-1-tan-10.1177_1756286420982134 for Efficacy and safety of alemtuzumab over 6 years: final results of the 4-year CARE-MS extension trial by Alasdair J. Coles, Douglas L. Arnold, Ann D. Bass, Aaron L. Boster, D. Alastair S. Compston, Óscar Fernández, Eva Kubala Havrdová, Kunio Nakamura, Anthony Traboulsee, Tjalf Ziemssen, Alan Jacobs, David H. Margolin, Xiaobi Huang, Nadia Daizadeh, Madalina C. Chirieac and Krzysztof W. Selmaj in Therapeutic Advances in Neurological Disorders
Acknowledgments
The authors and Sanofi thank the patients for their participation in the trials, as well as the CARE-MS I and II Steering Committees, along with the CAMMS03409 investigators. Critical review of the manuscript was provided by Darren P. Baker, PhD, Ericka M Bueno, PhD, and Colin Mitchell, PhD, of Sanofi. Editorial and writing assistance was provided by Rebecca L. Orndorff, PhD, and Richard J. Hogan, PhD, of Eloquent Scientific Solutions, and was funded by Sanofi. The CARE-MS I and II studies and their extension study were sponsored by Sanofi and Bayer HealthCare Pharmaceuticals. Alasdair J. Coles was supported by the NIHR Cambridge Biomedical Research Centre.
Footnotes
Conflict of interest statement: AJC reports receiving consulting fees, lecture fees, and institutional grant support from Sanofi prior to 2017. DLA reports receiving consulting fees and/or grants from Acorda, Adelphi, Alkermes, Biogen, Celgene, Frequency Therapeutics, Genentech, Genzyme, Hoffmann-La Roche, Immune Tolerance Network, Immunotec, MedDay, Merck Serono, Novartis, Pfizer, Receptos, Roche, Sanofi-Aventis, Canadian Institutes of Health Research, MS Society of Canada, International Progressive MS Alliance, and an equity interest in NeuroRx Research. ADB reports receiving research funding, compensation for medical advisory boards, and compensation for speakers bureaus from Actelion, Biogen, EMD Serono, Genentech-Roche, Mallinckrodt, Novartis, Sanofi, and TG Therapeutics. ALB reports receiving consulting fees and/or speaking fees for non-CME services from Biogen, Mallinckrodt, Medtronic, Novartis, Sanofi, and Teva. DASC reports receiving consulting fees, grant, and research support from Sanofi, and lecture fees from Bayer-Schering Pharma and Sanofi, prior to 2017. ÓF reports receiving speaking and/or consulting fees for Allergan, Almirall, Bayer-Schering Pharma, Biogen, Merck Serono, Novartis, Sanofi, and Teva, and research support from the Hospital Foundation FIMABIS; he also serves as editor of the Revista Española de Esclerosis Múltiple. EKH reports receiving honoraria and grant support from Actelion, Biogen, Celgene, Merck Serono, Novartis, Roche, Sanofi, and Teva, and is supported by the Ministry of Education of the Czech Republic, project PROGRES Q27/LF1. KN reports speaking fees and research support from Biogen and Sanofi, along with royalty fees for licenses with Biogen. AT reports receiving consulting and/or speaking fees, along with grant and research support, from Biogen, Chugai, Roche, Sanofi, and Teva. TZ reports receiving consulting and/or speaking fees from Almirall, Bayer, Biogen, Merck, Novartis, Roche, Sanofi, and Teva, along with grant and research support from Biogen, Novartis, Sanofi, and Teva. AJ, DHM, XH, and MCC report being employees of Sanofi during the time of the analysis. ND reports receiving personal compensation as an employee of Sanofi. KWS reports receiving consulting and/or speaking fees from Biogen, Merck, Novartis, Roche, Sanofi, and Synthon.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by Sanofi and Bayer HealthCare Pharmaceuticals.
ORCID iD: Tjalf Ziemssen  https://orcid.org/0000-0001-8799-8202
https://orcid.org/0000-0001-8799-8202
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Alasdair J. Coles, Department of Clinical Neurosciences, University of Cambridge, Box 165, Addenbrooke’s Hospital, Cambridge CB2 0QQ, UK.
Douglas L. Arnold, NeuroRx Research, Montréal, Québec, Canada Department of Neurology and Neurosurgery, Montréal Neurological Institute, McGill University, Montréal, Québec, Canada.
Ann D. Bass, Neurology Center of San Antonio, San Antonio, TX, USA
Aaron L. Boster, The Boster MS Center, Columbus, OH, USA
D. Alastair S. Compston, Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK
Óscar Fernández, Instituto de Investigación Biomédica de Málaga (IBIMA), Málaga, Spain.
Eva Kubala Havrdová, Department of Neurology and Center for Clinical Neuroscience, First Medical Faculty, Charles University, Prague, Czech Republic.
Kunio Nakamura, Department of Biomedical Engineering, Cleveland Clinic, Cleveland, OH, USA.
Anthony Traboulsee, The University of British Columbia, Vancouver, British Columbia, Canada.
Tjalf Ziemssen, Center of Clinical Neuroscience, Carl Gustav Carus University Hospital, Dresden, Germany.
Alan Jacobs, Immunovant, Inc., New York, NY, USA; Sanofi, Cambridge, MA, USA.
David H. Margolin, Cerevance, Inc., Boston, MA, USA Sanofi, Cambridge, MA, USA.
Xiaobi Huang, Sanofi, Cambridge, MA, USA; Biogen, Cambridge, MA, USA.
Nadia Daizadeh, Sanofi, Cambridge, MA, USA.
Madalina C. Chirieac, Vertex Pharmaceuticals, Inc., Boston, MA, USA Sanofi, Cambridge, MA, USA.
Krzysztof W. Selmaj, Department of Neurology, University of Warmia and Mazury, Olsztyn, Poland
References
- 1. Gajofatto A, Benedetti MD. Treatment strategies for multiple sclerosis: when to start, when to change, when to stop? World J Clin Cases 2015; 3: 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cox AL, Thompson SA, Jones JL, et al. Lymphocyte homeostasis following therapeutic lymphocyte depletion in multiple sclerosis. Eur J Immunol 2005; 35: 3332–3342. [DOI] [PubMed] [Google Scholar]
- 3. Hu Y, Turner MJ, Shields J, et al. Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology 2009; 128: 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012; 380: 1819–1828. [DOI] [PubMed] [Google Scholar]
- 5. Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012; 380: 1829–1839. [DOI] [PubMed] [Google Scholar]
- 6. Lemtrada (alemtuzumab). Prescribing information: Genzyme Corporation, Cambridge, MA, 2020. [Google Scholar]
- 7. Lemtrada. Summary of product characteristics: Sanofi Belgium, Diegem, Belgium, 2020. [Google Scholar]
- 8. Coles AJ, Jones J, Vermersch P, et al. Autoimmunity and other long-term safety and efficacy of alemtuzumab treatment of multiple sclerosis. In preparation. [Google Scholar]
- 9. Holmoy T, von der Lippe H, Leegaard TM. Listeria monocytogenes infection associated with alemtuzumab – a case for better preventive strategies. BMC Neurol 2017; 17: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Canham LJW, Manara A, Fawcett J, et al. Mortality from Listeria monocytogenes meningoencephalitis following escalation to alemtuzumab therapy for relapsing-remitting multiple sclerosis. Mult Scler Relat Disord 2018; 24: 38–41. [DOI] [PubMed] [Google Scholar]
- 11. Holmoy T, Fevang B, Olsen DB, et al. Adverse events with fatal outcome associated with alemtuzumab treatment in multiple sclerosis. BMC Res Notes 2019; 12: 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clerico M, De Mercanti S, Artusi CA, et al. Active CMV infection in two patients with multiple sclerosis treated with alemtuzumab. Mult Scler 2017; 23: 874–876. [DOI] [PubMed] [Google Scholar]
- 13. Russo CV, Sacca F, Paternoster M, et al. Post-mortem diagnosis of invasive pulmonary aspergillosis after alemtuzumab treatment for multiple sclerosis. Mult Scler 2020; 26: 123–126. [DOI] [PubMed] [Google Scholar]
- 14. Penkert H, Delbridge C, Wantia N, et al. Fulminant central nervous system nocardiosis in a patient treated with alemtuzumab for relapsing-remitting multiple sclerosis. JAMA Neurol 2016; 73: 757–759. [DOI] [PubMed] [Google Scholar]
- 15. Sheikh-Taha M, Corman LC. Pulmonary nocardia beijingensis infection associated with the use of alemtuzumab in a patient with multiple sclerosis. Mult Scler 2017; 23: 872–874. [DOI] [PubMed] [Google Scholar]
- 16. Yiannopoulou KG, Papadimitriou D, Anastasiou AI, et al. Neutropenia with fatal outcome in a multiple sclerosis patient 23 days after alemtuzumab infusion. Mult Scler Relat Disord 2018; 23: 15–16. [DOI] [PubMed] [Google Scholar]
- 17. Saarela M, Senthil K, Jones J, et al. Hemophagocytic lymphohistiocytosis in 2 patients with multiple sclerosis treated with alemtuzumab. Neurology 2018; 90: 849–851. [DOI] [PubMed] [Google Scholar]
- 18. Pfeuffer S, Beuker C, Ruck T, et al. Acute cholecystitis during treatment with alemtuzumab in 3 patients with RRMS. Neurology 2016; 87: 2380–2381. [DOI] [PubMed] [Google Scholar]
- 19. Croteau D, Flowers C, Kulick CG, et al. Acute acalculous cholecystitis: a new safety risk for patients with MS treated with alemtuzumab. Neurology 2018; 90: e1548–e1552. [DOI] [PubMed] [Google Scholar]
- 20. Ferraro D, Camera V, Vitetta F, et al. Acute coronary syndrome associated with alemtuzumab infusion in multiple sclerosis. Neurology 2018; 90: 852–854. [DOI] [PubMed] [Google Scholar]
- 21. Azevedo CJ, Kutz C, Dix A, et al. Intracerebral haemorrhage during alemtuzumab administration. Lancet Neurol 2019; 18: 329–331. [DOI] [PubMed] [Google Scholar]
- 22. Havrdova E, Arnold DL, Cohen JA, et al. Alemtuzumab CARE-MS I 5-year follow-up: durable efficacy in the absence of continuous MS therapy. Neurology 2017; 89: 1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coles AJ, Cohen JA, Fox EJ, et al. Alemtuzumab CARE-MS II 5-year follow-up: efficacy and safety findings. Neurology 2017; 89: 1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ziemssen T, Thomas K. Alemtuzumab in the long-term treatment of relapsing-remitting multiple sclerosis: an update on the clinical trial evidence and data from the real world. Ther Adv Neurol Disord 2017; 10: 343–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014; 83: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coles AJ, Fox E, Vladic A, et al. Alemtuzumab versus interferon beta-1a in early relapsing-remitting multiple sclerosis: post-hoc and subset analyses of clinical efficacy outcomes. Lancet Neurol 2011; 10: 338–348. [DOI] [PubMed] [Google Scholar]
- 27. Rudick RA, Fisher E, Lee JC, et al. Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS. Multiple Sclerosis Collaborative Research Group. Neurology 1999; 53: 1698–1704. [DOI] [PubMed] [Google Scholar]
- 28. Liu GF, Wang J, Liu K, et al. Confidence intervals for an exposure adjusted incidence rate difference with applications to clinical trials. Stat Med 2006; 25: 1275–1286. [DOI] [PubMed] [Google Scholar]
- 29. Phelps R, Winston JA, Wynn D, et al. Incidence, management, and outcomes of autoimmune nephropathies following alemtuzumab treatment in patients with multiple sclerosis. Mult Scler 2019; 25: 1273–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown JWL, Coles A, Horakova D, et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA 2019; 321: 175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Horakova D, Boster A, Bertolotto A, et al. Alemtuzumab-treated patients with relapsing-remitting MS show low rates of conversion to secondary progressive MS: 6-year follow-up of CARE-MS I and II. Mult Scler 2017; 23: P1195. [Google Scholar]
- 32. Cuker A, Bass AD, Nadj C, et al. Immune thrombocytopenia in alemtuzumab-treated MS patients: incidence, detection, and management. Mult Scler 2020; 26: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. US Food and Drug Administration. FDA warns about rare but serious risks of stroke and blood vessel wall tears with multiple sclerosis drug Lemtrada (alemtuzumab), https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-warns-about-rare-serious-risks-stroke-and-blood-vessel-wall-tears-multiple-sclerosis-drug#:~:text=On%20November%2029%2C%202018%20FDA,permanent%20disability%20and%20even%20death (accessed 17 September 2020).
- 34. European Medicines Agency. Measures to minimise risk of serious side effects of multiple sclerosis medicine Lemtrada [press release], https://www.ema.europa.eu/en/news/measures-minimise-risk-serious-side-effects-multiple-sclerosis-medicine-lemtrada (accessed 17 September 2020).
- 35. Jones JL, Thompson SA, Loh P, et al. Human autoimmunity after lymphocyte depletion is caused by homeostatic T-cell proliferation. Proc Natl Acad Sci U S A 2013; 110: 20200–20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Turner MJ, Lamorte MJ, Chretien N, et al. Immune status following alemtuzumab treatment in human CD52 transgenic mice. J Neuroimmunol 2013; 261: 29–36. [DOI] [PubMed] [Google Scholar]
- 37. Gross CC, Ahmetspahic D, Ruck T, et al. Alemtuzumab treatment alters circulating innate immune cells in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2016; 3: e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim Y, Kim G, Shin HJ, et al. Restoration of regulatory B cell deficiency following alemtuzumab therapy in patients with relapsing multiple sclerosis. J Neuroinflammation 2018; 15: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chan JK, Taylor C, Traboulsee A, et al. Durable neuroprotective effects of alemtuzumab over 5 years on retinal nerve fibre layer and ganglion cell layers in relapsing-remitting multiple sclerosis (RRMS) patients. Mult Scler 2017; 23: P1082. [Google Scholar]
- 40. Vavasour IM, Tam R, Li DK, et al. A 24-month advanced magnetic resonance imaging study of multiple sclerosis patients treated with alemtuzumab. Mult Scler 2019; 25: 811–818. [DOI] [PubMed] [Google Scholar]
- 41. Button T, Altmann D, Tozer D, et al. Magnetization transfer imaging in multiple sclerosis treated with alemtuzumab. Mult Scler 2013; 19: 241–244. [DOI] [PubMed] [Google Scholar]
- 42. Brown JWL, Prados Carrasco F, Eshaghi A, et al. The effect of alemtuzumab on periventricular magnetisation transfer ratio gradients. Mult Scler 2017; 23: P1877. [DOI] [PubMed] [Google Scholar]
- 43. Ziemssen T, Bass AD, Berkovich R, et al. Efficacy and safety of alemtuzumab through 9 years of follow-up in patients with highly active disease: post hoc analysis of CARE-MS I and II patients in the TOPAZ extension study. CNS Drugs 2020; 34: 973–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tan-10.1177_1756286420982134 for Efficacy and safety of alemtuzumab over 6 years: final results of the 4-year CARE-MS extension trial by Alasdair J. Coles, Douglas L. Arnold, Ann D. Bass, Aaron L. Boster, D. Alastair S. Compston, Óscar Fernández, Eva Kubala Havrdová, Kunio Nakamura, Anthony Traboulsee, Tjalf Ziemssen, Alan Jacobs, David H. Margolin, Xiaobi Huang, Nadia Daizadeh, Madalina C. Chirieac and Krzysztof W. Selmaj in Therapeutic Advances in Neurological Disorders