Significance
Bone is the main source of fibroblast growth factor 23 (FGF23), which is important for phosphate and vitamin D homeostasis. In acute kidney injury (AKI), high blood levels of FGF23 are positively correlated with disease progression and increased risk of mortality. Reducing adverse plasma FGF23 levels in AKI patients is favorable. We showed here that hepatocytes are the major source of circulating FGF23, and orphan nuclear receptor ERR-γ is a novel transcriptional regulator of hepatic FGF23 production in AKI. Liver-specific depletion of ERR-γ or ERR-γ inverse agonist, GSK5182, significantly reduced plasma levels of FGF23 in AKI. This study reveals liver is the source of FGF23 and a therapeutic strategy to control pathologically adverse plasma FGF23 levels in AKI.
Keywords: acute kidney injury, ERR-γ, FGF23, interleukin 6, orphan nuclear receptor
Abstract
Fibroblast growth factor 23 (FGF23), a hormone generally derived from bone, is important in phosphate and vitamin D homeostasis. In acute kidney injury (AKI) patients, high-circulating FGF23 levels are associated with disease progression and mortality. However, the organ and cell type of FGF23 production in AKI and the molecular mechanism of its excessive production are still unidentified. For insight, we investigated folic acid (FA)-induced AKI in mice. Interestingly, simultaneous with FGF23, orphan nuclear receptor ERR-γ expression is increased in the liver of FA-treated mice, and ectopic overexpression of ERR-γ was sufficient to induce hepatic FGF23 production. In patients and in mice, AKI is accompanied by up-regulated systemic IL-6, which was previously identified as an upstream regulator of ERR-γ expression in the liver. Administration of IL-6 neutralizing antibody to FA-treated mice or of recombinant IL-6 to healthy mice confirms IL-6 as an upstream regulator of hepatic ERR-γ–mediated FGF23 production. A significant (P < 0.001) interconnection between high IL-6 and FGF23 levels as a predictor of AKI in patients that underwent cardiac surgery was also found, suggesting the clinical relevance of the finding. Finally, liver-specific depletion of ERR-γ or treatment with an inverse ERR-γ agonist decreased hepatic FGF23 expression and plasma FGF23 levels in mice with FA-induced AKI. Thus, inverse agonist of ERR-γ may represent a therapeutic strategy to reduce adverse plasma FGF23 levels in AKI.
FGF23, a phosphotropic hormone generally produced from bone (1), is a 251 amino acid–glycosylated peptide, which plays an important role in mineral homeostasis (2, 3). The 30 to 32-kDa biologically active intact FGF23 (iFGF23) is cleaved by protein convertases between arginine 179 and serine 180 into 18-kDa N-terminal and 12-kDa C-terminal (cFGF23) fragments (4). O-glycosylation at T178 by polypeptide N-acetylgalactosaminyltransferase 3 (GalNT3) protects FGF23 from proteolytic cleavage. Conversely, phosphorylation at S180 via the secretory protein kinase family with sequence similarity-20 member C (FAM20C) inhibits GalNT3-mediated O-glycosylation and thereby promotes proteolytic cleavage of FGF23 (5). FGF23 binds to single-pass transmembrane FGF receptors (FGFR 1 to 4) that are expressed in many tissue types (6). FGF23 binding to FGFR1 and its coreceptor α-klotho, the latter being mainly expressed in the kidney and parathyroid gland (7), increases urinary phosphate excretion by downregulating sodium-phosphate cotransporters NPT2a and NPT2c expression in proximal tubular cells of the kidney (8). FGF23 also reduces circulatory vitamin D levels by inhibiting the 1,25-dihydroxyvitamin D3 (1,25(OH)2D) synthesizing enzyme Cyp27b1 and enhancing inactivating enzyme Cyp24a1 in the kidney (3). A downstream effect of FGF23-mediated vitamin D inhibition is reduction of circulating phosphate levels by dropping intestinal absorption of dietary phosphate. FGF23 extends its inhibitory action to parathyroid hormone (PTH) secretion from the parathyroid gland (9). PTH exhibits the same effect as FGF23 on phosphate reabsorption but has an opposite effect on vitamin D synthesis (10). Interestingly, vitamin D and PTH are both reported to increase FGF23 expression, indicating the existence of a number of negative feedback loops among PTH, vitamin D, and FGF23 (11–13). FGF23 circulatory levels are altered by many factors including, but not limited to, PTH, 1,25(OH)2D, phosphate, erythropoietin, tumor necrosis factor-α (TNF-α), transforming growth factor β2 (TGF-β2), insulin, iron status, and inflammation (12–14).
In patients suffering from acute kidney injury (AKI), FGF23 circulatory levels are markedly increased and positively correlate with disease progression, prolonged hospitalization, and severe sepsis, thereby increasing the risk of mortality (15). FGF23 production in folic acid–induced AKI (FA-AKI) mice is not induced by its classical regulators like PTH, vitamin D, or phosphate. Moreover, excessively high-circulating FGF23 levels in FA-AKI mice cannot be explained by the magnitude of increase in bone FGF23 messenger RNA (mRNA) expression (16). FGF23 mRNA levels are increased in multiple organs of FA-AKI mice. Interestingly, nonosseous tissues like the spleen, thymus, and heart showed more increase in FGF23 mRNA levels compared to bone (17). Finally, acute inflammation, one of the main features of AKI, markedly increases FGF23 mRNA levels in multiple organs of mice (18), suggesting that there must be other sources than bone during AKI, placing in additional mechanisms for FGF23 production.
Estrogen-related receptors (ERRs) belong to a subfamily of orphan nuclear receptors (NR3B) containing three distinct isoforms (ERR-α, -β, and -γ) that share their highest percentage of similarity with the estrogen receptor (19). ERRs display constitutive activity without binding to a known ligand and exert their transcriptional activity via binding as dimers or monomers to the core sequence TCAAGGTCA, referred to as an ERR response element (ERRE) that is present in the regulatory regions of ERR target genes (20). Transcriptional activity of ERR-γ is conferred by complex formation with coactivator or corepressor proteins (21). ERR-γ gene expression is highly induced and tightly controlled by various membrane receptors signaling, like endocannabinoid, IL-6, and glucagon in the liver (21, 22). We previously reported that ERR-γ is up-regulated in mouse liver during fasting to increase hepatic gluconeogenesis (23), whereas in the fed state, insulin inhibits this activity by PKB/Akt-mediated phosphorylation of ERR-γ (24). Additionally, ERR-γ inhibits Salmonella typhimurium infection by modulating host iron homeostasis and influences lipid metabolism in the liver (25, 26).
In this study, we provide evidence for a mechanism of ERR-γ–regulated hepatic FGF23 production in FA-AKI mice. We found that IL-6 is an upstream regulator of ERR-γ–mediated hepatic FGF23 production. Moreover, liver-specific ERR-γ knockout (ERR-γ-LKO) or ERR-γ–specific inverse agonist GSK5182 inhibits FA-induced hepatic FGF23 production and reduces circulatory FGF23 levels.
Results
Hepatic ERR-γ and FGF23 Gene Expression Increased in FA-AKI.
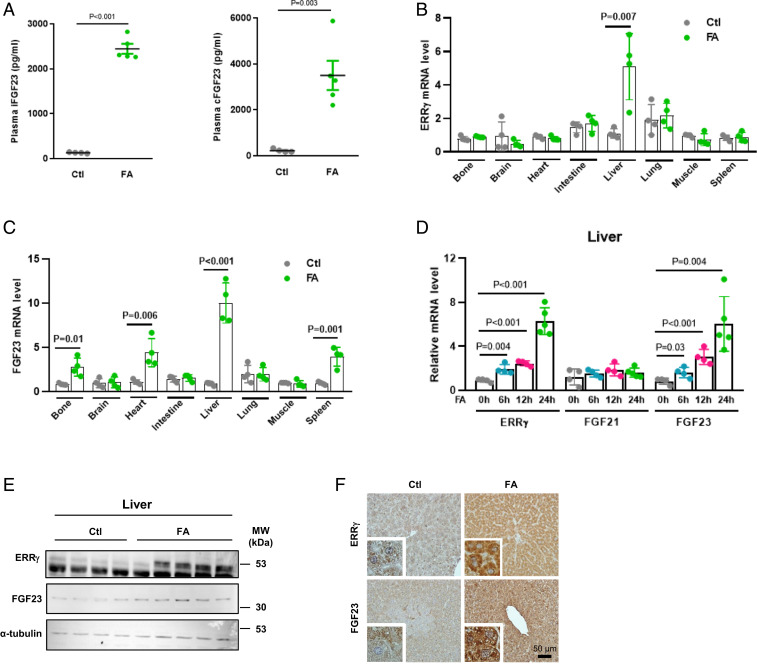
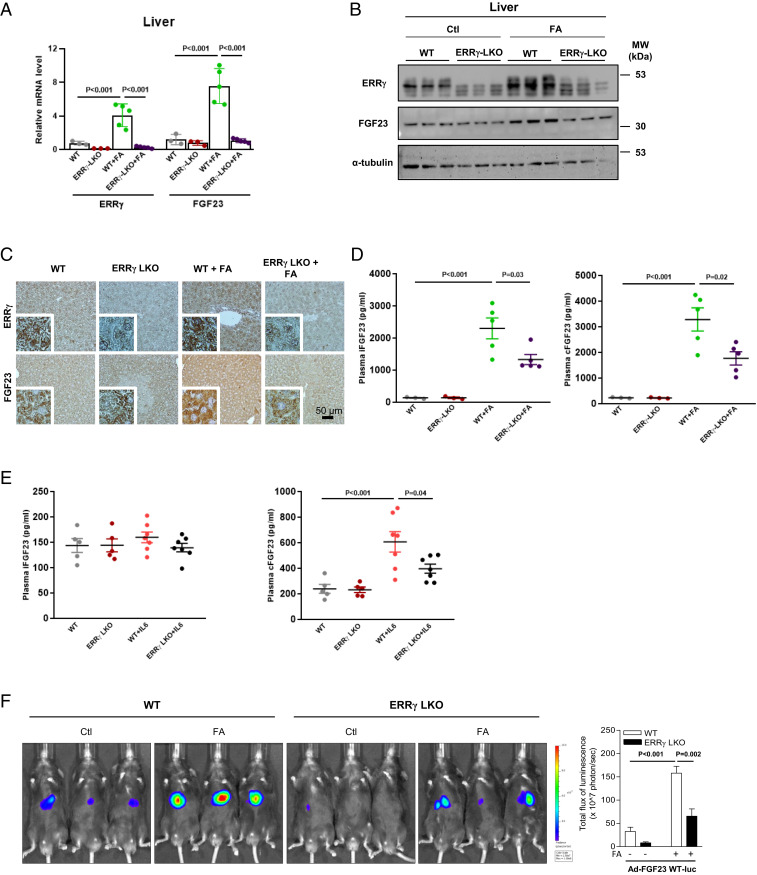
It has been previously suggested that proinflammatory cytokines, especially IL-1β, IL-6, and TNF-α, are up-regulated in AKI patients (27, 28) as well as in animal disease models (29) and are the potential mediators of organ-to-organ communication (30). The liver, especially hepatocytes, is highly sensitive and responsive to proinflammatory cytokines, including IL-6. Furthermore, we have shown that another member of the fibroblast growth factor family and stress-inducible hepatokine, FGF21, is transcriptionally induced in hepatocytes by ERR-γ and then secreted from this cell type (31). This result and our observation that IL-6 induces expression of ERR-γ in hepatocytes (22, 26) prompted us to hypothesize a mechanistic link between circulating IL-6, ERR-γ expression, and FGF23 production. To find out more about the source of high FGF23 levels in kidney disease, we treated C57BL/6J mice with a high dose of FA to induce AKI, which was confirmed by hematoxylin and eosin staining of kidney sections, as well as by increased plasma levels of blood urea nitrogen (BUN), creatinine, phosphate, 1,25(OH)2D, and PTH (SI Appendix, Fig. S1 A–F). Consistent with previous reports, iFGF23 and cFGF23 plasma levels were also increased by FA treatment, thereby phenocopying human AKI (Fig. 1A). We then examined ERR-γ and FGF23 mRNA levels in multiple organs of FA-treated mice and found FGF23 mRNA level was highly induced in the liver and coincided with ERR-γ expression (Fig. 1 B and C). We further found that ERR-γ and FGF23 mRNA levels continuously increased with time in FA-treated mouse liver, whereas FGF21 mRNA remained unchanged (Fig. 1D). Moreover, hepatic ERR-γ and FGF23 protein levels were also increased in FA-treated mice, as investigated by Western blot and immunohistochemistry (Fig. 1 E and F). Analysis of liver injury markers showed no damage in the liver at 24 h in response to FA treatment (SI Appendix, Fig. S1 G and H). We analyzed mRNA levels of entire ERR family members and found ERR-β level also increased along with ERR-γ in response to FA treatment, whereas ERR-α expression was not changed (SI Appendix, Fig. S2). These results suggest that the liver produces FGF23 in response to FA-AKI.
Fig. 1.
Hepatic ERR-γ and FGF23 gene expression increased in FA-AKI. (A) Plasma intact FGF23 (Left) and C-terminal FGF23 (Right) levels were measured by ELISA in control or FA- (24 h) treated mice (control n = 4 and FA n = 5). (B and C) qPCR analysis of total RNAs isolated from different tissues of FA-treated mice. C57BL/6J mice were intraperitoneally injected with a high dose of FA and euthanized after 24 h. The ERR-γ and FGF23 mRNA expressions were measured from different tissues of mice treated with control or FA (n = 4). (D) qPCR analysis of total RNAs isolated from control or FA time-course–treated mice livers (0, 6, and 12 h n = 4 and 24 h n = 5). (E) Western blot analysis of total protein isolated from livers of mice treated with control or FA and euthanized after 24 h (control n = 4 and FA n = 5). (F) Representative images of immunohistochemistry analysis in control and FA- (24 h) treated mouse-liver sections. Data represent mean ± SEM. All data were analyzed using two-tailed Student’s t test.
Overexpression of ERR-γ Increases Hepatic FGF23 Expression and Secretion.
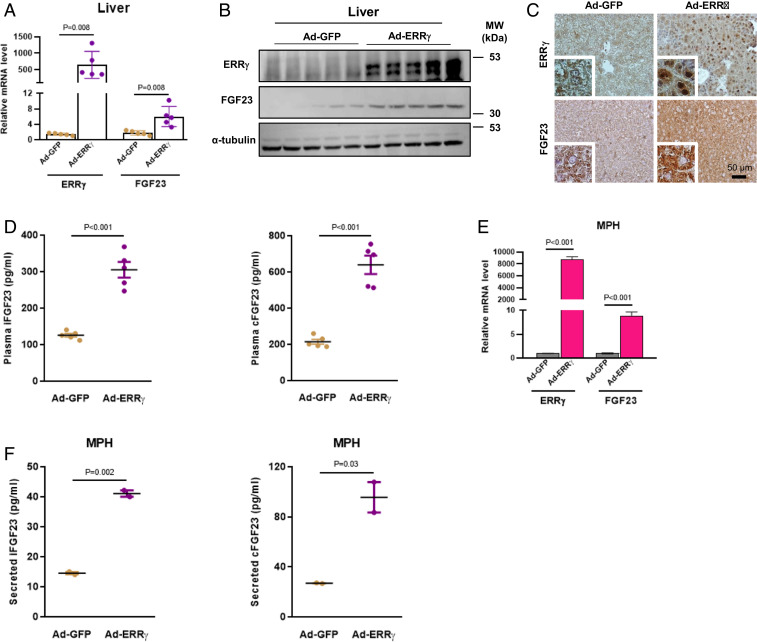
To delineate the connection between ERR-γ and FGF23 in detail, we overexpressed hepatic ERR-γ in mice using an adenoviral construct (Ad-ERR-γ). FGF23 mRNA and protein expression in the liver were significantly increased upon ERR-γ overexpression (Fig. 2 A–C). Moreover, plasma levels of FGF23 were also increased (Fig. 2D). We also showed that Ad-ERR-γ infection in mice induced FGF23 mRNA expression specifically in hepatocytes (Fig. 2E). Next, we measured secreted FGF23 levels and found both iFGF23 and cFGF23 levels were increased in mouse primary hepatocyte (MPH) medium by ERR-γ overexpression (Fig. 2F). Since we found ERR-β mRNA levels increased along with ERR-γ in FA-AKI condition (SI Appendix, Fig. S2), we wanted to know whether ERR-β can also regulate FGF23 gene expression. For this purpose, we performed luciferase reporter assays by transiently transfecting an FGF23 wild-type (WT) promoter construct along with ERR-α, ERR-β, and ERR-γ expression vectors into 293T cells. FGF23 gene promoter activity was specifically induced by ERR-γ, whereas ERR-α and ERR-β had no effect (SI Appendix, Fig. S3A). This result was further confirmed by overexpressing ERR-β in AML12 cells using an adenoviral construct (Ad-ERR-β), as FGF23 mRNA levels were not increased in this setting (SI Appendix, Fig. S3B). These results indicate that ERR-γ induces FGF23 gene expression in hepatocytes and increases plasma FGF23 levels.
Fig. 2.
Overexpression of ERR-γ increases hepatic FGF23 expression and secretion. (A) qPCR analysis of total RNAs and (B) Western blot analysis of total protein isolated from Ad-GFP– or Ad-FLAG-ERR-γ–infected mice livers (n = 5). (C) Representative images of immunohistochemistry analysis in Ad-GFP– or Ad-FLAG-ERR-γ–infected mice-liver sections. (D) Plasma intact FGF23 (Left) and C-terminal FGF23 (Right) levels measured by ELISA in Ad-GFP– or Ad-FLAG-ERR-γ–infected mice (n = 5). (E) qPCR analysis of total RNAs isolated from cultured MPH infected with Ad-GFP or Ad-FLAG-ERR-γ. (F) Secreted intact FGF23 (Left) and C-terminal FGF23 (Right) levels were measured in Ad-GFP– or Ad-FLAG-ERR-γ–infected MPH culture medium. All cell culture experiments were performed as three independent replicates. Data represent mean ± SEM. All data were analyzed using two-tailed Student’s t test.
IL-6 Is Correlated with FGF23 Levels and AKI Development in Human Patients following Cardiac Surgery and Is an Upstream Regulator of FA-Mediated Hepatic FGF23 Production.
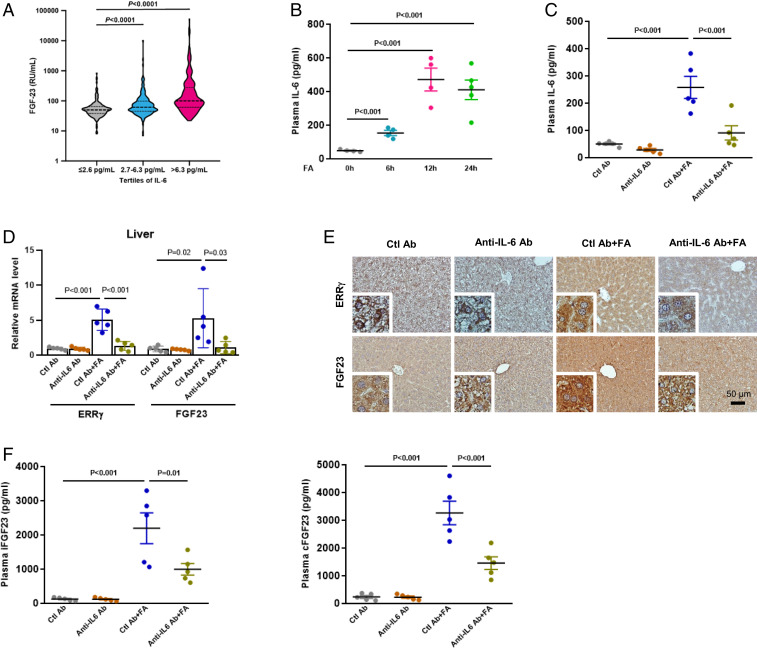
IL-6 is a known upstream regulator of ERR-γ expression and has been shown as increased in human AKI (22, 27, 28). To correlate IL-6 with FGF23, we measured IL-6 levels in a cohort of 709 patients that underwent cardiac surgery, from which FGF23 levels were available and a subgroup of patients (26%) developed AKI as a complication (32) (SI Appendix, Table S1). We could show that in this cohort IL-6 and FGF23 levels were highly correlated with each other (R2 = 0.448, P < 0.0001) (SI Appendix, Table S2). Notably, there was no correlation between FGF23 and high sensitivity C-reactive protein (hsCRP), an acute-phase protein, which underlines the specificity of the association between IL-6 and FGF23. FGF23 plasma levels were significantly higher in patients with high IL-6 within the second and third tertile, as mentioned above (P < 0.0001, Fig. 3A). Using logistic regression analyses, we found that higher preoperative IL-6 is associated with a significantly higher risk for postoperative AKI (SI Appendix, Table S3). This association persisted even after adjusting for several covariates. To assess the interaction between IL-6 and FGF23, we introduced an interaction term between both parameters in the respective models. This revealed a significant (P < 0.001) interaction between both parameters to predict the risk of AKI, suggesting that in humans, IL-6 and FGF23 are interconnected.
Fig. 3.
IL-6 is an upstream regulator of FA-mediated hepatic FGF23 production. (A) Violin plot on the association between tertiles of IL-6 and FGF23 in patients at high risk for AKI. (B) Plasma IL-6 level measured by ELISA in mice treated with FA in time-dependent manner (0, 6, and 12 h n = 4 and 24 h n = 5). (C) Plasma IL-6 level measured in C57BL/6J mice injected with control antibody or IL-6 neutralizing (anti–IL-6) antibody in presence or absence of FA for 24 h (n = 5). (D) qPCR analysis of total RNAs isolated from livers of mice injected with control or anti–IL-6 antibody in the presence or absence of FA. (E) Representative images of immunohistochemistry analysis in liver sections of mice injected with control or anti–IL-6 antibody in presence or absence of FA. (F) Plasma intact FGF23 (Left) and C-terminal FGF23 (Right) levels were measured by ELISA in mice injected with control or anti–IL-6 antibody in presence or absence of FA. Data represent mean ± SEM. Data in B was analyzed by two-tailed Student’s t test. Data in C, D, and F were analyzed by one-way ANOVA with Tukey’s multiple comparisons test.
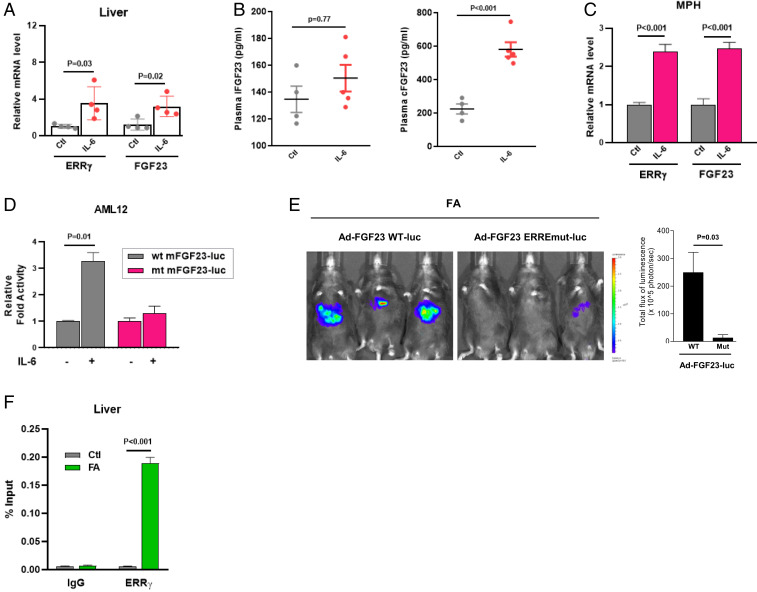
To functionally prove that up-regulated IL-6 levels, as present in FA-induced AKI (29) (Fig. 3B), are the trigger of hepatocyte ERR-γ induction and subsequent FGF23 production, we blocked IL-6 using an IL-6 neutralizing antibody in mice treated or not with FA (Fig. 3C). IL-6 neutralization resulted in reduced mRNA and protein expressions of hepatic ERR-γ and FGF23 (Fig. 3 D and E). Plasma iFGF23 and cFGF23 levels were also significantly reduced by 55% in IL-6 neutralized mice, as compared to controls (Fig. 3F). However, IL-6 neutralization did not alter plasma levels of FA-induced AKI markers (SI Appendix, Fig. S4). The role of IL-6 was further confirmed by administration of recombinant IL-6 in normal mice, which increased hepatic ERR-γ and FGF23 mRNA (Fig. 4A), as well as plasma cFGF23 levels, but not iFGF23 levels (Fig. 4B). Next, we showed that IL-6 increased ERR-γ and FGF23 mRNA levels in MPH (Fig. 4C). IL-6 increased WT FGF23 promoter activity but failed to activate the ERRE mutant FGF23 promoter in AML12 cells (Fig. 4D), suggesting that the FGF23 gene-activating signal is mediated via ERR-γ. Next, we performed an imaging analysis in mice to cross verify that ERR-γ acts on the FGF23 promoter in response to FA treatment. Adenovirus-carrying luciferase reporter construct fused with mouse FGF23 promoter, either WT (Ad-FGF23 WT-luc) or ERRE mutant (Ad-FGF23 ERRE mut-luc), was injected into mice via tail vein. FA treatment increased WT FGF23 promoter activity in the liver, as evidenced by the increased signals, whereas mice injected with the ERRE mutant promoter failed to respond to FA treatment (Fig. 4E). Finally, we did chromatin immunoprecipitation (ChIP) assay in control and FA-treated mice livers, which showed ERR-γ was recruited to the ERRE region of the FGF23 promoter in response to FA treatment (Fig. 4F). Soluble chromatin precipitated with ERR-γ or histone H3 antibodies and then analyzed with primers for a non-ERRE comprising region of the mouse FGF23 gene promoter, or primers for intron 2 of the mouse RPL30 gene served as negative and positive controls, respectively (SI Appendix, Fig. S5). These results confirm that IL-6 in FA-AKI is the upstream signal to increase ERR-γ gene expression in the liver, which in turn transcriptionally regulates hepatic FGF23 expression.
Fig. 4.
IL-6 injection increased hepatic FGF23 expression and secretion through direct binding of ERR-γ on FGF23 gene promoter. (A) qPCR analysis of total RNAs isolated from livers of mice injected with control or 2.5 μg/kg of IL-6 and euthanized after 1 h (n = 4). (B) Plasma intact FGF23 (Left) and C-terminal FGF23 (Right) levels measured by ELISA in mice intraperitoneally injected with control or IL-6 and euthanized after 3 h (control n = 4 and IL-6 n = 5). (C) qPCR analysis of total RNAs isolated from MPH treated with IL-6 (20 ng/mL). (D) AML12 cells were transfected with vectors expressing WT or ERRE mutant FGF23 promoter luciferase constructs and stimulated with 20 ng/mL of IL-6. (E) In-vivo imaging of hepatic FGF23 promoter WT-luciferase (Ad-FGF23 WT-luc) and FGF23 promoter ERRE mut-luciferase (Ad-FGF23 ERREmut-luc) activity in mice injected with FA (n = 3). (F) In-vivo ChIP assay showing the binding of ERR-γ to FGF23 gene promoter in response to FA. C57BL/6J mice were treated with control or FA (24 h), and soluble chromatin was immunoprecipitated with ERR-γ antibody. qPCR analysis of purified DNA with primers corresponding to ERRE region of mouse FGF23 gene promoter. All cell culture experiments were performed as three independent replicates. Data represent mean + SEM. All data were analyzed using two-tailed Student’s t test.
Liver-Specific ERR-γ Knockout Mice Fail to Induce Hepatic FGF23 Production in Response to FA and IL-6.
To further confirm the role of ERR-γ in FA-induced hepatic FGF23 production, we generated liver-specific ERR-γ knockout mice (ERR-γ-LKO) (SI Appendix, Fig. S6A) (22). FA-induced FGF23 mRNA and protein levels were significantly decreased upon depletion of ERR-γ in hepatocytes (Fig. 5 A–C). Plasma iFGF23 and cFGF23 levels were also markedly reduced in ERR-γ-LKO mice (Fig. 5D), whereas plasma levels of the typical AKI markers did not differ in FA-treated WT and ERR-γ-LKO mice (SI Appendix, Fig. S6 B–F). To confirm whether repressing hepatic FGF23 production by the ERR-γ knockout leads to enhanced production of FGF23 by other tissues, we examined FGF23 mRNA expression from the bone, brain, heart, intestine, liver, lungs, muscle, and spleen of FA-treated WT and ERR-γ-LKO mice. Except liver, none of the tissues showed a significant difference in FGF23 mRNA levels between FA-treated WT and ERR-γ-LKO groups (SI Appendix, Fig. S7). This result implies that repressing hepatic FGF23 production did not lead to a compensatory effect by other tissues. Moreover, administration of IL-6 increased plasma cFGF23 level in WT mice, whereas it was significantly reduced in ERR-γ-LKO mice (Fig. 5 E, Right). Consistent with the previous result (Fig. 4B), there was no change in plasma iFGF23 level by IL-6 in both WT and ERR-γ-LKO mice (Fig. 5 E, Left). Finally, we performed imaging analysis in WT and ERR-γ-LKO mice injected with Ad-FGF23 WT-luc to emphasize the importance of ERR-γ in FA-mediated hepatic FGF23 expression in vivo. FGF23 promoter activity was increased by FA treatment in WT mice, which was significantly blunted in ERR-γ-LKO mice (Fig. 5F). These results suggest that ERR-γ is a key regulator of hepatic FGF23 production and plasma FGF23 levels in FA-induced AKI.
Fig. 5.
Liver-specific ERR-γ knockout mice fail to induce hepatic FGF23 production in response to FA and IL-6. (A) qPCR analysis of total RNAs isolated from livers of WT and ERR-γ-LKO mice treated with FA and euthanized after 24 h (WT and ERR-γ-LKO n = 3; WT+FA and ERR-γ-LKO+FA n = 5). (B) Western blot analysis of total protein isolated from control or FA-treated WT and ERR-γ-LKO mice livers (n = 3). (C) Representative images of ERR-γ and FGF23 immunohistochemistry in liver sections of WT and ERR-γ-LKO mice treated with control or FA. (D) Plasma intact FGF23 (Left) and C-terminal FGF23 (Right) levels measured by ELISA in control or FA-treated WT and ERR-γ-LKO mice (WT and ERR-γ-LKO n = 3; WT+FA and ERR-γ-LKO+FA n = 5). (E) Intact FGF23 (Left) and C-terminal FGF23 (Right) levels measured by ELISA in control or 2.5 μg/kg (3 h) of IL-6–treated WT and ERR-γ-LKO mice (WT and ERR-γ-LKO n = 5; WT+IL-6 and ERR-γ-LKO+IL-6 n = 7). (F) Representative images for in-vivo imaging of hepatic FGF23 promoter WT-luciferase (Ad-FGF23 WT-luc) activity in WT and ERR-γ-LKO mice injected with or without FA (n = 4 for WT-Control (Ctl) and ERR-γ-LKO Ctl; n = 6 for WT-FA and ERR-γ-LKO FA group). Data represent mean ± SEM. All data were analyzed by one-way ANOVA with Tukey’s multiple comparisons test.
GSK5182 Inhibits Hepatic FGF23 Production in Response to IL-6 and FA.
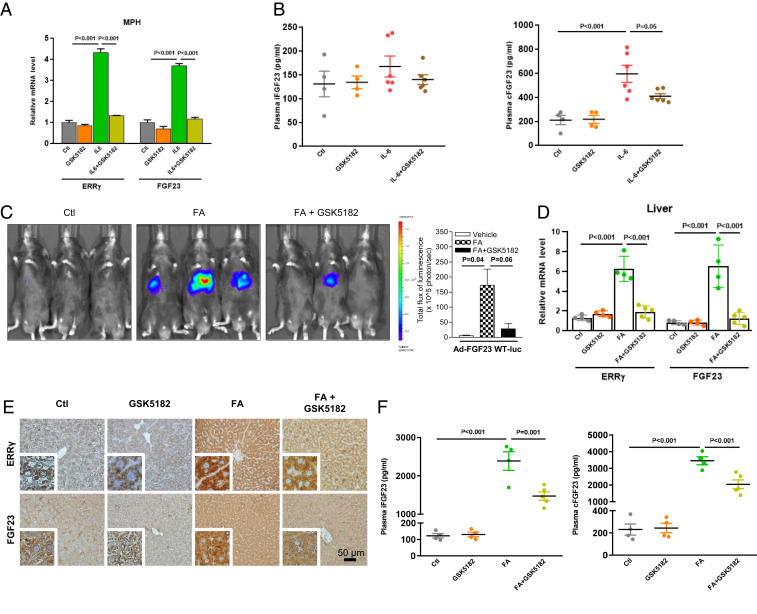
As from the above, we provide strong evidence that ERR-γ is important for FA-mediated FGF23 production in hepatocytes, so we tested pharmacological inhibition of ERR-γ by GSK5182, which acts as an inverse agonist of ERR-γ by inhibiting its transactivation, thereby reducing ERR-γ target gene expression (33–35). We found that GSK5182 treatment inhibited IL-6 mediated up-regulation of FGF23 mRNA levels in MPH (Fig. 6A). Moreover, plasma cFGF23 levels induced by recombinant IL-6 in mice are significantly inhibited by GSK5182 (Fig. 6 B, Right), while iFGF23 level was not changed (Fig. 6 B, Left). We also tested if GSK5182 treatment is sufficient to reduce hepatic FGF23 gene expression in the FA-induced AKI condition. We performed imaging analysis in mice tail-vein injected with Ad-FGF23 WT-luc treated with FA that were treated or not with GSK5182. FGF23 promoter activity was induced in the liver by FA treatment, which was significantly blunted in the presence of GSK5182 (Fig. 6C). GSK5182 treatment also inhibited FA-mediated up-regulation of hepatic ERR-γ and FGF23 mRNA and protein expression (Fig. 6 D and E), whereas no change was found for plasma IL-6 levels (SI Appendix, Fig. S8A). Consistent with the results in AKI mice with ERR-γ depletion in hepatocytes, pharmacological inhibition of ERR-γ by GSK5182 markedly reduced plasma levels of iFGF23 and cFGF23 by 38% and 41%, respectively (Fig. 6F). As expected from our other data, GSK5182 treatment had no effect on blood levels of AKI markers (SI Appendix, Fig. S8 B–F). Taken together, these results indicate that an inverse agonist-mediated inactivation of ERR-γ transcriptional activity significantly inhibits hepatic FGF23 production and reduces plasma FGF23 levels in FA-induced AKI mice.
Fig. 6.
GSK5182 inhibits hepatic FGF23 production in response to IL-6 and FA. (A) ERR-γ and FGF23 mRNA expression levels analyzed by qPCR in total RNAs isolated from cultured MPH treated with IL-6 (20 ng/mL) in the presence or absence of GSK5182 (10 μM). (B) Plasma intact FGF23 (Left) and C-terminal FGF23 (Right) levels measured by ELISA in mice injected with IL-6 (2.5 μg/kg) with or without GSK5182 (40 mg/kg) and euthanized after 3 h (control and GSK5182 n = 4; IL-6 and GSK5182+IL-6 n = 6). (C) In-vivo imaging of hepatic FGF23 promoter WT-luciferase (Ad-FGF23 WT-luc) activity in mice injected with FA in the presence or absence of GSK5182 (40 mg/kg) (n = 3). (D) qPCR analysis of total RNAs isolated from livers of mice injected with FA with or without GSK5182 (Control, GSK5182 and FA n = 4; FA+GSK5182 n = 5). (E) Representative images of immunohistochemical analysis in liver sections of control or FA-treated mice in the presence or absence of GSK5182. (F) Plasma intact FGF23 (Left) and C-terminal FGF23 (Right) levels measured by ELISA in mice treated with FA with or without GSK5182. All cell culture experiments were performed as three independent replicates. Data represent mean ± SEM. All data were analyzed by one-way ANOVA with Tukey’s multiple comparisons test.
Discussion
Our study discloses orphan nuclear receptor ERR-γ as a regulator of FGF23 production via expression up-regulation in hepatocytes. FA-AKI induces hepatic FGF23 production and secretion, which is blunted upon hepatocyte-specific ERR-γ knockout. ERR-γ overexpression is sufficient to induce hepatic FGF23 production in the absence of FA intoxication. We further identified IL-6 as an upstream signal to induce ERR-γ expression in FA-treated mice. Finally, an ERR-γ inverse agonist, GSK5182, was effective in reducing hepatic production and systemic levels of FGF23 in the FA mouse model of AKI.
Hepatic production of FGF23 was previously reported in patients with autosomal dominant polycystic kidney disease and in childhood biliary atresia (36–38). Activation of the Janus kinase (JAK)-signal transducer and activator of transcription 3 (STAT3) signaling pathway leads to liver inflammation and increased hepatic FGF23 synthesis in mice (39). Plasma FGF23 levels increased in about 63% of patients with end-stage liver disease and with diethyl-nitrosamine–treated mice; the study revealed that chronic liver lesions were the source of circulatory FGF23 (40). Here, we showed that unharmed liver produces FGF23 in response to a systemic signal from FA-AKI. Genetic abrogation or pharmacological inhibition of ERR-γ was sufficient to blunt FA-induced hepatic FGF23 production and decrease but not completely deplete circulatory FGF23 levels. From a recent report showing increased FGF23 expression in multiple organs of FA-treated mice, including the bone, we speculate that other organs besides the liver represent additional sources for the remaining FGF23 levels in the circulation (17). Hence, the liver is a major source of FGF23 in FA-AKI as it contributes a significant amount of circulating FGF23.
IL-6 increased FGF23 transcription through STAT3 signaling in bone, thereby contributing to the high levels of systemic FGF23 in both AKI and chronic kidney disease (CKD) mice (29). We previously reported that IL-6 can induce ERR-γ expression through JAK-STAT3 signaling in hepatocytes (22, 26). In the present study, we showed that (i) IL-6 blood levels were up-regulated in the FA-AKI model (ii), treatment with IL-6 neutralizing antibody inhibited hepatic ERR-γ and FGF23 gene expression, and reduced plasma FGF23 levels, (iii) whereas systemic delivery of recombinant IL-6 in healthy mice was sufficient to induce FGF23 transcription through ERR-γ in hepatocytes and increase circulatory FGF23 levels. Since it is recently reported that FGF23 can induce hepatic IL-6 secretion (41), there possibly exists a positive feedback loop between IL-6 and FGF23 within the liver. Upon recombinant IL-6 treatment, hepatic FGF23 mRNA and plasma cFGF23 levels are significantly increased in mice, whereas iFGF23 levels are unchanged. We reason that iFGF23 levels are not increased due to the fact that the dosage of recombinant IL-6 as used is much less, as compared to the FA-induced endogenous IL-6 levels, which are approximately seven- to ninefold induced as compared to controls. Moreover, circulatory levels of iFGF23 are tightly controlled by posttranslational modifications, such as phosphorylation at S180 by FAM20C, which then leads to proteolytic cleavage of iFGF23 into N-terminal and cFGF23 fragments (4, 5). To test if FAM20C activity may be relevant for the measured iFGF23 levels in the IL-6 treatment condition, we used MPH culture, where the FAM20C-mediated proteolytic cleavage of iFGF23 is not taking place, as compared to the in-vivo condition. We treated MPHs with recombinant IL-6 in the presence of adenoviral shERR-γ (Ad-shERR-γ) or GSK5182 and measured FGF23 levels in the culture medium. Both iFGF23 and cFGF23 levels are significantly increased by IL-6 treatment, and both are reduced in the MPH culture medium in Ad-shERR-γ and GSK5182 treatment conditions. This result shows that in the absence of FAM20C-mediated posttranslation control, both iFGF23 and cFGF23 levels are similarly and significantly increased by IL-6 treatment (SI Appendix, Fig. S9). These results support our claim that iFGF23 levels are not increased by IL-6 treatment due to FAM20C-mediated proteolytic cleavage. On the other hand, recombinant IL-6 treatment is sufficient to induce hepatic FGF23 transcription through ERR-γ, thereby increasing cFGF23 blood levels; however, it is not strong enough, as compared to FA treatment, to induce high levels of iFGF23 to overcome the posttranslational control machinery.
Several transcription factors like vitamin D receptor (VDR), hypoxia-inducible factor-1α (HIF-1α), forkhead box protein O1 (FOXO1), nuclear receptor related 1 protein (Nurr1), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) were reported to regulate FGF23 expression in bone (13, 42–45). Here, we report the transcription factor, orphan nuclear receptor ERR-γ, as a regulator of FGF23 expression in the liver. We previously reported the existence of variants of the classical ERRE consensus sequence TCAAGGTCA with significant ERR-γ binding activity to regulate target gene expression (22, 23, 25). We now found a functional ERRE motif AGGTCA in the FGF23 promoter at 251 bp upstream of the transcription start site that was responsible for ERR-γ–mediated FGF23 expression in hepatocytes and in mouse livers. Other nuclear receptors, Nurr1 and VDR, were previously reported to regulate FGF23 expression in bone in response to PTH and 1,25(OH)2D, respectively (13, 44). Thereby, the Nurr1 binding site overlaps with the ERRE. Interestingly, this coinciding region is conserved in humans, mice, and rats that denote the biological significance of this region.
Translation of our findings in the FA-AKI mouse model to humans is hampered by the fact that patients suffering from AKI do not undergo liver biopsy, and thus no samples are available to correlate FGF23 levels with liver ERR-γ activity. On the other hand, we were thinking that defining systemic IL-6 levels in an AKI patient cohort is indirect evidence that the liver of patients responds to the increased systemic IL-6 levels, as shown in the mice. The patient cohort that we investigated has been described previously (32) and comprises patients that underwent elective cardiac surgery, in which a subgroup of patients developed an AKI. Indeed, we found in these patients that IL-6 levels are associated with higher FGF23 levels. Furthermore, presurgery determined IL-6 is associated with an increased risk of acute renal failure, both parameters IL-6 and FGF23 influence each other, and we suggest that they can be used to predict the risk for an AKI in these patients. From these findings, we confirm the AKI-IL-6-FGF23 axis in patients and hypothesize that increased systemic IL-6 levels may activate the ERR-γ/FGF23 axis in human liver, as shown in our mouse model. In future studies, with Acute-on-Chronic Liver Failure patients that present with AKI and undergo liver transplantation, we may investigate blood samples and liver explants to further confirm our findings.
In conclusion, we report that FA-induced AKI mice up-regulate systemic IL-6 levels to induce expression of orphan nuclear receptor ERR-γ in hepatocytes, which is the key regulator of hepatic FGF23 production. Hepatocyte-specific genetic depletion or pharmacological inhibition of ERR-γ is sufficient to reduce hepatic FGF23 expression and plasma FGF23 levels in AKI mice. We propose that in pathological condition, acute renal injury induces plasma IL-6 levels, which in turn increases hepatic ERR-γ and FGF23 gene expression, thereby contributing to increased levels of plasma FGF23. This excessively high-circulating FGF23 level may result in renal disease progression and increased incidence of mortality in AKI (SI Appendix, Fig. S10). Therefore, we suggest the ERR-γ inverse agonist as small molecule–based therapy to reduce adverse plasma FGF23 levels in AKI.
Materials and Methods
DNA Cloning and Recombinant Adenovirus Construction.
Mouse FGF23 gene promoter (−1 kb) was PCR amplified from mouse genomic DNA (Promega) using the following primer: forward, 5′-CGCGGGGCTAGCCAGCAGTCTGCCTTCCAATG-3′ and reverse, 5′-CGCGGGCTCGAGGAGTGGCTAATGCTGAGTTTG-3′ inserted into the pGL3 basic vector (Promega) using the NheI and XhoI restriction enzyme sites. QuikChange Lightning Site-Directed mutagenesis kit (Catalog No. 210518, Agilent Technologies) was used to generate ERRE mutant constructs. Ad-GFP and Ad-FLAG-ERR-γ as well as the vector expressing ERR-γ were described previously (26, 34). The adenovirus-expressing mouse FGF23 promoter (Ad-FGF23-luc) was generated using pAdTrack-CMV and pAdEasy-1 system described by He et al. (46). Adenovirus-expressing mouse ERR-β (Ad-ERR-β) was purchased from Vector Biolabs.
Animal Experiments.
All animal experiments were performed with 8 to 12 wk old male C57BL/6J mice. WT and floxed ERR-γ exon 2 (ERR-γf/f)-containing mice were attained from the Korea Research Institute of Biosciences and Biotechnology (KRIBB) and PHENOMIN-iCS, PHENOMIN, the French National Infrastructure in Biology and Health, respectively. Liver-specific ERR-γ knockout mice (ERR-γ-LKO) were produced as previously described (22). WT mice were injected with Ad-GFP or Ad-FLAG-ERR-γ via tail vein for 5 d, and WT or ERR-γ-LKO mice were intraperitoneally administered with GSK5182 (40 mg/kg). All animal procedures were approved by the Animal Care and Use Committee of Chonnam National University (CNU-IACUC-YB-2017-40) and by the KRIBB Institutional Animal Care and Use Committee (KRIBB-AEC-19128). All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the NIH (47).
Induction of AKI.
AKI was induced in WT and ERR-γ-LKO mice by single intraperitoneal injection of FA (240 mg/kg dissolved in vehicle: 0.15 M NaHCO3 with pH 7.4). Control animals were injected with vehicle alone. Animals were euthanized at the indicated time period after FA treatment, and organs are collected within minutes after sacrifice. Where indicated, GSK5182 (40 mg/kg in 30% PEG400) were injected intraperitoneally 24 h before and at that time of FA injection. IL-6 neutralizing antibody or control antibody (rat IgG1) was injected (200 µg/200 μL/mice) intraperitoneally 30 min before and 12 h after FA treatment, then animals were euthanized after 24 h of FA treatment.
In Vivo Imaging.
C57BL/6J mice were infected with adenovirus fused with mouse FGF23 promoter WT-luciferase (Ad-FGF23WT-luc) or mouse FGF23 promoter ERRE mut-luciferase (Ad-FGF23-ERREmut-luc) via tail-vein injections. Mice were treated 3 d postinjection with either FA (240 mg/kg) or vehicle for 24 h in the presence or absence of GSK5182 (40 mg/kg). Mice were imaged using an IVIS Lumina II imaging system (Caliper Life Sciences) as described previously (48).
RNA Extraction, cDNA Synthesis, and qPCR.
Organs were resected and immediately frozen using liquid nitrogen and stored at -80 °C. Frozen tissues were homogenized using IKA T10 basic Ultra-Turrax homogenizer or liquid nitrogen cooled mortar and pestle. Total RNA was isolated using TriZol reagent and QIAGEN-RNeasy mini kit according to the manufacturer’s instructions. Purity and concentration of extracted total RNA was analyzed using Quick Drop spectrophotometer (Molecular Devices). One microgram of extracted total RNA was used as a template to synthesize cDNA using TOPscript RT DryMix (dT18 plus, enzynomics). Reverse transcription was performed with temperature set as 37 °C for 5 min, 45 °C for 60 min, and 95 °C for 5 min on a thermocycler (TaKaRa). qPCR was performed with StepOnePlus real-time PCR system (Applied Biosystems) using the following mouse primer sequences: FGF23 forward, 5′-ATGCTAGGGACCTGCCTTAGA-3′ and reverse, 5′-AGCCAA-GCAATGGGGAAGTG-3′; FGF21 forward, 5′- CTGCTGGGGGTCTACCAAG-3′ and reverse, 5′-CTGCGCCTACCACTGTTCC-3′; ERR-α forward, 5′-CTCAGCTCTCTACCC-AAACGC-3′ and reverse, 5′-CCGCTTGGTGATCTCACACTC-3′; ERR-β forward, 5′-GCACCTGGGCTCTAGTTGC-3′ and reverse, 5′-TACAGTCCTCGTAGCTCTTGC; ERR-γ forward, 5′-AAGATCGACACATTGATTCCAGC-3′ and reverse, 5′-CATGGT-TGAACTGTAACTCCCAC-3′; and L32 forward, 5′-GTGAAGCCCAAGATCGTCAA-3′ and reverse, 5′-TGTCAATGCCTCTGGGTTTC-3′. qPCR reactions were performed with TOPreal qPCR 2× PreMix (enzynomics), and all data were normalized to ribosomal protein L32 expression.
ChIP Assay.
SimpleChIP Plus Enzymatic Chromatin IP kit (Catalog No. 25268S) was used for ChIP analyses as previously described (23). Real-time qPCR analysis of ChIP eluted and input DNA was performed using the following primers. Mouse FGF23 ERRE site, forward 5′-AAACAAGGACACTGGAGGGAGATG-3′ and reverse, 5′-CCTCAATTTCAAGCCAGTGCTCC-3′; Mouse FGF23 nonbinding site, forward 5′-GGATGCTTCCTGCCCTCTG-3′ and reverse, 5′-GATCTGGTTTCTCAG-TTTCCCTCG-3′; Mouse Hepcidin ERRE site, forward 5′-GAGCCACAGTGTGAC-ATCAC-3′ and reverse, 5′-GTCTAGGAGCCAGTCCCAGT-3′; and primer specific to intron 2 of the mouse RPL30 gene was provided in the kit. Primary antibodies used include mouse monoclonal anti–ERR-γ (Perseus Proteomics, Clone No. H6812, Catalog No. PP-H6812-00) and Histone H3 (D2B12) XP rabbit monoclonal antibody (ChIP formulated and provided in the kit).
Western Blot Analysis.
Total protein from mouse livers were prepared using radioimmunoprecipitation assay (RIPA) buffer (Elpis-Biotech) as previously described (49). Proteins from liver lysates were separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. The membranes were probed with specified antibody. Immunoreactive proteins were visualized using an Amersham ECL kit (GE Healthcare) or using iBright CL1000 imaging system (Invitrogen) according to the manufacturer’s instructions.
Biochemical Measurements.
Enzyme-linked immunosorbent assay (ELISA) kits from Immutopics (Carlsbad) were used to measure circulating levels of PTH (Catalog No. 60-2305), intact FGF23 (Catalog No. 60-6800), and C-terminal FGF23 (Catalog No. 60-6300). Plasma 1,25(OH)2D level was measured by immunoassay kit from immunodiagnostic systems (Boldon). Plasma phosphate level was measured by QuantiChrom phosphate assay kit (DIPI-500, BioAssay Systems). AbFrontier Cymax mouse IL-6 ELISA kit (Catalog No. LF-EK0270) was used to measure mouse plasma IL-6 levels. Plasma BUN and creatinine levels were determined using an automated blood chemistry analyzer (AU480; Beckman Coulter).
Immunohistochemistry.
Immunohistochemistry of 10% neutral buffered formalin-fixed liver sections with anti–ERR-γ and anti-FGF23 antibodies were performed as described previously (22).
Histopathology.
Kidney samples were fixed in 10% neutral buffered formalin, embedded in paraffin, and cut into 5 μm thick sections. Kidney sections were stained with hematoxylin and eosin stain or picrosirius red.
Statistical Analysis.
Prism 8 (GraphPad Software) software was used to analyze the data, and all data are expressed as mean ± SEM. For statistical analysis between two groups, we used two-tailed Student’s t test and one-way ANOVA with Tukey’s multiple comparisons test used to analyze statistical significance between multiple treatment groups. P value of < 0.05 is considered statistically significant.
Supplementary Material
Acknowledgments
We thank Professors David Moore and Seok-Yong Choi for critical reading of the manuscript. We also acknowledge support from Frank Lammert, Department of Medicine II, Saarland University Medical Center, Saarland University, Homburg, Germany, for connecting us with Saarland University, Department of Internal Medicine IV, Nephrology and Hypertension, and therewith to get access to the clinical cohort. H.-S.C. is supported by National Research Foundation (NRF) of Korea (NRF-2021R1A2C3004923) and National Creative Research Initiatives Grant (20110018305) funded by the Korean government, Ministry of Science and ICT (MIST). C.-H.L. is supported by NRF grant funded by the Korean government (MSIT) (NRF-2020R1A2C3006952), and the KRIBB Research Initiative Program of the Republic of Korea. Y.-H.K. is supported by the NRF grant funded by the Korean government (MSIT) (NRF-2019R1C1C1005319). H.E.C. was supported by the NRF grant funded by the Korea government, MSIT (No. 2018R1A5A2024181). T.S. and D.F. are supported by the Deutsche Forschungsgemeinschaft (DFG, SFB-TRR 219). S.D. is supported by the Federal Ministry of Education and Research-Liver Systems Medicine Program, Grant PTJ-031L0043.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2022841118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
Change History
July 12, 2021: The Acknowledgments have been updated.
References
- 1.Mirams M., Robinson B. G., Mason R. S., Nelson A. E., Bone as a source of FGF23: Regulation by phosphate? Bone 35, 1192–1199 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Shimada T., et al., Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Invest. 113, 561–568 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimada T., et al., FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 19, 429–435 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Francis C., David V., Inflammation regulates fibroblast growth factor 23 production. Curr. Opin. Nephrol. Hypertens. 25, 325–332 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tagliabracci V. S., et al., Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc. Natl. Acad. Sci. U.S.A. 111, 5520–5525 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu X., et al., Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology 146, 4647–4656 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurosu H., et al., Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 281, 6120–6123 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gattineni J., et al., FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am. J. Physiol. Renal Physiol. 297, F282–F291 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben-Dov I. Z., et al., The parathyroid is a target organ for FGF23 in rats. J. Clin. Invest. 117, 4003–4008 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergwitz C., Jüppner H., Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu. Rev. Med. 61, 91–104 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavi-Moshayoff V., Wasserman G., Meir T., Silver J., Naveh-Many T., PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: A bone parathyroid feedback loop. Am. J. Physiol. Renal Physiol. 299, F882–F889 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Saito H., et al., Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J. Biol. Chem. 280, 2543–2549 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Meir T., et al., Parathyroid hormone activates the orphan nuclear receptor Nurr1 to induce FGF23 transcription. Kidney Int. 86, 1106–1115 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Bär L., Stournaras C., Lang F., Föller M., Regulation of fibroblast growth factor 23 (FGF23) in health and disease. FEBS Lett. 593, 1879–1900 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Christov M., Neyra J. A., Gupta S., Leaf D. E., Fibroblast growth factor 23 and klotho in AKI. Semin. Nephrol. 39, 57–75 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Christov M., et al., Plasma FGF23 levels increase rapidly after acute kidney injury. Kidney Int. 84, 776–785 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egli-Spichtig D., Zhang M. Y. H., Perwad F., Fibroblast growth factor 23 expression is increased in multiple organs in mice with folic acid-induced acute kidney injury. Front. Physiol. 9, 1494 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onal M., et al., A novel distal enhancer mediates inflammation-, PTH-, and early onset murine kidney disease-induced expression of the mouse Fgf23 gene. JBMR Plus 2, 32–47 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giguère V., Yang N., Segui P., Evans R. M., Identification of a new class of steroid hormone receptors. Nature 331, 91–94 (1988). [DOI] [PubMed] [Google Scholar]
- 20.Tremblay A. M., Giguère V., The NR3B subgroup: An ovERRview. Nucl. Recept. Signal. 5, e009 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misra J., Kim D. K., Choi H. S., ERRγ: A junior orphan with a senior role in metabolism. Trends Endocrinol. Metab. 28, 261–272 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Radhakrishnan K., Kim Y. H., Jung Y. S., Kim J., Kim D. K., Orphan nuclear receptor ERRγ is a novel transcriptional regulator of IL-6 mediated hepatic BMP6 gene expression in mice. Int. J. Mol. Sci. 21, 7148 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim D. K., et al., Orphan nuclear receptor estrogen-related receptor γ (ERRγ) is key regulator of hepatic gluconeogenesis. J. Biol. Chem. 287, 21628–21639 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim D. K., et al., PKB/Akt phosphorylation of ERRγ contributes to insulin-mediated inhibition of hepatic gluconeogenesis. Diabetologia 57, 2576–2585 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Kim D. K., et al., Estrogen-related receptor γ (ERRγ) is a novel transcriptional regulator of phosphatidic acid phosphatase, LIPIN1, and inhibits hepatic insulin signaling. J. Biol. Chem. 286, 38035–38042 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim D. K., et al., Inverse agonist of estrogen-related receptor γ controls Salmonella typhimurium infection by modulating host iron homeostasis. Nat. Med. 20, 419–424 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Simmons E. M.et al.; PICARD Study Group , Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int. 65, 1357–1365 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Alaynick W. A., et al., ERRgamma regulates cardiac, gastric, and renal potassium homeostasis. Mol. Endocrinol. 24, 299–309 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durlacher-Betzer K., et al., Interleukin-6 contributes to the increase in fibroblast growth factor 23 expression in acute and chronic kidney disease. Kidney Int. 94, 315–325 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Ologunde R., Zhao H., Lu K., Ma D., Organ cross talk and remote organ damage following acute kidney injury. Int. Urol. Nephrol. 46, 2337–2345 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Jung Y. S., et al., The orphan nuclear receptor ERRγ regulates hepatic CB1 receptor-mediated fibroblast growth factor 21 gene expression. PLoS One 11, e0159425 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schunk S. J., et al., Association between urinary dickkopf-3, acute kidney injury, and subsequent loss of kidney function in patients undergoing cardiac surgery: An observational cohort study. Lancet 394, 488–496 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Chao E. Y., et al., Structure-guided synthesis of tamoxifen analogs with improved selectivity for the orphan ERRgamma. Bioorg. Med. Chem. Lett. 16, 821–824 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Kim D. K., et al., Estrogen-related receptor γ controls hepatic CB1 receptor-mediated CYP2E1 expression and oxidative liver injury by alcohol. Gut 62, 1044–1054 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim D. K., et al., Inverse agonist of nuclear receptor ERRγ mediates antidiabetic effect through inhibition of hepatic gluconeogenesis. Diabetes 62, 3093–3102 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pavik I., et al., Patients with autosomal dominant polycystic kidney disease have elevated fibroblast growth factor 23 levels and a renal leak of phosphate. Kidney Int. 79, 234–240 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Bienaimé F., et al., Hepatic production of fibroblast growth factor 23 in autosomal dominant polycystic kidney disease. J. Clin. Endocrinol. Metab. 103, 2319–2328 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Wasserman H., et al., Two case reports of FGF23-induced hypophosphatemia in childhood biliary atresia. Pediatrics 138, e20154453 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Daryadel A., et al., Systemic Jak1 activation provokes hepatic inflammation and imbalanced FGF23 production and cleavage. FASEB J. 35, e21302 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Prié D., et al., Plasma fibroblast growth factor 23 concentration is increased and predicts mortality in patients on the liver-transplant waiting list. PLoS One 8, e66182 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh S., et al., Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. 90, 985–996 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bar L., et al., Insulin suppresses the production of fibroblast growth factor 23 (FGF23). Proc. Natl. Acad. Sci. U.S.A. 115, 5804–5809 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.David V., et al., Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 89, 135–146 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaneko I., et al., FGF23 gene regulation by 1,25-dihydroxyvitamin D: Opposing effects in adipocytes and osteocytes. J. Endocrinol. 226, 155–166 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang B., et al., NFκB-sensitive orai1 expression in the regulation of FGF23 release. J. Mol. Med. (Berl.) 94, 557–566 (2016). [DOI] [PubMed] [Google Scholar]
- 46.He T. C., et al., A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. U.S.A. 95, 2509–2514 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Research Council , Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, ed. 8, 2011). [Google Scholar]
- 48.Lee M. W., et al., Regulation of hepatic gluconeogenesis by an ER-bound transcription factor, CREBH. Cell Metab. 11, 331–339 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Jung Y. S., et al., An inverse agonist of estrogen-related receptor γ regulates 2-arachidonoylglycerol synthesis by modulating diacylglycerol lipase expression in alcohol-intoxicated mice. Arch. Toxicol. 94, 427–438 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.