Significance
Opioids acting at the mu-opioid receptor (MOR) are the gold standard for pain relief. Unfortunately, these drugs have severe side effects, including addiction liability and respiratory depression. Consequently, safe pain management remains an unmet medical need. Here, we show that a positive allosteric modulator of MOR (a mu-PAM) does not directly activate MOR but produces analgesia in mouse models by enhancing the ability of endogenous opioid peptides, released in pain states, to activate MOR. Because the mu-PAM does not activate MOR directly, this raises the possibility of analgesia without the typical opioid side effects of addiction liability, respiratory depression, or constipation. The study provides proof of principle for mu-PAMs as an innovative and potentially safer approach to pain management.
Keywords: analgesia, endogenous opioid peptides, allostery, mu-opioid receptor, signaling bias
Abstract
Positive allosteric modulators (PAMs) of the mu-opioid receptor (MOR) have been hypothesized as potentially safer analgesics than traditional opioid drugs. This is based on the idea that PAMs will promote the action of endogenous opioid peptides while preserving their temporal and spatial release patterns and so have an improved therapeutic index. However, this hypothesis has never been tested. Here, we show that a mu-PAM, BMS-986122, enhances the ability of the endogenous opioid Methionine-enkephalin (Met-Enk) to stimulate G protein activity in mouse brain homogenates without activity on its own and to enhance G protein activation to a greater extent than β-arrestin recruitment in Chinese hamster ovary (CHO) cells expressing human mu-opioid receptors. Moreover, BMS-986122 increases the potency of Met-Enk to inhibit GABA release in the periaqueductal gray, an important site for antinociception. We describe in vivo experiments demonstrating that the mu-PAM produces antinociception in mouse models of acute noxious heat pain as well as inflammatory pain. These effects are blocked by MOR antagonists and are consistent with the hypothesis that in vivo mu-PAMs enhance the activity of endogenous opioid peptides. Because BMS-986122 does not bind to the orthosteric site and has no inherent agonist action at endogenously expressed levels of MOR, it produces a reduced level of morphine-like side effects of constipation, reward as measured by conditioned place preference, and respiratory depression. These data provide a rationale for the further exploration of the action and safety of mu-PAMs as an innovative approach to pain management.
Mu-opioid receptor (MOR) agonists are the most effective treatments for moderate to severe acute and chronic pain, yet their use is limited by serious side effects, including constipation, respiratory depression, and physical and psychological dependence. These side effects are on-target effects (MOR-mediated) and result from the wide distribution of MORs across the central nervous system (CNS) (1, 2). Safer pain therapies are desperately needed. However, because of the efficacy of MOR agonists in blocking pain, this receptor continues to be a primary target for the discovery of novel pain therapies. Unfortunately, most drug discovery programs involve designing compounds that bind to the orthosteric site on MOR—the site that binds endogenous opioid peptides as well as exogenous opioids. Not surprisingly, these newer drugs tend to exhibit qualitatively similar side effect profiles to traditional opioid analgesics.
As an alternative, we have discovered small molecule, positive allosteric modulators of MOR [mu-PAMs (3)], including BMS-986122 (SI Appendix, Fig. S1). Such compounds interact with a site on MOR that is spatially distinct from the orthosteric site (3–7). Across a variety of in vitro assays, mu-PAMs increase the affinity and/or potency of orthosteric agonists at MOR, including exogenous MOR agonists as well as the endogenous opioid peptides Leucine- and Methionine-enkephalin, endomorphin-1, and β-endorphin (3, 8).
These in vitro studies have led to development of a so-far untested hypothesis that in vivo, mu-PAMs will promote the activity of endogenous opioid peptides released during pain (9–11). If this hypothesis is correct, mu-PAMs could replace traditional opioids by boosting the body’s own natural response to pain to provide clinically meaningful analgesia. In support of this concept, so called “enkephalinase inhibitors” that prolong the lifetime of endogenous opioid peptides are effective in the management of pain in preclinical and clinical studies (12–14), although such compounds are not selective for opioid peptides. Since mu-PAMs do not alter peptide release or metabolism, they should be more selective than enkephalinase inhibitors and also preserve the natural spatial and temporal release of the peptides in vivo following injury and/or during pain. To test this hypothesis, we examined the antinociceptive effects of BMS-986122 in mouse models of acute and inflammatory pain using measures of pain-evoked and pain-depressed behaviors as well as opioid side effects and the potential role of endogenous opioid peptides in these responses.
Results
BMS-986122 Enhances the Activity of Orthosteric MOR Agonists In Vitro.
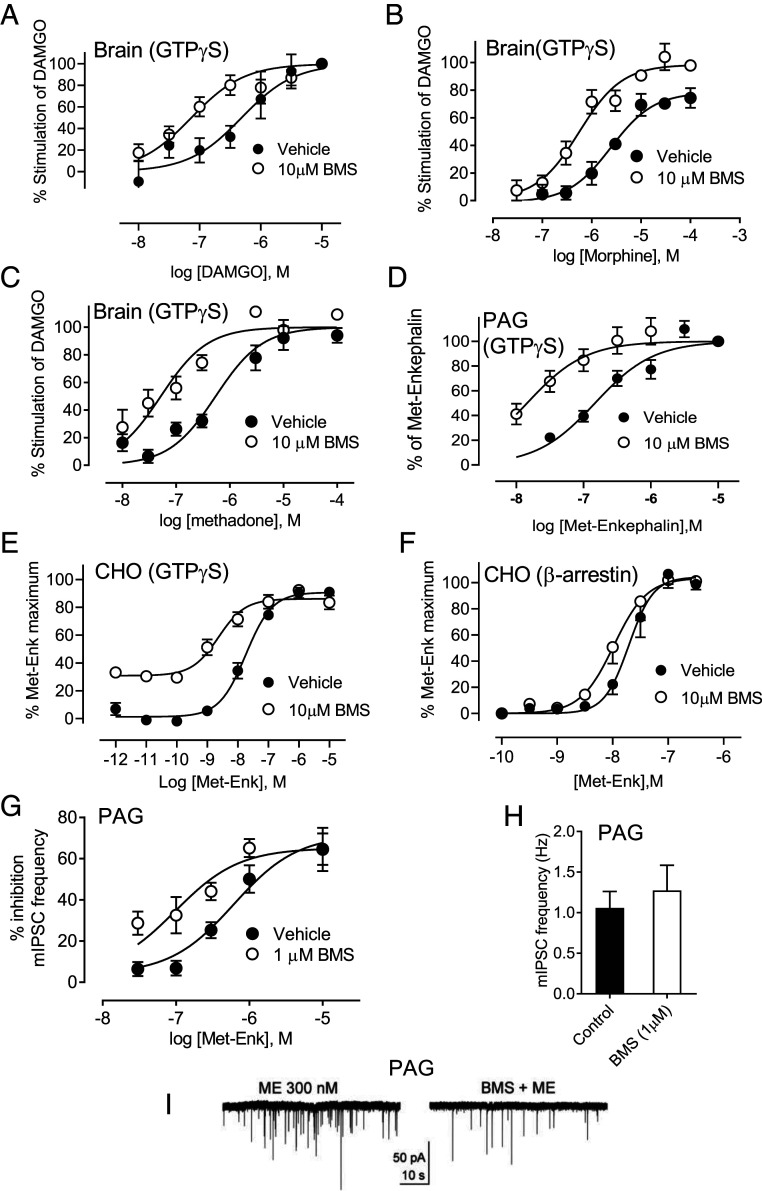
In heterologous cell systems, BMS-986122 (SI Appendix, Fig. S1) enhances the activity of orthosteric MOR agonists by an allosteric mechanism (3–5). Here, we show that this effect translates to MORs expressed at endogenous levels in homogenates from brains of C57BL/6 mice. In whole brain homogenates from wild-type mice, the selective MOR agonist DAMGO ([D-Ala2,N-MePhe4,Gly-ol]-enkephalin) activated heterotrimeric G protein, as determined by binding of the slowly hydrolyzable guanosine triphosphate (GTP) analog [35S]GTPγS, with a potency (effective concentration, EC50) and 95% C.I. value of 484 (255 to 906) nM. The potency of DAMGO was increased to 73.1 (41.4 to 125) nM in the presence of 10 μM BMS-986122, an enhancement of 6.6-fold (Fig. 1A). BMS-986122 had no effect on its own in the absence of DAMGO, and neither DAMGO nor the PAM, alone or together, stimulated [35S]GTPγS binding in brain homogenates from MOR knockout mice (SI Appendix, Fig. S2 A and B). Similarly, BMS-986122 significantly increased the potency of morphine in this assay by 4.8-fold, from 2,592 (1,470 to 4,571) nM to 543 (343 to 869) nM, and its maximal response from 79% (69 to 89) to 98% (90 to 107) (Fig. 1B), and it produced a significant eightfold improvement in the potency of methadone (EC50 447 (263 to 760) nM to 55 (31 to 98) nM (Fig. 1C). We were unable to accurately study the effect of BMS-986122 on the endogenous opioid Met-Enk in whole brain homogenates because this peptide also activates the delta-opioid receptor (15, 16), resulting in shallow concentration–response curves (SI Appendix, Fig. S3A). However, in homogenates of the periaqueductal gray (PAG), which is an important locus for opioid-mediated descending control of pain (17) and which expresses a high level of MOR (18, 19), Met-Enk stimulated [35S]GTPγS binding, with a potency of 148 (112 to 195) nM. This was increased by 10-fold to 14.5 (8.2 to 24.3) nM in the presence of 10 μM BMS-986122 (Fig. 1D). BMS 986122 caused no stimulation of [35S]GTPγS binding alone in homogenates of PAG (SI Appendix, Fig. S3C).
Fig. 1.
BMS-986122 (BMS) enhances activity of orthosteric agonists in vitro. (A–C) BMS increased the activity of (A) DAMGO, (B) morphine, and (C) methadone in the [35S]GTPγS binding assay in whole brain homogenates from C57BL/6 mice. (D) Potency of Met-Enk to stimulate [35S]GTPγS binding in homogenates of PAG from C57BL/6 mice increased in the presence of BMS. (E). The effect of BMS on Met-Enk-stimulation of [35S]GTPγS in CHO cell membranes expressing hMOR. (F) The effect of BMS on β-arrestin recruitment in Pathunter CHO cells expressing hMOR. All [35S]GTPγS data (A–E) are from at least three separate experiments in duplicate. β-arrestin data are from four separate experiments in triplicate. (G) BMS-986122 affords a sixfold increase in the potency of Met-Enk to inhibit presynaptic GABA release, measured as mIPSCs, in rat PAG slices (n = 4 to 9). (H) BMS alone did not alter mIPSC frequency, F[11,11] = 2.337, P = 0.17. (I) Representative traces showing increased inhibition by Met-Enk (ME) in the presence of BMS.
It is known that agonists can bias signaling downstream of G-protein-coupled receptors (GPCRs) (20–22), including MOR (23–25), to different intracellular pathways, particularly those activated by G protein or those downstream of β-arrestin (22). The endogenous opioid peptide Met-Enk is considered to be a neutral, nonbiased ligand (26–28). However, it can be hypothesized that by binding to an allosteric site on MOR, a PAM could not only alter the potency of agonists like Met-Enk but may do so in a biased fashion. To examine this possibility, we studied the ability of Met-Enk to activate G protein or recruit β-arrestin in CHO (Chinese hamster ovary) cells expressing human MOR (hMOR-CHO). Met-Enk stimulated [35S]GTPγS binding in homogenates of hMOR-CHO cells, giving an EC50 of 18.2 (12.6 to 26.9) nM. In the presence of BMS-986122, the potency was significantly enhanced by 7.8-fold to give an EC50 value of 2.3 (1.1 to 5.3) nM (Fig. 1E). In the β-arrestin recruitment assay in PathHunter hMOR-CHO cells, the EC50 of Met-Enk was similar at 11.4 (7.73 to 16.7) nM, but this was only shifted a nonsignificant 1.8-fold in the presence of 10 μM BMS-986122, giving an EC50 value of 6.2 (4.14 to 9.25) nM. Thus, the ratio of β-arrestin/G protein potency is 0.63 in the absence of BMS-986122 and 2.7 in the presence of BMS-986122, giving a modest bias calculated by the method of Kenakin (29) of 4.3 compared with Met-Enk alone. A similar but reduced biased effect of BMS-986122 was seen when DAMGO was used as the orthosteric ligand (SI Appendix, Fig. S4 A and B). Together, these data suggest that BMS-986122 enhances the preference of these two MOR peptides for G protein over β-arrestin pathways. It should be noted that unlike our previous work in C6 cells, or current results in brain tissue, in the CHO cells expressing high levels of hMOR, a direct agonist effect of BMS-986122 was observed in the [35S]GTPγS assay, although this is only seen at high concentrations with an EC50 value of 7.4 μM (SI Appendix, Fig. S4C) and was not observed in the β-arrestin assay (SI Appendix, Fig. S4 C and D).
A critical action contributing to the opioid modulation of pain is disinhibition of GABA-mediated inhibition of descending pathways by blockade of presynaptic GABA release in the PAG (30). Using slices of rat PAG, we found that BMS-986122 caused a 6.3-fold potentiation of the potency of Met-Enk to inhibit spontaneous presynaptic GABA release, as measured by a decrease in the frequency of miniature inhibitory postsynaptic potentials (mIPSCs). The potency of Met-Enk shifted significantly from 612 (305 to 1,228) nM to 97.7 (45 to 212) nM (Fig. 1 E and F). BMS-986122 alone did alter the frequency of mIPSCs (Fig. 1G).
BMS-986122 Enhances Exogenous Opioid Antinociception.
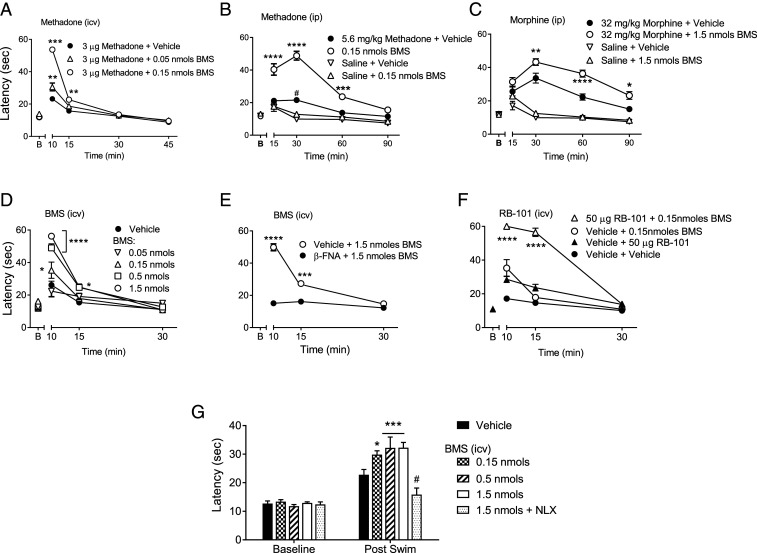
We next examined target engagement of BMS-986122 with MOR in vivo by testing the hypothesis that BMS-986122 would promote the antinociceptive action of the MOR agonist methadone, which we found to be the most sensitive agonist in vitro [Fig. 1C (4)]. BMS-986122 was administered directly into the cerebral ventricles (intracerebroventricular [i.c.v.] injection) of C57BL/6 mice in the presence of an i.c.v. dose of methadone (3 µg) that alone failed to produce a significant antinociception on the hot plate test. Coadministration with 0.05 or 0.15 nmol (23 or 67 ng) of BMS-986122 together with methadone gave a robust antinociception at 10 and 15 min postinjection (Fig. 2A).
Fig. 2.
Antinociceptive activity of BMS-986122 (BMS) in the hot-plate test in C57BL/6 mice. (A) Enhanced antinociceptive effect of methadone (3 µg, i.c.v.) with coadministration of BMS (i.c.v). Interaction: F(6,51) = 52.01, P < 0.0001, n = 6. ***P < 0.001, **P < 0.001 versus methadone alone. (B) Enhancement of a 5.6 mg/kg systemic (i.p.) dose of methadone coadministered with 0.15 nmol BMS (i.c.v.). Interaction: F(9,60) = 19.12, P < 0.0001, n = 6. ****P < 0.0001. ***P < 0.001 compared with methadone alone. (C) Administration of 1.5 nmol BMS (i.c.v.) enhances the antinociceptive effects of 32 mg/kg morphine. Interaction: F(9,60) = 14.47, P < 0.0001], n = 6. ****P < 0.0001, **P < 0.01, and *P < 0.05 compared with morphine alone. (D) Dose- and time-dependent effects of BMS (i.c.v): F(8,54) = 17.15, P < 0.0001, n = 6 to 8. ****P < 0.0001, *P < 0.05 compared with vehicle. (E) Pretreatment (24 h; 5.0 mg/kg) with the irreversible antagonist β-FNA prevented the effect of BMS (1.5 nmol, i.c.v.). Interaction: F(2,30) = 63.34, P < 0.0001, n = 6. ****P < 0.0001, ***P < 0.001 versus β-FNA–treated mice. (F) Enhanced antinociceptive effects of the enkephalinase inhibitor RB-101 (50 μg, i.c.v.) by BMS (0.15 nmol, i.c.v.) given simultaneously. Interaction: F(6,42) = 27.51, P < 0.0001, n = 6 to 7. ****P < 0.0001 compared with RB101 alone or BMS alone. (G) BMS (0.15 to 1.5 nmol, i.c.v.) administered 15 min before the swim significantly improved the antinociceptive effects of swim stress. Interaction: F(4,40) = 10.9 P < 0.0001, n = 6. ***P < 0.001, *P < 0.05 compared with vehicle; #the effect was prevented by 10 mg/kg naloxone (NLX; i.p.), P < 0.0001 versus 1.5 nmol BMS. y-axis labels in A and D refer to all figures in the row; 1 nmol BMS = 447 ng; 50 μg RB101 = 86 nmol.
The antinociceptive effect was short lived, which may be due to the rapid clearance of the methadone or the BMS-986122. To determine this, BMS-986122 (0.15 nmol [67 ng], i.c.v.) was given concurrently with a low dose (5.6 mg/kg) of systemically (intraperitoneal [i.p.]) administered methadone. BMS-986122 did not produce an antinociceptive effect on its own. However, with a dose of 5.6 mg/kg methadone that produced only a low level of antinociception, a robust antinociception was observed in the presence of BMS-986122, which lasted for 60 min (Fig. 2B). Similarly, the combination of BMS-986122 with morphine produced an enhanced antinociceptive effect, although a higher dose of BMS-986122 was required (Fig. 2C). When pretreatment baseline values are subtracted, the effects of methadone in animals cotreated with BMS-986122 are greater than the corresponding additive value of the methadone-alone and BMS-986122-alone treatment groups by more than two SDs at both the 30 min and 60 min time points, suggesting synergy; the same is true of morphine at the 60 min time point (SI Appendix, Fig. S5 A and B).
BMS-986122 Is Antinociceptive when Administered Alone by an Action that Requires Occupation of MOR by Endogenous Opioid Peptides.
BMS-986122 alone, given i.c.v., produced a very short-lived (<15 min) dose-dependent antinociception even in the absence of orthosteric agonist (Fig. 2D). Comparing the effect of i.c.v. BMS-986122 alone with its combined action with i.c.v methadone, we were not able to confirm a synergy between the two compounds (SI Appendix, Fig. S5). Nonetheless, this effect was absent in mice pretreated with 5.0 mg/kg of the MOR-selective, irreversible orthosteric antagonist, β-funaltrexamine (β-FNA), 24 h prior to BMS-986122 administration (Fig. 2E), confirming a MOR-mediated response. In vitro, we were unable to see direct MOR agonist activity of BMS-986122, except for weak activity in cells overexpressing MOR (SI Appendix, Fig. S3C) and using a highly amplified signaling measure (3); yet we did observe an enhancement of the Met-Enk response in the PAG (Fig. 1D). These factors, together with the rapid time course of its antinociceptive effect (Fig. 2D) compared with its longer duration in modulating systemic opioids (Fig. 2 B and C), led us to conclude that the antinociceptive activity produced by BMS-986122 did not result from direct activation of MOR but rather from potentiation of the action of endogenous opioid peptides released during stress caused by handling of the mice and/or the i.c.v. administration procedure (Fig. 2D). It is also possible the short duration of BMS-986122 administered alone was due to potentiation of the anesthetic (isoflurane), although the data show that if true, this action is MOR mediated.
To test a role for endogenous opioid peptides, we administered the enkephalinase inhibitor RB-101 (12, 13) i.c.v. in the presence or absence of BMS-986122. RB-101 prolongs the lifetime of endogenous peptides and on its own produced a small antinociceptive effect (Fig. 2F), which is thought to be due to increased levels of Met-Enk (31). The antinociceptive action of RB-101 was amplified by the administration of 0.15 nmol (67 ng) BMS-986122 and was especially evident at 15 min postinjection. The effects of the combination of RB-101 with BMS-986122 followed the time course of RB-101 alone, supporting the suggestion that BMS-986122 enhances the activity of endogenous opioid peptides rather than producing agonist effects by directly activating MOR. As with the exogenous opioids, the effect of coadministered RB-101 and BMS-986122 was greater by more than two SDs than the additive values of either compound administered alone (SI Appendix, Fig. S5C), indicating a synergistic interaction. To further corroborate our hypothesis, we used a model of swim stress–induced antinociception that has been shown to be mediated by endogenous opioids (32). Under control conditions, a 15 min swim stress produced a significant antinociceptive response. Treatment with BMS-986122 (i.c.v., 0.15 to 1.5 nmol; 67 to 670 ng) administered 15 min before the swim stress significantly improved the antinociceptive effects of swim stress in a naloxone-reversible manner (Fig. 2G). BMS-986122 had no effect in the absence of swim stress.
BMS-986122 Is Active following Systemic (i.p.) Injection in 129S1/SvlmJ Mice.
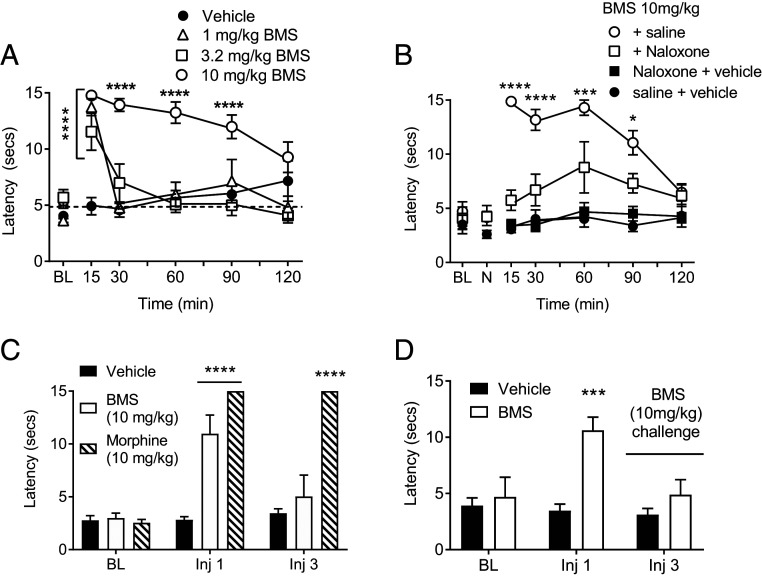
The above data demonstrate that BMS-986122 produces antinociception in the hot-plate test by enhancing exogenous and endogenous opioid activity after i.c.v. administration in C57BL/6 mice. However, in the C57BL/6 mice, we were unable to demonstrate an antinociceptive effect of BMS-986122 by systemic, i.p., injection up to its maximal solubility (10 mg/kg, equivalent to 2.2 μM) using the hot-plate or tail-withdrawal tests; nor was i.p. BMS-986122 able to enhance the action of systemic methadone. In contrast, in the 129S1/SvlmJ strain of mice, BMS-986122 administered i.p. did produce an antinociceptive effect in the warm water tail-withdrawal test, which, at 10 mg/kg, was maintained for 60 min (Fig. 3A); vehicle injections did not significantly elevate the basal responses. This effect of BMS-986122 was inhibited by naloxone (10 mg/kg) pretreatment, confirming a role for opioid receptors and occupation of the orthosteric site by endogenous opioid peptides (Fig. 3B). No effect was seen in the hot plate test. Consequently, all subsequent experiments were performed in 129S1/SvlmJ mice using the effective systemic dose of 10 mg/kg.
Fig. 3.
BMS-986122 (BMS) produces antinociception in 129S1/SvlmJ mice in the warm water tail-withdrawal test. (A) Antinociception produced by increasing doses of BMS. Interaction: F(15,135) = 7.170, P < 0.0001, n = 6. ****P < 0.0001 compared with vehicle. The dotted line indicates the mean basal response before treatment. (B) Inhibition of BMS-induced antinociception by 10 mg/kg naloxone (NLX). Interaction: F(18,144) = 6.533, P < 0.0001, n = 6. ****P < 0.0001, ***P < 0.001, and *P < 0.05 compared with BMS with NLX; NLX alone was not different from vehicle. Time indicates minutes after BMS injection. (C) Tail-withdrawal latencies after one and three administrations (i.p., over 2 d) of BMS or morphine. Interaction: F(4,30) = 15.94, P < 0.0001, n = 6. BMS produced antinociception after one but not three injections. Morphine was effective at both one and three injections; ****P < 0.00001 compared with vehicle. (D) Antinociceptive effect of BMS is lost after three injections of either vehicle or BMS. Interaction: F(2,30) = 4.30, P = 0.023, n = 6. ***P < 0.001 compared with vehicle. In C and D, data were not collected after the second injection.
The antinociceptive action of BMS-986122 in the tail-withdrawal test was lost after a third i.p. injection, whereas morphine administered in the same manner remained active (Fig. 3C). However, when animals were administered saline under the same regime and on the third day received BMS-986122, BMS-986122 again failed to produce antinociception despite the fact that it was the animals’ first exposure to the drug, whereas morphine-mediated antinociception was retained (Fig. 3D). This suggests habituation to the injection and handling, leading to reduced endogenous opioid peptide release and so a loss of BMS-986122 activity.
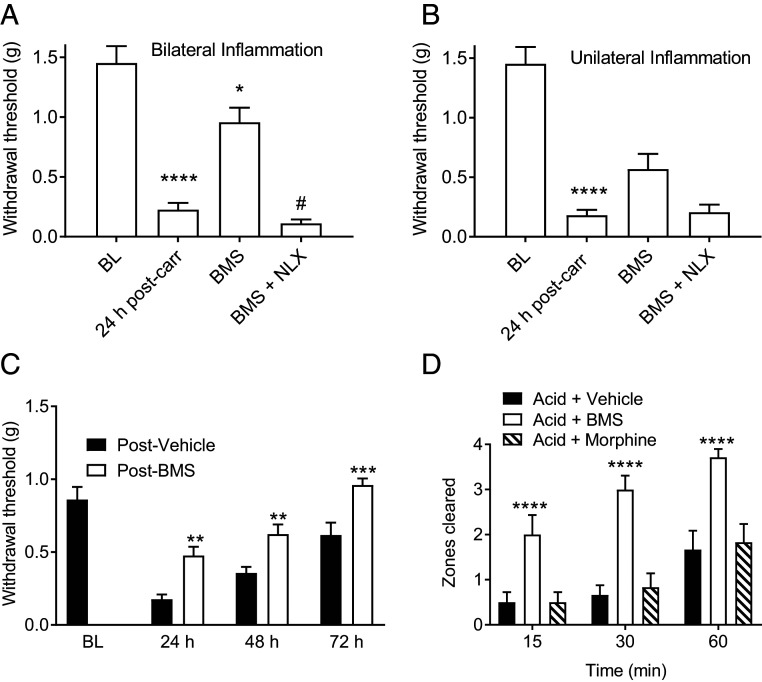
Using the 129S1/SvlmJ mice, we determined the action of BMS-986122 in the more clinically relevant model of chronic inflammatory pain (33, 34). Mice received either bilateral or unilateral intraplantar injections of carrageenan, and mechanical allodynia was assessed in response to von Frey filaments (35). Carrageenan produced a robust mechanical allodynia in the right hindpaw 24 h after bilateral or unilateral injections (Fig. 4 A and B). A single systemic injection of BMS-986122 significantly inhibited mechanical allodynia in mice with bilateral inflammation (Fig. 4A). There was inhibition of unilateral allodynia, although this did not reach significance (Fig. 4B). This antinociceptive activity was fully prevented by pretreatment with naloxone (Fig. 4 A and B).
Fig. 4.
BMS-986122 (BMS) produces antinociception against inflammatory pain in 129S1/Svlmj mice. Paw withdrawal thresholds were assessed in the right hindpaw 30 min after BMS (10 mg/kg, i.p.). Mice received either bilateral (A) or unilateral (B) intraplantar injections of carrageenan, and mechanical allodynia was assessed in the right hindpaw by sensitivity to von Frey filaments. Carrageenan produced robust mechanical allodynia in the right hindpaw 24 h after injection, compared with preinjury baseline (BL), after both unilateral [main effect of treatment: F(3,50) = 22.07, P < 0.0001, n = 6 ****P < 0.0001] and bilateral injections [main effect of treatment: F(3,46) = 19.05, P < 0.0001, n =6, ****P < 0.0001]. A single systemic injection of BMS reversed allodynia in mice with bilateral inflammation (*P < 0.05, n = 6) in a naloxone (NLX) reversible manner (#P < 0.05). (C) Sustained antiallodynic effects of BMS against CFA-induced unilateral inflammation after twice daily injections for 3 d. Main effect of treatment: F(1,10) = 69.51, P < 0.0001; n = 6. **P < 0.01, ***P < 0.001. (D) Reversal of pain-depressed nesting behavior following i.p. injections of BMS. i.p. injection of dilute acetic acid depressed nesting, which was restored by BMS. Main effect of treatment: F(2,16) = 24.58, P < 0.0001, n = 6. ****P < 0.0001 versus vehicle or morphine.
To produce a more persistent inflammatory pain, mice received unilateral intraplantar injections of Complete Freund’s Adjuvant (CFA). CFA produced robust mechanical allodynia 24 h after injection (Fig. 4C). Systemic administration of BMS-986122 (10 mg/kg) reversed this mechanical allodynia (Fig. 4C). The effect was observed for three daily injections over 72 h, indicating that endogenous opioid peptide release continues and that tolerance to BMS-986122 does not develop within this time frame.
Pain-depressed behaviors, as opposed to pain-evoked behaviors, more closely model pain measures used in clinical situations (36, 37). Nesting is an instinctive behavior in mice that is suppressed by pain (38). An i.p. injection of dilute acetic acid depressed nesting behavior in mice for at least 60 min (Fig. 4D). Pretreatment with BMS-986122 restored nesting behavior. In contrast, 10 mg/kg morphine failed to restore nesting behavior in mice (Fig. 4D).
BMS-986122 Exhibits Reduced MOR Side Effects Compared with Morphine.
We evaluated the effects of systemic (i.p.) BMS-986122 on three common on-target side effects of morphine in the 129S1/SvlmJ mice, using the maximal dose (10 mg/kg) possible due to solubility constraints.
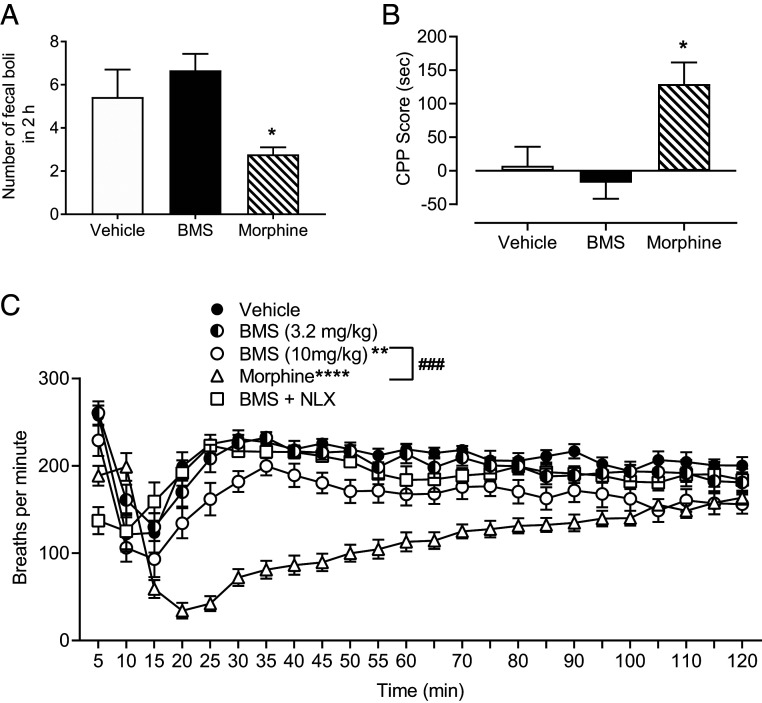
Constipation was measured by determining the number of fecal boli produced during the 2 h following i.p. administration of either 10 mg/kg BMS-988122 or morphine. Animals treated with morphine deposited significantly fewer fecal boli compared with animals treated with either vehicle or BMS-986122 (Fig. 5A).
Fig. 5.
BMS-986122 (BMS) produces a reduced level of MOR-mediated side effects compared with morphine in 129S1/Svlmj mice. (A) The number of fecal boli deposited 2 h following i.p. administration of 10 mg/kg BMS or morphine. Main effect of treatment: F(2,22) = 13.10, P = 0.0002; n = 6. *P < 0.05 versus BMS or vehicle. (B) CPP of 10 mg/kg BMS compared with 10 mg/kg morphine following 5 d of conditioning. CPP score refers to the difference in time spent on the drug-paired side on test day compared with preconditioning. Animals conditioned with morphine showed significant preference for the drug-paired chamber compared with animals conditioned with either vehicle or BMS. Main effect of treatment: F(2,15) = 7.65, P = 0.005, n = 6. *P < 0.05 versus BMS or vehicle. (C) Respiration was assessed immediately after i.p. injection of morphine or BMS. Interaction: F(69,1,012) = 10.75, P < 0.0001, n = 12. Respiratory depression was seen with morphine (****P < 0.0001) and 10 mg/kg BMS (**P < 0.01), but the effect of morphine was significantly greater than BMS (###P < 0.001). The effect of BMS was reversed by naloxone (NLX).
Conditioned place preference was used to determine whether BMS-986122 produces rewarding effects in mice. Animals that were conditioned with 10 mg/kg morphine for 5 d showed significant preference for the drug-paired chamber compared with animals conditioned with either vehicle or 10 mg/kg BMS-986122 (Fig. 5B).
Respiratory depression was monitored by measuring breathing rate using the Comprehensive Lab Animal Monitoring System. After i.p. injection, mice, whether given vehicle, 10 mg/kg morphine, or 10 mg/kg, but not 3.2 mg/kg, BMS-986122, showed an initial change in rate when returned to the apparatus that lasted for 20 min. Compared with vehicle, morphine produced significantly greater respiratory rate depression that was maintained for 100 min (Fig. 5C). A systemic injection of BMS-986122 produced a much-reduced degree of respiratory depression compared with morphine, but this small effect was reversed by a pretreatment with naloxone (Fig. 5C), suggesting an action at MOR.
Brain and Plasma Levels of BMS-986122.
Levels of BMS-986122 were determined in brain and plasma 1 h after i.p. injection. The mean plasma concentration was 397 ± 100 ng/g (n = 9), and the mean brain concentration was 127 ± 16 ng/mL (n = 15). These values correspond to 900 nM and 280 nM, respectively, and a plasma:brain ratio of 3:1. BMS-986122 at 300 nM did produce a significant 3.9-fold shift the concentration–response curve for Met-Enk in the [35S]GTPγS assay using membranes from hMOR-CHO cells from 10.2 (7.3 to 14.2) nM to 2.6 (1.9 to 3.6) nM (SI Appendix, Fig. S6). We have previously reported that 300 nM BMS-986122 produces a similar shift in the methadone concentration–response curve in membranes from C6 cells expressing MOR (4).
Discussion
In this report, we demonstrate that allosteric modulation of MOR by the small molecule mu-PAM BMS-986122 affords antinociception in mouse models of acute and persistent pain in a manner consistent with potentiation of the activity of released endogenous opioid peptides acting at MOR. We provide proof of target engagement using the exogenous MOR agonists methadone and morphine as well as the antagonists naloxone and β-FNA to block the access of endogenous opioid peptide to MOR. The enhancing effects of BMS-986122 on the opioids as well as on the enkephalinase inhibitor RB101 appear to be synergistic rather than additive, in agreement with studies by Pasternak and colleagues of a synergy between methadone and other mu-opioids, including morphine (39). The antinociception afforded by BMS-986122 is seen in the absence of exogenous opioid drug administration and occurs without, or with reduced, side effects of constipation, respiratory depression, and reward. The work reveals the in vivo function of mu-PAMs and identifies positive allosteric modulation of MOR as a potential new mechanism for therapeutic intervention for the management of pain, possibly with reduced side effects.
In vitro, BMS-986122 had no direct agonist activity at endogenously expressed MOR, as shown by its inability to stimulate G protein in membranes from mouse brain, including the PAG, or inhibit GABA release in slices of PAG. This agrees with findings in heterologous cell systems expressing MOR, where the compound is unable to activate heterotrimeric G protein or recruit β-arrestin (3, 10); however, it does show low activity (EC50 ∼0.1 mM) at the more amplified downstream effector, adenylate cyclase (3), and we did observe weak activity in CHO cells overexpressing hMOR. Yet, there was a dose-dependent antinociception when BMS-986122 was given centrally (i.c.v.) to C57BL/6 mice. This action was short lived but reversed by the orthosteric MOR antagonist β-FNA. It is feasible, even with its very low potency and efficacy, that BMS-986122 is acting directly to stimulate the receptor. However, since BMS-986122 does not bind to the orthosteric site on MOR (3, 4) but does enhance the action of Met-Enk to activate [35S]GTPγS binding in PAG membranes and inhibit GABA release in the rat PAG, we hypothesized that the reversal by β-FNA was due to blockade of endogenous opioid peptides binding to the orthosteric site on MOR. These opioid peptides would be released during the pain and/or stress of the experimental procedures and be rapidly metabolized. To confirm this, we used the enkephalinase inhibitor RB-101, a compound that prolongs the lifetime of endogenous opioid peptides, including Met-Enk (31), and thereby increases their concentration (12, 13). We chose a low dose of RB-101 that was minimally effective alone but afforded a robust antinociceptive effect in the presence of BMS-986122, supporting a synergistic interaction. In addition, BMS-986122 promotion of swim-stress antinociception was naloxone reversible, again implicating endogenous opioid peptides and MOR in the action of the modulator. Consequently, these data suggest that by increasing the activity of released endogenous opioid peptides, BMS-986122 affords antinociception.
The antinociceptive action of BMS-986122 against noxious heat was observed in two strains of mice, C57BL/6 and 129S1/SvlmJ. However, we only observed activity after systemic administration in the 129S1/SvlmJ strain and then only in the tail-withdrawal assay. BMS-986122 does access the brain after i.p. administration in the 129S1/SvlmJ mice; however, these discrepancies could still be due to pharmacokinetic factors, as the hot-plate assay involves supraspinal sites compared with the spinal reflex action in the tail-withdrawal test (40), allied with the higher agonist efficacy requirement of the hot-plate assay (41), or they may be due to spatial release of opioid peptides. Additionally, it is known that 129 strains of mice are more susceptible to higher levels of anxiety-like behaviors (42, 43) and have higher responsiveness to acoustic and tactile stimuli (44). Moreover, they are generally more resistant to pain that involves a stressor (45), which could indicate increased levels of endogenous opioids released during testing and handling in this strain; the effects of these peptides would be enhanced by BMS-986122. In support of this idea, the antinociceptive effect of BMS-986122 in the tail-withdrawal test was lost after repeated handling and vehicle injections, suggesting this habituation leads to reduced stress and so reduced release of opioid peptides.
The results indicate that BMS-986122 can facilitate efficacious antinociception in the absence of traditional opioid drugs, in agreement with the suggestion that continual release of opioid peptides is needed for the modulator to be effective. To confirm this, we used the CFA model of inflammatory pain in the mouse, which provides an increase in nociceptive behavior and is longer lasting. Several lines of evidence implicate endogenous opioid peptide release as modulating the nociceptive response to inflammation. Opioid peptides are released into inflamed tissue from immune cells (46), and levels of the opioid peptides Met-Enk and dynorphin remain high for at least 18 d after CFA in the rat paw (47). In addition, in this model, increased levels of Met-Enk are seen in brainstem areas involved in the control of pain, including the ventrolateral region of the PAG, nucleus raphe magnus, and parabrachial nucleus, that persist for at least 14 d (48). Finally, the antagonist naltrexone, but not its peripherally selective analog N-methyl naltrexone, prolongs hyperalgesia in the model (49). After CFA-induced inflammation in the mouse paw, BMS-986122 maintained its effectiveness over several days. We also observed an antinociceptive effect of BMS-986122 against carrageenan-induced inflammatory pain. This effect was enhanced with bilateral administration of carrageenan, which we interpret to be due to increased opioid peptide release under the more severe conditions and so further implicates a role for central MORs.
As with the inflammation models, i.p. injection of acetic acid causes the release of endogenous opioid peptides. In rats, Met-Enk is produced in the spinal cord (50), and the nociceptive responses can be reduced by enkephalinase inhibitors in mice and rats (51, 52) and enhanced by naloxone (52). Moreover, both peripheral and central opioid receptors appear to play a role in antinociception in this test (53, 54). Thus, the reversal of i.p. acetic acid inhibition of nesting adds further support to the suggestion that BMS-986122 enhances the action of endogenous opioid peptides. Morphine at 10 mg/kg, a dose that prevents a writhing response following intraperitoneal acetic acid (55), failed to restore nesting, possibly because sedation and/or dysphoria prevents the animal from functioning normally (56). This was not the case with BMS-986122, suggesting a lack of behavioral disruption with the modulator. While mice generally show an enhanced locomotor activity to morphine, this action is biphasic with inhibition at lower doses (57), and the 129 strain is not especially sensitive to the locomotor-stimulating action of morphine (58).
The above findings support the premise that continual peptide release is needed for effectiveness of BMS-986122 and that the endogenous opioid tone in resting states is not high enough to be affected by allosteric modulators unless there is a nociceptive insult. As a consequence of this, BMS-986122 produced no constipation or rewarding effects in the conditioned place preference (CPP) assay compared with morphine. This lack of effect in CPP is perhaps surprising given that naloxone has been shown to have an aversive action in this assay. However, this action of naloxone is strain dependent (59), and the 129 strain is reportedly less sensitive to morphine CPP (60). Moreover, it is feasible, given the findings with repeated BMS-986122 administration in the tail-withdrawal assay, that the mice become habitual during the 5 d conditioning paradigm. There was a small reduction in respiration compared with vehicle that was reversed by naloxone. Since BMS-986122 does distribute to the brain, this suggests a central, rather than peripheral, effect. Moreover, because BMS-986122 does not bind to the orthosteric site on MOR (3, 4), this can be ascribed to the enhancement of endogenous opioid peptides released during the handling, drug injections, and reintroduction of the animals into the environment of the respiration chamber.
The results of the current study are consistent with the hypothesis that BMS-986122 enhances the antinociceptive activity of endogenous opioid peptides in vivo and so should translate to analgesic activity in human pain states. Moreover, mu-PAMs can be predicted to enhance the effectiveness of nondrug interventions such as placebo analgesia and acupuncture, both of which are thought to involve opioid peptides (11, 61–69).
Overall, the present findings in mice demonstrate that by enhancing the activity of endogenous opioid peptides, mu-PAMs afford antinociception without the requirement for traditional, and problematic, opioid drugs. The ideal mu-PAM would be silent in the absence of orthosteric ligand but would boost endogenous opioid peptide action at MOR during pain and nondrug pain-relieving interventions, thus providing efficacious pain relief and a specificity of action not seen with opioid drugs or the enkephalinase inhibitors. Further development could take advantage of the probe dependence of mu-PAMs (4, 70) to tailor activity toward particular opioid peptides and away from traditional opioid drugs and to the stimulation of specific signaling pathways. In particular, there is evidence from studies using β-arrestin 2 knockout mice that morphine side effects, including respiratory depression, may be mediated downstream of β-arrestin rather than G protein (71). Recent studies have not successfully repeated these experiments (72), although compounds with bias for G protein pathways, especially when there is a considerable separation, show reduced respiratory depression and constipation in preclinical models (73, 74). The fact that BMS-986122 affords a shift toward G protein activation in the signaling profile of Met-Enk, albeit modest, raises the possibility that this may play a role in the observed mu-PAM profile. Enhancement of this aspect of BMS-986122 in newer analogs could contribute to understanding the physiological consequences of bias, especially as it relates to endogenous opioid ligands (15). Moreover, now that MOR signaling from intracellular organelles has been established, an added advantage of mu-PAMs is that they will maintain the spatiotemporal intracellular signaling pattern of opioid peptides rather than the different signaling pattern seen with small molecule opioids (75). Taken together, the results support a rationale for additional investigation and further safety evaluation of mu-PAMs—in particular, unwanted side effects and studies in combination with opioid drugs—for the management of pain as efficacious replacements for the current clinically used, and abused, opioid analgesics.
Materials and Methods
Animals.
Behavioral experiments, [35S]GTPγS binding assays and pharmacokinetic experiments were conducted using equal numbers of male and female C57BL/6 (wild-type and mu-opioid knockout) and 129S1/SvlmJ mice between 8 and 16 wk of age, bred in house or purchased from Envigo or Jackson Laboratories respectively. Electrophysiology experiments were conducted using adult male Sprague–Dawley rats purchased from Envigo. All animals were group-housed by sex on a 12 h light/dark cycle (lights on 0700 hours). All testing was performed during the light phase. Mice and rats had free access to food and water while in their home cage. Experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (76) and were approved by the Institutional Animal Care and Use Committees of the University of Michigan, University of Florida, and Oregon Health and Science University.
Drugs and Chemicals.
Details and sources of drugs and chemicals are provided in SI Appendix, Materials and Methods. For behavioral experiments, drugs were dissolved as follows: BMS-986122 and methadone (when administered i.c.v.) were dissolved in 1% dimethyl sulfoxide, 1% alkamuls, and 98% sterile H2O; RB-101 was dissolved in 20% (2-hydroxypropyl)-β-cyclodextrin; methadone, morphine sulfate, and naloxone were dissolved in sterile saline (0.9% NaCl); and β-FNA was dissolved in sterile H2O. For administration by the i.p. route, BMS-986122 was dissolved in 60% sterile H2O, 20% ethanol, and 20% ethoxylated castor oil. Methadone, morphine, and naloxone were dissolved in saline.
In Vitro Assays.
Additional details are provided in SI Appendix, Materials and Methods.
[35S]GTPγS binding assay.
Assays were performed in membrane homogenates from CHO cells expressing hMOR (kindly provided by Dr. L. Toll, Department of Biomedical Science, Florida Atlantic University, Boca Raton, FL) from mouse brain (minus olfactory bulb and cerebellum) or from 1 mm sections of midbrain containing the PAG. Homogenates were prepared in ice cold 50 mM Tris base, pH 7.4, as previously described (77).
β-arrestin 2 recruitment.
β-arrestin 2 recruitment was determined using the commercially available kit PathHunter Detection kit and CHO-hMOR β-Arrestin cells from DiscoverX (https://www.discoverx.com/home).
Brain slice electrophysiological recordings.
Coronal slices (220 μm) of brains from male Sprague–Dawley rats (postnatal day >30), containing ventrolateral PAG, were cut and maintained at 32 °C in physiological saline equilibrated with 95% O2 and 5% CO2 for electrophysiological recording, as previously described (78). Spontaneous mIPSCs were obtained in the presence of tetrodotoxin (500 nM) and (2,3-dioxo-6-nitro-7-sulfamoyl-benzo[f]quinoxaline [NBQX, 5 μM]), filtered at 2 kHz and sampled at 5 kHz.
Behavioral Assays.
Additional details are provided in SI Appendix, Materials and Methods.
Animal numbers per group for behavioral tests are indicated in the figure legends. All behavioral assays included controls (vehicle) run at the same time. The i.c.v. injections were not blinded, but these assays were performed by four different individuals trained to observe the same endpoint. All behavioral assays involving the peripheral (i.p.) administration of BMS-986122 were performed blinded to the drug, vehicle, or pretreatment condition.
Hot-plate test.
This was used to measure supraspinally mediated antinociception in C57BL/6 and 129S1/SvlmJ mice following i.p. or i.c.v. administration of compounds. A hot plate temperature of 52 °C was employed and latency to lick or shake the forepaw(s) or hindpaw(s) or jump was measured. A cutoff time of 60 s was used to prevent tissue damage (79).
Warm water tail withdrawal.
Withdrawal of the mouse tail from 55 °C water was used as a measure of spinally mediated nociception in C57BL/6 and 129S1/SvlmJ mice following i.p. or i.c.v. administration of compounds. A cutoff time of 15 s was used to prevent tissue damage (78).
Swim stress–induced antinociception.
This was measured after a 15 min swim stress in 25 °C (13) water using the hot-plate test as described above.
Pain-depressed nesting.
This was performed as described (38). Animals were allowed to nest for 120 min, and the number of zones cleared was counted every 15 min.
Inflammatory pain testing using CFA or carrageenan.
Inflammatory pain was induced via unilateral intraplantar administration of either 2.5% carrageenan (20 μL) or CFA (5 μL). von Frey filaments were used to assess mechanical allodynia in the inflamed paw using a modified up–down method (80).
Conditioned Place Preference (CPP).
Mice were conditioned to one side of a two-compartment chamber with morphine, BMS-986122, or vehicle. After 5 d of conditioning, mice were allowed to roam freely for 30 min. CPP scores were calculated as the difference between time spent on the drug-paired side on test day compared with the time spent on the future drug-paired side on day one.
Constipation.
Mice were injected i.p. with morphine, BMS-986122, or vehicle and immediately placed in individual cages with a mesh bottom. The number of fecal boli was counted for 2 h.
Respiratory depression.
Respiration rates were measured using the respiratory monitor component of the Comprehensive Lab Animal Monitoring System (Columbus Instruments), as described previously (81). Respiration frequency of each occupant mouse was collected sequentially every 30 s and grouped into 5 min bins until the completion of the test.
Plasma and brain levels of BMS-986122.
These were determined following i.p administration of BMS-986122 (10 mg/kg) in 129S1/SvlmJ mice using the same vehicle as for the behavioral assays. The measurements were performed in the Pharmacokinetics and Mass Spectrometry Core of the College of Pharmacy, University of Michigan (https://pharmacy.umich.edu/pkcore). Additional details are provided in SI Appendix, Materials and Methods.
Data Analysis.
For in vitro experiments, best fit lines were calculated for using nonlinear regression analysis (Hill slope = 1) using GraphPad Prism Version 6.0 (GraphPad Software) to provide potency (EC50) and maximal effect and expressed as mean (95% CI). Bias calculations were performed as described by Kenakin (29), as follows: Log (max/EC50) values were calculated for each agonist on each pathway and compared as Δlog (max/EC50). Bias was calculated as ΔΔlog (Δlog (max/EC50). For behavioral experiments, mixed-factor ANOVAs were used with drug treatment or dose as the between-subject factor and time as the within-subject factor for all pain and side-effect experiments. A significant ANOVA was followed by a Tukey post-hoc test, and the criterion for significance was set at P < 0.05. Synergy was determined if the difference between cotreatment with two compounds was more than two SDs greater than the additive group mean of each compound alone.
Supplementary Material
Acknowledgments
This work was funded by the NIH Grant (R37 DA039997 to J.R.T.). R.K. and T.M.H. were supported by NIH Grant T32 DA007268 (to J.R.T.); K.E.L. by NIH Grant T32 DA007267 and an Endowment for the Development of Graduate Education award from the Endowment for the Basic Sciences, University of Michigan; and K.E.K. by NIH Grant T32 GM007767.
Footnotes
Competing interest statement: N.T.B. and A.A. have a patent related to this work: “Positive allosteric modulators and silent allosteric modulators of the μ-opioid receptor.” Publication number: WO2014/107344 A1. Publication date: July 10, 2014.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2000017118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Valentino R. J., Volkow N. D., Untangling the complexity of opioid receptor function. Neuropsychopharmacology 43, 2514–2520 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mansour A., Fox C. A., Thompson R. C., Akil H., Watson S. J., mu-Opioid receptor mRNA expression in the rat CNS: Comparison to mu-receptor binding. Brain Res. 643, 245–265 (1994). [DOI] [PubMed] [Google Scholar]
- 3.Burford N. T., et al., Discovery of positive allosteric modulators and silent allosteric modulators of the μ-opioid receptor. Proc. Natl. Acad. Sci. U.S.A. 110, 10830–10835 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livingston K. E., Traynor J. R., Disruption of the Na+ ion binding site as a mechanism for positive allosteric modulation of the mu-opioid receptor. Proc. Natl. Acad. Sci. U.S.A. 111, 18369–18374 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livingston K. E., et al., Pharmacologic evidence for a putative conserved allosteric site on opioid receptors. Mol. Pharmacol. 93, 157–167 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartuzi D., Kaczor A. A., Matosiuk D., Interplay between two allosteric sites and their influence on agonist binding in human μ opioid receptor. J. Chem. Inf. Model. 56, 563–570 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Shang Y., et al., Proposed mode of binding and action of positive allosteric modulators at opioid receptors. ACS Chem. Biol. 11, 1220–1229 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bisignano P., et al., Ligand-based discovery of a new scaffold for allosteric modulation of the μ-Opioid receptor. J. Chem. Inf. Model. 55, 1836–1843 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burford N. T., Traynor J. R., Alt A., Positive allosteric modulators of the mu-opioid receptor: A novel approach for future pain medications. Br. J. Pharmacol. 172, 277–286 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livingston K. E., Traynor J. R., Allostery at opioid receptors: Modulation with small molecule ligands. Br. J. Pharmacol. 175, 2846–2856 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine J. D., Gordon N. C., Fields H. L., The mechanism of placebo analgesia. Lancet 2, 654–657 (1978). [DOI] [PubMed] [Google Scholar]
- 12.Fournié-Zaluski M. C., et al., “Mixed inhibitor-prodrug” as a new approach toward systemically active inhibitors of enkephalin-degrading enzymes. J. Med. Chem. 35, 2473–2481 (1992). [DOI] [PubMed] [Google Scholar]
- 13.Noble F., et al., Inhibition of the enkephalin-metabolizing enzymes by the first systemically active mixed inhibitor prodrug RB 101 induces potent analgesic responses in mice and rats. J. Pharmacol. Exp. Ther. 261, 181–190 (1992). [PubMed] [Google Scholar]
- 14.Noble F., et al., Pain-suppressive effects on various nociceptive stimuli (thermal, chemical, electrical and inflammatory) of the first orally active enkephalin-metabolizing enzyme inhibitor RB 120. Pain 73, 383–391 (1997). [DOI] [PubMed] [Google Scholar]
- 15.Gomes I., et al., Biased signaling by endogenous opioid peptides. Proc. Natl. Acad. Sci. U.S.A. 117, 11820–11828 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paterson S. J., Robson L. E., Kosterlitz H. W., Classification of opioid receptors. Br. Med. Bull. 39, 31–36 (1983). [DOI] [PubMed] [Google Scholar]
- 17.Morgan M. M., Sohn J. H., Liebeskind J. C., Stimulation of the periaqueductal gray matter inhibits nociception at the supraspinal as well as spinal level. Brain Res. 502, 61–66 (1989). [DOI] [PubMed] [Google Scholar]
- 18.Loyd D. R., Wang X., Murphy A. Z., Sex differences in mu-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J. Neurosci. 28, 14007–14017 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H., Wessendorf M. W., Mu- and delta-opioid receptor mRNAs are expressed in periaqueductal gray neurons projecting to the rostral ventromedial medulla. Neuroscience 109, 619–634 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Wootten D., Christopoulos A., Marti-Solano M., Babu M. M., Sexton P. M., Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 19, 638–653 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Rajagopal S., et al., Quantifying ligand bias at seven-transmembrane receptors. Mol. Pharmacol. 80, 367–377 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reiter E., Ahn S., Shukla A. K., Lefkowitz R. J., Molecular mechanism of β-arrestin-biased agonism at seven-transmembrane receptors. Annu. Rev. Pharmacol. Toxicol. 52, 179–197 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raehal K. M., Schmid C. L., Groer C. E., Bohn L. M., Functional selectivity at the μ-opioid receptor: Implications for understanding opioid analgesia and tolerance. Pharmacol. Rev. 63, 1001–1019 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grim T. W., Acevedo-Canabal A., Bohn L. M., Toward directing opioid receptor signaling to refine opioid therapeutics. Biol. Psychiatry 87, 15–21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehrlich A. T., et al., Biased signaling of the mu opioid receptor revealed in native neurons. iScience 14, 47–57 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McPherson J., et al., μ-opioid receptors: Correlation of agonist efficacy for signalling with ability to activate internalization. Mol. Pharmacol. 78, 756–766 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivero G., et al., Endomorphin-2: A biased agonist at the mu opioid receptor. Mol. Pharmacol. 82, 178–188 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson G. L., et al., Biased agonism of endogenous opioid peptides at the μ-Opioid receptor. Mol. Pharmacol. 88, 335–346 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Kenakin T., A scale of agonism and allosteric modulation for assessment of selectivity, bias, and receptor mutation. Mol. Pharmacol. 92, 414–424 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Basbaum A. I., Fields H. L., Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annu. Rev. Neurosci. 7, 309–338 (1984). [DOI] [PubMed] [Google Scholar]
- 31.Daugé V., Mauborgne A., Cesselin F., Fournié-Zaluski M. C., Roques B. P., The dual peptidase inhibitor RB101 induces a long-lasting increase in the extracellular level of Met-enkephalin-like material in the nucleus accumbens of freely moving rats. J. Neurochem. 67, 1301–1308 (1996). [DOI] [PubMed] [Google Scholar]
- 32.Parikh D., et al., Stress-induced analgesia and endogenous opioid peptides: The importance of stress duration. Eur. J. Pharmacol. 650, 563–567 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reis E. F., et al., Lipophilic amino alcohols reduces carrageenan-induced paw edema and anti-OVA DTH in BALB/c mice. Int. Immunopharmacol. 17, 727–732 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Sammons M. J., et al., Carrageenan-induced thermal hyperalgesia in the mouse: Role of nerve growth factor and the mitogen-activated protein kinase pathway. Brain Res. 876, 48–54 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Ignatowska-Jankowska B. M., et al., A cannabinoid CB1 receptor-positive allosteric modulator reduces neuropathic pain in the mouse with no psychoactive effects. Neuropsychopharmacology 40, 2948–2959 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Negus S. S., et al., Preclinical assessment of candidate analgesic drugs: Recent advances and future challenges. J. Pharmacol. Exp. Ther. 319, 507–514 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Tappe-Theodor A., King T., Morgan M. M., Pros and cons of clinically relevant methods to assess pain in rodents. Neurosci. Biobehav. Rev. 100, 335–343 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Negus S. S., et al., Effects of ketoprofen, morphine, and kappa opioids on pain-related depression of nesting in mice. Pain 156, 1153–1160 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolan E. A., Tallarida R. J., Pasternak G. W., Synergy between mu opioid ligands: Evidence for functional interactions among mu opioid receptor subtypes. J. Pharmacol. Exp. Ther. 303, 557–562 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Callahan B. L., Gil A. S., Levesque A., Mogil J. S., Modulation of mechanical and thermal nociceptive sensitivity in the laboratory mouse by behavioral state. J. Pain 9, 174–184 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Gårdmark M., Höglund A. U., Hammarlund-Udenaes M., Aspects on tail-flick, hot-plate and electrical stimulation tests for morphine antinociception. Pharmacol. Toxicol. 83, 252–258 (1998). [DOI] [PubMed] [Google Scholar]
- 42.Rodgers R. J., Boullier E., Chatzimichalaki P., Cooper G. D., Shorten A., Contrasting phenotypes of C57BL/6JOlaHsd, 129S2/SvHsd and 129/SvEv mice in two exploration-based tests of anxiety-related behaviour. Physiol. Behav. 77, 301–310 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Homanics G. E., Quinlan J. J., Firestone L. L., Pharmacologic and behavioral responses of inbred C57BL/6J and strain 129/SvJ mouse lines. Pharmacol. Biochem. Behav. 63, 21–26 (1999). [DOI] [PubMed] [Google Scholar]
- 44.Logue S. F., Owen E. H., Rasmussen D. L., Wehner J. M., Assessment of locomotor activity, acoustic and tactile startle, and prepulse inhibition of startle in inbred mouse strains and F1 hybrids: Implications of genetic background for single gene and quantitative trait loci analyses. Neuroscience 80, 1075–1086 (1997). [DOI] [PubMed] [Google Scholar]
- 45.Mogil J. S., Nessim L. A., Wilson S. G., Strain-dependent effects of supraspinal orphanin FQ/nociceptin on thermal nociceptive sensitivity in mice. Neurosci. Lett. 261, 147–150 (1999). [DOI] [PubMed] [Google Scholar]
- 46.Stein C., Schäfer M., Machelska H., Attacking pain at its source: New perspectives on opioids. Nat. Med. 9, 1003–1008 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Jiang Y. L., et al., Analgesic roles of peripheral intrinsic met-enkephalin and dynorphin A in long-lasting inflammatory pain induced by complete Freund’s adjuvant in rats. Exp. Ther. Med. 9, 2344–2348 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hurley R. W., Hammond D. L., Contribution of endogenous enkephalins to the enhanced analgesic effects of supraspinal mu opioid receptor agonists after inflammatory injury. J. Neurosci. 21, 2536–2545 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corder G., et al., Constitutive μ-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science 341, 1394–1399 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cesselin F., et al., Spontaneous and evoked release of methionine-enkephalin-like material from the rat spinal cord in vivo. Brain Res. 339, 305–313 (1985). [DOI] [PubMed] [Google Scholar]
- 51.Kita A., et al., Antinociceptive and antidepressant-like profiles of BL-2401, a novel enkephalinase inhibitor, in mice and rats. Jpn. J. Pharmacol. 75, 337–346 (1997). [DOI] [PubMed] [Google Scholar]
- 52.Costentin J., et al., Dissociated effects of inhibitors of enkephalin-metabolising peptidases or naloxone on various nociceptive responses. Eur. J. Pharmacol. 123, 37–44 (1986). [DOI] [PubMed] [Google Scholar]
- 53.Labuz D., Mousa S. A., Schäfer M., Stein C., Machelska H., Relative contribution of peripheral versus central opioid receptors to antinociception. Brain Res. 1160, 30–38 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez-Gaztelumendi A., Spahn V., Labuz D., Machelska H., Stein C., Analgesic effects of a novel pH-dependent μ-opioid receptor agonist in models of neuropathic and abdominal pain. Pain 159, 2277–2284 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller L. L., Picker M. J., Schmidt K. T., Dykstra L. A., Effects of morphine on pain-elicited and pain-suppressed behavior in CB1 knockout and wildtype mice. Psychopharmacology (Berl.) 215, 455–465 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kandasamy R., Calsbeek J. J., Morgan M. M., Analysis of inflammation-induced depression of home cage wheel running in rats reveals the difference between opioid antinociception and restoration of function. Behav. Brain Res. 317, 502–507 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patti C. L., et al., Behavioral characterization of morphine effects on motor activity in mice. Pharmacol. Biochem. Behav. 81, 923–927 (2005). [DOI] [PubMed] [Google Scholar]
- 58.Murphy N. P., Lam H. A., Maidment N. T., A comparison of morphine-induced locomotor activity and mesolimbic dopamine release in C57BL6, 129Sv and DBA2 mice. J. Neurochem. 79, 626–635 (2001). [DOI] [PubMed] [Google Scholar]
- 59.Solecki W., Turek A., Kubik J., Przewlocki R., Motivational effects of opiates in conditioned place preference and aversion paradigm–A study in three inbred strains of mice. Psychopharmacology (Berl.) 207, 245–255 (2009). [DOI] [PubMed] [Google Scholar]
- 60.Dockstader C. L., van der Kooy D., Mouse strain differences in opiate reward learning are explained by differences in anxiety, not reward or learning. J. Neurosci. 21, 9077–9081 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eippert F., et al., Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63, 533–543 (2009). [DOI] [PubMed] [Google Scholar]
- 62.Zubieta J. K., et al., Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J. Neurosci. 25, 7754–7762 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wager T. D., Scott D. J., Zubieta J. K., Placebo effects on human mu-opioid activity during pain. Proc. Natl. Acad. Sci. U.S.A. 104, 11056–11061 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lipman J. J., et al., Peak B endorphin concentration in cerebrospinal fluid: Reduced in chronic pain patients and increased during the placebo response. Psychopharmacology (Berl.) 102, 112–116 (1990). [DOI] [PubMed] [Google Scholar]
- 65.Mayer D. J., Price D. D., Rafii A., Antagonism of acupuncture analgesia in man by the narcotic antagonist naloxone. Brain Res. 121, 368–372 (1977). [DOI] [PubMed] [Google Scholar]
- 66.Dougherty D. D., et al., A combined [11C]diprenorphine PET study and fMRI study of acupuncture analgesia. Behav. Brain Res. 193, 63–68 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harris R. E., et al., Traditional Chinese acupuncture and placebo (sham) acupuncture are differentiated by their effects on mu-opioid receptors (MORs). Neuroimage 47, 1077–1085 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han J. S., Acupuncture analgesia: Areas of consensus and controversy. Pain 152 (suppl.3), S41–S48 (2011). [DOI] [PubMed] [Google Scholar]
- 69.Ernst E., Re: Han J-S. Acupuncture analgesia: Areas of consensus and controversy. Pain 2011; 152(3S): S41-8. Pain 152, 1439 (2011). [DOI] [PubMed] [Google Scholar]
- 70.Livingston K. E., Mahoney J. P., Manglik A., Sunahara R. K., Traynor J. R., Measuring ligand efficacy at the mu-opioid receptor using a conformational biosensor. eLife 7, e32499 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raehal K. M., Walker J. K., Bohn L. M., Morphine side effects in beta-arrestin 2 knockout mice. J. Pharmacol. Exp. Ther. 314, 1195–1201 (2005). [DOI] [PubMed] [Google Scholar]
- 72.Kliewer A., et al., Morphine-induced respiratory depression is independent of β-arrestin2 signalling. Br. J. Pharmacol. 177, 2923–2931 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmid C. L., et al., Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell 171, 1165–1175.e13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DeWire S. M., et al., A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J. Pharmacol. Exp. Ther. 344, 708–717 (2013). [DOI] [PubMed] [Google Scholar]
- 75.Stoeber M., et al., A genetically encoded biosensor reveals location bias of opioid drug action. Neuron 98, 963–976.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.National Research Council , Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, ed. 8, 2011).
- 77.Traynor J. R., Nahorski S. R., Modulation by mu-opioid agonists of guanosine-5′-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol. Pharmacol. 47, 848–854 (1995). [DOI] [PubMed] [Google Scholar]
- 78.Lamberts J. T., et al., Differential control of opioid antinociception to thermal stimuli in a knock-in mouse expressing regulator of G-protein signaling-insensitive Gαo protein. J. Neurosci. 33, 4369–4377 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lamberts J. T., Jutkiewicz E. M., Mortensen R. M., Traynor J. R., μ-Opioid receptor coupling to Gα(o) plays an important role in opioid antinociception. Neuropsychopharmacology 36, 2041–2053 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chaplan S. R., Bach F. W., Pogrel J. W., Chung J. M., Yaksh T. L., Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 (1994). [DOI] [PubMed] [Google Scholar]
- 81.Cirino T. J., et al., Characterization of sigma 1 receptor antagonist CM-304 and its analog, AZ-66: Novel therapeutics against allodynia and induced pain. Front. Pharmacol. 10, 678 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.