Abstract
Gait provides a sensitive measurement for signs of aging and neurodegenerative conditions. Measurement of gait is transitioning from the laboratory environment to the clinic with the use of inertial measurement units, providing a simple and cost-effective assessment tool. However, such assessments first needs validation against reference systems. The aim of this study was to validate the APDM Mobility Lab (ML) system (version 2) against a pressure sensor walkway in younger adults (n=18), older adults (n=18) and people with mild Parkinson’s disease (n=21) in the laboratory. Participants completed a two-minute walk over a pressure sensor walkway whilst wearing three sensors (strapped to the lumbar spine and both feet). Comparison of output from the systems was then performed. Overall, we identified that ML provided good to excellent agreement (ICC >0.75) for gait velocity, stride length, stride length SD, cadence, stride time and stride time SD. Measures of double support time, single support time and swing time had moderate to poor agreement (ICC 0.213–0.725), particularly for younger adults and PD. Overall, Mobility Lab provides a valid system for gait data collection for clinical and research application.
Keywords: Gait, validation, Parkinson’s disease, inertial measurement unit, instrumented walkway
Introduction
Poor walking ability is considered a clinical marker of increased risk of falls, decline in cognitive ability and disease progression for neurodegenerative conditions such as Parkinson’s disease (PD) (Verghese et al., 2007, Morris et al., 2017, Mc Ardle et al., 2018, Pang et al., 2018, Chen et al., 2005). Traditionally, assessments of comprehensive measures of gait have been conducted in laboratory environments that require expensive equipment (e.g., 3D motion capture systems, force-plates etc.), trained staff, and large allocated space (Del Din et al., 2016a). In addition, participant performance, particularly in the case of PD, is often optimized in these laboratory settings and can affect one-off performance (Robles-García et al., 2015). To overcome these challenges, and with the latest miniaturization and accessibility of technology, objective gait assessment is transitioning into clinics as well as patient homes and community settings. These ecological settings provide more natural gait assessment over longer durations, providing a more complete overview of daily-life walking and enabling other relevant clinical factors to be explored (e.g. medication related fluctuations in mobility).
Inertial measurement units (IMUs), incorporating tri-axial accelerometers, gyroscopes and magnetometers have been successfully used to measure mobility, and provide a cost-effective and lightweight solution for comprehensive gait assessment in laboratory, clinical, and community environments. Mobility Lab™ (ML, APDM, Inc., Portland, OR, USA), is a commercially available system that allows for six IMU’s to be wirelessly synchronized (Opal V1, APDM Inc., Portland, OR, USA) and software that allows for easy data collection and automatic analysis, without gait research knowledge or expert data processing (Mancini et al., 2011, Mancini and Horak, 2016). The ML commercial software package allows the user a choice of instrumented tests of balance and gait and automatically generates a report for each participant. This simplicity and automatic output allows for simple data collection for clinical settings and/or large clinical trials. However, evaluations of the validity, reliability and sensitivity of gait measures through commercial systems are often sparse. ML (version 1) has previously been validated against a pressure sensor walkway in both healthy controls and clinical populations (Schmitz-Hübsch et al., 2016). Progression in commercial system development (ML version 2) has led to novel algorithm development applying sensors to the feet rather than shanks, thus allowing for more accurate and additional gait measures. However, system developments require further validation and assessment to other specific populations of interest. Such systems need validation against robust previously validated reference systems, such as instrumented walkways (Webster et al., 2005, McDonough et al., 2001) in controlled environments (i.e. laboratory) before being validated and implemented in clinical settings or randomized controlled trials (Din et al., 2016, Washabaugh et al., 2017, Schmitz-Hübsch et al., 2016).
Therefore, this study aimed to validate comprehensive gait output in the laboratory from ML system (version 2), compared to a validated reference system for gait assessment, specifically the GAITRite™ (GR) instrumented walkway in healthy young adults (YA) and older adults (OA), as well as people with PD.
Methods
Study design
This study was a cross-sectional design for which participants consented to a one-time visit to complete a two-minute gait assessment using GR and ML simultaneously, synchronized using external synchronization.
Participants
To cover a spectrum of normal and pathological gait patterns, we recorded comprehensive gait measurements from YA (aged 20–40 years), OA (aged over 50 years) and people with a diagnosis of idiopathic PD. YA and OA were recruited if they were able to; i) walk independently without an ambulatory aid and ii) had no diagnosis of a neurological disorder and iii) without a musculoskeletal, peripheral or central nervous system disorder that would significantly affect balance or gait. Idiopathic PD participants were recruited if they were; i) aged over 50 years old, ii) diagnosed with idiopathic PD by a Movement Disorder Specialist, iii) without a musculoskeletal, peripheral or central nervous system disorder (other than PD) that would significantly affect balance or gait, and iv) cognitive impairment causing an inability to consent and participate to testing procedures. All participants with PD were tested in their practical ‘Off’ medication state, defined as withholding their levodopa medication for at least 12 hours (Langston et al., 1992). All participants gave their informed consent. Recruitment procedures and experimental protocols were approved by the Oregon Health & Science University (OHSU) institutional review board.
Demographic and Clinical Assessments
Age, height and body mass were collected for all participants. For those with idiopathic PD, disease duration was recorded, as well as motor severity using the Movement Disorders Society Unified Parkinson’s disease Rating Scale part III (MDS-UPDRS III) and Hoehn and Yahr (H & Y).
Gait Assessment
Participants were asked to walk barefoot for two minutes at their preferred walking speed back and forth over an instrumented walkway (GR; 6m × 0.6m, v3.8, sampling frequency of 60Hz) completing 180° degree turns at either end (stepping off the mat to turn). Participants walked 0.5m (approximately one step) at each end of the walkway before turning. Simultaneously, participants wore three IMUs (Opals V1; APDM Inc, sampling frequency 128Hz) attached with straps bilaterally on both feet as well as the fifth lumbar vertebrae. The IMU’s combined accelerometer, gyroscope and magnetometer technology within each sensor, and were wirelessly synchronized with a separate laptop through the ML (version 2) software, which was used to initialize and collect the data (alternatively, data can be collected offline and later processed if necessary). Turning steps are not included within the gait measurement of the ML system, therefore only straight ahead gait between the two systems was used for comparison.
Data Processing
For the GR, there was visual inspection of raw footfall data and manual removal of partial footfalls, once processing was completed, data was exported in a CSV-file. In addition, ML provided gait outcomes that could be exported into an additional CSV-file. For both GR and ML, a range of spatio-temporal gait characteristics were calculated that i) assessed a comprehensive range of characteristics measuring different aspects of gait, and ii) were available as automatic output from both systems. Gait measures were derived from GR and ML and processed using manufacturer provided software, and finally averaged values were exported for further analysis. The following characteristics were exported: measures of spatial; mean gait velocity (m/s), mean stride length (m), measures of temporal; cadence (steps per minute), mean stride time (s), mean swing time (% gait cycle time [GCT]), single support time (% GCT) and double support time (%GCT) and measures of variability; stride length SD (m) and stride time SD (s).
Statistical Analysis
All data analysis was completed using SPSS® (version 24, IBM). Initially, data was inspected using boxplots and histograms to check for normality of data, meeting criteria for parametric analysis. Demographic characteristics and descriptive characteristics for all gait characteristics were calculated as means and standard deviations (SD).
Absolute agreement between the two systems was assessed by: intra-class correlation coefficients (ICC2,1), Pearson correlations were used to assess the strength of the relationship between the two measures and Bland-Altman plots were used to visualize bias with limits of agreement (LoA) expressed as a percentage and 95% prediction value (Martin Bland and Altman, 1986). The ICC findings were interpreted as follows; excellent (>0.90), good (0.75–0.89), moderate (0.50–0.74) and poor (<0.50) (Field, 2013). For LoA, <5% are considered very good, between 5 and 10% good, between 10% and 20% moderate, between 20 and 50% poor, and >50% very poor (Godfrey et al., 2015). Statistical significance was set at p<0.05.
Results
Participant Characteristics
In total, 21 participants with idiopathic PD, 18 OA and 18 YA were recruited to the study. Demographic and clinical characteristics are shown for all participants in Table 1. The OA and PD cohort were similar in age. Those with PD had mild to moderate disease with an average (SD) disease duration of 4.7 (3.2) years, an average (SD) MDS-UPDRS III of 36.8 (10.6) with the majority of participants categorized as HY stage II (Table 1).
Table 1.
Demographic and clinical details of all participants. Mean (SD).
| Young Adults (n=18) | Older Adults (n=18) | Parkinson’s Disease (n=21) |
||||
|---|---|---|---|---|---|---|
| Age (years) | 27.0 (4.4) | 63.4 (9.5) | 67.5 (8.8) | |||
| Gender (M/F) | 8/10 | 8/10 | 12/9 | |||
| Height (m) | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) | |||
| Mass (kg) | 68.0 (12.1) | 78.1 (12.9) | 79.9 (15.5) | |||
| Disease Duration (years) | N/A | N/A | 4.7 (3.2) | |||
| MDS-UPDRS III | N/A | N/A | 36.8 (10.6) | |||
| H & Y | N/A | N/A | H&Y I: 1 (4.8%) H&Y II: 17 (81.0%) H&Y III: 2 (9.5%) H&Y IV: 1 (4.8%) |
[MDS-UPDRS= Movement Disorders Society- Unified Parkinson’s disease Rating Scale, H & Y= Hoehn and Yahr]
Gait Validation
Table 2 contains descriptive gait data from GR and the ML, as well as agreement between the two systems. The agreement between GR and ML is additionally visualized using Bland-Altman plots in Figures 1 and 2.
Table 2.
ICC2,1, mean difference (p), Pearson correlations (r), and limits of agreement (LoA%) between GAITRite™ and Mobility Lab™ for gait characteristics in younger adults, older adults and people with Parkinson’s disease
| Gait Characteristics | Group | GAITRite | Mobility Lab | Agreement | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | ICC | Mean difference ± LoA95% | p | r | LOA (%) | ||||
| Spatial | Gait Velocity (m/sec) | YA | 1.34 (0.16) | 1.22 (0.14) | .861 | −0.111 ± 0.073 | <.01 | .978 | 5.7 | |
| OA | 1.24 (0.25) | 1.13 (0.25) | .934 | −0.111 ± 0.141 | <.01 | .960 | 11.9 | |||
| PD | 1.11 (0.17) | 1.03 (0.14) | .920 | −0.080 ± 0.068 | <.01 | .992 | 6.3 | |||
| Overall | 1.23 (0.21) | 1.12 (0.20) | .928 | −0.100 ± 0.101 | <.01 | .972 | 8.6 | |||
| Stride Length (m) | YA | 1.37 (0.11) | 1.25 (0.10) | .741 | −0.118 ± 0.054 | <.01 | .973 | 4.2 | ||
| OA | 1.31 (0.23) | 1.20 (0.20) | .939 | −0.103 ± 0.074 | <.01 | .990 | 5.9 | |||
| PD | 1.17 (0.14) | 1.08 (0.12) | .880 | −0.085 ± 0.059 | <.01 | .990 | 5.2 | |||
| Overall | 1.29 (0.18) | 1.17 (0.16) | .908 | −0.101 ± 0.067 | <.01 | .989 | 5.5 | |||
| Temporal | Cadence (steps per min) | YA | 117.56 (8.89) | 117.54(8.86) | .998 | −0.033 ± 1.545 | .860 | .996 | 1.3 | |
| OA | 112.20 (12.57) | 111.50 (13.06) | .996 | −0.527 ± 3.163 | .184 | .994 | 2.8 | |||
| PD | 114.15 (8.82) | 113.64 (8.81) | .996 | −0.505 ± 2.141 | .046 | .992 | 1.9 | |||
| Overall | 114.89 (10.01) | 114.15(10.52) | .996 | −0.363 ± 2.365 | .027 | .994 | 2.1 | |||
| Stride Time (sec) | YA | 1.02 (0.08) | 1.03 (0.08) | .998 | 0.006 ± 0.015 | .004 | .997 | 1.5 | ||
| OA | 1.08 (0.12) | 1.09 (0.13) | .998 | 0.009 ± 0.021 | .003 | .999 | 1.9 | |||
| PD | 1.05 (0.08) | 1.06 (0.08) | .992 | 0.012 ± 0.027 | .001 | .990 | 2.5 | |||
| Overall | 1.05 (0.10) | 1.07 (0.10) | .996 | 0.009 ± 0.022 | <.01 | .996 | 2.1 | |||
| Swing Time (%GCT) | YA | 37.92 (1.26) | 41.17 (0.94) | .220 | −0.092 ± 2.452 | .760 | .680 | 6.0 | ||
| OA | 36.16(2.60) | 39.28 (2.60) | .712 | 3.114 ± 1.830 | <.01 | .938 | 4.9 | |||
| PD | 35.43 (1.39) | 39.54 (1.84) | .281 | 4.107 ± 2.564 | <.01 | .706 | 6.8 | |||
| Overall | 36.54 (1.98) | 39.96 (2.07) | .517 | 2.467 ± 4.203 | <.01 | .845 | 10.9 | |||
| Single Support (%GCT) | YA | 37.83 (1.26) | 41.13 (0.90) | .215 | 3.199 ± 1.837 | <.01 | .669 | 4.6 | ||
| OA | 36.15 (2.60) | 39.17 (2.63) | .725 | 3.002 ± 1.931 | <.01 | .932 | 5.1 | |||
| PD | 35.43 (1.38) | 39.44 (1.88) | .290 | 4.013 ± 2.626 | <.01 | .702 | 7.0 | |||
| Overall | 36.54 (1.97) | 39.88 (2.09) | .528 | 3.437 ± 2.324 | <.01 | .840 | 6.1 | |||
| Double Support (%GCT) | YA | 24.30 (2.47) | 17.70 (1.84) | .213 | −6.594 ± 3.495 | <.01 | .693 | 16.6 | ||
| OA | 27.85 (5.30) | 21.55 (5.22) | .716 | −6.272 ± 3.612 | <.01 | .940 | 14.6 | |||
| PD | 29.34 (2.81) | 21.02 (3.71) | .285 | −8.310 ± 5.031 | <.01 | .723 | 20.0 | |||
| Overall | 27.09 (3.99) | 20.17 (4.15) | .518 | −7.125 ± 4.469 | <.01 | .853 | 18.8 | |||
| Variability | Stride Length SD (m) | YA | 0.035 (0.011) | 0.037 (0.011) | .836 | 0.002 ± 0.016 | .246 | .723 | 44.2 | |
| OA | 0.036 (0.009) | 0.044 (0.013) | .658 | 0.008 ± 0.020 | .005 | .641 | 50.1 | |||
| PD | 0.042 (0.014) | 0.048 (0.013) | .905 | 0.005 ± 0.013 | .002 | .880 | 29.5 | |||
| Overall | 0.038 (0.012) | 0.043 (0.013) | .835 | 0.005 ± 0.017 | <.01 | .777 | 41.3 | |||
| Stride Time SD (sec) | YA | 0.019 (0.011) | 0.021 (0.004) | .701 | 0.002 ± 0.008 | .072 | .583 | 39.6 | ||
| OA | 0.024 (0.011) | 0.032 (0.020) | .804 | 0007 ± 0.023 | .019 | .868 | 82.7 | |||
| PD | 0.026 (0.010) | 0.035 (0.016) | .690 | 0.009 ± 0.022 | .001 | .714 | 72.2 | |||
| Overall | 0.023 (0.010) | 0.029 (0.016) | .773 | 0.006 ± 0.020 | <.01 | .800 | 74.8 | |||
Figure 1.
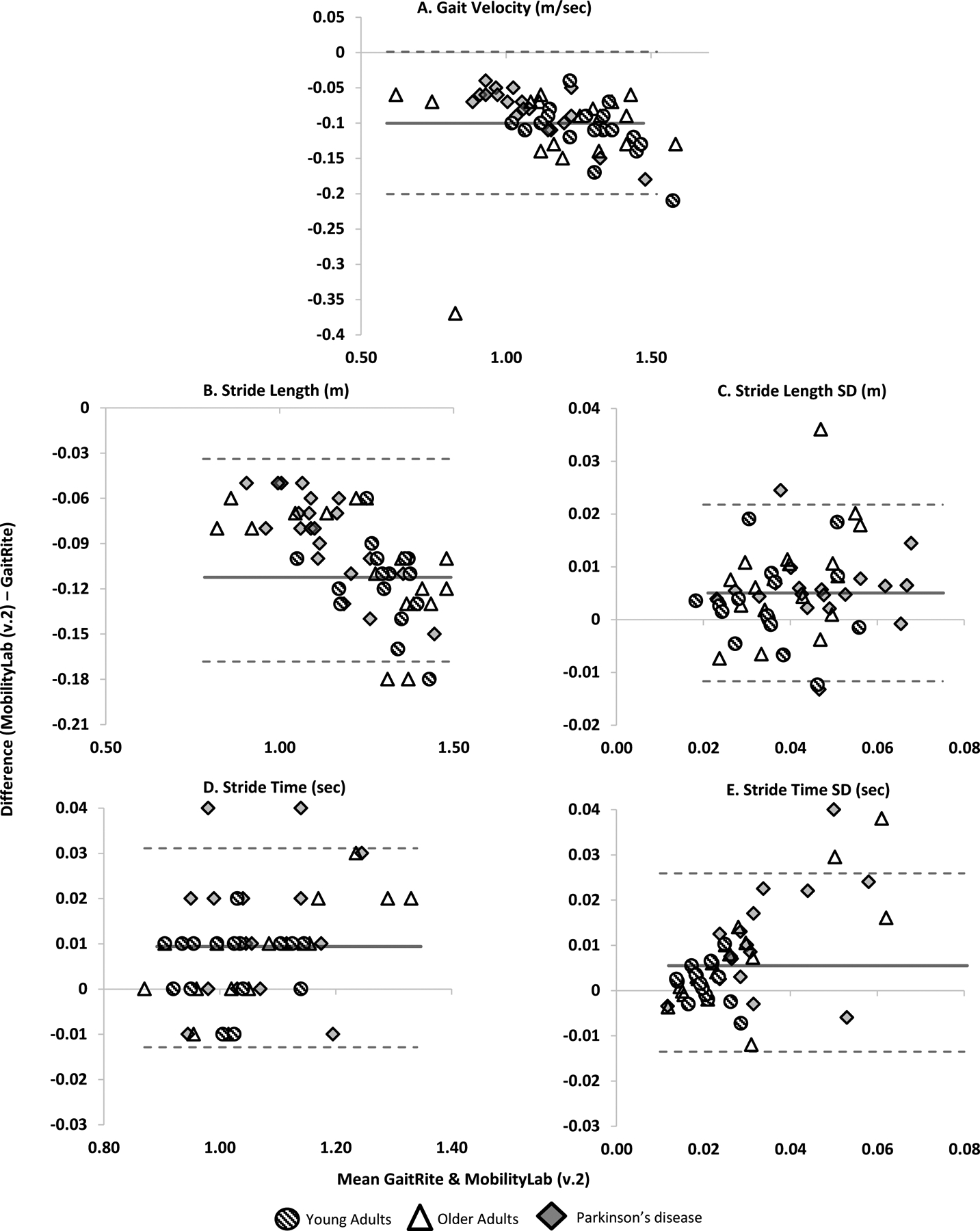
Bland Altman plots demonstrating agreement between GaitRite and MobilityLab version 2 for A) Gait velocity, B) Stride length, C) Stride length SD, D) Stride time and E) Stride time SD. Dashed lines represent LoA. Solid line represents mean difference.
Figure 2.
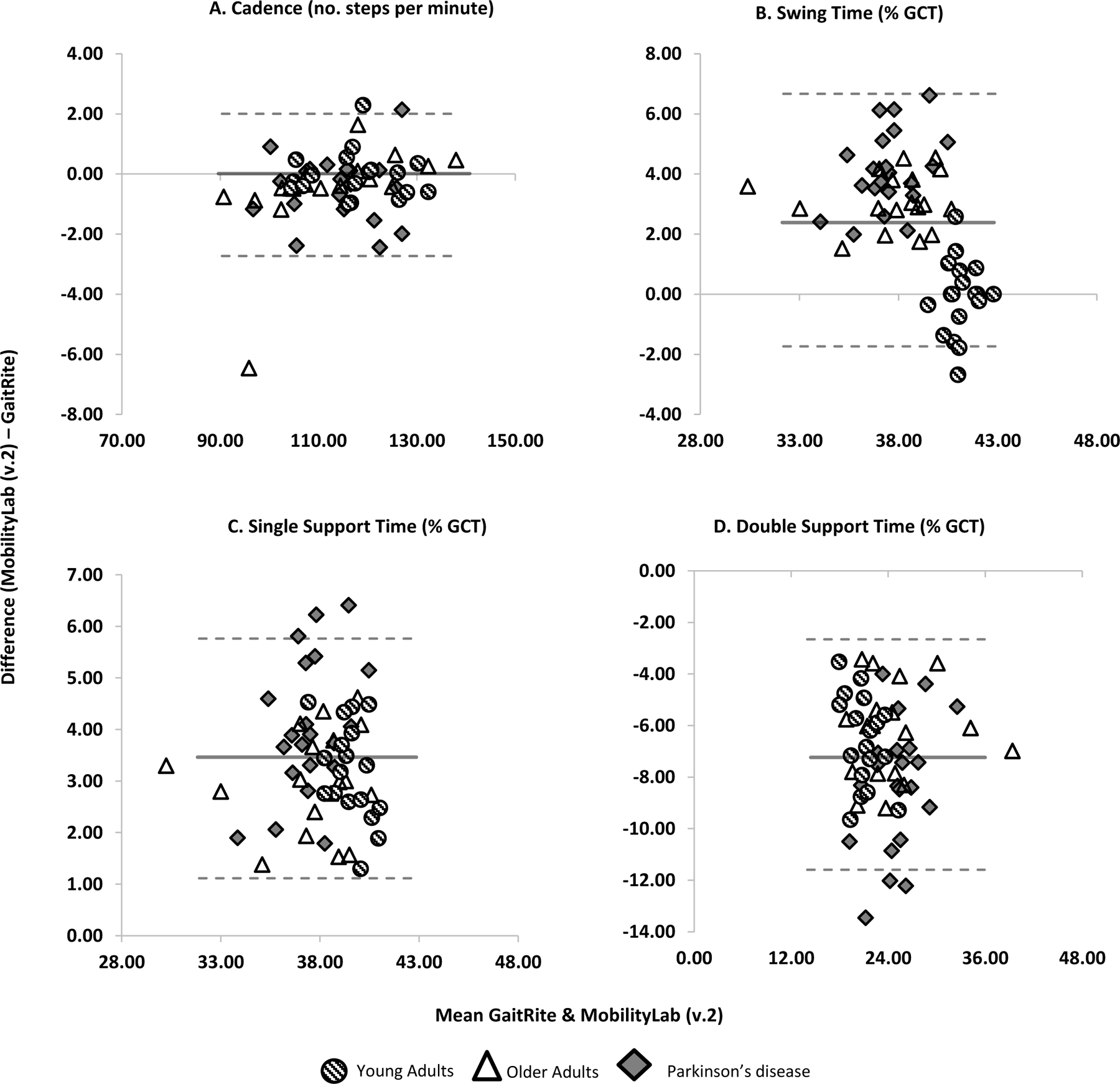
Bland Altman plots demonstrating agreement between GaitRite and MobilityLab version 2 for A) Cadence, B) Swing Time, C) Single support time and D) double support time. Dashed lines represent LoA. Solid line represents mean difference.
Spatial
Overall gait velocity and stride length showed excellent agreement (ICC2,1 .908 to .928, LoA%; 5.5 to 8.6%) between GR and ML. At the group level, agreement was slightly lower in the YA for gait velocity and stride length (ICC2,1 .741 to .861, LoA%; 4.2 to 5.7%) compared to OA (ICC2,1 .934 to .939, LoA%; 5.9 to 11.9%) and PD (ICC2,1 .880 to .920, LoA%; 5.2 to 6.3%).
Temporal
For cadence and stride time, agreement was excellent both overall and at group level (ICC2,1 .992 to .998, LoA%; 1.3 to 2.8%). Overall, the measures expressed in % of GCT, such as double support, single support and swing time, showed poor to moderate agreement (ICC2,1 .213 to.725, LoA% 4.6 to 20%). For measures of double support, single support and swing time, agreement was poor in YA and PD (ICC2,1 .213 to .290, LoA%; 4.6 to 20.0%), and moderate in OA (ICC2,1 .712 to .725, LoA%; 4.9 to 14.6%).
Variability
Measures of gait variability (stride length SD and stride time SD) showed good agreement overall (ICC2,1 .773 to .835, LoA% 41.3 to 74.8). At the group level, agreement was more varied, for stride length SD, YA demonstrated good agreement (ICC2,1 .836 LoA%; 44.2), OA demonstrated moderate agreement (ICC2,1 .658, LoA%; 50.1%), and PD excellent agreement (ICC2,1 .905, LoA%; 29.5%). For stride time SD, YA demonstrated moderate agreement ICC2,1 .701 LoA%; 39.6), OA demonstrated good agreement (ICC2,1 .804, LoA%; 82.7%), and PD moderate agreement (ICC2,1 .690, LoA%; 72.2%). For gait variability measures, LoA% were poorer than for measures of pace and rhythm.
Discussion
The aim of this study was to validate the ML (version 2) system against a pressure sensor walkway laboratory reference system in populations with different walking abilities in controlled laboratory conditions. We identified that ML provided good to excellent agreement for the majority of gait characteristics in three different groups: YA, OA and people with PD but there was poorer agreement for measures expressed as percentage of the gait cycle.
Excellent agreement was evident between GR and ML for all three groups overall for mean characteristics of gait velocity, cadence, stride time and stride length, which is similar to previous studies using diverse IMUs or a single accelerometer configuration (Greene et al., 2012, Din et al., 2016, Godfrey et al., 2015, Schmitz-Hübsch et al., 2016, Washabaugh et al., 2017). In contrast, other mean characteristics of swing time, double-support time and single-support time had poor agreement between GR and ML. When separating groups, agreement was good to excellent for characteristics of gait velocity, cadence, stride time and stride length. In comparison, for characteristics of mean swing time, double and single-support times the agreement was better in older adults compared to both YA and PD younger groups, which has been shown before in previous IMU validation studies (Din et al., 2016, Godfrey et al., 2015, Schmitz-Hübsch et al., 2016). Although speculative, these differences may be dependent on differences in gait speed and pathology between groups, but this needs further investigation.
Our findings for measures of gait variability are partly in contrast with previous studies showing poor agreement for measures of gait variability between GR and IMUs that were possibly attributed to inherent differences between the two systems (Din et al., 2016). The moderate to good agreement for variability metrics found in the current study may be due to a similarity among the algorithm (ML and GR), or sensor placement (i.e. on the foot in the current study vs ankle/lumbar spine in previous studies), as this has been found to affect agreement between IMUs and laboratory reference systems (Washabaugh et al., 2017). It has to be addressed that for measures of variability, the LoA were poorer. Overall there was a trend for ML to consistently overestimate compared to GR which increased as gait variability increased (Figure 1), as seen in previous studies validating IMU’s (Godfrey et al., 2015, Din et al., 2016). Future studies should compare gait measurement between various IMUs placed in different locations as well as different algorithms to derive gait measures, as previous studies have identified sensor placement impacts measures of gait variability and asymmetry (Del Din et al., 2016b).
For gait measures other than variability, ML tended to underestimate gait characteristics compared to GR in this study. Data trends were seen for a number of characteristics (gait velocity, stride length, stride time SD and swing time) in which the larger the measurement, the greater the difference between devices was demonstrated. Previous studies of gait measurement with IMUs and comparisons to GR have shown a mixture of under- and over-estimation by IMUs (Schmitz-Hübsch et al., 2016, Din et al., 2016). Therefore the intrinsic nature of our findings is unclear as it could be due to a range of technological or device application factors, e.g. device sampling frequency, placement, algorithm features, gait measurement protocols etc. Future studies are required to investigate these factors.
Limitations
This study had a number of limitations that need to be considered. First, both systems used in this study do not automatically provide the same gait characteristic output, and therefore characteristics from some gait domains (e.g. asymmetry and some variability measures) were not compared. Second, we compared the gait data automatically generated by ML and GR, rather than step-by-step comparison between the systems. Step-by-step comparison, although possible after extracting the stride-by-stride detailed output of ML, was not performed because the focus here was to take the direct output of the software, as would be commonly done for large trials. Future studies should perform step-by-step extrapolation from the detailed ML output if interested in step-by-step based parameters. Third, we only assessed the use of the ML system within YA, OA and PD, and findings may not be applicable to other populations. Finally, we only validated the IMU measurement of gait in controlled laboratory settings, gait assessment using ML (version 2) now needs to be validated in the home and community environment to provide habitual outcome measures.
Conclusions
This study identified that gait characteristics measured by ML (version 2) software is able to replicate measurements from an instrumented walkway in healthy young and older adults, as well as people with PD in the laboratory. Absolute agreement between the systems for gait characteristics ranged from excellent (gait velocity, stride length, cadence and stride time) to poor (double support time, single support time and swing time) and gait variability demonstrating poor limits of agreement. Validation of gait measurements using ML (version 2) is now required in the home and community environment to provide habitual outcome measures.
Acknowledgements
The authors would like to acknowledge Makena Strand (Research Assistant) for her help with data collection/processing and the research participants for their involvement in this study. This work has been supported by grants from the National Institute of Health via NIH 2R01AG006457, 1R44AG055388-01, 5R00HD078492-04 (PI: Mancini), VA Merit 5I01RX001075, and the Medical Research Foundation or Oregon (PI: Stuart).
References
- CHEN J, KWONG K, CHANG D, LUK J & BAJCSY R Wearable Sensors for Reliable Fall Detection. 2005. IEEE Engineering in Medicine and Biology 27th Annual Conference, 17–18 January. 2006 2005. 3551–3554. [DOI] [PubMed] [Google Scholar]
- DEL DIN S, GODFREY A, MAZZA C, LORD S & ROCHESTER L 2016a. Free-living monitoring of Parkinson’s disease: Lessons from the field. Mov Disord, 31, 1293–313. [DOI] [PubMed] [Google Scholar]
- DEL DIN S, HICKEY A, HURWITZ N, MATHERS JC, ROCHESTER L & GODFREY A 2016b. Measuring gait with an accelerometer-based wearable: influence of device location, testing protocol and age. Physiol Meas, 37, 1785–1797. [DOI] [PubMed] [Google Scholar]
- DIN SD, GODFREY A & ROCHESTER L 2016. Validation of an Accelerometer to Quantify a Comprehensive Battery of Gait Characteristics in Healthy Older Adults and Parkinson’s Disease: Toward Clinical and at Home Use. IEEE Journal of Biomedical and Health Informatics, 20, 838–847. [DOI] [PubMed] [Google Scholar]
- FIELD A 2013. Discovering Statistics using IBM SPSS statistics, Sage. [Google Scholar]
- GODFREY A, DEL DIN S, BARRY G, MATHERS J, ROCHESTER LJME & PHYSICS 2015. Instrumenting gait with an accelerometer: a system and algorithm examination. 37, 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENE BR, FORAN TG, MCGRATH D, DOHENY EP, BURNS A & CAULFIELD BJJOAB 2012. A comparison of algorithms for body-worn sensor-based spatiotemporal gait parameters to the GAITRite electronic walkway. 28, 349–355. [DOI] [PubMed] [Google Scholar]
- LANGSTON J, WIDNER H, GOETZ C, BROOKS DJ, FAHN S, FREEMAN T & WATTS R 1992. Core assessment program for Intracerebral Transplantations (CAPIT). Movement Disorders, 7, 2–13. [DOI] [PubMed] [Google Scholar]
- MANCINI M & HORAK FB 2016. Potential of APDM mobility lab for the monitoring of the progression of Parkinson’s disease. Expert Review of Medical Devices, 13, 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANCINI M, KING L, SALARIAN A, HOLMSTROM L, MCNAMES J & HORAK FB 2011. Mobility Lab to Assess Balance and Gait with Synchronized Body-worn Sensors. Journal of bioengineering & biomedical science, Suppl 1, 007–007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN BLAND J & ALTMAN D 1986. Statistical methods for assessing agreement between two methods of clinical measurement The Lancet, 327, 307–310. [PubMed] [Google Scholar]
- MC ARDLE R, MORRIS R, HICKEY A, DEL DIN S, KOYCHEV I, GUNN RN, LAWSON J, ZAMBONI G, RIDHA B, SAHAKIAN BJ, ROWE JB, THOMAS A, ZETTERBERG H, MACKAY C, LOVESTONE S, ROCHESTERON L, DEEP & FREQUENT PHENOTYPING STUDY, T. 2018. Gait in Mild Alzheimer’s Disease: Feasibility of Multi-Center Measurement in the Clinic and Home with Body-Worn Sensors: A Pilot Study. J Alzheimers Dis, 63, 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDONOUGH AL, BATAVIA M, CHEN FC, KWON S & ZIAI J 2001. The validity and reliability of the GAITRite system’s measurements: A preliminary evaluation. Arch Phys Med Rehabil, 82, 419–25. [DOI] [PubMed] [Google Scholar]
- MORRIS R, LORD S, LAWSON RA, COLEMAN S, GALNA B, DUNCAN GW, KHOO TK, YARNALL AJ, BURN DJ & ROCHESTER L 2017. Gait Rather Than Cognition Predicts Decline in Specific Cognitive Domains in Early Parkinson’s Disease. The Journals of Gerontology: Series A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANG I, OKUBO Y, STURNIEKS D, LORD SR & BRODIE MA 2018. Detection of Near Falls Using Wearable Devices: A Systematic Review. J Geriatr Phys Ther. [DOI] [PubMed] [Google Scholar]
- ROBLES-GARCÍA V, CORRAL-BERGANTIÑOS Y, ESPINOSA N, JÁCOME MA, GARCÍA-SANCHO C, CUDEIRO J & ARIAS P 2015. Spatiotemporal Gait Patterns During Overt and Covert Evaluation in Patients With Parkinsońs Disease and Healthy Subjects: Is There a Hawthorne Effect? Journal of applied biomechanics, 31, 189–194. [DOI] [PubMed] [Google Scholar]
- SCHMITZ-HÜBSCH T, BRANDT AU, PFUELLER C, ZANGE L, SEIDEL A, KÜHN AA, PAUL F, MINNEROP M & DOSS S 2016. Accuracy and repeatability of two methods of gait analysis – GaitRite™ und Mobility Lab™ – in subjects with cerebellar ataxia. Gait & Posture, 48, 194–201. [DOI] [PubMed] [Google Scholar]
- VERGHESE J, WANG C, LIPTON RB, HOLTZER R & XUE X 2007. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry, 78, 929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASHABAUGH EP, KALYANARAMAN T, ADAMCZYK PG, CLAFLIN ES & KRISHNAN C 2017. Validity and repeatability of inertial measurement units for measuring gait parameters. Gait & Posture, 55, 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBSTER KE, WITTWER JE & FELLER JA 2005. Validity of the GAITRite walkway system for the measurement of averaged and individual step parameters of gait. Gait Posture, 22, 317–21. [DOI] [PubMed] [Google Scholar]


