Abstract
Introduction
MicroRNAs (miRNAs) are short noncoding RNA molecules that regulate gene expression and are related to endothelial dysfunction (EnD). Recently, miRNAs have also been explored as potential biomarkers and target molecular therapy of erectile dysfunction (ED). Could the miRNAs be the tip of the iceberg of chronic arterial disease foreshadowed by the ED?
Aim
To investigate the expression of miR-15b, miR-16, miR-138, miR-221, and miR-222 in corpus cavernosum (CC) and peripheral blood in a rat model of endothelium dysfunction secondary to diabetes (DM) and alcohol consumption to assess potential endothelial lesion biomarkers.
Methods
Twenty males Wistar rats were divided into 4 groups: control group (C), alcohol consumption group (A), diabetic group (D), diabetic-alcohol consumption group (D + A). DM was alloxan-induced and alcohol consumption was through progressive increase of ethanol concentration in drinkable water. After 7 weeks, miRNAs expressions from CC and blood sample were evaluated by real-time PCR. Functional assessment of CC was performed in an acetylcholine endothelium-dependent relaxation pharmacological study.
Main Outcome Measure
miRNA expression in CC and blood were evaluated; pharmacological study in CC strips was conducted to validate EnD.
Results
We found that 3 miRNAs (miR-16, miR-221, and miR-222) were downregulated in the CC in the D+A group, while all 5 miRNAs were downregulated in the blood of D and D + A groups. The endothelium-dependent relaxation induced by acetylcholine was significantly decreased in groups A, D, and D + A. Diagnostic accuracy estimated by AUC, to discriminating groups A, D, and D + A from controls, was superior to >0.9 in all plasmatic miRNAs.
Conclusion
miRNAs downregulation was identified in both CC and blood notably in DM associated with alcohol consumption animals (D + A), the greatest endothelial injury potential group. Serum miRNAs have also demonstrated high diagnostic accuracy properties in predicting CC relaxation dysfunction labeling EnD. RB Tiraboschi, FSL Neto, DP da Cunha Tirapelli, et al. Expression of MicroRNAs (miR-15b, miR-16, miR-138, miR-221, and miR-222) as Biomarkers of Endothelial Corpus Cavernosum Dysfunction in a Diabetic Alcoholic Murine Model. Sex Med 2021;9:100326.
Key Words: Alcoholism, Biomarkers, Diabetes mellitus, Endothelium, Erectile dysfunction, MicroRNAs
Introduction
Penile erection involves the integration and coordination of multiple regulatory systems such as neurological, endocrinological, vascular and psychological.1 Erectile dysfunction (ED) is defined as the inability to achieve and maintain an erection sufficient to permit satisfactory sexual intercourse.2 ED is a major health problem that has implications in self-esteem, relationships and overall quality of life.3 Studies have shown that ED may precede the onset of future cardiovascular disease (CVD), coronary heart disease, stroke, and has recently been labeled as an independent risk factor of CVD that seemed to predict reduced longevity.4,5
Diabetes mellitus (DM) is one of the most critical health problems in the world, triggering various unintended complications, including ED. It is associated with a wide variety of cardiovascular conditions, which is the main cause of morbidity and mortality among these patients. In 2019, the estimated prevalence of more than 400 million adults, with projections of 700 million by 2045.6 Furthermore, it is known that patients with DM are 3 times more likely to develop ED, especially older individuals and those with inadequate disease control.7
Alcoholism, similarly to DM, is also a prevalent condition with known negative consequences to physical and mental health. Excessive alcohol consumption not only negatively impacts diabetes self-care adherence but also affects the course of DM. Diabetes patients who are at-risk drinkers are likely to have poor diabetes treatment adherence, leading to increased morbidity and mortality.8 Interestingly, clinical and experimental studies have also associated alcoholism with endothelial dysfunction, impaired corpus cavernosum smooth muscle (CSM) relaxation capacity, hypertension, oxidative stress and release of inflammatory mediators.9, 10, 11, 12, 13
MicroRNAs (MiRNAs) are small noncoding RNAs of 19 to 25 nucleotides that have emerged in recent decades as important regulators of gene expression, since a single miRNA is able to modulate the expression of hundreds of genes.14 Thus, the dysregulation of their expression can influence multiple cellular processes, with known repercussion to numerous diseases such as cancer, DM, and cardiovascular events and ED.15,16 Therefore, it has been postulated that MiRNAs could potentially be useful as biomarkers in different medical conditions both for diagnosis and follow-up.17
Lizarte et al demonstrated that chronic alcohol consumption associated with diabetes impairs erectile function in rats.18 Further, the same group provided evidence that excessive alcohol consumption leads to impaired corpus CSM relaxation capacity as a result of decreased activity of the nitric oxide pathway linked to endothelium-dependent mechanisms.19 The same research group also demonstrated that changes in the endothelinergic system (ETA and ETB) in the CSM that can play a role in the pathogenesis of ED.20 miR-15b, miR-16, miR-138, miR-221, and miR-222 are miRNAs whose expressions have been related to endothelial cells, vascular physiology, angiogenesis, and vascular remodeling.21, 22, 23 According to the MiRNA database and target prediction tool index (DIANA-miRPath v3.0), such miRNAs have also been related to regulatory pathways of angiogenesis, nitric oxide synthase activity and the mechanism of cell death by apoptosis.24
We hypothesized that specific MiRNAs reached in peripheral blood may be associated with endothelial dysfunction in the corpora cavernosa of diabetic rats exposed to alcohol, turning them possible liquid biopsy.
AIM
By using our research group's experience with the animal model of diabetic and alcohol consumption rats, we aimed at studying the expression of miRNAs 15b, 16, 138, 221, and 222 in the corpus cavernosum (CC) and peripheral blood to assess their potential as biomarkers of endothelial dysfunction in the CC.
Material and Methods
Animal Groups and Study Design
Animal studies were approved by the Institutional Animal Care and Use Committee of our institution. Twenty male Wistar rats (Rattus norvegicus) were divided into 4 groups: control group (C), alcohol consumption group (A), diabetic group (D), diabetes associated with alcohol consumption group (D + A) with 5 animals allocated to each one. Experimental animals had free acess to food and water, and were maintained on a 12-hour dark-light cycle for 7 weeks. The sample size in each group was defined considering previous studies that assessed genes and miRNAs expression in an animal model of diabetic and alcoholic rats.19,20
Animals belonging to alcohol consumption groups (A, D + A) were submitted to semi-volunteer alcohol consumption through progressive increase of ethanol concentration in drinkable water. The alcoholic concentration started at 5% and was doubled every week up to to 20% as proposed by Tirapelli et al.25 Similarly, on the first day all rats were weighed and the diabetes was developed by 1 injection of alloxan (Sigma Aldrich Chemical - Saint Louis, MO, USA) (dissolved in 0.1 citrate buffer, 45 mg/kg) into the tail vein.18, 19, 20 The control group received 1 injection of citrate buffer only into the tail vein and drinkable water replaced at the same time as did the others. Blood samples were drawn every week from a tail stab to measure the level of blood glucose using reagent strips (BM-Accutest and an auto-recorder, Accutrend, Boehring Mannheim, UK).
All animals were followed 7 weeks long, weighted again at the end of the experimental period, anesthetized, and then euthanized. A blood sample was drawn from the vena cava, and the penis was dissected to harvest the CC.
Validations of the Murine Model System
Previous studies have found that rats with alloxan-induced DM have significant increases in blood glucose levels, and the association with alcohol comsumption leads to an impaired endothelial-dependent vasoreactivity in the corporal tissue. Endothelium-dependent relaxation was assessed using acetylcholine (Ach) on corporal stripes.11,18
After the CSM chamber was set, the penis was harvested. The corporal bodies were detached at the level of the ischium bone and they were immersed in Krebs solution. The strips of CSM (1 × 1 × 10 mm) were mounted into the 10-mL organ chamber containing Krebs solution at 37°C continuously bubbled with a gas mixture of 95% O2 and 5% CO2 (pH 7.4), and vertically suspended and supported with 2 metal hooks. One hook was connected to a force transducer (Ugo Basile, Varese, Italy), and the others worked as a fixed attachment point. The strips were stretched to a resting tension of 5 mN and allowed to equilibrate for 45 minutes. Changes in isometric force were recorded using a PowerLab 400 Data Acquisition System (Chart, version 4.2, AD Instruments, Colorado Springs, USA). Endothelial-dependent relaxation was tested by cumulatively adding ACh(0.01–1000 μmol/) to the medium of the precontracted segments with phenylephrine (10 μmol/L).
Expression Profile of MiRNAs
The expression profile of the miR-15b, miR-16, miR -138, miR -221, and miR -222 were analyzed in blood and cavernous tissue samples from each animal. Total cellular RNA was extracted with Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA) to cavernous tissue samples and RNeasy Mini Kit (QIAGEN, Hilden, Germany) to the blood from each animal, according to the manufacturer's instructions. For real-time PCR assessment, reverse transcription of RNA samples were performed using a High-Capacity cDNA kit (Thermo Fisher Scientific) also according to the manufacturer's protocol. For quantitative analysis of the miRNAs we used the commercially available system TaqMan Assay-on demand (Thermo Fisher Scientific). Reverse transcription was performed using 5 ng total RNA for each sample in 7.5 µL of the total reaction mixture. The cDNA obtained was diluted 1:4 and 4.5 µL was used for each 10 µL of the quantitative real-time polymerase chain reaction mixture using the TaqMan Master Mix (Thermo Fisher Scientific). All reactions were carried out in duplicate and analyzed with the 7500 Sequence Detection System apparatus (Thermo Fisher Scientific). Data were analyzed using the ABI-7500 SDS software. The total RNA absorbed was normalized based on the Ct value for U6 (000391). The variation in expression among samples was calculated by the 2-∆∆Ct method, with the mean ∆Ct value for a group of 5 samples from control rats used as a calibrator.
Bioinformatic Analysis of MiRNAs Targets
The selected miRNAs were analyzed using the mirPath V.3 tool, available online at Diana Tools (http://snf-515788.vm.okeanos.grnet.gr/). This tool identifies the signaling pathways in the KEGG database (Kyoto Encyclopedia of Genes and Genomes) (http://www.genome.jp/kegg/pathway.html) that are potentially regulated by the expression of miRNAs.24 Five miRNAs were identified in the analysis: miR-15b; miR-16; miR-138; miR-221, and miR-222 that target the regulatory pathways for the activity of nitric oxide synthases, the mechanism of cell death by apoptosis and angiogenesis. The targets were confirmed based on the miRTarBase database (http://mirtarbase.mbc.nctu.edu.tw/php/download.php).
Statistical Analysis
Quantitative variables were presented as medians and interquartile ranges. Kruskall Wallis, with Dunn's post-test, were used to compare continuous variables between groups. ROC curves were constructed to compare the diagnostic performance of miRNA. P values < .05 were considered statistically significant, and 95% confidence intervals were presented as a measure of precision. GraphPad Prism, version 8.4.3, San Diego-CA, USA, was used for data analysis.
Results
Validation of Endothelial Dysfunction Following DM and Alcoholism Induction
Median animal weight was 515 g (495–525 g) in the control group, 320g (306–332 g) in group D, 430g (417–454 g) in the group A, and 360 g (355–371 g) in the D + A group. There was a significant difference in groups A, D, and D + A compared to C (Figure 1A). Blood glucose values were significantly higher in D and D + A rats when compared to controls and A rats (Figure 1B). Vasoreactivity evaluated by the response to Ach revealed that groups A, D, and D + A had significantly impaired endothelial-dependent vasoreactivity, and that the maximal responses of CSM to ACh were significantly reduced in groups A, D, and D + A compared to C. (Figure 1C)
Figure 1.
A, Representation of weight median [interquartile range]: Control (C), Alcohol Consumption (A), Diabetic (D) and Diabetic-Alcohol Consumption (D + A). There was a significant difference in groups A, D and D+A compared to C. *P < .001. (Kruskal-Wallis test and Dunn's post test). B, Representation of fasting blood glucose levels median [interquartile range]: Control (C), Alcohol Consumption (A), Diabetic (D) and Diabetic-Alcohol Consumption (D + A). There was a significant difference in groups D and D+A compared to C. *P < .001. (Kruskal-Wallis test and Dunn's post test). C, Relaxant effects induced ACh (0.01–1000 μmol/L) on CSM strips precontracted by PE (10μmol/L)Experimental values represent a rate to maximal changes from contraction produced by PE in each preparation (taken as 100%). Values are median *P < .001 vs control (Kruskal-Walis and Dunn's post test).
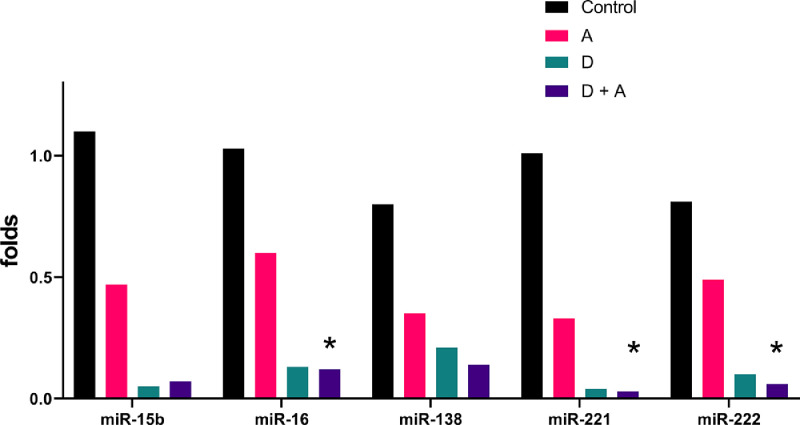
RT-PCR Analysis of MiRNA Expression in the CC and Blood of Rats
In the tissue of the CC (Figure 2) a significant downregulation of the miR-16, miR-221 e miR-222 in the D+A group was found, and nonsignificant downregulation in the A and D groups compared to the C group was observed. When analyzing the blood MiRNA profile (Figure 3), a significant downregulation of all studied miRNAs was found in groups D and D+A, and nonsignificant downregulation was observed in group A compared to the C group. In the blood, diagnostic accuracy estimated by ROC curve was very similar to all miRNA (Figure 4). The cutoff values representedin ROC curve of miR-15b, miR-16, miR-138, miR-221, and miR-222 were respectively: 0.49; 0.22; 0.23; 0.09; 0.10.
Figure 2.
Relative folds of expression miRNAs median in corporal tissue of the studied groups.*P < .05 compared with control group. (Kruskal-Wallis test and Dunn's post test).
Figure 3.
Relative folds of expression miRNAs median in blood of the studied groups.*P < .05 compared with control group. (Kruskal-Wallis test and Dunn's post test).
Figure 4.
ROC curves and AUC for miR-15b, miR-16, miR-138, miR-221, and miR-222.
Discussion
Endothelial dysfunction has been recognized as the central event that links ED and cardiovascular complications in patients with DM.1,26 ED has been considered as an independent predictor of cerebral and cardiac vascular events.These issues, regarding endothelial health, have motivated the development of experimental studies recently. In the present study, we found that the downregulation of MiRNAs expressed in CC was confirmed in the blood. Group D and D+A were at greater risk for endothelial injury and have demonstrated statistically significant miRNA downregulation. Such effect was particularly important in the D + A group, perhaps due to the additional detrimental effect of alcohol in the physiopathology of DM. The 5 chosen miRNAs may be implicated in the regulation of endothelium function.21, 22, 23 miR-15b and miR-16 have been referred as angiogenesis modulation and expression control of the vascular endothelial growth factor.21 miR-138 mediates endothelial cell dysfunction and is involved in cardiomyocytes apoptosis when subjected to hypoxia conditions.27,28 Finally, miR-221 and miR-222, have been implicated to shut off migration, proliferation, angiogenesis of endothelial cells and indirectly regulate the expression of endothelial nitric oxide synthase.22,29 By using bioinformatic analysis of targets prediction tools, miR-15b, miR-16, miR-138, miR-221, and miR-222 have been identified as possibly involved in nitric oxide synthases activity regulatory pathways and in mechanisms of cell death by apoptosis and angiogenesis.24 Therefore, we have hypothesized that these miRNAs could refer to proper endothelial function pointing eventual endothelial dysfunction. The abundance of endothelial cells in the CC has supported our experimental model.
It has been demonstrated that reduced activity in the nitric oxide pathway/endothelial-dependent mechanisms was linked to a possible regulation in eNOS and iNOS isoforms by miRNA-27b expression, which contributes to losing CSM histological traits.18,19 In another study, miR-155 and miR-199 downregulation were associated with protein expression of endothelin's receptors upregulation which showed possible impaired endothelial function in diabetic rats exposed to high levels of alcohol intake.20
Several studies in animal models have shown that altered expression of miRNAs is involved in ED.30, 31, 32 By using a mouse model of diet-induced ED, Barbery et al quantified miRNA isolated from corporal tissue by a NanoString analysis and concluded that miRNAs play a significant role on vasculogenic ED, as miR‑1937c and miR‑151‑5p had upregulation, and miR‑153 and miR‑425 had downregulation by qRT-PCR analysis.30 In fact the majority of miRNAs had down-regulation while the minority of them upregulation.30
In another study designed with an animal model of aging rats with ED, the expression profiles of miRNAs in CC were identified using GeneChip array by Pan et al The authors identified 428 dysregulated miRNAs, in which 105 miRNAs had upregulation while 323 miRNAs had downregulation.31 Similarly, another study from the same group in a murine model of type 2 DM‑associated ED, 161 miRNAs had above 2.0-fold change in expression (56 miRNAs upregulated and105 miRNAs downregulated). Four differentially expressed miRNAs (miR-18a, miR-206, miR-122, and miR-133) had their expression confirmed by qRT-PCR. Therefore, such miRNAs have been implicated in the mechanism of type 2 DM‑associated ED via regulating different genes and pathways, including IGF-1 and apoptosis, nitric oxide, and vascular smooth muscle contraction process.32
GamalEl Din SF et al studied the expression of miR 200a and miR206 in blood and cavernosus tissue from patients with refractory veno-occlusive ED scheduled for penile implant and compared them with men that had emergency surgery to penile fracture; they found these 2 miRNAs were upregulated in tissue and blood of ED patients. Interesting to note that men with diabetes, hypertension, CVDs or obesity were excluded from this study.33
In the present study we have demonstrated a significant downregulation in the expression of all analysed MiRNAs. It is also reasonable to infer that all miRNAs expression were more severely downregulated in the D+A group.These findings indicate a potential association between miRNA expressions and endothelial injury. It is also a sign that alcohol has an additional negative effect on endothelial changes generated by DM. We hypothesize that it is a result of both a direct cell toxicicity and poorer DM control.
MiRNAs were first established as biomarkers for cancer in 2008, when Lawrie et al utilized them for the examination of diffuse large B-cell lymphoma in the serum of patients. Afterwards, their potential use as biomarkers have been mentioned in literature for numerous diseases.34,35 Since it has been discovered that miRNA could be detected in both extra and intracellular environments, their potential application as biomarkers became an important focus of research worldwide.35
We have chosen to investigate the role of miR-15b, miR-16, miR-138, miR-221, and miR-222 on the endothelial function through blood and CC assessment because endothelial dysfunction may preceed more seriously cardiovascular events and its evaluation might be of clinical relevance.
Experimental models evaluating neurogenic ED without known endothelial lesion have demonstrated a significant upregulation of miRNA-138 expression.36 We have found an unique miRNAs downregulation pattern in CC and peripheric blood, more prominent on D and D+A groups, respectively. Although some authors advocate that the ideal biomarker would be more likely to have an upregulation pattern, we do confess our results turned us somewhat disappointed, despite other studies have found a predominance of miRNA downregulation either.30,32
ED is an early harbinger of generalized vascular systemic disorder, therefore an evaluation of endothelial health in ED patients should be of prime relevance.37 In this scenario, the study of miRNAs might gain increasing relevance in the evaluation and follow-up of patients with ED. However, there are some doubts about the real relevance of miRNAs' targets and its biological and clinical significances, especially because the mechanisms involved in gene expression and the synthesis of the target protein remain largely unknown. Another source of criticism is the fact that miRNA can be involved in multiple biological events and that the potential influence of epigenetic effects have not been identified yet.
This study is not without limitations. It is a known fact that experimental models using rats not always represent the pathophysiology of ED in humans. Although a small number of animals was used in the study, this number was calculated to meet the standards of NC3Rs of the ARRIVE guidelines. So far, the use of miRNA in the setting of endothelial dysfunctions in animals with DM and increased alcohol consumption has demonstrated great discriminatory power and high accuracy of MiRNAs expression in the blood.
Conclusions
miR-16, miR-221, and miR-222 had significantly downregulation in CC in the (diabetic-alcohol consumption) group. In blood analysis, all miRNAs had significantly downregulation in diabetic and diabetic-alcohol consumption groups. ROC curves demonstrated excellent diagnostic accuracy for all blood miRNAs in experimental groups, suggesting that these miRNAs could be considered potential blood markers of structural and functional endothelial dysfunction within the CC in this model.
STATEMENT OF AUTHORSHIP
RBT, CAFM, DPCT, LFT, STJ conceived and designed the study. RBT, FSLN, MLAC,DPCT, LFT carried out experimental study. RBT, JBJ , FSLN, MLAC, DPCT, analyzed the data. JBJ, EPM statistical analysis. RBT, CAFL, EPM, JBJ wrote the manuscript and critical review.
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
Funding: FAPESP # 11/15167-2.
REFERENCES
- 1.Bivalacqua TJ, Usta MF, Champion HC. Endothelial dysfunction in erectile dysfunction: Role of the endothelium in erectile physiology and disease. J Androl. 2003;24(Suppl. 6):S17–S37. doi: 10.1002/j.1939-4640.2003.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 2.NIH Consensus Conference Impotence. NIH Consensus Development Panel on Impotence. JAMA J Am Med Assoc. 1993;270:83–90. doi: 10.1001/jama.270.1.83. [DOI] [PubMed] [Google Scholar]
- 3.Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013;381:153–165. doi: 10.1016/S0140-6736(12)60520-0. [DOI] [PubMed] [Google Scholar]
- 4.Thompson IM, Tangen CM, Goodman PJ. Erectile dysfunction and subsequent cardiovascular disease. JAMA J Am Med Assoc. 2005;294:2996–3002. doi: 10.1001/jama.1993.03510020054014. [DOI] [PubMed] [Google Scholar]
- 5.Zhao B, Hong Z, Wei Y. Erectile dysfunction predicts cardiovascular events as an independent risk factor: A systematic review and meta-analysis. J Sex Med. 2019;16:1005–1017. doi: 10.1016/j.jsxm.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 6.International Diabetes Federation . 9th ed. IDF Diabetes Atlas; 2019. IDF Diabetes Atlas.https://www.diabetesatlas.org/en/ Available at: Accessed May 3, 2020. [Google Scholar]
- 7.U.S.Department of Health and Human Services Sexual and urologic problems of diabetes. Natl Inst Diabetes Dig kidney Dis. 2008;9:1–8. https://www.niddk.nih.gov/health-information/diabetes Available at: Accessed May 3, 2020. [Google Scholar]
- 8.Engler PA, Ramsey SE, Smith RJ. Alcohol use of diabetes patients: The need for assessment and intervention. Acta Diabetol. 2013;50:93–99. doi: 10.1007/s00592-010-0200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hijmering ML, De Lange DW, Lorsheyd A. 2007. Binge drinking causes endothelial dysfunction, which is not prevented by wine polyphenols: A small trial in healthy volunteers. [PubMed] [Google Scholar]
- 10.Oda N, Kajikawa M, Maruhashi T. Endothelial function is impaired in relation to alcohol intake even in the case of light alcohol consumption in Asian men; Flow-mediated Dilation Japan (FMD-J) Study. Int J Cardiol. 2017;230:523–528. doi: 10.1016/j.ijcard.2016.12.065. [DOI] [PubMed] [Google Scholar]
- 11.Lizarte FS, Claudino MA, Tirapelli CR. Chronic ethanol consumption induces cavernosal smooth muscle dysfunction in rats. Urology. 2009;74:1250–1256. doi: 10.1016/j.urology.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 12.Muniz JJ, Leite LN, De Martinis BS. Chronic ethanol consumption induces erectile dysfunction: Role of oxidative stress. Life Sci. 2015;141:44–53. doi: 10.1016/j.lfs.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Leite LN, do Vale GT, Simplicio JA. Ethanol-induced erectile dysfunction and increased expression of pro-inflammatory proteins in the rat cavernosal smooth muscle are mediated by NADPH oxidase-derived reactive oxygen species. Eur J Pharmacol. 2017;804:82–93. doi: 10.1016/j.ejphar.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Chen J, Sen S. MicroRNA as biomarkers and diagnostics. J Cell Physiol. 2016;231:25–30. doi: 10.1002/jcp.25056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C, Wang R. 2017. The role of microRNA in erectile dysfunction. [DOI] [Google Scholar]
- 17.Huang W. MicroRNAs: Biomarkers, diagnostics, and therapeutics. Methods Mol Biol. 2017;1617:57–67. doi: 10.1007/978-1-4939-7046-9_4. [DOI] [PubMed] [Google Scholar]
- 18.Lizarte FS, Morgueti M, Tirapelli CR. Chronic alcoholism associated with diabetes impairs erectile function in rats. BJU Int. 2010;105:1592–1597. doi: 10.1111/j.1464-410X.2009.09084.x. [DOI] [PubMed] [Google Scholar]
- 19.da Cunha JP, Lizarte Neto FS, Novais PC. Expression profiles of eNOS, iNOS and microRNA-27b in the corpus cavernosum of rats submitted to chronic alcoholism and diabetes mellitus. Acta Cir Bras. 2017;32:38–45. doi: 10.1590/s0102-865020170105. [DOI] [PubMed] [Google Scholar]
- 20.Gonçalves FZ, Lizarte Neto FS, Novais PC. Expression profile of endothelin receptors (ETA and ETB) and microRNAs-155 and -199 in the corpus cavernosum of rats submitted to chronic alcoholism and diabetes mellitus. Brazilian J Med Biol. 2018;51:e6329. doi: 10.1590/1414-431X20176329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chamorro-Jorganes A, Araldi E, Suárez Y. MicroRNAs as pharmacological targets in endothelial cell function and dysfunction. Pharmacol Res. 2013;75:15–27. doi: 10.1016/j.phrs.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dentelli P, Rosso A, Orso F. MicroRNA-222 controls neovascularization by regulating signal transducer and activator of transcription 5A expression. Arterioscler Thromb Vasc Biol. 2010;30:1562–1568. doi: 10.1161/ATVBAHA.110.206201. [DOI] [PubMed] [Google Scholar]
- 23.Sen A, Most P, Peppel K. Induction of microRNA-138 by pro-inflammatory cytokines causes endothelial cell dysfunction. FEBS Lett. 2014;588:906–914. doi: 10.1016/j.febslet.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vlachos IS, Zagganas K, Paraskevopoulou MD. DIANA-miRPath v3.0: deciphering microRNA function with experimental support. Web Serv issue Publ online. 2015;43 doi: 10.1093/nar/gkv403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tirapelli LF, Tirapelli CR, Tirapelli DPC. Morphometric analysis of seromucous acini and granular ducts of submandibular glands from rats (rattus norvegicus) submitted to experimental chronic alcoholism. Rev Chil anatomía. 2001 doi: 10.4067/s0716-98682001000300006. [DOI] [Google Scholar]
- 26.Hadi HAR, Al Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag. 2007;3:853–876. doi: 10.1055/s-2000-13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sen A, Ren S, Lerchenmüller C. MicroRNA-138 regulates hypoxia-induced endothelial cell dysfunction by targeting S100A1. PLoS One. 2013;8:e78684. doi: 10.1371/journal.pone.0078684. Ma X-L, ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He S, Liu P, Jian Z. MiR-138 protects cardiomyocytes from hypoxia-induced apoptosis via MLK3/JNK/c-jun pathway. Biochem Biophys Res Commun. 2013;441:763–769. doi: 10.1016/j.bbrc.2013.10.151. [DOI] [PubMed] [Google Scholar]
- 29.Poliseno L, Tuccoli A, Mariani L. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 30.Barbery CE, Celigoj FA, Turner SD. Alterations in microRNA expression in a murine model of diet-induced vasculogenic erectile dysfunction. J Sex Med. 2015;12:621–630. doi: 10.1111/jsm.12793. [DOI] [PubMed] [Google Scholar]
- 31.Pan F, Xu J, Zhang Q. Identification and characterization of the microrna profile in aging rats with erectile dysfunction. J Sex Med. 2014;11:1646–1656. doi: 10.1111/jsm.12500. [DOI] [PubMed] [Google Scholar]
- 32.Pan F, You J, Liu Y. Differentially expressed microRNAs in the corpus cavernosum from a murine model with type 2 diabetes mellitus ‑ associated erectile dysfunction. Mol Genet Genomics. 2016 doi: 10.1007/s00438-016-1250-8. [DOI] [PubMed] [Google Scholar]
- 33.GamalEl Din SF, Rashed LA, Alghobary HA. Are the cavernous tissue and serum levels of micro RNAs 200a and 206 elevated in patients with refractory veno-occlusive erectile dysfunction? A comparative study. Urology. 2017;108:108–113. doi: 10.1016/j.urology.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 34.Lawrie CH, Gal S, Dunlop HM. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. 2008;141:672–675. doi: 10.1111/j.1365-2141.2008.07077.x. [DOI] [PubMed] [Google Scholar]
- 35.Condrat CE, Thompson DC, Barbu MG. miRNAs as biomarkers in disease: latest findings regarding their role in diagnosis and prognosis. Cells. 2020;9:276. doi: 10.3390/cells9020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu C, Cao Y, Ko TC. The changes of microRNA expression in the corpus cavernosum of a rat model with cavernous nerve injury. J Sex Med. 2018;15:958–965. doi: 10.1016/j.jsxm.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Costa C, Virag R. The endothelial-erectile dysfunction connection: An essential update. J Sex Med. 2009;6:2390–2404. doi: 10.1111/j.1743-6109.2009.01356.x. [DOI] [PubMed] [Google Scholar]