Significance
Epilepsy, a neurological disorder, is caused by abnormal brain activity due to genetic variances or acquired brain injuries. However, the underlying molecular mechanisms remain elusive. We demonstrate that NEO1, a member of the deleted in colorectal cancer (DCC) family transmembrane proteins, is decreased in human hippocampi of patients with epilepsy. By using a combination of genetic, electrophysiological, biochemical, and molecular biological methods, we show that KO Neo1 in hippocampal astrocytes, but not in neurons, increased epileptiform spikes and seizure susceptibility in mice. Astrocyte-specific KO of Neo1 reduced inhibitory synaptic vesicles and GABAergic synaptic transmission in the hippocampus by impairing the GLAST-mediated glutamate–glutamine cycle. Together, this study reveals a function of NEO1 in hippocampal astrocytes to protect the brain from epilepsy.
Keywords: epilepsy, astrocyte, neogenin, GLAST, glutamate uptake
Abstract
Epilepsy, a common neurological disorder, is featured with recurrent seizures. Its underlying pathological mechanisms remain elusive. Here, we provide evidence for loss of neogenin (NEO1), a coreceptor for multiple ligands, including netrins and bone morphological proteins, in the development of epilepsy. NEO1 is reduced in hippocampi from patients with epilepsy based on transcriptome and proteomic analyses. Neo1 knocking out (KO) in mouse brains displays elevated epileptiform spikes and seizure susceptibility. These phenotypes were undetectable in mice, with selectively depleted NEO1 in excitatory (NeuroD6-Cre+) or inhibitory (parvalbumin+) neurons, but present in mice with specific hippocampal astrocytic Neo1 KO. Additionally, neurons in hippocampal dentate gyrus, a vulnerable region in epilepsy, in mice with astrocyte-specific Neo1 KO show reductions in inhibitory synaptic vesicles and the frequency of miniature inhibitory postsynaptic current(mIPSC), but increase of the duration of miniature excitatory postsynaptic current and tonic NMDA receptor currents, suggesting impairments in both GABAergic transmission and extracellular glutamate clearance. Further proteomic and cell biological analyses of cell-surface proteins identified GLAST, a glutamate–aspartate transporter that is marked reduced in Neo1 KO astrocytes and the hippocampus. NEO1 interacts with GLAST and promotes GLAST surface distribution in astrocytes. Expressing NEO1 or GLAST in Neo1 KO astrocytes in the hippocampus abolishes the epileptic phenotype. Taken together, these results uncover an unrecognized pathway of NEO1-GLAST in hippocampal GFAP+ astrocytes, which is critical for GLAST surface distribution and function, and GABAergic transmission, unveiling NEO1 as a valuable therapeutic target to protect the brain from epilepsy.
Epilepsy affects over 70 million people worldwide and interferes with people’s daily activities (1). Patients with epilepsy usually display unprovoked, recurrent seizures and sometimes loss of awareness. Temporal lobe epilepsy (TLE) is the most common form of focal epilepsy, and seizures usually occur in the hippocampus. Although anti-epileptic drugs could help control seizures in most patients, ∼30% of them still fail to respond to the medical treatments. Studies in patients with epilepsy and epileptic animal models have shown many structural and functional changes in the brain, which include reduced tissue volume (2), impaired blood–brain barrier (BBB) (3), hippocampal sclerosis (4), neurodegeneration (5), dysregulated neural circuit (6), and abnormal neurogenesis (7). It is of considerable interest to investigate the underlying mechanisms of epileptogenesis.
Epilepsy is often due to the disruption of physiological balance between excitatory (E) and inhibitory (I) activities in the brain (8). Both genetic factors and acquired injuries could disturb E/I balance, resulting in epilepsy (9). Many proteins have been identified to regulate epileptogenesis, including dysregulation of voltage-gated sodium channels (e.g., Nav1.2 and Nav1.6) in excitatory pyramidal neurons, which contributes to febrile seizures through modulating neuronal excitability (10). Loss-of-function mutations in the Nav1.1 channel that impairs action potential (AP) firing in GABAergic inhibitory neurons and thus induce hyperexcitability of dentate granule neurons and severe myoclonic epilepsy in infancy (11). Additionally, numerous astrocytic proteins have been found to regulate epileptogenesis, including Aquaporin 4 (AQP4), ATP-sensitive inward rectifier potassium channel 10 (Kir4.1), glutamine synthetase (GS), and glutamate transporters (12). Two glutamate transporters, glutamate-aspartate transporter (GLAST) and glutamate transporter-1 (GLT1), are predominantly expressed in astrocytes (13, 14). The deficits in astrocytic glutamate uptake mediated by GLAST/GLT1 elevate extracellular glutamate levels, impair synaptic functions, and increase the risk of epileptogenesis (15–17). While mounting evidence support a critical role of astrocytic glutamate uptake in preventing epileptogenesis, how glutamate uptake is regulated remains largely unclear.
By RNA-seq and proteomic analyses of brain samples from patients with or without epilepsy, we have identified neogenin (NEO1), which is reduced in patients’ samples with epilepsy. NEO1, a member of deleted in colorectal cancer family transmembrane proteins, serves as a receptor or coreceptor for multiple ligands, including netrins, repulsive guidance molecules (RGMs), and bone morphogenetic proteins (BMPs) (18–23). Thus, NEO1 plays diverse functions in both central nervous system and periphery tissues, including RGM-regulated axonal guidance (18) and neural tube formation (24), BMP-regulated endochondral bone formation (25), digit patterning (26), and ion homeostasis (27). In the brain, NEO1 is abundantly expressed in neural stem cells in the subventricular zone and subgranular zone, where it promotes neocortical astrogliogenesis and adult hippocampal neurogenesis, respectively (28, 29). NEO1 is also expressed in glutamatergic neurons at the amygdala, where it regulates neuronal activity and fear memory (30), and in embryonic neural crest cells, where it prevents persistent hyperplastic primary vitreous formation (31). Intriguingly, at young adult age, NEO1 is mainly expressed in astrocytes and promotes blood vessel homeostasis and function within the mouse cortex (28, 32). However, little is known about astrocytic NEO1’s function in epilepsy.
Here, we provide evidence that the astrocytic, but not neuronal, NEO1 plays a key role in preventing epileptic response. Mice with Neo1 knockout (KO) in hippocampal GFAP+ astrocytes exhibited epileptiform spikes and enhanced seizure susceptibility induced by pentylenetetrazole (PTZ), as well as displayed longer durations of dentate gyrus (DG)-kindled seizures (>stage 3) than that of control mice. Further electrophysiological studies showed an impairment in GABAergic synaptic transmission in DG granule neurons of astrocytic Neo1 KO mice. Molecular and cellular studies in hippocampal astrocytes identified GLAST, whose surface distribution requires astrocytic NEO1 expression. Finally, overexpressing GLAST or NEO1 in Neo1 KO astrocytes restored GLAST’s cell surface level and its glutamate uptake activity, and attenuated epileptiform activities in the Neo1 KO mice. Taken together, these results suggest that astrocytic NEO1 in the hippocampus plays a critical role in preventing epilepsy, likely through increasing GLAST-mediated glutamate uptake.
Results
Decreased NEO1 Expression in Hippocampus of Patients with Epilepsy.
To screen for the potential risk factors that may underlie epilepsy, we carried out both RNA-seq and proteomic analyses of human hippocampal samples from patients with or without epilepsy (SI Appendix, Fig. S1 A and D). As shown in SI Appendix, Fig. S1B and Dataset S1, 716 genes were down-regulated and 137 genes were up-regulated in patients with epilepsy, as compared with that of controls, by RNA-seq analysis. Interestingly, among the down-regulated genes, in addition to previously identified epilepsy-associated genes (e.g., CLCN2, KCNJ10) (33), several unrecognized genes, including Neo1, were identified (SI Appendix, Fig. S1C).
The proteomic analysis of human hippocampal samples was performed by tandem mass tagging–based quantitative proteomics (SI Appendix, Fig. S1D). Eighty-seven proteins were down-regulated and 116 were up-regulated in patients with epilepsy (over that of controls) (SI Appendix, Fig. S1 E and F and Dataset S2). Intriguingly, comparing the down-regulated proteins with those identified by RNA-seq analysis, nine proteins were found to be decreased in patients with epilepsy by both analyses, and NEO1 is one of these proteins (SI Appendix, Fig. S1G). We thus further examined NEO1’s expression by Western blot (WB) analysis. Indeed, NEO1 protein levels were lower in the hippocampal homogenates of patients with epilepsy (both males and females, from the ages of 8 to 83 y old) than those of controls (SI Appendix, Fig. S1 H and I). These results suggest that the hippocampal NEO1 expression is decreased in patients with epilepsy, which is likely due to its reduced transcription, implicating hippocampal NEO1-deficiency in epilepsy.
Increased Epileptiform Spikes and Seizure Susceptibility in NeoGFAP-Cre Mice.
To investigate if NEO1-deficiency contributes to the epilepsy, NeoGFAP-Cre mice were generated by crossing floxed Neo1 (Neof/f) with GFAP-Cre mice that express Cre under the control of human GFAP (hGFAP) promoter (SI Appendix, Fig. S2A). As the Cre is expressed in multiple types of brain cells, including radial glial cells, neural progenitor cells, and their derivatives, including neurons and astrocytes (34, 35), ∼90% NEO1 protein was depleted in homogenates of the cortex or hippocampus of young adult NeoGFAP-Cre mice as compared with that of control mice (Neof/f) (SI Appendix, Fig. S2 B and C). We then determined if NeoGFAP-Cre mice show any epileptic-like response by intracranial electroencephalographic (EEG) recordings, which monitor the local field potential. An electrode was implanted in the dorsal hippocampus of each control (Neof/f) or NeoGFAP-Cre mouse (at the age of 8 wk old); after 1 wk recovery, the EEG recordings were performed for more than 4 h (SI Appendix, Fig. S2D). Interestingly, obvious epileptiform spikes, an EEG activity associated with latent preseizure period (36), were recorded in NeoGFAP-Cre, but not control, mice (SI Appendix, Fig. S2 E and F). Next, we examined whether NeoGFAP-Cre mice show more sensitivity to PTZ-induced epileptic response. To this end, both NeoGFAP-Cre and control mice received a single injection of PTZ at a subthreshold dose (30 mg/kg, i.p.) (37). As shown in SI Appendix, Fig. S2 G and H, NeoGFAP-Cre mice displayed a significant increase in EEG power spectrum, a feature of epilepsy (38), as compared with that in control mice. We further examined PTZ-induced epileptic behavior response in NeoGFAP-Cre and control mice. Upon 30 mg/kg or 40 mg/kg of PTZ treatments, the seizure responses were scored according to Racine’s stage description (39, 40) (SI Appendix, Fig. S2I). Indeed, higher seizure scores were observed in NeoGFAP-Cre mice than that of control mice (SI Appendix, Fig. S2J). Together, these results suggest that loss of NEO1 expression within the mouse brain promotes seizure susceptibility.
Increased Epileptic Response in Astrocytic, but Not Neuronal, Neo1 KO Mice.
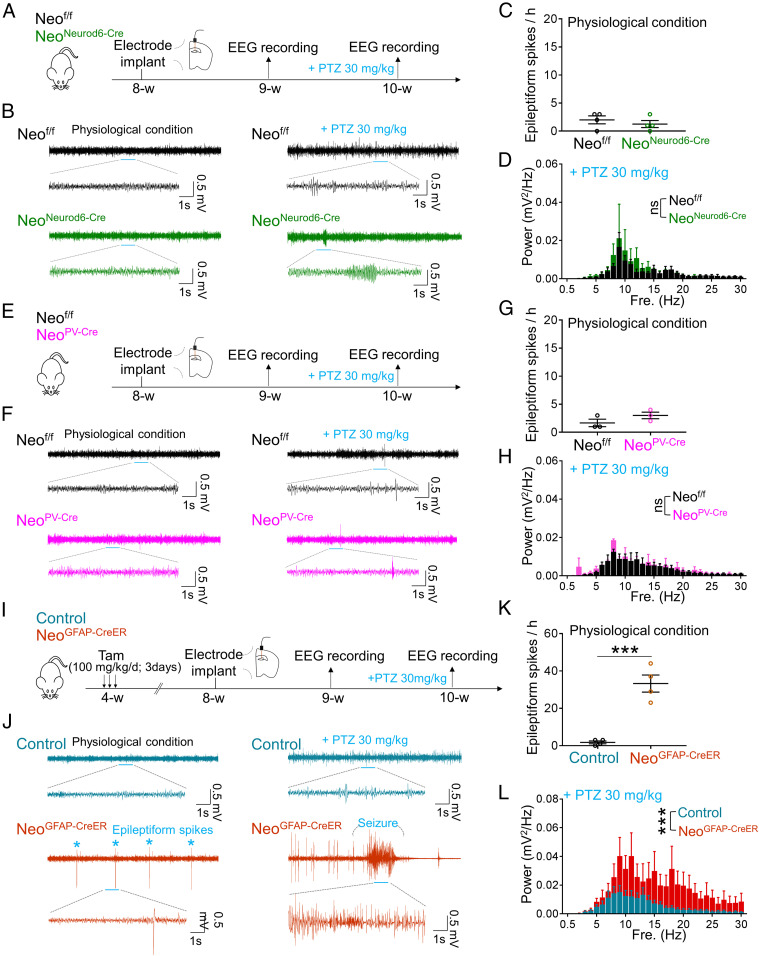
Given the observations that both GFAP-Cre and NEO1 are expressed in multiple types of brain cells, including neurons and astrocytes (28, 29, 34, 35), we asked in which type of brain cells that NEO1 is necessary for preventing seizure susceptibility. To this end, NeoNeurod6-Cre, NeoPV-Cre, and NeoGFAP-CreER mice were generated by breeding Neof/f with Neurod6-Cre, PV-Cre, and GFAP-CreER, respectively. Thus, the NEO1 expression in the forebrain (especially the cortex and hippocampus) of NeoNeurod6-Cre, NeoPV-Cre, and NeoGFAP-CreER mice was depleted in Neurod6-Cre+ pyramidal neurons, PV-Cre+ interneurons, and GFAP+ astrocytes, respectively. EEG recordings in the hippocampus of these mice showed normal epileptiform activities with or without PTZ treatment in NeoNeurod6-Cre or NeoPV-Cre mice as compared with those of control mice (Fig. 1 A–H), suggesting little to no effect of NEO1 in Neurod6-Cre+ pyramidal neurons or PV-Cre+ interneurons in this event. However, in NeoGFAP-CreER mice, upon tamoxifen treatment (100 mg/kg/d, once/day for 3 d, at age of 4 wk), NEO1 protein was selectively depleted in astrocytes of NeoGFAP-CreER mice (SI Appendix, Fig. S3), due to the Cre activity (viewed by tdTomato of Ai9 reporter mice) was turned on predominantly in GFAP+ astrocytes (SI Appendix, Fig. S3B). Intriguingly, increases in epileptiform spikes and seizure susceptibility induced by low dose (30 mg/kg) of PTZ were recorded in NeoGFAP-CreER mice (Fig. 1 I–L), which resembled the epileptic responses in NeoGFAP-Cre mice. Next, to determine whether astrocyte-specific KO of Neo1 increases seizure susceptibility in a chronic epilepsy model, we performed DG-kindled seizures in control and NeoGFAP-CreER mice. As shown in SI Appendix, Fig. S4A, an electrode was implanted in the DG of control and NeoGFAP-CreER mice at the age of 8 wk upon tamoxifen treatment; after 1 wk recovery, all the mice underwent kindling stimulations (a 3 s train of 50 Hz monophasic pulses at the duration of 0.5 ms and intensity of afterdischarge threshold) three times per day until stage 5 seizures were elicited over three consecutive days, which was considered as kindled mice. Whereas control and NeoGFAP-CreER mice showed comparable development of kindling seizures, longer duration of seizures (> stage3) and larger increase in epileptiform spikes were observed in NeoGFAP-CreER mice as compared with those of control mice (SI Appendix, Fig. S4 B–F). Together, these results suggest that the astrocytic, but not neuronal, NEO1 is likely to be responsible for its role in preventing seizure susceptibility.
Fig. 1.
Increased epileptic response in astrocytic, but not neuronal, Neo1 KO mice. (A) Schematic diagram of experimental design in NeoNeurod6-Cre for EEG recordings. (B) Representative EEG traces of NeoNeurod6-Cre mice with or without PTZ treatment. Traces indicated by blue lines were zoomed out below. (C) Quantification of the data in B, the number of epileptiform spikes per hour. n = 4 mice. Student’s t test. (D) Quantification of the data in B, the EEG power spectrum of different frequency after PTZ treatment. n = 4 mice. (E) Schematic diagram of experimental design in NeoPV-Cre mice for EEG recordings. (F) Representative EEG traces of NeoPV-Cre mice with or without PTZ treatment. Traces indicated by blue lines were zoomed out below. (G) Quantification of the data in F, the number of epileptiform spikes per hour. n = 3 mice. Student’s t test. (H) Quantification of the data in F, the EEG power spectrum of different frequency after PTZ treatment. n = 3 mice. (I) Schematic diagram of experimental design in NeoGFAP-CreER mice for EEG recordings. (J) Representative EEG traces of NeoGFAP-CreER mice with or without PTZ treatment. Traces indicated by blue lines were zoomed out below. (K) Quantification of the data in J, the number of epileptiform spikes per hour. n = 4 mice. Student’s t test. (L) Quantification of the data in J, the EEG power spectrum of different frequency after PTZ treatment. n = 5 mice for control group; and n = 4 mice for NeoGFAP-CreER group. Two-way ANOVA test, genotype factor: F(1, 217) = 60.09, P < 0.0001. Data in C, D, G, H, K, and L are presented as the mean ± SEM. ***P < 0.001.
Necessity of Hippocampal Astrocytic NEO1 in Preventing Epileptic Response.
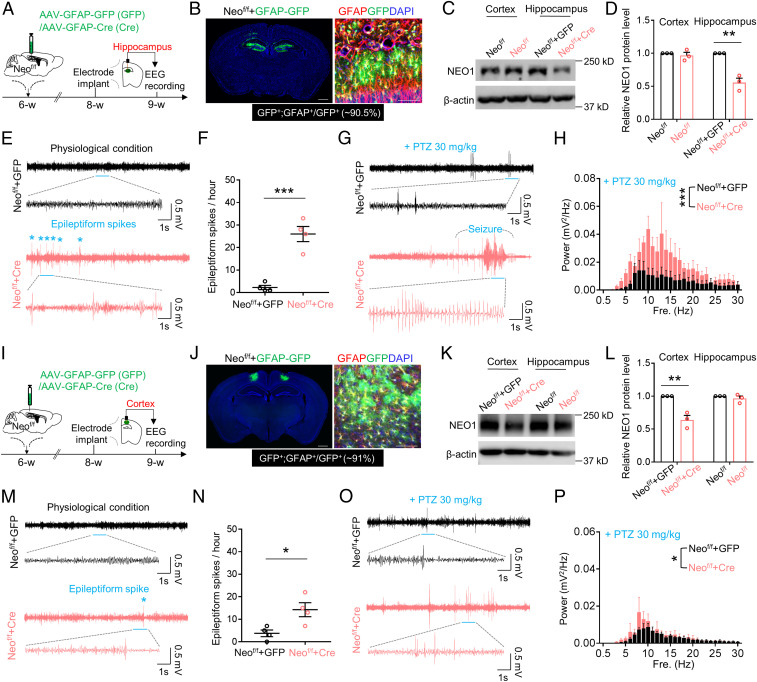
We further addressed whether astrocytic NEO1 prevents seizure susceptibility in a brain region (hippocampus and/or cortex) specific manner. To this end, AAV-GFAP-GFP/Cre viruses, which expressed GFP/Cre under the control of GFAP promotor, were bilaterally injected into the hippocampus or lateral parietal association cortex of Neof/f mice to KO Neo1 in astrocytes in a brain region specific manner (Fig. 2 A and I). As expected, ∼90% of GFAP promotor driven GFP/Cre expression was detected in GFAP+ astrocytes (Fig. 2 B and J). Neof/f brain regions (hippocampus or cortex) injected with AAV-GFAP-Cre showed significant decreases in NEO1 protein levels in the injected regions as compared with those injected with the control viruses (AAV-GFAP-GFP) (Fig. 2 C, D, K, and L). Interestingly, EEG recordings showed increased epileptiform activities in the hippocampus, where astrocytic NEO1 was depleted, at a comparable degree as those epileptiform activities detected in NeoGFAP-Cre or NeoGFAP-CreER mice (Fig. 2 E and F). In addition to the increased epileptiform spikes, the seizure susceptibility induced by PTZ was also enhanced in astrocytic Neo1 KO hippocampus (Fig. 2 G and H). However, in astrocytic Neo1 KO cortex (Neof/f cortex injected with AAV-GFAP-Cre), whereas more epileptiform spikes were detected by EEG recordings, the seizure susceptibility induced by PTZ was comparable to that of control cortex (Neof/f cortex injected with AAV-GFAP-GFP) (Fig. 2 M–P). These results thus suggest that hippocampal astrocytic NEO1 plays a key role in preventing seizure susceptibility.
Fig. 2.
Increased epileptiform spikes and seizure susceptibility in mice with Neo1 KO in hippocampal astrocytes. (A) Schematic diagram of experimental design in Neof/f mice with virus injection in the hippocampus for EEG recordings. (B) Representative images of Neof/f mice with virus injection in the hippocampus. GFAP (red) and GFP (green) indicate GFAP promoter mediated protein expression in astrocytes. DAPI (4′,6-diamidino-2-phenylindole) (blue) was used for staining cell nucleus. (Scale bar, 200 μm.) (C) Western blot analysis of NEO1 protein level in Neof/f mice injected with AAV-GFAP-GFP (Neof/f+GFP) or AAV-GFAP-Cre (Neof/f+Cre) in the hippocampus. (D) Quantification of the data in C, the NEO1 band intensity was normalized by β-actin; values of Neof/f mice injected with AAV-GFAP-GFP (Neof/f+GFP) were taken as 1. n = 3 mice. Student’s t test. (E) Representative EEG traces of Neof/f mice injected with AAV-GFAP-GFP or AAV-GFAP-Cre virus in the hippocampus. Traces indicated by blue lines were zoomed out below. (F) Quantification of the data in E, the number of epileptiform spikes per hour. n = 4 mice. Student’s t test. (G) Representative EEG traces of Neof/f mice injected with AAV-GFAP-GFP or AAV-GFAP-Cre virus in the hippocampus after PTZ treatment. Traces indicated by blue lines were zoomed out below. (H) Quantification of the data in G, the EEG power spectrum of different frequency. n = 4 mice for Neof/f+GFP group and n = 5 mice for Neof/f+Cre group. Two-way ANOVA test, genotype factor: F(1, 217) = 42.1, P < 0.0001. (I) Schematic diagram of experimental design in Neof/f mice with virus injection in the cortex for EEG recordings. (J) Representative images of Neof/f mice with virus injection in the cortex. GFAP (red) and GFP (green) indicate GFAP promoter mediated protein expression in astrocytes. DAPI (blue) was used for staining cell nucleus. (Scale bar, 200 μm.) (K) Western blot analysis of NEO1 protein level in Neof/f mice injected with AAV-GFAP-GFP (Neof/f +GFP) or AAV-GFAP-Cre (Neof/f +Cre) in the cortex. (L) Quantification of the data in K, the NEO1 band intensity was normalized by β-actin; values of Neof/f mice injected with AAV-GFAP-GFP (Neof/f+GFP) were taken as 1. n = 3 mice. Student’s t test. (M) Representative EEG traces of Neof/f mice injected with AAV-GFAP-GFP or AAV-GFAP-Cre virus in the cortex. Traces indicated by blue lines were zoomed out below. (N) Quantification of the data in M, the number of epileptiform spikes per hour. n = 4 mice. Student’s t test. (O) Representative EEG traces of Neof/f mice injected with AAV-GFAP-GFP or AAV-GFAP-Cre virus in the cortex after PTZ treatment. Traces indicated by blue lines were zoomed out below. (P) Quantification of the data in O, the EEG power spectrum of different frequency. n = 4 mice for Neof/f+GFP group and n = 3 mice for Neof/f+Cre group. Two-way ANOVA test, genotype factor: F(1, 155) = 6.045, P = 0.015. Data in D, F, H, L, N, and P are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
Decreased GABAergic Synaptic Transmission in Astrocytic Neo1 KO Mice.
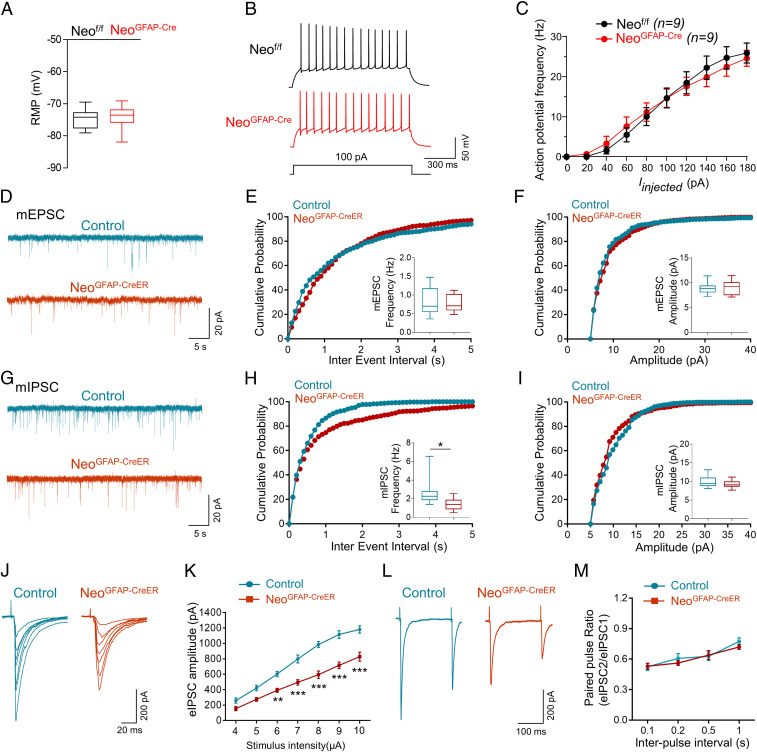
Epileptic response is often due to alterations in excitatory/inhibitory balance (e.g., increase in glutamate-induced excitatory neurotransmission and/or decrease in GABAergic inhibitory activity) (41, 42) and/or elevation in excitatory neuronal excitability (43). Notice that DG is highly associated with temporal lobe epilepsy, and it appears to be a “gate” in preventing seizures from entering CA3 and CA1 (44, 45). Thus, we examined DG granule neurons’ activities in control and Neo1 KO mice by electrophysiological (ePhys-) recordings. As shown in Fig. 3 A–C, DG granule neurons in NeoGFAP-Cre mice showed little changes in resting membrane potential and AP firing rates as compared with those of controls, eliminating the possible contribution to the epileptic response by increasing intrinsic neuronal excitability. Examining the frequency and amplitude of glutamate-induced excitatory neurotransmission (miniature excitatory postsynaptic currents [mEPSCs]) showed little to no change in NeoGFAP-Cre mice (SI Appendix, Fig. S5 A–C). However, recording and analyzing GABAergic inhibitory activity (miniature inhibitory postsynaptic currents [mIPSCs]) showed a significant decrease in the frequency, but not the amplitude of mIPSCs in NeoGFAP-Cre DG granule neurons (SI Appendix, Fig. S5 D–F). Additionally, this specific deficit in GABAergic, but not glutamatergic, synaptic transmission was also observed in NeoGFAP-CreER mice after tamoxifen treatment (Fig. 3 D–I). We further examined the evoked IPSC (eIPSC) and found that the amplitudes of eIPSCs in NeoGFAP-Cre and NeoGFAP-CreER mice were significantly decreased as compared with those of control mice (Fig. 3 J and K and SI Appendix, Fig. S5 G and H). These results suggest an impaired GABAergic synaptic transmission in astrocytic Neo1 KO mice.
Fig. 3.
Impaired GABAergic transmission in DG neurons of Neo1 KO mice. (A) Comparable resting membrane potentials of DG neurons in Neof/f and NeoGFAP-Cre mice. n = 15 neurons, 3 mice for Neof/f group; n = 19 neurons, 3 mice for NeoGFAP-Cre group. Student’s t test. (B) Representative traces of spikes evoked by injecting depolarizing currents. (C) Firing rate plotted against increasing injected currents. n = 9 neurons, 3 mice for each genotype. (D) Representative traces of mEPSCs in DG neurons of control and NeoGFAP-CreER mice. (E and F) Quantification of the data in D, the frequency (E) and amplitude (F) of mEPSCs. n = 8 neurons, 3 mice for each genotype. Student’s t test. (G) Representative traces of mIPSCs in DG neurons of control and NeoGFAP-CreER mice. (H and I) Quantification of the data in G, the frequency (H) and amplitude (I) of mIPSCs. n = 8 neurons, 3 mice for control group; n = 10 neurons, 3 mice for NeoGFAP-CreER group. Student’s t test. (J) Representative traces of eIPSCs in DG neurons of control and NeoGFAP-CreER mice after different intensity of stimulations. (K) Quantification of the data in J, the amplitude of eIPSCs. n = 9 neurons, 3 mice for control group; n = 10 neurons, 3 mice for NeoGFAP-CreER group. Student’s t test. (L) Representative traces of pair-pulse stimulation. (M) PPRs plotted against interstimulus intervals. n = 10 neurons, 3 mice for each group. Data in C and M are presented as the mean ± SEM; data in A, E, F, H, I, and K are presented as median with interquartile range, whiskers are the minimum and maximum. *P < 0.05, ***P < 0.001.
Reduced GABAergic, but Not Glutamatergic, Vesicles in DG Neurons of NeoGFAP-CreER Mice.
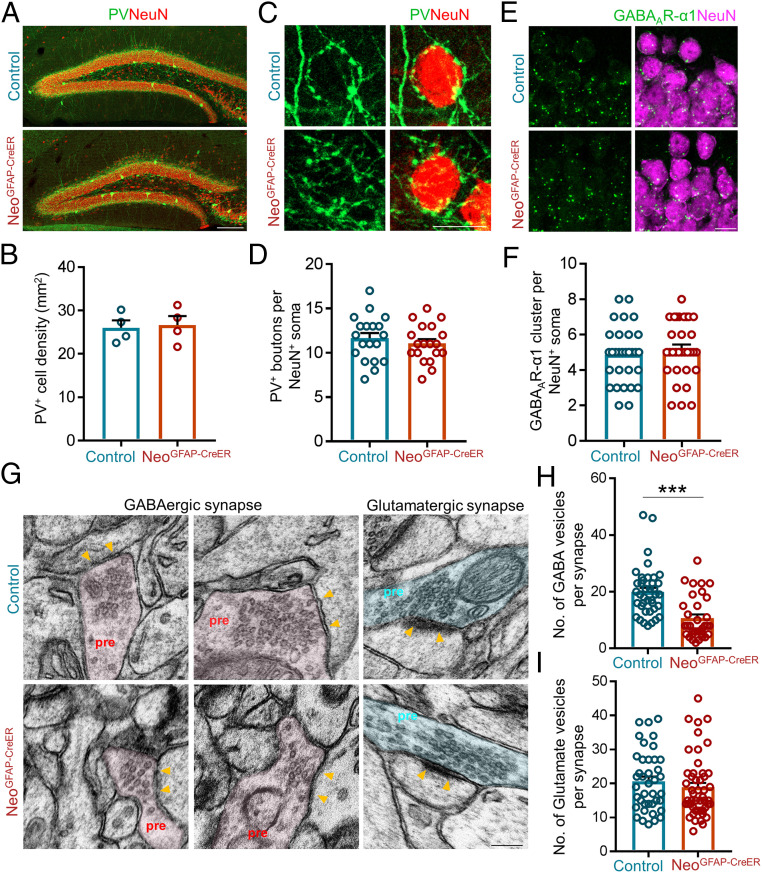
The reduced frequency, but not amplitude, of mIPSCs in Neo1 mutant mice implicate potential deficits in presynaptic GABA release probability, number of GABAergic synapses, and/or number of presynaptic vesicles of GABAergic neurons. To test these possibilities, we first examined the paired-pulse ratio (PPR) of eIPSC in DG neurons, which measures the presynaptic release probability (46). As shown in Fig. 3 L and M and SI Appendix, Fig. S5 I and J, the PPR in the mutant DG neurons was comparable to that of control mice, excluding this possibility for the deficit of the GABAergic transmission in Neo1 mutant mice. Second, we examined GABAergic interneuron numbers and their synapses in DG of NeoGFAP-CreER mice by immunostaining analyses. Using antibody against parvalbumin (PV), a marker for major group of GABAergic neurons in the hippocampus, a comparable level in PV+ interneuron density was detected in Neo1 mutant DG to those of controls (Fig. 4 A and B). Additionally, similar levels of GABAergic presynaptic terminals (boutons) that surround DG neurons and GABAA-receptors at the postsynaptic sites of DG neurons were detected in Neo1 mutant mice to those of controls (Fig. 4 C–F). These results thus suggest a normal number of GABAergic synapses in Neo1 mutant mice. Third, we examined the presynaptic vesicle numbers in hippocampal neurons of control and Neo1 mutant mice by electromicroscopy. Remarkably, a decrease in vesicles in the inhibitory/GABAergic, but not excitatory/glutamatergic, synapses was detected in Neo1 mutant mice as compared with those of controls (Fig. 4 G–I). Taken together, these results suggest that the reduced GABAergic vesicles might be the major mechanism for the decreased frequency of mIPSCs or the impaired GABAergic transmission in astrocyte-specific Neo1 KO mice.
Fig. 4.
Reduced GABAergic, but not glutamatergic, vesicles in DG neurons of astrocytic Neo1 KO mice. (A) Coimmunostaining of PV (green) and NeuN (red) in DG of control and NeoGFAP-CreER mice. (Scale bar, 100 μm.) (B) Quantification of the data in (A), the density of PV+ interneurons in DG. n = 4 mice for each genotype. Student’s t test. (C) Coimmunostaining of PV+ (green) presynaptic terminals and NeuN (red) in DG of control and NeoGFAP-CreER mice. (Scale bar, 10 μm.) (D) Quantification of the data in (C), the number of PV+ boutons surrounding the NeuN+ soma. n = 19 neurons, 3 mice for each genotype. Student’s t test. (E) Coimmunostaining of NeuN (green) and GABAAR-α1 (red) in DG of control and NeoGFAP-CreER mice. DAPI staining indicates cell nucleus. (Scale bar, 10 μm.) (F) Quantification of the data in E, the number of GABAAR-α1+ cluster per NeuN+ soma. n = 32 neurons, 3 mice for each genotype. Student’s t test. (G) Representative EM images of GABAergic and glutamatergic synapses in control and NeoGFAP-CreER mice. Yellow arrows indicate the postsynaptic areas. (H and I) Quantification of data in G, the number of GABAergic vesicles per synapse (H) and the number of glutamatergic vesicles per synapse (I). n = 39 synapses for control group and n = 34 synapses for NeoGFAP-CreER group in H; n = 40 synapses for control group and n = 49 synapses for NeoGFAP-CreER group in I. Student’s t test. Data in B, D, F, H, and I are presented as the mean ± SEM.
Requirement of NEO1 for GLAST Surface Distribution.
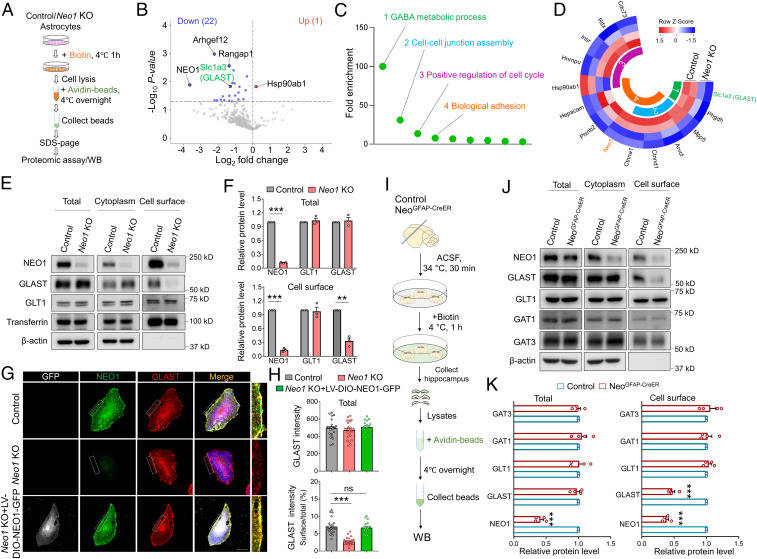
To understand how astrocytic NEO1 deficiency regulates GABAergic transmission and epileptic response, the proteomic analysis by liquid chromatography-tandem mass spectrometry was carried out to screen for altered plasma membrane proteins in primary cultured Neo1 KO astrocytes. As shown in Fig. 5A, both control and Neo1 KO astrocytes were cultured in vitro and incubated with biotin for 1 h at 4 °C to label cell surface membrane proteins. The surface proteins labeled by biotin were pulled down by avidin beads and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-Page) gel and proteomic analyses. Among a total of 344 proteins identified, 22 proteins were down-regulated and 1 protein was up-regulated in Neo1 KO astrocytes, as compared with those of controls (Fig. 5B). Interestingly, gene ontology (GO) enrichment analysis of these dysregulated proteins exhibited different biological processes, including GABA metabolic process (Fig. 5C). This view was supported by the observation that GLAST, a glutamate-aspartate transporter abundantly expressed in astrocytes, was markedly reduced in Neo1 KO astrocytes as compared with that of controls (Fig. 5D). The reduction of cell surface GLAST in Neo1 KO astrocytes was further confirmed by WB analysis of lysates of membrane and cytosol fractions of astrocytes, which showed a marked decrease of GLAST in cell surface, but not total, lysates of Neo1 KO astrocytes (Fig. 5 E and F). In contrast, GLT1, the other glutamate transporter in astrocytes, was unaffected by Neo1 KO (Fig. 5 E and F). The reduced cell surface level of GLAST in Neo1 KO astrocytes was further verified by immunostaining analysis, and GLAST surface levels in Neo1 KO astrocytes were restored upon infection with LV-DIO-NEO1-GFP (Fig. 5 G and H). We then wondered whether GLAST level was changed in vivo; to this end, cell surface biotinylation of fresh hippocampal slices from NeoGFAP-CreER and control mice were performed as illustrated in Fig. 5I. Again, GLAST in the cell surface, but not total, lysates was significantly decreased in NeoGFAP-CreER mice as compared with that of control mice (Fig. 5 J and K). However, GLT1 and GABA transporters, including GAT1 and GAT3, were not changed by astrocyte-specific KO of Neo1 (Fig. 5 J and K).
Fig. 5.
Decreased GLAST cell surface level in Neo1 KO astrocytes and hippocampus of NeoGFAP-CreER mice. (A) Schematic diagram of quantitative proteomic analysis. Proteomic data were obtained from surface proteins of control and Neo1 KO astrocytes. (B) Volcano plots of differentially expressed proteins in the cell surface of control and Neo1 KO astrocytes. (C) GO enrichment analysis of dysregulated proteins in biological processes. (D) Heat map of selected differentially expressed proteins in the first four biological processes of C. (E) Western blot analyses of lysates of total and cell surface fractions of control and Neo1 KO astrocytes. (F) Quantification of the data in E, the relative band intensity of total (up) and cell surface (down) NEO1, GLT1, and GLAST. n = 3 for each group. Student’s t test. (G) Coimmunostaining of GFP (white), NEO1 (green), GLAST (red), and GFAP (blue) in cultured control, Neo1 KO and Neo1 KO+LV-DIO-NEO1-GFP astrocytes. (Scale bar, 20 μm.) (H) Quantification of the data in G, the fluorescent intensity of total GLAST (up) and the ratio of cell surface GLAST over total GLAST (down). n = 23 cells for control group; n = 24 cells for Neo1 KO group; n = 17 cells for Neo1 KO+LV-DIO-NEO1-GFP group. One-way ANOVA test, F(2, 61) = 81.06, P < 0.0001. (I) Schematic diagram of biotinylation of cell surface proteins in hippocampus of control and NeoGFAP-CreER mice. (J) Western blot analyses of homogenates of total and cell surface fractions of control and NeoGFAP-CreER hippocampus. (K) Quantification of the data in J, the relative band intensity of total (Left) and cell surface (Right) NEO1, GLT1, GLAST, GAT1, and GAT3. n = 4 for each group. Student’s t test. Data in F, H, and K are presented as the mean ± SEM. **P < 0.01; ***P < 0.001.
Next, to investigate how NEO1 regulates GLAST surface distribution, we wondered whether NEO1 interacts with GLAST. To this end, hippocampal homogenates of adult mouse were subjected to coimmunoprecipitation (co-IP) assay. NEO1 was indeed detected in the immunocomplex precipitated by GLAST antibody, but not control IgG (SI Appendix, Fig. S6A), suggesting that GLAST and NEO1 formed a complex in mouse hippocampus. The interaction between NEO1 and GLAST was further confirmed in HEK293T cells cotransfected with plasmids of Flag-NEO1 and human GLAST (hGLAST) (SI Appendix, Fig. S6B). To address whether the interaction affects GLAST trafficking or stabilization, we coimmunostained GLAST with GM130 (a Golgi marker) or EEA1 (an early endosome marker) in 293T cells cotransfected plasmids of Flag-NEO1 and hGLAST. Remarkably, overexpression of Flag-NEO1 resulted in a significant increase of GLAST within the cell surface but a decrease in GM130+ trans-Golgi, without a change in EEA1+ early endosomes, as compared with those in 293T cells cotransfected with Flag control plasmids and hGLAST (SI Appendix, Fig. S6 C–H). Together, these results suggest that NEO1 interacting with GLAST are likely to promote GLAST trafficking from trans-Golgi to the cell surface. Together, these results indicate that NEO1 is required and sufficient for GLAST cell surface distribution.
Impaired Glutamate Uptake and Glutamine Synthesis, Accompanied with Increased Extracellular Glutamate Levels, by Depleting Astrocytic Neo1.
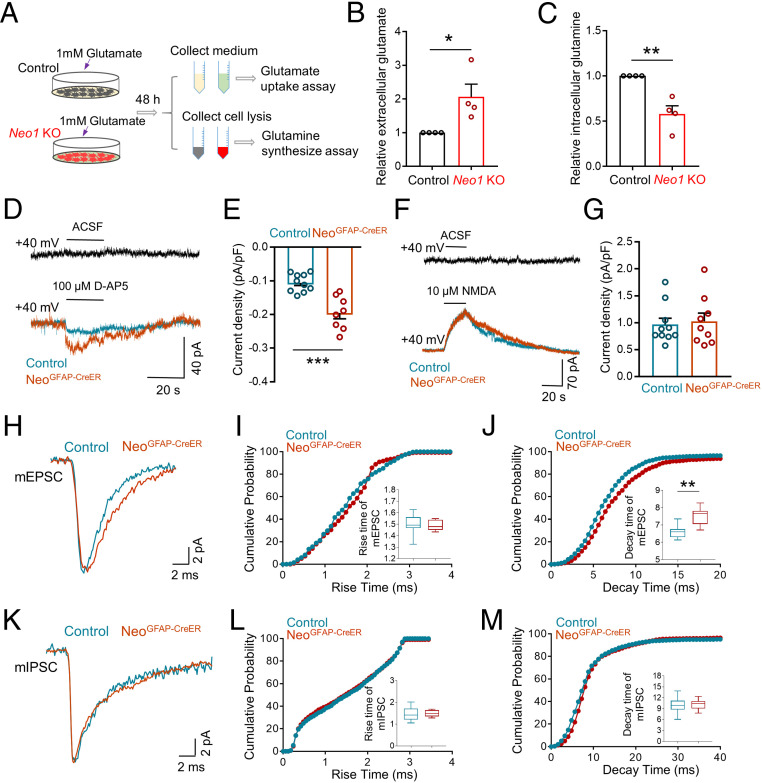
Given that GLAST is one of the key glutamate transporters in astrocytes (47) and its surface level was reduced in Neo1 KO astrocytes and NeoGFAP-CreER hippocampus (Fig. 5), we examined whether Neo1 KO astrocytes show an impairment in glutamate uptake. To this end, both control and Neo1 KO astrocytes were incubated with 1mM glutamate for 48 h; and the glutamate levels in the culture medium were measured by colorimetric assay (Fig. 6A). In line with the reduction of cell surface GLAST in Neo1 KO astrocytes, the concentration of glutamate in the medium was much higher in the mutant cultures than that of controls, indicating a deficit in glutamate uptake (Fig. 6B). Additionally, the intracellular glutamine, which were synthesized from glutamate (48), were also measured. Decreased glutamine levels were detected in Neo1 KO astrocytes, as compared with that of controls (Fig. 6C), suggesting that depletion of Neo1 in astrocytes impairs their glutamate uptake and glutamine synthesis.
Fig. 6.
Impaired glutamate uptake and glutamine synthesis, accompanied with increased extracellular glutamate levels, by depleting astrocytic Neo1. (A) Schematic diagram of glutamate uptake assay and glutamine synthesis assay in control and Neo1 KO astrocytes. (B and C) Quantification of the data in A, the relative extracellular glutamate (B) and intracellular glutamine level (C). Values of control astrocytes were taken as 1. n = 4 for each group. Student’s t test. (D) Representative traces of DG neurons after adding 100 µM D-AP5 in control and NeoGFAP-CreER brain slices. ACSF: Artificial cerebrospinal fluid. (E) Quantification of the data in D, the current density elicited by 100 µM D-AP5. n = 10 neurons, 3 mice for control group; n = 8 neurons, 3 mice for NeoGFAP-CreER group. Student’s t test. (F) Representative traces of DG neurons after adding 10 µM NMDA in control and NeoGFAP-CreER brain slices. ACSF: Artificial cerebrospinal fluid. (G) Quantification of the data in F, the current density evoked by 10 µM NMDA. n = 10 neurons, 3 mice for control group; n = 9 neurons, 3 mice for NeoGFAP-CreER group. Student’s t test. (H) Representative trace of a single mEPSC in DG neurons of control and NeoGFAP-CreER mice after tamoxifen treatment. (I and J) Quantification of the data in H, the rise time (I) and decay time (J) of mEPSC. n = 8 neurons, 3 mice for each genotype. Student’s t test. (K) Representative trace of a single mIPSC in DG neurons of control and NeoGFAP-CreER mice after tamoxifen treatment. (L and M) Quantification of the data in K, the rise time (L) and decay time (M) of mIPSC. n = 8 neurons, 3 mice for control group; n = 10 neurons, 3 mice for NeoGFAP-CreER group. Student’s t test. Data in B, C, E, and G are presented as the mean ± SEM; data in I, J, L, and M are presented as median with interquartile range; whiskers are the minimum and maximum. *P < 0.05, **P < 0.01, ***P < 0.001.
Next, to determine whether extracellular glutamate concentration was changed in NeoGFAP-CreER mice after tamoxifen treatment, we recorded high-affinity NMDA receptors (NMDARs)-mediated tonic currents in DG neurons, as this recording uses NMDARs to detect extracellular glutamate as reported previously (49, 50). In brief, neuronal membrane potential was held at +40 mV to relieve the magnesium block of NMDARs. After a stable baseline holding current was achieved, D-AP5 (100 µM), a competitive NMDAR antagonist, was applied and the tonic NMDARs currents were revealed by changes in the holding current. As shown in Fig. 6 D and E, the density of tonic NMDARs currents in NeoGFAP-CreER mice was much larger than that of control mice. This could result from a higher extracellular glutamate level and/or a higher expression of cell surface NMDAR. To distinguish both possibilities, a saturated high concentration of NMDA (10 µM) was applied to activate all NMDARs and the same level of NMDA-induced outward current was observed, excluding the possibility that NeoGFAP-CreER mice expressed more NMDARs (Fig. 6 F and G) and suggesting that the larger NMDARs current density in NeoGFAP-CreER mice was likely to be induced by higher concentration of extracellular glutamate. Additionally, we also analyzed the rise time and decay time of mEPSC and mIPSC in control and NeoGFAP-CreER mice after tamoxifen treatment. Intriguingly, a significant slower decay time, but not the rise time, of glutamate-induced mEPSC was observed in NeoGFAP-CreER mice than that of control mice (Fig. 6 H–J), in line with the view for an impairment of extracellular glutamate clearance in the mutant mice. Notice that the Neo1 mutant mice had little effect on the rise and decay time of mIPSC (Fig. 6 K–M), implicating normal clearance of extracellular GABA in the mutant mice. Taken together, these results suggest that astrocyte-specific KO of Neo1 reduced glutamate uptake, and thus increased extracellular glutamate, and impaired glutamate–glutamine cycle.
Restore of Glutamate Uptake in Neo1 KO Astrocytes and Diminish of Epileptic Response in NeoGFAP-CreER Mice by Expressing GLAST or NEO1.
We then addressed whether increasing astrocytic GLAST expression could restore glutamate uptake deficits in Neo1 KO astrocytes. Both control and Neo1 KO astrocytes were infected with LV-GLAST-GFP viruses that encode mouse GLAST protein fused with GFP (SI Appendix, Fig. S7A). Interestingly, compared to control astrocytes infected with LV-GFP, overexpression of GLAST with LV-GLAST-GFP viruses significantly increased GLAST surface level in control astrocytes, as well as recruited a number of GLAST onto the cell membrane in Neo1 KO astrocytes (SI Appendix, Fig. S7 B and C). The glutamate uptake and glutamine synthesis deficits were also diminished in Neo1 KO astrocytes by LV-GLAST-GFP (Fig. 7 A and B).
Fig. 7.
Restore of glutamate uptake in Neo1 KO astrocytes and diminish of epileptic response in NeoGFAP-CreER mice by expressing GLAST. (A) Schematic diagram of glutamate uptake assay and glutamine synthesis assay in control and Neo1 KO astrocytes infected with LV-GFP or LV-GLAST-GFP, respectively. (B) Quantification of the data in A, the relative extracellular glutamate (Left) and intracellular glutamine level (Right). Values of control astrocytes infected with LV-GFP were taken as 1. n = 4 for each group. Two-way ANOVA test for extracellular glutamate level, interaction: F(1, 12) = 8.907, P = 0.0114; genotype factor: F(1, 12) = 59.24, P < 0.0001; virus factor: F(1, 12) = 30.66, P = 0.0001. Two-way ANOVA test for intracellular glutamine level, F(1, 12) = 0.3113, P = 0.5872; genotype factor: F(1, 12) = 61.67, P < 0.0001; virus factor: F(1, 12) = 126.5, P < 0.0001. (C) Schematic diagram of experimental design for EEG recordings in hippocampus of control and NeoGFAP-CreER mice injected with LV-GFP, LV-GLAST-GFP, or LV-DIO-NEO1-GFP, respectively. Mice injected LV-DIO-NEO1-GFP received additional tamoxifen treatment (100 mg/kg/d, 3 d) at age of 7 wk. (D) Representative EEG traces of control and NeoGFAP-CreER mice injected with LV-GFP, LV-GLAST-GFP, or LV-DIO-NEO1-GFP in hippocampus. (E) Quantification of the data in D, the number of epileptiform spikes per hour. n = 3 mice for each group. Two-way ANOVA test, interaction: F(2, 12) = 68.41, P < 0.0001; genotype factor: F(1, 12) = 87.17, P < 0.0001; virus factor: F(2, 12) = 75.17, P < 0.0001. (F) Representative EEG traces of control and NeoGFAP-CreER mice injected with LV-GFP, LV-GLAST-GFP, or LV-DIO-NEO1-GFP in hippocampus after PTZ treatment. (G) Quantification of the data in F, the EEG power spectrum of different frequency. n = 3 mice for Control + LV-GFP, Control + LV-GLAST-GFP, Control + LV-DIO-NEO1-GFP and NeoGFAP-CreER + LV-DIO-NEO1-GFP group; n = 4 mice for NeoGFAP-CreER + LV-GFP and NeoGFAP-CreER + LV-GLAST-GFP group. Two-way ANOVA test, interaction: F(150, 434) = 1.545, P = 0.0004; genotype factor: F(5, 434) = 74.11, P < 0.0001. (H) Summary of results and a working model for hippocampal astrocytic NEO1 regulates glutamate uptake and IPSCs, and prevents epileptic response. Data in B, E, and G are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001; ns, no significant difference.
Next, we determined whether expression of GLAST in the hippocampus could ameliorate epileptic response in Neo1 mutant mice. To this end, LV-GFP or LV-GLAST-GFP viruses were bilaterally injected into the hippocampus of control and NeoGFAP-CreER mice after tamoxifen treatment, and 3 wk later, each group of mice were recorded by EEG (Fig. 7C). Indeed, significant decreases of epileptiform spikes and seizure susceptibility were observed in NeoGFAP-CreER mice injected with LV-GLAST-GFP viruses as compared with those of NeoGFAP-CreER mice injected with LV-GFP viruses (Fig. 7 D–G). Additionally, the epileptiform activities observed in NeoGFAP-CreER mice were also rescued by injecting LV-DIO-NEO1-GFP (Fig. 7 C–G), which restored NEO1 protein level in NeoGFAP-CreER hippocampus under the control of Cre with a GFP reporter (SI Appendix, Fig. S8). Taken together, these results support the view that hippocampal astrocytic NEO1 prevents epileptic response likely by promoting GLAST-mediated glutamate uptake.
Discussion
Astrocytes are known to be key players in balancing the brain activity, and deficits in astrocytes could contribute to the development of various neurological diseases, including epilepsy. However, the detailed mechanisms underlying astrocyte regulation of epilepsy remain elusive. Here, we provide evidence for NEO1’s function in hippocampal astrocytes to prevent epileptiform activities. NEO1 expression is decreased in hippocampus of patients with epilepsy. Loss of Neo1 in mouse hippocampal GFAP+ astrocytes results in epileptiform activities. To understand how astrocytic NEO1 regulates epileptic response, a working model is depicted in Fig. 7H, in which, NEO1 maintains GLAST cell surface distribution and glutamate uptake activity in hippocampal astrocytes and thus promoting extracellular glutamate clearance and GABA synthesis and preventing epileptic activity. These results reveal a function of hippocampal astrocytic NEO1 in preventing epileptic response and uncover NEO1 as a valuable therapeutic target to protect the brain from epilepsy.
Epilepsy is often due to the disruption of physiological excitatory/inhibitory balance in the brain (8), which could be caused by both genetic factors and acquired injuries. To date, a number of genes have been identified and associated with epilepsy by genetic technology, which provide an important basis for clinical diagnosis and treatment of epilepsy. Mutations in some neuronal genes (e.g., SCN1A, SCN2A, and SCN8A) cause epilepsy through impairing GABAergic interneurons’ function or modulating excitatory neuronal excitability (10, 11). Additionally, dysregulation of some astrocytic genes (e.g., KCNJ10, AQP4, and GLUL) cause epilepsy through disturbing ion (K+) homeostasis and/or reducing synaptic release of GABA (12). Intriguingly, by RNA-seq and proteomic analyses of the hippocampus from patients with epilepsy, in addition to these previously identified epilepsy-associated genes, we have detected changes in several unrecognized genes in patients with epilepsy as compared with that of controls; Neo1 (NEO1) is one of these genes (SI Appendix, Fig. S1).
NEO1 has been found to play multiple roles in the brain, including adult neurogenesis (29, 51), olfactory epithelium development (22), prevention of hydrocephalus-like deficit (52), and persistent hyperplastic primary vitreous formation (31). We have demonstrated that NEO1 in NSCs promotes neocortical astrogliogenesis (28) but inhibits astrocytic differentiation in the hippocampus (29), suggesting a brain region specific function of NEO1 in astrogliogenesis during development. However, astrocytic NEO1’s function in the young adult brain, particularly the hippocampus, remains elusive. Here, we have observed several lines of evidence for an essential role of hippocampal astrocytic NEO1 to prevent epileptic response. First, significant decreases of Neo1/NEO1 at both mRNA and protein levels were found in the hippocampus from patients with epilepsy as compared with that of controls (SI Appendix, Fig. S1). Second, KO Neo1 in hippocampal GFAP+ astrocytes resulted in epileptiform spikes and enhanced seizure susceptibility induced by PTZ, as well as prolonged generalized seizure durations (> stage 3) in DG-kindled seizures and increased more epileptiform spikes in kindled mice (Figs. 1 and 2 and SI Appendix, Fig. S4). Third, significant decreases in GABAergic synaptic transmission and increases in extracellular glutamate levels were observed in astrocytic Neo1 KO hippocampus (Figs. 3 and 6), which may contribute to the epileptiform activities.
How does astrocytic NEO1 regulate GABAergic synaptic transmission? Although astrocytes have multiple functions, one of their critical roles is to medicate the clearance of extracellular glutamate by glutamate uptake, which not only reduces excitotoxicity due to the accumulation of excessive glutamate in the extracellular space, but also converts intracellular glutamate into glutamine that constitutes the major precursor for the biosynthesis of GABA (53–55). Indeed, our observations that decreases in glutamate uptake and glutamine levels in Neo1 KO astrocytes and reduced GABAergic vesicles in DG neurons of astrocytic Neo1 KO hippocampus (Figs. 6 A–C and 4 G–I) are in line with the above view and suggest an impairment of glutamate–glutamine cycle by depletion of astrocytic Neo1. Also in agreement with the report that impaired glutamate–glutamine cycle could reduce GABAergic transmission (54), the frequency of mIPSCs and the amplitude of eIPSCs were significantly decreased in Neo1 KO mice as compared with those of controls (Fig. 3 G–K and SI Appendix, Fig. S5 D–H). In light of these observations, we speculate that the reduced GABAergic transmission in astrocytic Neo1 KO mice may result from their impaired glutamate–glutamine cycle with decreased GABAergic vesicles (Figs. 6 A–C and 4 G–I). Notice that the frequency and amplitude of glutamatergic transmission (mEPSCs) were unaffected (Fig. 3 D–F and SI Appendix, Fig. S5 A–C), supporting the view that excitatory synaptic transmission is independent of the glutamate–glutamine cycle (56), which may be explained by alternative sources of vesicular glutamate in excitatory neurons and/or increasing neuronal glutamate reuptake induced by elevated extracellular glutamate concentration.
How does NEO1 regulate astrocytic glutamate uptake? We propose that NEO1 regulates GLAST-mediated glutamate uptake by increasing and/or maintaining GLAST surface levels. Although total GLAST protein levels were not changed in epilepsy patients (Dataset S2) and Neo1 mutant mice (Fig. 5), the cell surface levels of GLAST, but not GLT1, were significantly decreased in both Neo1 KO astrocytes and NeoGFAPcreER hippocampus as compared with those of controls (Fig. 5). Additionally, while GLAST protein levels were markedly increased in the cell surface, its distribution in Golgi compartment was decreased upon co-expressing with Flag-NEO1 in 293T cells (SI Appendix, Fig. S6 C–F), suggesting that Flag-NEO1 may promote GLAST trafficking from trans-Golgi to the cell surface. However, we could not eliminate the possibility of NEO1 to maintain GLAST surface levels by its interaction with GLAST.
Notice that both GLT1 and GLAST are key glutamate transporters predominantly expressed in astrocytes (13, 14); GLAST appears to be more abundant than GLT-1 in the early postnatal ages, and GLT1 expression is gradually increased and maintains at high levels in adult ages (57–59). GLT1 KO mice show lethal spontaneous seizures (15), thus demonstrating a key function of GLT1 in regulating glutamate uptake in adult mouse. However, several lines of evidence suggest that the astrocytic GLAST in young adult age also plays a critical role in regulating glutamate uptake. First, GLAST expression remains at a high level in adult age, without a decline as compared with that in neonatal ages (57–59). Second, GLAST KO mice (10 to 11 wk of age) display higher seizure susceptibility with more severe stages of PTZ-induced seizures than control mice (16), suggesting the indispensable role of GLAST in preventing seizure. Third, the epileptic phenotypes in both NeoGFAP-Cre and NeoGFAPcreER mice were remarkably similar to that in GLAST KO mice, but different from that in GLT1 KO mice, supporting our model for NEO1-GLAST pathway to be involved in regulating epileptic response.
In summary, our findings suggest that NEO1-defciency is a risk factor for epilepsy. Loss of Neo1 in hippocampal GFAP+ astrocytes impairs astrocytic glutamate uptake by decreasing GLAST surface distribution, which causes a significant decrease in GABAergic synaptic transmission and increases in extracellular glutamate concentration and epileptiform activities.
Materials and Methods
Animals and Mouse Breeding.
Neogeninf/f (Neof/f) mice were maintained and genotyped as described previously (28). GFAP-Cre (#004600), PV-Cre (#017320), and Ai9 (#007907) mice were purchased from the Jackson Laboratory. Neurod6-Cre mice were kindly provided by Dr. KA Nave (60). GFAP-CreER mice were a gift from K.D. McCarthy (University of North Carolina, Chapel Hill, NC) 61). NeoGFAP-Cre, NeoNeurod6-Cre, and NeoPV-Cre mice were generated by crossing Neof/f with GFAP-Cre, Neurod6-Cre, and PV-Cre mice, respectively. Neowt/wt; GFAP-CreER (control), and NeoGFAP-CreER mice were generated as described in SI Appendix, Fig. S3A. NeoGFAP-CreER mice were crossed with Ai9 mice to get NeoGFAP-CreER—Ai9 mice. All the mouse lines were maintained in C57BL/6 strain background for >6 generations and confirmed by genotyping analysis with PCR. Mice were maintained on a 12 h light–dark cycle with ad libitum access to water and food. Male mice were used for all the studies. All the experiments with animals were performed with the approval of the Institutional Animal Care and Use Committee of Case Western Reserve University.
Detailed methods and materials regarding human patient samples, reagents, EEG, electrophysiological recordings, EM, virus injections, and biochemical experiments are provided in SI Appendix.
Supplementary Material
Acknowledgments
This work is supported in part by the start-up fund from Case Western Reserve University and NIH (AG045781) to W.C.X. We thank the members in Drs. Xiong’s and Dr. Mei’s laboratories for helpful discussions and suggestions. Hippocampal samples of patients with or without epilepsy were obtained from the NIH Neurobiobank at the University of Maryland, Baltimore, MD. We are grateful to the patients’ families and all the members that helped with the sample request.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2022921118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Thijs R. D., Surges R., O’Brien T. J., Sander J. W., Epilepsy in adults. Lancet 393, 689–701 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Hogan R. E., Epileptic seizures, brain volume changes, and “brain damage”: What do we know so far? Epilepsy Curr. 18, 224–226 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marchi N., Granata T., Ghosh C., Janigro D., Blood-brain barrier dysfunction and epilepsy: Pathophysiologic role and therapeutic approaches. Epilepsia 53, 1877–1886 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thom M., Review: Hippocampal sclerosis in epilepsy: A neuropathology review. Neuropathol. Appl. Neurobiol. 40, 520–543 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrell J. S., Wolff M. D., Teskey G. C., Neurodegeneration and pathology in epilepsy: Clinical and basic perspectives. Adv. Neurobiol. 15, 317–334 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Chen B., et al., A disinhibitory nigra-parafascicular pathway amplifies seizure in temporal lobe epilepsy. Nat. Commun. 11, 923 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jessberger S., Parent J. M., Epilepsy and adult neurogenesis. Cold Spring Harb. Perspect. Biol. 7, a020677 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scharfman H. E., The neurobiology of epilepsy. Curr. Neurol. Neurosci. Rep. 7, 348–354 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stafstrom C. E., Carmant L., Seizures and epilepsy: An overview for neuroscientists. Cold Spring Harb. Perspect. Med. 5, a022426 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye M., et al., Differential roles of NaV1.2 and NaV1.6 in regulating neuronal excitability at febrile temperature and distinct contributions to febrile seizures. Sci. Rep. 8, 753 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu F. H., et al., Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat. Neurosci. 9, 1142–1149 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Coulter D. A., Steinhäuser C., Role of astrocytes in epilepsy. Cold Spring Harb. Perspect. Med. 5, a022434 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothstein J. D., et al., Localization of neuronal and glial glutamate transporters. Neuron 13, 713–725 (1994). [DOI] [PubMed] [Google Scholar]
- 14.Chaudhry F. A., et al., Glutamate transporters in glial plasma membranes: Highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron 15, 711–720 (1995). [DOI] [PubMed] [Google Scholar]
- 15.Tanaka K., et al., Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276, 1699–1702 (1997). [DOI] [PubMed] [Google Scholar]
- 16.Watanabe T., et al., Amygdala-kindled and pentylenetetrazole-induced seizures in glutamate transporter GLAST-deficient mice. Brain Res. 845, 92–96 (1999). [DOI] [PubMed] [Google Scholar]
- 17.Demarque M., et al., Glutamate transporters prevent the generation of seizures in the developing rat neocortex. J. Neurosci. 24, 3289–3294 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajagopalan S., et al., Neogenin mediates the action of repulsive guidance molecule. Nat. Cell Biol. 6, 756–762 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Wilson N. H., Key B., Neogenin interacts with RGMa and netrin-1 to guide axons within the embryonic vertebrate forebrain. Dev. Biol. 296, 485–498 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Zhang A. S., Yang F., Wang J., Tsukamoto H., Enns C. A., Hemojuvelin-neogenin interaction is required for bone morphogenic protein-4-induced hepcidin expression. J. Biol. Chem. 284, 22580–22589 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Vries M., Cooper H. M., Emerging roles for neogenin and its ligands in CNS development. J. Neurochem. 106, 1483–1492 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Kam J. W., et al., RGMB and neogenin control cell differentiation in the developing olfactory epithelium. Development 143, 1534–1546 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Schmidt E. R., Pasterkamp R. J., van den Berg L. H., Axon guidance proteins: Novel therapeutic targets for ALS? Prog. Neurobiol. 88, 286–301 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Mawdsley D. J., et al., The Netrin receptor Neogenin is required for neural tube formation and somitogenesis in zebrafish. Dev. Biol. 269, 302–315 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Zhou Z., et al., Neogenin regulation of BMP-induced canonical Smad signaling and endochondral bone formation. Dev. Cell 19, 90–102 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong M., Schachter K. A., Jiang G., Krauss R. S., Neogenin regulates Sonic Hedgehog pathway activity during digit patterning. Dev. Dyn. 241, 627–637 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee D. H., et al., Neogenin inhibits HJV secretion and regulates BMP-induced hepcidin expression and iron homeostasis. Blood 115, 3136–3145 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Z., et al., Neogenin promotes BMP2 activation of YAP and Smad1 and enhances astrocytic differentiation in developing mouse neocortex. J. Neurosci. 36, 5833–5849 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun D., et al., Neogenin, a regulator of adult hippocampal neurogenesis, prevents depressive-like behavior. Cell Death Dis. 9, 8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun X. D., et al., Neogenin in amygdala for neuronal activity and information processing. J. Neurosci. 38, 9600–9613 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin S., et al., Neogenin-loss in neural crest cells results in persistent hyperplastic primary vitreous formation. J. Mol. Cell Biol. 12, 17–31 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao L. L., et al., Astrocytic neogenin/netrin-1 pathway promotes blood vessel homeostasis and function in mouse cortex. J. Clin. Invest. 130, 6490–6509 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J., et al., Epilepsy-associated genes. Seizure 44, 11–20 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Zhuo L., et al., hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis 31, 85–94 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Doetsch F., The glial identity of neural stem cells. Nat. Neurosci. 6, 1127–1134 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Milikovsky D. Z., et al., Electrocorticographic dynamics as a novel biomarker in five models of epileptogenesis. J. Neurosci. 37, 4450–4461 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhir A., Pentylenetetrazol (PTZ) kindling model of epilepsy. Curr. Protoc. Neurosci. Chapter 9, Unit9 37 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Adebimpe A., Aarabi A., Bourel-Ponchel E., Mahmoudzadeh M., Wallois F., EEG resting state analysis of cortical sources in patients with benign epilepsy with centrotemporal spikes. Neuroimage Clin. 9, 275–282 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ziemann A. E., et al., Seizure termination by acidosis depends on ASIC1a. Nat. Neurosci. 11, 816–822 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Racine R. J., Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 32, 281–294 (1972). [DOI] [PubMed] [Google Scholar]
- 41.De Fusco A., et al., Acute knockdown of Depdc5 leads to synaptic defects in mTOR-related epileptogenesis. Neurobiol. Dis. 139, 104822 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi M., Buckmaster P. S., Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J. Neurosci. 23, 2440–2452 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reid C. A., et al., Reduced dendritic arborization and hyperexcitability of pyramidal neurons in a Scn1b-based model of Dravet syndrome. Brain 137, 1701–1715 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Lothman E. W., Stringer J. L., Bertram E. H., The dentate gyrus as a control point for seizures in the hippocampus and beyond. Epilepsy Res. Suppl. 7, 301–313 (1992). [PubMed] [Google Scholar]
- 45.Heinemann U., et al., The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res. Suppl. 7, 273–280 (1992). [PubMed] [Google Scholar]
- 46.Cho J. H., Askwith C. C., Presynaptic release probability is increased in hippocampal neurons from ASIC1 knockout mice. J. Neurophysiol. 99, 426–441 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Pajarillo E., Rizor A., Lee J., Aschner M., Lee E., The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: Potential targets for neurotherapeutics. Neuropharmacology 161, 107559 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schousboe A., Scafidi S., Bak L. K., Waagepetersen H. S., McKenna M. C., Glutamate metabolism in the brain focusing on astrocytes. Adv. Neurobiol. 11, 13–30 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herman M. A., Jahr C. E., Extracellular glutamate concentration in hippocampal slice. J. Neurosci. 27, 9736–9741 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le Meur K., Galante M., Angulo M. C., Audinat E., Tonic activation of NMDA receptors by ambient glutamate of non-synaptic origin in the rat hippocampus. J. Physiol. 580, 373–383 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Leary C. J., et al., The Netrin/RGM receptor, Neogenin, controls adult neurogenesis by promoting neuroblast migration and cell cycle exit. Stem Cells 33, 503–514 (2015). [DOI] [PubMed] [Google Scholar]
- 52.O’Leary C. J., et al., Neogenin recruitment of the WAVE regulatory complex to ependymal and radial progenitor adherens junctions prevents hydrocephalus. Cell Rep. 20, 370–383 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Tani H., et al., A local glutamate-glutamine cycle sustains synaptic excitatory transmitter release. Neuron 81, 888–900 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang S. L., Carlson G. C., Coulter D. A., Dynamic regulation of synaptic GABA release by the glutamate-glutamine cycle in hippocampal area CA1. J. Neurosci. 26, 8537–8548 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fricke M. N., Jones-Davis D. M., Mathews G. C., Glutamine uptake by system A transporters maintains neurotransmitter GABA synthesis and inhibitory synaptic transmission. J. Neurochem. 102, 1895–1904 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Kam K., Nicoll R., Excitatory synaptic transmission persists independently of the glutamate-glutamine cycle. J. Neurosci. 27, 9192–9200 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Furuta A., Rothstein J. D., Martin L. J., Glutamate transporter protein subtypes are expressed differentially during rat CNS development. J. Neurosci. 17, 8363–8375 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kugler P., Schleyer V., Developmental expression of glutamate transporters and glutamate dehydrogenase in astrocytes of the postnatal rat hippocampus. Hippocampus 14, 975–985 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Schreiner A. E., et al., Laminar and subcellular heterogeneity of GLAST and GLT-1 immunoreactivity in the developing postnatal mouse hippocampus. J. Comp. Neurol. 522, 204–224 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Goebbels S., et al., Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis 44, 611–621 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Casper K. B., Jones K., McCarthy K. D., Characterization of astrocyte-specific conditional knockouts. Genesis 45, 292–299 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.