Significance
Keratinocyte carcinomas are the most common malignancies in fair-skinned patients. Increased age and sun exposure are important skin cancer risk factors and have been associated with a loss of DNA methylation in the human epidermis. Complete Dnmt3a deficiency in mouse skin creates a premalignant state, but the mechanisms responsible for this phenomenon are not clear. This study demonstrates that Dnmt3a deficiency in murine epidermis causes focal, canonical DNA hypomethylation and an increased proportion of cells with a proliferative gene expression signature. Understanding the mechanisms responsible for the premalignant state may be important for defining novel preventative or therapeutic strategies for skin cancers.
Keywords: DNMT3A, DNA methylation, epigenetic, epidermis, premalignant
Abstract
DNA hypomethylation is a feature of epidermal cells from aged and sun-exposed skin, but the mechanisms responsible for this methylation loss are not known. Dnmt3a is the dominant de novo DNA methyltransferase in the skin; while epidermal Dnmt3a deficiency creates a premalignant state in which keratinocytes are more easily transformed by topical mutagens, the conditions responsible for this increased susceptibility to transformation are not well understood. Using whole genome bisulfite sequencing, we identified a focal, canonical DNA hypomethylation phenotype in the epidermal cells of Dnmt3a-deficient mice. Single-cell transcriptomic analysis revealed an increased proportion of cells with a proliferative gene expression signature, while other populations in the skin were relatively unchanged. Although total DNMT3A deficiency has not been described in human disease states, rare patients with an overgrowth syndrome associated with behavioral abnormalities and an increased risk of cancer often have heterozygous, germline mutations in DNMT3A that reduce its function (Tatton-Brown Rahman syndrome [TBRS]). We evaluated the DNA methylation phenotype of the skin from a TBRS patient with a germline DNMT3AR882H mutation, which encodes a dominant-negative protein that reduces its methyltransferase function by ∼80%. We detected a focal, canonical hypomethylation phenotype that revealed considerable overlap with hypomethylated regions found in Dnmt3a-deficient mouse skin. Together, these data suggest that DNMT3A loss creates a premalignant epigenetic state associated with a hyperproliferative phenotype in the skin and further suggest that DNMT3A acts as a tumor suppressor in the skin.
Aging and sun exposure are the most important epidemiologic risk factors for the development of skin cancer in populations with lightly pigmented skin. Although there are no registries to accurately account for the incidence of nonmelanoma skin cancers, treatment utilization for keratinocyte carcinomas sharply increases with advancing age (1). The accumulation of genetic mutations from UV light exposure clearly contributes to age-dependent increases in skin cancer; however, sun-damaged skin can harbor a remarkable number of driver mutations while remaining clinically normal (2). This suggests that nonmutational factors may also influence skin cancer susceptibility. The contribution of epigenetic perturbations (including alterations in DNA methylation) to cancer susceptibility is incompletely understood (3, 4). Aged, sun-exposed epidermal skin cells in older people develop DNA methylation changes, compared to the sun-protected skin of younger individuals (5). It is not yet clear whether altered activity of DNA methyltransferases or demethylases, cellular population shifts, or changes in cellular signaling or other modifying factors are responsible for this change.
DNA methyltransferases are responsible for CpG methylation and comprise two functional groups: 1) the maintenance methyltransferase DNMT1, which copies methylation marks to the CpGs of newly synthesized DNA; and 2) the de novo methyltransferases DNMT3A and DNMT3B, which are responsible for modifying unmethylated cytidine residues in a CpG dinucleotide context as well as some aspects of maintenance methylation (6, 7). DNA methylation is important for normal skin development and function. Mice with Dnmt1 deficiency in skin develop progressive alopecia, resulting in >50% hair loss over a year, while DNMT1 knockdown in human keratinocytes causes premature differentiation (8, 9). Similarly, the knockdown of either DNMT3A or DNMT3B reduces the clonogenic potential of cultured human keratinocytes (10). Importantly, Dnmt3a (but not Dnmt3b) has tumor suppressor activity in the classic 7,12-dimethylbenz[a]anthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA) chemical carcinogenesis model, with shortened latency and a higher tumor burden in Dnmt3a-deficient skin (11); however, the precise epigenetic changes that contribute to the premalignant, susceptible state have not yet been clearly defined.
In humans, there is considerable evidence that alterations in DNA methylation machinery may play a role in skin cancer pathogenesis. Both precancerous actinic keratoses (AKs) and squamous cell carcinomas (SCCs) exhibit hypomethylation of CpGs in lamina-associated domains, hypermethylation of CpG islands, and increased DNMT3B expression (12). DNMT3A is expressed at lower levels in AKs and SCCs, compared to healthy skin (11). Finally, somatic DNMT3A mutations have been identified in 11% (33/304) of patients from three studies of basal cell carcinoma (BCC) and SCC (cbioportal.org); DNMT3B was mutated in 8% (12/146) of cases and DNMT1 in 15% (45/304) (13–15). Many of the mutations in DNMT3A in these tumors were localized to the methyltransferase domain, or were predicted to cause protein truncations that would be expected to disrupt its function and potentially cause haploinsufficiency.
Dnmt3a deficiency in murine bone marrow cells results in a focal, canonical DNA hypomethylation phenotype (16–18) that is associated with the development of hematopoietic malignancies (17, 19, 20). DNA hypomethylation in Dnmt3a-deficient marrow is reversible with DNMT3A reexpression, and remethylation occurs in an orderly fashion with predictable kinetics and high fidelity (18). Germline mutations in DNMT3A are strongly associated with an overgrowth syndrome (Tatton-Brown-Rahman syndrome [TBRS]) that is associated with a potentially increased risk of acute myeloid leukemia (AML) (21, 22). Finally, acquired mutations of DNMT3A in older individuals are the most common cause of hematopoietic clonal expansion, which is associated with an increased risk of AML development (23–26). Indeed, DNMT3A mutations are among the most common initiating mutations in adults with de novo AML; mutations at amino acid position R882 (most frequently R882H) represent the most common mutational hot spot in this gene (27).
In this study, we evaluate the premalignant epigenetic phenotype of Dnmt3a-deficient skin and identify changes that could potentially contribute to its role in increasing susceptibility to transformation. We then relate these findings to the skin methylation phenotype of a TBRS patient with a germline DNMT3AR882H mutation.
Results
Dnmt3a Deficiency in Epidermal Keratinocytes Results in a Focal, Canonical DNA Hypomethylation Phenotype.
Mice with homozygous Dnmt3a deficiency develop normally but die within 3 wk of birth (28). To study the effects of Dnmt3a deficiency in the skin, we therefore used transgenic mice expressing Cre recombinase under the control of the Keratin 14 (Krt14) promoter, which exhibits a well-established pattern of expression that affects the epidermal skin and its appendages (29). With appropriate crosses, we obtained mice that were wild type (WT) for Dnmt3a (Krt14-Cre− × Dnmt3aflox/flox, hereafter “Dnmt3aWT”), heterozygous (Krt14-Cre+ × Dnmt3aflox/+, hereafter “Dnmt3aHET”), or homozygous for the floxed allele (Krt14-Cre+ × Dnmt3aflox/flox, hereafter “Dnmt3aKO”). The floxing efficiency of the Dnmt3aflox/flox alleles in epidermal cells with Krt14-Cre was >90%, resulting in a complete loss of Dnmt3a protein expression (SI Appendix, Fig. S1A).
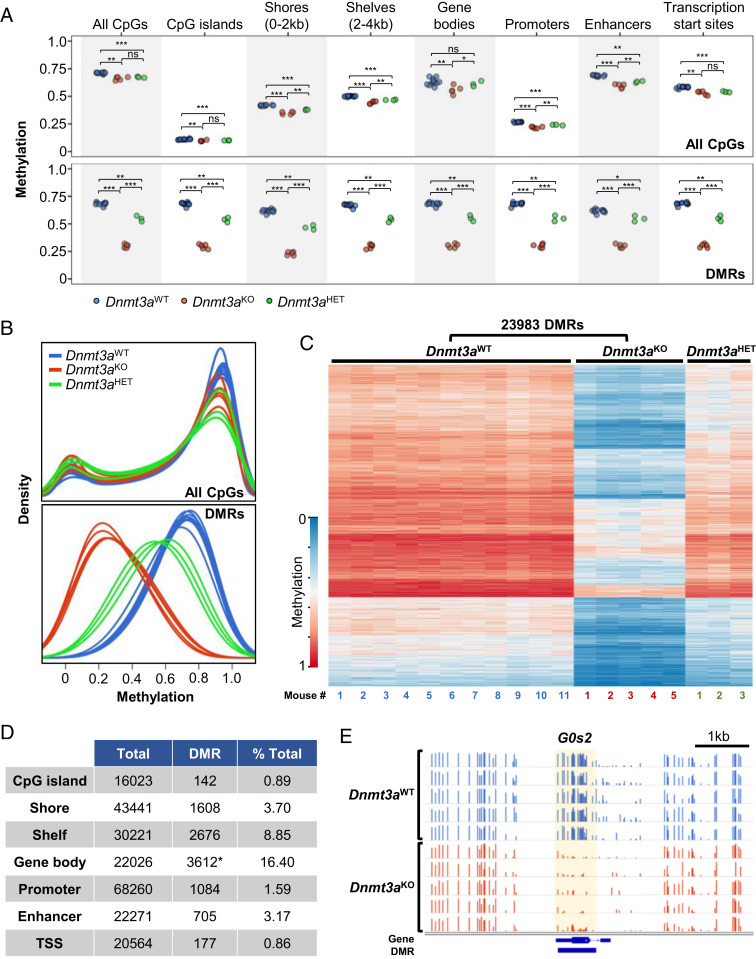
Whole genome bisulfite sequencing (WGBS) was used to assess DNA methylation of isolated murine epidermal cells. Dnmt3aKO mice exhibited a small but significant decrease in global DNA methylation in all annotated regions examined, including CpG islands, island shores, island shelves, gene bodies, promoters, enhancers, and transcriptional start sites (Fig. 1 A, Top). Loss of one allele of Dnmt3a (Dnmt3aHET) resulted in a statistically significant change in methylation from the Dnmt3aWT in all regions except gene bodies. On a global scale, the overall decrease in CpG methylation was very small, similar to what has been observed in hematopoietic tissues (Fig. 1 B, Top) (18, 30).
Fig. 1.
Dnmt3a deficiency results in focal, canonical CpG methylation loss in murine epidermal cells. (A) WGBS of isolated murine epidermal cells, comparing independent samples from 11 Dnmt3aWT vs. 5 Dnmt3aKO vs. 3 Dnmt3aHET mice. Dot plots represent average methylation values over annotated regions for all CpGs (Top) and for DMRs within each annotated region (Bottom). Significance was determined by Student’s t test with denotation of “ns” for not significant and levels of significance as * adjusted P < 0.05, **P < 0.01, ***P < 0.001. (B) Methylation density plots for the indicated genotypes across all genomic CpGs (Top) and at DMRs (Bottom). (C) Targeted deficiency of Dnmt3a (Krt14-Cre+ × Dnmt3afl/fl, Dnmt3aKO) results in 23,983 DMRs in epidermal cells compared to wild-type control, Dnmt3aWT. Heatmap representation of WGBS data demonstrates DMRs defined by differences between Dnmt3aWT vs. Dnmt3aKO epidermal cells. Heterozygosity for Dnmt3a (Krt14-Cre+ × Dnmt3afl/+, Dnmt3aHET) results in an intermediate phenotype. Each column represents an independent mouse. (D) Annotated regions associated with DMRs. *Of 16,315 DMRs involving gene bodies, 3,612 unique genes are involved. (E) Integrated genome viewer (IGV) view of the G0s2 gene CpG methylation of Dnmt3aWT versus Dnmt3aKO epidermal cells. Normalized methylated CpG reads are represented in bars (scale 0 to 1.0 for each row). Differentially methylated regions are indicated in the bottom track and highlighted in yellow.
We identified differentially methylated regions (DMRs) between Dnmt3aWT and Dnmt3aKO samples using previously described approaches (18, 30). DMRs were defined as having greater than 10 CpGs with a mean methylation difference of more than 0.2 (i.e., 20%) between Dnmt3aWT and Dnmt3aKO skin, with a false discovery rate (FDR) of less than 0.05. Adjacent DMRs within 50 base pairs were merged. Based on these criteria, we identified 23,983 DMRs with an average size of 0.95 kilobases (Kb), encompassing 23.53 megabases (Mb) of DNA, which represents 0.87% of the genome (Dataset S1). Virtually all DMRs were hypomethylated (23,981/23,983; 99.99%), and the degree of hypomethylation did not vary based on their location in annotated genomic regions (Fig. 1 A, Bottom). Skin from Dnmt3aHET mice demonstrated a more subtle methylation phenotype at these DMRs (Fig. 1 B, Bottom). The DMRs in Dnmt3a-deficient skin are represented by a heatmap, where each column represents an independent mouse, and each row, the mean methylation value of an individual DMR for all samples (Fig. 1C). There is remarkable consistency (i.e., canonicality) among the methylation phenotypes at each DMR among independent mice with the same genotypes. The methylation values of the Dnmt3aHET samples are plotted passively and exhibit an intermediate level of methylation at many DMRs (Fig. 1C), demonstrating that haploinsufficiency for Dnmt3a also has a methylation phenotype in the skin (Fig. 1 A and B).
Dnmt3a-dependent DMRs occur most commonly within gene bodies; 16.4% of all annotated protein-coding genes were found to have at least one DMR. DNA methylation within most CpG islands is low in Dnmt3aWT skin, as expected. A small fraction of these islands (142/16,923; 0.89%) exhibit significantly higher methylation than total CpG islands in Dnmt3aWT skin, and these rare islands generally become hypomethylated in Dnmt3aKO skin (Fig. 1 D and A, Bottom). These DMRs usually represent intragenic CpG islands, of which ∼65% are methylated in human embryonic stem cells (31). Indeed, 129/142 (91%) Dnmt3aKO DMRs associated with CpG islands are located within gene bodies. The shores and shelves flanking CpG islands exhibited greater proportions of DMRs (3.7% and 8.85%, respectively), while the methylation of transcriptional start sites (TSSs) was minimally affected by Dnmt3a deficiency (0.86%, Fig. 1D). The methylation pattern near the G0s2 gene is shown as an example of a Dnmt3aKO DMR that occurs in a gene body (Fig. 1E). This gene was found to be up-regulated in Dnmt3aKO skin tumors (11).
Dnmt3a Deficiency Results in a Distinct Transcriptional Phenotype.
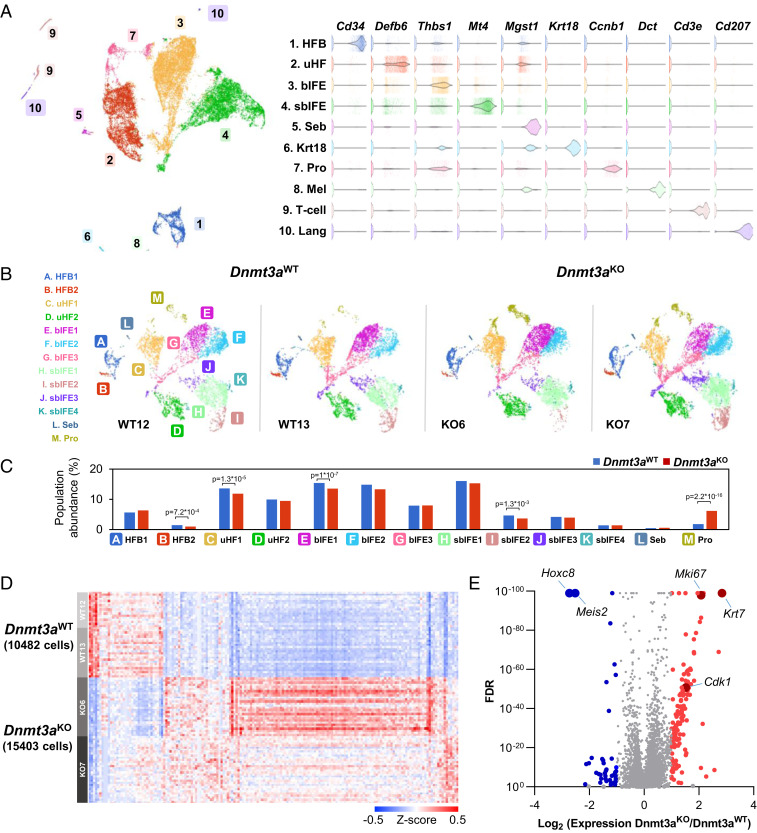
Dnmt3a deficiency does not result in any gross abnormalities in unperturbed skin (11). Further, flow cytometric evaluation of cellular populations within the skin revealed minimal differences between Dnmt3aWT and Dnmt3aKO (SI Appendix, Fig. S1B). To interrogate the transcriptomic consequences of Dnmt3a deficiency, we employed single-cell RNA sequencing to analyze adult murine epidermis in telogen (the resting phase of the hair cycle). We analyzed two mice from each genotype (Dnmt3aWT and Dnmt3aKO) at 9 to 10 wk of age; 10,482 cells from Dnmt3aWT and 15,403 cells from Dnmt3aKO met quality standards. Unbiased clustering based on expression signatures defined subpopulations of cells in the skin (Fig. 2A) that were remarkably similar among all four samples (Fig. 2B).
Fig. 2.
Single-cell RNA sequencing reveals that Dnmt3a deficiency causes discrete gene expression changes in murine keratinocytes. (A) UMAP representation of single-cell RNA sequencing data from whole epidermis of two pairs of Krt14-Cre− × Dnmt3afl/fl (Dnmt3aWT; 10,783 cells) and Krt14-Cre+ × Dnmt3afl/fl mice (Dnmt3aKO;16,324 cells) with unbiased graph-based clustering demonstrating known skin populations represented by lineage defining genes (Right), including HFB, uHF, bIFE, sbIFE, and sebaceous gland (Seb), Krt18-expressing cells (Krt18), proliferative cells (Pro), melanocytes (Mel), T cells (T-cell), and Langerhan’s cells (Lang). (B) Comparison of keratinocyte lineage cells in the Dnmt3aWT versus Dnmt3aKO mice, excluding inflammatory cells and melanocytes (excluded clusters 6, 8, 9, and 10). UMAP projections for each pair of samples with the indicated genotypes are shown. (C) No major population shifts are observed in epidermal lineages affected by Krt14-Cre mediated Dnmt3a deficiency, with the exception that the cells dominated by a proliferative signature (Pro, cluster M) are increased (6.1% in Dnmt3aKO versus 1.8% in Dnmt3aWT), as indicated by the bar graphs of keratinocyte lineage subclusters. Subclusters of the hair follicle bulge (A and B), upper hair follicle (C and D), basal interfollicular epidermis (E–G), suprabasal interfollicular epidermis (H–K), and sebocytes (L) are relatively unchanged. (D) Canonical gene expression changes were noted in single-cell RNA sequencing comparing keratinocyte lineage cells in Dnmt3aWT versus Dnmt3aKO epidermis (n = 2 mice per genotype). (E) There are 174 differentially expressed genes between Dnmt3aWT and Dnmt3aKO keratinocyte lineage cells with 137 up-regulated in the Dnmt3aKO and 37 down-regulated genes with fold change >2 and FDR <0.01.
Unique populations segregated into distinct clusters with graph-based clustering and are represented by uniform manifold approximation and projection (UMAP) dimension reduction plots shown in Fig. 2A (32). In total, 19 clusters were identified by unbiased methods (Louvain method graph-based clustering, Dataset S3), which were subsequently categorized into 10 populations based on well-established, experimentally validated markers representing known populations in the epidermis (Fig. 2 A, Right). The hair follicle bulge (HFB) contains follicular stem cells and is marked by Cd34 expression (33), while the upper hair follicle (uHF) is marked by beta defensin (Defb6) expression (34, 35). Subdivided into the isthmus and infundibulum, the upper hair follicle bridges the follicle with the interfollicular epidermis and sebaceous gland, which is marked by the expression of Mgst1 (34). The basal interfollicular epithelium (bIFE) contains slow cycling, proliferative cells that act as an epidermal stem cell compartment (36), and is marked by “basal” keratin gene expression (Krt5 and Krt14), as well as Thbs1 (37). The suprabasal interfollicular epithelium (sbIFE) is postmitotic and marked by the expression of the genes that encode keratins Krt1 and Krt10 and an early differentiation marker metallothionein Mt4 (34). Melanocytes were marked by expression of the genes encoding tyrosinase (Tyr) and dopachrome tautomerase (Dct). Inflammatory cells, including T cells and Cd207-expressing Langerhans cells, comprised 1.3% and 1.1% of total cells in the samples, respectively. Finally, one population was dominated by expression of several genes associated with cellular proliferation (“Pro”), including genes encoding cyclins, cyclin-dependent kinases, and later cell cycle components like Ube2c, which encodes part of the anaphase promoting complex (see Fig. 4 below); this suggests that cells committed to mitosis have a signature dominated by components necessary for execution of the cell cycle (38).
Fig. 4.
Dnmt3a-deficient keratinocytes exhibit a proliferative phenotype. (A) UMAP of Dnmt3aWT and Dnmt3aKO single-cell RNA sequencing data representing a pair of samples for each indicated genotype, highlighting the population (in red) identified by unbiased clustering as dominated by expression of genes involved in cell cycling (cluster M). (B) Proliferative gene expression in the proliferative cluster (cluster M) versus the aggregate of the remaining keratinocyte linage clusters (clusters A–L). These data represent the aggregate of Dnmt3aWT and Dnmt3aKO cells. Each dot is an individual cell. Expression values are represented by least-squares (LS) means. (C) The number of cells expressing the proliferative marker gene Mki67 increases from 298/10,485 (2.8%) cells in Dnmt3aWT to 1,509/14,815 (10.2%) cells in the Dnmt3aKO. (D) Single-cell RNA-sequencing expression data of multiple cell cycle relevant genes, comparing the proliferative cells (cluster M) versus the rest (cluster A–L). *FDR <0.01 and ns, not significant. Expression values are represented by LS means. (E) BrdU uptake in vivo in keratinocytes assessed by flow cytometry (Cd45-negative, single-cell epidermal suspension) comparing Dnmt3aWT (n = 7) and Dnmt3aKO (n = 5) mice injected 24 h earlier with BrdU. BrdU uptake is significantly higher in Dnmt3aKO epidermal keratinocytes (5.6% BrdU+ in Dnmt3aWT vs. 7.5% BrdU+ in Dnmt3aKO, P = 0.023).
The Krt14-Cre conditional knockout of Dnmt3a targets the epidermal keratinocyte lineages; we therefore excluded skin-infiltrating hematopoietic cells from subsequent analyses. Keratinocyte lineage UMAP representations of single-cell data demonstrate a high degree of similarity among samples, and unbiased clustering demonstrated similar distributions between populations (Fig. 2B, clusters A–M). Small, but statistically significant differences in population sizes between Dnmt3aWT and Dnmt3aKO epidermal keratinocytes were noted in the follicular bulge cluster 2 and upper hair follicle 1 (clusters B and C), as well as basal and suprabasal interfollicular epidermis (clusters E and I); however, the most dramatic difference was in the proliferative (Pro) cluster (cluster M), which is expanded 3.4-fold in Dnmt3aKO skin (1.8% vs. 6.1%, P = 2.2*10−16, Fig. 2C). In addition to this population shift, there were canonical gene expression changes associated with Dnmt3a deficiency (Fig. 2D). In total, there were 174 differentially expressed genes (DEGs) exhibiting a fold change of 2 or greater with a FDR of 0.01 or less. Most of these genes (137) were more highly expressed in the Dnmt3aKO cells, while 37 were expressed at lower levels (Fig. 2 D and E).
Differentially Methylated Regions Are Associated with Gene Expression Changes.
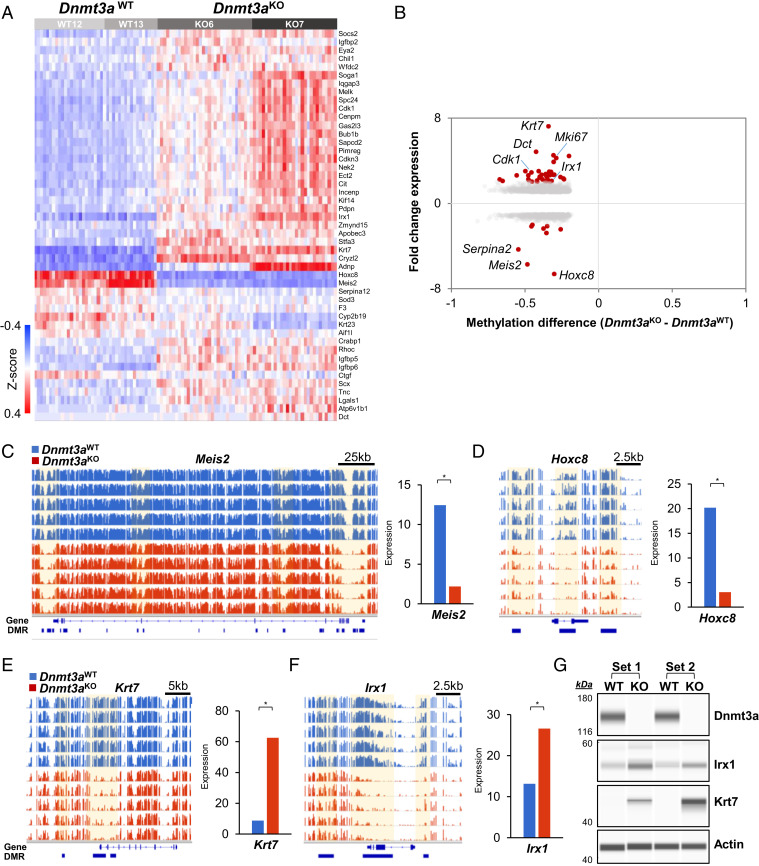
Of the 23,983 total DMRs identified by WGBS, 16,772 were within 10 kb of a gene, representing 5,573 unique genes. Of these, 2,181 genes (39%) were differentially expressed, as defined by an FDR of <0.01 and fold change >1, while 47 of these genes had an expression change of greater than twofold (38 up-regulated, and 9 down-regulated in Dnmt3aKO cells, Fig. 3A); however, the magnitude of methylation change was not predictive of expression changes. Further, some genes (e.g., Krt7 and Meis2) exhibited dramatic but opposing expression changes, despite associated DMRs that were hypomethylated to a similar extent (Fig. 3B). The location of DMRs within annotated regions (e.g., enhancers, CpG islands, etc.) did not predict the magnitude or direction of expression changes (SI Appendix, Fig. S2).
Fig. 3.
Differentially methylated regions are associated with differentially expressed genes. (A) Heatmap from single-cell RNA sequencing represents the 47 differentially expressed genes between Dnmt3aWT and Dnmt3aKO keratinocyte lineage cells that are associated with a Dnmt3a DMR and exhibit an expression fold change >2 with an FDR <0.01. Grayscale bars represent cells from independent samples, two from each genotype. (B) Relationship between gene expression and methylation changes between Dnmt3aKO and Dnmt3aWT keratinocytes. There are 5,968 differentially expressed genes with an FDR <0.01, of these there are 2,181 genes associated with DMRs. This plot represents expression versus methylation of these 2,181 genes. Significant differential expression, with fold change >2 denoted as red dots. Gray dots represent fold change between 1 and 2. (C) IGV views of differentially methylated regions associated with Meis2, which has annotated DMRs in CpG islands, shelves, shores, and gene body. Gene expression of Meis2 changes by −5.77-fold (FDR 5.7*10−249). Dnmt3aKO expresses Meis2 in 172/15,403 (1.11%) of cells compared to Dnmt3aWT 1,106/10,482 (10.6%). (D) Differentially methylated regions associated with Hoxc8, which has DMRs in shores and gene body. Hoxc8 expression changes by −6.72-fold (FDR 2.51*10−300) and is expressed in 234/15,403 (1.52%) Dnmt3aKO cells compared to 1,375/10,482 (13.1%) Dnmt3aWT. (E) Differentially methylated regions associated with Krt7, which has DMRs in shores, shelves, promoter, and gene body. Expression is changed by 7.19-fold in Dnmt3aKO (FDR 9.2*10−182), and cells expressing increase to Dnmt3aKO 2,551/15,403 (16.6%) from Dnmt3aWT 573/10,482 (5.47%). (F) Differentially methylated regions associated with Irx1, which has DMRs in CpG islands, shores, promoter, and gene body. Expression is changed by 2.02-fold (FDR 1.16*10−109) in Dnmt3aKO keratinocyte lineage cells, and cells expressing increases from 2,873/15,403 (18.7%) Dnmt3aKO cells to 885/10,482 (8.44%) Dnmt3aWT. (G) Simple Western demonstrates protein level expression changes in Irx1 and Krt7 from epidermal cells isolated from the indicated Dnmt3aWT and Dnmt3aKO littermates (bracketed). Actin serves as a loading control.
Meis2 is a homeobox transcription factor involved in developmental processes and regulation of tumor proliferation (39), and Hoxc8 modulates Wnt signaling in the hair follicle niche to control hair follicle regeneration (40). Both are DMR-associated genes and are expressed at significantly lower levels in most Dnmt3aKO epidermal cells (Fig. 3 C and D and SI Appendix, Fig. S3A), suggesting that the normal expression of these two genes may be linked to DNA methylation marks added by Dnmt3a. In contrast, some genes are differentially expressed only in subpopulations of skin cells. For example, Keratin 7 (Krt7), which is frequently overexpressed in aggressive subtypes of cutaneous squamous cell carcinoma, and associated with poor survival in patients with esophageal squamous cell carcinoma (41, 42), is predominantly expressed in the upper hair follicle in Dnmt3aWT mice; in Dnmt3aKO mice, its expression increases in the upper hair follicle as well as the basal and suprabasal interfollicular epidermis compartments (Fig. 3E and SI Appendix, Fig. S3B). Similarly, Irx1 is primarily expressed in the hair follicle bulge and upper hair follicle cells of Dnmt3aWT mice, but its expression increases in all compartments in Dnmt3aKO mice (Fig. 3F and SI Appendix, Fig. S3B), a finding that was validated by Western blotting (Fig. 3G). Decreased methylation in the IRX1 promoter is associated with increased expression and metastatic capability in osteosarcoma (43).
Deficiency in Dnmt3a Is Associated with an Increased Fraction of Cycling Cells in the Skin.
The most significant population shift between Dnmt3aWT and Dnmt3aKO keratinocyte lineage cells occurred in a cluster that was dominated by the expression of genes associated with cell division (Figs. 2 B and C and 4A). This population (cluster M) was defined by the expression of late interphase cyclins (e.g., Ccna2 and Ccnb2), as well as later cell cycle genes like Cenpf; these genes are rarely expressed in the cells of any other clusters (Fig. 4B). The size of this “proliferative cluster” expanded from 1.8% of total epidermal cells in Dnmt3aWT mice to 6.1% in Dnmt3aKO mice (3.4-fold, Fig. 4A), similar to the increase in the fraction of cells expressing Mki67 (from 2.8 to 10.2%, 3.6-fold, Fig. 4C). The expression of cell cycle genes in cluster M was equally high in Dnmt3aWT and Dnmt3aKO cells, as expected, since all cells in this cluster are likely to be actively dividing, regardless of their genotype (Fig. 4D); however, Dnmt3aKO cells in clusters A–L demonstrated increased expression levels of cell cycle genes, including Mki67 (although the expression levels of these genes were far lower than that of proliferating cells), as well as the essential cyclin-dependent kinase Cdk1, which is a differentially expressed gene associated with a DMR (Fig. 3A). To validate the proliferative phenotype with an orthogonal method, we injected 5-bromo-2′-deoxyuridine (BrdU) intraperitoneally into young (8 to 9 wk old) Dnmt3aWT and Dnmt3aKO mice, and generated single-cell suspensions of epidermal cells 24 h later for flow cytometric analysis of BrdU uptake. As predicted by the single-cell RNA-sequencing (scRNA-seq) data, the fraction of actively cycling, Cd45-negative epidermal cells was significantly increased in the Dnmt3aKO mice (Fig. 4E). Epidermal subpopulations likewise demonstrated significantly increased BrdU incorporation in Dnmt3aKO basal interfollicular epidermis cells (and trended upwards in the upper hair follicle); however, no change was detected in the follicular bulge stem cell compartment, or the postmitotic suprabasal interfollicular epidermis, neither of which proliferates in the telogen state (SI Appendix, Fig. S4 A and B).
Focal, Canonical DNA Hypomethylation in the Skin of a Patient with a Dominant-Negative DNMT3AR882H Mutation.
Patients with TBRS have germline mutations in DNMT3A and exhibit several common clinical features, including overgrowth, characteristic facies, and neuropsychiatric disorders (44, 45), as well as an increased risk of developing AML and perhaps other malignancies. We previously demonstrated that the peripheral blood cells from a nonleukemic patient with a heterozygous germline DNMT3AR882H allele exhibited a focal, canonical hypomethylation phenotype similar to that of AML samples initiated by the same mutation, suggesting that hypomethylation precedes transformation (30).
To determine whether this mutation causes a methylation phenotype in human skin, we analyzed DNA methylation in two skin samples from the same TBRS patient (at ages 9 and 14) and his unaffected brother (at ages 13 and 17). Global DNA methylation levels were minimally altered (similar to Dnmt3aKO skin). Nearly all of the 1,234 DMRs identified (1,221/1,234; 98.9%) were hypomethylated (Fig. 5B); the smaller number of DMRs (compared to Dnmt3aKO mouse skin) probably reflects the fact that the R882H mutation is dominant negative (80% reduced activity) rather than null, and also the reduced power to detect DMRs with only two samples from each genotype. Plotting the mean methylation values of each DMR between both samples from the two brothers demonstrated the canonical nature of most of these DMRs (Fig. 5C). These DMRs were most likely to occur in gene bodies, similar to that seen in Dnmt3aKO mice (Figs. 1D and 5E).
Fig. 5.
A germline, dominant-negative DNMT3A mutation causes focal DNA methylation alterations in human skin. (A) Whole genome bisulfite sequencing analysis for whole skin samples comparing two samples from a TBRS patient and his brother, comparing CpG methylation globally (Top) or at DMRs (Bottom). Level of significance * adjusted P < 0.05 and **P < 0.01. (B) Density plots demonstrate global CpG methylation (Top) and CpG methylation at DMRs (Bottom) in the TBRS patient and his unaffected sibling. (C) Heatmap illustrates 1,234 differentially methylated regions in the skin of the TBRS patient, compared with his sibling. (D) IGV visualization of CpG methylation at the IRX3/Irx3 and FZD1/Fzd1 loci. Each row represents an independent sample with Dnmt3aWT vs. Dnmt3aKO represented in blue and red (Top) and sibling vs. TBRS patient in purple and yellow (Bottom). Gray boxes highlight areas of differential methylation. (E) Gene bodies are the most frequently involved annotated region by TBRS DMRs. *There are 963 DMRs that reside in annotated gene bodies, representing 637 unique genes. (F) There are 23,983 DMRs in the Dnmt3aKO, 16,315 of them are within 10 kb of 3,612 unique genes. Of the 1,234 TBRS DMRs, 1,086 are located within 10 kb of genes in the human genome. Of the 1,086 TBRS-DMR associated genes, 707 have a mouse homolog, with 432 genes overlapping DMR-associated genes in Dnmt3aKO mice and the TBRS patient.
At many loci, there was a striking similarity in DNA methylation patterns between the DMRs of homologous genes in mice and humans. For example, DMRs near the homeobox gene IRX3 in human TBRS skin are similar to DMRs near Irx3 in mouse skin (Fig. 5 D, Left). Another gene important for development, FZD1, shares a similar methylation phenotype between the TBRS skin and mouse Dnmt3aKO skin (Fig. 5 D, Right). The Wnt signaling pathway is important both for skin and bone development, and multiple genes of the Wnt pathway also exhibited similar Dnmt3a-dependent methylation patterns between mouse and human skin (SI Appendix, Fig. S5). Of the 1,034 DMR-associated genes identified in the TBRS patient, 432 overlapped with Dnmt3aKO DMR-associated genes identified in mouse skin (Fig. 5F).
Discussion
The purpose of this study was to define the baseline epigenetic characteristics of Dnmt3a-deficient skin. Using WGBS, we defined the focal, canonical DNA methylation phenotype of Dnmt3a-deficient mouse epidermal cells and of a human patient with a germline DNMT3AR882H mutation. The small changes in the global methylation state of epidermal cells reflects the restricted places in the genome where Dnmt3a acts, and the small sizes of these regions (on average, less than 1 Kb). There were 1,234 DMRs identified in the skin of the TBRS patient, which affected 3.19% of annotated gene bodies. Similarly, Dnmt3a-dependent methylation in murine epidermis occurred most often in gene bodies—more than in promoters or CpG island shores, which were the next most common regions affected. However, the magnitude and location of these differentially methylated regions were not predictive of gene expression changes detected by scRNA-seq. Overall, there were 5,968 DEGs in Dnmt3aKO skin cells, but the number of DEGs that were close to a DMR (i.e., within 10 Kb) represented a subset of these genes (2,181/5,968; 36.5%). Only a small subset of these exhibited an expression fold change of >2 (2.15% of DMR associated DEGs, 0.8% of all DEGs). Like multiple previous studies (18, 30, 46), the relationship of DMRs to gene expression patterns was unpredictable at a global level, again demonstrating that DNA methylation is but one factor in determining the expression of a specific gene in a specific cellular context. Prior studies demonstrated accurate remethylation of Dnmt3a-dependent DMRs in Dnmt3aKO bone marrow with induced expression of wild-type DNMT3A, as well as correction of a subset of gene expression changes (18). However, it is not yet clear whether remethylation was directly responsible for altering the expression of these differentially expressed genes.
Aging and sun exposure have been demonstrated to cause DNA methylation changes, which were particularly apparent comparing UV-exposed skin in older patients to UV-protected skin in younger patients. Large, predominantly hypomethylated blocks were found to cover 99 Mb of the genome in older, UV-exposed skin (5). Although the cause of this phenotype is unknown, a number of possibilities exist, including a progressive decline in expression (or function) of the DNA methyltransferases, or increased activity of the demethylases (i.e., TET gene family members). In mice, Dnmt1 and Dnmt3a are expressed in the basal layer of keratinocytes during development; however, in adults, the expression of Dnmt1 is restricted to the hair follicle, and Dnmt3a expression is restricted to the interfollicular epidermis (8, 11). Notably, Dnmt3a expression decreases in this compartment in adult mice (11), though it is not yet clear whether these findings contribute to the increased risk of skin cancers in elderly patients.
To begin to address this question, Rinaldi et al. demonstrated that epidermal Dnmt3a deficiency resulted in decreased tumor latency and increased tumor numbers in mouse skin treated with DMBA/TPA, suggesting that the threshold for oncogenic transformation is lower in mice with Dnmt3a deficiency (11). In the same study, mice with Dnmt3b deficiency had no phenotype, and compound deficiency of Dnmt3a and Dnmt3b (double knockout/DKO) resulted in tumor latency that was indistinguishable from that of Dnmt3a deficiency; however, a broader spectrum of tumors arose in the DKO mice, including basal cell carcinomas, as well as more aggressive tumors (including squamous cell and spindle cell carcinomas). These tumors developed a reliance on Pparg; we did not detect up-regulation of Pparg in the premalignant state, suggesting that dysregulation may occur during or after transformation. In contrast, certain gene expression changes (i.e., increased expression of G0s2) persisted from the Dnmt3a-deficient preneoplastic state to the resulting tumors (11) (Fig. 1E and Dataset S2). Together, these data strongly suggested that Dnmt3a deficiency creates a premalignant state for keratinocyte carcinomas in mice, with some gene expression changes that persist in tumors. We observed that Dnmt3b deficiency causes only 20 DMRs in the skin, and that Dnmt3a and Dnmt3b compound deficiency (DKO) adds little to the Dnmt3aKO methylation phenotype (SI Appendix, Fig. S6). Therefore, in mouse skin, Dnmt3a is the dominant de novo DNA methyltransferase, and the loss of its function in aging skin could likewise be relevant for human skin cancer pathogenesis.
Although Dnmt3a deficiency results in minimal skin population changes, it is associated with an expanded number of cells with a proliferative signature in the Dnmt3aKO epidermis. Despite this, the skin of young mice is not measurably thickened, and spontaneous skin cancers do not occur, suggesting that robust compensatory mechanisms are active. Multiple critical cell cycle genes, including Ccna2 or Cdk1, are up-regulated in both proliferating and nonproliferating cells as well; conversely, the cyclin-dependent kinase inhibitor Cdkn1a is down-regulated in nonproliferating Dnmt3a-deficient cells (SI Appendix, Fig. S7A). The expression of Mki67 is tightly regulated and absent in quiescent (G0) cells and more highly expressed in G2 through M phase cells. Cells that are more frequently in the cell cycle express higher levels of Mki67 than quiescent cells (47). Indeed, we found that Mki67 expression is elevated even in the nonproliferating cells of Dnmt3aKO mice, which may reflect the proliferative history of these cells. Together, these results suggest that Dnmt3a deficiency may prime epidermal keratinocytes to enter the cell cycle, or remove antiproliferative “brakes” from mitogenic signals. While proliferative signaling was implicated, Cdk1 was the only direct proliferative driver associated with a DMR and expressed with a fold change >2.
Enhanced proliferation has been observed in the setting of DNMT3A deficiency in other premalignant and malignant conditions. Decreased DNMT3A expression has been associated with nonmalignant adenomyosis, where ectopic endometrial cells invade the myometrium. These cells exhibit increased proliferation with DNMT3A knockdown, and decreased proliferation with overexpression (48). Additionally, a model of Dnmt3a deficiency in KRASG12D driven lung tumors demonstrated that at 24 wk after induction, Dnmt3aKO tumors were six times larger than their Dnmt3aWT counterparts and exhibited a markedly higher proliferative index (49). Together, these findings suggest that epigenetic changes caused by Dnmt3a deficiency can result in a hyperproliferative state in several cellular contexts. The specific genes responsible for initiating this phenotype are not yet clear, but several good candidates (i.e., Cdk1, Hras, E2f1, and Cdkn1a) are defined in this study (SI Appendix, Fig. S7 B–D). Proliferative priming may therefore represent one mechanism that is relevant for the premalignant state associated with DNMT3A haploinsufficiency or deficiency in multiple tissues. The resulting expanded proliferative capacity may exist in equilibrium until cooperating mutations occur, which appear to be essential for transformation in many model systems.
In addition to clonal hematopoiesis, premalignant mutation-driven clonal expansion has now been shown to occur in many other systems, including the skin (2), liver (50), esophagus (51, 52), and bronchial epithelium (53). Mutationally driven, expanded clones usually do not progress to frank malignancies, suggesting that additional epigenetic or genetic alterations are required to cause transformation. In clonal hematopoiesis, the expansion of hematopoietic stem/progenitor cells is usually caused by mutations in epigenetic regulatory genes, most commonly in DNMT3A (23, 24, 26, 54). These clonally expanded cells are at increased risk of transformation to myelodysplastic syndromes or AML, likely by altering the epigenetic “fitness” of these cells for transformation (55–57). Similar epigenetic fitness alterations may modify tumor susceptibility in skin and other organs by a variety of mechanisms.
We identified similarities between the methylation phenotypes of mice and humans with decreased DNMT3A activity: DMRs in the epidermis of Dnmt3aKO mice shared considerable gene-level similarity with a TBRS patient with a germline DNMT3AR882H mutation. In fact, 432 out of the 1,086 gene-associated DMRs identified in the skin of this patient overlapped with gene-associated DMRs found in Dnmt3aKO skin. Several of these overlapping genes have known roles in development, including IRX3/Irx3; this locus shares a high degree of evolutionary conservation in noncoding sequences between human, mouse, and zebrafish, suggesting the presence and conservation of both functional and regulatory elements (58). Additionally, important pathways in skin development (like the WNT pathway) have multiple members with similar DNMT3A/Dnmt3a-dependent methylation patterns (SI Appendix, Fig. S5). Recent descriptions of AMLs and central nervous system (CNS) tumors in young patients with germline loss-of-function DNMT3A mutations strongly suggest that DNMT3A acts as a tumor suppressor in both hematopoietic and CNS cells (59). Although the skin phenotype and skin cancer susceptibility of children with TBRS has not yet been described, our data suggest that these patients may be at elevated risk, and that appropriate primary prevention measures should be taken to protect these patients from sustained UV light exposure. Finally, these data also suggest a role for DNMT3A in keratinocyte carcinomas arising in aging and sun-exposed skin; additional studies to define this role are in progress.
Materials and Methods
Human Studies.
Samples were obtained as part of a study approved by the Human Research Protection Office at Washington University School of Medicine (approval #201011766), which explicitly allows for potentially identifying genomic studies, including whole genome sequencing. Written informed consent was obtained from the parents of the patient with TBRS (and his unaffected brother) for whole genome bisulfite sequencing studies, in accordance with the institutional review board approved protocol at Washington University School of Medicine.
Animals and Epidermal Cell Isolation.
All mouse work was performed in accordance with institutional guidelines and approved by the Animal Studies Committee at Washington University. For all studies, we harvested single-cell suspensions of dorsal epidermis in second telogen (7 to 10 wk of age), or in older mice, when skin is >90% telogen based on pale or pink skin color. Details for mouse strain and epidermal single-cell suspensions can be found in SI Appendix, Supplementary Materials and Methods.
Whole Genome Bisulfite Sequencing and Analysis.
WGBS was performed as described previously (17) with the exception that DNA was generated from epidermal cells. Alignment and DMR calling details are in SI Appendix, Supplementary Materials and Methods.
Single-Cell RNA Sequencing and Analysis.
Single-cell suspensions of murine epidermis were freshly prepared and libraries generated using the 10× Chromium Controller system. Data analysis was performed using the standard tools available in the Partek Flow software suite (Partek, Inc.). Full details of the analysis are in SI Appendix, Supplementary Materials and Methods.
Immunoblotting and Flow Cytometry.
Freshly isolated single-cell suspensions of murine epidermis were lysed in radioimmunoprecipitation assay (RIPA) buffer or suspended in staining buffer for further analysis by flow cytometry. Lysis and staining conditions (as well as antibody information) are described in SI Appendix, Supplementary Materials and Methods.
Supplementary Material
Acknowledgments
This study was supported by grants from the Dermatology Foundation (Dermatologist Investigator Research Fellowship and Physician Scientist Career Development Award) and NIH Grants CA237727 (to D.Y.C.), CA211782 (to C.A.M.), and CA101937 and CA197561, and a Barnes Jewish Foundation Award #5169 (to T.J.L.). The Siteman Cancer Center Flow Cytometry Core (National Cancer Institute P30CA91842) provided expert support for all flow sorting studies. We thank Mieke Hoock for excellent animal husbandry.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2022760118/-/DCSupplemental.
Data Availability
DNA and RNA sequences (mouse data) have been deposited in NCBI Sequence Read Archive (BioProject PRJNA674614). Anonymized DNA sequences (human data) have been deposited in dbGaP (https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000159.v10.p5).
References
- 1.Rogers H. W., Weinstock M. A., Feldman S. R., Coldiron B. M., Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 151, 1081–1086 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Martincorena I., et al., Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 348, 880–886 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baylin S., Bestor T. H., Altered methylation patterns in cancer cell genomes: Cause or consequence? Cancer Cell 1, 299–305 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Timp W., Feinberg A. P., Cancer as a dysregulated epigenome allowing cellular growth advantage at the expense of the host. Nat. Rev. Cancer 13, 497–510 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandiver A. R., et al., Age and sun exposure-related widespread genomic blocks of hypomethylation in nonmalignant skin. Genome Biol. 16, 80 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen T., Ueda Y., Dodge J. E., Wang Z., Li E., Establishment and maintenance of genomic methylation patterns in mouse embryonic stem cells by Dnmt3a and Dnmt3b. Mol. Cell. Biol. 23, 5594–5605 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones P. A., Liang G., Rethinking how DNA methylation patterns are maintained. Nat. Rev. Genet. 10, 805–811 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J., et al., Progressive alopecia reveals decreasing stem cell activation probability during aging of mice with epidermal deletion of DNA methyltransferase 1. J. Invest. Dermatol. 132, 2681–2690 (2012). Corrected in: J. Invest. Dermatol.133, 859 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sen G. L., Reuter J. A., Webster D. E., Zhu L., Khavari P. A., DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature 463, 563–567 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rinaldi L., et al., Dnmt3a and Dnmt3b associate with enhancers to regulate human epidermal stem cell homeostasis. Cell Stem Cell 19, 491–501 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Rinaldi L., et al., Loss of Dnmt3a and Dnmt3b does not affect epidermal homeostasis but promotes squamous transformation through PPAR-γ. eLife 6, e21697 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodríguez-Paredes M., et al., Methylation profiling identifies two subclasses of squamous cell carcinoma related to distinct cells of origin. Nat. Commun. 9, 577 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickering C. R., et al., Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin. Cancer Res. 20, 6582–6592 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y. Y., et al., Genomic analysis of metastatic cutaneous squamous cell carcinoma. Clin. Cancer Res. 21, 1447–1456 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonilla X., et al., Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat. Genet. 48, 398–406 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Jeong M., et al., Large conserved domains of low DNA methylation maintained by Dnmt3a. Nat. Genet. 46, 17–23 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole C. B., et al., Haploinsufficiency for DNA methyltransferase 3A predisposes hematopoietic cells to myeloid malignancies. J. Clin. Invest. 127, 3657–3674 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ketkar S., et al., Remethylation of Dnmt3a-/- hematopoietic cells is associated with partial correction of gene dysregulation and reduced myeloid skewing. Proc. Natl. Acad. Sci. U.S.A. 117, 3123–3134 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayle A., et al., Dnmt3a loss predisposes murine hematopoietic stem cells to malignant transformation. Blood 125, 629–638 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Celik H., et al., Enforced differentiation of Dnmt3a-null bone marrow leads to failure with c-Kit mutations driving leukemic transformation. Blood 125, 619–628 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollink I. H. I. M., et al., Acute myeloid leukaemia in a case with Tatton-Brown-rahman syndrome: The peculiar DNMT3A R882 mutation. J. Med. Genet. 54, 805–808 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Kosaki R., Terashima H., Kubota M., Kosaki K., Acute myeloid leukemia-associated DNMT3A p.Arg882His mutation in a patient with Tatton-Brown-Rahman overgrowth syndrome as a constitutional mutation. Am. J. Med. Genet. A. 173, 250–253 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Xie M., et al., Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat. Med. 20, 1472–1478 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaiswal S., et al., Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genovese G., et al., Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 371, 2477–2487 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bick A. G.et al.; NHLBI Trans-Omics for Precision Medicine Consortium , Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature 586, 763–768 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ley T. J., et al., DNMT3A mutations in acute myeloid leukemia. N. Engl. J. Med. 363, 2424–2433 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okano M., Bell D. W., Haber D. A., Li E., DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99, 247–257 (1999). [DOI] [PubMed] [Google Scholar]
- 29.Dassule H. R., Lewis P., Bei M., Maas R., McMahon A. P., Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 127, 4775–4785 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Spencer D. H., et al., CpG island hypermethylation mediated by DNMT3A is a consequence of AML progression. Cell 168, 801–816.e13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeziorska D. M., et al., DNA methylation of intragenic CpG islands depends on their transcriptional activity during differentiation and disease. Proc. Natl. Acad. Sci. U.S.A. 114, E7526–E7535 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becht E., et al., Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 37, 38–44 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Trempus C. S., et al., Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J. Invest. Dermatol. 120, 501–511 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Joost S., et al., Single-cell transcriptomics reveals that differentiation and spatial signatures shape epidermal and hair follicle heterogeneity. Cell Syst. 3, 221–237.e9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Füllgrabe A., et al., Dynamics of Lgr6+ progenitor cells in the hair follicle, sebaceous gland, and interfollicular epidermis. Stem Cell Reports 5, 843–855 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blanpain C., Fuchs E., Epidermal homeostasis: A balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 10, 207–217 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varani J., et al., Thrombospondin-induced adhesion of human keratinocytes. J. Clin. Invest. 81, 1537–1544 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitfield M. L., et al., Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol. Biol. Cell 13, 1977–2000 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groß A., et al., Tumorigenic and anti-proliferative properties of the TALE-transcription factors MEIS2D and MEIS2A in neuroblastoma. Cancer Res. 78, 1935–1947 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Yu Z., et al., Hoxc-dependent mesenchymal niche heterogeneity drives regional hair follicle regeneration. Cell Stem Cell 23, 487–500.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Pulitzer M., Desman G., Busam K. J., CK7 expression in primary cutaneous squamous cell carcinoma. J. Cutan. Pathol. 37, 966–972 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Yamada A., et al., Expression of cytokeratin 7 predicts survival in stage I/IIA/IIB squamous cell carcinoma of the esophagus. Oncol. Rep. 20, 1021–1027 (2008). [PubMed] [Google Scholar]
- 43.Lu J., et al., IRX1 hypomethylation promotes osteosarcoma metastasis via induction of CXCL14/NF-κB signaling. J. Clin. Invest. 125, 1839–1856 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tatton-Brown K.et al.; Childhood Overgrowth Consortium , Mutations in the DNA methyltransferase gene DNMT3A cause an overgrowth syndrome with intellectual disability. Nat. Genet. 46, 385–388 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen W., et al., The spectrum of DNMT3A variants in Tatton-Brown-Rahman syndrome overlaps with that in hematologic malignancies. Am. J. Med. Genet. A. 173, 3022–3028 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Russler-Germain D. A., et al., The R882H DNMT3A mutation associated with AML dominantly inhibits wild-type DNMT3A by blocking its ability to form active tetramers. Cancer Cell 25, 442–454 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller I., et al., Ki67 is a graded rather than a binary marker of proliferation versus quiescence. Cell Rep. 24, 1105–1112.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zou Y., et al., Downregulation of DNA methyltransferase 3 alpha promotes cell proliferation and invasion of ectopic endometrial stromal cells in adenomyosis. Gene 604, 41–47 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Gao Q., et al., Deletion of the de novo DNA methyltransferase Dnmt3a promotes lung tumor progression. Proc. Natl. Acad. Sci. U.S.A. 108, 18061–18066 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brunner S. F., et al., Somatic mutations and clonal dynamics in healthy and cirrhotic human liver. Nature 574, 538–542 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martincorena I., et al., Somatic mutant clones colonize the human esophagus with age. Science 362, 911–917 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yokoyama A., et al., Age-related remodelling of oesophageal epithelia by mutated cancer drivers. Nature 565, 312–317 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Yoshida K., et al., Tobacco smoking and somatic mutations in human bronchial epithelium. Nature 578, 266–272 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Genovese G., Jaiswal S., Ebert B. L., McCarroll S. A., Clonal hematopoiesis and blood-cancer risk. N. Engl. J. Med. 372, 1071–1072 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Watson C. J., et al., The evolutionary dynamics and fitness landscape of clonal hematopoiesis. Science 367, 1449–1454 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Miles L. A., et al., Single-cell mutation analysis of clonal evolution in myeloid malignancies. Nature 587, 477–482 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morita K., et al., Clonal evolution of acute myeloid leukemia revealed by high-throughput single-cell genomics. Nat. Commun. 11, 5327 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smemo S., et al., Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature 507, 371–375 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sweeney K. J., et al., The first case report of medulloblastoma associated with Tatton-Brown-Rahman syndrome. Am. J. Med. Genet. A. 179, 1357–1361 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DNA and RNA sequences (mouse data) have been deposited in NCBI Sequence Read Archive (BioProject PRJNA674614). Anonymized DNA sequences (human data) have been deposited in dbGaP (https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000159.v10.p5).