Significance
Macrophages play a key role in shaping tumor immunity. CCRL2 was originally cloned from LPS-stimulated macrophages; however, whether CCRL2 influences tumor immunity by regulating macrophage function remains unknown. In this study, we identify CCRL2 as a predictive indicator of robust antitumor immunity in human cancers and report the predominant expression of CCLR2 in TAM with immunostimulatory phenotypes. We also reveal the functional role of CCRL2 in potentiating antitumor immunity. Specifically, CCRL2 that is primarily induced by TLR4 agonists in turn interacts with TLR4 to facilitate its retainment in cell surface, thereby amplifying membrane TLR4-mediated downstream inflammatory signaling, finally leading to optimal activation of immunostimulatory macrophages and subsequent antitumor CD8+ T-cell responses.
Keywords: CCRL2, tumor associated macrophages, antitumor T-cell immunity, TLR4
Abstract
Macrophages are the key regulator of T-cell responses depending on their activation state. C-C motif chemokine receptor-like 2 (CCRL2), a nonsignaling atypical receptor originally cloned from LPS-activated macrophages, has recently been shown to regulate immune responses under several inflammatory conditions. However, whether CCRL2 influences macrophage function and regulates tumor immunity remains unknown. Here, we found that tumoral CCRL2 expression is a predictive indicator of robust antitumor T-cell responses in human cancers. CCRL2 is selectively expressed in tumor-associated macrophages (TAM) with immunostimulatory phenotype in humans and mice. Conditioned media from tumor cells could induce CCRL2 expression in macrophages primarily via TLR4, which is negated by immunosuppressive factors. Ccrl2−/− mice exhibit accelerated melanoma growth and impaired antitumor immunity characterized by significant reductions in immunostimulatory macrophages and T-cell responses in tumor. Depletion of CD8+ T cells or macrophages eliminates the difference in tumor growth between WT and Ccrl2−/− mice. Moreover, CCRL2 deficiency impairs immunogenic activation of macrophages, resulting in attenuated antitumor T-cell responses and aggravated tumor growth in a coinjection tumor model. Mechanically, CCRL2 interacts with TLR4 on the cell surface to retain membrane TLR4 expression and further enhance its downstream Myd88-NF-κB inflammatory signaling in macrophages. Similarly, Tlr4−/− mice exhibit reduced CCRL2 expression in TAM and accelerated melanoma growth. Collectively, our study reveals a functional role of CCRL2 in activating immunostimulatory macrophages, thereby potentiating antitumor T-cell response and tumor rejection, and suggests CCLR2 as a potential biomarker candidate and therapeutic target for cancer immunotherapy.
The central role of T cells, particularly cytotoxic CD8+ T cells (CTL), in anti-tumor immunity has been highlighted by the clinical success of cancer immunotherapies. Melanoma is known as an immunogenic tumor with abundant tumor-infiltrating T cells and is susceptible to immune checkpoint blockades (1). However, many types of cancer are not responsive to immunotherapy, and even for melanoma, less than 40% of patients could benefit from these therapies, possibly due to insufficient activation of tumor-specific CTL or their failure to infiltrate tumors (2).
Macrophages constitute the largest fraction of tumor-infiltrating immune cells and act as an important regulator during cancer progression (3–6). The abundance of tumor-associated macrophages (TAM) is generally associated with impaired anti-tumor T-cell immunity and poor clinical outcome and response to treatment in solid tumors (7–10). However, in some cases, macrophages can be associated with a good prognosis; for example, high frequencies of HLA-DR+ macrophages within tumors have been associated with good outcomes (11–13). It has become clear that TAM consist of a continuum of phenotypes, ranging from an immunostimulatory M1-like phenotype to an immunosuppressive M2-like phenotype (14, 15). M1-like macrophages predominate at sites of early oncogenesis, mediating anti-tumor effects including direct killing and activation of anti-tumor T-cell immunity (5, 7, 16–18). Over tumor progression, macrophages can be shifted toward M2-like phenotype by responding to cues within the tumor microenvironment (TME) (19–21). M2-like macrophages predominate in established tumors, mediating protumor effects including the induction of immunosuppression, promotion of angiogenesis, and tumor cell biology (5, 7). Thus, targeting macrophages has become an attracting strategy to complement the existing cancer immunotherapy. Instead of depletion of all macrophages which contain both anti- and protumor subsets, induction of immunostimulatory phenotype or reprograming TAM from protumor into anti-tumor phenotype could be more efficient to control tumor progression primarily by enhancing anti-tumor T-cell responses (7). Thus, identification of the key factors that regulate the activation state of macrophages, particularly those enforcing anti-tumor M1-like phenotype, could facilitate the development of new therapeutic targets to improve the efficacy of anti-cancer immunotherapy.
C-C motif chemokine receptor-like 2 (CCRL2) was originally cloned from LPS-stimulated macrophages and first named as a LPS inducible C-C chemokine receptor related gene (l-CCR) (22). CCRL2 is absent in resting immune cells and induced in activated myeloid cells, but not T cells, under certain pathological conditions (23–27). CCRL2 was later identified as a nonsignaling atypical receptor to enrich and present its ligand chemerin to the functional receptor, CMKLR1 (24). Further studies demonstrated that CCRL2 expressed in endothelial cells promotes CMKLR1-dependent dendritic cell (DC) and natural killer (NK) cell transmigration (28, 29). In addition, CCRL2 expression in activated neutrophils regulates CXCR2-dependent neutrophil chemotaxis toward CXCL8 (25). Surprisingly, the role of CCRL2 in macrophages remains unknown. Preclinical mouse studies demonstrated that CCRL2 is involved in several inflammatory diseases (25, 27, 30). However, the involvement of CCRL2 in tumors has been reported until very recently. CCRL2 expression in nonhematopoietic cells inhibits lung tumors by facilitating NK cell migration (29), while CCRL2 expression in human breast cancer tissues positively correlates to tumor-infiltrating immune cells (31).
Here, we demonstrate that CCLR2 expression is not only a predictive indicator of robust anti-tumor immunity in human cancers but also plays a functional role in the activation of immunostimulatory macrophages via interacting with surface TLR4 and amplifying its downstream inflammatory signaling, finally leading to optimal anti-tumor T-cell responses.
Results
Tumoral CCRL2 Expression Is Positively Associated with Robust Anti-Tumor T-Cell Immunity in Cancer Patients.
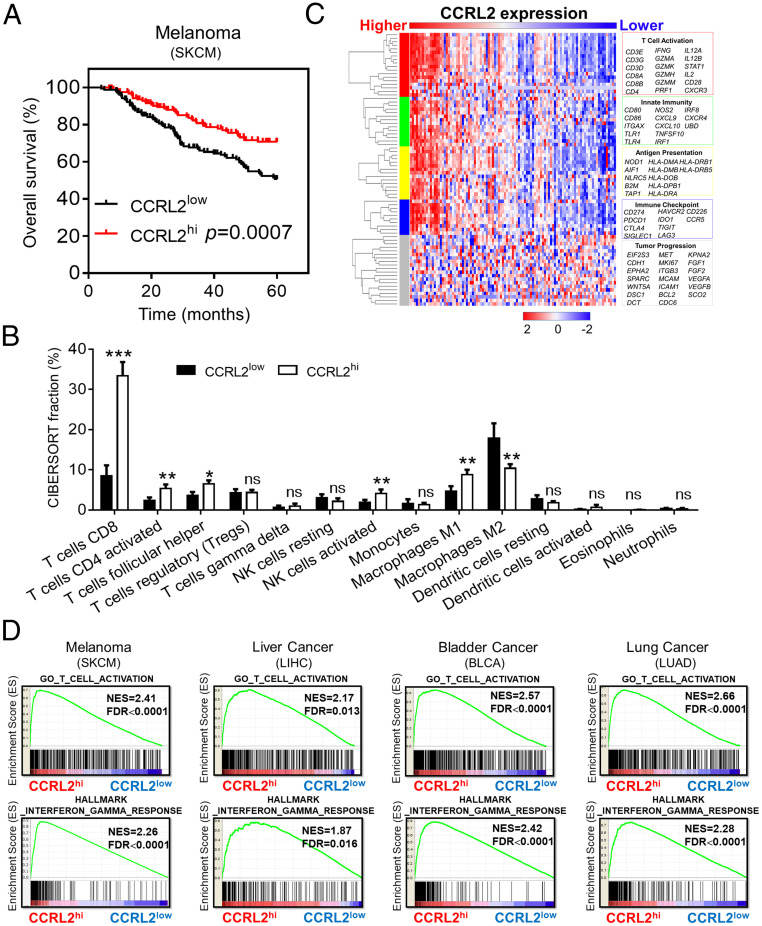
We first evaluated the clinical relevance of tumoral CCLR2 expression and found that metastatic melanoma (SKCM) patients with high tumoral CCRL2 expression (CCRL2hi) had significantly longer survival than those with low CCRL2 expression (CCRL2low) (Fig. 1A). Moreover, CCRL2hi melanoma had significantly increased intratumoral anti-tumor immunity characterized by increased infiltrating CD8+ T cells, activated CD4+ T cells, activated NK cells, and M1 macrophages, but decreased M2 macrophages. Of note, CD8+ T cells were the most obviously increased immune cell type in CCRL2hi melanoma (Fig. 1B). Consistently, strong correlation patterns were observed for the up-regulation of genes involved in T-cell activation, innate immunity, antigen presentation, and immune checkpoint related pathway among CCRL2hi melanoma, but not for those in tumor progression (Fig. 1C). Gene Set Enrichment Analysis (GSEA) also revealed that genes associated with T-cell activation and IFN-γ responses were highly enriched in CCRL2hi melanoma (Fig. 1D). Similar results were obtained from patients with liver cancer, bladder cancer, and lung cancer (Fig. 1D). Together, these results suggest that tumoral CCRL2 expression could be a useful marker for monitoring the magnitude of anti-tumor immunity in cancer patients.
Fig. 1.
Tumoral CCRL2 expression is positively associated with robust anti-tumor T-cell immunity in cancer patients. (A) Overall survival rates of patients with metastatic melanoma (SKCM) from The Cancer Genome Atlas (TCGA) database with high or low CCRL2 expression as defined by the median value, n = 369. (B) Characterization of the immune cell composition in primary melanoma of TCGA datasets by CIBERSORT; data represent mean ± SEM, n = 103. (C) The heatmap showing the z-score normalized log-cpm values of signature immune gene sets based on CCRL2 expression levels. n = 103. (D) GSEA demonstrating the enrichment of signature genes of T-cell activation and IFN-γ responses in CCRL2hi tumors. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
CCRL2 Is Selectively Expressed in TAM with Immunostimulatory Phenotype.
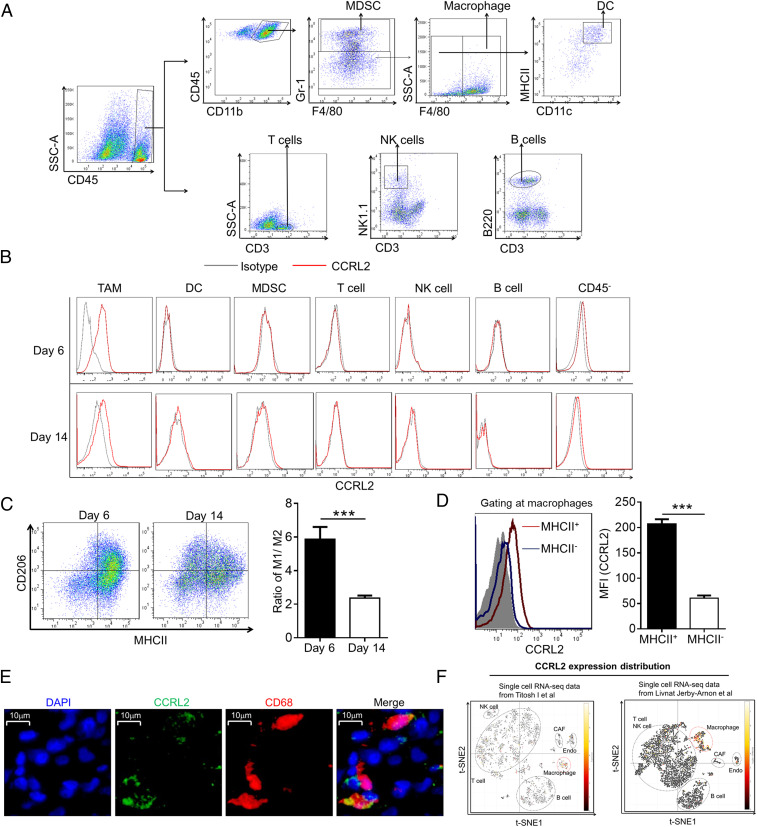
As expected, CCRL2 was undetectable in all investigated immune cells from different sites of naive WT mice (SI Appendix, Fig. S1A). Since little is known about cellular distribution of CCRL2 and how tumor growth influences its expression, we examined the cellular expression pattern of CCRL2 in a murine B16F10 melanoma model. Flow cytometric analysis revealed CCRL2 expression was detected in tumor-infiltrating macrophages, but not in other types of immune cells including myeloid-derived suppressor cells (MDSCs), DCs, NK cells, T cells, and B cells on days 6 and 14 after B16F10 cell inoculation (Fig. 2 A and B). CCRL2 was detected in CD45− nonimmune cells at low levels, but not in B16F10 cells (Fig. 2B and SI Appendix, Fig. S1B). CCRL2 expression was undetectable in circulating immune cells of tumor-bearing mice (SI Appendix, Fig. S1C). Interestingly, we noted lower CCRL2 levels in TAM on day 14 than those on day 6 (Fig. 2B). Given a significantly lower ratio of MHCII+CD206− M1-like macrophages and MHCII−CD206+ M2-like macrophages in tumors from day 14 (Fig. 2C), we speculated that lower CCRL2 expression could be due to the shift from immunostimulatory M1-like to immunosuppressive M2-like macrophages over tumor progression. MHCII+ macrophages were previously reported to exhibit immunostimulatory M1-like phenotype and positively correlate with tumor regression (32, 33). Accordingly, CCRL2 was predominately expressed in MHCII+ macrophages but not MHCII− macrophages (Fig. 2D). Moreover, CCRL2 expression in TAM was confirmed in human melanoma. As shown in Fig. 2E, CCLR2 was expressed in tumor-infiltrating CD68+ macrophages in human melanoma tissue section. Furthermore, analysis of two independently published single-cell RNA sequencing datasets of human melanoma tumors (34, 35) showed high enrichment of CCRL2 expression in macrophages from both studies (Fig. 2F).
Fig. 2.
CCRL2 is selectively expressed in TAM with immunostimulatory phenotype. (A) Gating strategy for tumor-infiltrating immune cells. (B) Representative histograms measuring CCRL2 expression. (C) Representative dot plots of macrophages and the ratio of M1 to M2 macrophages in B16F10 tumors. (D) Representative histograms measuring CCRL2 expression in MHCII+ and MHCII− macrophages and average mean fluorescence intensity (MFI) values of CCRL2. (E) Representative images of immunostaining of human melanoma tissues for expression of CCRL2 (green) and CD68 (red). Tissues were counterstained with DAPI (blue) to detect nuclei. (F) t-SNE plot generated from two sets of published data via Single Cell Portal (singlecell.broadinstitute.org) showing relative expression levels of CCRL2 in different clusters of nonmalignant cells of human melanoma. Data represent mean ± SEM (n = 5) in C and D. Representative data from three experiments are shown. ***P < 0.001.
The Immunostimulatory Factors Induce CCRL2 Expression in Macrophages, which Is Antagonized by Immunosuppressive Factors.
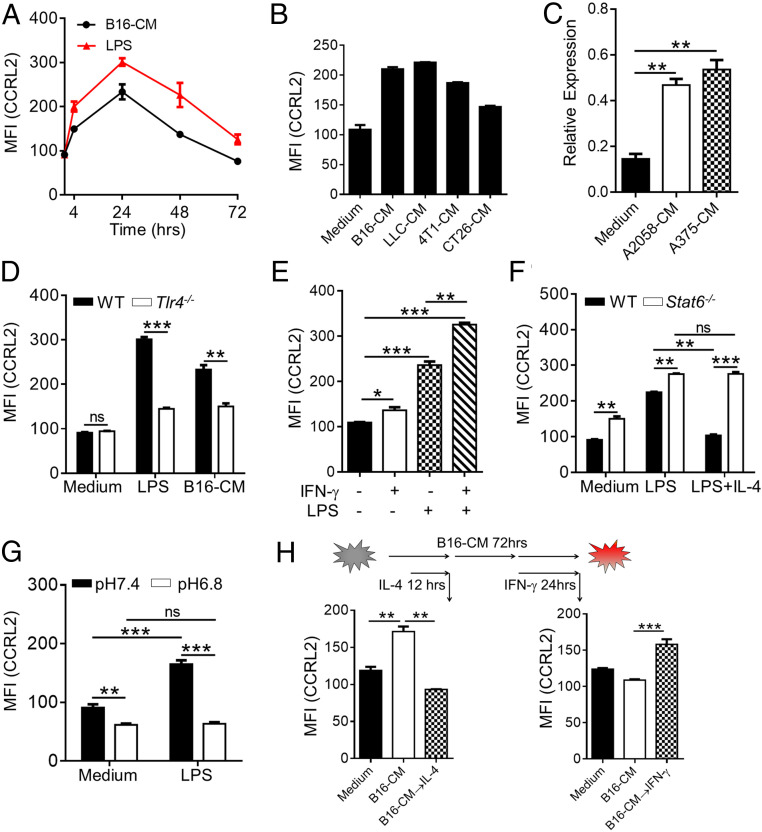
To investigate the factors that regulate CCRL2 expression in macrophages, bone marrow-derived macrophages (BMDM) were generated and treated with different stimuli. CCRL2 expression was undetectable in resting BMDM (SI Appendix, Fig. S1A) but induced by LPS stimulation as early as 4 h and reached peak at 24 h followed by returning to baseline levels at 72 h (Fig. 3A). Notably, incubation with conditioned media (CM) of B16F10 cells induced CCRL2 expression in a similar pattern, albeit at relatively lower levels (Fig. 3A). CM from different types of murine tumor cell lines also induced CCRL2 expression (Fig. 3B). The same phenomenon was observed in human THP-1–derived macrophages stimulated with CM from human melanoma cell lines (Fig. 3C). In contrast to LPS being reported to induce CCRL2 expression in DCs and neutrophils (25, 27), B16F10 CM failed (SI Appendix, Fig. S1D), which was consistent with undetectable CCLR2 expression in tumor-infiltrating DC and MDSCs (Fig. 2B). Moreover, Tlr4−/− BMDM had significantly decreased CCLR2 expression in response to B16F10 CM (Fig. 3D), suggesting that some TLR4 ligands released by tumor cells could be responsible for CCRL2 expression in TAM. IFN-γ slightly up-regulated CCRL2 expression, but together with LPS-induced significantly higher CCRL2 levels in BMDM than those stimulated with LPS alone (Fig. 3E). In contrast, IL-4 markedly abrogated LPS-induced CCRL2 expression, which was absent in Stat6−/− BMDM (Fig. 3F). Notably, significantly higher CCRL2 levels were observed in Stat6−/− BMDM regardless of LPS stimulation (Fig. 3F), suggesting STAT6 as a negative regulator of CCRL2 expression. Acidosis was reported to be a characteristic of tumors, including melanoma, and involved in the induction of immunosuppressive macrophages (36). LPS-induced CCRL2 expression in BMDM was significantly abrogated in culture media with pH6.8 (Fig. 3G). Interestingly, addition of IL-4 to the culture of BMDM at 12 h following B16F10 CM stimulation down-regulated the peak expression of CCRL2 at 24 h (Fig. 3H). In contrast, addition of IFN-γ to the culture of BMDM at 48 h following B16F10 CM stimulation prevented the reduction of CCRL2 expression to the baseline levels at 72 h (Fig. 3H). Together, these results suggest that CCRL2 expression in macrophages is preferably induced by immunostimulatory factors, particularly TLR4 ligands, which could be antagonized by immunosuppressive factors.
Fig. 3.
The immunostimulatory factors induce CCRL2 expression in macrophages, which is antagonized by immunosuppressive factors. (A and B) CCRL2 MFI in WT bone marrow-derived macrophages (BMDM) stimulated with LPS (100 ng/mL) or condition media (CM) of B16F10 cells (B16-CM) (A), and in WT BMDM with CM from different tumor cell lines for 24 h (B). (C) Quantitative polymerase chain reaction (qPCR) analysis of CCRL2 expression in THP1-derived human macrophages stimulated with CM of human melanoma cell lines. (D–G) CCRL2 MFI expression in WT and Tlr4−/− BMDM following stimulation with LPS or B16-CM (D), in WT BMDM with LPS, IFN-γ or LPS, and IFN-γ together (E), in WT and Stat6−/− BMDM with LPS alone or LPS and IL-4 (20 ng/mL) together (F), and in WT BMDM with LPS (in medium with pH 7.4 or pH 6.8) (G). (H) CCRL2 MFI in WT BMDM that were stimulated with B16-CM for 12 h or 48 h, and then treated with/without IL-4 for another 12 h or with/without IFN-γ for another 24 h along with B16-CM. Data represent mean ± SEM of triplicate wells from a representative of three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
Hematopoietic CCRL2 Is Responsible for Inhibiting Melanoma Growth in a CD8+ T Cell–dependent Manner.
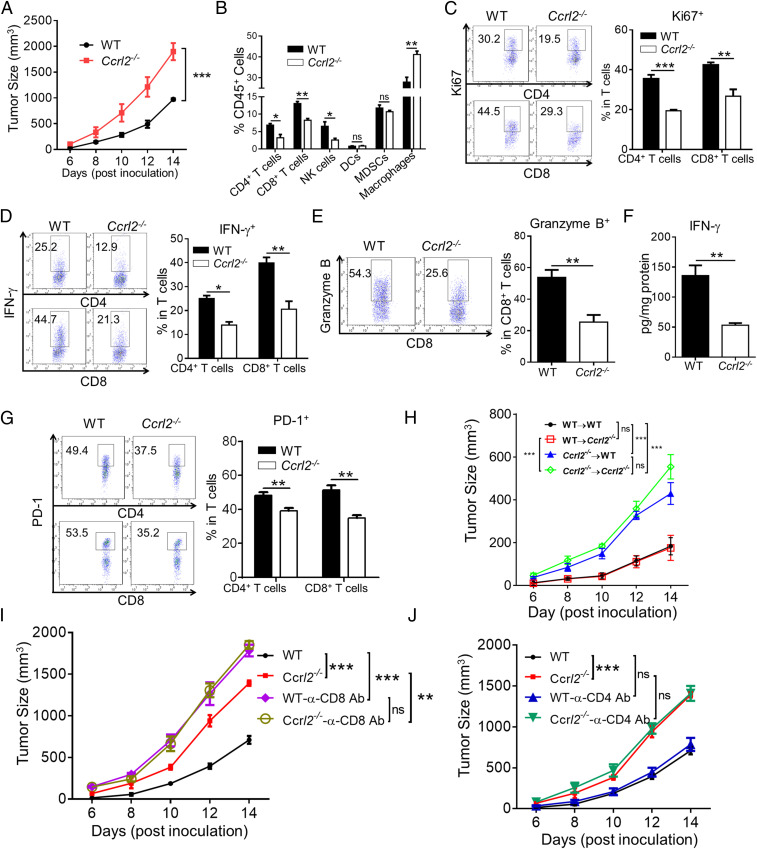
We next investigated whether CCRL2 plays a causal role in murine melanoma growth. To this end, we generated Ccrl2−/− mice, in which CCRL2 was not detectable in lung stromal cells which were reported to constitutively express CCRL2 (23), while CCRL2 was readily detected in WT controls (SI Appendix, Fig. S1E). Ccrl2−/− mice are viable and fertile and developed normally (SI Appendix, Fig. S1 F–H). We showed that tumors grew faster and larger in Ccrl2−/− mice than in WT mice (Fig. 4A). Consistent with the previous findings (24), increased serum chemerin levels were detected in Ccrl2−/− mice (SI Appendix, Fig. S1I). Previous studies from us and others demonstrated chemerin, a reported ligand for CCRL2, inhibited melanoma via CMKLR1-dependent NK cell recruitment (37, 38). However, we ruled out the possible involvement of chemerin in the tumor-inhibitory effects of CCRL2, as chemerin overexpression effectively inhibited melanoma growth accompanied by increased frequencies of NK cells and CD8+ T cells in Ccrl2−/− mice (SI Appendix, Fig. S2 A–C). We further found that CCRL2 deficiency markedly decreased frequencies of CD4+ T, CD8+ T cells, and NK cells, while possessing significantly increased macrophages, with no effect on DCs and MDSCs in B16 tumors (Fig. 4B). CCRL2 deficiency also caused less activated T cells, as evidenced by significant decreases in frequencies of proliferating CD4+ T cells and CD8+ T cells, IFN-γ-expressing CD4+ T cells and CD8+ T cells, and granzyme B-expressing CD8+ T cells (Fig. 4 C–E). Consistently, markedly decreased IFN-γ levels were detected in Ccrl2−/− tumor homogenates (Fig. 4F). We also noted lower frequencies of PD-1-expressing CD4+ and CD8+ T cells in Ccrl2−/− tumors (Fig. 4G), further confirming insufficient T-cell activation in the absence of CCRL2. Consistently, weakened intratumoral ovalbumin (OVA)-specific T-cell responses were observed in Ccrl2−/− mice that were inoculated with OVA-B16 cells compared to WT controls (SI Appendix, Fig. S3)
Fig. 4.
Hematopoietic CCRL2 is responsible for inhibiting melanoma growth in a CD8+ T cell–dependent manner. (A) Tumor growth curve. (B) Average percentages of different types of infiltrating immune cells in tumors of WT and Ccrl2−/− mice on day 14. (C and D) CD4+ and CD8+ T cells were gated and examined for expression of Ki67 (C) and IFN-γ (D). (E) Expression of Granzyme B. (F) The concentrations of IFN-γ in tumor homogenates. (G) Expression of PD-1. (H–J) Tumor growth curve in bone marrow chimeras (H), WT and Ccrl2−/− mice treated with neutralizing antibodies (Abs) against CD8 (I) or CD4 (J). Data represent mean ± SEM (n = 5 to 7). Similar results were obtained from three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
Since we found that nonimmune cells also express CCRL2 in B16F10 melanoma, bone marrow chimeras were generated to determine whether the melanoma-inhibitory effect of CCRL2 is mediated by hematopoietic or nonhematopoietic compartments or both. Ccrl2−/−→WT mice, which are deficient in hematopoietic CCRL2, exhibited similar tumor growth to those observed in Ccrl2−/−→ Ccrl2−/− mice (Fig. 4H). In contrast, CCRL2 WT→Ccrl2−/− mice, which are deficient in nonhematopoietic CCRL2, exhibited similar tumor growth to those observed in WT→WT mice (Fig. 4H). These results demonstrate that nonhematopoietic CCRL2 expression is dispensable for controlling melanoma growth in our model. We were then prompted to investigate whether T cells are required by the tumor-inhibitory effects of CCLR2. As expected, CD8+ T-cell depletion caused faster tumor growth and larger tumor size in WT mice, confirming the critical role of CD8+ T cells in controlling melanoma growth (Fig. 4I). Notably, CD8+ T-cell depletion completely eliminated the differences in tumor growth between WT and Ccrl2−/− mice (Fig. 4I). In contrast, CD4+ T-cell depletion had no such effects, suggesting a dispensable role of CD4+ T cells (Fig. 4J). Together, these results demonstrate that the tumor-inhibitory effect of CCRL2 is dependent on CD8+ T cell–mediated anti-tumor immunity.
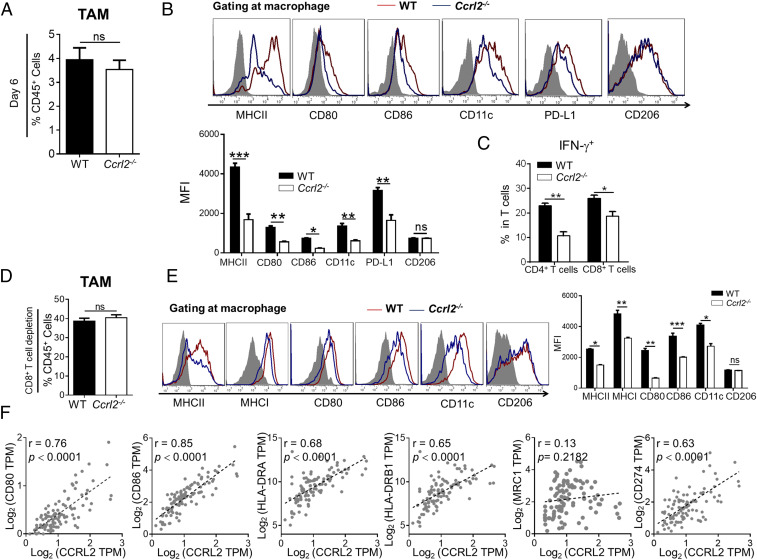
CCRL2 Deficiency Impairs Immunostimulatory Phenotype of Macrophages in Melanoma.
Since CD8+ T cells do not express CCRL2 but are required by the melanoma-inhibitory effect of CCRL2, we reasoned that CCRL2 could influence the activation state of macrophages, thereby regulating intratumoral T-cell responses. To test it, we first compared the expression of phenotype markers in WT and Ccrl2−/− TAM on day 6 when immunostimulatory M1-like macrophages were dominated. Comparable frequencies of total macrophages were detected in WT and Ccrl2−/− tumors on day 6, which were much lower than those detected in tumor on day 14 (Figs. 5A and 4B). Of note, an overall decrease in expression of immunostimulatory surface markers but unchanged CD206 expression were observed in Ccrl2−/− TAM (Fig. 5B). Accordingly, Ccrl2−/− mice had significantly lower frequencies of IFN-γ-expressing CD4+ T and CD8+ T cells (Fig. 5C), suggesting CCRL2 deficiency impairs anti-tumor T-cell immunity at an early stage of melanoma growth. Lower levels of PD-L1 were detected in Ccrl2−/− TAM (Fig. 5B), which could have resulted from IFN-γ, the potent stimulus for PD-L1 expression, being down-regulated in the Ccrl2−/− tumor microenvironment. We also compared the activation state of macrophages in WT and Ccrl2−/− mice with depleted CD8+ T cells, in which tumor size were comparable to exclude the potential influence on macrophage activation. Despite comparable frequencies of total macrophages in both genotypes (Fig. 5D), macrophages from CD8+ T cell–depleted Ccrl2−/− mice had an overall decrease in expression of immunostimulatory surface markers but unchanged CD206 expression (Fig. 5E), suggesting that CCRL2 directly promotes the immunostimulatory phenotypes of TAM. Consistently, positive correlations between gene expression of CCRL2 and CD80, CD86, HLA-DR or PD-L1, but no correlation between CCRL2 and CD206/MRC1, were found in melanoma patients (Fig. 5F). Together, these results indicate that CCRL2 is involved in activation of immunostimulatory macrophages, thereby facilitating subsequent activation of anti-tumor T cells in melanoma.
Fig. 5.
CCRL2 deficiency impairs immunostimulatory phenotype of macrophages in melanoma. (A–C) Average percentages of macrophages (A), representative histogram measuring expressions of surface markers and average MFI of each marker in macrophages (B), and average percentages of IFN-γ+ cells in CD4+ T and CD8+ T (C) from WT and CCRL2−/- tumors on day 6. (D and E) Average percentages of macrophages (D), representative histogram measuring expressions of surface markers and average MFI of each marker in macrophages (E) in WT and Ccrl2−/− tumors with depleted CD8+ T cells on day 14. (F) Linear regression analysis between CCRL2 expression and surface markers in human melanoma based on TCGA dataset. (n = 103). Data represent mean ± SEM (n = 5 to 7). Similar results were obtained from three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
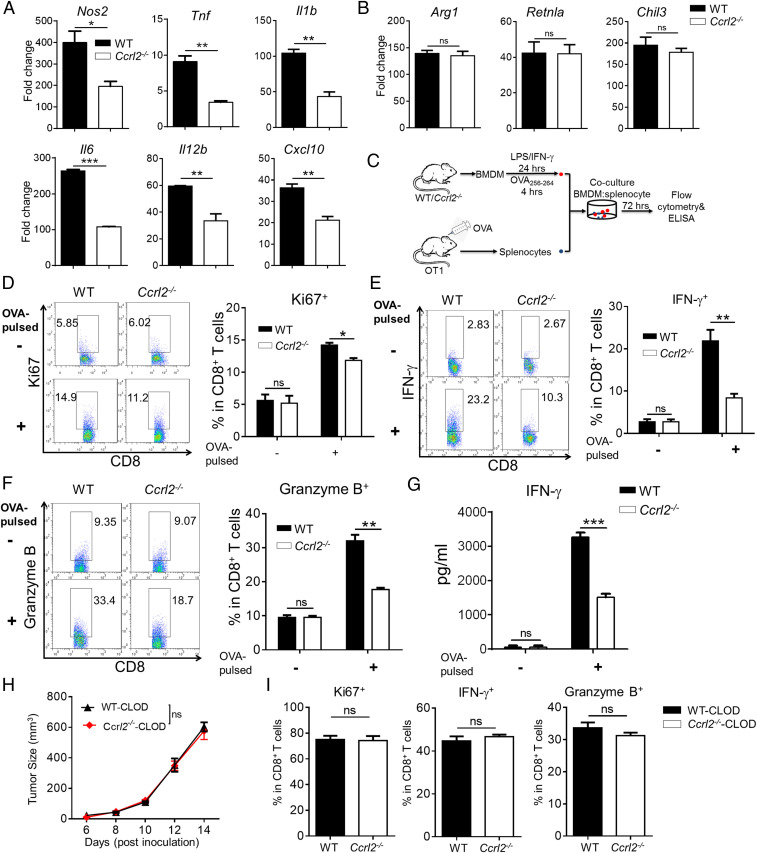
CCRL2 Promotes Activation of M1 Macrophages to Potentiate Anti-Tumor CD8+ T-Cell Responses.
To seek the direct evidence that CCRL2 promotes immunostimulatory macrophage activation, we used the classical in vitro M1 macrophage polarization model by stimulating WT and Ccrl2−/− BMDM with LPS/IFN-γ. Ccrl2−/− BMDM exhibited markedly impaired M1 activation, as evidenced by significantly reduced gene expression of proinflammatory mediators when compared to WT BMDM (Fig. 6A). In contrast, CCRL2 had no effect on IL-4–induced M2 macrophage polarization, as gene expression of M2-related molecules were comparable to Ccrl2−/− and WT BMDM (Fig. 6B). To investigate whether CCRL2 expression in classically activated macrophages is required for reactivating antigen-specific CD8+ T-cell response, WT and Ccrl2−/− BMDM were first stimulated with LPS/IFN-γ, and then pulsed with OVA257–264 peptides followed by coculturing with splenocytes from OVA-sensitized OT1 mice (Fig. 6C). Compared with similarly-treated WT BMDM, Ccrl2−/− BMDM had decreased ability to stimulate proliferation and effector function of CD8+T cells (Fig. 6 D–G). We further investigated the anti-tumor effect of CCRL2 expression in LPS/IFN-γ-activated BMDM in vivo using a coinjection model (SI Appendix, Fig. S4A). Although coinjection of LPS/IFN-γ-activated WT or Ccrl2−/− BMDM both slowed down B16F10 tumor growth in Ccrl2−/− mice, coinjection of Ccrl2−/− BMDM had significantly diminished tumor-inhibitory effect and decreased proliferation and activation of CD4+ and CD8+ T cells (SI Appendix, Fig. S4 B–F). To further determine whether the anti-tumor effect of CCRL2 requires macrophages in vivo, macrophages were depleted by injecting clodronate-conjugated liposomes. Macrophage depletion completely eliminated the difference in tumor growth between WT and Ccrl2−/− mice (Fig. 6H), which caused no differences in the proliferation and activation of CD8+ T cells (Fig. 6I). Together, these results demonstrate that CCRL2 expression in macrophages is critical for restraining tumor growth by potentiating anti-tumor T-cell response.
Fig. 6.
CCRL2 promotes activation of M1 macrophages to potentiate anti-tumor CD8+ T-cell responses. (A and B) qPCR analysis of M1-related genes (A) and M2-related genes (B) in WT and Ccrl2−/− BMDM stimulated with LPS/IFN-γ and IL-4, respectively. (C) Schematic protocol for coculture of WT or Ccrl2− BMDM and OT1 splenocytes. (D–F) CD8+ T cells were gated in OT1 splenocytes that were cocultured with LPS/IFN-γ-activated WT or Ccrl2−/− BMDM without or with SIINFEKL (OVA257–264) pulse and examined for expression of Ki67 (D), IFN-γ (E), and Granzyme B (F). (G) The concentrations of IFN-γ in the cocultures determined by ELISA. Data represent mean ± SEM of triplicate wells from a representative of two independent experiments. (H–I) Tumor growth curve (H) and expression of Ki67, IFN-γ, and Granzyme B in CD8+ T cells (I) from clodronate liposomes (CLOD)-treated WT or Ccrl2−/− mice on day 14. In A and B, data represent mean ± SEM of triplicate wells from a representative of three independent experiments. In D–I, data represent mean ± SEM (n = 5 to 6) and similar results were obtained from two to three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
CCRL2 Retains Membrane TLR4 Expression to Amplify Downstream Myd88-NF-κB Signaling.
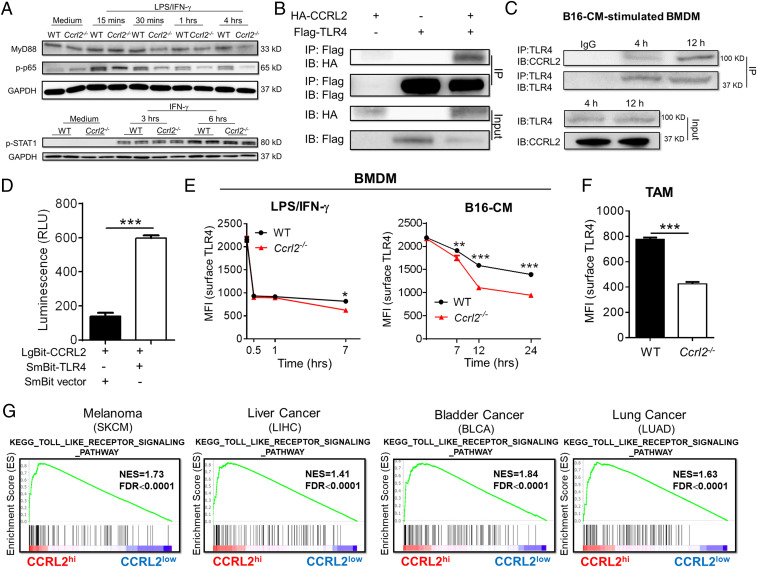
We then sought to explore the underlying molecular mechanism of how CCRL2 promotes immunostimulatory macrophage activation. Ccrl2−/− BMDM exhibited reduced protein levels of Myd88 and phosphorylated p65 (p-p65) but similar levels of p-STAT1 compared with WT controls in response to LPS/IFN-γ (Fig. 7A), indicating that CCRL2 could amplify Myd88-NFκB signaling in classically activated macrophages. Given the importance of membrane TLR4 in mediating Myd88-NFκB signaling, we hypothesized that CCRL2 that is primarily induced by TLR4 ligands may interact with surface TLR4 to influence its downstream signaling. We first demonstrated the interaction between CCRL2 and TLR4 in HEK 293T cells and BMDM stimulated with B16F10 CM by coimmunoprecipitation assay (Fig. 7 B and C and SI Appendix, Fig. S5). Such interaction was further confirmed in live cells using NanoBit proximity assay system. As shown in Fig. 7D, HEK 293T cells cotransfected with expression vector LgBit-CCRL2 and SmBit-TLR4 showed an obviously elevated luminescence signal compared with negative control. We then examined whether induced CCRL2 expression is able to retain surface TLR4 expression in activated macrophages. Stimulation with LPS or B16F10 CM both reduced surface TLR4 levels in BMDM over time, and the former caused the greater and faster loss of surface TLR4 (Fig. 7E). Notably, significantly lower levels of surface TLR4 levels were detected in Ccrl2−/− BMDM (Fig. 7E). We also noted the differences in surface TLR4 levels were more obvious between WT and Ccrl2−/− BMDM stimulated with B16F10-CM, suggesting that CCLR2 may be more efficient in retaining surface TLR4 expression in TAM. There were no differences in surface TLR4 expression between WT and Ccrl2−/− BMDM at least within 1 h after stimulation (Fig. 7E), which was paralleled with undetectable CCRL2 expression at this time point (SI Appendix, Fig. S6A). Moreover, Ccrl2−/− TAM had significantly less surface TLR4 levels than WT controls (Fig. 7F). Despite lower surface TLR4 levels in Ccrl2−/− BMDM, comparable TLR4 expressions at both mRNA and protein levels were detected in classically activated WT and Ccrl2−/− BMDM (SI Appendix, Fig. S6 B and C), suggesting that decreased surface TLR4 is not due to the transcription and translation. Moreover, the TLR signaling pathway was enriched in human cancers with high CCRL2 expression, underscoring the positive correlation between CCRL2 expression and TLR4 signaling (Fig. 7G). Thus, these results demonstrate that CCRL2 interacts with TLR4 to retain its surface expression, thereby amplifying downstream inflammatory Myd88-NFκB signaling in macrophages.
Fig. 7.
CCRL2 retains membreane TLR4 expression to amplify downstream Myd88-NF-κB signaling. (A) Representative Western blots showing protein levels of Myd88, phosphorylated p65 (p-p65), and phosphorylated STAT1 (p-STAT1) in WT and Ccrl2−/− BMDM. (B and C) Coimmunoprecipitation (co-IP) assay showing the interaction between TLR4 and CCRL2 in HEK 293T cells (B) and in BMDM stimulated with B16-CM for indicated time (C). (D) NanoBit proximity assay system showing the interaction between TLR4 and CCRL2 in living cells. Luminescence was assayed using Nano-Glo Live cell substrate. (E and F) Surface TLR4 levels in WT and Ccrl2−/− BMDM (E), and in macrophages from WT and Ccrl2−/− tumors on day 14. Data represent mean ± SEM, n = 4 (F). (G) GSEA demonstrating the enrichment of signature genes of TLR signaling pathway in CCRL2hi tumors. In A–C, representative data from two to three experiments are shown. In D and E, data represent mean ± SEM of triplicate wells from a representative of three independent experiments. **P < 0.01; ***P < 0.001.
TLR4 Deficiency Reduces CCRL2 Expression in TAM and Anti-Tumor T-Cell Responses in Murine Melanoma.
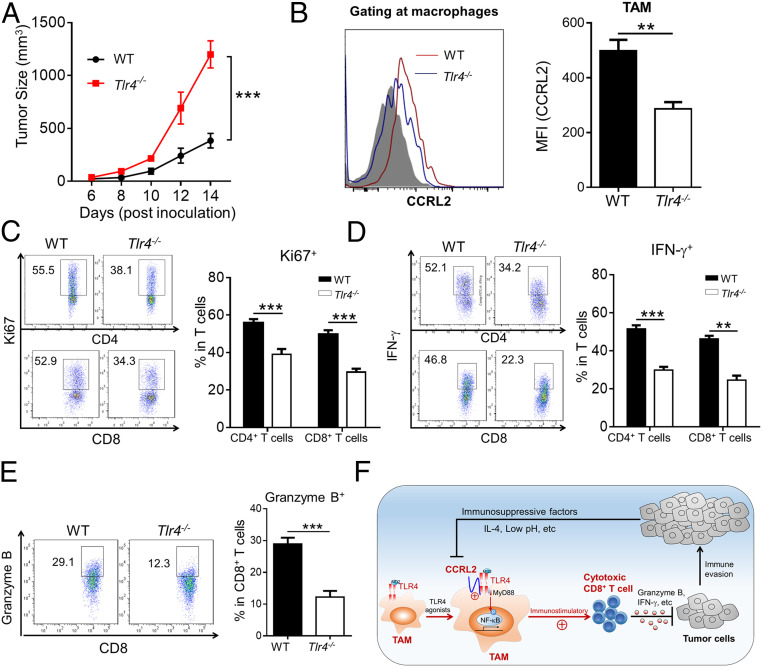
Finally, we investigated the direct effect of TLR4 on CCLR2 expression in TAM and anti-tumor T-cell responses. Compared with WT controls, Tlr4−/− mice had increased tumor growth (Fig. 8A), which resembled Ccrl2−/− mice. Importantly, significantly decreased CCRL2 expression was detected in Tlr4−/− TAM compared to WT controls (Fig. 8B), which was consistent with the in vitro data (Fig. 3D). Accordingly, TLR4 deficiency resulted in significantly decreased proliferation and activation of T cells in tumors (Fig. 8 C–E). Together, these results demonstrate the presence of TLR4 could up-regulate CCRL2 expression in TAM and facilitate anti-tumor T-cell immunity.
Fig. 8.
TLR4 deficiency reduces CCRL2 expression in TAM and anti-tumor T-cell responses in murine melanoma. (A) Tumor growth curve. (B–E) Surface CCRL2 levels in macrophages (B), CD4+ and CD8+ T cells were gated and examined for expression of Ki67 (C) and IFN-γ (D), as well as expression of Granzyme B in CD8+ T cells (E) from tumors of WT and Tlr4−/− mice on day 14. (F) Schematic representation of the functional role of CCRL2 in macrophages to potentiate anti-tumor immunity. Data represent mean ± SEM (n = 5 to 6). **P < 0.01; ***P < 0.001.
Discussion
Despite emerging evidence demonstrating the regulatory role of CCLR2 in inflammatory diseases, little is known about its role in anti-tumor immunity. Here, we report a positive association between tumoral CCRL2 expression and the intensity of anti-tumor T-cell immunity in human cancer. Moreover, CCRL2 is found to be selectively induced in immunostimulatory M1-like macrophages and functions to potentiate anti-tumor CD8+ T-cell responses, thereby restraining melanoma growth. Mechanistically, CCRL2 could interact with membrane TLR4 to retain its expression in cell surface of classically activated macrophages, thereby amplifying membrane TLR4-mediated inflammatory signaling to enhance their immunostimulatory phenotype (Fig. 8F).
Our study revealed that CCRL2 is not only a predictive indicator of robust anti-tumor T-cell immunity in cancer patients, but functional in potentiating anti-tumor T-cell responses. In contrast to the recent finding that nonhematopoietic CCRL2 is critical for NK cell–mediated anti-tumor immunity (29), we found that CCRL2 expression in hematopoietic cells, but not nonhematopoietic cells, was required for inhibiting melanoma growth. Moreover, the melanoma-inhibitory effect of CCRL2 is primarily dependent on CD8+ T cells via facilitating activation of immunostimulatory macrophages. Among tumor-infiltrating leukocytes, CCRL2 was exclusively expressed in TAM, particularly in those with immunostimulatory phenotypes. We found that B16F10 CM could induce CCLR2 expression in BMDM which was primarily dependent on TLR4, suggesting that some danger signals that serve as TLR4 ligands in the TME could be responsible. Further investigation needs to identify the specific factors that up-regulate CCRL2 expression in TAM. In contrast to CCLR2 expression in neutrophils and DCs in several inflammatory diseases (22, 25, 27), we failed to detect its expression in tumor-infiltrating DCs and neutrophils or B16F10 CM-stimulated DCs and neutrophils. The differential cellular distribution of CCRL2 in models of tumor and immune-related inflammatory disease could be due to the differences in the nature or the intensity of inflammatory signaling that are present in the microenvironment. Further, we demonstrated that CCRL2 is functional in the activation of immunostimulatory macrophages, thereby potentiating anti-tumor T-cell responses. This is supported by several lines of evidence. First, impaired activation of M1-like immunostimulatory macrophages was observed in Ccrl2−/− tumors, which was independent of tumor size or T-cell responses. Second, Ccrl2−/− BMDM had impaired LPS/IFN-γ-induced M1 macrophage polarization with decreased ability to reactivate antigen-specific OT1 CD8+ T cells in vitro or to stimulate anti-tumor T-cell responses and restrain tumor growth in Ccrl2−/− mice. Lastly, macrophage depletion completely eliminated the differences in tumor growth and anti-tumor CD8+ T-cell responses between WT and Ccrl2−/− mice. Together, our results reveal a nonredundant role of CCRL2 in activation of immunostimulatory macrophages and subsequent stimulation of anti-tumor T-cell immunity. Thus, the induction and maintenance of CCRL2 expression in macrophages within the TME could be a promising strategy to potentiate anti-tumor immunity.
Accumulating evidence has demonstrated that various factors influence cancer progression by regulating the balance between M1-like and M2-like macrophages (5, 7). Although increased total macrophages were found in Ccrl2−/− tumors on day 14, a tumor-inhibiting effect of CCRL2 is unlikely due to the limitation of immunosuppressive M2-like macrophage activation. In support of it, we demonstrated that IL-4-induced M2 activation was intact in Ccrl2−/− BMDM. We also found lower PD-L1 expression in Ccrl2−/− TAM, indicating weaker anti-tumor T-cell responses being initiated in Ccrl2−/− mice. Interestingly, induced CCRL2 expression in immunostimulatory M1-like macrophages could be negated by the immunosuppressive factors, which may represent a strategy used by tumors to antagonize the immunostimulatory states of TAM. Together, these data suggest that increased immunosuppression in established Ccrl2−/− tumors could result from aggravated tumor growth secondary to impaired activation of immunostimulatory M1-like macrophages during early onset of tumor growth.
Finally, we elucidated the underlying molecular mechanism of how CCRL2 contributes to classical activation of immunostimulatory macrophages. We found that CCRL2 interacted with membrane TLR4, which was important for retaining amounts of cell surface TLR4. CCRL2 deficiency caused greater loss of surface TLR4 expression in Ccrl2−/− TAM or classically activated BMDM, leading to decreased membrane TLR4-dependent Myd88-NF-κB signaling and proinflammatory cytokine expression. The enhancement of TLR4 signaling in innate immune response is known to be important for potentiation of anti-tumor T-cell response (39). We further demonstrated that Tlr4−/− mice had obviously decreased CCRL2 expression in TAM and impaired anti-tumor T-cell responses, suggesting the important role of TLR4 in orchestrating host anti-tumor immune responses. This is consistent with a recent study showing that TLR4 activation in macrophages is important to control tumor growth (40). Therefore, we propose that CCRL2 that is induced by TLR4 agonists could in turn facilitate the retainment of surface TLR4 expression in macrophages, thereby amplifying membrane TLR4-mediated downstream inflammatory signaling, finally leading to optimal activation of immunostimulatory macrophages. Given a previous study that reported that CCRL2 interacting with CXCR2 on the surface of neutrophils increased their chemotactic activity toward CXCL-8 (25), cell surface expression of nonsignaling CCRL2 is likely involved in the regulation of different immune cell functions via interacting with corresponding functional surface receptors in a cell context-dependent way.
In summary, our study identifies CCRL2 as a predictive indicator of robust anti-tumor immunity in human cancers and further reveals the functional role of CCRL2 in activation of immunostimulatory macrophages and subsequent anti-tumor T-cell response. Therefore, our study suggests that CCLR2 could be a potential biomarker candidate and therapeutic target for cancer immunotherapy.
Materials and Methods
Animals.
Ccrl2−/− mice were generated by Cyagen Company using a TALEN knockout strategy. Stat6−/−, Tlr4−/−, and OT1 mice were purchased from The Jackson Laboratory. Six- to eight-week-old WT mice were purchased from the Chinese Academy of Sciences. All mice were maintained in a specific pathogen-free environment. All animal experiments were approved by the Animal Care and Use Committee at Fudan University, Shanghai, China.
Cell Depletion.
Neutralizing anti-mouse CD8α antibody (200 μg/mouse, YTS169.4, BioXell) or anti-mouse CD4 antibody (200 μg/mouse, GK1.5, BioXell) were intraperitoneally (IP) injected once a week from the day of B16F10 cell inoculation. Clodronate liposomes (50 μL/mouse, FormuMAX) were IP injected into mice every 3 d to deplete macrophages. The efficiencies of cell depletion were determined by flow cytometric analysis (SI Appendix, Fig. S7).
Statistics.
Comparisons between groups were performed using unpaired Student's t test and one-way ANOVA or two-way ANOVA (Sidak’s comparison test with selected pairs). Survival was determined by Kaplan–Meier method and survival curves between different groups were calculated by log-rank test. A P value <0.05 was considered statistically significant. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
Supplementary Material
Acknowledgments
This work was supported by the Program of Shanghai Academic/Technology Research Leader Grant (19XD1400200 to R.H.), the Science and Technology Innovation Plan of Shanghai Science and Technology Commission Grant (18140903300 to R.H.), the National Natural Science Foundation of China Grants (81771679 to R.H. and 91942313 to Y. Lin), and the Major Special Projects of the Ministry of Science and Technology Grant (2018ZX10302207 to Y. Lin).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2024171118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Wolchok J. D., et al., Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ribas A., Wolchok J. D., Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gajewski T. F., Schreiber H., Fu Y. X., Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 14, 1014–1022 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabrilovich D. I., Ostrand-Rosenberg S., Bronte V., Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noy R., Pollard J. W., Tumor-associated macrophages: From mechanisms to therapy. Immunity 41, 49–61 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y., Cao X., The origin and function of tumor-associated macrophages. Cell. Mol. Immunol. 12, 1–4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeNardo D. G., Ruffell B., Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 19, 369–382 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medrek C., Pontén F., Jirström K., Leandersson K., The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 12, 306 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen P., et al., Tumor-associated macrophages promote angiogenesis and melanoma growth via adrenomedullin in a paracrine and autocrine manner. Clin. Cancer Res. 17, 7230–7239 (2011). [DOI] [PubMed] [Google Scholar]
- 10.DeNardo D. G., et al., Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 1, 54–67 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohri C. M., Shikotra A., Green R. H., Waller D. A., Bradding P., Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur. Respir. J. 33, 118–126 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Ino Y., et al., Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br. J. Cancer 108, 914–923 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forssell J., et al., High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin. Cancer Res. 13, 1472–1479 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Mantovani A., Sozzani S., Locati M., Allavena P., Sica A., Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23, 549–555 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Movahedi K., et al., Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 70, 5728–5739 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Wang B., et al., Transition of tumor-associated macrophages from MHC class II(hi) to MHC class II(low) mediates tumor progression in mice. BMC Immunol. 12, 43 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu M., et al., Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47-mediated ‘don’t-eat-me’ signal. Nat. Immunol. 20, 265–275 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baer C., et al., Suppression of microRNA activity amplifies IFN-γ-induced macrophage activation and promotes anti-tumour immunity. Nat. Cell Biol. 18, 790–802 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Zhou W., et al., Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat. Cell Biol. 17, 170–182 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ubil E., et al., Tumor-secreted Pros1 inhibits macrophage M1 polarization to reduce antitumor immune response. J. Clin. Invest. 128, 2356–2369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colegio O. R., et al., Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimada T., Matsumoto M., Tatsumi Y., Kanamaru A., Akira S., A novel lipopolysaccharide inducible C-C chemokine receptor related gene in murine macrophages. FEBS Lett. 425, 490–494 (1998). [DOI] [PubMed] [Google Scholar]
- 23.Monnier J., et al., Expression, regulation, and function of atypical chemerin receptor CCRL2 on endothelial cells. J. Immunol. 189, 956–967 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zabel B. A., et al., Mast cell-expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-mediated passive cutaneous anaphylaxis. J. Exp. Med. 205, 2207–2220 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Prete A., et al., The atypical receptor CCRL2 is required for CXCR2-dependent neutrophil recruitment and tissue damage. Blood 130, 1223–1234 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Brouwer N., et al., Induction of glial L-CCR mRNA expression in spinal cord and brain in experimental autoimmune encephalomyelitis. Glia 46, 84–94 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Otero K., et al., Nonredundant role of CCRL2 in lung dendritic cell trafficking. Blood 116, 2942–2949 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalvo-Feo S., et al., Endothelial cell-derived chemerin promotes dendritic cell transmigration. J. Immunol. 192, 2366–2373 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Del Prete A., et al., The atypical receptor CCRL2 is essential for lung cancer immune surveillance. Cancer Immunol. Res. 7, 1775–1788 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazzon C., et al., CCRL2 regulates M1/M2 polarization during EAE recovery phase. J. Leukoc. Biol. 99, 1027–1033 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Sarmadi P., Tunali G., Esendagli-Yilmaz G., Yilmaz K. B., Esendagli G., CRAM-A indicates IFN-γ-associated inflammatory response in breast cancer. Mol. Immunol. 68, 692–698 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Georgoudaki A. M., et al., Reprogramming tumor-associated macrophages by antibody targeting inhibits cancer progression and metastasis. Cell Rep. 15, 2000–2011 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Racioppi L., et al., CaMKK2 in myeloid cells is a key regulator of the immune-suppressive microenvironment in breast cancer. Nat. Commun. 10, 2450 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tirosh I., et al., Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jerby-Arnon L., et al., A cancer cell program promotes T cell exclusion and resistance to checkpoint blockade. Cell 175, 984–997.e24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bohn T., et al., Tumor immunoevasion via acidosis-dependent induction of regulatory tumor-associated macrophages. Nat. Immunol. 19, 1319–1329 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Song Y., et al., Chemerin partly mediates tumor-inhibitory effect of all-trans retinoic acid via CMKLR1-dependent natural killer cell recruitment. Immunology 157, 248–256 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pachynski R. K., et al., The chemoattractant chemerin suppresses melanoma by recruiting natural killer cell antitumor defenses. J. Exp. Med. 209, 1427–1435 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Awasthi S., Toll-like receptor-4 modulation for cancer immunotherapy. Front. Immunol. 5, 328 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wanderley C. W., et al., Paclitaxel reduces tumor growth by reprogramming tumor-associated macrophages to an M1 profile in a TLR4-dependent manner. Cancer Res. 78, 5891–5900 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.