Abstract
Viral infections affecting the liver have had an important impact on humanity, as they have led to significant morbidity and mortality in patients with acute and chronic infections. Once an unknown etiology, the discovery of the viral agents triggered interest of the scientific community to establish the pathogenesis and diagnostic modalities to identify the affected population. With the rapid scientific and technological advances in the last centuries, controlling and even curing the infections became a possibility, with a large focus on preventive medicine through vaccination. Hence, a comprehensive understanding of hepatitis A, B, C, D and E is required by primary care physicians and gastroenterologists to provide care to these patients. The review article describes the epidemiology, pathogenesis, clinical presentation, diagnostic tools and current medication regimens, with a focus on upcoming treatment options and the role of liver transplantation.
Keywords: Hepatitis A, Hepatitis B, Hepatitis C, Hepatitis D, Hepatitis E, Treatment
Core Tip: Viral hepatitis (A, B, C, D and E) diagnosis and treatment have evolved through the last decades, with recent investigations aiming to cure and prevent them pharmacologically or through liver transplantation. This state-of-art review focuses on the epidemiology, pathogenesis, clinical presentation, with a special focus on upcoming diagnostic tools and treatments.
INTRODUCTION
Viral hepatitis has been a formidable challenge eliciting epidemic dating back to ancient times, with documented outbreaks 5000 years ago in China and similar jaundice descriptions by Hippocrates in the fifth century BC in the island of Thassos[1,2]. Technological advancements over the modern era allowed for the viruses to be identified and subsequent scientific inquiry revolutionized the morbidity and mortality caused by these viral pathogens. We aim to provide an overview of viral hepatitis with discussion of current and prospective therapies. Though most remain dominant in certain parts of the world, globalization demands a fundamental understanding of each virus as we all have the daily potential of encountering any of them at our doorstep.
HEPATITIS A
Epidemiology
First discovered in 1973 by Feinstone, a spherical 27 nanometer particle was seen on immune electron microscopy in the fecal sample of hepatitis A patients[3]. A member of the picornavirus family, the hepatitis A virus (HAV) is an RNA virus responsible for 1.4 million cases per year globally[4], with an estimated 7134 deaths in 2016; almost half of these cases were reported in Asia[5]. In the United States, the annual incidence rate was reported to be 2 cases per 100000 people, in 2006. Recent outbreaks of the disease have shown a 294% increase in infections between 2016-2018 compared to 2013-2015[6,7].
Recent outbreaks
In 1988, about 300000 people in Shanghai reported symptoms of HAV after consumption of raw clams, described as one of the largest outbreaks in the modern era[8,9]. Several outbreaks related to specific food products affecting over 300 patients from multiple states in the United States have been reported in the recent years (2013-2019). The outbreaks have been related to consumption of fresh blackberries, frozen strawberries, raw scallops, and pomegranate seeds. A recent study in 2017 conducted over 4 states (California, Kentucky, Michigan, and Utah) has shown evidence for shift towards large community outbreaks with person-to-person transmission. The majority of these infections have been reported among persons with injection or non-injection drug use and/or persons experiencing homelessness[10].
Current data from state health departments shows, over 37000 new outbreak related cases with greater than 22600 hospital admissions and approximately 350 HAV deaths reported between July 2016 and December 2020[11].
On the contrary, 19947 cases of HAV from 24 European Union countries have been reported between January and December 2017, which is four times higher when compared to similar time periods between 2012 and 2015[12]. The number of outbreak related cases from 22 European Union countries was 4475 in 2016 and most outbreaks were related to men who have sex with men.
Pathogenesis
The transmission of HAV occurs via fecal-oral route, which includes consumption of contaminated food or water and person to person contact. Polymerase chain reaction testing for blood donors is performed as transmission through blood transfusion is noted on rare occasions[13]. The dissemination of the HAV into the liver occurs via the portal vein after the virus traverses the mucosa of the small intestinal wall. The virus particles subsequently replicate and are secreted into the biliary canaliculi, reaching back to the small intestine through the bile ducts and being re-excreted in the feces. Until the body responds with appropriate immune reaction in antibodies, the HAV enterohepatic cycle continues. Human leukocyte antigen-restricted, HAV-specific CD8+ T lymphocytes and natural killer cells have been implicated in the damage and destruction of infected hepatocytes[14-16].
Clinical presentation
The usual HAV incubation period is about 2-4 wk[17]. Fever, malaise, jaundice have been described as the most common presenting symptoms for HAV infection[18]. Other common symptoms include weakness, fatigue, nausea, vomiting, abdominal pain, arthralgias, myalgias, diarrhea and anorexia[12]. Patients rarely enter a prolonged cholestatic phase through recovery, while relapsing infections have been described as well[19].
About 10%-15% of patients present with a relapsing course within a 6-mo period of the initial infection[20]. The symptoms during the relapse are usually less severe than the initial infection. Notably, on extremely rare occasions a type 1 autoimmune hepatitis has been observed in genetically predisposed patients[21]. The spectrum of infections can range from asymptomatic patients without jaundice, symptomatic patients with jaundice, cholestasis with prolonged jaundice, to relapsing infections or acute liver failure[19].
Serum aminotransferases above 1000 U/dL are usually noted, with total bilirubin typically ≤ 10 mg/dL, and alkaline phosphatase below 400 U/L. Usually the serum alanine aminotransferase (ALT) is higher than the aspartate aminotransferase (AST)[22,23]. In general, older patients are more likely to have severe hepatocellular derangements, hospital admissions and higher mortality[24]. These findings can be attributed to an impaired regeneration capacity of the liver and a relatively weaker immune system in the older population[25]. In addition to old age, higher mortality has been reported in males[26]. Old age, underlying liver pathology and chronic viral hepatitis are reported risk factors for acute liver failure. In patients who develop acute liver failure, higher mortality has been associated with creatinine > 2 mg/dL (strongest predictor) total bilirubin > 9.6 mg/dL and albumin < 2.5 g/L[18].
Diagnosis
Specific antibodies against HAV (anti-HAV) in the serum can be detected. The diagnosis is confirmed by the presence of immunoglobulin (Ig) M anti-HAV. The antibodies can be detected at the time of onset of symptoms. Serum IgM levels peak during the acute infection and remain positive for up to 4 mo on an average from the onset of symptoms[27]. Immunity is usually tested with HAV total antibody to determine HAV natural exposure or secondary to vaccination[28]. The presence of IgM antibodies without any clinical symptoms is indicative of HAV infection in the past with persistent antibodies, asymptomatic infection or false positive test[29].
Liver biopsy or imaging studies are not required to make a diagnosis. If performed, a liver biopsy may show marked portal inflammation with typically a lesser degree of necrosis, Kupffer cell proliferation, acidophil bodies, or ballooning when compared to non-HAV viral hepatitis[30].
Management
No specific treatment is available for HAV and the management is mainly symptomatic. The primary focus remains on improving sanitary conditions to minimize the transmission in the community. Historically, immunoglobulins have been used in the prevention of HAV infections. With the availability of an effective vaccine, the use of immunoglobulins has been largely abandoned except in infants below the age of 12 mo. Post exposure prophylaxis with hepatitis A vaccine has been approved since 2007 for immunocompetent patients without chronic liver disease, who are between the ages of 12 mo and 40 years of age[11].
An inactivated HAV vaccine has been licensed in Europe since 1991, while a live attenuated hepatitis A vaccine has been in use in China since 1992[31]. The inactivated virus vaccine was first approved for use in United States in 1995. The vaccine is given in 2 to 3 dose series at least 6 mo apart, depending on the vaccine formulation used. In 1996, the Advisory Committee on Immunization Practices (ACIP) recommended vaccination for people at high risk for HAV infection or adverse consequences of the infection[32]. In 2006, ACIP recommended routine vaccination of all children 12-23 mo of age; and in 2019, the committee recommended routine HAV vaccination of people experiencing homelessness[33,34]. Other recommendations for groups that would benefit from vaccination are described in Table 1[35].
Table 1.
Centers for Disease Control and Prevention recommendations for hepatitis A vaccination
|
|
Recommendations
|
| Children | Children age 12-23 mo |
| Age 2-18 yr who have not received the vaccine (catch up vaccination) | |
| High risk population | International travelers |
| Men who have sex with men | |
| Illicit drug users | |
| People at occupational risk of exposure | |
| People anticipating close personal contact with international adoptee | |
| People experiencing Homeless | |
| Population at high risk of severe hepatitis A | People with chronic liver disease |
| HIV+ people | |
| Others | Pregnant women at high risk or at risk of severe hepatitis A |
| Any person requesting the vaccine |
HIV: Human immunodeficiency virus.
The vaccine has had significant effect on the decrease in HAV infections, although coverage rates remain lower when compared to other childhood vaccines. These rates were noted to be around 87% for first dose and 57% for the second dose. Long term immune response up to 40 years in noted in over 90% patients who receive both doses of the 2-vaccine series[36].
Role of liver transplant
Acute liver failure occurs in less than 1% of acute HAV infections[6]. From these patients, only 31% require emergent liver transplant for treatment of fulminant disease, while the remaining patients recover spontaneously with symptomatic management[37]. In a study comparing liver transplant outcomes in patients with HAV vs hepatitis B infection, the patients with HAV were found to have lower 1- and 5-year survival rates. Presence of acute pancreatitis and HAV recurrence in this population was identified as risk factors for shorter graft and patient survival. Following transplant, patients should be carefully monitored for HAV recurrence as it is common and is associated with poor outcomes[38].
HEPATITIS B
Epidemiology
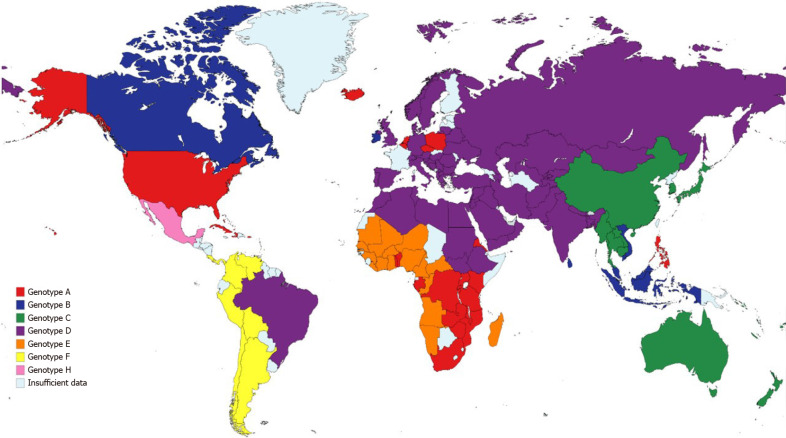
Chronic hepatitis B virus (HBV) infection had an estimated prevalence of 257 million people in 2015 worldwide, with the highest proportion of cases in Western Pacific and African regions (68% of the cases) and with the lowest prevalence in North America[39]. An estimated 29% of cirrhosis-related deaths worldwide were due to HBV in 2017[40], while also contributing to the majority of liver cancer-deaths the same year. There are ten identified HBV genotypes (A-J) collectively, with 35 identified sub-genotypes; the distribution of the genotypes varies broadly worldwide (Figure 1)[41]. The variation in the genotype distribution and risk factors relies on common factors in high-risk populations, such as vertical transmission which is associated to higher risk of chronic disease and hepatocellular carcinoma (HCC)[42]. Risk factors for HBV transmission include a history of blood transfusion, intravenous drug or paraphernalia use[43], contaminated piercing instruments[44], sexual intercourse with an infected person and organ transplantation from HBV positive donors[45].
Figure 1.
Global geographic distribution of most common hepatitis B virus genotypes per country.
Pathogenesis
The HBV is a double-stranded DNA virus belonging to the Hepadnaviridae family, first identified in 1963 and named the Australian Antigen due to the protein reacting to antibodies from a hemophiliac patient. The virus has a special trophism for hepatocytes, to which it adheres and integrates upon initial infection[46]. After viral uncoating, the DNA integrates to the host nucleus as a covalently closed circular DNA (cccDNA) that can persist indefinitely in the hepatocytes; this explains the possibility of HBV reactivation in chronic inactive disease[47]. Ultimately, through a process driven by reverse transcriptase, new viral molecules are assembled from the cccDNA and are released by exocytosis. This process leads to immune-mediated liver injury as antigens are recognized by the immunologic system, with a lesser effect from direct viral cytotoxicity. Importantly, the DNA expresses important proteins for its survival including two capsid core proteins—core antigen and e antigen (hepatitis B e antigen, HBeAg), and the surface antigen (hepatitis B surface antigen, HBsAg), all of which are relevant for diagnosis and surveillance. Variations in genotypes have been described, with differences clinically relevant for pegylated (PEG)-interferon response and HCC risk[48].
Clinical presentation
Acute HBV infection can range from a subclinical disease to an icteric hepatitis, the former being the most common presentation[49]. Patients may present fatigue, nausea, vomiting and right upper quadrant pain before or during jaundice onset. Notably, less than 1% of cases may present fulminant hepatitis. During the acute phase, there is a rapid HBV viral load increase in the 10000-100000 ng/mL range, along with ALT and AST elevation in the 1000-2000 IU/L range, and total bilirubin elevation[50]. At this point, HBsAg and IgM core antibody (HBcAb) become positive, supporting an acute HBV diagnosis. Although a subset of acute HBV can have resolution of the infection with liver enzyme normalization, if the ALT remains elevated after 6 mo from initial presentation, a chronic HBV phase is established.
Chronic HBV may develop in patients exposed at a younger age[51], with genetic predisposition or in those that did not develop symptoms when acute HBV infection occurred[52]. These patients are usually asymptomatic for years, unless there is HBV exacerbation[53] or development of complications[54]. The occurrence of HBV exacerbations is best explained by four phases reflecting the disease activity[55]. The first phase (known as immune tolerant) shows markedly elevated HBV titers and positive HBeAg, without ALT elevation or liver inflammation for which patients are asymptomatic. The second phase (immune clearance) is characterized by activation of the immune system, leading to elevation of ALT at least five-fold the upper limit, HBV DNA decrease and histological evidence of inflammation, possibly leading to fatigue, jaundice, and right upper quadrant pain. The third phase (immune control) shows negative HBeAg, as there is seroconversion to positive HBeAb (hepatitis B e antibody) and undetectable to low HBV DNA titers. The fourth phase (immune active) is characterized by ALT and HBV DNA elevation due to triggers such as hepatitis D virus (HDV) superinfection or immunosuppression, leading to similar symptoms seen in the immune clearance phase with potential risk for acute liver failure. Importantly, the disease can fluctuate between the third and fourth phases.
Diagnosis
Patients that present with acute HBV present with serologies as previously discussed. However, some patients will be in the “window period”, where the immune system has cleared the HBsAg, but no HBV surface antibody (HBsAb) is present; in this scenario, the only positive marker is an elevated IgM HBcAb. Ultimately, once the patient clears the infection, both positive IgG HBcAb and HBsAb will be present. On the other hand, if the patient develops chronic HBV infection, no HBsAb will be detected and HBsAg will persist, even if IgG HBcAb is present. Also, the HBeAg status, HBV DNA and aminotransferases level help determining the phase of the disease and tailor treatment considerations. Finally, the population that has been vaccinated and has never been exposed to HBV will only show positive HBsAb[56].
Complications
Due to parenchymal inflammation and fibrosis with long-term disease, patients with chronic HBV are predisposed to develop liver cirrhosis. Also, patients with HBV have an increased risk to develop HCC, regardless of the presence of cirrhosis; this risk is well established, but the mechanisms of carcinogenicity are hypothesized to be multifactorial including length[57] and severity[58] of HBV DNA elevation, ethnicity of the patients and more recently has been associated to the actual genome strain of the virus, especially patients with genotype C[59].
Current treatment options
Based on consensus from multiple societies, treatment with viral suppression therapy is recommended if patients have extrahepatic manifestations, pregnancy, family history of HCC, hepatitis C virus (HCV)/HDV co-infection, immunosuppression prophylaxis, compensated/decompensated liver cirrhosis[60], or if in the immune active phase and meeting specific criteria established by liver society guidelines (Table 2)[61-63].
Table 2.
Treatment criteria in patients with chronic hepatitis B virus and immune active phase per society guidelines
|
Criteria
|
American Association for the Study of Liver Disease[61]
|
European Association for the Study of the Liver[63]
|
Asian Pacific Association for the study of Liver Diseases[62]
|
| ALT | ≥ 2 times ULN—males 35 U/L, females 25 U/L | > 1 time ULN—40 U/L | > 2 times ULN—40 U/L |
| HBV DNA viral load | > 2000 IU/mL if HBeAg negative or > 20000 IU/mL if HBeAg positive | > 2000 IU/mL, regardless of HBeAg status | > 2000 IU/mL if HBeAg negative or > 20000 IU/mL if HBeAg positive |
| Degree of liver fibrosis/inflammation | Liver biopsy with moderate-severe inflammation or advanced liver fibrosis (F3-F4). Liver elastography or serum markers showing advanced liver fibrosis (F3-F4) | Liver biopsy, liver elastography or non-invasive testing consistent with moderate-severe fibrosis (F3-F4) | Liver biopsy with moderate-severe inflammation or advanced liver fibrosis (F3-F4). Fibroscan or serum markers showing advanced liver fibrosis (F3-F4) |
ALT: Alanine aminotransferase; HBV: Hepatitis B virus; HBeAg: Hepatitis B e antigen; ULN: Upper limit of normal.
Current HBV treatments include nucleoside/nucleotide analogs and PEG-interferon. Overall, the HBsAg seroconversion is higher with PEG-interferon regimen compared to other medications, but its efficacy is limited due to poor patient tolerance[64]. Available nucleoside/nucleotide analogs include lamivudine, adefovir, telvidudine, entecavir, tenofovir fumarate and tenofovir alafenamide[61,63]. From these medications, the first line regimen for treatment consists of entecavir, tenofovir fumarate or tenofovir alafenamide monotherapy due to high genetic resistance barrier, while the other medications can be considered as options based on medication availability and poor access to first line agents.
Role for liver transplant
Patients with HBV that develop acute liver failure, decompensated liver cirrhosis or HCC can potentially undergo liver transplantation as ultimate therapy. In the setting of posttransplant immunosuppression, the rate of HBV recurrence is high with survival rates < 50% after 2 years if no preventive measures are taken[65]. Hence, based on donor and recipient serologies different strategies have been developed including the use of combination of HBV immunoglobulin during the anhepatic and postoperative phase when indicated, with indefinite use of high-genetic barrier nucleoside/nucleotide analogs demonstrating the lowest rates of HBV recurrence and post-transplant decompensation[66].
Ongoing research and future directions
Current treatment options have changed the prognosis for HBV patients, but have been limited by its low cure rates. Hence, other targets in the viral life cycle have been evaluated to improve HBsAg seroconversion and potential cure. For example, inhibition of the HBV entry into the uninfected hepatocytes by blockage of the sodium taurocholate co-transporting peptide (NTCP) receptor in the HBV capsid has been studied with three drugs in different trial stages. Bulevirtide is a NTCP antagonist found to inhibit infection in mice injected with HBV[67]; in a phase 1 study it showed to be safe without occurrence of serious side effects in 36 healthy subjects at a max dose of 20 mg[68]. Subsequently, a multicenter phase 2b randomized trial in 60 HBV/HDV patients receiving PEG-interferon, bulevirtide 2 mg or both with bulevirtide at 2 mg and 5 mg dosing for 48 wk showed a higher proportion of HBsAg decline or loss in patients with combination therapy[69]. Other potential NTCP inhibitor are the cyclosporin derivatives such as SCY450 and SCY995[70], which have been found to inhibit hepatocyte HBV entry in vitro without affecting bile acid uptake, opening a new therapeutic window. Other experimental medications from the cyclophilin inhibitor family like alisporivir[71] and CRV431[72], have shown reduction of HBV DNA and HBsAg in lab models possibly through a similar mechanism with promising results.
Another potential target is at the level of capsid assembly, where core assembly modulators have been found to disrupt the assembly by producing abnormal capsid proteins (Type I) or virions without DNA (Type II)[73]. In vitro evaluation of infected hepatocytes showed a decrease in HBV DNA with different investigational molecules such as JNJ-6379, RO7049389, EDP 514, GLS4 and BAY 41-4109. From these, JNJ-6379, R07019389 and GLS4 have advanced to phase 2 and 3 clinical studies[64].
High interest has focused on the use of interfering RNA molecules. These are small molecules administered intravenously, with the capacity of covalently binding to RNA before assembly and leading to RNA degradation, which could decrease the HBV DNA, HBeAg, HBsAg and HBcAg titers while increasing the seroconversion rates. A few molecules have advanced to phase 2 studies with no delivery method concern, with most of the studies presented at major hepatology conferences. The use of GSK3389404 in a phase IIa study showed a dose-dependent reduction in HBsAg, with an adequate safety profile in 66 patients[74]. Similarly, JNJ-3989 Led to sustained HBsAg reduction in 56% of 39 patients in a phase II study[75]. Also, a phase 2a randomized study evaluating GSK3228836 showed several log HBsAg reduction among 17 HBV patients[76]. Further phase 3 studies are needed to evaluate the seroconversion and functional cure rates in larger populations.
Other relevant target has been the inhibition of HBsAg release from infected hepatocytes to decrease the immunogenic tolerance induced by this molecule. A phase 2 trial study evaluating the combination of PEG-interferon and REP 2139 in 12 patients with HDV coinfection showed a titer reduction to < 50 IU/mL in 6 subjects and HBV DNA suppression in 10 subjects[77]. A second study on REP 2139 and a derivative (REP 2165) in combination with tenofovir and PEG-interferon showed functional cure rates up to 40% in HBeAg negative HBV patients[78].
Lastly, newer nucleoside/nucleotide analogs affecting the HBV life cycle have been developed. Besifovir is a molecule with similar chemical structure to tenofovir fumarate. These two medications were compared in a phase 3 randomized trial including 197 HBV patients receiving the medications for 48 wk. There was no difference in HBV DNA suppression rate with the use of besifovir (80.9%) compared to tenofovir (84.9%), showing equivalence in treatment rates between both arms[79].
On another hand, modulation of the immune system to increase HBV clearance has been a hot topic since the introduction of interferon-based treatments. Recently, the use of immune checkpoint inhibitor via programmed death receptor 1 (PD-1) blockage has been explored as a mechanism to overcome the T-cell anergy seen in chronic HBV. Nivolimumab is a PD-1 inhibitor that was studied in a phase I trial along with and without a therapeutic vaccine in 24 patients with HBeAg negative chronic HBV, showing a significant HBV DNA log reduction but no significant HBsAg seroconversion rates (only seen in one patient)[80]. Other studied mechanisms include the innate immunity stimulation through stimulation of toll-like receptors (TLR). However, in phase 1 studies neither TLR-7 nor TLR-8 agonists have shown to decrease significantly the HBsAg titers in the short term[81]; further phase 2 studies with longer treatment duration may help elucidate its role in HBV treatment.
Finally, HBV vaccination has become revolutionary in public health infection prevention by reducing vertical transmission and decreasing HCC incidence in younger populations[82]. While the currently widespread regimen with recombinant HBsAg (Engerix-B/Recombivax HB) includes three doses for immunization, a recent regimen utilizing an adjuvant in conjunction with recombinant HBsAg (Heplisav) has shown to be as efficacious after two doses, with consideration to administer in non-compliant patients or patients with HBsAb titers < 10 IU/mL. Special interest has been directed to therapeutic vaccines to achieve functional HBV cure. Even though different therapeutic vaccines have been studied, the HeberNavac HBsAg-HBcAg is the only vaccine that has shown significant HBeAg seroconversion rates compared to PEG-interferon in a randomized phase III trial[83]. Although promising, immunotherapy remains a territory to be explored and similar to HCV the goal of viral eradication remains at the core of research efforts.
HEPATITIS C
Epidemiology
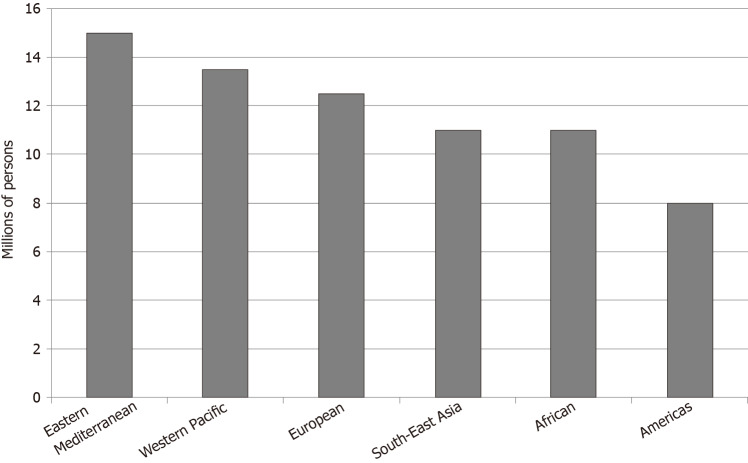
Greater than 71 million persons worldwide are infected with HCV[84]. Prevalence has increased from 1990 to 2010[85]. The Eastern Mediterranean region has an HCV prevalence greater than 2%, considered to be the highest in the world. The continents of Africa and Europe have a prevalence of 1%-2%. North and South America and the Western Pacific region have a prevalence of less than 1% (Figure 2)[84].
Figure 2.
World Health Organization estimated chronic hepatitis C infection prevalence worldwide per region in 2015.
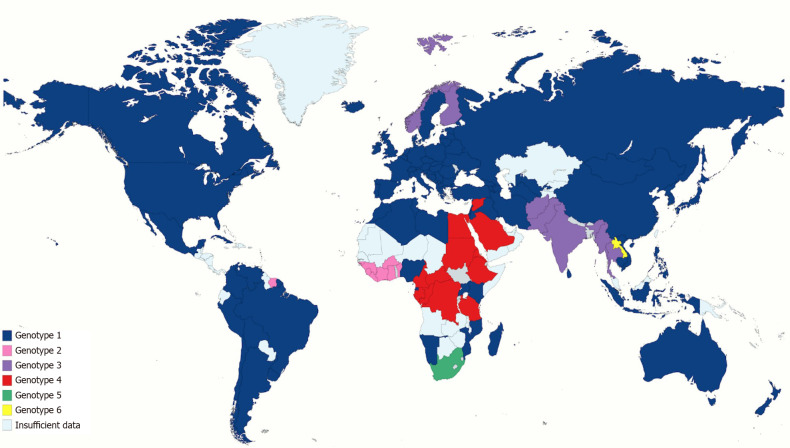
There are seven main genotypes and 67 subtypes. HCV genotype 1 represents about half of all HCV infections, making it the most prevalent genotype in the world. The second most common is HCV genotype 3, involving approximately one-third of HCV infections; it is found more commonly in south Asia, Australia, and several European countries. Genotypes 2 is found in Asia and West Africa, while genotype 4 is found in central and eastern sub-Saharan Africa, North Africa, and the Middle East. Genotypes 2 and 4 represent about 9%-13% of all HCV cases. Genotypes 5, 6, and 7 are restricted to South Africa, South-east Asia, and Democratic Republic of Congo, respectively[86-88] (Figure 3).
Figure 3.
Global geographic distribution of most common hepatitis C virus genotype per country.
Pathogenesis
Hepatitis C is a small, enveloped, positive single-stranded RNA virus that was first discovered in 1989 as a member of the Flaviridae family and is the only member of genus Hepacivirus. It enters the body percutaneously through needlestick injuries (including healthcare or intravenous drug use) containing contaminated blood. It may also enter non-percutaneously through organ transplantation, blood transfusions, sexual intercourse, perinatal transmission, hemodialysis, religious scarification, body piercings, tattoos, and immunoglobulin injection[89]. There are 4 host-derived factors that facilitate the entry of HCV into the bloodstream: Scavenger receptor class B type I, Occludin, Claudin-I and CD81[90]. Additionally, CD81 binds with the viral particle, either through viral envelope protein (E)2 or other molecules and facilitates its entry into the hepatocyte[91].
Although it is considered a non-cytopathic virus, once HCV enters the hepatocyte, it replicates and causes cell necrosis via immune-mediated cytolysis (innate immunity and adaptive immunity) and metabolism-mediated inflammation (hepatic steatosis, oxidative stress, and insulin resistance)[90].
Non-structural protein 5A (NS5A) and E2 regions of HCV also play an important role in the pathogenesis. NS5A inactivates RNA dependent protein kinase (PKR) in hepatocytes, inhibiting the apoptotic pathway and inducing anti-inflammatory interleukin secretion, allowing for viral replication. E2 protein inhibits PKR, allowing the evasion of HCV. The role that different HCV genotypes play in the progression of liver disease is still controversial, but genotype 1b seems to have a more aggressive course, as it has been more associated to cirrhosis progression and liver disease decompensation requiring transplantation[92].
HCV core proteins leads to insulin resistance by direct receptor effect or through increased secretion of tumor necrosis factor-alpha; this leads to further hepatic steatosis, inflammation, and fibrosis. HCV associated oxidative stress is mainly caused by HCV-core protein which induces oxidation of glutathione and increase in reactive oxygen species[93]. NS5A promotes production of reactive oxygen species in the membrane of the endoplasmic reticulum[94]. NS3 is also thought to directly induce oxidative stress[95]. Finally, HCV causes steatosis through impaired secretion of lipids from liver cells, increased production of free fatty acids, and impaired fatty acid breakdown[90].
Clinical presentation
Chronic HCV infection can occur in 50%-85% of patients, while 15%-45% of patients may present spontaneous clearance of the virus[96]. Most patients with HCV infection are asymptomatic or have nonspecific symptoms. These may include fatigue, sleep disturbances, nausea, diarrhea, abdominal pain, anorexia, myalgia, arthralgia, weakness, depression, anxiety, and weight loss[97]. Patients who develop cirrhosis may develop jaundice, ascites, and other stigmata of cirrhosis.
Serum aminotransferase levels remain relatively stable over time in chronic HCV patients. One in three patients have a normal ALT; only one fourth have a serum ALT more than twice the upper limit of normal; the rest of patients have slight enzyme elevations, usually less than twice the upper limit of normal. Rarely, ALT elevations more than 10-fold the upper limit of normal may occur[98]. There is little correlation between aminotransferase levels and liver histologic findings[99].
On the other hand, it is estimated that acute HCV accounts for 15%-20% of cases of acute hepatitis[100]. More than two thirds of acute HCV infections are usually asymptomatic[101]. Symptomatic patients present similarly to other acute hepatitis, with jaundice, nausea, dark urine, and right upper quadrant pain. These symptoms develop 2-26 wk after exposure and lasts 2-12 wk[102]. Aminotransferase levels tend to be greater than 10 times to 20 times the upper limit of normal but fluctuate widely and may even normalize[103]. Acute liver failure due to acute HCV infection is extremely rare, but patients with chronic HBV[104] or other underlying chronic liver conditions may be predisposed to this outcome.
Extrahepatic manifestations are found in up to 38% of patients with chronic HCV[105]. These may include hematologic abnormalities, such as essential mixed cryoglobulinemia and lymphoma; glomerular renal disease, most notably membranoproliferative glomerulonephritis and cryoglobulinemia; dermatologic disorders such as porphyria cutanea tarda and lichen planus; rheumatologic complaints such as arthralgias/myalgias; neurological involvement with sensory or motor neuropathy; and autoimmune conditions such as thyroiditis. Resolution of these manifestations can be expected with treatment.
Complications
Spontaneous clearance of HCV occurs in about one fourth to one half of patients[106]. This usually occurs within 12 wk of exposure. A C/C type allele polymorphism in the chromosomal locus close to interleukin-28B has been associated with higher spontaneous clearance (50%) compared to the 15% rate of spontaneous viral clearance seen with the T/T allele[107]. Other favorable factors associated with higher rates of clearance include HLA-DRB1 and DQB1 alleles, white race, female sex, childhood infection, symptomatic acute infection, and high titers of neutralizing antibodies[106].
Rate of progression of chronic HCV infection to cirrhosis varies widely among the literature, but pooled data estimates that 16% of patients develop cirrhosis within 20 years[108]. It estimated that an HCV infected liver develops cirrhosis after approximately 20-30 years[109,110] with approximately 700000 deaths annually worldwide[111]. Risk factors for progression of hepatic fibrosis in patients with chronic HCV infection include age > 40 years[110], alcohol consumption, HBV coinfection human immunodeficiency virus coinfection, immunosuppressed stage, marijuana use, obesity, diabetes mellitus[112], schistosomiasis, severe hepatic necroinflammation, smoking, male sex[113], and white race[114]. The viral load has not correlated with risk of progression to cirrhosis. Studies have described a 3.9% risk of decompensation per year with ascites presence representing the most common form of decompensation[115]. Once patients develop cirrhosis, there is a 1.4%-4.9% yearly incidence rate of HCC[115-117]. It is estimated that HCV accounts for 25% of HCC cases worldwide[118].
Diagnosis
Third generation enzyme immunoassays (EIAs) detect antibodies against different HCV antigens, including HCV core, NS3, NS4, and NS5 as early as 8 wk, with sensitivity and specificity of 99% after 2-6 mo after exposure[119]. False negatives may arise in patients on hemodialysis and immunocompromised patients. These are considered indirect assays.
Quantitative, HCV RNA tests are a type of direct immunoassays that may detect HCV viremia in patients with at last 10-15 IU/mL[120]. HCV core antigen immunoassay is an alternative to HCV RNA testing but cannot be used to monitor response to antiviral therapy as it has limitations in sensitivity[121].
In patients with low pre-test probability for HCV infection, a negative EIA for anti-HCV antibody effectively excludes HCV infection. However, in patients suspected acute HCV infection, HCV RNA viral load by polymerase chain reaction (PCR) must also be obtained as antibodies may not become positive until 2-6 mo on average. A positive HCV antibody and positive HCV RNA confirms HCV infection but cannot distinguish between acute and chronic infection. Acute infection can be confirmed if a patient previously tested negative in the 6 mo prior to infection and has not tested positive for both RNA and antibody, or if the RNA and antibody become negative within 6 mo. Chronic HCV infection is defined as positive HCV RNA of at least 6 mo duration.
For patients with suspected exposure to HCV, HCV RNA, anti-HCV antibody, and serum aminotransferases are obtained within 48 h to obtain baseline values. If HCV RNA is positive, infection is confirmed, and acuity will depend on whether HCV antibody is positive (chronic) or negative (acute). However, if HCV RNA is negative, it should be rechecked in 4 wk and if positive it is considered an acute HCV infection. If HCV RNA is negative 4 wk after exposure, it should be re-checked at 12-16 wk. If negative again, HCV should be checked once more at 6 mo.
Currently available treatments
Antiviral therapy should be contemplated for almost all patients with HCV. Clinicians should consider withholding treatment in patients with decompensated cirrhosis listed for liver transplant with a high Model for End-Stage Liver Disease (MELD) score, pregnant patients, and patients with less than 12 mo of life expectancy.
The intent of HCV treatment is to eradicate the virus to mitigate cirrhosis and associated complications, such as portal hypertension, HCC, need for liver transplantation, and death. The goal is to obtain sustained viral remission (SVR), which is the absence of virus in the blood 12 wk after treatment completion. SVR has been associated with improvement in elastography fibrosis scores[122], while decreasing hepatic decompensation[123], HCC[124], and liver-related deaths[125].
The major groups of therapy include interferon-alpha, ribavirin, and direct acting antivirals (DAAs). Interferon was the first treatment available for HCV in the 1980s. More recently, interferon has been bound to PEG, which increases its half-life and has increased SVR rates[126]. Ribavirin, an oral guanosine analog, may be used in a synergistic combination interferon to increase response and reduce relapse. Ribavirin is usually well tolerated but may produce hemolytic anemia, especially in patients with renal disease given its excretion in the kidney. It should be avoided in patients with a creatinine clearance less than 50 mL/min. In addition, ribavirin is teratogenic[127].
DAAs inhibit replication of HCV by targeting the NS3/NS4 protease, NS5A protein, and the NS5B polymerase. NS3/NS4 protease inhibitors end in the suffix “-previr” and include boceprevir, telaprevir, simeprevir, paritaprevir, grazoprevir, glecaprevir, and voxilaprevir. NS5A inhibitors end in suffix “-asvirs” and include daclatasvir, ledipasvir, ombitasvir, and elbasvir, velpatasvir, and pibrentasvir. NS5B polymerase inhibitors end in “-buvirs” and are either nucleoside, nucleotide (sofosbuvir), or non-nucleoside (dasabuvir).
The classes of DAAs are typically used in combination: sofosbuvir/lepidasvir, elbasvir/grazoprevir, sofosbuvir/velpatasvir, sofosbuvir/velpatasvir/voxilaprevir, and glecaprevir/pribrentasvir. The choice of regimen and duration depend on the patient’s genotype, renal function, and concurrent medications. The genotypic coverage provided by each regimen is outside of the scope of this review, but importantly sofusbuvir/velpatasvir and glecaprevir/pibrentasvir are considered pangenotypic regimens. Protease inhibitors are primarily hepatically excreted and thus are contraindicated in Child-Pugh B and C cirrhosis. Nucleoside NS5B inhibitor sofosbuvir is primarily renally excreted and should be avoided in patients an estimated glomerular filtration rate less than 30 mL/min per 1.73 m2.
Role of liver transplant
Liver transplantation frequently provides destination treatment for HCV decompensated cirrhosis or HCC in candidates who are not deemed appropriate for surgical resection. HCV infection remains one of the leading etiologies for liver transplantation worldwide[128]. Since 2012, the percentage of liver transplants allocated for chronic HCV infection has steadily decreased in United States[129] due to the use of DAAs. The use of liver transplant for acute liver failure secondary to HCV infection has not been a common phenomenon.
Virtually all recipients who are serologically positive for HCV at the time of transplant develop recurrent HCV infection during reperfusion of the transplanted liver graft[130]. The main consequence is the development of post-transplant liver injury in the form of fibrosis in up to 40%[130], recurrent cirrhosis in up to 30% after 5 years[131], and fibrosing cholestatic HCV in up to 10%[132]. This has been associated with lower overall survival in HCV infected recipients in comparison to HCV negative recipients[133]. To counteract this, therapy with DAAs have been used and have increased post-transplant one-year survival rates in HCV viremic recipients from 90%-92%[129]. DAAs may be used to cure HCV prior to transplant, eliminating the chance of HCV recurrence[134], or can be used post-transplant. Reasons why HCV treatment with DAAs may be delayed until after transplant include: improvement of MELD score leading to de-listing or increased wait time on transplant list, lower likelihood of SVR, and limited access to available regimens for advanced cirrhosis[135].
Utilization of HCV infected organs has been one of the major advancements aimed at increasing the donor organ pool and decreasing waitlist time. Initially, this was primarily performed in recipients already infected with chronic HCV. Between 2010 to 2015, the proportion of HCV positive livers transplanted into HCV seropositive recipients increased 2.4-fold[136]. More recently, the transplantation of HCV infected organs including livers into HCV seronegative recipients has been utilized with similarly good outcomes[137,138]. Donors positive for HCV RNA have a high risk of transmitting infection to HCV negative recipients but this may also occur with HCV antibody positive HCV RNA negative donors in up to 16% of cases[139].
HCV negative recipients who receive HCV positive organs may receive DAA therapy prophylactically or reactively after they are found to be positive for HCV. In liver transplantation, DAA therapy is typically given for at least 12 wk to recipients once they are found to have positive HCV RNA, usually within 3 mo. Thus far, studies have shown SVR of up to 100%[140,141]. Questions are easing surrounding the concern whether the risk of rejection is increased in recipients of HCV organs, either from HCV or DAA therapy[140]. Initial 2-year outcomes of HCV positive organ transplantation in HCV negative recipients have shown promising similar patient and graft survival[138]. Short duration of DAA has been considered but not extensively studied in HCV positive liver transplantation.
In non-hepatic transplantation, prophylactic DAA therapy is favored before viremia to reduce the chances of complications and the duration of DAA therapy[142]. Some authors have even limited duration of DAA therapy to as few as 8 d, but this is not currently recommended outside a clinical trial setting[143]. A pangenotypic regimen is typically utilized if prophylactic DAA therapy is used. Thus far, outcomes of transplantation of HCV positive nonhepatic organs in HCV negative patients have been promising, but data is limited. There is still some concern regarding risks such as DAA treatment failure with evolution of resistance associated substitutions and fibrosing cholestatic hepatitis[144].
Ongoing research and future directions
Much of the future research and guidelines will aim to answer questions regarding the safety, ethical, and practical concerns regarding the transplantation of HCV viremic organs into HCV negative recipients. Several factors related to the HCV donor organ remain nebulous. It is unclear whether duration of HCV infection, viral load, genotype, and failed prior treatment of the donor will affect SVR rates post-transplant in this patient population as studies have not controlled for these factors[145]. In addition, several optimal donor factors, specifically age and fibrosis grade, are not clear. Previous studies showed that HCV infected livers of donors 50 years or older have been found to have increased fibrosis progression and worse graft survival[146], but this data is prior to the advent of DAAs and should be re-examined. Similarly, data prior to the widespread use of DAAs showed that HCV positive donor kidneys had worse outcomes; because of this, the kidney donor profile index includes HCV status as a relevant factor but more recent data from the DAA era shows similar outcomes in these HCV positive donor kidneys[147]. Further studies are needed to establish its effects in the outcomes and limit the number of discarded HCV infected organs.
Recipient selection to match donor characteristics when utilizing HCV organs need further investigation to establish best practice guidelines for HCV organ utilization. For now, HCV infected donor allografts are used mainly in patients whose waitlist duration is expected to exceed their duration of transplant-free survival, but no studies have explicitly examined this. Another scarcely studied but relevant risk is how healthy the recipient’s liver condition factors to receive an HCV infected non-liver organ, and what the long-term outcomes of this are. Timing of DAA therapy, optimal regimen, duration, cost effectiveness of different strategies, interactions between DAAs and calcineurin inhibitors, and other drug interactions with DAAs will also need to be investigated. Finally, data on long-term outcomes, specifically efficacy and safety, following transplantation of HCV viremic organs into HCV negative recipients is lacking, as current data is limited to 1-2 years.
The ongoing pursuit for a HCV vaccine remains prudent, especially since DAAs do not prevent against reinfection. To date, there have been a few clinical trials studying different methods for active immunity, including viral vectors[148], DNA-plasmid, recombinant proteins, and prime-boost vaccination strategies[149]. However, thus far, many of these trials are still in the early phases or have not conferred consistent long-term immunity.
In 2020, the United States Center for Disease Control and Prevention increased the previous screening expanse by recommending one-time universal screening for all adults at least 18 years of age. Similarly, the United States Preventive Services Task Force recommended one-time universal screening for patients 18-79 years old. The World Health Organization (WHO), the American Association for the Study of Liver Diseases/Infectious Diseases Society of America joint guideline group, the European Association for the Study of the Liver, the National Health Service in the United Kingdom, the Canadian Task Force on Preventive Health Care, the Canadian Association for the Study of the Liver, and other groups have yet to recommend universal screening.
Given the vast prevalence of HCV infections worldwide, there has been an increased effort in the last five years to encourage primary care physicians to treat HCV. This has been aided by the ease of using DAAs and the advent of pangenotypic drugs. However, studies have shown that up to 59% of primary care physicians still refer all of their HCV patients to specialists, citing needing additional training to feel comfortable treating the infection[150]. Thus, further studies investigating the best methods in educating and involving primary care physicians to treat HCV are needed.
HEPATITIS D
Epidemiology
In a recent large meta-analysis by Chen et al[58] with 182 articles included from 61 countries, it was estimated that 62-72 million individuals around the globe are infected with HDV. Approximately 10.58% of HBsAg carriers were coinfected with HDV, which is two-fold higher than what has been estimated previously[150,151]. Areas of highest HDV infection carriage include western and middle Africa, the Amazon Basin, eastern and Mediterranean Europe, the Middle East, and parts of Asia. Notably, a recent epidemiological study examining a cohort of HBV-positive intravenous drug users revealed an alarming increase in HDV seroprevalence from 29% in 1988-1989 to 50% in 2005-2006[152]. Nonetheless, the lack of HDV RNA validation in many of these studies hinders a true estimation of HDV prevalence.
Pathogenesis
HDV is a single-stranded RNA virus, considered a defective virus that requires the existence of HBV for full expression and replication. It was first described in 1977 by Rizzetto et al[153] in Italy, when they detected a new antigen-antibody system associated with but immunologically distinct from HBV by direct immunofluorescence. Although there are 8 different described HDV genotypes, HDV-1 is responsible for most cases in North America, Europe, and the Middle East[154].
Like HBV, HDV is transmitted via the parenteral route through exposure to infected blood or body fluids with an exceedingly small inoculum needed to transmit infection[155].
Clinical presentation
The clinical spectrum of HDV infection ranges from inactive asymptomatic carrier to acute liver failure[156]. Concomitant infection of HBV and HDV typically leads to mild self-limited disease or less likely severe acute hepatitis with spontaneous resolution of both infections[157]. In contrast, HDV superinfection in chronic HBV carriers usually results in a protracted clinical course[158]. Nevertheless, patients exhibit heterogeneous clinical presentations with more severe disease reported in genotypes 1 and 3[159], intravenous drug users, and older patients in European cohorts. This could be due to the variable direct cytotoxic effect and immunogenic response to HDV on the host hepatocytes[160-162].
Screening for HDV should be considered in all HBsAg-positive patients especially those presenting with worsening liver disease. Initially, testing for total anti-HDV antibody (IgM and IgG) should be attempted by EIAs or radioimmunoassays. A positive test warrants confirmation by serum reverse transcriptase-PCR assays for HDV RNA. Quantification of serum HDV RNA is important in evaluating the need for and efficacy of antiviral therapy.
Currently available treatments
Current evidence supports treating chronic HDV infection in those with detectable viral RNA and evidence of biochemical or histological active liver disease, particularly if significant fibrosis exists[61]. On the contrary, asymptomatic patients with normal liver enzymes do not require therapy.
With nearly 34 years lapsed since the introduction of interferon for the treatment of chronic HDV infection, it remains the only available treatment option of proven benefit[163]. Nonetheless, sustained virologic response is low even with the newer PEG-interferon and rarely exceeds 25%[164]. A meta-analysis of five trials comparing standard 48-wk PEG-interferon with no treatment confirmed not only a modest benefit to PEG-interferon but such benefit was not sustained[165].
Ongoing research and future directions
The past decade has witnessed the evolution of multiple novel antiviral agents that target various critical steps of HDV life cycle. Three new drugs have emerged for the treatment of chronic HDV infection. Among them is the specific inhibitor of HDV prenylation lonafarnib which showed promising results as a monotherapy or when combined with ritonavir or PEG-interferon. Reduction in viral RNA load and improvement in liver enzymes were observed at variable endpoints[166]. Although observed therapeutic benefits correlated with lonafarnib dose used, that was challenged by its universal limiting adverse effects like nausea, diarrhea, abdominal bloating, and weight loss.
Other new therapies included HDV entry inhibitors such as bulevirtide. As mentioned above, this medication was evaluated in patients with HBV coinfection for 24 wk as a monotherapy or in combination with PEG-interferon or tenofovir. The majority of bulevirtide treated patients experienced a > 1 log10 reduction in HDV RNA after 24 wk of therapy with combination therapy being more effective than monotherapy. Finally, as mentioned in the hepatitis B section, REP 2139 was introduced as an inhibitor of virion secretion with potential for viral load suppression in patients with HBV-HDV coinfection. However, marked ALT elevations were noted in these studies[77,78]. Combination of inhibitors over an enduring treatment regimen are likely required. These novel agents are being considered in combination with PEG-interferon, which carries known side effects that may be prohibitive in its use with HDV cirrhosis.
Animal studies suggest that vaccine strategies for preventing HDV superinfection may be feasible[167,168]. Those studies used groundhogs as animal models and focused on inducing active immunity by injecting the animal with a full-sized HDV antigen or synthetic peptides. These investigations, although promising, were limited by their small size and heterogenous results. As of today, vaccination against HBV remains the most cost-effective means to prevent HDV infection.
Role of liver transplant
Liver transplantation is indicated in patients with fulminant liver failure. Communication and arrangement for transfer to a transplant facility are paramount in the ability to rescue these individuals. Post liver transplantation recurrence of HDV infection despite appropriate medical therapy was estimated to be 13.4%[169] with comparable outcomes between patients with and without recurrence.
HEPATITIS E
Epidemiology
The WHO reported an estimated 20 million new hepatitis E virus (HEV) infections every year globally, leading to 3.3 million symptomatic cases of acute hepatitis and in another report to over 44000 deaths in 2015[39]. Although the use of current antibody assays in seroprevalence studies remains debatable, the overall seroprevalence of the HEV in the United States between 1988 and 1994 was projected to be 21%[170]. Other more recent analyses reported a lower but rising overall seroprevalence in the United States born individuals from 4.5% in 2013-2014 to 8.1% in 2015-2016, with increasing age, female sex, and Asian ethnicity being significant predictors of HEV seropositivity[171].
Pathogenesis
The HEV is a small, single-stranded RNA virus that is considered one of the most common, yet underdiagnosed etiologies of acute viral hepatitis with the earliest epidemic going back to 1955 in New Delhi[172]. While it stands as the only member of the genus Hepevirus in the family Hepeviridaea, a plethora of genotypes were identified to date with four of particular clinical significance; genotypes 1 and 2 being confined to humans, while genotypes 3 and 4 can infect humans and animals largely via the fecal-oral route[173,174].
Clinical presentation
Most HEV infected patients are either asymptomatic or may develop mild, self-limited, HAV-like illness. Typically preceded by an incubation period ranging between 2 wk to 10 wk[175]. This is essentially true of HEV genotypes 1 and 2. However, the zoonotic genotypes HEV 3 and 4 have been increasingly reported as causes of chronic hepatitis almost exclusively in those who are immunocompromised[176,177]. Less than 5% of acutely infected HEV patients will progress to acute liver failure and those patients are more likely to be pregnant, malnourished, or have a preexisting liver condition[178,179].
Although a uniform diagnostic approach for HEV infection is limited by the lack of standardized antibody assays, patients with consistent clinical presentation should be tested for anti-HEV IgM antibody assay with a confirmatory HEV RNA viral load if positive.
Currently available treatments
Similar to HAV infection, the majority of patients with acute HEV infection require no specific treatment and the management is mostly supportive. However, patients who develop acute liver failure may warrant liver transplantation[180]. A rising body of literature investigated the role of ribavirin in treating chronic HEV infection in immunosuppressed patients with variable success[181]. Whenever possible, treatment should be accompanied by reducing immunosuppressive therapy and followed by testing for clearance to confirm eradication[182]. A 3-mo course of ribavirin has been proposed for the above indication, while its use in acute fulminant hepatitis appear promising[183], more robust evidence is yet awaited.
Prevention remains the most effective approach to combat HEV-related disease. This can be achieved by maintaining quality standards for public water supplies and maintaining hygienic practices as per the WHO recommendation[175].
In 2011, a recombinant vaccine to prevent HEV infection was registered in China. Although it is not approved yet in other countries, few studies have emerged to evaluate efficacy and safety of this vaccine. In a large, randomized trial from China including 112604 healthy adults that evaluated different doses of HEV vaccine compared to placebo, a protective effect of 100% was reported at 1 year following 30 d from the last dose received. Another trial revealed a protective efficacy of the vaccine against HEV to be 86.8% after 4.5 years of the first vaccination[184]. Other preventive options such as immune globulin remain experimental[185].
Ongoing research and future directions
For chronic HEV patients who fail ribavirin therapy, some alternatives such as PEG-interferon[186] and sofosbuvir are being considered pending more evidence[187]. Their routine use is being hindered by the lack of safety and efficacy profile in this setting besides the perceived higher risk of acute rejection with PEG-interferon due to its immunostimulatory effect in organ transplant recipients.
Role of liver transplant
Although liver transplant recipients may develop chronic HEV in the setting of prolonged immunosuppression, acute HEV infection may rarely result in acute liver failure necessitating liver transplant. Accumulating epidemiologic evidence described significant heterogeneity in HEV infection prevalence and outcomes. For example, HEV remains the most common cause of acute viral hepatitis and acute liver failure (up to 44%) in some Asian countries such as India and Bangladesh[187,188]. This contrasts with Western Europe and the United States where it is a rare cause of acute liver failure[189].
CONCLUSION
The recognition of the epidemiological and clinical features of hepatitis A, B, C, D and E is crucial to guide the diagnosis of these conditions. Fortunately, the medical advances in the last centuries have allowed to effectively diagnose them and comprehensively establish its multisystemic impact in this population. With the advent of new therapies, the possibility of achieving control of the disease is a reality, while reaching the cure and eradication of chronic hepatitis B, C and D is around the corner.
Footnotes
Conflict-of-interest statement: None of the authors have any compelling conflict of interest.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology, No. 46861; American Society for Gastrointestinal Endoscopy, No. 164369; and American Gastroenterological Association.
Peer-review started: January 25, 2021
First decision: February 27, 2021
Article in press: April 9, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng H, Shayesteh AA S-Editor: Gao CC L-Editor: A P-Editor: Liu JH
Contributor Information
Daniel Castaneda, Digestive Disease Institute, Cleveland Clinic Florida, Weston, FL 33331, United States. daniel.castaneda.m@gmail.com.
Adalberto Jose Gonzalez, Digestive Disease Institute, Cleveland Clinic Florida, Weston, FL 33331, United States.
Mohammad Alomari, Digestive Disease Institute, Cleveland Clinic Florida, Weston, FL 33331, United States.
Kanwarpreet Tandon, Digestive Disease Institute, Cleveland Clinic Florida, Weston, FL 33331, United States.
Xaralambos Bobby Zervos, Transplant Department, Cleveland Clinic Florida, Weston, FL 33331, United States.
References
- 1.Fonseca JC. [History of viral hepatitis] Rev Soc Bras Med Trop. 2010;43:322–330. doi: 10.1590/s0037-86822010000300022. [DOI] [PubMed] [Google Scholar]
- 2.Martin NA. The discovery of viral hepatitis: a military perspective. J R Army Med Corps. 2003;149:121–124. doi: 10.1136/jramc-149-02-04. [DOI] [PubMed] [Google Scholar]
- 3.Koff RS. Feinstone SM, Kapikian AZ, Purcell RH. Hepatitis A: detection by immune electron microscopy of a virus like antigen associated with acute illness [Science 1973;182:1026-1028] J Hepatol. 2002;37:2–6. doi: 10.1016/s0168-8278(02)00169-1. [DOI] [PubMed] [Google Scholar]
- 4.Melnick JL. Properties and classification of hepatitis A virus. Vaccine. 1992;10 Suppl 1:S24–S26. doi: 10.1016/0264-410x(92)90536-s. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Hepatitis A. [cited 19 January 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-a .
- 6.Wasley A, Fiore A, Bell BP. Hepatitis A in the era of vaccination. Epidemiol Rev. 2006;28:101–111. doi: 10.1093/epirev/mxj012. [DOI] [PubMed] [Google Scholar]
- 7.Foster MA, Hofmeister MG, Kupronis BA, Lin Y, Xia GL, Yin S, Teshale E. Increase in Hepatitis A Virus Infections - United States, 2013-2018. MMWR Morb Mortal Wkly Rep. 2019;68:413–415. doi: 10.15585/mmwr.mm6818a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooksley WG. What did we learn from the Shanghai hepatitis A epidemic? J Viral Hepat. 2000;7 Suppl 1:1–3. doi: 10.1046/j.1365-2893.2000.00021.x. [DOI] [PubMed] [Google Scholar]
- 9.Halliday ML, Kang LY, Zhou TK, Hu MD, Pan QC, Fu TY, Huang YS, Hu SL. An epidemic of hepatitis A attributable to the ingestion of raw clams in Shanghai, China. J Infect Dis. 1991;164:852–859. doi: 10.1093/infdis/164.5.852. [DOI] [PubMed] [Google Scholar]
- 10.Foster M, Ramachandran S, Myatt K, Donovan D, Bohm S, Fiedler J, Barbeau B, Collins J, Thoroughman D, McDonald E, Ballard J, Eason J, Jorgensen C. Hepatitis A Virus Outbreaks Associated with Drug Use and Homelessness - California, Kentucky, Michigan, and Utah, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:1208–1210. doi: 10.15585/mmwr.mm6743a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Widespread person-to-person outbreaks of hepatitis A across the United States. [cited 28 July 2020]. In: Centers for Disease Control and Prevention [Internet]. Available from: https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm .
- 12.European Centre for Disease Prevention and Control. Epidemiological update: Hepatitis A outbreak in the EU/EEA mostly affecting men who have sex with men. [cited 28 July 2020]. In: European Centre for Disease Prevention and Control [Internet]. https://www.ecdc.europa.eu/en/news-events/epidemiological-update-hepatitis-outbreak-eueea-mostly-affecting-men-who-have-sex-men-2 .
- 13.Manka P, Verheyen J, Gerken G, Canbay A. Liver Failure due to Acute Viral Hepatitis (A-E) Visc Med. 2016;32:80–85. doi: 10.1159/000444915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baba M, Hasegawa H, Nakayabu M, Fukai K, Suzuki S. Cytolytic activity of natural killer cells and lymphokine activated killer cells against hepatitis A virus infected fibroblasts. J Clin Lab Immunol. 1993;40:47–60. [PubMed] [Google Scholar]
- 15.Fleischer B, Fleischer S, Maier K, Wiedmann KH, Sacher M, Thaler H, Vallbracht A. Clonal analysis of infiltrating T lymphocytes in liver tissue in viral hepatitis A. Immunology. 1990;69:14–19. [PMC free article] [PubMed] [Google Scholar]
- 16.Vallbracht A, Fleischer B, Busch FW. Hepatitis A: hepatotropism and influence on myelopoiesis. Intervirology. 1993;35:133–139. doi: 10.1159/000150304. [DOI] [PubMed] [Google Scholar]
- 17.Richardson M, Elliman D, Maguire H, Simpson J, Nicoll A. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J. 2001;20:380–391. doi: 10.1097/00006454-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Mackinney-Novelo I, Barahona-Garrido J, Castillo-Albarran F, Santiago-Hernández JJ, Méndez-Sánchez N, Uribe M, Chávez-Tapia N. Clinical course and management of acute hepatitis A infection in adults. Ann Hepatol. 2012;11:652–657. [PubMed] [Google Scholar]
- 19.Gordon SC, Reddy KR, Schiff L, Schiff ER. Prolonged intrahepatic cholestasis secondary to acute hepatitis A. Ann Intern Med. 1984;101:635–637. doi: 10.7326/0003-4819-101-5-635. [DOI] [PubMed] [Google Scholar]
- 20.Matheny SC, Kingery JE. Hepatitis A. Am Fam Physician. 2012;86:1027–34; quiz 1010. [PubMed] [Google Scholar]
- 21.Tagle Arrospide M, León Barúa R. [Viral hepatitis A as a triggering agent of autoimmune hepatitis report of a case and review of literature] Rev Gastroenterol Peru. 2003;23:134–137. [PubMed] [Google Scholar]
- 22.Koff RS. Clinical manifestations and diagnosis of hepatitis A virus infection. Vaccine. 1992;10 Suppl 1:S15–S17. doi: 10.1016/0264-410x(92)90533-p. [DOI] [PubMed] [Google Scholar]
- 23.Tong MJ, el-Farra NS, Grew MI. Clinical manifestations of hepatitis A: recent experience in a community teaching hospital. J Infect Dis. 1995;171 Suppl 1:S15–S18. doi: 10.1093/infdis/171.supplement_1.s15. [DOI] [PubMed] [Google Scholar]
- 24.Carrion AF, Martin P. Viral hepatitis in the elderly. Am J Gastroenterol. 2012;107:691–697. doi: 10.1038/ajg.2012.7. [DOI] [PubMed] [Google Scholar]
- 25.Ponnappan S, Ponnappan U. Aging and immune function: molecular mechanisms to interventions. Antioxid Redox Signal. 2011;14:1551–1585. doi: 10.1089/ars.2010.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Center for Health Statistics. Health Data Interactive. [cited 30 December 2020]. In: Centers for Disease Control and Prevention [Internet]. In: Available from: https://www.cdc.gov/nchs/hdi.htm .
- 27.Liaw YF, Yang CY, Chu CM, Huang MJ. Appearance and persistence of hepatitis A IgM antibody in acute clinical hepatitis A observed in an outbreak. Infection. 1986;14:156–158. doi: 10.1007/BF01645253. [DOI] [PubMed] [Google Scholar]
- 28.Lemon SM, Binn LN, Marchwicki R, Murphy PC, Ping LH, Jansen RW, Asher LV, Stapleton JT, Taylor DG, LeDuc JW. In vivo replication and reversion to wild type of a neutralization-resistant antigenic variant of hepatitis A virus. J Infect Dis. 1990;161:7–13. doi: 10.1093/infdis/161.1.7. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Positive test results for acute hepatitis A virus infection among persons with no recent history of acute hepatitis--United States, 2002-2004. MMWR Morb Mortal Wkly Rep. 2005;54:453–456. [PubMed] [Google Scholar]
- 30.Kryger P, Christoffersen P. Liver histopathology of the hepatitis A virus infection: a comparison with hepatitis type B and non-a, non-b. J Clin Pathol. 1983;36:650–654. doi: 10.1136/jcp.36.6.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werzberger A, Kuter B, Shouval D, Mensch B, Brown L, Wiens B, Lewis J, Miller W, Sitrin R, Provost P. Anatomy of a trial: a historical view of the Monroe inactivated hepatitis A protective efficacy trial. J Hepatol. 1993;18 Suppl 2:S46–S50. doi: 10.1016/s0168-8278(05)80378-2. [DOI] [PubMed] [Google Scholar]
- 32.Prevention of hepatitis A through active or passive immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1996;45:1–30. [PubMed] [Google Scholar]
- 33.Advisory Committee on Immunization Practices (ACIP) Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55:1–23. [PubMed] [Google Scholar]
- 34.Doshani M, Weng M, Moore KL, Romero JR, Nelson NP. Recommendations of the Advisory Committee on Immunization Practices for Use of Hepatitis A Vaccine for Persons Experiencing Homelessness. MMWR Morb Mortal Wkly Rep. 2019;68:153–156. doi: 10.15585/mmwr.mm6806a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Hepatitis A Questions and Answers for Health Professionals. [cited 28 July 2020]. In: Centers for Disease Control and Prevention [Internet]. Available from: https://www.cdc.gov/hepatitis/hav/havfaq.htm#vaccine .
- 36.Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kolasa M. National, State, and Selected Local Area Vaccination Coverage Among Children Aged 19-35 Months - United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:889–896. doi: 10.15585/mmwr.mm6433a1. [DOI] [PubMed] [Google Scholar]
- 37.Taylor RM, Davern T, Munoz S, Han SH, McGuire B, Larson AM, Hynan L, Lee WM, Fontana RJ US Acute Liver Failure Study Group. Fulminant hepatitis A virus infection in the United States: Incidence, prognosis, and outcomes. Hepatology. 2006;44:1589–1597. doi: 10.1002/hep.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung DH, Hwang S, Lim YS, Kim KH, Ahn CS, Moon DB, Ha TY, Song GW, Park GC, Lee SG. Outcome comparison of liver transplantation for hepatitis A-related vs hepatitis B-related acute liver failure in adult recipients. Clin Transplant. 2018;32 doi: 10.1111/ctr.13140. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. Global hepatitis report, 2017. [cited 19 January 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/publications/i/item/global-hepatitis-report-2017 .
- 40.Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the Global Burden of Chronic Liver Diseases From 2012 to 2017: The Growing Impact of NAFLD. Hepatology. 2020;72:1605–1616. doi: 10.1002/hep.31173. [DOI] [PubMed] [Google Scholar]
- 41.Velkov S, Ott JJ, Protzer U, Michler T. The Global Hepatitis B Virus Genotype Distribution Approximated from Available Genotyping Data. Genes (Basel) 2018;9 doi: 10.3390/genes9100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meireles LC, Marinho RT, Van Damme P. Three decades of hepatitis B control with vaccination. World J Hepatol. 2015;7:2127–2132. doi: 10.4254/wjh.v7.i18.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Busch MP, Bloch EM, Kleinman S. Prevention of transfusion-transmitted infections. Blood. 2019;133:1854–1864. doi: 10.1182/blood-2018-11-833996. [DOI] [PubMed] [Google Scholar]
- 44.Jafari S, Buxton JA, Afshar K, Copes R, Baharlou S. Tattooing and risk of hepatitis B: a systematic review and meta-analysis. Can J Public Health. 2012;103:207–212. doi: 10.1007/BF03403814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bixler D, Annambholta P, Abara WE, Collier MG, Jones J, Mixson-Hayden T, Basavaraju SV, Ramachandran S, Kamili S, Moorman A. Hepatitis B and C virus infections transmitted through organ transplantation investigated by CDC, United States, 2014-2017. Am J Transplant. 2019;19:2570–2582. doi: 10.1111/ajt.15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scaglioni PP, Melegari M, Wands JR. Recent advances in the molecular biology of hepatitis B virus. Baillieres Clin Gastroenterol. 1996;10:207–225. doi: 10.1016/s0950-3528(96)90003-2. [DOI] [PubMed] [Google Scholar]
- 47.Rybicka M, Bielawski KP. Recent Advances in Understanding, Diagnosing, and Treating Hepatitis B Virus Infection. Microorganisms. 2020;8 doi: 10.3390/microorganisms8091416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Revill PA, Tu T, Netter HJ, Yuen LKW, Locarnini SA, Littlejohn M. The evolution and clinical impact of hepatitis B virus genome diversity. Nat Rev Gastroenterol Hepatol. 2020;17:618–634. doi: 10.1038/s41575-020-0296-6. [DOI] [PubMed] [Google Scholar]
- 49.Liaw YF, Tsai SL, Sheen IS, Chao M, Yeh CT, Hsieh SY, Chu CM. Clinical and virological course of chronic hepatitis B virus infection with hepatitis C and D virus markers. Am J Gastroenterol. 1998;93:354–359. doi: 10.1111/j.1572-0241.1998.00354.x. [DOI] [PubMed] [Google Scholar]
- 50.Jindal A, Kumar M, Sarin SK. Management of acute hepatitis B and reactivation of hepatitis B. Liver Int. 2013;33 Suppl 1:164–175. doi: 10.1111/liv.12081. [DOI] [PubMed] [Google Scholar]
- 51.Chu CM, Liaw YF. Chronic hepatitis B virus infection acquired in childhood: special emphasis on prognostic and therapeutic implication of delayed HBeAg seroconversion. J Viral Hepat. 2007;14:147–152. doi: 10.1111/j.1365-2893.2006.00810.x. [DOI] [PubMed] [Google Scholar]
- 52.Yan ZH, Fan Y, Wang XH, Mao Q, Deng GH, Wang YM. Relationship between HLA-DR gene polymorphisms and outcomes of hepatitis B viral infections: a meta-analysis. World J Gastroenterol. 2012;18:3119–3128. doi: 10.3748/wjg.v18.i24.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang ML, Liaw YF. Hepatitis B flares in chronic hepatitis B: pathogenesis, natural course, and management. J Hepatol. 2014;61:1407–1417. doi: 10.1016/j.jhep.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 54.Lapointe-Shaw L, Chung H, Holder L, Kwong JC, Sander B, Austin PC, Janssen HLA, Feld JJ. Diagnosis of Chronic Hepatitis B Peri-Complication: Risk factors and Trends over Time. Hepatology. 2020 doi: 10.1002/hep.31557. [DOI] [PubMed] [Google Scholar]
- 55.Croagh CM, Lubel JS. Natural history of chronic hepatitis B: phases in a complex relationship. World J Gastroenterol. 2014;20:10395–10404. doi: 10.3748/wjg.v20.i30.10395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prasidthrathsint K, Stapleton JT. Laboratory Diagnosis and Monitoring of Viral Hepatitis. Gastroenterol Clin North Am. 2019;48:259–279. doi: 10.1016/j.gtc.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seo SI, Kim HS, Yang BK, Kang JG, Shin WG, Lee JH, Kim HY, Jang MK. Predictive factors for risk of hepatocellular carcinoma in immune inactive chronic hepatitis B. Clin Res Hepatol Gastroenterol. 2020;44:711–717. doi: 10.1016/j.clinre.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 58.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 59.Chan HL, Hui AY, Wong ML, Tse AM, Hung LC, Wong VW, Sung JJ. Genotype C hepatitis B virus infection is associated with an increased risk of hepatocellular carcinoma. Gut. 2004;53:1494–1498. doi: 10.1136/gut.2003.033324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jeng WJ, Lok AS. Should Treatment Indications for Chronic Hepatitis B Be Expanded? Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.04.091. [DOI] [PubMed] [Google Scholar]
- 61.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 64.Tao Y, Wu D, Zhou L, Chen E, Liu C, Tang X, Jiang W, Han N, Li H, Tang H. Present and Future Therapies for Chronic Hepatitis B. Adv Exp Med Biol. 2020;1179:137–186. doi: 10.1007/978-981-13-9151-4_6. [DOI] [PubMed] [Google Scholar]
- 65.Jothimani D, Venugopal R, Vij M, Rela M. Post liver transplant recurrent and de novo viral infections. Best Pract Res Clin Gastroenterol. 2020;46-47:101689. doi: 10.1016/j.bpg.2020.101689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park JS, Gayam V, Pan CQ. Review article: preventing hepatitis B graft infection in hepatitis B patients after liver transplantation: immunoglobulin vs anti-virals. Aliment Pharmacol Ther. 2020;52:944–954. doi: 10.1111/apt.15999. [DOI] [PubMed] [Google Scholar]
- 67.Volz T, Allweiss L, Ben MBarek M, Warlich M, Lohse AW, Pollok JM, Alexandrov A, Urban S, Petersen J, Lütgehetmann M, Dandri M. The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus. J Hepatol. 2013;58:861–867. doi: 10.1016/j.jhep.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 68.Blank A, Markert C, Hohmann N, Carls A, Mikus G, Lehr T, Alexandrov A, Haag M, Schwab M, Urban S, Haefeli WE. First-in-human application of the novel hepatitis B and hepatitis D virus entry inhibitor myrcludex B. J Hepatol. 2016;65:483–489. doi: 10.1016/j.jhep.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 69.Weldemeyer H, Shoneweis L, Bogomolov P, Voronka N, Stepanova T, Bremer B, Allweiss L, Dandri M, Burhenne J, Haefeli WE, Ciesek S, Dittmer U, Alexandrov A, Urban S. GS-13-Final results of a multicenter open lebel phase 2 clinical trial (MYR203) to assess safety and efficacy of Myrcludex B with Peg interferon a-2a in Patients with Chronic Hepatitis HBV/HDV Co-Infection. J Hepatol . 2019;70:e81. [Google Scholar]
- 70.Shimura S, Watashi K, Fukano K, Peel M, Sluder A, Kawai F, Iwamoto M, Tsukuda S, Takeuchi JS, Miyake T, Sugiyama M, Ogasawara Y, Park SY, Tanaka Y, Kusuhara H, Mizokami M, Sureau C, Wakita T. Cyclosporin derivatives inhibit hepatitis B virus entry without interfering with NTCP transporter activity. J Hepatol. 2017;66:685–692. doi: 10.1016/j.jhep.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Phillips S, Chokshi S, Chatterji U, Riva A, Bobardt M, Williams R, Gallay P, Naoumov NV. Alisporivir inhibition of hepatocyte cyclophilins reduces HBV replication and hepatitis B surface antigen production. Gastroenterology 2015; 148: 403-14. :e7. doi: 10.1053/j.gastro.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gallay P, Ure D, Bobardt M, Chatterji U, Ou J, Trepanier D, Foster R. The cyclophilin inhibitor CRV431 inhibits liver HBV DNA and HBsAg in transgenic mice. PLoS One. 2019;14:e0217433. doi: 10.1371/journal.pone.0217433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alexopoulou A, Vasilieva L, Karayiannis P. New Approaches to the Treatment of Chronic Hepatitis B. J Clin Med. 2020;9 doi: 10.3390/jcm9103187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yuen MF, Heo J, Kumada H, Suzuki F, Suzuki Y, Xie Q, Jia J, Karino Y, Hou J, Chayama K, Imamura M, Lao-Tan JY, Lim SG, Tanaka Y, Xie W, Yoon JH, Duan Z, Kurosaki M, Park SJ, Labio E, Kumar R, Kweon YO, Yim HJ, Cremer J, Elston R, Chen S, Davies M, Baptiste-Brown S, Han K, Campbell FM, Paff M, Theodore D .Results after 12 wk treatment of multiple doses of GSK3389404 in chronic hepatitis B subjects on stable nucleos(t)ide therapy in a phase 2a double-blind, placebo-controlled study. Hepatology . 2019;70:433A. [Google Scholar]
- 75.Gane E, Locarnini S, Lim TH, Strasser S, Sievert W, Cheng W, Thompson A, Given B, Schluep T, Hamilton J, Biermer M, Kalmeijer R, Beumont-Mauviel M, Lenz O, Cloherty G, Wong DKH, Schwabe C, Jackson K, Ferrari C, Lai CL, Gish RG, Yuen MF. Short-term treatment with RNA interference therapy, JNJ-3989, results in sustained hepatitis B surface antigen supression in patients with chronic hepatitis B receiving nucleos(t)ide analogue treatment. J Hepatol . 2020;73:S20. [Google Scholar]
- 76.Yuen MF, Heo J, Jang JW, Yoon JH, Kweon YO, Park SJ. Hepatitis B virus (HBV) surface antigen (HBsAg) inhibition with is 505358 in chronic hepatitis B (CHB) patients on stable nucleos (t)ide analogue (NA) regimen and in NA -naive CHB patients: phase 2a, randomized, double-blind, placebo-controlled study. J Hepatol . 2020;73:S49–S50. [Google Scholar]
- 77.Bazinet M, Pântea V, Cebotarescu V, Cojuhari L, Jimbei P, Albrecht J, Schmid P, Le Gal F, Gordien E, Krawczyk A, Mijočević H, Karimzadeh H, Roggendorf M, Vaillant A. Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus co-infection (REP 301 and REP 301-LTF): a non-randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2017;2:877–889. doi: 10.1016/S2468-1253(17)30288-1. [DOI] [PubMed] [Google Scholar]
- 78.Bazinet M, Pantea V, Placinta G, Moscalu I, Cebotarescu V, Cojuhari L, Jimbei P, Iarovoi L, Smesnoi V, Musteata T, Jucov A, Krawczyk A, Vaillant A. FRI-210 Establishment of high rates of functional cure of HBeAg negative chronic HBV infection with REP 2139-Mg based combination therapy: Ongoing follow-up results from the REP 401 study. J Hepatol . 2019;70:E486. [Google Scholar]
- 79.Ahn SH, Kim W, Jung YK, Yang JM, Jang JY, Kweon YO, Cho YK, Kim YJ, Hong GY, Kim DJ, Um SH, Sohn JH, Lee JW, Park SJ, Lee BS, Kim JH, Kim HS, Yoon SK, Kim MY, Yim HJ, Lee KS, Lim YS, Lee WS, Park NH, Jin SY, Kim KH, Choi W, Han KH. Efficacy and Safety of Besifovir Dipivoxil Maleate Compared With Tenofovir Disoproxil Fumarate in Treatment of Chronic Hepatitis B Virus Infection. Clin Gastroenterol Hepatol 2019; 17: 1850-1859. :e4. doi: 10.1016/j.cgh.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 80.Gane E, Verdon DJ, Brooks AE, Gaggar A, Nguyen AH, Subramanian GM, Schwabe C, Dunbar PR. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study. J Hepatol. 2019;71:900–907. doi: 10.1016/j.jhep.2019.06.028. [DOI] [PubMed] [Google Scholar]
- 81.Boni C, Vecchi A, Rossi M, Laccabue D, Giuberti T, Alfieri A, Lampertico P, Grossi G, Facchetti F, Brunetto MR, Coco B, Cavallone D, Mangia A, Santoro R, Piazzolla V, Lau A, Gaggar A, Subramanian GM, Ferrari C. TLR7 Agonist Increases Responses of Hepatitis B Virus-Specific T Cells and Natural Killer Cells in Patients With Chronic Hepatitis B Treated With Nucleos(T)Ide Analogues. Gastroenterology 2018; 154: 1764-1777. :e7. doi: 10.1053/j.gastro.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 82.Lin CL, Kao JH. Hepatitis B: Immunization and Impact on Natural History and Cancer Incidence. Gastroenterol Clin North Am. 2020;49:201–214. doi: 10.1016/j.gtc.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 83.Fernández G, L Sanchez A, Jerez E, E Anillo L, Freyre F, A Aguiar J, Leon Y, Cinza Z, A Diaz P, Figueroa N, Muzio V, G Nieto G, Lobaina Y, Aguilar A, Penton E, C Aguilar J. Five-year Follow-up of Chronic Hepatitis B Patients Immunized by Nasal Route with the Therapeutic Vaccine HeberNasvac. Euroasian J Hepatogastroenterol. 2018;8:133–139. doi: 10.5005/jp-journals-10018-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.World Health Organization. Web Annex B. WHO estimates of the prevalence and incidence of hepatitis C virus infection by WHO region, 2015. [cited 19 January 2021]. In: World Health Organization [Internet]. Available from: https://apps.who.int/iris/bitstream/handle/10665/277005/WHO-CDS-HIV-18.46-eng.pdf .
- 85.Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 86.Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, Simmonds P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318–327. doi: 10.1002/hep.26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45–S57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 88.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 89.Murphy EL, Bryzman SM, Glynn SA, Ameti DI, Thomson RA, Williams AE, Nass CC, Ownby HE, Schreiber GB, Kong F, Neal KR, Nemo GJ. Risk factors for hepatitis C virus infection in United States blood donors. Hepatology. 2000;31:756–762. doi: 10.1002/hep.510310329. [DOI] [PubMed] [Google Scholar]
- 90.Irshad M, Mankotia DS, Irshad K. An insight into the diagnosis and pathogenesis of hepatitis C virus infection. World J Gastroenterol. 2013;19:7896–7909. doi: 10.3748/wjg.v19.i44.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zeisel MB, Felmlee DJ, Baumert TF. Hepatitis C virus entry. Curr Top Microbiol Immunol. 2013;369:87–112. doi: 10.1007/978-3-642-27340-7_4. [DOI] [PubMed] [Google Scholar]
- 92.Zein NN. Clinical significance of hepatitis C virus genotypes. Clin Microbiol Rev. 2000;13:223–235. doi: 10.1128/cmr.13.2.223-235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clément S, Pascarella S, Negro F. Hepatitis C virus infection: molecular pathways to steatosis, insulin resistance and oxidative stress. Viruses. 2009;1:126–143. doi: 10.3390/v1020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum HE, Pircher H, Thimme R. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6:e1000947. doi: 10.1371/journal.ppat.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dionisio N, Garcia-Mediavilla MV, Sanchez-Campos S, Majano PL, Benedicto I, Rosado JA, Salido GM, Gonzalez-Gallego J. Hepatitis C virus NS5A and core proteins induce oxidative stress-mediated calcium signalling alterations in hepatocytes. J Hepatol. 2009;50:872–882. doi: 10.1016/j.jhep.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 96.Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58–S68. doi: 10.1016/j.jhep.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 97.Evon DM, Stewart PW, Amador J, Serper M, Lok AS, Sterling RK, Sarkar S, Golin CE, Reeve BB, Nelson DR, Reau N, Lim JK, Reddy KR, Di Bisceglie AM, Fried MW . A comprehensive assessment of patient reported symptom burden, medical comorbidities, and functional well being in patients initiating direct acting antiviral therapy for chronic hepatitis C: Results from a large US multi-center observational study. PLoS One . 2018;13:e0196908. doi: 10.1371/journal.pone.0196908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Conry-Cantilena C, VanRaden M, Gibble J, Melpolder J, Shakil AO, Viladomiu L, Cheung L, DiBisceglie A, Hoofnagle J, Shih JW. Routes of infection, viremia, and liver disease in blood donors found to have hepatitis C virus infection. N Engl J Med. 1996;334:1691–1696. doi: 10.1056/NEJM199606273342602. [DOI] [PubMed] [Google Scholar]
- 99.Haber MM, West AB, Haber AD, Reuben A. Relationship of aminotransferases to liver histological status in chronic hepatitis C. Am J Gastroenterol. 1995;90:1250–1257. [PubMed] [Google Scholar]
- 100.Mosley JW, Operskalski EA, Tobler LH, Andrews WW, Phelps B, Dockter J, Giachetti C, Busch MP. Viral and host factors in early hepatitis C virus infection. Hepatology. 2005;42:86–92. doi: 10.1002/hep.20742. [DOI] [PubMed] [Google Scholar]
- 101.Seeff LB. Natural history of hepatitis C. Hepatology. 1997;26:21S–28S. doi: 10.1002/hep.510260704. [DOI] [PubMed] [Google Scholar]
- 102.Marcellin P. Hepatitis C: the clinical spectrum of the disease. J Hepatol. 1999;31 Suppl 1:9–16. doi: 10.1016/s0168-8278(99)80368-7. [DOI] [PubMed] [Google Scholar]
- 103.Loomba R, Rivera MM, McBurney R, Park Y, Haynes-Williams V, Rehermann B, Alter HJ, Herrine SK, Liang TJ, Hoofnagle JH, Heller T. The natural history of acute hepatitis C: clinical presentation, laboratory findings and treatment outcomes. Aliment Pharmacol Ther. 2011;33:559–565. doi: 10.1111/j.1365-2036.2010.04549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chu CM, Yeh CT, Liaw YF. Fulminant hepatic failure in acute hepatitis C: increased risk in chronic carriers of hepatitis B virus. Gut. 1999;45:613–617. doi: 10.1136/gut.45.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cacoub P, Renou C, Rosenthal E, Cohen P, Loury I, Loustaud-Ratti V, Yamamoto AM, Camproux AC, Hausfater P, Musset L, Veyssier P, Raguin G, Piette JC. Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. The GERMIVIC. Groupe d'Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l'Hepatite C. Medicine (Baltimore) 2000;79:47–56. doi: 10.1097/00005792-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 106.Grebely J, Raffa JD, Lai C, Krajden M, Conway B, Tyndall MW. Factors associated with spontaneous clearance of hepatitis C virus among illicit drug users. Can J Gastroenterol. 2007;21:447–451. doi: 10.1155/2007/796325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O'Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G, Goedert JJ, Kirk GD, Donfield SM, Rosen HR, Tobler LH, Busch MP, McHutchison JG, Goldstein DB, Carrington M. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–431. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 109.Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332:1463–1466. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 110.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 111.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huang YW, Yang SS, Fu SC, Wang TC, Hsu CK, Chen DS, Hu JT, Kao JH. Increased risk of cirrhosis and its decompensation in chronic hepatitis C patients with new-onset diabetes: a nationwide cohort study. Hepatology. 2014;60:807–814. doi: 10.1002/hep.27212. [DOI] [PubMed] [Google Scholar]
- 113.Marabita F, Aghemo A, De Nicola S, Rumi MG, Cheroni C, Scavelli R, Crimi M, Soffredini R, Abrignani S, De Francesco R, Colombo M. Genetic variation in the interleukin-28B gene is not associated with fibrosis progression in patients with chronic hepatitis C and known date of infection. Hepatology. 2011;54:1127–1134. doi: 10.1002/hep.24503. [DOI] [PubMed] [Google Scholar]
- 114.Wiley TE, Brown J, Chan J. Hepatitis C infection in African Americans: its natural history and histological progression. Am J Gastroenterol. 2002;97:700–706. doi: 10.1111/j.1572-0241.2002.05555.x. [DOI] [PubMed] [Google Scholar]
- 115.Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, Nevens F, Solinas A, Mura D, Brouwer JT, Thomas H, Njapoum C, Casarin C, Bonetti P, Fuschi P, Basho J, Tocco A, Bhalla A, Galassini R, Noventa F, Schalm SW, Realdi G. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–472. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 116.Hu KQ, Tong MJ. The long-term outcomes of patients with compensated hepatitis C virus-related cirrhosis and history of parenteral exposure in the United States. Hepatology. 1999;29:1311–1316. doi: 10.1002/hep.510290424. [DOI] [PubMed] [Google Scholar]
- 117.Lok AS, Seeff LB, Morgan TR, di Bisceglie AM, Sterling RK, Curto TM, Everson GT, Lindsay KL, Lee WM, Bonkovsky HL, Dienstag JL, Ghany MG, Morishima C, Goodman ZD HALT-C Trial Group. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–148. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tanaka Y, Kurbanov F, Mano S, Orito E, Vargas V, Esteban JI, Yuen MF, Lai CL, Kramvis A, Kew MC, Smuts HE, Netesov SV, Alter HJ, Mizokami M. Molecular tracing of the global hepatitis C virus epidemic predicts regional patterns of hepatocellular carcinoma mortality. Gastroenterology. 2006;130:703–714. doi: 10.1053/j.gastro.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 119.Alborino F, Burighel A, Tiller FW, van Helden J, Gabriel C, Raineri A, Catapano R, Stekel H. Multicenter evaluation of a fully automated third-generation anti-HCV antibody screening test with excellent sensitivity and specificity. Med Microbiol Immunol. 2011;200:77–83. doi: 10.1007/s00430-010-0171-0. [DOI] [PubMed] [Google Scholar]
- 120.Cobb B, Pockros PJ, Vilchez RA, Vierling JM. HCV RNA viral load assessments in the era of direct-acting antivirals. Am J Gastroenterol. 2013;108:471–475. doi: 10.1038/ajg.2012.248. [DOI] [PubMed] [Google Scholar]
- 121.Mederacke I, Wedemeyer H, Ciesek S, Steinmann E, Raupach R, Wursthorn K, Manns MP, Tillmann HL. Performance and clinical utility of a novel fully automated quantitative HCV-core antigen assay. J Clin Virol. 2009;46:210–215. doi: 10.1016/j.jcv.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 122.Singh S, Facciorusso A, Loomba R, Falck-Ytter YT. Magnitude and Kinetics of Decrease in Liver Stiffness After Antiviral Therapy in Patients With Chronic Hepatitis C: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2018; 16: 27-38. :e4. doi: 10.1016/j.cgh.2017.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hutchinson S, Valerio H, Dillon J, Fox R, Innes H, Weir A, Barclay S, Mcdonald S, Kennedy N, Fraser A, Stanley A, Bramley P, Hayes P, Goldberg D. Reduction in the incidence of hepatitis C related decompensated cirrhosis associated with national scale up of direct-acting antiviral therapies targeting patients with advanced liver fibrosis. J Hepatol. 2018;68:S67. [Google Scholar]
- 124.Backus LI, Belperio PS, Shahoumian TA, Mole LA. Impact of sustained virologic response with direct-acting antiviral treatment on mortality and hepatocellular carcinoma. Hepatology. 2017;66:46A. doi: 10.1002/hep.29408. [DOI] [PubMed] [Google Scholar]
- 125.Kim KR, Osinusi A, Mannalithara A, Aguilar R, Brainard D. Survival benefit of direct acting antiviral therapy in patients with decompensated cirrhosis. J Hepatol. 2018;68:S84. [Google Scholar]
- 126.Dusheiko G, Wedemeyer H. New protease inhibitors and direct-acting antivirals for hepatitis C: interferon's long goodbye. Gut. 2012;61:1647–1652. doi: 10.1136/gutjnl-2012-302910. [DOI] [PubMed] [Google Scholar]
- 127.Thomas E, Ghany MG, Liang TJ. The application and mechanism of action of ribavirin in therapy of hepatitis C. Antivir Chem Chemother. 2012;23:1–12. doi: 10.3851/IMP2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Younossi ZM, Stepanova M, Ong J, Trimble G, AlQahtani S, Younossi I, Ahmed A, Racila A, Henry L. Nonalcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States. Clin Gastroenterol Hepatol 2021; 19: 580-589. :e5. doi: 10.1016/j.cgh.2020.05.064. [DOI] [PubMed] [Google Scholar]
- 129.Cholankeril G, Ahmed A. Alcoholic Liver Disease Replaces Hepatitis C Virus Infection as the Leading Indication for Liver Transplantation in the United States. Clin Gastroenterol Hepatol. 2018;16:1356–1358. doi: 10.1016/j.cgh.2017.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dickson RC, Caldwell SH, Ishitani MB, Lau JY, Driscoll CJ, Stevenson WC, McCullough CS, Pruett TL. Clinical and histologic patterns of early graft failure due to recurrent hepatitis C in four patients after liver transplantation. Transplantation. 1996;61:701–705. doi: 10.1097/00007890-199603150-00005. [DOI] [PubMed] [Google Scholar]
- 131.Berenguer M. Natural history of recurrent hepatitis C. Liver Transpl . 2002;8:S14–S18. doi: 10.1053/jlts.2002.35781. [DOI] [PubMed] [Google Scholar]
- 132.Verna EC, Abdelmessih R, Salomao MA, Lefkowitch J, Moreira RK, Brown RS Jr. Cholestatic hepatitis C following liver transplantation: an outcome-based histological definition, clinical predictors, and prognosis. Liver Transpl. 2013;19:78–88. doi: 10.1002/lt.23559. [DOI] [PubMed] [Google Scholar]
- 133.Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889–896. doi: 10.1053/gast.2002.32418. [DOI] [PubMed] [Google Scholar]
- 134.Curry MP, Forns X, Chung RT, Terrault NA, Brown R Jr, Fenkel JM, Gordon F, O'Leary J, Kuo A, Schiano T, Everson G, Schiff E, Befeler A, Gane E, Saab S, McHutchison JG, Subramanian GM, Symonds WT, Denning J, McNair L, Arterburn S, Svarovskaia E, Moonka D, Afdhal N. Sofosbuvir and ribavirin prevent recurrence of HCV infection after liver transplantation: an open-label study. Gastroenterology 2015; 148: 100-107. :e1. doi: 10.1053/j.gastro.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 135.Verna EC. The dynamic landscape of liver transplant in the era of effective hepatitis C virus therapy. Hepatology. 2017;65:763–766. doi: 10.1002/hep.29054. [DOI] [PubMed] [Google Scholar]
- 136.Bowring MG, Kucirka LM, Massie AB, Luo X, Cameron A, Sulkowski M, Rakestraw K, Gurakar A, Kuo I, Segev DL, Durand CM. Changes in Utilization and Discard of Hepatitis C-Infected Donor Livers in the Recent Era. Am J Transplant. 2017;17:519–527. doi: 10.1111/ajt.13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kapila N, Menon KVN, Al-Khalloufi K, Vanatta JM, Murgas C, Reino D, Ebaid S, Shaw JJ, Agrawal N, Rhazouani S, Navas V, Sheffield C, Rahman AU, Castillo M, Lindenmeyer CC, Miller C, Quintini C, Zervos XB. Hepatitis C Virus NAT-Positive Solid Organ Allografts Transplanted Into Hepatitis C Virus-Negative Recipients: A Real-World Experience. Hepatology. 2020;72:32–41. doi: 10.1002/hep.31011. [DOI] [PubMed] [Google Scholar]
- 138.Cotter TG, Paul S, Sandıkçı B, Couri T, Bodzin AS, Little EC, Sundaram V, Charlton M. Increasing Utilization and Excellent Initial Outcomes Following Liver Transplant of Hepatitis C Virus (HCV)-Viremic Donors Into HCV-Negative Recipients: Outcomes Following Liver Transplant of HCV-Viremic Donors. Hepatology. 2019;69:2381–2395. doi: 10.1002/hep.30540. [DOI] [PubMed] [Google Scholar]
- 139.Bari K, Luckett K, Kaiser T, Diwan T, Cuffy M, Schoech MR, Safdar K, Blackard JT, Apewokin S, Paterno F, Sherman KE, Zucker SD, Anwar N, Shah SA. Hepatitis C transmission from seropositive, nonviremic donors to non-hepatitis C liver transplant recipients. Hepatology. 2018;67:1673–1682. doi: 10.1002/hep.29704. [DOI] [PubMed] [Google Scholar]
- 140.Kwong AJ, Wall A, Melcher M, Wang U, Ahmed A, Subramanian A, Kwo PY. Liver transplantation for hepatitis C virus (HCV) non-viremic recipients with HCV viremic donors. Am J Transplant. 2019;19:1380–1387. doi: 10.1111/ajt.15162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bethea E, Arvind A, Gustafson J, Andersson K, Pratt D, Bhan I, Thiim M, Corey K, Bloom P, Markmann J, Yeh H, Elias N, Kimura S, Dageforde LA, Cuenca A, Kawai T, Safa K, Williams W, Gilligan H, Sise M, Fishman J, Kotton C, Kim A, Rogers CC, Shao S, Cote M, Irwin L, Myoung P, Chung RT. Immediate administration of antiviral therapy after transplantation of hepatitis C-infected livers into uninfected recipients: Implications for therapeutic planning. Am J Transplant. 2020;20:1619–1628. doi: 10.1111/ajt.15768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Woolley AE, Singh SK, Goldberg HJ, Mallidi HR, Givertz MM, Mehra MR, Coppolino A, Kusztos AE, Johnson ME, Chen K, Haddad EA, Fanikos J, Harrington DP, Camp PC, Baden LR DONATE HCV Trial Team. Heart and Lung Transplants from HCV-Infected Donors to Uninfected Recipients. N Engl J Med. 2019;380:1606–1617. doi: 10.1056/NEJMoa1812406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Feld JJ, Cypel M, Kumar D, Dahari H, Pinto Ribeiro RV, Marks N, Kamkar N, Bahinskaya I, Onofrio FQ, Zahoor MA, Cerrochi O, Tinckam K, Kim SJ, Schiff J, Reichman TW, McDonald M, Alba C, Waddell TK, Sapisochin G, Selzner M, Keshavjee S, Janssen HLA, Hansen BE, Singer LG, Humar A. Short-course, direct-acting antivirals and ezetimibe to prevent HCV infection in recipients of organs from HCV-infected donors: a phase 3, single-centre, open-label study. Lancet Gastroenterol Hepatol. 2020;5:649–657. doi: 10.1016/S2468-1253(20)30081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kapila N, Al-Khalloufi K, Bejarano PA, Vanatta JM, Zervos XB. Fibrosing cholestatic hepatitis after kidney transplantation from HCV-viremic donors to HCV-negative recipients: A unique complication in the DAA era. Am J Transplant. 2020;20:600–605. doi: 10.1111/ajt.15583. [DOI] [PubMed] [Google Scholar]
- 145.Weinfurtner K, Reddy KR. Hepatitis C viraemic organs in solid organ transplantation. J Hepatol. 2021;74:716–733. doi: 10.1016/j.jhep.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 146.Khapra AP, Agarwal K, Fiel MI, Kontorinis N, Hossain S, Emre S, Schiano TD. Impact of donor age on survival and fibrosis progression in patients with hepatitis C undergoing liver transplantation using HCV+ allografts. Liver Transpl. 2006;12:1496–1503. doi: 10.1002/lt.20849. [DOI] [PubMed] [Google Scholar]
- 147.Potluri VS, Goldberg DS, Mohan S, Bloom RD, Sawinski D, Abt PL, Blumberg EA, Parikh CR, Sharpe J, Reddy KR, Molnar MZ, Sise M, Reese PP. National Trends in Utilization and 1-Year Outcomes with Transplantation of HCV-Viremic Kidneys. J Am Soc Nephrol. 2019;30:1939–1951. doi: 10.1681/ASN.2019050462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Swadling L, Capone S, Antrobus RD, Brown A, Richardson R, Newell EW, Halliday J, Kelly C, Bowen D, Fergusson J, Kurioka A, Ammendola V, Del Sorbo M, Grazioli F, Esposito ML, Siani L, Traboni C, Hill A, Colloca S, Davis M, Nicosia A, Cortese R, Folgori A, Klenerman P, Barnes E. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci Transl Med. 2014;6:261ra153. doi: 10.1126/scitranslmed.3009185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Masavuli MG, Wijesundara DK, Underwood A, Christiansen D, Earnest-Silveira L, Bull R, Torresi J, Gowans EJ, Grubor-Bauk B. A Hepatitis C Virus DNA Vaccine Encoding a Secreted, Oligomerized Form of Envelope Proteins Is Highly Immunogenic and Elicits Neutralizing Antibodies in Vaccinated Mice. Front Immunol. 2019;10:1145. doi: 10.3389/fimmu.2019.01145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Doshi RK, Ruben M, Drezner K, Lachmann A, Kuo I, Chanes-Mora P, Varga L, Saafir-Callaway B, Visconti A, Kharfen M. Knowledge, Attitudes, and Behaviors Related to Hepatitis C Screening and Treatment among Health Care Providers in Washington, DC. J Community Health. 2020;45:785–794. doi: 10.1007/s10900-020-00794-z. [DOI] [PubMed] [Google Scholar]
- 151.Farci P. Delta hepatitis: an update. J Hepatol. 2003;39 Suppl 1:S212–S219. doi: 10.1016/s0168-8278(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 152.Kucirka LM, Farzadegan H, Feld JJ, Mehta SH, Winters M, Glenn JS, Kirk GD, Segev DL, Nelson KE, Marks M, Heller T, Golub ET. Prevalence, correlates, and viral dynamics of hepatitis delta among injection drug users. J Infect Dis. 2010;202:845–852. doi: 10.1086/655808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rizzetto M, Canese MG, Aricò S, Crivelli O, Trepo C, Bonino F, Verme G. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut. 1977;18:997–1003. doi: 10.1136/gut.18.12.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rizzetto M, Smedile A. Pegylated interferon therapy of chronic hepatitis D: in need of revision. Hepatology. 2015;61:1109–1111. doi: 10.1002/hep.27585. [DOI] [PubMed] [Google Scholar]
- 155.Ponzetto A, Hoyer BH, Popper H, Engle R, Purcell RH, Gerin JL. Titration of the infectivity of hepatitis D virus in chimpanzees. J Infect Dis. 1987;155:72–78. doi: 10.1093/infdis/155.1.72. [DOI] [PubMed] [Google Scholar]
- 156.Govindarajan S, De Cock KM, Redeker AG. Natural course of delta superinfection in chronic hepatitis B virus-infected patients: histopathologic study with multiple liver biopsies. Hepatology. 1986;6:640–644. doi: 10.1002/hep.1840060415. [DOI] [PubMed] [Google Scholar]
- 157.Smedile A, Farci P, Verme G, Caredda F, Cargnel A, Caporaso N, Dentico P, Trepo C, Opolon P, Gimson A, Vergani D, Williams R, Rizzetto M. Influence of delta infection on severity of hepatitis B. Lancet. 1982;2:945–947. doi: 10.1016/s0140-6736(82)90156-8. [DOI] [PubMed] [Google Scholar]
- 158.Smedile A, Dentico P, Zanetti A, Sagnelli E, Nordenfelt E, Actis GC, Rizzetto M. Infection with the delta agent in chronic HBsAg carriers. Gastroenterology. 1981;81:992–997. [PubMed] [Google Scholar]
- 159.Su CW, Huang YH, Huo TI, Shih HH, Sheen IJ, Chen SW, Lee PC, Lee SD, Wu JC. Genotypes and viremia of hepatitis B and D viruses are associated with outcomes of chronic hepatitis D patients. Gastroenterology. 2006;130:1625–1635. doi: 10.1053/j.gastro.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 160.Bockmann JH, Grube M, Hamed V, von Felden J, Landahl J, Wehmeyer M, Giersch K, Hall MT, Murray JM, Dandri M, Lüth S, Lohse AW, Lütgehetmann M, Schulze Zur Wiesch J. High rates of cirrhosis and severe clinical events in patients with HBV/HDV co-infection: longitudinal analysis of a German cohort. BMC Gastroenterol. 2020;20:24. doi: 10.1186/s12876-020-1168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cole SM, Gowans EJ, Macnaughton TB, Hall PD, Burrell CJ. Direct evidence for cytotoxicity associated with expression of hepatitis delta virus antigen. Hepatology. 1991;13:845–851. [PubMed] [Google Scholar]
- 162.Guilhot S, Huang SN, Xia YP, La Monica N, Lai MM, Chisari FV. Expression of the hepatitis delta virus large and small antigens in transgenic mice. J Virol. 1994;68:1052–1058. doi: 10.1128/jvi.68.2.1052-1058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rizzetto M, Rosina F, Saracco G, Bellando PC, Actis GC, Bonino F, Smedile A, Trinchero P, Sansalvadore F, Pintus C. Treatment of chronic delta hepatitis with alpha-2 recombinant interferon. J Hepatol. 1986;3 Suppl 2:S229–S233. doi: 10.1016/s0168-8278(86)80125-8. [DOI] [PubMed] [Google Scholar]
- 164.Wedemeyer H, Yurdaydìn C, Dalekos GN, Erhardt A, Çakaloğlu Y, Değertekin H, Gürel S, Zeuzem S, Zachou K, Bozkaya H, Koch A, Bock T, Dienes HP, Manns MP HIDIT Study Group. Peginterferon plus adefovir vs either drug alone for hepatitis delta. N Engl J Med. 2011;364:322–331. doi: 10.1056/NEJMoa0912696. [DOI] [PubMed] [Google Scholar]
- 165.Abbas Z, Khan MA, Salih M, Jafri W. Interferon alpha for chronic hepatitis D. Cochrane Database Syst Rev. 2011:CD006002. doi: 10.1002/14651858.CD006002.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yurdaydin C, Keskin O, Kalkan Ç, Karakaya F, Çalişkan A, Karatayli E, Karatayli S, Bozdayi AM, Koh C, Heller T, Idilman R, Glenn JS. Optimizing lonafarnib treatment for the management of chronic delta hepatitis: The LOWR HDV-1 study. Hepatology . 2018;67:1224–1236. doi: 10.1002/hep.29658. [DOI] [PubMed] [Google Scholar]
- 167.Karayiannis P, Saldanha J, Jackson AM, Luther S, Goldin R, Monjardino J, Thomas HC. Partial control of hepatitis delta virus superinfection by immunisation of woodchucks (Marmota monax) with hepatitis delta antigen expressed by a recombinant vaccinia or baculovirus. J Med Virol. 1993;41:210–214. doi: 10.1002/jmv.1890410308. [DOI] [PubMed] [Google Scholar]
- 168.Ponzetto A, Eckart M, D'Urso N, Negro F, Silvestro M, Bonino F, Wang KS, Chien D, Choo QL, Houghton M. Towards a vaccine for the prevention of hepatitis delta virus superinfection in HBV carriers. Prog Clin Biol Res. 1993;382:207–210. [PubMed] [Google Scholar]
- 169.Serin A, Tokat Y. Recurrence of Hepatitis D Virus in Liver Transplant Recipients With Hepatitis B and D Virus-Related Chronic Liver Disease. Transplant Proc. 2019;51:2457–2460. doi: 10.1016/j.transproceed.2019.01.163. [DOI] [PubMed] [Google Scholar]
- 170.Kuniholm MH, Purcell RH, McQuillan GM, Engle RE, Wasley A, Nelson KE. Epidemiology of hepatitis E virus in the United States: results from the Third National Health and Nutrition Examination Survey, 1988-1994. J Infect Dis. 2009;200:48–56. doi: 10.1086/599319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cangin C, Focht B, Harris R, Strunk JA. Hepatitis E seroprevalence in the United States: Results for immunoglobulins IGG and IGM. J Med Virol. 2019;91:124–131. doi: 10.1002/jmv.25299. [DOI] [PubMed] [Google Scholar]
- 172.Gupta DN, Smetana HF. The histopathology of viral hepatitis as seen in the Delhi epidemic (1955-56) Indian J Med Res. 1957;45:101–113. [PubMed] [Google Scholar]
- 173.Xia J, Zeng H, Liu L, Zhang Y, Liu P, Geng J, Wang L, Zhuang H. Swine and rabbits are the main reservoirs of hepatitis E virus in China: detection of HEV RNA in feces of farmed and wild animals. Arch Virol. 2015;160:2791–2798. doi: 10.1007/s00705-015-2574-0. [DOI] [PubMed] [Google Scholar]
- 174.Andonov A, Robbins M, Borlang J, Cao J, Hatchette T, Stueck A, Deschambault Y, Murnaghan K, Varga J, Johnston L. Rat Hepatitis E Virus Linked to Severe Acute Hepatitis in an Immunocompetent Patient. J Infect Dis. 2019;220:951–955. doi: 10.1093/infdis/jiz025. [DOI] [PubMed] [Google Scholar]
- 175.World Health Organization. Hepatitis E. [cited 19 January 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e#:~:text=Symptoms,%20after%20onset%20of%20the%20disease .
- 176.Perumpail RB, Ahmed A, Higgins JP, So SK, Cochran JL, Drobeniuc J, Mixson-Hayden TR, Teo CG. Fatal Accelerated Cirrhosis after Imported HEV Genotype 4 Infection. Emerg Infect Dis. 2015;21:1679–1681. doi: 10.3201/eid2109.150300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Behrendt P, Steinmann E, Manns MP, Wedemeyer H. The impact of hepatitis E in the liver transplant setting. J Hepatol. 2014;61:1418–1429. doi: 10.1016/j.jhep.2014.08.047. [DOI] [PubMed] [Google Scholar]
- 178.Aggarwal R. Hepatitis E: clinical presentation in disease-endemic areas and diagnosis. Semin Liver Dis. 2013;33:30–40. doi: 10.1055/s-0033-1338112. [DOI] [PubMed] [Google Scholar]
- 179.Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, Wang H, Yang CL, Jiang HM, Cai JP, Wang YJ, Ai X, Hu YM, Tang Q, Yao X, Yan Q, Xian YL, Wu T, Li YM, Miao J, Ng MH, Shih JW, Xia NS. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376:895–902. doi: 10.1016/S0140-6736(10)61030-6. [DOI] [PubMed] [Google Scholar]
- 180.Wedemeyer H, Pischke S, Manns MP. Pathogenesis and treatment of hepatitis e virus infection. Gastroenterology 2012; 142: 1388-1397. :e1. doi: 10.1053/j.gastro.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 181.Dalton HR, Kamar N. Treatment of hepatitis E virus. Curr Opin Infect Dis. 2016;29:639–644. doi: 10.1097/QCO.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 182.Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, Dumortier J, Cannesson A, Cassuto-Viguier E, Thervet E, Conti F, Lebray P, Dalton HR, Santella R, Kanaan N, Essig M, Mousson C, Radenne S, Roque-Afonso AM, Izopet J, Rostaing L. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481–1489. doi: 10.1053/j.gastro.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 183.Bhatia V, Singhal A, Panda SK, Acharya SK. A 20-year single-center experience with acute liver failure during pregnancy: is the prognosis really worse? Hepatology. 2008;48:1577–1585. doi: 10.1002/hep.22493. [DOI] [PubMed] [Google Scholar]
- 184.Zhang J, Zhang XF, Huang SJ, Wu T, Hu YM, Wang ZZ, Wang H, Jiang HM, Wang YJ, Yan Q, Guo M, Liu XH, Li JX, Yang CL, Tang Q, Jiang RJ, Pan HR, Li YM, Shih JW, Ng MH, Zhu FC, Xia NS. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914–922. doi: 10.1056/NEJMoa1406011. [DOI] [PubMed] [Google Scholar]
- 185.Tsarev SA, Tsareva TS, Emerson SU, Govindarajan S, Shapiro M, Gerin JL, Purcell RH. Successful passive and active immunization of cynomolgus monkeys against hepatitis E. Proc Natl Acad Sci USA. 1994;91:10198–10202. doi: 10.1073/pnas.91.21.10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kamar N, Rostaing L, Abravanel F, Garrouste C, Esposito L, Cardeau-Desangles I, Mansuy JM, Selves J, Peron JM, Otal P, Muscari F, Izopet J. Pegylated interferon-alpha for treating chronic hepatitis E virus infection after liver transplantation. Clin Infect Dis. 2010;50:e30–e33. doi: 10.1086/650488. [DOI] [PubMed] [Google Scholar]
- 187.Dao Thi VL, Debing Y, Wu X, Rice CM, Neyts J, Moradpour D, Gouttenoire J. Sofosbuvir Inhibits Hepatitis E Virus Replication In Vitro and Results in an Additive Effect When Combined With Ribavirin. Gastroenterology 2016; 150: 82-85. :e4. doi: 10.1053/j.gastro.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 188.Khuroo MS, Kamili S. Aetiology and prognostic factors in acute liver failure in India. J Viral Hepat. 2003;10:224–231. doi: 10.1046/j.1365-2893.2003.00415.x. [DOI] [PubMed] [Google Scholar]
- 189.Fontana RJ, Engle RE, Scaglione S, Araya V, Shaikh O, Tillman H, Attar N, Purcell RH, Lee WM US Acute Liver Failure Study Group. The role of hepatitis E virus infection in adult Americans with acute liver failure. Hepatology. 2016;64:1870–1880. doi: 10.1002/hep.28649. [DOI] [PMC free article] [PubMed] [Google Scholar]